Abstract
Solasodine is a main active component isolated from Solanum incanum L. that performs a wide range of functions containing anti‐oxidant, anti‐infection, and neurogenesis promotion. In this study, we explored the influence of solasodine on three types of human colorectal cancer (CRC) cell lines. The results show that solasodine prohibited CRC cell proliferation dose‐ and time‐dependently and impeded CRC cell motility by downregulating MMPs. Solasodine was also found to fuel caspase‐cascade reaction and increase the ratio between Bax and Bcl‐2 so as to induce CRC cell apoptosis. When cells were pretreated with AKT activator (insulin‐like growth factor‐1) followed by solasodine, the solasodine‐induced apoptosis was partially abrogated by insulin‐like growth factor‐1. Moreover, solasodine hindered tumor development and stimulated similar mechanisms in vivo. In general, our study provides the first evidence that solasodine has a suppressive effect on CRC cells and that this agent may be a novel therapeutic drug for CRC treatment.
Keywords: Apoptosis, colorectal cancer, metastasis, solasodine, β‐Catenin
Colorectal cancer is the third most commonly diagnosed malignancy, causing 694 000 deaths per year, globally.1 Despite the increase in 5‐year survival rates from 51% to 65% in CRC patients, benefitting from early diagnostic approaches and progressive therapeutics, half will eventually develop inoperable metastatic colorectal cancer, which is always fatal.2, 3 Therefore, developing new antitumor therapies against CRC is necessary for preventing metastasis and increasing the number of long‐term survivors.
Carcinogenesis of CRC is accompanied by a series of genetic variations, and several signaling pathways have been singled out as key factors. The activation of the Wnt/β‐catenin and PI3K/AKT/GSK‐3β signaling pathways is the crucial event in CRC.4, 5, 6, 7 Accumulated cytoplasmic β‐catenin transfers into the nucleus and binds to nuclear partners to create a transcriptional activation of downstream genes. Of note, phosphorylation of GSK‐3β at Ser9 by phosphorylated AKT has been verified to induce GSK‐3β inactivation and inhibit the degradation of β‐catenin.8, 9, 10 Allowing for these essential molecules, AKT/GSK‐3β/β‐catenin signaling could be a promising target for CRC treatment.
Natural products have been considered prominent sources for anticancer drug discovery with mild side‐effects.11, 12 Solasodine, the aglycone of solamargine or solasonine after hydrolysis, displays a variety of pharmacological activities including anti‐oxidant, neurogenesis promotion, and inhibition of fungal infection.13, 14, 15 As a primary active ingredient of the traditional herb Solanum incanum L., recent studies have reported that solasodine has powerful anticancer properties by inducing apoptosis and cell cycle arrest in a wide variety of tumor cells including breast cancer, oral epidermoid carcinoma, chronic myelogenous leukemia, prostate cancer, and basal cell carcinoma, as well as stimulating persistent immunity against cancer such as sarcoma 180.16, 17, 18 However, the effects and mechanisms of solasodine on human CRC cell lines have never been clarified. Our research indicated that solasodine suppresses the proliferation and motility of three types of CRC cells efficiently through inhibition of the AKT/GSK‐3β/β‐catenin signaling pathway. These findings were further investigated in vivo, suggesting that solasodine is a potential therapeutic agent for CRC treatment.
Materials and Methods
Reagents and antibodies
Solasodine was purchased from Sigma‐Aldrich (St. Louis, MO, USA) (Fig. 1a), dissolved in 100% DMSO, and stored at −20°C. Both MTT and monoclonal mouse β‐actin antibody were also obtained from Sigma‐Aldrich. Rabbit anti‐human antibodies against MMP‐9, MMP‐2, E‐cadherin, apoptosis kits, AKT, p‐AKT, GSK‐3β, p‐GSK‐3β, β‐catenin, p‐β‐catenin, and secondary antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). Matrix metalloproteinase‐14 was from Abcam (Cambridge, MA, USA). The apoptosis detection kit was from BD Biosciences (San Diego, CA, USA). TRIzol reagent and Power SYBR Green PCR Master Mix were from Life Technologies (Grand Island, NY, USA). The PrimeScript RT reagent kit with gDNA Eraser was from TaKaRa (Dalian, China).
Figure 1.
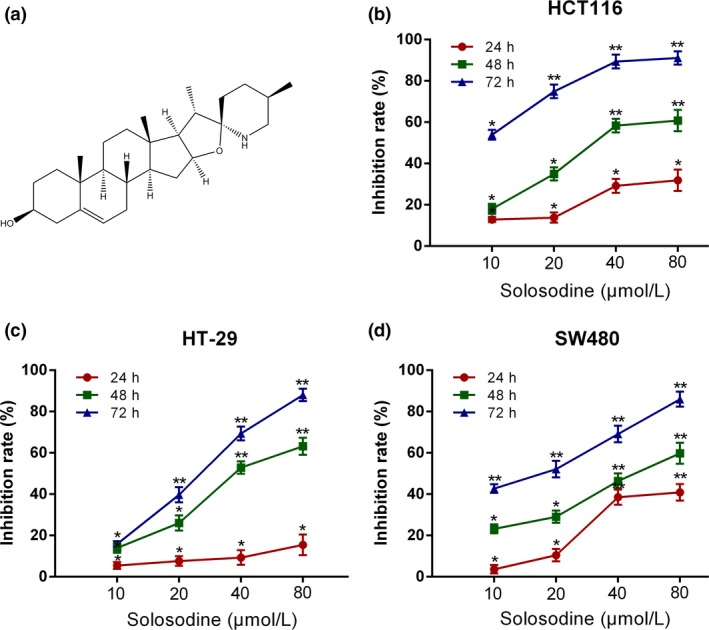
Solasodine suppresses colorectal cancer cell growth. (a) Chemical structure of solasodine. (b–d) HCT116 (b), HT‐29 (c), and SW480 (d) cells were incubated with different concentrations of solasodine for 24, 48, and 72 h. Cell inhibitive rates were measured by MTT assay. The values were presented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 versus control group.
Cell culture and treatment
The human CRC cell lines HCT116, HT‐29, and SW480 were purchased from the Type Culture Collection, Chinese Academy of Sciences (Shanghai, China). All cells were cultivated in RPMI‐1640 medium with 10% FBS (both from Gibco‐BRL, Gaithersburg, MD, USA) in a humidified incubator at 37°C containing 5% CO2.
Cell proliferation assay
Human CRC cell lines (cell density, 7 × 103 cells per well for all) were seeded into 96‐well plates followed by treatment with various concentrations of solasodine (0, 20, 40, and 80 μmol/L) for 24, 48, or 72 h. Then 20 μL MTT solution (5 mg/mL) was added to incubate the cells at 37°C for 4 h, followed by 150 μL DMSO per well. The absorbance was detected at an OD of 490 nm using a microplate reader (Bio‐Tek, Winooski, VT, USA). Cell growth inhibitive rates were calculated using the following formula: 1−ODexperiment/ODcontrol.
Cell cycle assay
Cells were seeded into a 100‐mm Petri dish for incubation overnight and then synchronized by serum‐free media. Cells were treated with different doses of solasodine for 48 h and then harvested and fixed with 70% cold ethanol at 4°C overnight. Fixed cells were then resuspended in 100 μg/mL RNase and incubated with 50 μg/mL PI at 37°C for 30 min in the dark for FCM analysis.
Apoptosis assay
The annexin V/PI method was used to monitor the cell apoptotic rate. Cells were seeded in 6‐well plates for exposure to solasodine (0, 40, or 80 μmol/L) for 48 h, then collected after trypsinization and washed twice with cold PBS. Cells were resuspended in 500 μL binding buffer and finally stained with 5 μL annexin V‐FITC and 5 μL PI at room temperature for 15 min in the dark. The apoptotic rate analysis was carried out by FCM.
Hoechst 33258 staining
Three types of cells were treated with different concentrations of solasodine for 48 h, then fixed with 4% paraformaldehyde and washed once with PBS. Subsequently, cells were stained with 50 ng⁄mL Hoechst 33342 for 30 min. Nuclear apoptotic changes were observed using an Axioplan2 fluorescence microscope (Zeiss, Jena, Germany).
Transwell assay
Cell invasion ability was examined by Transwell membrane filter inserts (8‐μm pore size; Costar, Corning, NY, USA) in 24‐well dishes. Cells (1 × 104) suspended in 200 μL serum‐free medium with solasodine were seeded into the upper chambers; 500 μL complete medium was added to the lower chamber. Invaded cells were fixed in 4% paraformaldehyde and stained with 0.05% crystal violet for observation under an inverted microscope (Bio‐Tek).
Scratch wound assay
All cells were seeded into 6‐well plates as confluent monolayers and then scratched by a pipette tip. The cells were then washed twice with PBS to remove detached cells and underwent incubation with various doses of solasodine for 48 h. Wound images were acquired by use of an inverted microscope.
Immunofluorescence staining
After being treated with solasodine, cells were permeated in 0.5% Triton X‐100 for 20 min, blocked in 5% BSA for 30 min, and then anchored in 4% paraformaldehyde for 15 min. Cells were incubated with antibody against β‐catenin (1:100 dilution) overnight at 4°C. Cells were then incubated for 1 h with Cy3‐labeled anti‐rabbit IgG (1:200 dilution; Boster, Wuhan, China) secondary antibody. Laser scanning confocal microscope (LSM710; Zeiss) was used for image capture.
β‐Catenin siRNA transient transfection
Colorectal cancer cells were transiently transfected with β‐catenin siRNA (sense, 5′‐GUUAUGGUCCAUCAGCUUU‐3′; antisense, 5′‐AAAGCUGAUGGACCAUAAC‐3′) with Lipofectamine RNAiMAX Transfection Reagent and used in experiments 48 h later. The knockdown efficiency was confirmed by RT‐PCR.
Animals and in vivo tumor xenograft assay
BALB/c/nu/nu nude mice (6–8 weeks old, 18–22 g body weight) were from Beijing Vital River Laboratory Animal Technology (Beijing, China). HCT116 cells (1 × 106) were suspended in 100 μL PBS and injected s.c. into the right flank of all mice. Mice were randomly assigned to four groups (PBS, 30 or 50 mg/kg solasodine, or 20 mg/kg 5‐Fu) with six animals in each group. When the tumors reached a volume of approximately 150 mm3, each group received i.p. injections of PBS, solasodine, or 5‐Fu once every day for 5 weeks. The mean tumor volumes were measured weekly using the formula: volume = (length × width2)/2. All mice were killed and tumors were excised and weighed on the last day. Tumors were stored at −80°C for RNA or protein isolation. All animal studies complied with the NIH guide for the care and use of laboratory animals and approved by the Animal Ethics and Research Committee of Nanjing University of Chinese Medicine (Nanjing, China).
Total RNA extraction and RT‐qPCR
Total RNA was extracted from human CRC cells and tumor tissues with TRIzol reagent. The RNA was then reverse transcribed into cDNA using a TaKaRa RT reagent kit. Gene expression levels were quantitatively measured by an ABI 7500 fast RT‐qPCR System (ABI; Applied Biosystems, Waltham, MA, USA) through DNA‐binding dye SYBR‐Green by the ΔΔCt method, and the internal reference was ACTB. The primer sequences are described in Table 1.
Table 1.
Primers for RT–quantitative PCR
| Target | Forward | Reverse | Size (bp) |
|---|---|---|---|
| Bax | 5′‐TTTGCTTCAGGGTTTCATCC‐3′ | 5′‐GCCACTCGGAAAAAGACCTC‐3′ | 213 |
| Bak | 5′‐ACGCTATGACTCAGAGTTCC‐3′ | 5′‐CTTCGTACCACAAACTGGCC‐3′ | 360 |
| Bcl‐2 | 5′‐TCGCCCTGTGGATGACTGAG‐3′ | 5′‐CAGAGTCTTCAGAGACAGCCAGGA‐3′ | 143 |
| Bcl‐xl | 5′‐ATGAACTCTTCCGGGATGG‐3′ | 5′‐TGGATCCAAGGCTCTAGGTG‐3′ | 166 |
| Survivin | 5′‐TTCTCAAGGACCACCGCATC‐3′ | 5′‐GCCAAGTCTGGCTCGTTCTC‐3′ | 127 |
| MMP‐2 | CTCCCGGAAAAGATTGATG | GGTGCTGGCTGAGTAGAT | 96 |
| MMP‐9 | 5′‐TCTATGGTCCTCGCCCTGAA‐3′ | 5′‐CATCGTCCACCGGACTCAAA‐3′ | 219 |
| MMP‐14 | 5′‐ATCTGCCTCTGCCTCACCTA‐3′ | 5′‐AAGCCCCATCCAAGGCTAAC‐3′ | 126 |
| AKT | 5′‐GGATTTGATGAGGAGTTCACG‐3′ | 5′‐GAGTAGGAGAACTGGGGAAGT‐3′ | 117 |
| GSK‐3β | 5′‐GACTAAGGTCTTCCGACCCC‐3′ | 5′‐AAGAGTGCAGGTGTGTCTCG‐3′ | 177 |
| β‐Catenin | 5′‐CAACTAAACAGGAAGGGATGGA ‐3′ | 5′‐CTATACCACCCACTTGGCAGAC ‐3′ | 159 |
| β‐Actin | 5′‐GGCCAACCGCGAGAAGAT ‐3′ | 5′‐CGTCACCGGAGTCCATCA ‐3′ | 134 |
Western blot analysis
Cells and xenograft tumor tissues were lysed by RIPA buffer which contains protease inhibitor cocktail. The lysates were separated by SDS‐PAGE and transferred to PVDF membranes (Millipore, Bedford, MA, USA). After incubation with BSA for 1 h at room temperature, membranes were probed with primary antibodies directed against corresponding proteins overnight at 4°C. After washing three times with TBST, membranes were incubated with secondary antibodies and detected by an ECL detection kit.
Immunohistochemical staining
Paraffin‐embedded tumor tissues were cut into successive 4‐mm transverse planes in order to analyze immunohistochemical staining of Bax, cleaved PARP1, MMP‐9, vascular endothelial growth factor, E‐cadherin, and β‐catenin in the guidance of the routine protocols.19
Statistical analysis
Results of experiments are presented as means ± SD. Statistical analysis was carried out using spss 20.0 software (SPSS, Chicago, IL, USA) with one‐way anova followed by Duncan's test to distinguish treatment groups from control groups. Statistical significance was identified as P‐value < 0.05 or 0.01.
Results
Solasodine prohibits proliferation of CRC cells
The MTT assay was carried out with the purpose of investigating the effects of solasodine on CRC cell growth in vitro. Solasodine dramatically inhibited the proliferative capability of three types of CRC cell lines dose‐ and time‐dependently (Fig. 1b–d). According to the results, we chose 48 h as the optimal treatment time for the next studies as the IC50 values of HCT116, HT‐29, and SW480 were 39.43, 44.56, and 50.09 μmol/L, respectively. Additionally, cytotoxicities of a number of established antineoplastics, including 5‐Fu, oxaliplatin, and folinic acid, against three types of CRC cells were compared with solasodine. Of them, solasodine showed the greatest suppressive effect, particularly when its concentration exceeded 40 μmol/L. Moreover, synergistic cytotoxic efficiencies were studied by combining solasodine and these chemotherapeutic drugs. The results revealed that co‐treatment showed stronger cytotoxicity, depending primarily on the concentration of solasodine (Fig. 2).
Figure 2.
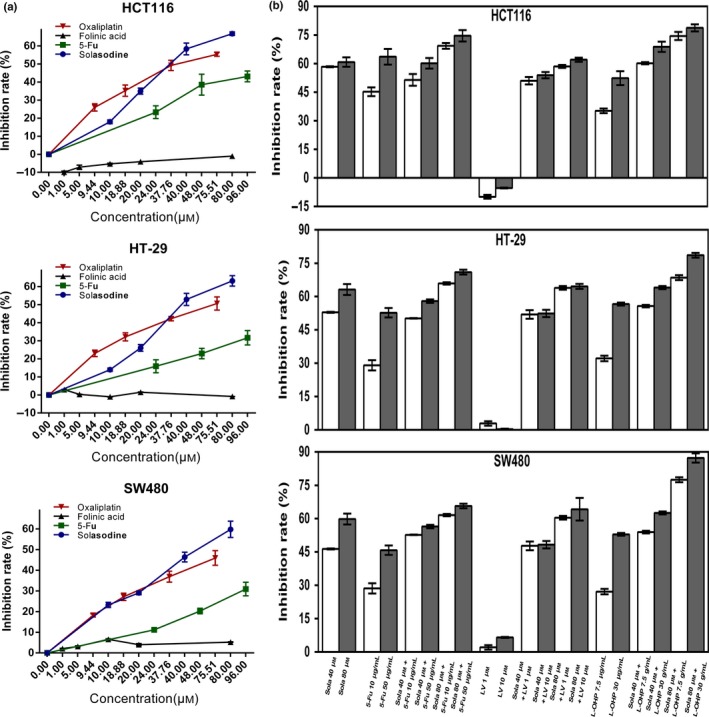
Solasodine shows cytotoxicity against colorectal cancer cells and synergistic effects with established chemotherapeutic agents. (a,b) Three types of cells were treated with solasodine, 5‐fluorouracil (5‐Fu), oxaliplatin, and folinic acid alone or in combination for 48 h. Cell viability was tested by MTT assay. Values are shown as mean ± SD of three independent assays. *P < 0.05, **P < 0.01 versus control group.
Solasodine induces G2/M‐phase cell cycle arrest in CRC cells
We used FACS analyses with PI staining to further assess the influence of different concentrations of solasodine on the cell cycle. The results showed that the percentage of all three types of cells in G2/M phase increased in response to solasodine treatment for 48 h, suggesting the ability of solasodine to induce G2/M‐phase cell cycle arrest in CRC cells dose‐dependently (Fig. 3).
Figure 3.
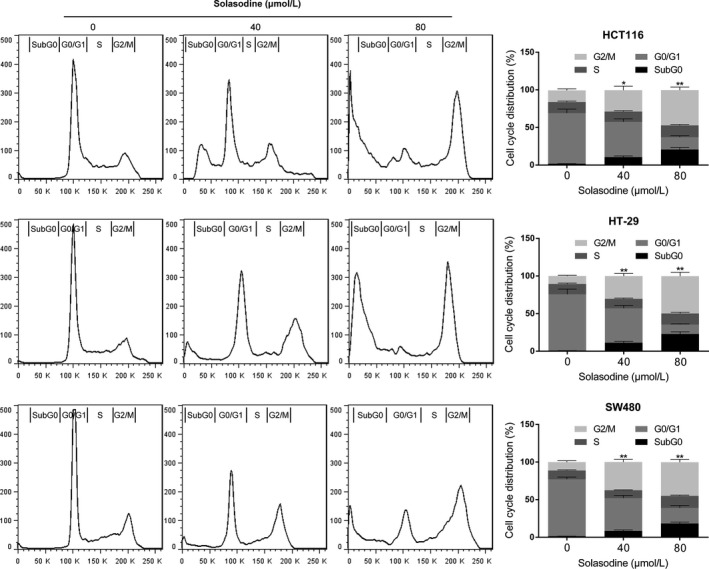
Solasodine induces G2/M‐phase cell cycle arrest in colorectal cancer cells. Cells were incubated with RNase, treated with solasodine for 48 h, stained with propidium iodide, and then analyzed by flow cytometry. Data are expressed as the mean ± SD of three experiments. *P < 0.05, **P < 0.01 versus control.
Solasodine induces apoptosis in human CRC cells
To evaluate the effects of solasodine treatment on CRC cell apoptosis, flow cytometric and Hoechst 33258 staining experiments were carried out. Compared with the control group, the apoptosis rate of CRC cells in solasodine‐treated groups (40 or 80 μmol/L for 48 h) was significantly improved (Fig. 4a). In addition, the solasodine‐treated cells with small, condensed, and fragmented nuclear variations were visualized by fluorescence microscopy after staining with Hoechst 33258, which indicated typical apoptotic morphology (Fig. 4b). It was noted that the powerful apoptotic influence of solasodine is comparable to 5‐Fu. Both results revealed that solasodine might have greater cytotoxicity than conventional chemotherapeutic agents, and that solasodine‐induced cell death is based on apoptosis.
Figure 4.
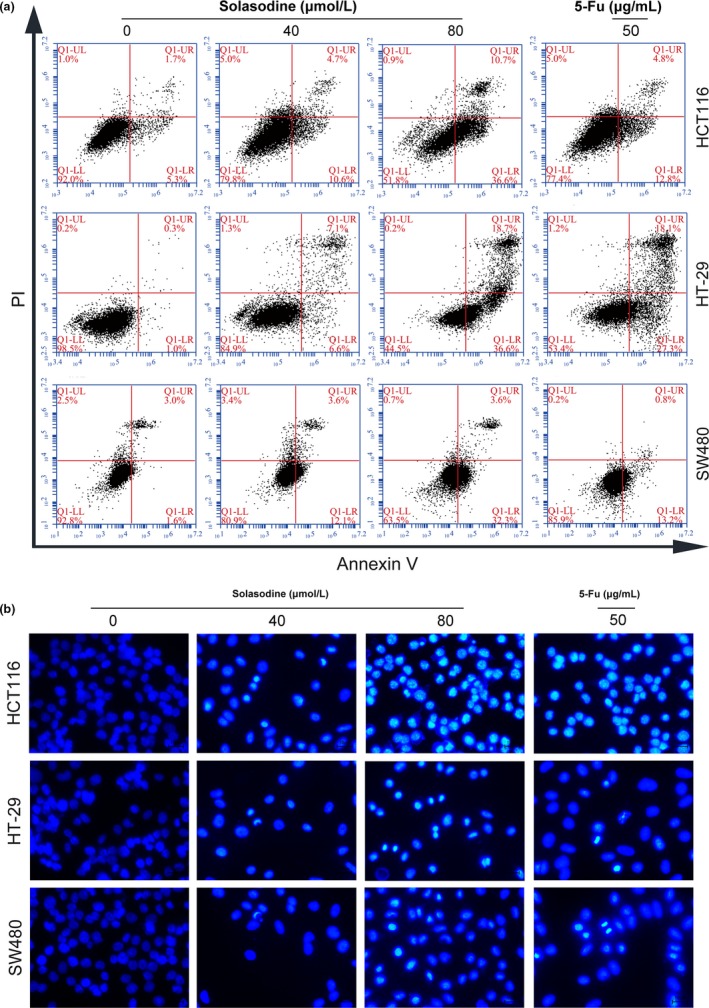
Solasodine induces apoptosis in colorectal cancer cells. (a) Three types of colorectal cancer cells were treated with solasodine and then analyzed by annexin V/propidium iodide (AV/PI) staining. Lower left (LL) quadrants, living cells (AV negative/PI negative). Lower right (LR) quadrants, early apoptotic cells (AV positive/PI negative). Upper right (UR) quadrants, late apoptotic cells (AV positive/PI positive). Upper left (UL) quadrants, necrotic cells (AV negative/PI positive). (b) Variations in nuclear morphology were examined by Hoechst 33258 staining and captured by fluorescence microscopy. 5‐Fu, 5‐fluorouracil.
Solasodine regulates apoptosis‐related genes in human CRC cells
Reverse transcription–qPCR was carried out to investigate the influences of solasodine on mRNA changes in molecules related to apoptosis. Solasodine treatment resulted in a dose‐dependent increase in the mRNA levels of Bax and Bak but decrease of Bcl‐2, Bcl‐xl, and Survivin (Fig. 5a). Western blot analysis was used to validate the apoptotic protein expressions. After incubation with solasodine for 48 h, protein levels of Bcl‐2, Bcl‐xl, and caspase‐9 decreased, while those of Bax, cleaved caspase‐8, cleaved caspase‐3, and cleaved PARP1 increased in a dose‐dependent way (Fig. 5b,c). These results revealed that solasodine‐induced CRC cell apoptosis occurs through stimulating caspase‐cascade activation.
Figure 5.
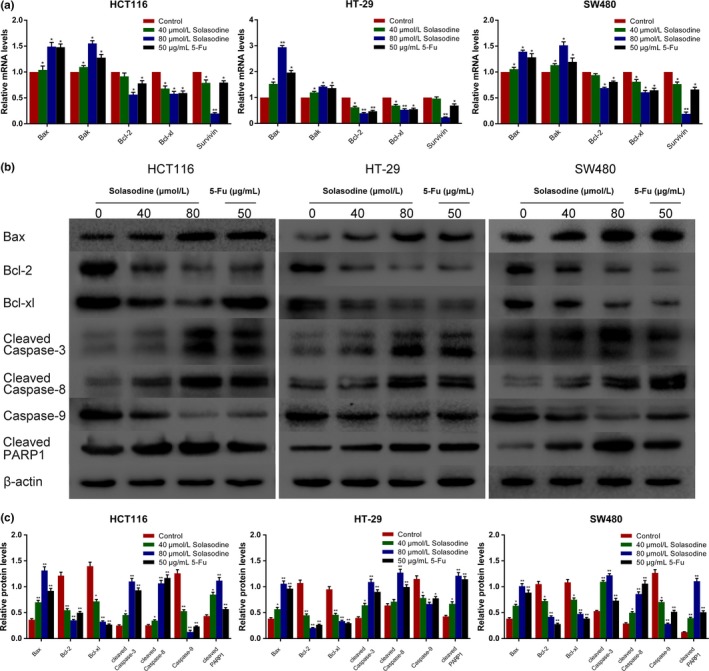
Solasodine regulates apoptosis‐associated genes. (a) RT–quantitative PCR analysis of apoptosis‐related mRNA. β‐Actin was selected as internal control. (b,c) Western blot analysis of apoptosis‐related proteins in all three cell lines. β‐Actin was chosen as loading control. Data are represented as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared to control. 5‐Fu, 5‐fluorouracil; PARP1, poly (ADP‐ribose) polymerase 1.
Solasodine inhibits CRC cell invasion and migration
Transwell assay was used to verify whether solasodine inhibits CRC cell invasive ability. As shown in (Fig. 6a,b), the number of cells that invaded the lower chamber was clearly reduced in response to solasodine for 48 h. The scratch wound assay also showed that solasodine‐treated cells migrated into the wound region more slowly than cells in the control group (Fig. 6c,d). Suppression of CRC cell invasion and migration by solasodine both showed a dose‐dependent trend.
Figure 6.
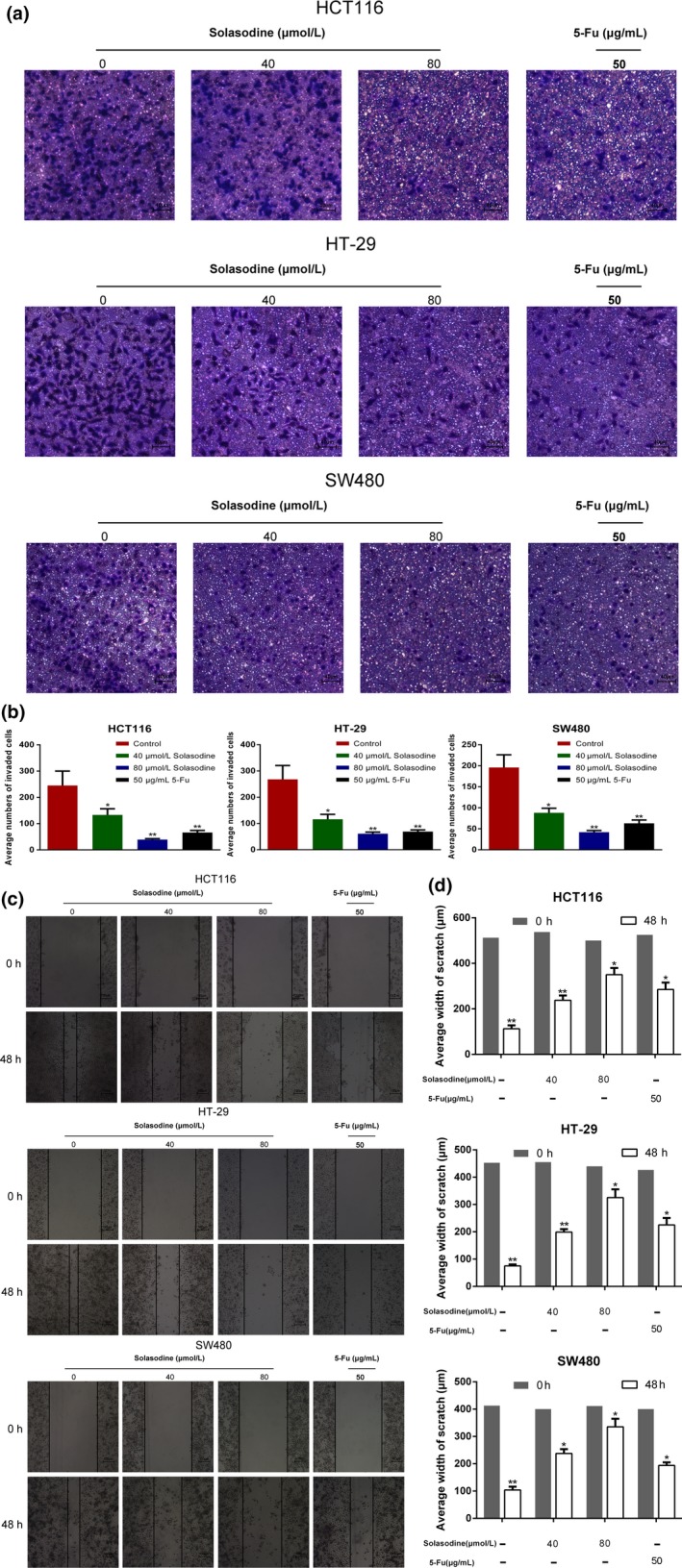
Solasodine inhibits colorectal cancer cell invasion and migration. (a,b) Transwell assay was carried out to test changes in colorectal cancer cell invasive ability by solasodine. (c,d) Wound healing assay assessed the inhibitive role of solasodine in cell migration. The average width of each wound region was observed under an inverted microscope. Results are expressed as mean ± SD. *P < 0.05, **P < 0.01. 5‐Fu, 5‐fluorouracil.
Solasodine modifies the expression of invasion‐ and adhesion‐related genes in human CRC cells
For the purpose of exploring the underlying mechanism of solasodine‐mediated suppression in cell motility, RT‐qPCR and Western blot analyses were applied to evaluate the expression of MMPs and E‐cadherin. As shown in Figure 7, MMP‐2, MMP‐9, and MMP‐14 decreased at both transcriptional and translational levels as the concentration of solasodine increased. Furthermore, solasodine treatment led to a gradual increase of E‐cadherin, suggesting its potential to block CRC cells from adhering to fibronectin.
Figure 7.
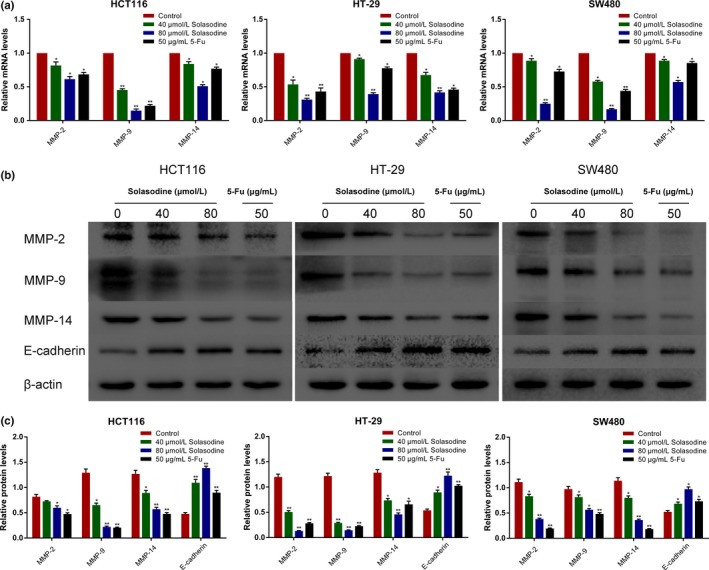
Solasodine modifies invasion‐associated genes. (a) Analysis of mRNA levels of MMPs using RT–quantitative PCR. β‐Actin was elected as internal control. (b,c) Western blot analysis of invasion‐ and adhesion‐related proteins. β‐Actin was chosen as loading control. Data are shown as the mean ± SD. *P < 0.05, **P < 0.01 versus control group. 5‐Fu, 5‐fluorouracil.
Solasodine suppresses CRC cells through regulation of the AKT/GSK‐3β/β‐catenin signaling pathway
Because the AKT/GSK‐3β/β‐catenin axis has been acknowledged to play a vital role in several malignancies, the regulative effect of solasodine on this pathway was examined. A prominent downregulation of p‐PI3K, PI3K, p‐AKT, AKT, mTOR, p‐GSK‐3β, and β‐catenin as well as upregulation of GSK‐3β and p‐β‐catenin were detected after treatment with solasodine (Fig. 8). Of note, solasodine reduced AKT/GSK‐3β/β‐catenin activity in a dose‐dependent manner, whereas the inhibitive degree in the high concentration group was similar to the 5‐Fu group and even stronger. To further substantiate that the inhibitive role of solasodine is based on regulation of AKT/GSK‐3β/β‐catenin signaling, specific activation of AKT by IGF‐1 (100 ng/mL for 4 h) was used ahead of treatment with 40 μmol/L solasodine for 48 h. Western blot analyses showed that IGF‐1 not only leads to stimulation of AKT/GSK‐3β/β‐catenin signaling but also partially repairs solasodine‐induced apoptosis (Fig. 9). In general, solasodine participates in attenuating the AKT/GSK‐3β/β‐catenin pathway to suppress CRC cells.
Figure 8.
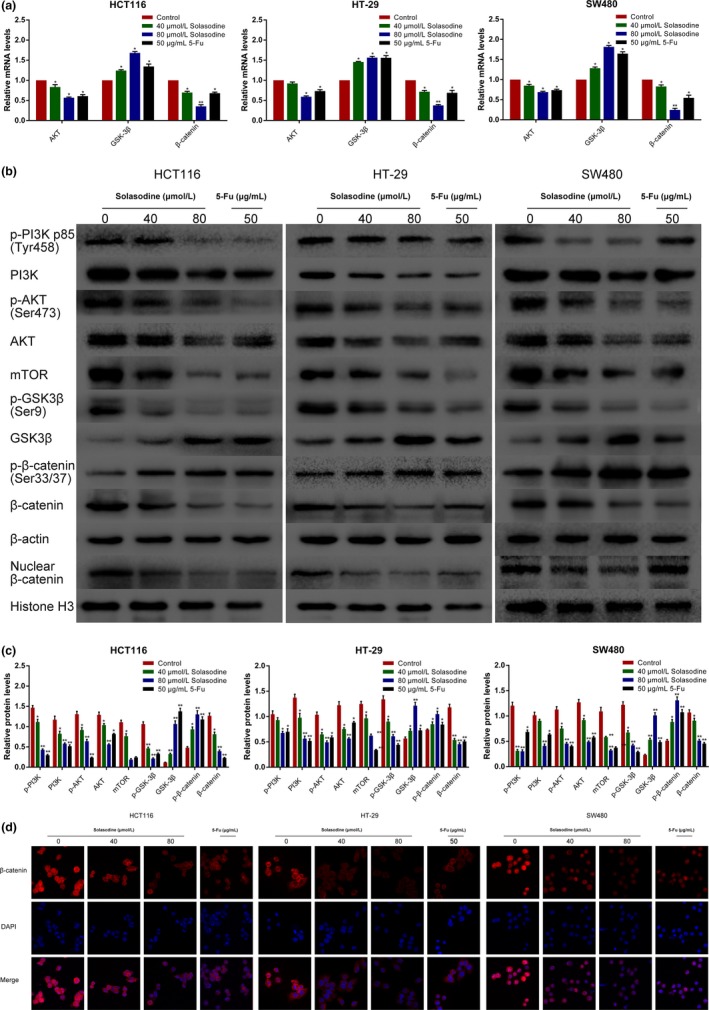
Solasodine regulates the AKT/glycogen synthase kinase‐3β (GSK‐3β)/β‐catenin signaling and restrains nuclear translocation of β‐catenin. (a) RT–quantitative PCR analysis was carried out to test mRNA expression of AKT/GSK‐3β/β‐catenin elements. (b,c) Western blot analysis of AKT/GSK‐3β/β‐catenin‐related proteins. Data are shown as the mean ± SD. *P < 0.05, **P < 0.01 versus control group. (d) Immunofluorescence staining analysis of cytoplasmic and nuclear expression of β‐catenin. Magnification, ×200.5‐Fu, 5‐fluorouracil; mTOR, mammalian target of rapamycin.
Figure 9.
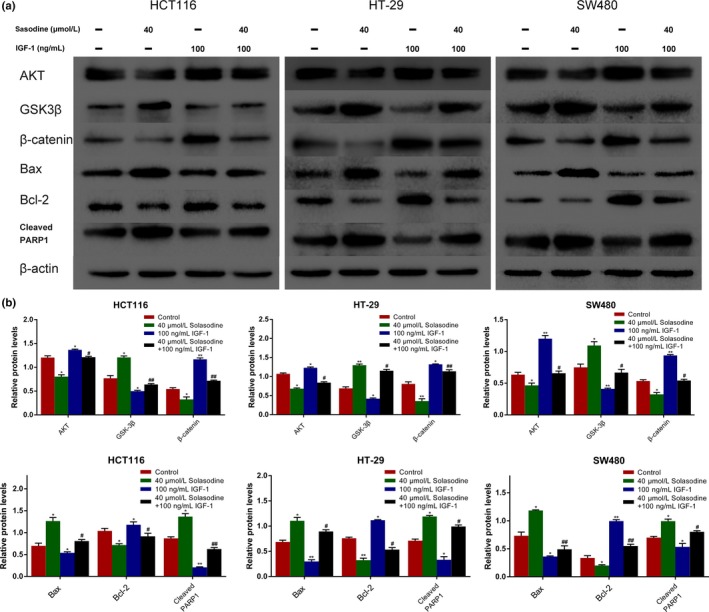
Solasodine‐induced apoptosis is regulated by the AKT/glycogen synthase kinase‐3β (GSK‐3β)/β‐catenin pathway. (a, b) Western blot analysis was applied to test whether AKT/GSK‐3β/β‐catenin is associated with apoptotic protein expression regulated by solasodine and insulin‐like growth factor 1 (IGF‐1). Data are presented as the mean ± SD. *P < 0.05, **P < 0.01 versus control. # P < 0.05, ## P < 0.01 versus solasodine‐treated group. PARP1, poly (ADP‐ribose) polymerase 1.
Solasodine terminates nuclear translocation of β‐catenin in human CRC cells
It is widely accepted that nuclear β‐catenin is the dominant initiator that triggers downstream genes, paving the way for carcinogenesis. Therefore, the nuclear translocation of β‐catenin after treatment with solasodine was monitored by Western blot analyses and immunofluorescence assays. The protein expression of nuclear β‐catenin was remarkably reduced, and immunofluorescence studies revealed that both cytoplasmic and nuclear β‐catenin were lessened when treated with solasodine (Fig. 8b,d). As β‐catenin was downregulated by solasodine when suppressing CRC cells, we applied β‐catenin siRNA and specific inhibitor XAV939 to suppress its activity and assess the variations in cell proliferation, movement, and apoptosis. The results showed that both β‐catenin siRNA and XAV939 not only inhibited growth and motility but also induced apoptosis effectively in all of these three cells (Figs 10, 11), indicating that the antitumor activity of solasodine might be based on prompting the degradation of cytoplasmic β‐catenin and hindering β‐catenin gathering in the nucleus.
Figure 10.
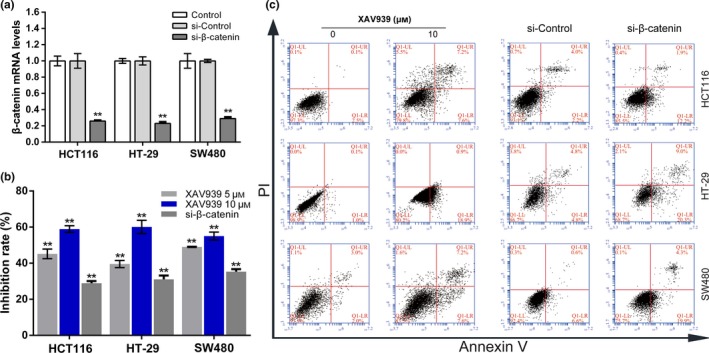
Role of β‐catenin in proliferation and apoptosis in colorectal cancer cells. (a) Cells were transfected with β‐catenin siRNA and incubated for 48 h. β‐Catenin mRNA expression was determined using RT‐PCR. (b) MTT assay was used to study inhibitive rates of transfected cells or cells incubated with XAV939 for 24 h. (c) Transfected cells and cells treated with 10 μM XAV939 for 24 h were analyzed by annexin V/propidium iodide (PI) staining.
Figure 11.
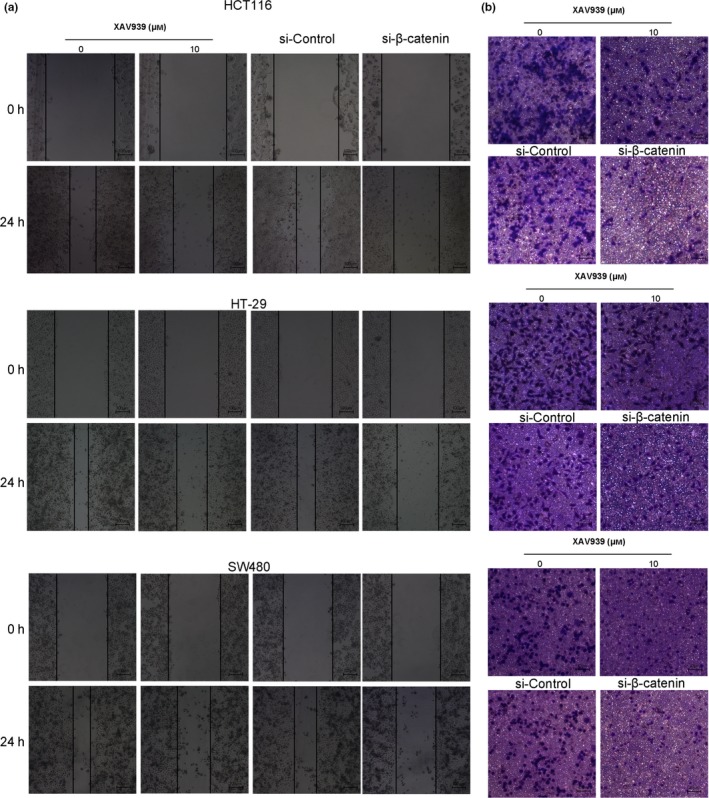
XAV939 inhibits colorectal cancer cell migration and invasion. (a) Wound healing assay tested the variation of cell migration and the average width of wound area was observed under an inverted microscope. (b) Transwell assay was undertaken to study the changes in cell invasion ability.
Solasodine suppresses xenograft tumor growth
We established a xenograft model with HCT116 cells in order to further research the influence of solasodine on tumor development in vivo. Tumors in both treated groups developed more slowly and their final volume and weight were conspicuously lower compared to the control group (Fig. 12). In addition, we investigated several molecules from tumor tissues. Both RT‐qPCR and Western blot studies indicated upregulation of apoptotic proteins but downregulation of metastasis‐associated genes and elements of the AKT/GSK‐3β/β‐catenin pathway (Fig. 13). In accordance, immunohistochemistry analysis showed apparent induction of Bax, cleaved PARP1, and E‐cadherin and reduction of MMP‐9, vascular endothelial growth factor, and β‐catenin (Fig. 14). These findings verified that solasodine antagonized CRC development in vivo.
Figure 12.
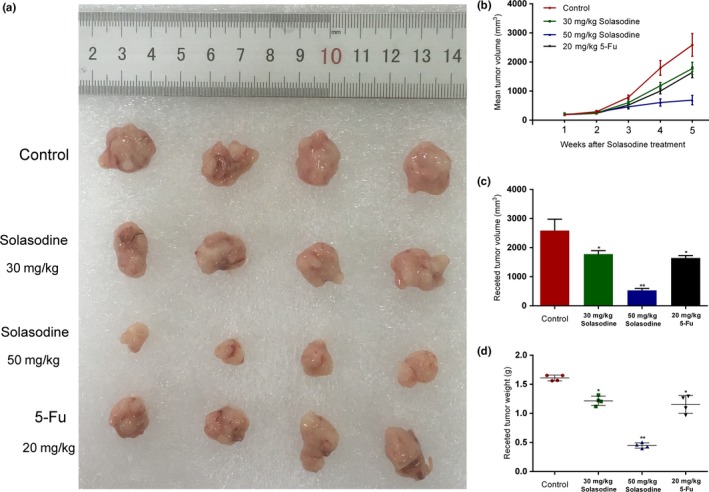
Solasodine confines tumor growth of mouse xenograft colorectal cancer in vivo. (a) Differences in excised tumor volume after the last treatment with solasodine. (b,c) Tumor volume was calculated after solasodine application. (d) Variations in tumor weight in the solasodine‐treated group compared to the control group. *P < 0.05, **P < 0.01. 5‐Fu, 5‐fluorouracil.
Figure 13.
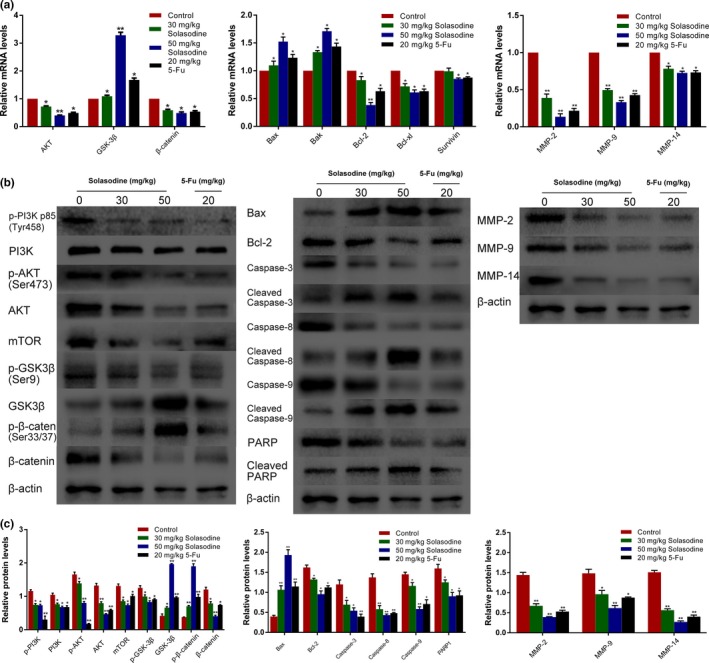
Solasodine induces apoptosis and prohibits AKT/glycogen synthase kinase‐3β (GSK‐3β)/β‐catenin signaling in HCT116 xenograft tumors. Tumors were abscised after the last solasodine i.p. injection and mRNA or protein was extracted. (a–c) RT–quantitative PCR and Western blot studies were undertaken to evaluate the levels of AKT/GSK‐3β/β‐catenin, apoptotic, and invasive molecules. 5‐Fu, 5‐fluorouracil; PARP, poly (ADP‐ribose) polymerase.
Figure 14.
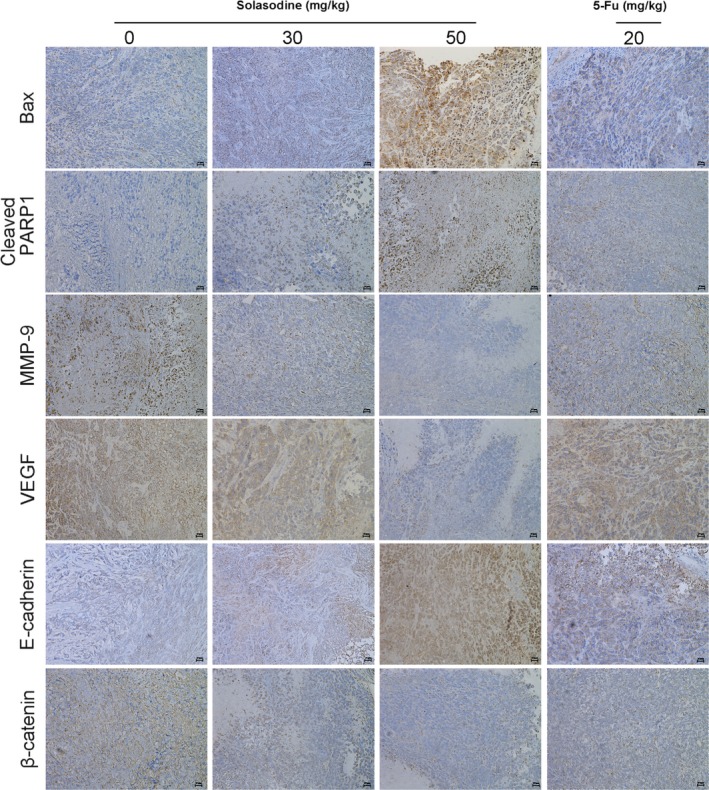
Solasodine varies apoptotic‐ and invasion‐relevant proteins and β‐catenin expression in mouse xenograft colorectal cancer. Immunohistochemical analysis of tumor specimens revealed the modifications between solasodine‐treated and control groups. 5‐Fu, 5‐fluorouracil; PARP1, poly (ADP‐ribose) polymerase 1; VEGF, vascular endothelial growth factor.
Discussion
In spite of recent progress in CRC diagnosis and treatments, the prognosis of advanced disease is still poor on account of the high frequency of recurrence and metastasis to distant organs,2, 3 highlighting the necessity of the search for new effective anticancer agents. Solasodine is a natural product that is present in solanaceous plants that has potent antitumor properties, as well as relatively fewer side‐effects. Solasodine has been reported to initially interact with free sterols to diffuse within the cancer cell wall, resulting in the expression of tissue necrosis factor receptors.20 Additionally, specific receptors on the cancer cell surface, such as endogenous sugar receptor and rhamnose‐specific binding lectin receptor, selectively bounded by the rhamnose moiety of solasodine to regulate signal transduction or drug transportation, can increase the bioavailability of solasodine to tumor cells while enhanced plasma or tissue enzymatic activity could lead to lack of toxicity towards normal cells.21, 22 However, the cytotoxicity of solasodine towards CRC cells and related mechanisms has not yet been illustrated. In our present study, the results indicated that solasodine could not only suppress CRC cell viability or synergistically with chemotherapeutic drugs, but also induce G2/M‐phase cell cycle arrest and inhibit CRC proliferation in vivo. As apoptosis resistance and invasion have been regarded as the most fundamental features of tumors, we examined the efficiency of solasodine on these essential biological behaviors.
The first serious analysis and discussion of apoptosis emerged in 1972 with the achievement made by Kerr et al.23 Of late, a considerable number of published works have implied the paramount role of caspase‐cascade during apoptosis, which is acknowledged to be induced by a cell death receptor‐mediated or mitochondrion‐dependent pathway.24 Investigators have shown that solasodine has great antineoplastic capability against several malignancies through both pathways as well as sensitizing cancer cells to chemotherapeutics.25, 26 In regard to the death receptor pathway, reciprocity between death ligands and receptors leads to intracellular structural variations, which recruits the activation of pro‐caspase‐8 and the succeeding cascade reaction. Endogenous apoptosis is marked by the release of cytochrome C followed by pro‐caspase‐9 activation, both of which participate in constituting the apoptosome and activating several executioners. Finally, two pathways result in the cleavage of caspase‐3 and PARP1, recognized to be the executants of cell apoptosis.27, 28 The Bcl‐2 family also plays a vital role in either prompting (Bax and Bak) or suppressing (Bcl‐2 and Bcl‐xl) apoptosis.29 Bcl‐2 has been verified to be able to terminate apoptosis by attenuating the release of cytochrome C to inactivate caspase.30 However, Bax can transport from the cytosol to mitochondria and nuclear membrane when inducing apoptosis. Overexpression of Bax is capable of antagonizing the protective effect of Bcl‐2 and accordingly impels cell death.31 In addition, as an important upstream protein of the Bcl‐2 family, p53 is able to lead to cell apoptosis based on its transcription‐dependent and ‐independent functions, which have been reported to be involved in solasodine‐induced apoptosis.32 In our present research, the results of annexin V–FITC/PI staining showed that solasodine induces apoptosis in three types of CRC cells as its concentration increases. Moreover, Hoechst 33258 analysis detected evident nuclear morphological variations, including condensation and fragmentation. The RT‐qPCR and Western blot studies further explored the molecular mechanisms underlying solasodine‐mediated apoptosis. They showed that solasodine activated caspase‐3, caspase‐8, caspase‐9, and PARP1 and led to increases of Bax and Bak, but decreases of Bcl‐2, Bcl‐xl, and Survivin, dose‐dependently. Additionally, we detected similar functions of solasodine in an HCT116 xenograft model, in which the tumor development was significantly restrained in vivo. Expression of apoptotic mRNA and protein extracted from tumors also showed the same tendencies as the results obtained in vitro. These results showed that solasodine efficiently induces CRC cell apoptosis through stimulating a caspase‐cascade reaction.
In recent years, an increasing number of reports have described the influential role of the interaction between the PI3K/AKT and Wnt/β‐catenin pathways, through the AKT/GSK‐3β/β‐catenin axis, in tumor progression.33, 34 Protein kinase B is a decisive molecule of PI3K/AKT signaling, and p‐AKT can phosphorylate the serine‐9 of GSK‐3β phosphorylated by p‐AKT can limit1the activity of GSK‐3β. Inactivated GSK‐3β loses the capability to phosphorylate and degrade cytoplasmic β‐catenin, accordingly improves nuclear translocation of β‐catenin to stimulate downstream genes. Our present study showed that treatment with solasodine weakens the expression of p‐PI3K, PI3K, p‐AKT, AKT, mTOR, p‐GSK‐3β, and β‐catenin in CRC cells, whereas the levels of GSK‐3β and p‐β‐catenin were strengthened. Moreover, Western blot and immunofluorescence analyses provide further evidence that solasodine suppresses both cytoplasm aggregation and nuclear localization of β‐catenin. We next chose specific inhibitor XAV939 and transfected cells with β‐catenin siRNA to suppress β‐catenin activity; subdued cell proliferation and motility, as well as increased apoptotic rates, were assessed in response. Research on the expression of related mRNAs and proteins from in vivo tumors revealed parallel outcomes. Mechanically, pretreatment with a specific activator of AKT (IGF‐1) partly blocked solasodine‐induced variations of apoptotic protein levels in all three types of CRC cells, indicating that solasodine initiates apoptosis through abrogation of the AKT/GSK‐3β/β‐catenin pathway indeed. Protein kinase B is important for the anticancer effects of solasodine and our results have verified the suppression of its activation as a critical event when inhibiting CRC cells. At the transcriptional level, solasodine might decrease the stability of AKT mRNA and activate its degradation mechanism through regulation of several molecules, including RNA helicase of the DEAD box family, histone deacetylase, and the β‐galactoside binding protein cytokine.35, 36, 37 At the translation level, PI3K is one of the primary upstream molecules of AKT, which is a heterodimeric enzyme consisted of p110 catalytic and p85 regulatory subunits. Several reports have shown that these two subunits modify PI3K activity to further regulate its target molecule AKT,38, 39 supporting our hypothesis that solasodine probably targets p110α/β/δ or p85α/β to resist PI3K/AKT signal transduction. Protein kinase B is composed of three parts from the amino to the carboxyl terminal, including PH, kinase, and regulatory domains. Phosphorylation of Thr308 and Ser473 is meaningful while Ser473 activation is a necessary condition for stimulating AKT, which can be regulated by choline kinase.40 We accordingly consider that the inhibition of choline kinase to dephosphorylate AKT at Ser473 might be part of the potential mechanism involved in solasodine‐associated termination of AKT phosphorylation. Mammalian target of rapamycin is an important downstream component of PI3K/AKT signal and contains two protein complexes (mTORC1 and mTORC2); mTORC2 participates in regulating AKT Ser473 phosphorylation, whereas S6K, one of the substrate molecules of mTORC1, is able to initiate negative feedback loops to restrain AKT.41, 42 Based on this, we speculate that solasodine could control the activation of mTORCs to further suppress AKT. Moreover, lipid rafts located in the cell membrane, which have been reported to facilitate AKT recruitment, might be another target for solasodine.43 All of these remain to be researched for further insight into solasodine‐mediated AKT inactivation in CRC cells.
Infiltrative and metastatic characters of malignancies are the most important factors determining severity and the degradation of the ECM as well as the basement membrane is the prerequisite of tumor invasion. Matrix metalloproteases are a family of enzymes that can destroy the activity of the ECM; among them, MMP‐2, MMP‐9, and MMP‐14 are crucial elements that give rise to CRC progression.44 E‐cadherin is a transmembrane molecule that mediates adhesion between adjacent cells, whose degree of expression has an inverse association with invasive capability in CRC.45 Our results of wound‐healing and Transwell assays showed that solasodine significantly reduces CRC cell migration and invasion. Solasodine was also found to be capable of lowering MMP‐2, MMP‐9, and MMP‐14 and raising E‐cadherin levels, which was further investigated in vivo experiments. This may provide a meaningful mechanism underlying solasodine‐related termination of colorectal cancer cell motility.
In summary, our current study presents assertive evidence that solasodine induces CRC cell apoptosis and hinders cell migration and invasion by regulating the AKT/GSK‐3β/β‐catenin signaling pathway. These antiproliferative and antimetastatic properties imply that solasodine could provide new insight into the research and development for valid therapeutic applications for human CRC.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- 5‐Fu
5‐fluorouracil
- ACTB
β‐actin
- CRC
colorectal cancer
- FCM
flow cytometry
- GSK‐3β
glycogen synthase kinase‐3β
- IGF‐1
insulin‐like growth factor‐1
- mTORC
mammalian target of rapamycin complex
- mTOR
mammalian target of rapamycin
- OD
optical density
- PARP1
poly (ADP‐ribose) polymerase 1
- PI
propidium iodide
- qPCR
quantitative PCR
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81473605, 81202954, 81303124), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and Scientific Research Innovation for Graduates from Jiangsu Higher Education Institutions (SJZZ16_0177, SJLX15_0440).
Cancer Sci 108 (2017) 2248–2264
Funding Information
National Natural Science Foundation of China (no. 81473605, 81202954, 81303124); Priority Academic Program Development of Jiangsu Higher Education Institutions; Scientific Research Innovation for Graduates from Jiangsu Higher Education Institutions (SJZZ16_0177, SJLX15_0440).
Contributor Information
Shen‐lin Liu, Email: lsljsszyy@163.com.
Hui Cai, Email: njzyy_caihui@163.com.
References
- 1. Ferlay J, Ervik M, Dikshit R et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Ece E, Suayib Y. Maintenance strategy in metastatic colorectal cancer: a systematic review. Cancer Treat Rev 2016; 42: 82–90. [DOI] [PubMed] [Google Scholar]
- 3. Kim KY, Cha IH, Ahn JB et al Estimating the adjuvant chemotherapy effect in elderly stage II and III colon cancer patients in an observational study. J Surg Oncol 2013; 107: 613–8. [DOI] [PubMed] [Google Scholar]
- 4. Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med 2009; 361: 2449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol 2000; 18: 1967–79. [DOI] [PubMed] [Google Scholar]
- 6. Samuels Y, Wang Z, Bardelli A et al High frequency of mutations of the PIK3CA gene in human cancers. Science 2004; 304: 554. [DOI] [PubMed] [Google Scholar]
- 7. Parsons DW, Wang TL, Samuels Y et al Colorectal cancer: mutations in a signalling pathway. Nature 2005; 436: 792. [DOI] [PubMed] [Google Scholar]
- 8. Wang G, Feng CC, Chu SJ et al Toosendanin inhibits growth and induces apoptosis in colorectal cancer cells through suppression of AKT/GSK‐3β/β‐catenin pathway. Int J Oncol 2015; 47: 1767–74. [DOI] [PubMed] [Google Scholar]
- 9. Cross DA, Alessi DR, Cohen P et al Inhibition of glycogen synthase kinase‐3 by insulin mediated by protein kinase B. Nature 1995; 378: 785–9. [DOI] [PubMed] [Google Scholar]
- 10. Kolligs FT, Bommer G, Goke B. Wnt/beta‐catenin/tcf signaling: a critical pathway in gastrointestinal tumorigenesis. Digestion 2002; 66: 131–44. [DOI] [PubMed] [Google Scholar]
- 11. Catalani E, Proietti F, Zecchini S et al Natural products from aquatic eukaryotic microorganisms for cancer therapy: perspectives on anti‐tumour properties of ciliate bioactive molecules. Pharmacol Res 2016; 113: 409–20. [DOI] [PubMed] [Google Scholar]
- 12. Crotti S, Posocco B, Marangon E et al Mass spectrometry in the pharmacokinetic studies of anticancer natural products. Mass Spectrom Rev 2017; 36: 213–51. [DOI] [PubMed] [Google Scholar]
- 13. Sharma T, Airao V, Panara N et al Solasodine protects rat brain against ischemia/reperfusion injury through its antioxidant activity. Eur J Pharmacol 2014; 725: 40–6. [DOI] [PubMed] [Google Scholar]
- 14. Lecanu L, Hashim A, McCourty A et al The naturally occurring steroid solasodine induces neurogenesis in vitro and in vivo . Neuroscience 2011; 183: 251–64. [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Chang WQ, Zhang M et al Natural Product solasodine‐3‐O–D‐glucopyranoside inhibits the virulence factors of Candida albicans. FEMS Yeast Res 2015; 15: 1–8. [DOI] [PubMed] [Google Scholar]
- 16. Cui CZ, Wen XS, Cui M et al Synthesis of solasodine glycoside derivatives and evaluation of their cytotoxic effects on human cancer cells. Drug Discov Ther 2012; 6: 9–17. [PubMed] [Google Scholar]
- 17. Punjabi S, Cook L, Kersey P et al Solasodine glycoalkaloids: a novel topical therapy for basal cell carcinoma. A double‐blind, randomized, placebo‐controlled, parallel group, multicenter study. Int J Dermatol 2008; 47: 78–82. [DOI] [PubMed] [Google Scholar]
- 18. Cham BE, Chase TR. Solasodine rhamnosyl glycosides cause apoptosis in cancer cells. Do they also prime the immune system resulting in long‐term protection against cancer? Planta Med 2012; 8: 349–53. [DOI] [PubMed] [Google Scholar]
- 19. Drake JM, Graham NA, Stoyanova T et al Oncogene‐specific activation of tyrosine kinase networks during prostate cancer progression. Proc Natl Acad Sci USA 2012; 109: 1643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Punjabi S, Cook LJ, Kersey P et al Solasodine glycoalkaloids: a novel topical therapy for basal cell carcinoma. A double‐blind, randomized, placebo‐controlled, parallel group, multicenter study. Int J Dermatol 2008; 1: 78–82. [DOI] [PubMed] [Google Scholar]
- 21. Cham BE, Daunter B. Solasodine glycosides. Selective cytotoxicity for cancer cells and inhibition of cytotoxicity by rhamnose in mice with sarcoma 180. Cancer Lett 1990; 3: 221–5. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Gao J, Gu G et al In situ RBL receptor visualization and its mediated anticancer activity for solasodine rhamnosides. ChemBioChem 2011; 16: 2418–20. [DOI] [PubMed] [Google Scholar]
- 23. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide‐ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matthews GM, Newbold A, Johnstone RW. Intrinsic and extrinsic apoptotic pathway signaling as determinants of histone deacetylase inhibitor antitumor activity. Adv Cancer Res 2012; 116: 165–97. [DOI] [PubMed] [Google Scholar]
- 25. Kuo KW, Hsu SH, Li YP et al Anticancer activity evaluation of the solanum glycoalkaloid solamargine. Triggering apoptosis in human hepatoma cells. Biochem Pharmacol 2000; 12: 1865–73. [DOI] [PubMed] [Google Scholar]
- 26. Shiu LY, Chang LC, Liang CH et al Solamargine induces apoptosis and sensitizes breast cancer cells to cisplatin. Food Chem Toxicol 2007; 11: 2155–64. [DOI] [PubMed] [Google Scholar]
- 27. Prenek L, Boldizsár F, Kugyelka R et al The regulation of the mitochondrial apoptotic pathway by glucocorticoid receptor in collaborationwith Bcl‐2 family proteins in developing T cells. Apoptosis 2017; 22: 239–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang G, Ding L, Renegar R. Hydroxycamptothecin‐loaded Fe3O4 nanoparticles induce human lung cancer cell apoptosisthrough caspase‐8 pathway activation and disrupt tight junctions. Cancer Sci 2011; 102: 1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Basanez G, Soane L, Hardwick JM. A new view of the lethal apoptotic pore. PLoS Biol 2012; 10: e1001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Novais PC, Paula AA, Rodrigues AA Jr et al Apoptotic genes as a prognostic factor in penile epidermoid carcinoma. J Clin Oncol 2009; 27: e22208. [Google Scholar]
- 31. Gahl RF, Dwivedi P, Tjandra N. Bcl‐2 proteins bid and bax form a network to permeabilize the mitochondria at the onset of apoptosis. Cell Death Dis 2016; 7: e2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Zhao Y, Wu WK et al Solamargine induces apoptosis associated with p53 transcription‐dependent and transcription‐independent pathways in human osteosarcoma U2OS cells. Life Sci 2011; 7–8: 314–21. [DOI] [PubMed] [Google Scholar]
- 33. Wu K, Fan J, Zhang L et al PI3K/Akt to GSK3β/β‐catenin signaling cascade coordinates cell colonization for bladder cancer bone metastasis through regulating ZEB1 transcription. Cell Signal 2012; 24: 2273–82. [DOI] [PubMed] [Google Scholar]
- 34. Arqués O, Chicote I, Puig I et al Tankyrase Inhibition Blocks Wnt/β‐Catenin Pathway and Reverts Resistance to PI3K and AKT Inhibitors in the Treatment of Colorectal Cancer. Clin Cancer Res 2016; 22: 644–56. [DOI] [PubMed] [Google Scholar]
- 35. Sarkar M, Khare V, Guturi KK et al The DEAD box protein p68: a crucial regulator of AKT/FOXO3a signaling axis in oncogenesis. Oncogene 2015; 47: 5843–56. [DOI] [PubMed] [Google Scholar]
- 36. Thomas S, Thurn KT, Raha P et al Efficacy of histone deacetylase and estrogen receptor inhibition in breast cancer cells due to concerted down regulation of Akt. PLoS One 2013; 7: e68973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wells V, Mallucci L. Phosphoinositide 3‐kinase targeting by the beta galactoside binding protein cytokine negates akt gene expression and leads aggressive breast cancer cells to apoptotic death. Breast Cancer Res 2009; 1: R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jaiswal BS, Janakiraman V, Kljavin NM et al Somatic mutations in p85α promote tumorigenesis through class IA PI3K activation. Cancer Cell 2009; 6: 463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Q, Liu P, Spangle JM et al PI3K‐p110α mediates resistance to HER2‐targeted therapy in HER2 + , PTEN‐deficient breast cancers. Oncogene 2016; 27: 3607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chua BT, Gallego‐Ortega D, Ramirez de Molina A et al Regulation of Akt(ser473) phosphorylation by Choline kinase in breast carcinoma cells. Mol Cancer 2009; 8: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarbassov DD, Guertin DA, Ali SM et al Phosphorylation and Regulation of Akt/PKB by the Rictor‐mTOR Complex. Science 2005; 5712: 1098–101. [DOI] [PubMed] [Google Scholar]
- 42. Harrington LS, Findlay GM, Gray A et al The TSC1‐2 tumor suppressor controls insulin–PI3K signaling via regulation of IRS proteins. J Cell Biol 2004; 2: 213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lasserre R, Guo XJ, Conchonaud F et al Raft nanodomains contribute to Akt/PKB plasma membrane recruitment and activation. Nat Chem Biol 2008; 9: 538–47. [DOI] [PubMed] [Google Scholar]
- 44. Schwegmann K, Bettenworth D, Hermann S et al Detection of Early Murine Colorectal Cancer by MMP‐2/‐9‐Guided Fluorescence Endoscopy. Inflamm Bowel Dis 2016; 22: 82–91. [DOI] [PubMed] [Google Scholar]
- 45. Sugiyama M, Oki E, Nakaji Y et al High expression of the Notch ligand Jagged‐1 is associated with poor prognosis after surgery for colorectal cancer. Cancer Sci 2016; 107: 1705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


