Abstract
Background
Blocking of TREM-1 signaling improves survival of mice with sepsis induced by Pseudomonas aeruginosa. However, whether TREM-1 blockade has beneficial effects in polymicrobial sepsis is poorly understood. Here, we aimed to investigate the effect of modulation of the TREM-1 pathway in rats with polymicrobial sepsis induced by cecal ligation and puncture (CLP).
Material/Methods
Normal Sprague-Dawley (SD) rats with sepsis induced by CLP were allocated randomly to received scramble peptide or LP17 via the jugular vein. Serum level of sTREM-1, IL6, TNF-α, and IL-1β were detected by ELISA assay. The mRNA and protein levels of JAK2 and STAT3 were detected by real-time PCR and Western blot analysis.
Results
STREM-1 concentration was greatly and progressively increased in rats with CLP-induced sepsis, and the increase was attenuated by TREM-1 inhibitory peptide LP17. More than 60% survival was observed in rats at the experiment endpoint after LP17 treatment. TREM-1 blockade also attenuated the increased level of pro-inflammatory cytokines TNF-α, IL-6, and IL-1β, and thus attenuated systematic and distant inflammatory responses. Furthermore, TREM-1 blockade significantly attenuated the increased levels of pJAK2 and pSTAT3.
Conclusions
TREM-1 blockade by the use of an inhibitory peptide LP17 could prolong survival of rats with polymicrobial sepsis and attenuate systematic inflammatory responses through the JAK2/STAT3 signaling pathway. Our results suggest that modulation of TREM-1 by a synthetic peptide might be a potential therapeutic option for polymicrobial sepsis.
MeSH Keywords: Cytokines; Inflammation; Intracellular Signaling Peptides and Proteins; Proteinase Inhibitory Proteins, Secretory; Sepsis
Background
Sepsis is a leading cause of death in intensive care units (ICU), with life-threatening infectious complications [1,2]. Rapid progression to circulatory collapse, multi-organ failure, and eventually causing death often caused by late diagnosis and treatment of sepsis [3]. Sepsis is a collection of disorders associated with infection by bacteria, viruses, or fungi. It often leads to an overwhelming response of innate inflammation [4], which results in a release of pro-inflammatory factors such as IL-1, IL-6, and TNF-α [5].
The triggering receptors expressed on myeloid cells (TREM)-1 is an additional family of innate immune receptors, which is mainly expressed on murine and human granulocytes and monocyte/macrophages. TREM-1 plays a vital role in the triggering of inflammatory responses [4,6–8]. It can induce cytokine production through binding with its adaptor molecule TYRO protein tyrosine kinase-binding protein (TYROBP) [7,9,10]. In addition, TREM-1 can also synergize with various Toll-like receptors (TLRs), causing amplified inflammatory responses [5,7,9,10]. It is generally accepted that TREM-1 upregulation is specifically associated with an anti-microbial immune response, whereas TREM-1 is not increased in conditions with noninfectious inflammatory disorders such as vasculitis or auriasis [6,11]. Soluble TREM-1 has been reported to be upregulated in patients with septic shock, and may serve as a diagnostic marker in patients with sepsis. Moreover, clinical studies revealed that it can also reflect the severity of sepsis and predict prognosis of patients [12–15]. Elevation of TREM-1 expression has been observed in pneumonia, mesenteric ischemia-reperfusion, and chronic obstructive nephropathy in murine models [16–18]. In addition, it has been reported that blocking TREM-1 signaling can prolong the survival of mice with sepsis induced solely by Pseudomonas aeruginosa [19]. However, less is known about the role of TREM-1 blockade in polymicrobial sepsis. A murine model with sepsis induced by cecal ligation and puncture (CLP) is more representative of clinical features of sepsis. Thus, we used this model to evaluate the effect of TREM-1 blockade.
TREM-1 has been reported to lack known signaling motifs and its natural ligand is currently unknown. It has been reported that TREM-1 can trigger the Janus kinase 2 (JAK2) signaling pathway, and thus leads to the phosphorylation of signal transducers of activation of transcription 3 (STAT3) [20]. The transcription factors upregulate the relative genes involved in the inflammatory response. Sepsis is present when there is clinical evidence of systemic inflammation, and the most common site of infection is the lungs, accounting for 40% of infection [21,22]. Sepsis has been also reported to be able to induce acute kidney injury from experimental and clinical data, where the degree of inflammation and/or the tolerance to inflammation play vital roles [23,24]. Thus, in order to further elucidate the possible mechanism underlying the effect of TREM-1 blockade, we further evaluate the expression level of JAK2, STAT3, pJAK2, and pSTAT3 in the kidneys and lungs.
Material and Methods
Animals
Adult male Sprague-Dawley rats (250–300 g) were purchased from SPF Laboratory Biotechnology, Inc. (Beijing, China). Rats had free access to food and water. The animals were housed and handled according to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication Number 80-23) and the study protocol was approved by the Ethics Committee of Tianjin First Central Hospital.
TREM-1 inhibitors
LP17, which spanned the complementary region loop 3 of TREM-1, was synthesized by Ribobio, LTD (Guangzhou, China) as previously described [25]. The sequence of the LP17 was LQVTDSGLYRCVIYHPP [25]. The purity of LP17 (99%) was determined by mass spectrometry (MS) and reversed-phase high-performance liquid chromatography (RP-HPLC). A scrambled peptide, consisting of the same amino acids but in an absolutely different sequence, was synthesized and acted as a control peptide (Ribobio, LTD).
Cecal ligation and puncture model and experimental design
Polymicrobial sepsis was induced using the cecal ligation and puncture (CLP) method as described previously. Briefly, rats were anesthetized with intraperitoneal injection of 40 mg/kg thiopental [26]. A small midline incision was made to expose the cecum through the skin and peritoneum under aseptic conditions. Approximately 1.5 cm of the cecal appendage was ligated midway between the cecal base and the distal pole, using 4/0 surgical silk. Then, double punctures were made using an 18-gauge needle and expelling a small amount of feces. After cecum repositioning and skin closure, resuscitation was achieved through subcutaneous injection of 1 ml pre-warmed saline (0.9% saline) [27].
Normal SD Rats receiving 0.1 ml saline (1 mg in 0.1 ml of saline) served as the normal control group (NC). Rats with sepsis induced by CLP were allocated randomly to received scramble peptide (3.5 mg/kg; CLP-Scrambled) or LP17 (3.5 mg/kg; CLP-LP17) via the jugular vein. At each time point of 12, 24, and 48 h, 8 rats were killed for the detection of sTREM-1, TNF-α, IL-6 and IL-1β, JAK2, and STAT3.
Enzyme-linked immunosorbent assay (ELISA) for sTREM-1, TNF-α, IL-6, and IL-1β
Blood specimens (2 ml) were obtained and centrifuged at 3000 rpm for 10 min. The supernatant was collected and stored at −80°C for further use. The levels of sTREM-1, TNF-α, IL-6, and IL-1β in serum were determined by ELISA using commercial kits, following the manufacturers’ protocol. The human sTREM-1 kit was obtained from Abcam (Cambridge, UK). TNF-α, IL-6, and IL-1β were purchased from eBioscience (R&D Systems).
Real-time PCR
Total RNA was extracted from rat lung and kidney tissues using TRIzol regent (Invitrogen). Reverse transcription was carried out and obtained cDNA was used as the template for real-time PCR. Quantitative real-time PCR was performed based on SYBR Green I (Power SYBR Green Master Mix, Roche, IN, USA). GAPDH was used as an internal control to normalize for the RNA abundance. Relative expression levels of the gene was calculated using the 2−ΔΔCt method after normalization with the GAPDH gene [28]. The experiments were repeated in triplicate.
Western blot analysis
Western blot analysis was performed as previously described [20,28]. The primary antibodies used for Western blot were rabbit-anti-rat JAK2 monoclonal antibody (Cell Signaling Technology, Inc.), rabbit-anti-rat phospho-JAK2 monoclonal antibody (Cell Signaling Technology, Inc.), rabbit-anti-rat STAT3 monoclonal antibody (Cell Signaling Technology, Inc.), rabbit-anti-rat phospho-STAT3 monoclonal antibody (Cell Signaling Technology, Inc.), and rabbit-anti-rat β-actin polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Statistical analysis
Results are expressed as mean ±SD (standard deviation). Data were recorded and assessed using SPSS 19.0 (Chicago, IL). Statistical significance was calculated by one-way ANOVA analysis followed by the LSD post hoc test. The probability value p<0.05 was considered as a significant difference.
Results
LP17 attenuates the increased level of soluble TREM-1 in rats with sepsis
We found that the concentration of sTREM-1 was progressively and greatly increased in rats with CLP-induced sepsis, (Figure 1). Moreover, a significant attenuation of sTREM-1 concentration was observed after the administration of LP17 (Figure 1). Our results suggest that LP17 attenuates the increased level of sTREM-1 in rats with sepsis induced by CLP.
Figure 1.
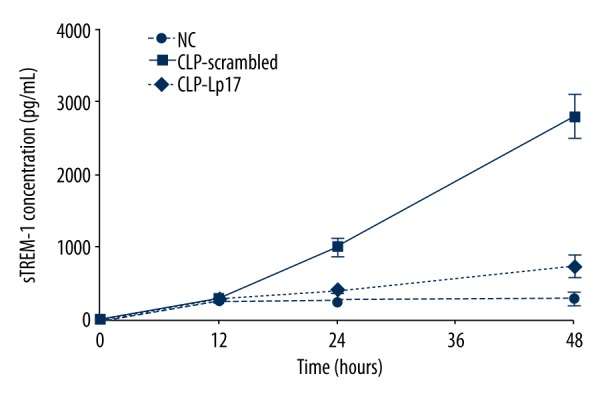
LP-17 decreased the concentration of triggering receptor expressed on soluble myeloid cells (sTREM-1). Serum sTREM-1 was detected 12, 24, and 48 h after the first administration of saline, scrambled peptides, or LP17. NC, normal rats receiving saline; CLP-Scrambled peptides, rats with sepsis receiving scrambled peptide; CLP-LP17, rats with sepsis receiving LP17.
LP17 prolongs survival of rats with sepsis induced by CLP
Considering that TREM-1 was highly expressed during microbial inflammations [6], we wanted to test whether TREM-1 blockade had an effect on the survival of rats with CLP-induced sepsis. As shown in Figure 2, after administration of LP17, more than 60% survival was observed in rats at the experiment endpoint, but none of the rats survived after the treatment with scrambled peptide. Our results indicate that LP17 significantly prolonged the survival of rats with CLP-induced sepsis and might serve as a potential therapeutic option for sepsis.
Figure 2.
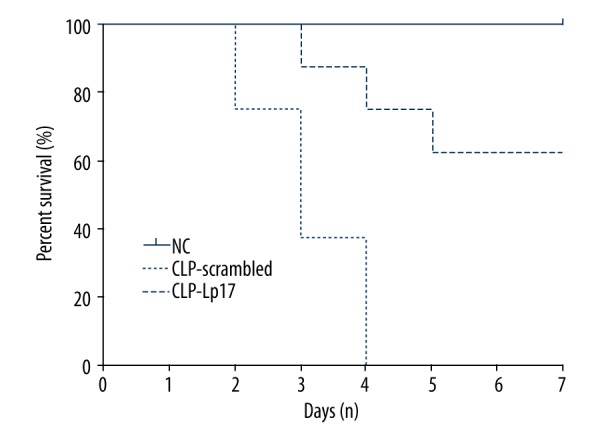
LP17 prolongs survival of rat with sepsis induced by CLP. Sprague-Dawley rats with sepsis induced by CLP were treated with scrambled peptides (CLP-Scrambled) or LP17 (CLP-LP17). Normal SD rats receiving saline served as normal controls (NC). There were 8 rats in each group.
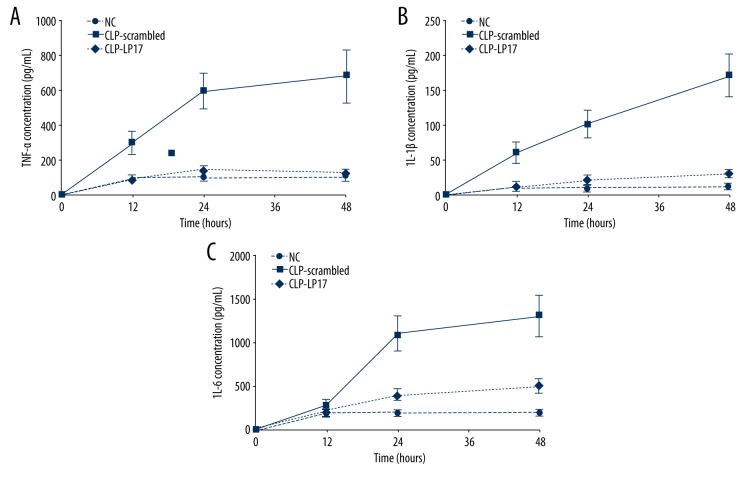
LP17 attenuates systematic inflammatory responses
The concentration of TNF-α, IL-6, and IL-1β were measured in the serum at time points of 12 h, 24 h, and 48 h after the first treatment. As compared with the normal rats receiving saline, TNF-α, IL-6, and IL-1β were all significantly increased in rats with sepsis (Figure 3). Moreover, serum concentrations of all these 3 cytokines were all strikingly attenuated after LP17 administration as compared with scrambled peptide. No significant difference was found between the CLP-LP17 group and normal control group (Figure 3). These results indicate that LP17 administration attenuates the increase of TNF-α, IL-6 and IL-1β, and thus attenuates systematic and distant inflammatory responses.
Figure 3.
LP17 attenuates systematic inflammatory response. The concentration of TNF-α (A), IL-6 (B), and IL-1β (C) in serum was determined by ELISA assay in triplicate 12, 24, and 48 h after the first administration of saline, scrambled, or LP17. There were 8 rats in each group. NC, normal rats receiving saline; CLP-Scrambled peptides, rats with sepsis receiving scrambled peptide; CLP-LP17, rats with sepsis receiving LP17.
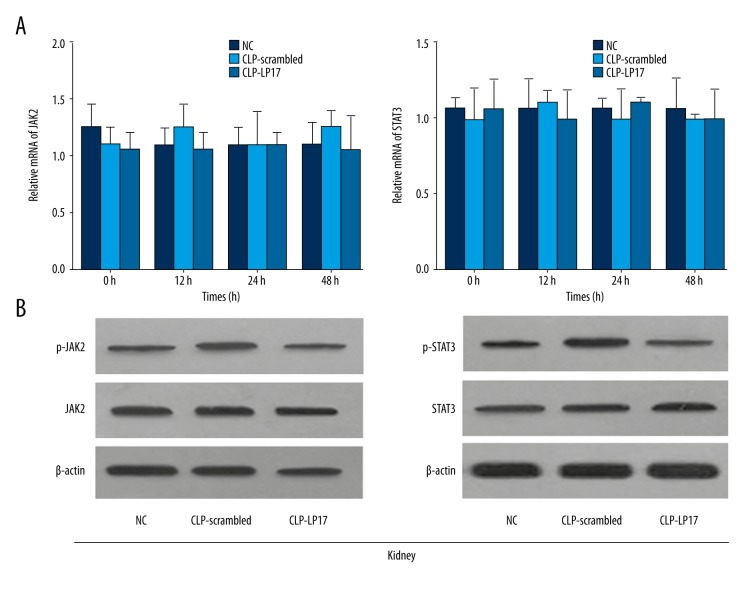
LP17 attenuated the increased level of pJAK2 and pSTAT3
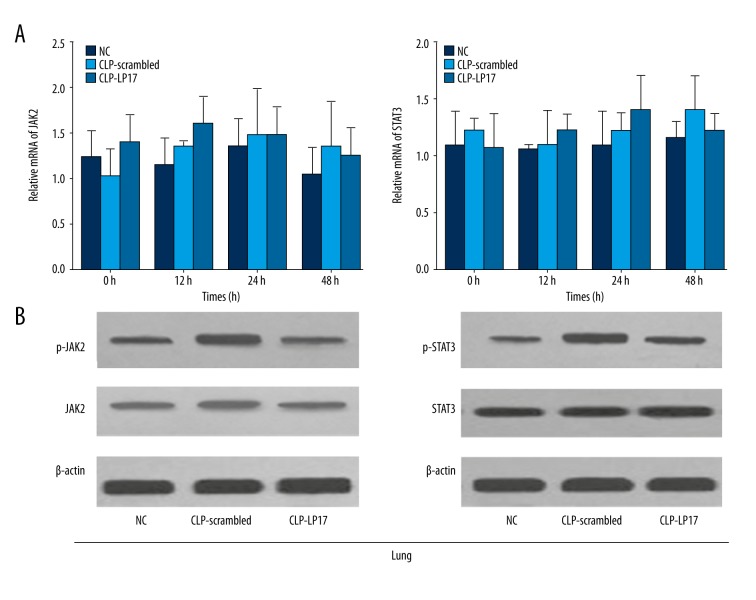
To elucidate the underlining mechanism, we further evaluated the expression level of JAK2, STAT3, pJAK2, and pSTAT3 in kidney and lung. Our results showed no significant changes in the mRNA and protein levels of JAK2 and STAT3 in kidney after LP17 administration and the scrambled peptide (Figure 4). However, the increased levels of pJAK2 and pSTAT3 were significantly attenuated by the administration of LP17 (Figure 4). Similar results were obtained in the lung (Figure 5). These results indicate that LP17 may prolong survival of rat and attenuate systematic inflammatory responses through the JAK2/STAT3 signaling pathway.
Figure 4.
LP17 attenuated the increased level of pJAK2 and pSTAT3 in kidney. (A) Relative mRNA of JAK2 (left) and STAT3 (right) were detected by real-time PCR at the time point of 0 h, 12 h, 24 h, and 48 h. (B) The protein levels of JAK2/pJAK2 (left) and STAT3/pSTAT3 (right) were measured by Western blotting 48 h after first administration of saline, scrambled peptide, or LP17. NC, normal rats receiving saline; CLP-Scrambled peptides, rats with sepsis receiving scrambled peptide; CLP-LP17, rats with sepsis receiving LP17.
Figure 5.
LP17 attenuated the increased level of pJAK2 and pSTAT3 in lung. (A) Relative mRNA of JAK2 (left) and STAT3 (right) were detected by real-time PCR at the time points of 0 h, 12 h, 24 h, and 48 h. (B) The protein levels of JAK2/pJAK2 (left), STAT3/pSTAT3 (right) were measured by Western blotting 48 h after first administration of saline, scrambled peptide, or LP17. NC, normal rats receiving saline; CLP-Scrambled peptides, rats with sepsis receiving scrambled peptide; CLP-LP17, rats with sepsis receiving LP17.
Discussion
Soluble TREM-1 (sTREM-1) has been considered to be a sensitive and specific marker of infection, and elevated levels of sTREM-1 have been reported in critically ill patients with sepsis [2,4,6,8,10,13], as well as in murine models of sepsis [19]. Our study revealed that sTREM-1 concentration was greatly increased in rats with CLP-induced spesis, and TREM-1 inhibitory peptide (LP17) can significantly attenuate the elevated sTREM-1 concentration and prolong survival of rats. It has been widely reported TREM-1 is highly expressed during microbial inflammations [4,6,19]. Wang et al. reported that the TREM-1 expression was found on blood neutrophils and peritoneal macrophages in mice with sepsis induced solely by a strain of gram-negative bacteria (Pseudomonas aeruginosa) [19]. They also found that the proportion of TREM-1-positive cells was increased [19]. Many studies also indicated that increased protien concentrations of circulating sTREM-1 are detected in plasm during infection and inflammation [29–31]. In our study, we evaluated the concentration of soluble TREM-1 in rats with polymicrobial sepsis induced by CLP. Our results showed a remarkble and progressive increase of sTREM-1 concentration in rats with CLP-induced sepsis. Moreover, the increase was attenuated by LP17, a TREM-1 inhibitor spanning the complementary region loop 3 of TREM-1. Our results confirmed upregulation of soluble TREM-1 in polymicrobibial spesis, and the increase of soluble TREM-1 could also be atteneuated by its specific inhibitor LP17.
It has been reported that blockade of TREM-1 signaling can prolong survival of mice with LPS-induced septic shock [6]. Wang et al. also revealed that TREM-1 blockade protects the animals from death in a murine model of P. aeruginosa-induced spesis [19]. However, less is known about whether TREM-1 blockade has any beneficial effects in sepsis with polymicrobial infection, which is closer to the clinical features of sepsis. In the present study, we tested the effect of TREM-1 blockade under the condition of polymicrobial infection in a murine model of CLP-induced sepsis. Our results showed that more than 60% survival was observed in rats at the experiment endpoint after administration of LP17, but none of the rats survived after treatment with scrambled peptide. Our results partially agree with those from Wang et al., who observed 80% survival in mice with P. aeruginosa-induced spesis treated with TREM-1 Fc fusion protein [19].
It has been reported that LP17 successfully attenuates the inflammatory responses in a variety of diseases such as hemorrhagic shock, autoimmune arthritis, and mesenteric IR-induced injury [17,18,32–37]. Similar results were obtained in our study. Given that TREM-1 can trigger inflammatory responses, and that TREM-1 inhibitors may prevent pathology and mortality caused by excessive inflammations, we further investigated whether TREM-1 blockade through inhibitory peptide LP17 could attenuate the inflammatory response. Serum concentrations of pro-inflammatory factors such as TNF-α, IL-6, and IL-1β were all strikingly attenuated after LP17 treatment as compared with the administrations of scrambled peptide.
Previous reports imply that the activation of JAK/STAT signaling pathway could regulate inflammatory responses and inhibits the level of pro-inflammatory factors, including cytokines and chemokines [38–40]. It has also been reported that TREM-1 activation triggers the JAK2 pathway, thus leading to the phosphorylation of STAT3 [20]. The transcription factors upregulate the relative genes involved in the inflammatory response. To further study the potential underlying mechanism of the decrease of pro-inflammatory factors by LP17, we evaluated the mRNA and protein levels of JAK2 and STAT3 in 2 main organs, the kidneys and lungs. In aggreement with previous reports, the increased levels of pJAK2 and pSTAT3 were significantly attenuated by the administration of LP17. These results indicate that LP17 may prolong survival of rats and attenuate systematic inflammatory responses through inhibition of the JAK2/STAT3 signaling pathway.
Conclusions
In conclusion, TREM-1 blockade by the use of an inhibitory peptide LP17 could prolong survival of rat and attenuate systematic inflammatory responses through the JAK2/STAT3 signaling pathway. Our results suggest that modulation of TREM-1 by a synthetic peptide might be a potential therapeutic option for polymicrobial sepsis. However, it should be noted that we only detected the effect of LP17 in rats. Further experiments with larger animals should be carried out to evaluate the role of TREM-1 blockade in sepsis.
Footnotes
Source of support: Departmental sources
Confict of interest
The authors declare no conflict of interest.
References
- 1.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–29. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Wax RS. Epidemiology of sepsis: An update. Crit Care Med. 2001;29(7 Suppl):S109–16. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 3.Warren HS. Strategies for the treatment of sepsis. New Engl J Med. 1997;336(13):952–53. doi: 10.1056/NEJM199703273361311. [DOI] [PubMed] [Google Scholar]
- 4.Bouchon A, Dietrich J, Colonna M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164(10):4991–95. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 5.Bleharski JR, Kiessler V, Buonsanti C, et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. 2003;170(7):3812–18. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- 6.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410(6832):1103–7. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 7.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3(6):445–53. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- 8.Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): A new player in acute inflammatory responses. J Infect Dis. 2003;187(Suppl 2):S397–401. doi: 10.1086/374754. [DOI] [PubMed] [Google Scholar]
- 9.Sharif O, Knapp S. From expression to signaling: Roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008;213(9–10):701–13. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Arts RJ, Joosten LA, van der Meer JW, Netea MG. TREM-1: Intracellular signaling pathways and interaction with pattern recognition receptors. J Leukoc Biol. 2013;93(2):209–15. doi: 10.1189/jlb.0312145. [DOI] [PubMed] [Google Scholar]
- 11.Weber B, Schuster S, Zysset D, et al. TREM-1 deficiency can attenuate disease severity without affecting pathogen clearance. PLoS Pathog. 2014;10(1):e1003900. doi: 10.1371/journal.ppat.1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Wang H, Liu J, et al. Serum soluble triggering receptor expressed on myeloid cells-1 and procalcitonin can reflect sepsis severity and predict prognosis: A prospective cohort study. Mediators Inflamm. 2014;2014:641039. doi: 10.1155/2014/641039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Zhu Z, Chen J, et al. Diagnostic value of soluble triggering receptor expressed on myeloid cells-1 in critically-ill, postoperative patients with suspected sepsis. Am J Med Sci. 2013;345(3):178–84. doi: 10.1097/MAJ.0b013e318253a1a6. [DOI] [PubMed] [Google Scholar]
- 14.Halim B, Ozlem T, Melek C, et al. Diagnostic and prognostic value of procalcitonin and sTREM-1 levels in sepsis. Turk J Med Sci. 2015;45(3):578–86. [PubMed] [Google Scholar]
- 15.Brenner T, Uhle F, Fleming T, et al. Soluble TREM-1 as a diagnostic and prognostic biomarker in patients with septic shock: An observational clinical study. Biomarkers. 2017;22(1):63–69. doi: 10.1080/1354750X.2016.1204005. [DOI] [PubMed] [Google Scholar]
- 16.Tammaro A, Kers J, Emal D, et al. Effect of TREM-1 blockade and single nucleotide variants in experimental renal injury and kidney transplantation. Sci Rep. 2016;6:38275. doi: 10.1038/srep38275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibot S, Massin F, Alauzet C, et al. Effects of the TREM-1 pathway modulation during mesenteric ischemia-reperfusion in rats. Crit Care Med. 2008;36(2):504–10. doi: 10.1097/01.CCM.0B013E318161FAF3. [DOI] [PubMed] [Google Scholar]
- 18.Gibot S, Alauzet C, Massin F, et al. Modulation of the triggering receptor expressed on myeloid cells-1 pathway during pneumonia in rats. J Infect Dis. 2006;194(7):975–83. doi: 10.1086/506950. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Liu S, Wu S, et al. Blocking TREM-1 signaling prolongs survival of mice with Pseudomonas aeruginosa induced sepsis. Cell Immunol. 2012;272(2):251–58. doi: 10.1016/j.cellimm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Fan D, He X, Bian Y, et al. Triptolide modulates TREM-1 signal pathway to inhibit the inflammatory response in rheumatoid arthritis. Int J Mol Sci. 2016;17(4):498. doi: 10.3390/ijms17040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: The CUB-Rea Network. Am J Respir Crit Care Med. 2003;168(2):165–72. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- 22.Minasyan H. Sepsis and septic shock: Pathogenesis and treatment perspectives. J Crit Care. 2017;40:229–42. doi: 10.1016/j.jcrc.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Angeli P, Tonon M, Pilutti C, et al. Sepsis-induced acute kidney injury in patients with cirrhosis. Hepatol Int. 2016;10(1):115–23. doi: 10.1007/s12072-015-9641-1. [DOI] [PubMed] [Google Scholar]
- 24.Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibot S, Kolopp-Sarda MN, Bene MC, et al. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200(11):1419–26. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferro MM, Angelucci ME, Anselmo-Franci JA, et al. Neuroprotective effect of ketamine/xylazine on two rat models of Parkinson’s disease. Braz J Med Biol Res. 2007;40(1):89–96. doi: 10.1590/s0100-879x2007000100012. [DOI] [PubMed] [Google Scholar]
- 27.Walker J, Dichter E, Lacorte G, et al. Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock. 2011;36(4):410–16. doi: 10.1097/SHK.0b013e31822798c1. [DOI] [PubMed] [Google Scholar]
- 28.Truong AD, Hong Y, Thanh Hoang C, et al. Chicken IL-26 regulates immune responses through the JAK/STAT and NF-kappaB signaling pathways. Dev Comp Immunol. 2017;73:10–20. doi: 10.1016/j.dci.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Gibot S, Cravoisy A, Levy B, et al. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. New Engl J Med. 2004;350(5):451–58. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 30.Gibot S, Kolopp-Sarda MN, Bene MC, et al. Plasma level of a triggering receptor expressed on myeloid cells-1: Its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med. 2004;141(1):9–15. doi: 10.7326/0003-4819-141-1-200407060-00009. [DOI] [PubMed] [Google Scholar]
- 31.Tzivras M, Koussoulas V, Giamarellos-Bourboulis EJ, et al. Role of soluble triggering receptor expressed on myeloid cells in inflammatory bowel disease. World J Gastroenterol. 2006;12(21):3416–19. doi: 10.3748/wjg.v12.i21.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiechl G, Brunner SM, Kesselring R, et al. Inhibition of innate co-receptor TREM-1 signaling reduces CD4(+) T cell activation and prolongs cardiac allograft survival. Am J Transplant. 2013;13(5):1168–80. doi: 10.1111/ajt.12186. [DOI] [PubMed] [Google Scholar]
- 33.Kamei K, Yasuda T, Ueda T, et al. Role of triggering receptor expressed on myeloid cells-1 in experimental severe acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17(3):305–12. doi: 10.1007/s00534-009-0191-6. [DOI] [PubMed] [Google Scholar]
- 34.Wiersinga WJ, Veer C, Wieland CW, et al. Expression profile and function of triggering receptor expressed on myeloid cells-1 during melioidosis. J Infect Dis. 2007;196(11):1707–16. doi: 10.1086/522141. [DOI] [PubMed] [Google Scholar]
- 35.Gibot S, Massin F, Alauzet C, et al. Effects of the TREM 1 pathway modulation during hemorrhagic shock in rats. Shock. 2009;32(6):633–37. doi: 10.1097/SHK.0b013e3181a53842. [DOI] [PubMed] [Google Scholar]
- 36.Schenk M, Bouchon A, Seibold F, Mueller C. TREM-1 – expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest. 2007;117(10):3097–106. doi: 10.1172/JCI30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami Y, Akahoshi T, Aoki N, et al. Intervention of an inflammation amplifier, triggering receptor expressed on myeloid cells 1, for treatment of autoimmune arthritis. Arthritis Rheum. 2009;60(6):1615–23. doi: 10.1002/art.24554. [DOI] [PubMed] [Google Scholar]
- 38.Darnell JE., Jr STATs and gene regulation. Science. 1997;277(5332):1630–35. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 39.Fahmy RG, Waldman A, Zhang G, et al. Suppression of vascular permeability and inflammation by targeting of the transcription factor c-Jun. Nat Biotechnol. 2006;24(7):856–63. doi: 10.1038/nbt1225. [DOI] [PubMed] [Google Scholar]
- 40.Takeda K, Clausen BE, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10(1):39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]