Abstract
Identifying early life-stage transitions limiting seagrass recruitment could improve our ability to target demographic processes most responsive to management. Here we determine the magnitude of life-stage transitions along gradients in physical disturbance limiting seedling establishment for the marine angiosperm, Posidonia australis. Transition matrix models and sensitivity analyses were used to identify which transitions were critical for successful seedling establishment during the first year of seed recruitment and projection models were used to predict the most appropriate environments and seeding densities. Total survival probability of seedlings was low (0.001), however, transition probabilities between life-stages differed across the environmental gradients; seedling recruitment was affected by grazing and bioturbation prevailing during the first life-stage transition (1 month), and 4–6 months later during the third life-stage transition when establishing seedlings are physically removed by winter storms. Models projecting population growth from different starting seed densities showed that seeds could replace other more labour intensive and costly methods, such as transplanting adult shoots, if disturbances are moderated sufficiently and if large numbers of seed can be collected in sufficient quantity and delivered to restoration sites efficiently. These outcomes suggest that by improving management of early demographic processes, we could increase recruitment in restoration programs.
Introduction
Restoration of terrestrial vegetation has moved from transplanting or seeding target plant species to restoration of biodiverse plant communities, building demographic resilience and replacing ecological function1. This is not the case for restoration of the marine vascular plants, the seagrasses2. Recent reviews show that most seagrass restoration programs are little more than pilot-scale transplantation exercises where the resilience and recovery of the transplants are only followed for a short period and are mostly unsuccessful2–6. In addition, these restoration efforts come at a high cost, from $US33 000 ha−1 to more than $US3.3 million ha−1 depending on the project, species being transplanted and geographic region4. More importantly, the scale of transplanting (<10’s of ha; e.g.7) cannot hope to alleviate the massive scale of restoration compounded by the increasing rate of seagrass loss (100’s km2 per decade8). While seeding has recently emerged as an effective tool to restore large areas of degraded seagrass habitat for one species, Zostera marina in the USA (100’s–1000’s ha9), and at a reasonable cost (annual re-seeding $US10 000 ha−1 R. J. Orth Pers. Comm), the science behind seed-based approaches is largely underdeveloped for the majority of seagrass species and severely lags behind their terrestrial counterparts. Clearly it is time to move from this situation to applying terrestrial plant models of seed-based restoration to seagrasses.
Life-cycle population models have been valuable in the development of terrestrial restoration theory and practice. In particular these models allow for the quantification of early life-stage transitions from seed, to germinant, to emerged seedling, identifying which of these transitions are the most limiting in plant recruitment10. Armed with this knowledge restoration practitioners can target those life-stage transitions most responsive to management10,11. However, only recently have seagrass ecologists investigated the importance of early life-history elements for seed-based recruitment in seagrasses, challenging the paradigm that seed recruitment is of little consequence to the persistence, maintenance and recovery of seagrasses12–14. As a result, early life-history demographic studies are remarkably few among seagrass species. This is a crucial research gap given that our ability to restore and manage seagrass populations effectively is limited since we have little understanding of the demography of early life-history components.
Determining the magnitude of life-stage transitions limiting seedling establishment is the first step in understanding the demography of a plant species and the influence of the local environment on successful recruitment. Previous research on recruitment from seeds in seagrasses have found high mortalities between seed settlement and seedling establishment, where poor survival of seedlings was driven by physical disturbance associated with winter storms15. Similarly16, observed that losses of Zostera marina seedlings during winter in north-eastern USA were not related to poor germination rates (internal) but rather to physical forces (external) such as waves and currents disturbing sediments and removing seeds. Predation and herbivory also influence seedling establishment and their persistence. Seed predation can remove seeds from the recruitment pool17–19 and seedling defoliation from herbivory15 can reduce the photosynthetic area and depress plant growth and resilience.
For seed-based restoration programs utilizing one of the most widely distributed genera in Australia, Posidonia, initial population size is limited by a series of transitions from seeds to seedlings that initially are dependent on seed reserves, to seedlings independent of seed reserves then juveniles (>1 year old) to reproductive adults (Fig. 1). Although recruitment of a seed into the adult population depends on these early life-stage transitions20, few seagrass demographic models acknowledge these early life-history components (but see16). Instead current practices have quantified seed recruitment as the proportion of seeds that establish as seedlings, combining all the transitions a species undergoes into one (e.g.14). Without an understanding of the proportion of seeds transitioning through early life-stages leading up to seedling establishment, we cannot identify and manage the processes driving population dynamics (and recruitment failure) in restoration programs21.
Figure 1.
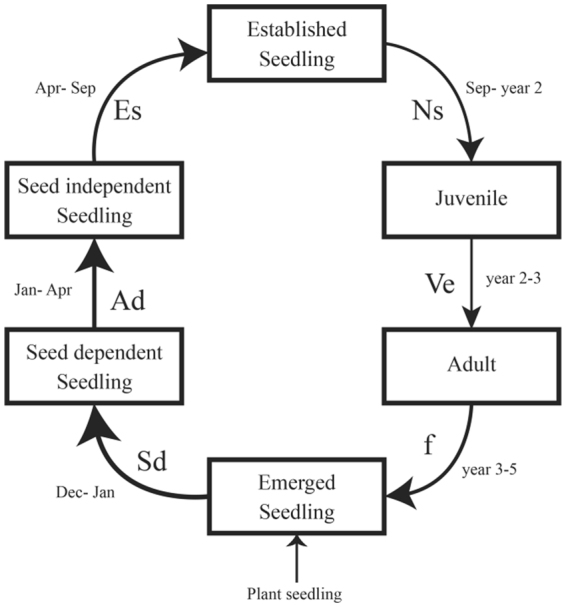
A life-cycle model describing the demographic stages (boxes) and transitions (arrows) Posidonia australis follows to adulthood and the months each transition spanned. The transitions included; Seed-dependency, when seedlings are highly dependent on seed reserves, Sd (Decemberyr1–Januaryyr1). Seedlings then undergo an extended period where they continue to draw nourishment from maternally-derived reserves but there is greater uptake and assimilation of resources from the environment due to production of photosynthetically active leaves and development of a small but functional root system; autonomous development, Ad (Januaryyr1–Aprilyr1). By the end of this period seedlings have exhausted the majority (~90%) of their seed reserves and are relatively independent of their seed. Seedlings then become fully integrated into their environment upon exhaustion of the seed reserves; seedling establishment, Es (Aprilyr1–Septemberyr1). Production of new shoots, Ns (Septemberyr1–year 2) typically occurs in the months following seedling establishment and seedlings become Juveniles. Juveniles transition into adults after plants undergo horizontal vegetative expansion, Ve (year 2–3). Adults typically become reproductively mature, f (fecundity), between years 3–5.
The genus Posidonia produces viviparous seeds that are released, settle and colonise sediments over a 2–3 month period in both the Northern (e.g. Posidonia oceanica 22), and Southern (e.g. Posidonia spp.23) hemispheres. Without the capacity to store seeds for later restoration as utilized in Zostera seed-based restoration24, Posidonia seeds need to be deployed immediately or grown ex situ in culture to transplantable seedlings (e.g. Posidonia australis 25,26). Previous seed-based restoration experiments have not been successful for Posidonia but the scale of seeding was limited to 100’s of seeds, the transplanting environment was inadequate and with little characterization of the physical disturbance regimes, and monitoring of recruitment success limited2,6. Here, we address these limitations by investigating the demographic consequences of seed-based restoration and the effect of seed density on survival for Posidonia australis in temperate Western Australia.
The specific objectives of this study were to determine through a large field-based seed restoration experiment: the mortality of early life-history transitions during establishment of seagrass seedlings within historically degraded sites, and; how they vary spatially along gradients in disturbance resulting from water motion, grazing and bioturbation. We then use a transition matrix model utilizing the outcomes of the experiment to investigate what life-stage transitions most limit seedling recruitment and population growth in the first year of seed recruitment, and; to make predictions from the matrix model as to the most appropriate environments and seeding densities required for seed-based restoration in the seagrass, Posidonia australis.
Results
Restoration locations and environmental characteristics
The locations varied in depth from ~2.4 m, recorded at the southern end (Southern Flats, SF) of the study area, to ~8.4 m (Parmelia Bank, PB), north of Cockburn Sound (Table S1, Fig. 2). There was a strong wave-exposure gradient, which was greatest in the west and north and least in the east and south. The shallow sites varied in exposure to wave action from protected in the south (SF), to exposed, in north-western locations, Carnac Island (CI) and Garden Island (GI). Owen Anchorage (OA) and Woodman Point (WP) had depths of ~4.5 m and were located close to the mainland at the northern end of the study area but had moderate exposure to waves. Deep locations also had contrasting exposure, the most exposed being Parmelia Bank (PB) and the least exposed was the eastern bank (~7.5 m) in Cockburn Sound (CS).
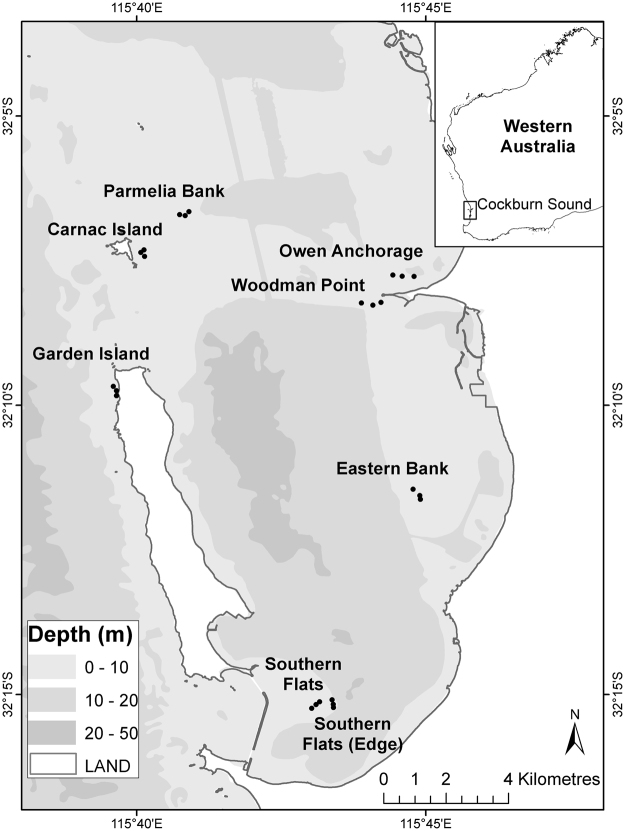
Figure 2.
Study area with position of all Locations and their Sites within bare sand in Cockburn Sound (Inset: Cockburn Sound, Western Australia). Figure created in ArcMap (ESRI 2011. ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute).
The sediment distribution across locations was normal, with mean grain size of medium sands (~0.3 mm) (Table S1). GI, CI and PB sediments were well-sorted while the southern and eastern locations were moderately sorted. Sites located at the southern end (SF) contained large pieces of biogenic material such as bivalves shells. The size distribution was symmetrical on all the sites but slightly skewed towards the coarse at WP. Organic matter content on surface sediments was ~2.7% (2.3–3.0%) of sediment dry weight, with some presence of seagrass matte (Table S1). The light availability on the sea-floor was simultaneously recorded at SF (shallow), OA (mid-depth) and PB (deep) (Table S1). SF recorded the highest daily light availability of ~10.6 mols photons m−2 d−1 (±1.96 mols photons m−2 d−1). There was a 70% decrease in light availability from shallow to deep, with a maximum of 3.28 mols photons m−2 d−1 recorded at PB (±0.91 mols photons m−2 d−1). Given that Posidonia seagrasses occupy this depth suggests that light availability is within a suitable range for this species across our study area.
During winter, when wave conditions were greater due to storm fronts moving from north west to south west, maximum significant wave height (Hsmax) recorded at the most exposed north-westerly site (PB) was 0.9 m (Table 1), with long periods from 8–25 s. Hsmax decreased by 45% at sites with medium exposure and 78% at the most sheltered site in southern Cockburn Sound (SF) (Table 1). In total ~6 large storm events were recorded over the two months of data collection during winter 2014. Storm events were defined as daily averaged Hsmax surpassing the 75th percentile. The longevity of these events varied from short extreme events to systems lasting up to ~3–4 days (Table 1).
Table 1.
Environmental wave conditions (significant wave height, Hs) from spectral analysis of the frequencies 8–25 s during Winter (Aug–Sep 2014).
| Location | Depth (m) | Hs (m) | HsMAX (m) | Hs 75th percentile (m) | Days > 75th percentile | Events > 75th Percentile |
|---|---|---|---|---|---|---|
| SF | 2.41 | 0.08 | 0.21 | 0.10 | 15 | 8 |
| CS | 7.48 | 0.14 | 0.28 | 0.17 | 14 | 6 |
| WP | 4.29 | 0.24 | 0.50 | 0.29 | 12 | 5 |
| OA | 4.55 | 0.19 | 0.47 | 0.23 | 13 | 6 |
| CI | 2.95 | 0.31 | 0.54 | 0.37 | 13 | 5 |
| PB | 8.43 | 0.46 | 0.92 | 0.56 | 13 | 6 |
Southern Flats (SF), Cockburn Sound east bank (CS), Woodman Point (WP), Owen Anchorage (OA), Carnac Island (CI), Parmelia Bank (PB).
In comparison to previous year’s storminess, our seeding year was a relatively harsh year with several storms arriving from the north, particularly in the month of July (Fig. S1). In 2010 and 2013, despite several storms originating in the southwest, these were considered relatively benign years in terms of storm activity arriving from the north (Fig. S1).
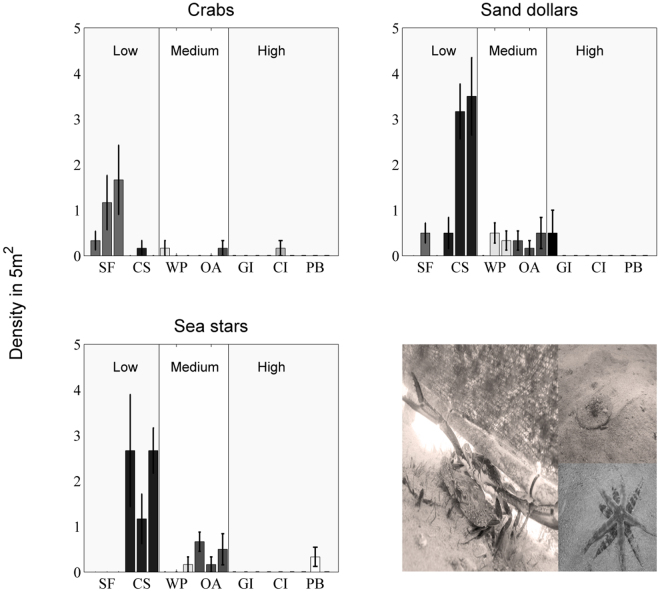
Benthic bioturbators and potential seed predators were more abundant in sheltered inshore sites than offshore wave-exposed sites. Blue-swimmer crabs (Portunus armatus), sand-dollars (Peronella lesueuri) and sand sea-stars (Archaster angulates, Fig. 3) were the most abundant benthic fauna at sheltered CS, SF and SFe, with densities of 1–4 individuals per 5 m−2 transect (Fig. 3). At moderate wave-exposed sites (WP & OA) there were lower densities of the bioturbators/grazers (<1 individuals per 5 m−2 transect), and few individuals were observed at offshore locations (GI, CI and PB).
Figure 3.
Faunal densities observed with 5 m2 (5 m × 1 m transect) at locations with low, medium and high wave-exposure. Columns are means (±1SE). Image on bottom right shows (clockwise); Blue swimmer crabs (male [top] and female [bottom]), sand-dollar and sand sea-star.
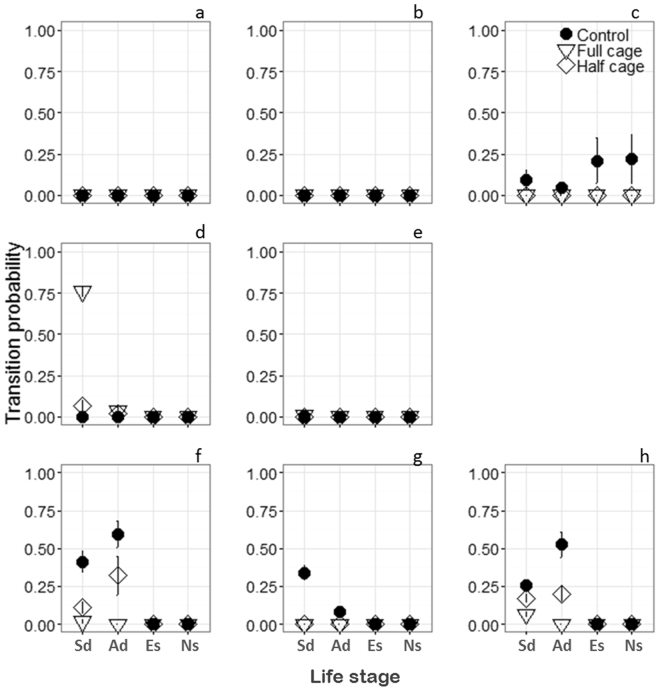
Life-stage transition probabilities at restoration locations
Across all locations the total survival probability of P. australis seedlings was low (0.001) with total survivorship driven by one location with low wave exposure and in deep water (CS, Fig. 4). Across low and moderately wave-exposed, shallow locations (SF, SFe, OA, and WP) seed-dependency (1 month, the life-stage where seedling growth is 100% dependent on seed reserves) was the transition with the lowest (100% loss) survival probability (p < 0.001, Fig. 4). In wave-exposed locations (CI, GI, PB) seedling survival was high during seed-dependency with transition probabilities of 0.25–0.41. The transition probability to seed-independent seedling was also high for CI and PB (0.50–0.60) but lower for GI (0.15), yet were not significantly different (p = 0.34). However, in wave-exposed locations during the seedling establishment life-stage, we recorded 100% losses of seedlings, resulting in no seedlings surviving past this life-stage transition for exposed locations.
Figure 4.
Life stage transition probabilities at the different Locations within Cockburn Sound; Southern Flats (a), Southern Flats edge (b), Cockburn Sound eastern bank (c), Owen Anchorage (d), Woodman Point (e), Carnac Island (f), Garden Island (g) and Parmelia Bank (h). Life-stage transitions - seed-dependency (Sd), autonomous development (Ad), seedling establishment (Es), survival to juveniles (Ns). Fauna exclusion cages were tested at all sites and included; full cage enclosures, half-cage enclosures (procedural control) and uncaged controls.
Limiting life-stage transitions at restoration locations
The use of transition matrices allowed us to assess the importance of the different life stages, and their effect on the stability of the population (λ). However, we are excluding clonal expansion from this analysis. This exclusion was purposely made to capture the effect of a population establishing specifically from seeds. For all the seedlings as a single population, we obtained a λ smaller than one (mean = 0.49, range = 0.2–0.7), meaning a decaying population. We obtained the greatest λ for the sheltered deep sites (λ = 0.71). The exposed scenarios on the other hand gave a λ of only 0.21, reflecting the general decline of the population across all the stages, with complete mortality during the establishment stage (Table 2).
Table 2.
population growth rate (λ), sensitivity (S) and elasticities (E) analyses for each life stage transition.
| λ | Sensitivities & Elasticities | |||||||
|---|---|---|---|---|---|---|---|---|
| Sd | Ad | Es | Ns | Ve | f | |||
| All sites as a single population | 0.49 | S= | 0.62 | 0.23 | 2.17 | 0.09 | 0.30 | 0.01 |
| E= | 0.18 | 0.18 | 0.18 | 0.18 | 0.12 | 0.18 | ||
| Low exposure; Shallow (SF, SFe) | 0.21 | S= | 8.24 | 2.29 | 0.00 | 0.00 | 0.91 | 0.00 |
| E= | 0.02 | 0.02 | 0.02 | 0.02 | 0.89 | 0.02 | ||
| Low exposure; Deep (CS) | 0.71 | S= | 1.37 | 0.68 | 0.15 | 0.13 | 0.26 | 0.02 |
| E= | 0.19 | 0.19 | 0.19 | 0.19 | 0.07 | 0.19 | ||
| Moderate exposure and depth (WP & OA) | 0.21 | S= | 4.12 | 4.58 | 0.00 | 0.00 | 0.91 | 0.00 |
| E= | 0.02 | 0.02 | 0.02 | 0.02 | 0.89 | 0.02 | ||
| High exposure; Shallow (GI,CI) | 0.21 | S= | 0.01 | 0.01 | 538.22 | 0.00 | 0.91 | 0.00 |
| E= | 0.02 | 0.02 | 0.02 | 0.02 | 0.89 | 0.02 | ||
| High exposure; Deep (PB) | 0.21 | S= | 0.02 | 0.01 | 581.74 | 0.00 | 0.91 | 0.00 |
| E= | 0.02 | 0.02 | 0.02 | 0.02 | 0.89 | 0.02 | ||
Note that the elasticity during the seedling stage is greater than the vegetative stage for two of the shallow scenarios (in bold). The sensitivities show the specific stages that have greater effect over the establishment (italic). Life stages: seed-dependency, Sd (Decemberyr1 –Januaryyr1); autonomous development, Ad (Januaryyr1–Aprilyr1; seedling establishment, Es (Aprilyr1–Septemberyr1); juveniles (new shoot production after Es), Ns (Septemberyr1–year 2); horizontal vegetative expansion, Ve (year 2–3). Adults typically become reproductively mature, f (fecundity), between years 3–5.
Sensitivity analysis showed for sheltered and deep sites, population growth was driven by early seedling life-stage transitions (Sd and Ad), while for exposed sites greater sensitivities occur during seedling establishment (Es) (Table 2). The sheltered and deep locations produced greater elasticity values during early seedling development (Sd-Ns) compared to adult life-stage transition (Ve) suggesting an increase in one of these survival transitions would produce a greater change in λ and consequently in all the population (Table 2). The exposed populations both east and west showed the opposite pattern on elasticities; for these populations there was a greater value on the elasticity on the adult stage (Ve = 0.89) than on the seedling transitions (Sd-Ns = 0.02). This suggests that increasing a single survival stage of seedlings would result in little change in overall recruitment success.
Modelling seeding densities at restoration locations
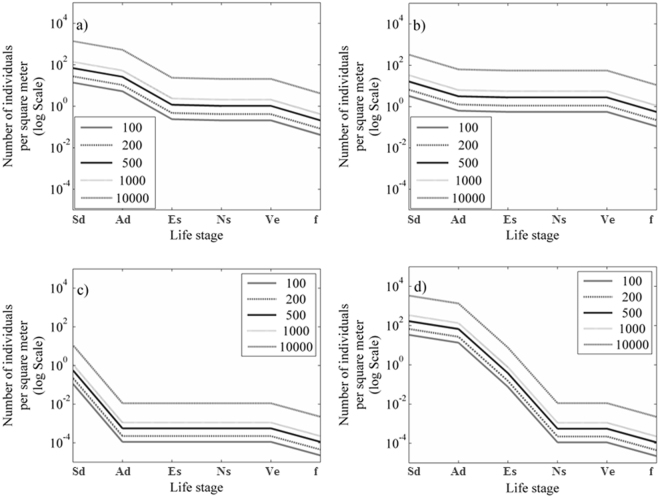
The transitions in survival of seedlings across all stages were evaluated with a hypothetical increase in the number of seeds planted (Fig. 5). Two to 40-fold increases in seeding rates (m−2) across sheltered sites increased the number of seedlings surviving to adulthood (Fig. 5a,b) with a proportional increase in survivorship up to 10 individuals m−2. In exposed locations the onset of winter storms resulted in complete mortality at any seeding density.
Figure 5.
Modelled projection of survivors throughout the transition stages with an increase on the number of seedlings planted. (a) All sites as a single population. (b) Individuals at the low exposure sites, including shallow and deep. (c) Individuals from the mid-exposure sites and (d) Individuals at the exposed sites (shallow and deep). Life-stage transitions - seed-dependency (Sd), autonomous development (Ad), seedling establishment (Es), survival to juveniles (Ns), adults (Ve), fecundity (f).
Discussion
Major recruitment bottlenecks to successful seedling establishment occurred in Posidonia australis during the experiment and the drivers of mortality varied at different life history transitions. In the seagrass Posidonia australis, successful establishment from seeds was strongly influenced at early life-stage transitions, by gradients in seed predation and dislodgement by bioturbators in summer and storms during winter. A similar approach was used by10, who found that seed ‘emergence’ was a major bottleneck in recruitment in arid grasslands. This study has identified that the early recruitment environment has important implications for successful P. australis establishment and for identifying what ecological processes need to be managed to increase seedling recruitment in seed restoration programs.
Seeds in the benthic sedimentary environment are in a dynamic state27 and therefore an understanding of seed movement13,28 and seed fates14 is essential for seagrass restoration and management efforts. Secondary dispersal agents (i.e. secondary dispersal is the dispersal after release from parent to sediment surface or after seeding interventions27 such as fauna and water movement), will likely drive the patterning of seedling establishment and population dynamics across a marine landscape29. Interactions with marine fauna can occur within days to weeks after a seed settling event. For example, in Rottnest Island, Perth, at least six different crustacean taxa predated on P. australis seeds within a day of seed release19. In Odense Fjord, Denmark, lugworms (Arenicola marina) buried 95% of seeds and 75% of seedlings of Z. marina below their critical depth within 4–8 weeks30. Despite the massive seed losses within the first month of our study, we know little about how the most abundant benthic fauna; Blue-swimmer crabs (Portunus armatus), sand-dollars (Peronella lesueuri) and sand sea-stars (Archaster angulates, Fig. 4) influence Posidonia seeds, and whether seeds were completely removed from the recruitment pool or just displaced. Based on the patterning of faunal tracks and pits we observed within our study locations we assume that seeds were dragged or pushed outside and away from our plots by sand-dollars and sea stars, or dislodged, buried or predated by crabs. Clearly, a better understanding of the mechanisms of faunal disturbance along with the movement dynamics and behavioural ecology of these benthic fauna will improve our ability to predict successful recruitment and/or develop strategies to overcome recruitment bottlenecks.
Hydrodynamic forces also have the potential to significantly influence the practice and outcomes of seagrass restoration and conservation by altering the biophysical setting in which a seed recruits. Under extreme storm events the spatial distribution of entire annual seagrass populations can be shifted31,32. In Cockburn Sound the water movement during summer tends to be driven by low tidal ranges and local wind circulation from the east and south-west such that most locations are relatively protected by land features (e.g. Garden Island attenuates south-westerly wind-driven seas and oceanic swells). Whereas in winter, locations near the northern boundary of Cockburn Sound tend to be wave-swept, particularly from storm events arriving from the west and north33,34. An analysis of hydrodynamic conditions during winter (2014) revealed that at least six major storms crossed the coast, with four such events arriving from the north. Subsequently, in moderate to high wave-exposed locations, we observed high seedling survival prior to the arrival of winter storms, but complete loss during winter.
Repeat seeding over successive years may be the most appropriate strategy to overcome recruitment bottlenecks and enhance Posidonia seagrass establishment. Despite our results suggesting that seedlings are less suited to locations with high faunal abundance and high wave-exposed environments, the natural existence of adult stands and small patches of P. australis within these environments suggests recruitment has occurred in the past (e.g.35) though potentially these events may be highly episodic. In addition, high genetic variation within populations of P. australis and high genetic connectivity across the northern section of our study area36 infers contemporary recruitment occurs. In this study, determining demographic rates across environmental gradients showed that successful recruitment required benign conditions. For P. australis, a species that is an effective seed disperser, high fecundity and no dormant seed bank13 could be interpreted as adaptations to the highly variable and disturbed environment they live. ‘Windows of opportunity’ for recruitment limiting seedling recruitment to more benign years37 may be driven by inter-annual variation in the strength and direction of winter storms (Fig. S1). What this may mean for restoring P. australis in these environments is that seeding may be an appropriate strategy for regenerating this species, but annual re-seeding over potentially a decade is recommended to capture inter-annual variation in both summer bottlenecks associated with bioturbators and grazers and winter bottlenecks from physical disturbances associated with winter storms (e.g.9).
We modelled the effect of seeding density on the number of surviving recruits across the entire study area and demonstrated that the probability of surviving from 100 seeds m−2 would be ~0.001. Although the probability of successful recruitment appears low and clearly below detectable limits for this seeding rate, it is comparable to recruitment success for terrestrial restoration species across a range of habitats at lower and higher seeding rates (Table 3). Simulating seeding densities 2- to 40-fold (Fig. 5a) resulted in a proportional increase in seedling establishment for P. australis, but assumes an unlimited carrying capacity. While increasing seeding densities may improve our power to detect recruitment success, seeding densities of 200–1 000 m−2 equate to what would be suitable based on seed size of Posidonia (2 cm). For other seagrass species with much smaller seeds, there have been contrasting effects of seeding density. For example38, found no consistent effects of seeding density on germination or emergence of Z. marina in field studies when seeded at rates as high as 1 250 seeds m−2, whereas controlled laboratory studies on Cymodocea nodosa found negative density dependence at much lower seeding rates, 44–666 seeds m−2 39. Under field conditions, aggregations of established Posidonia seedlings have been found at densities of 10’s–100’s of seedlings per 0.1–0.2 m−2 within naturally formed pits and troughs in the sediment. These field observations suggest that Posidonia may benefit from higher seeding densities under specific micro-site conditions.
Table 3.
Probability of seedling establishment within different habitat types.
| Species | Habitat | Seeding rate (m−2) | Probability of seedling establishment | Authors |
|---|---|---|---|---|
| Posidonia australis | Marine (seagrass) Cockburn Sound, Perth Australia | 100 | 0.001 | This study |
| Zostera marina | Marine (seagrass) Chesapeake Bay, Virginia, USA | 25–50 | 0.016–0.056 | 9 |
| Agropyron desertorum Elymus elymoides Pseudoroegenaria spicata | Arid grasslands Eastern Oregon, USA | 376 | 0–0.06 | 10 |
| Olea europaea | Dense scrubland Sierra Sur de Jaén (Spain) | ~37 | 0.002–0.009 | 51 |
| Rhamnus ludovici-salvatoris | Scrubland Balearic Islands (western Mediterranean) | — | 0.005–0.03 | 52 |
| Epilobium angustifolium Anaphalis margaritacea, | Pumice Plains, Mount St. Helens Washington, USA | ~285 | 0.014 | 53 |
| Vaccinium myrtillus | Coniferous forest Southeast Sweden | 1 250 | 0.003 | 37 |
Conclusions and management implications
Evaluating life cycle processes are the first steps towards optimizing seagrass restoration programs. Targeted monitoring after seeding is needed to observe these early life-history stages and transitions, and will be particularly important when alternative management strategies are applied that aim to enhance vital rates that have a bearing on subsequent seedling survival16,40. We conclude that seedling recruitment in Posidonia populations is affected by site environmental conditions and frequency of disturbances prevailing within one month after seeding and some 4–6 months later when established seedlings are physically removed by winter storms. The differences observed across the environmental gradients demonstrate that patterns of plant recruitment can vary notably at a spatial and temporal scale, and that the constant pressures of disturbance across these scales limit population growth and restoration outcomes. Where these disturbances are moderated sufficiently or can be overcome through intervention strategies, seed-based restoration could replace other more labour intensive re-planting methods for seagrasses in general.
When utilizing seed as a restoration tool, this study showed we can use demographic models effectively to identify how and when ecological processes could be managed in order to alter seagrass population dynamics. If benthic fauna and winter storms are major drivers of seedling survival, then there are potential management options that can be used to overcome these limitations. For example, removal, relocation or deflection of the most problematic benthic fauna in wave-sheltered restoration sites during the early stages of seedling establishment could be used to reduce the probability of biotic dislodgement or predation during this vulnerable and critical life-stage (Johnson et al. unpublished data). Alternatively, increasing the initial seeding density could offset the early heavy losses through an ‘escape by predator satiation’ strategy, though this has largely been untested in seagrasses and relies upon greater investment of valuable seed resources. In wave-exposed locations, introducing seed and seedling mixtures of species whose niche habitats overlap with turbulent and high exposure environments could be a more effective approach to re-establish seagrass back into such environments. While the specific bioturbating fauna and threshold water velocities inhibiting establishment still need to be identified, our results suggest advances in these areas are likely to substantially increase our ability to restore seagrasses across a range of environments.
The temporal resolution (1–3 months) and spatial scale (144 km2) in a highly wave impacted environment over which this study was undertaken has provided greater perspective on the importance of environmental conditions suitable for P. australis seedling establishment. Of greater interest to seagrass restoration ecologists and practitioners is an ability to predict or forecast persistence within a set of environmental conditions. Projection models based on measured demographic rates is a valuable predictor of appropriate locations for seed introductions. By simulating several potential restoration-scale starting seed densities across our study area, outcomes from projection models suggest that successful establishment of seedlings would be restricted to the most favourable of environmental scenarios tested in this study; low energy sites. Low exposure habitats support the later stages of seedling development, although there still remains a potential bottleneck incurred soon after seeding as a result of predation by fauna. Notwithstanding, those seedlings that successfully navigate these filters during early life-stages tend to show long-term persistence, and eventually ramify across the seafloor within two years of seeding. Under moderate exposure scenarios, heavy losses incurred early in the establishment process have a disproportionate influence on seedling establishment and thus increasing initial seeding density will have little effect. However, we have less certainty on what is driving these early losses in these locations. Although we recorded high initial seedling survival in high exposure sites, recruitment was constrained by winter storms which impose a significant barrier to middle life stages of seedlings transitioning to established plants.
Methodology
Life stages and study location
To address these objectives we examined the variation in survival, impacts of predation and disturbance of seeds by animals and physical dislodgement from storms using a large experiment that direct-seeded Posidonia australis seeds across an environmental gradient within and outside Cockburn Sound, Western Australia. Posidonia australis is a large, habitat-forming seagrass that is one of the dominate features of nearshore coastal sediments across temperate and subtropical Australia41. This species produces large (15–20 mm) seeds that lack any seed dormancy (ie. direct-development) (summarized in13). After seed settlement the seed and seedlings undergo distinct early life-history stages and transitions prior to establishment as an adult plant (Fig. 1).
The study area, Cockburn Sound, is a marine embayment (16 km long and 9 km wide, 144 km2) located on the temperate west coast of Western Australia (Fig. 2). Cockburn Sound consists of a deep central basin (17–22 m deep) surrounded by shallow (1–15 m deep) platforms (south, east and west), and banks to the north (Parmelia and Success Banks). Seagrass meadows typically inhabit these platforms and banks and have been mapped accurately to a depth of 5 m35,42. There were substantial losses of seagrass on these platforms and banks during the 1960’s and 70’s due to eutrophication and industrial development of the coast, with minimal natural recovery within Cockburn Sound42 but significant recovery across Success and Parmelia Banks to the north35. Cockburn Sound is further characterised by a spatial and temporal gradient in wind-wave energy. Local water circulation within the Sound is driven by south-west winds during summer (sea breeze) and northerly and westerly storms during winter34. The southern end of the Sound is protected from south-westerly swells by Garden Island and Carnac Island. Northern locations on Parmelia Bank and Carnac Island are not protected from northerly and westerly swells (summer and winter). Study locations and specific site characteristics are described in Table S1, Supporting Information.
Experimental design and measurements
In November 2013, approximately 22 000 Posidonia australis fruit were hand-collected from Woodman Point, Cockburn Sound. This location supports highly fecund and genetically diverse meadows of P. australis 36. This location represents a source of genetic material with proven dispersal capability to locations across the whole embayment and to the north36,43. Whole fruit were transported back to the lab where they were transferred into large (1800 L), temperature controlled (25 °C) recirculating aquaria. Vigorous aeration was applied to the aquaria to agitate floating fruit and promote fruit dehiscence and seed release25. Negatively buoyant seeds sink and were collected from the aquarium floor. Seedling viability was determined by the presence of an undamaged seed, intact prophyll and radical.
To determine how major abiotic and biotic characteristics, such as physical disturbance, depth and biotic interactions can potentially influence seed survival and recruitment we chose eight study locations with a gradient in wave exposure and depth (Table S1). Within each location three sites were selected. At each site, nine 1 m2 plots were established within a 5 m × 5 m area and plots were spaced 1 m apart. Three plots were randomly assigned full cage enclosures, three plots half-cage enclosures (procedural control) and three plots were uncaged controls. We direct-seeded 100 seedlings into each 1 m2 plot by gently inserting a developing seedling just below the sediment surface. This mimicked how seeds are buried naturally. Seeds were spaced equally in each plot. In total, 21 600 seedlings were planted into 216 m2 across an area encompassing 144 km2.
Seedling survival was monitored for 18 months at intervals that coincided with each life-stage transition. Life stages and transitions (Fig. 1) were defined by a changing dependency of the seedling on seed reserves during the first months of development25,44,45. Fruits mature and release an individual seed with an emerging radical and prophyll during late November. Subsequently, seedlings were planted in early December. Initially, seedlings are highly dependent on seed reserves; seed-dependent, Sd (Decemberyr1 - Januaryyr1). Seedlings then undergo an extended period where they continue to draw nourishment from maternally-derived reserves, however, during this stage there is greater uptake and assimilation of resources from the environment due to production of photosynthetically active leaves and development of a small but functional root system; autonomous development, Ad (Januaryyr1–Aprilyr1). By the end of this period seedlings have exhausted the majority (~90%) of their seed reserves and are relatively independent of their seed. Seedlings then become fully integrated into their environment upon exhaustion of the seed reserves; seedling establishment, Es (Aprilyr1–Septemberyr1). Production of new shoots, Ns (Septemberyr1–year 2) typically occurs in the months following seedling establishment and seedlings become Juveniles. Juveniles transition into adults after plants undergo horizontal vegetative expansion, Ve (year 2–3). Adults typically become reproductively mature, f (fecundity), between years 3–57.
During the experiment we monitored several biotic and abiotic characteristics at each location. A census on benthic faunal abundance was undertaken in February 2014, counting the number of animals present within a 5 m × 1 m transect, replicated six times at each site. The physical environment was also characterized (Table 1). Wave conditions were determined from pressure sensors (RBR Virtuoso, Dtide) deployed simultaneously over winter at different locations. Each sensor recorded pressure continuously at 1 Hz. Posterior analysis consisted of removing atmospheric pressure and converting recorded pressure to meters of water, we then separated the data into hourly bursts. A time series of water level was calculated and the trend removed to account only for events from the desired frequencies. A spectral density analysis for each burst was then calculated, focusing in the energy bands of the swell components with periods from 5 to 25 s (e.g.46,47). The significant wave height, Hs, defined as the highest third of the waves was calculated from the total spectral energy for the bursts across the mentioned frequencies. Sediment grain size distribution and sediment organic matter content (see Table S1) were determined from 9 cm diameter by 5 cm deep cores. Sediment cores were dried at 60 °C for 72 hours before compositional analysis. Sediment grain size distribution was analysed using settling velocities in a 2.2 m settling tube. The recorded settling velocity was transformed to sediment size using the Gibbs equation with the corresponding sand density of 2.56 g cm−3 measured by volumetric displacement. Sediment distribution was computed with the graphical logarithmic method from48. Sediment organic matter content was determined from loss on ignition at 550 °C following methods by49. Light (Photosynthetically Active Radiation, PAR) loggers (HOBO H21-002 Micro Station, recording every 5 minutes) and temperature loggers recording every minute (HOBO® UTBI-001 TidbiT, recording every 5 minutes) were deployed across the depth gradient; Southern Flats as the shallow site, Owen Anchorage was mid-depth and Parmelia Bank was the deep site (see Table S1).
Statistical analysis
Mortality of early life-history transitions and how they vary spatially along gradients in disturbance were tested for normality. For transition probabilities, each 1 m2 plot was considered a replicate with Location (8), caging Treatment (3) and Life-Stage (4) as fixed factors and Site (3) nested within Location. A Bartletts test revealed the data were not normally distributed (R Core Team, 2014), so we used a fixed factor PERMANOVA (‘vegan’, R package) to test for differences in transition probabilities. Data were transformed into a Euclidean resemblance matrix, with 9999 permutations. If a significant main effect or interaction was detected we tested additional hypotheses about the pairwise differences in the coefficients. Pairwise comparisons revealed that cages confounded seedling survival across all Locations with the only exception being Owen Anchorage, where full caging treatments resulted in a positive effect on seedling survival for the first life stage. Subsequently, we simplified the model by removing the factor Treatment and re-analyzed the data using only control plots (ie. no cages) with Location and Life-Stage as fixed factors and Site nested within Location.
We used population matrices for each location to determine the influence of life stage on the overall recruitment dynamics. Because complete mortality occurred in some locations resulting in a zero probability of transitioning from one life-stage to the next, we replaced these values with a value of 10−3 which was lower than the probability of a seedling surviving within 1 m2 plot and had little weight on population growth. Although we only have values for the first five stages of this matrix, we populated the sixth transition or mortality and the fecundity (R1, Fig. 1) from data collected at Rottnest Island on seed production and natural seedling mortality over multiple years (Kendrick unpublished). A value of 0.2 was assigned as mortality and remained constant across all models, and we used a value of 8 as a fecundity value based on seed production (Kendrick unpublished). While these mortality and fecundity values may not represent the sites tested in this study, we tested the conclusions drawn from our matrix and sensitivity analysis by varying adult transition rates within realistic values; fecundity from 3 to 15 and then mortality from 0.06 to 0.5 (Supplementary Information Tables S2–S18). Despite modifying fecundity and mortality within realistic values our conclusions regarding the early life-stages as bottlenecks to seed-based restoration success do not change. However, only when adult mortality reaches a threshold of 0.5 or greater (mortality of half the adult population each year) does the adult life-stage become a bottleneck. We then calculated the population growth rate (λ) as the dominant eigenvalue of each transition matrix and then calculated the sensitivity and elasticity of each transition using Matlab code “eigenall”. These values determine the overall importance of individual transitions to λ based on the left and right eigenvectors50. We finally modelled the transition matrices to determine what the outcome would have been if we increased starting seed densities across the gradient in environmental conditions and observed the outcome of seed survival. We modelled starting seed densities of 100 m2, 200 m2, 500 m2, 1000 m2 and 10,000 m2.
Electronic supplementary material
Acknowledgements
We would like to thank the large team of volunteers for their field assistance. This research was funded from an ARC Linkage Grant (LP130100155) supported by outstanding financial and logistical contributions from BMT Oceanica, Cockburn Cement Ltd., Shark Bay Resources, Botanic Gardens and Parks Authority and Virginia Institute of Marine Science. This is contribution no. xxxx from the Virginia Institute of Marine Science, The College of William and Mary.
Author Contributions
Conceived and designed the experiments: J.S., G.A.K., K.W.D., R.J.O. Performed the experiments: J.S., L.R.M., G.A.K., R.J.O. Analyzed the data: J.S., L.R.M., G.A.K. Contributed materials and analysis tools: J.S., L.R.M., G.A.K., K.W.D., R.J.O. All authors contributed critically to the drafts and gave final approval for publication.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-13833-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Temperton, V. M. Assembly rules and restoration ecology: bridging the gap between theory and practice. Vol. 5 (Island Press, 2004).
- 2.Katwijk MM, et al. Global analysis of seagrass restoration: the importance of large‐scale planting. Journal of Applied Ecology. 2016;53:567–578. doi: 10.1111/1365-2664.12562. [DOI] [Google Scholar]
- 3.Bell SS, Tewfik A, Hall MO, Fonseca MS. Evaluation of seagrass planting and monitoring techniques: implications for assessing restoration success and habitat equivalency. Restoration Ecology. 2008;16:407–416. doi: 10.1111/j.1526-100X.2007.00308.x. [DOI] [Google Scholar]
- 4.Paling, E. I., Fonseca, M., van Katwijk, M. M. & van Keulen, M. Seagrass restoration. Coastal wetlands: An integrated ecosystems approach 687–713 (2009).
- 5.Van Katwijk MM, et al. Guidelines for seagrass restoration: importance of habitat selection and donor population, spreading of risks, and ecosystem engineering effects. Marine pollution bulletin. 2009;58:179–188. doi: 10.1016/j.marpolbul.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Statton J, Dixon KW, Hovey RK, Kendrick GA. A comparative assessment of approaches and outcomes for seagrass revegetation in Shark Bay and Florida Bay. Marine and Freshwater Research. 2012;63:984–993. doi: 10.1071/MF12032. [DOI] [Google Scholar]
- 7.Bastyan GR, Cambridge ML. Transplantation as a method for restoring the seagrass Posidonia australis. Estuarine, Coastal and Shelf Science. 2008;79:289–299. doi: 10.1016/j.ecss.2008.04.012. [DOI] [Google Scholar]
- 8.Waycott M, et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences. 2009;106:12377–12381. doi: 10.1073/pnas.0905620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orth RJ, Moore KA, Marion SR, Wilcox DJ, Parrish DB. Seed addition facilitates eelgrass recovery in a coastal bay system. Marine Ecology Progress Series. 2012;448:177–195. doi: 10.3354/meps09522. [DOI] [Google Scholar]
- 10.James JJ, Svejcar TJ, Rinella MJ. Demographic processes limiting seedling recruitment in arid grassland restoration. Journal of Applied Ecology. 2011;48:961–969. doi: 10.1111/j.1365-2664.2011.02009.x. [DOI] [Google Scholar]
- 11.Dalgleish HJ, Koons DN, Hooten MB, Moffet CA, Adler PB. Climate influences the demography of three dominant sagebrush steppe plants. Ecology. 2011;92:75–85. doi: 10.1890/10-0780.1. [DOI] [PubMed] [Google Scholar]
- 12.Orth, R. J., Harwell, M. C. & Inglis, G. J. In Seagrasses: Biology, Ecology, and Conservation 111–133 (Springer, 2006).
- 13.Kendrick GA, et al. The central role of dispersal in the maintenance and persistence of seagrass populations. BioScience. 2012;62:56–65. doi: 10.1525/bio.2012.62.1.10. [DOI] [Google Scholar]
- 14.Kendrick, G. A. et al. Demographic and genetic connectivity: the role and consequences of reproduction, dispersal and recruitment in seagrasses. Biological Reviews92, 921–938 (2017). [DOI] [PubMed]
- 15.Kirkman H. Pilot experiments on planting seedlings and small seagrass propagules in Western Australia. Marine Pollution Bulletin. 1999;37:460–467. doi: 10.1016/S0025-326X(99)00146-0. [DOI] [Google Scholar]
- 16.Marion SR, Orth RJ. Seedling establishment in eelgrass: seed burial effects on winter losses of developing seedlings. Marine Ecology Progress Series. 2012;448:197–207. doi: 10.3354/meps09612. [DOI] [Google Scholar]
- 17.Fishman JR, Orth RJ. Effects of predation on Zostera marina L. seed abundance. Journal of Experimental Marine Biology and Ecology. 1996;198:11–26. doi: 10.1016/0022-0981(95)00176-X. [DOI] [Google Scholar]
- 18.Orth RJ, Heck KL, Jr., Tunbridge DJ. Predation on seeds of the seagrass Posidonia australis in Western Australia. Marine Ecology Progress Series. 2002;244:81–88. doi: 10.3354/meps244081. [DOI] [Google Scholar]
- 19.Orth RJ, Kendrick GA, Marion SR. Predation on Posidonia australis seeds in seagrass habitats of Rottnest Island, Western Australia: patterns and predators. Marine Ecology Progress Series. 2006;313:105–114. doi: 10.3354/meps313105. [DOI] [Google Scholar]
- 20.Harper, J. L. & Harper, J. L. Population biology of plants. Vol. 892 (JSTOR, 1977).
- 21.James JJ, et al. A systems approach to restoring degraded drylands. Journal of Applied Ecology. 2013;50:730–739. doi: 10.1111/1365-2664.12090. [DOI] [Google Scholar]
- 22.Piazzi L, Acunto S, Cinelli F. In situ survival and development of Posidonia oceanica (L.) Delile seedlings. Aquatic Botany. 1999;63:103–112. doi: 10.1016/S0304-3770(98)00115-6. [DOI] [Google Scholar]
- 23.Campey ML, Kendrick GA, Walker DI. Interannual and small-scale spatial variability in sexual reproduction of the seagrasses Posidonia coriacea and Heterozostera tasmanica, southwestern Australia. Aquatic Botany. 2002;74:287–297. doi: 10.1016/S0304-3770(02)00127-4. [DOI] [Google Scholar]
- 24.Marion SR, Orth RJ. Innovative Techniques for large‐scale seagrass restoration using Zostera marina (eelgrass) seeds. Restoration Ecology. 2010;18:514–526. doi: 10.1111/j.1526-100X.2010.00692.x. [DOI] [Google Scholar]
- 25.Statton J, Cambridge ML, Dixon KW, Kendrick GA. Aquaculture of Posidonia australis seedlings for seagrass restoration programs: effect of sediment type and organic enrichment on growth. Restoration Ecology. 2013;21:250–259. doi: 10.1111/j.1526-100X.2012.00873.x. [DOI] [Google Scholar]
- 26.Statton J, Kendrick GA, Dixon KW, Cambridge ML. Inorganic nutrient supplements constrain restoration potential of seedlings of the seagrass. Posidonia australis. Restoration ecology. 2014;22:196–203. doi: 10.1111/rec.12072. [DOI] [Google Scholar]
- 27.Chambers JC, MacMahon JA. A day in the life of a seed: movements and fates of seeds and their implications for natural and managed systems. Annual review of ecology and systematics. 1994;25:263–292. doi: 10.1146/annurev.es.25.110194.001403. [DOI] [Google Scholar]
- 28.McMahon K, et al. The movement ecology of seagrasses. Proceedings of the Royal Society of London B: Biological Sciences. 2014;281:20140878. doi: 10.1098/rspb.2014.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inglis GJ. Variation in the recruitment behaviour of seagrass seeds: implications for population dynamics and resource management. Pacific Conservation Biology. 2000;5:251–259. doi: 10.1071/PC000251. [DOI] [Google Scholar]
- 30.Valdemarsen T, Wendelboe K, Egelund JT, Kristensen E, Flindt MR. Burial of seeds and seedlings by the lugworm Arenicola marina hampers eelgrass (Zostera marina) recovery. Journal of Experimental Marine Biology and Ecology. 2011;410:45–52. doi: 10.1016/j.jembe.2011.10.006. [DOI] [Google Scholar]
- 31.Bell SS, Fonseca MS, Kenworthy WJ. Dynamics of a subtropical seagrass landscape: links between disturbance and mobile seed banks. Landscape Ecology. 2008;23:67–74. doi: 10.1007/s10980-007-9137-z. [DOI] [Google Scholar]
- 32.Fonseca MS, et al. Factors influencing landscape pattern of the seagrass Halophila decipiens in an oceanic setting. Estuarine, Coastal and Shelf Science. 2008;76:163–174. doi: 10.1016/j.ecss.2007.06.014. [DOI] [Google Scholar]
- 33.Symonds, G., Zhong, L. & Mortimer, N. A. C. C. Effects of wave exposure on circulation in a temperate reef environment. Journal of Geophysical Research: Oceans 116, n/a-n/a, doi:10.1029/2010jc006658 (2011).
- 34.Ruiz-Montoya L, Lowe RJ. Summer circulation dynamics within the Perth coastal waters of southwestern Australia. Continental Shelf Research. 2014;77:81–95. doi: 10.1016/j.csr.2014.01.022. [DOI] [Google Scholar]
- 35.Kendrick GA, Hegge BJ, Wyllie A, Davidson A, Lord DA. Changes in Seagrass Cover on Success and Parmelia Banks, Western Australia Between 1965 and 1995. Estuarine, Coastal and Shelf Science. 2000;50:341–353. doi: 10.1006/ecss.1999.0569. [DOI] [Google Scholar]
- 36.Sinclair EA, Krauss SL, Anthony J, Hovey R, Kendrick GA. The interaction of environment and genetic diversity within meadows of the seagrass Posidonia australis (Posidoniaceae) Marine Ecology Progress Series. 2014;506:87–98. doi: 10.3354/meps10812. [DOI] [Google Scholar]
- 37.Eriksson O, Fröborg H. Windows of opportunity” for recruitment in long-lived clonal plants: experimental studies of seedling establishment in Vaccinium shrubs. Canadian Journal of Botany. 1996;74:1369–1374. doi: 10.1139/b96-166. [DOI] [Google Scholar]
- 38.Orth RJ, Fishman JR, Harwell MC, Marion SR. Seed-density effects on germination and initial seedling establishment in eelgrass Zostera marina in the Chesapeake Bay region. Marine Ecology Progress Series. 2003;250:71–79. doi: 10.3354/meps250071. [DOI] [Google Scholar]
- 39.Balestri E, Vallerini F, Lardicci C. Effect of seed density and sediment nutrient heterogeneity on recruitment and early patch growth in the seagrass Cymodocea nodosa. Marine Ecology Progress Series. 2010;417:63–72. doi: 10.3354/meps08783. [DOI] [Google Scholar]
- 40.Irving AD, et al. Testing alternate ecological approaches to seagrass rehabilitation: links to life‐history traits. Journal of Applied Ecology. 2010;47:1119–1127. doi: 10.1111/j.1365-2664.2010.01852.x. [DOI] [Google Scholar]
- 41.Carruthers T, et al. Seagrasses of south–west Australia: A conceptual synthesis of the world’s most diverse and extensive seagrass meadows. Journal of Experimental Marine Biology and Ecology. 2007;350:21–45. doi: 10.1016/j.jembe.2007.05.036. [DOI] [Google Scholar]
- 42.Kendrick GA, et al. Changes in seagrass coverage in Cockburn Sound, Western Australia between 1967 and 1999. Aquatic Botany. 2002;73:75–87. doi: 10.1016/S0304-3770(02)00005-0. [DOI] [Google Scholar]
- 43.Ruiz-Montoya L, Lowe RJ, Kendrick GA. Contemporary connectivity is sustained by wind-and current-driven seed dispersal among seagrass meadows. Movement ecology. 2015;3:1. doi: 10.1186/s40462-015-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hocking P, Cambridge M, McComb A. The nitrogen and phosphorus nutrition of developing plants of two seagrasses, Posidonia australis and Posidonia sinuosa. Aquatic Botany. 1981;11:245–261. doi: 10.1016/0304-3770(81)90064-4. [DOI] [Google Scholar]
- 45.Kuo, J. & Kirkman, H. In Seagrass Biology: Proceedings of an international workshop Rottnest Island, Western Australia. 25–29.
- 46.Lowe RJ, et al. Spectral wave dissipation over a barrier reef. Journal of Geophysical Research: Oceans. 2005;110:C04001. [Google Scholar]
- 47.Dean, R. G. & Dalrymple, R. A. Coastal processes with engineering applications. (Cambridge University Press, 2004).
- 48.Folk, R. L. & Ward, W. C. Brazos River bar: a study in the significance of grain size parameters. Journal of Sedimentary Research27 (1957).
- 49.Sutherland R. Loss-on-ignition estimates of organic matter and relationships to organic carbon in fluvial bed sediments. Hydrobiologia. 1998;389:153–167. doi: 10.1023/A:1003570219018. [DOI] [Google Scholar]
- 50.Caswell H. Analysis of life table response experiments I. Decomposition of effects on population growth rate. Ecological Modelling. 1989;46:221–237. doi: 10.1016/0304-3800(89)90019-7. [DOI] [Google Scholar]
- 51.Rey PJ, Alcantara JM. Recruitment dynamics of a fleshy‐fruited plant (Olea europaea): connecting patterns of seed dispersal to seedling establishment. Journal of Ecology. 2000;88:622–633. doi: 10.1046/j.1365-2745.2000.00472.x. [DOI] [Google Scholar]
- 52.Traveset A, Gulías J, Riera N, Mus M. Transition probabilities from pollination to establishment in a rare dioecious shrub species (Rhamnus ludovici‐salvatoris) in two habitats. Journal of Ecology. 2003;91:427–437. doi: 10.1046/j.1365-2745.2003.00780.x. [DOI] [Google Scholar]
- 53.Wood, D. M. & Morris, W. F. Ecological constraints to seedling establishment on the pumice plains, Mount St. Helens, Washington. American Journal of Botany 1411–1418 (1990).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.