Abstract
Pyrroloquinoline quinone (PQQ), considered as an ROS scavenger,could protect mitochondrial activity from damage of oxidative stress. To determine the role of PQQ supplement in rescuing long bone osteoporosis in Bmi-1-/- mice. We fed Bmi-1 knockout mice a diet supplemented with PQQ (BKO+PQQ), BKO mice with normal diet (BKO) and wild type mice with normal diet (WT) as controls. We compared the differences of skeletal phenotype by means of imaging, histopathological and molecular biology methods in three groups of animals. Results showed that BKO+PQQ mice increased morphology of tibia, decreased X-ray transmittance, and increased bone density, thickness of cortical bone, width of growth plate and trabecular bone mass compared with BKO mice. Our study also investigated that, compared mice BKO, PCNA positive cells percentage of tibial growth plate areas significantly increased in BKO+PQQ mice, and TUNEL positive cells percentage was significantly decreased. To detect the effect of PQQ on osteoblast formation of tibiae. Our results showed, compared with BKO mice, osteogenic cell, osteoblast number areas, ALP, Col I and OCN positive areas significantly increased in tibia of BKO+PQQ mice. Further studies showed that supplemental PQQ played a role in anti-osteoporosis by up-regulating antioxidant capacity, inhibiting oxidative stress and reducing DNA damage, down-regulating CDKI proteins levels, and decreasing cell apoptosis. This study not only reveals the mechanism of PQQ supplementation in anti-osteoporosis, but also provides the experimental and theoretical basis for the clinical application of PQQ in osteoporosis.
Keywords: Osteoporosis, pyrroloquinoline quinone, Bmi-1, oxidative stress
Introduction
Osteoporosis, a degenerative skeletal disorder, known as “the silent thief” because the gradual loss of bone associated with this disease usually occurs over the years, and there are usually no noticeable symptoms until the bones are so fragile that a fracture occurs [1,2]. Approximately 1 in 2 women and 1 in 5 men older than 50 years will eventually experience osteoporotic fractures [3]. Osteoporosis is “a major public health threat” that is projected to lead to 8.1 million fractures (78% women, 22% men) during the period between 2010 and 2050 [4]. The condition costs our healthcare system $18 billion per year [5]. In recent years, the theory of oxidative stress and aging has become a hot study to explain mechanism for osteoporosis.
More than 50 years ago, Denham Harman proposed the free radical or oxidative stress theory of aging [6]. He hypothesized that free radicals and/or reactive oxygen species (ROS) can be produced endogenously from normal cellular metabolic processes. In this theory, imbalances between ROS and antioxidants can lead to oxidative stress that damages various macromolecules. A growing body of evidence demonstrated that increased production of ROS played an important role in the development of various age-related diseases.
B cell-specific Moloney MLV insertion site-1 (Bmi-1) belongs to the polycomb group (PcG) genes, which are transcriptional repressors that are essential for the maintenance of appropriate gene expression patterns during development [7]. It is well established that deletion of Bmi-1 mice exhibit a total body premature aging phenotype including osteoporosis [8]. Protection against oxidative stress and apoptosis emerges as an important Bmi-1-downstream pathway as well, either by reducing P53 levels via Bmi-1-mediated repression of the INK4a/Arf locus or via modulation of the oxidative stress response in an INK4a/Arf-independent manner. In Bmi1-/- mice, the increase in ROS coincided with an increase in DNA damage and an activation of the DNA damage repair pathways, and treatment with NAC or targeting of CHK2 at least partially restored some of the phenotypes [9].
Pyrroloquinoline quinone (PQQ) was first identified as a novel cofactor of ethanol and glucose dehydrogenase in the methylotrophic bacteria, now is considered an important nutritional growth factor [10,11]. There is mounting evidence that, PQQ is a 4,5-dihydro-4,5-dioxo-1H-Pyrrolo[2,3-f] quinoline-2,7,9-tricarboxylic acid, is thought to be bacterial glucose dehydrogenase redox cofactor, widely distributed in plants, bacteria, animal, food and many biological fluid, it is soluble in water and thermal stability, divided into the oxidized and reduced forms [12,13].
Previous studies showed that PQQ had multiple physiological functions, such as promoting growth and reproduction [14-16], neural and cardiovascular protection [17-19], radioprotective function [20] and enhancing immune function and resisting tumors [21]. However, the potential mechanism remains poorly understood. PQQ was also reported to be anti-oxidant and pro-oxidant to protect mitochondria from oxidative stress induced DNA damages [22-25]. Further studies defined PQQ as an ROS scavenger in oxidative stress [26].
In recent years, PQQ has become a hot research, because PQQ has been shown to act as an antioxidant by scavenging superoxide radicals and to protect mitochondria from oxidative stress-induced DNA damages. However, the mechanism underlying effect of PQQ on Bmi1-/- mice has not been elucidated.
To investigate the potential mechanism of PQQ, in the present study, we utilize Bmi-1 knockout mice induced typical premature aging and osteoporosis phenotype. We hypothesize whether PQQ could rescue deletion of Bmi-1 induced aging and osteoporosis through anti-oxidative stress pathway.
Materials and methods
Mice and genotyping
The Bmi-1 heterozygote (Bmi-1+/-) mice (129Ola/FVB/N hybrid back-ground) were backcrossed 10-12 times to the C57BL/6J background and mated to generate Bmi-1 homozygote (Bmi-1-/-) and their wild-type (WT) littermates genotyped by PCR, as described previously [8,27]. Mice were maintained in the Experimental Animal Center of Nanjing Medical University. This study was carried out in strict accordance with the guidelines of the Institute for Laboratory Animal Research of Nanjing Medical University. The protocol was approved by the Committee on the Ethics of Animal Experiments of Nanjing Medical University.
Animals treatment and supplementary of dietary PQQ
Purified PQQ was given free by Professor Chuanjun Wen in Academy of Life Science, Nanjing Normal University. PQQ-supplemented diet was made in Beijing cooperation Feed Co. Ltd., China. In vivo, mice were divided into 3 groups of 6 mice each and treated as following:
(1) Normal diet (WT) group: 3-week weaning littermate wild type mice were fed normal diet for 4 weeks.
(2) Normal diet (BKO) group: 3-week weaning littermate Bmi-1 knockout mice were fed normal diet for 4 weeks.
(3) PQQ-supplemented diet (BKO+PQQ) group: 3-week weaning littermate Bmi-1 knockout mice were fed PQQ-supplemented diet (4 mg PQQ/kg in normal diet) for 4 weeks [28]. Seven weeks later, 3 groups of 6 mice each were sacrificed for further analysis.
Analysis of mice phenotype, percent survival, and body weight
In order to investigate the effect of PQQ on Bmi-1-/- mice total body, In vivo, Other animals were divided into 3 groups of 6 mice each (WT, BKO, BKO+PQQ). We carried out statistic analysis of phenotype, body weight, and percent survival in different groups mice respectively.
Skeletal radiography
Tibiae were removed and dissected free of soft tissue. Contact radiographs were taken using a FaxitronModel 805 radiographic inspection system (Faxitron Contact, Faxitron, Germany; 22 kV and 4-minute exposure time). X-Omat TL film (Eastman Kodak Co., Rochester, NY, USA) was used and processed routinely.
Micro-computed tomography (μCT)
Tibiae obtained from 7-week-old mice were dissected free of soft tissue, fixed overnight in 70% ethanol, and analyzed by μCT with a SkyScan 1072 scanner and associated analysis software (SkyScan, Antwerp, Belgium) as described previously. Briefly, image acquisition was performed at 100 kV and 98 μA with a 0.9-degree rotation between frames. During scanning, the samples were enclosed in tightly fitting plastic wrap to prevent movement and dehydration. Thresholding was applied to the images to segment the bone from the background. 2D images were used to generate 3D renderings using the 3D creator software suppliedwith the instrument. The resolution of the μCT images is 18.2 μm.
Histology
Tibiae were removed and fixed in PLP fixative (2% paraformalde-hyde containing 0.075 M lysine and 0.01 M sodium periodate) overnight at 4°C and processed histologically as described previously [8]. Proximal ends of tibiae were decalcified in EDTA glycerol solution for 5 to 7 days at 4°C. Decalcified tibiae were dehydrated and embedded in paraffin, after which 5 μm sections were cut on a rotary microtome. The sections were stained with hematoxylin and eosin (H&E) or histochemically for total collagen (TCOL) or alkaline phosphatase activity (ALP), or tartrate-resistant acid phosphatase (TRAP) activity, or immunohistochemically as described below. Alternatively, undecalcified tibiae were embedded in LR White acrylic resin (London Resin Company, Ltd., London, UK), and 1 μm sections were cut on an ultramicrotome. These sections were observed under fluorescence microscopy.
Immunohistochemical staining
Immunohistochemical staining was carried out for PCNA, COL 1, OCN using the avidin-biotin-peroxidase complex technique with affinity-purified goat anti-rabbit PCNA antibody (Abcam, UK), affinity-purified goat anti-mouse COL 1 antibody (Santa Cruz, CA, USA), affinity-purified goat anti-mouse OCN (Cell Signal, China) [8]. Briefly, dewaxed and rehydrated paraffin-embedded sections were incubated with methanol-hydrogen peroxide (1:10) to block endogenous peroxidase activity and then washed in Tris-buffered saline (pH 7.6). The slides were then incubated with the primary antibodies overnight at room temperature. After rinsing with Tris-buffered saline for 15 min, sections were incubated with biotinylated secondary antibody (Sigma, St. Louis, MO, USA). Sections were then washed and incubated with the Vectastain Elite ABC reagent (Vector Laboratories, Burlington, Canada) for 45 min. After washing, brown pigmentation was likewise produced using 3,3-diaminobenzidine (DAB). Finally, the stained sections were counterstained with H&E staining. Images were acquired with a Leica microscope (Leica DM4000B, Solms, German) equipped with Leica software.
Evaluation of apoptotic cells by TUNEL staining
The apoptotic cell of tibiae was evaluated using the terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) technique (20 mg/mL, Cat. no. 21627, Chemicon International, Temecula, CA, USA) [29]. To count the TUNEL-positive cells in the parotid glands tissues, the ocular micrometer compatible with an Olympus BX51 microscope was used.
Western blot analysis
Proteins were extracted from long bones and quantitated by a kit (Bio-Rad, Mississauga, Ontario, Canada). Protein samples were fractionated by SDS-PAGE and transferred to nitro-cellulose membranes. Western blot was carried out as described previously (28) using antibodies against P16 (goat anti-mouse, M-156, Santa Cruz Biotechnology, USA), P19 (goat anti-mouse, Santa Cruz Biotechnology, USA), P21 (goat anti-mouse, M19, Santa Cruz Biotechnology, USA), P27 (goat anti-mouse, Zymed Laboratories, Santa Cruz, CA, USA), P53 (goat anti-mouse, Cell Signal, China), SOD1 and SOD2 (goat anti-rabbit, Abcam, UK), prdx I (goat anti-rabbit, Abcam, UK), prdx IV (goat anti-rabbit, Abcam, UK), Caspase-3 (goat anti-rabbit, Cell Signal, China), γH2AX (goat anti-mouse, Santa Cruz, CA, USA), and β-actin (goat anti-rabbit, Santa Cruz Biotechnology, USA). Bands were visualized using enhanced chemiluminescence (ECL, Amersham) and quantitated by Scion Image Beta 4.02 (Scion Corporation, Bethesda, MD, USA).
Detection of ROS levels
The femurs tissues were converted into single-cell suspensions containing 5×105 cells/mL. We used 2’,7’-dichlorofluorescein diacetate (DCFH-DA, Sigma, USA) for detection of intracellular ROS. Fluorescence intensity is proportional to oxidant production [30]. DCFH-DA was added to bone cell suspensions to yield final concentrations of 20 μmol/L. Then, the cells were incubated at 37°C for 30 min in the dark, washed twice with 0.01 mol/L phosphate-buffered saline (PBS), and centrifuged at ×300 g for 5 min. ROS levels were measured by mean fluorescence intensity (MFI) of 10,000 cells using a flow cytometer (Becton, Dickinson and Co, USA).
Computer-assisted image analysis
After H&E staining or histochemical or immunohistochemical staining of sections from 3 groups of 6 mice each, images of interested fields were photographed with a SONY digital camera. Images of micrographs from single section were digitally recorded using a rectangular template, and recordings were processed and analyzed using Northern Eclipse image analysis software as described previously [9].
Statistic analysis
All data were expressed as mean ± standard error. Statistical analysis of numeration data were performed using analysis of X2 text, while statistical analyses of measurement data were performed using analysis of student’s t-test. The significance level was set at P<0.05.
Results
Effect of PQQ on premature aging phenotype in Bmi-1-/- mice
To investigate whether PQQ-supplemented diet could rescue Bmi-1-/- mice premature aging phenotype, we performed statistic analysis of phenotype (Figure 1A), body weight (Figure 1B), and percent survival (Figure 1C) in different groups mice respectively. Compared with only normal diet feeding (WT) mice, BKO mice with normal diet significantly showed a premature aging phenotype, body weight loss, and shortened percent survival. Whereas BKO mice with PQQ-supplemented diet partially rescued total body size, increased body weight, and prolonged percent survival compared to BKO mice with only normal diet. These data supported that PQQ-supplemented diet partially rescued Bmi-1-/- mice premature aging phenotype compared with normal diet.
Figure 1.
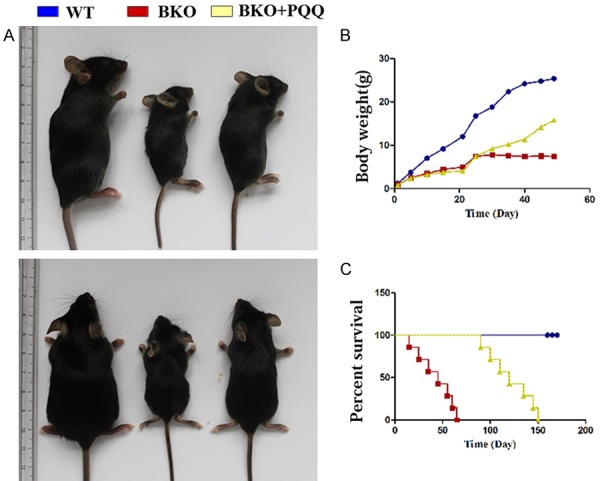
Effect of PQQ on phenotype, body weight, percent survival in BKO mice. A. Effect of PQQ-supplemented diet on BKO mice size. B. Effect of PQQ-supplemented diet on BKO mice body weight. C. Effect of PQQ-supplemented diet on BKO mice percent survival.
Effect of PQQ on bone growth retardation in Bmi-1-/- mice
To determine whether PQQ-supplemented diet could rescue bone growth retardation in Bmi-1-/- mice, We comparatively analysized related parameters of bone growth in different groups mice respectively through X-ray photography (Figure 2A), histology (Figure 2C), PCNA immunohistochemistry (Figure 2E) and TUNEL staining (Figure 2G). The results showed that: compared with BKO mice, the size and length of tibiae, bone density (Figure 2B), growth plate width (Figure 2D), PCNA positive cell percentage of cartilage (Figure 2F) were significantly increased in BKO+PQQ mice, however, the percentage of TUNEL positive cells (Figure 2H) were apparently decreased in BKO+PQQ mice compared to BKO mice. The results were well established that PQQ could improve bone growth retardation of Bmi-1 deficiency by promoting cell proliferation and inhibiting cell apoptosis.
Figure 2.
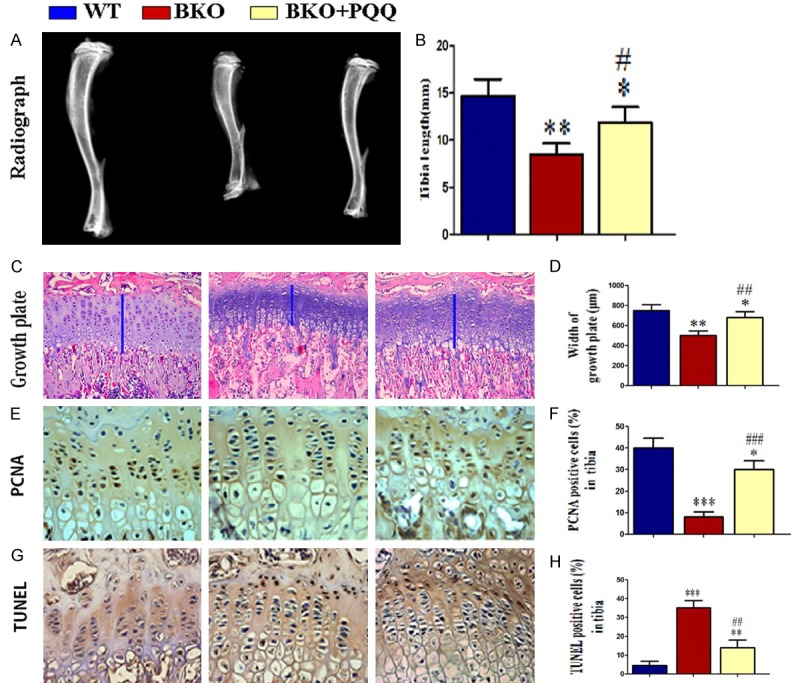
Effect of PQQ on bone growth and development in Bmi-1-/- mice. A. Radiographs of the tibiae of wild-type (WT), Bmi-1-/- mice (BKO) and BKO+BKO mice. B. Statistics of tibiae length. C. H&E staining of proximal tibial growth plate cartilage, the blue line represents growth plat cartilage (200x). D. Statistics of tibial growth plate cartilage width. E. PCNA immunohistochemical staining micrograhp of proximal tibial growth plate cartilage (400x). F. Statistics of PCNA positive cell percentage. G. TUNEL staining micrograhp of proximal tibial growth plate cartilage (400x). H. Statistics of TUNEL psitive cell percentage. Each value is the mean ± SEM of determinations in six animals of the same groups. *, P<0.05; **, P<0.01; ***, P<0.001, compared with WT mice; #, P<0.05; ##, P<0.01; ###, P<0.001, compared with BKO mice.
Effect of PQQ on trabecular bone volume of tibiae in Bmi1-/- mice
To determine whether long bones osteoporosis phenotype caused by Bmi-1 deficiency were improved by PQQ-supplemented diet, Phenotypic differences of tibiae between BKO+PQQ mice and littermates WT, BKO mice were analyzed by micro CT scanning (Figure 3A) and TCOL staining (Figure 3B). As shown in Figure 3C, the results showed that, compared with BKO mice, thickness of bone cortex was significantly increased in BKO+PQQ mice, and tibial trabecular bone volume increased apparently in BKO+PQQ mice. These results demonstrated that phenotype of osteoporosis induced by Bmi-1 deficiency was reversed partially by PQQ-supplemented diet.
Figure 3.
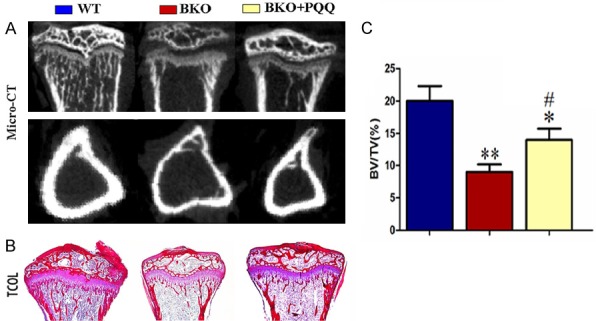
Effect of PQQ on trabecular bone volume of tibiae in Bmi1-/- mice. A. 3-dimensional reconstructed longitudinal sections of micro-CT scanning images. B. Micrographs of paraffin sections of the tibiae stained with Siries Red for total collagen (TCOL) from 7-week-old vehicle-treated wild-type (WT) and Bmi1-/- mice (BKO) and PQQ-treated Bmi1-/- mice (BKO+PQQ) (50x). C. Quantitation of trabecular bone volume relative to tissue volume (BV/TV, %) in metaphyseal regions. Each value is the mean ± SEM of determinations in six animals of the same groups. *, P<0.05; **, P<0.01; ***, P<0.001, compared with WT mice; #, P<0.05; ##, P<0.01; ###, P<0.001, compared with BKO mice.
Effect of PQQ on osteoblast formation of tibiae in Bmi1-/- mice
To detect the effect of PQQ on osteoblast formation of tibiae, we analyzed number of osteoblast of tibiae with H&E staining (Figure 4A), ALP staining (Figure 4B), Col I (Figure 4C) and OCN (Figure 4D) immunohistochemical staining. The results showed that, compared with BKO mice, osteogenic cell (Figure 4E), osteoblast number areas (Figure 4F), ALP (Figure 4G), Col I (Figure 4H) and OCN (Figure 4I) positive areas significantly increased in tibiae of BKO+PQQ mice. On the contrary, compared with the BKO mice, BKO+PQQ mice significantly decreased the number of osteoclasts in tibiae. All of the results suggested that PQQ was conducive to osteoblastic bone formation mice.
Figure 4.
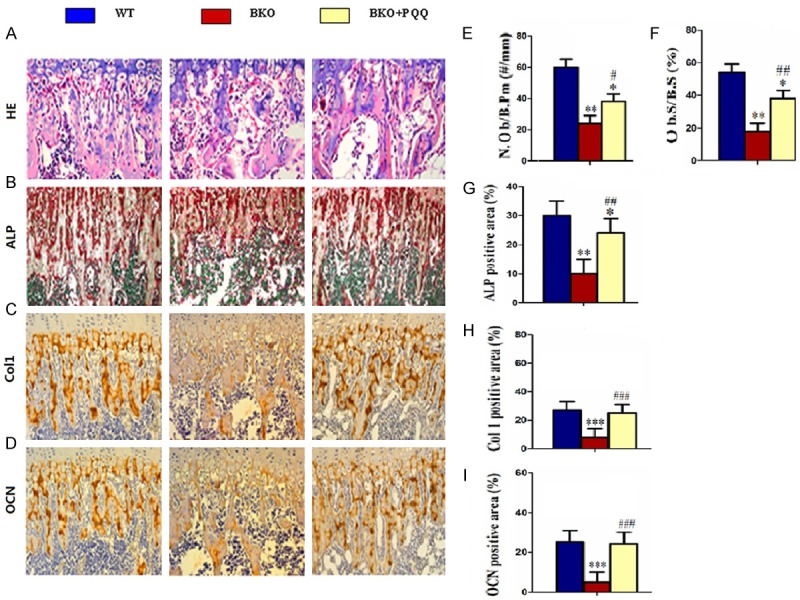
Effect of PQQ on osteoblast formation of tibiae in Bmi1-/- mice. A. Micrographs of H&E staining of tibiae (400x). B. Micrographs of ALP staining of the tibiae (400x). C. Micrographs of Col I immunohistochemical staining of tibiae (400x). D. Micrographs of OCN immunohistochemical staining of tibiae (400x). E. Number of positive osteoblasts of tibiae. F. Percentage of positive areas osteoblast of tibiae. G. ALP positive areas of tibiae. H. Col I positive areas percentage of tibiae. I. OCN positive areas percentage of tibiae. Each value is the mean ± SEM of determinations in six animals of the same groups. *, P<0.05; **, P<0.01; ***, P<0.001, compared with WT mice; #, P<0.05; ##, P<0.01; ###, P<0.001, compared with BKO mice. Number of positive osteoblasts (N.Ob/B.Pm, #/mm) . Percent ratio of positive areas osteoblasts (Ob.S/B.S, %).
Effect of PQQ on osteoclast bone resorption of tibiae in Bmi1-/- mice
To determine the effect of PQQ on osteoclast bone resorption of tibiae, we analyzed number of osteoclast of tibiae with TRAP staining (Figure 5A). The results showed that, compared with BKO mice, number of osteoclast positive cell (Figure 5B), osteoclast positive cell areas (Figure 5C) significantly decreased in tibiae of BKO+PQQ mice. All of the results suggested that PQQ attenuated osteoclast bone resorption abilities.
Figure 5.
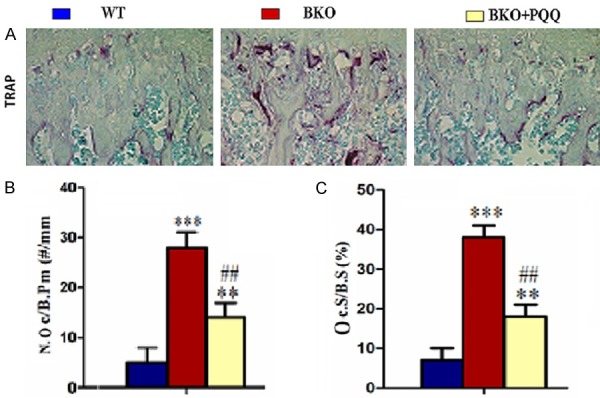
Effect of PQQ on osteoclasts bone resorption of tibiae in Bmi1-/- mice. A. Micrographs of TRAP staining of tibiae (400x). B. Number of TRAP-positive osteoclasts of tibiae. C. Surface of TRAP-positive osteoclasts of tibiae; Each value is the mean ± SEM of determinations in six animals of the same groups. *, P<0.05; **, P<0.01; ***, P<0.001, compared with WT mice; #, P<0.05; ##, P<0.01; ###, P<0.001, compared with BKO mice. Number of TRAP-positive osteoclasts (N.Oc/B.Pm, #/mm). Surface of TRAP-positive osteoclasts (Oc.S/B.S, %).
Effect of PQQ on DNA damage and cell apoptosis of tibiae in Bmi-1-/- mice
To determine whether PQQ reduces BKO mice skeleton DNA damage and cell apoptosis, we performed one most commonly used markers for double strand DNA breaks γH2AX and representative markers of apoptosis Caspase-3 (Figure 6A). The results found that BKO mice significantly induced cell DNA damages and cell apoptosis, as indicated by the protein levels of γH2AX and Caspase-3. The increased DNA damages and cell apoptosis were prevented by PQQ-supplemented diet (Figure 6B and 6C). Therefore, these results suggested PQQ partially played anti-DNA damages and anti-cell apoptosis roles of tibiae in Bmi-1-/- mice.
Figure 6.
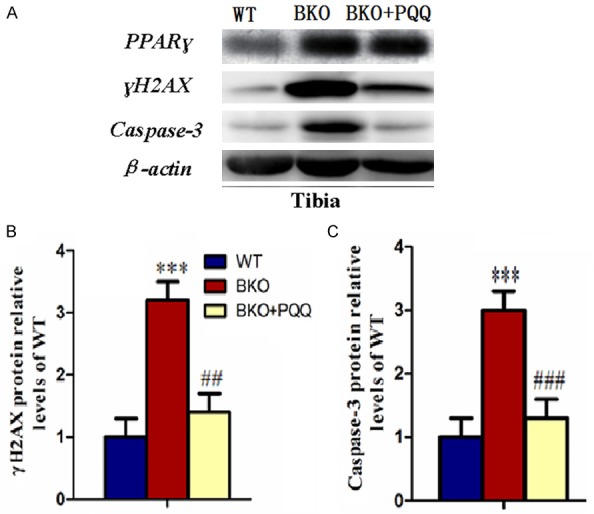
Effect of PQQ on DNA damage and cell apoptosis of tibiae in Bmi1-/- mice. (A) Representative tibiae western blot for expression of γH2AX and Caspase-3. β-actin was used as loading control for western blot in WT mice, BKO mice and BKO+PQQ mice respectively; γH2AX (B) and Caspase-3 (C) proteins levels relative to β-actin protein levels were assessed by densitometric analysis and expressed relative to levels of WT mice. Each value is the mean ± SEM of determinations in six animals of the same groups. *, P<0.05; **, P<0.01; ***, P<0.001, compared with WT mice; #, P<0.05; ##, P<0.01; ###, P<0.001, compared with BKO mice.
Effect of PQQ on antioxidant proteins of tibiae in Bmi-1-/- mice
To further explore whether the effect of PQQ was related to the enhanced expressions of various antioxidant proteins, we performed western blot to examine the expression of BCL-2, prdxI, prdx IV, SOD1 and SOD2 (Figure 7A). Results showed that all the antioxidant proteins were down-regulated in BKO mice compared to WT mice. Strikingly, the expression levels of all the antioxidant proteins were increased by viable levels in BKO+PQQ mice compared to BKO mice (Figure 7B-F). These data suggested that PQQ played antioxidant roles in protecting BKO mice from oxidative stress.
Figure 7.
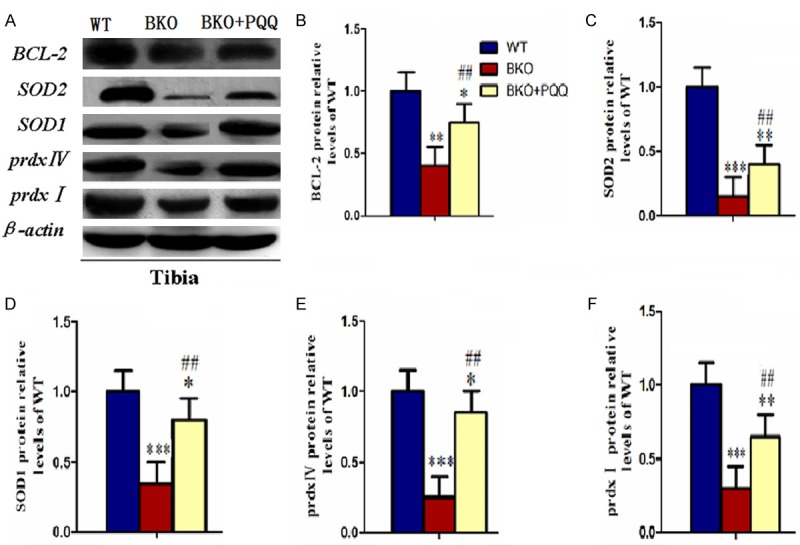
Effect of PQQ on antioxidant proteins of tibiae in Bmi1-/- mice. (A) Representative tibiae western blots for expression of BCL-2, SOD1, SOD2, prdx I, pxdx IV. β-actin was used as loading control for western blot in WT mice, BKO mice and BKO+PQQ mice respectively. BCL-2 (B), SOD1 (C), SOD2 (D), prdx I (E), prdx IV (F) proteins levels relative to β-actin protein levels were assessed by densitometric analysis and expressed relative to levels of WT mice. Each value is the mean ± SEM of determinations in six animals of the same groups. *, P<0.05; **, P<0.01; ***, P<0.001, compared with WT mice; #, P<0.05; ##, P<0.01; ###, P<0.001, compared with BKO mice.
Effect of PQQ on cell cycle proteins of tibiae in Bmi-1-/- mice
It was well elucidated that cell proliferation was mediated by the expression and activation of tumor-suppressor genes, we examined the expression of P16, P19, P21, P27, P53 (Figure 8A). Results showed that BKO mice promoted the expression of tumor-suppressor, P16, P19, P21, P27, P53. However, PQQ-supplemented diet on BKO mice decreased expression of P16, P19, P21, P27, P53 (Figure 8B-F).
Figure 8.
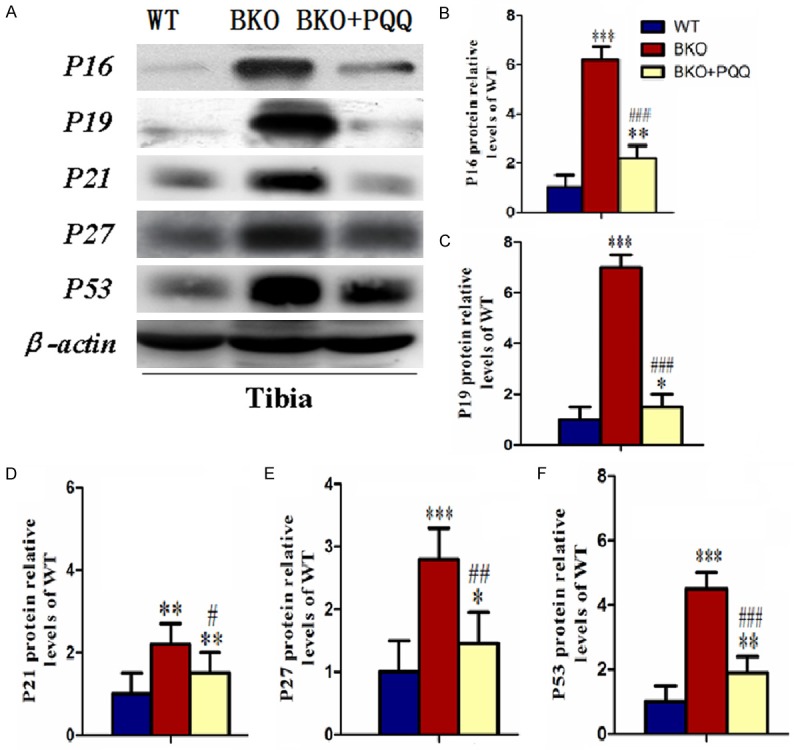
Effect of PQQ on cell cycle proteins of tibiae in Bmi1-/- mice. (A) Representative tibiae western blot for expression of P16, P19, P21, P27 and P53. β-actin was used as loading control for western blot in WT mice, BKO mice and BKO+PQQ mice respectively. P16 (B), P19 (C), P21 (D), P27 (E) and P53 (F) proteins levels relative to β-actin protein levels were assessed by densitometric analysis and expressed relative to levels of WT mice. Each value is the mean ± SEM of determinations in six animals of the same groups. *, P<0.05; **, P<0.01; ***, P<0.001, compared with WT mice; #, P<0.05; ##, P<0.01; ###, P<0.001, compared with BKO mice.
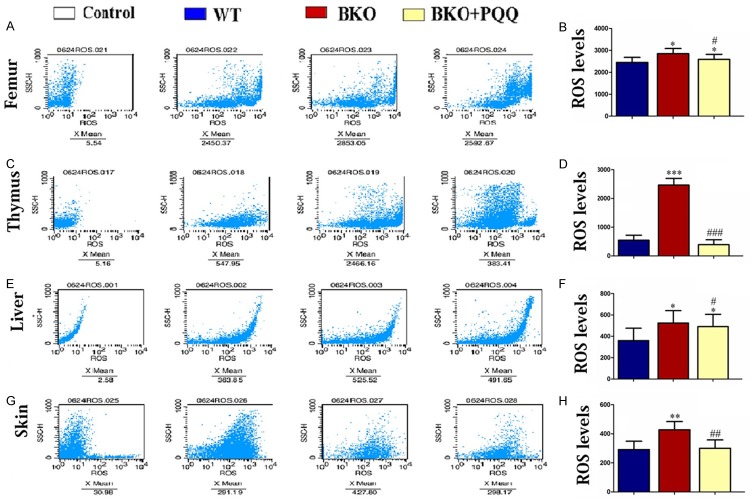
Effect of PQQ on ROS levels of Femur, Thymus, liver and skin in Bmi-1-/- mice
It was well established that BKO mice increased ROS levels and hydroxyl free radicals by oxidative stress, which subsequently resulted in the increase of DNA double strand breaks. Our results showed that PQQ-supplemented diet decreased BKO induced double strand DNA breaks in Femur, Thymus, liver and skin. To determine whether PQQ inhibits ROS formation, we examined ROS levels by flow cytometry (Figure 9A, 9C, 9E and 9G). The results showed that the increased ROS levels in BKO mice were inhibited by PQQ-supplemented diet (Figure 9B, 9D, 9F and 9H).
Figure 9.
Effect of PQQ on ROS levels of Femur, Thymus, liver and skin in Bmi1-/- mice. Flow cytometric analysis for ROS levels of Femur (A), Thymus (C), liver (E) and skin (G) in WT mice, BKO mice and BKO+PQQ mice respectively; ROS levels of Femur (B), Thymus (D), liver (F) and skin (H). Each value is the mean ± SEM of determinations in six animals of the same groups. *, P<0.05; **, P<0.01; ***, P<0.001 relative to WT mice; #, P<0.05; ##, P<0.01; ###, P<0.001, compared with BKO mice.
Discussion
Osteoporosis, a bone metabolic disease, is characterized by low bone mass (osteopenia) and microarchitectural deterioration of bone tissue that results in compromised bone strength and increased risk of fractures [2]. Osteoporosis is the result of an imbalance in the ratio with more resorption than formation. Enhancing the activity of osteoblasts, plus reducing that of the osteoclasts, may help restore the balance in bone metabolism and limit bone loss in the development of osteoporosis. Previous study [31] was well established that PQQ played treatment effect on defective teeth and mandible caused by Bmi-1 deficiency through promoting osteoblast bone formation, reducing osteoclast bone resorption, scavenging ROS and reducing DNA damages. As we know, osteogenesis way of teeth and mandible belong to intramembranous ossification, and which of long bones belong to cartilage ossification. In view of this, we put forward to the hypothesis that whether PQQ also could play a rescuing role of correcting in long bone osteoporosis induced by Bmi-1 deficiency.
In this study, we demonstrated that Bmi-1 deficiency resulted in growth retardation and premature aging because of decreased proliferation and increased apoptosis, impaired skeletal growth and development and premature osteoporosis associated with decreased osteoblastic bone formation, increased osteoblastic bone resorption and upregulated aging-associated molecules, and increased oxidative stress and DNA damages of multiple organs. Our results also demonstrated that these typical aging and osteoporosis phenotypes in Bmi-1 knockout mice were partially rescued by PQQ through anti-aging, anti-apoptosis, proliferating, up-regulating antioxidant abilities and scavenging ROS in Bmi-1-/- transplant recipients. These findings indicated that PQQ had preventative and therapeutic potential for aging and aging-associated degenerative diseases such as osteoporosis.
Bmi-1, derived from the polycomb family, inhibits space-specific and time-specific expression of the Hox gene in growth and development. Bmi-1 systematic deficiency leads to shortened life span and growth retardation [8,32]. Consistent with these results, we found that Bmi-1 deficiency led to shortened survival rates, and decreased body weight and overall size of the body. Our study testified that PQQ prolonged survival, increased overall sizes of body directly by promoting cell proliferation and inhibiting cell apoptosis in Bmi-1 knockout mice. Thus, PQQ rescued the shortened life span and growth retardation in a model of systematic aging and osteoporosis.
Miao, et al [8] demonstrated that aging-associated osteoporosis was induced in Bmi-1 knockout mice, as determined by down-regulated self-renewal capacity of bone marrow mesenchymal stem cells. The study had evidence suggesting that PQQ significantly slowed the loss of bone density and prolong the life span of Bmi-1-/- mice. Results from this study indicated that PQQ rescued aging-associated osteoporosis by promoting osteogenesis and inhibiting apoptosis.
Both osteoblastic and osteoclastic cells regulate bone metabolism, and both cell types are involved in the development of osteoporosis [33]. Osteoblasts are bone-forming cells located near the surface of the bone that produces cytokines. Cytokines, including macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB (NF-κB) ligand (RANKL), are both essential for osteoclast differentiation, function, and survival [34,35]. Osteoclasts are bone-resorbing multinucleated cells that become tightly attached to mineralized bone surfaces through their integrins and form resorption lacuna by secreting protons, proteases, and superoxide through ruffled borders [36-39]. Bone resorption by activated osteoclasts with subsequent deposition of a new matrix by osteoblasts causes the formation of bone structure and bone remodeling [33]. Imbalance between bone formation and bone resorption is the key pathophysiological event in many metabolic bone disorders in adult humans including osteoporosis [40]. In the study, our results investigated that PQQ promoted long bone formation by attenuating osteoclast bone resorption abilities. Oxidative stress is a pivotal pathogenic factor for age-related bone loss in mice and rats [41-43], leading to an increase in osteoblast and osteocyte apoptosis, among other changes, and a decrease in osteoblast numbers and the rate of bone formation via Wnt/β-catenin signaling [42]. Recent studies showed that oxidative stress inhibited osteoblastic differentiation [44,45] via extracellular signal-regulated kinases (ERKs) and ERK-dependent NF-κB signaling pathways [46]. Osteoblasts can produce antioxidants, such as glutathione peroxidase, to protect against ROS [47], as well as transforming growth factor β (TGF-β), which is involved in a reduction of bone resorption [48]. ROS are also involved in bone resorption with a direct contribution of osteoclast-generated superoxide to bone degradation, and oxidative stress increases differentiation and function of osteoclasts [49-51]. Our results tested that the increased ROS levels in BKO mice were inhibited by PQQ-supplemented diet. The above results showed that, in vivo, PQQ reduced DNA damages and promoted cell proliferation through scavenging ROS of oxygen free radicals and regulating cell cycle.
In conclusion, we demonstrated that oxidative stress due to ROS that are shown to cause the development of osteoporosis may be prevented by supplementation with PQQ. This study not only reveals the mechanism of PQQ supplementation in anti-osteoporosis, but also provides the experimental and theoretical basis for the clinical application of PQQ in osteoporosis.
Acknowledgements
The study was supported by grant from Hunan Province Natural Scientific Foundation Project of China (No. 2016JJ4063) and Hunan Province Education Department Scientific Research Youth Project of China (No. 14B141).
Disclosure of conflict of interest
None.
References
- 1.Kling JM, Clarke BL, Sandhu NP. Osteoporosis prevention, screening, and treatment: a review. J Womens Health (Larchmt) 2014;23:563–572. doi: 10.1089/jwh.2013.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–170. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woźniak-Holecka J, Sobczyk K. Nutritional education in the primary prevention of osteoporosis in perimenopausal andpostmenopausal women. Prz Menopauzalny. 2014;13:56–63. doi: 10.5114/pm.2014.41087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleibler F, Konnopka A, Benzinger P, Rapp K, König H. The health burden And costs of incident fractures attributable to osteoporosis from 2010 to 2050 in Germany-a demographic simulation model. Osteoporos Int. 2013;24:835–847. doi: 10.1007/s00198-012-2020-z. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay R, Burge RT, Strauss DM. One year outcomes and costs following a vertebral fracture. Osteoporosis Int. 2005;16:78–85. doi: 10.1007/s00198-004-1646-x. [DOI] [PubMed] [Google Scholar]
- 6.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 7.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 8.Zhang HW, Ding J, Jin JL, Guo J, Liu JN, Karaplis A, Goltzman D, Miao D. Defects in mesenchymal stem cell self-renewal and cell fate determination lead to an osteopenic phenotype in Bmi-1 null mice. J Bone Miner Res. 2010;25:640–652. doi: 10.1359/jbmr.090812. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY, Ahn BH, Kumar NG, Rovira II, Xu XL, van Lohuizen M, Motoyama N, Deng CX, Finkel T. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–392. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauge JG. Glucose dehydrogenase of bacterium anitratum: an enzyme with a novel prosthetic group. J Biol Chem. 1964;239:3630–3639. [PubMed] [Google Scholar]
- 11.Duine JA. Cofactor diversity in biological oxidations: implications and applications. Chem Rec. 2001;1:74–83. doi: 10.1002/1528-0691(2001)1:1<74::AID-TCR10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell AE, Jones AD, Mercer RS, Rucker RB. Characterization of pyrroloquinoline quinone Aminoacid derivatives by electrospray ionization mass spectrometry and detection in human milk. Anal Biochem. 1999;269:317–325. doi: 10.1006/abio.1999.4039. [DOI] [PubMed] [Google Scholar]
- 13.Kumazawa T, Sato K, Seno H, Ishii A, Suzuki O. Levels of pyrroloquinoline quinone in various foods. Biochem J. 1995;307:331–333. doi: 10.1042/bj3070331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stites TE, Mitchell AE, Rucker RB. Physiological importance of quinoenzymes and the O-quinone family of cofactors. J Nutr. 2000;130:719–727. doi: 10.1093/jn/130.4.719. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg FM, Gershwin ME, Rucker RB. Dietary pyrroloquinoline quinone: growth and immune response in BALB/c mice. J Nutr. 1994;124:744–753. doi: 10.1093/jn/124.5.744. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg F, Stites TE, Anderson P, Storms D, Chan I, Eghbali S, Rucker R. Pyrroloquinoline quinone improves growth and reproductive performance in mice fed chemically defined diets. Exp Biol Med. 2003;228:160–166. doi: 10.1177/153537020322800205. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Feustel PJ, Kimelberg HK. Neuroprotection by pyrroloquinoline quinone (PQQ) in reversible middle cerebral artery occlusion in the adult rat. Brain Res. 2006;1094:200–206. doi: 10.1016/j.brainres.2006.03.111. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Rosenberg PA. The essential nutrient pyrroloquinoline quinone may act as a neuroprotectant by suppressing peroxynitrite formation. Eur J Neurosci. 2002;16:1015–1024. doi: 10.1046/j.1460-9568.2002.02169.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhu BQ, Simonis U, Cecchini G, Zhou HZ, Li L, Teerlink JR, Karliner JS. Comparison of pyrroloquinoline quinone and/or metoprolol on myocardial infarct size and mitochondrial damage in a rat model of ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther. 2006;11:119–128. doi: 10.1177/1074248406288757. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Chen N, Miao D. Radioprotective effects of pyrroloquinoline quinone on parotid glands in C57BL/6J mice. Exp Ther Med. 2016;12:3685–3693. doi: 10.3892/etm.2016.3843. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Shankar BS, Pandey R, Amin P, Misra HS, Sainis KB. Role of glutathione in Augmenting the anticancer activity of pyrroloquinoline quinone (PQQ) Redox Rep. 2010;15:146–154. doi: 10.1179/174329210X12650506623762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouchi A, Nakano M, Nagaoka S, Mukai K. Kinetic study of the antioxidant activity of pyrroloquinolinequinol (PQQH(2), a reduced form of pyrroloquinoline quinone) in micellar solution. J Agric Food Chem. 2009;57:450–456. doi: 10.1021/jf802197d. [DOI] [PubMed] [Google Scholar]
- 23.Stites T, Storms D, Bauerly K, Mah J, Harris C, Fascetti A, Rogers Q, Tchaparian E, Satre M, Rucker RB. Pyrroloquinoline quinone modulates mitochondrial quantity and function in mice. J Nutr. 2006;136:390–396. doi: 10.1093/jn/136.2.390. [DOI] [PubMed] [Google Scholar]
- 24.Ishii T, Akagawa M, Naito Y, Handa O, Takagi T, Mori T, Kumazawa S, Yoshikawa T, Nakayama T. Pro-oxidant action of pyrroloquinoline quinone: characterization of protein oxidative modifications. Biosci Biotechnol Biochem. 2010;74:663–666. doi: 10.1271/bbb.90764. [DOI] [PubMed] [Google Scholar]
- 25.Tao R, Karliner JS, Simonis U, Simonis U, Zheng J, Zhang J, Honbo N, Alano CC. Pyrroloquinoline quinone preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes. Biochem Biophys Res Commun. 2007;363:257–262. doi: 10.1016/j.bbrc.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misra HS, Khairnar NP, Barik A, Indira Priyadarsini K, Mohan H, Apte SK. Pyrroloquinolinequinone: a reactive oxygen speciesscavenger in bacteria. FEBS Lett. 2004;578:26–30. doi: 10.1016/j.febslet.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 27.Cao G, Gu M, Zhu M, Gao J, Yin Y, Marshall C, Xiao M, Ding J, Miao D. Bmi-1 absence causes premature brain degeneration. PLoS One. 2012;7:e32015. doi: 10.1371/journal.pone.0032015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauerly K, Harris C, Chowanadisai W, Graham J, Havel PJ, Tchaparian E, Satre M, Karliner JS, Rucker RB. Altering pyrroloquinoline quinone nutritional status modulates mitochondrial, lipid, and energy metabolism in rats. PLoS One. 2011;6:e21779. doi: 10.1371/journal.pone.0021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kressel M, Groscurth P. Distinction of apoptotic and necrotic cell death by in situ labelling of fragmented DNA. Cell Tissue Res. 1994;278:549–556. doi: 10.1007/BF00331373. [DOI] [PubMed] [Google Scholar]
- 30.Xue Y, Karaplis AC, Hendy GN, Goltzman D, Miao D. Genetic models show that parathyroid hormone and 1,25-dihydroxyvitamin D3 play distinct and synergistic roles in postnatal mineral ion homeostasis and skeletal development. Hum Mol Genet. 2005;14:1515–1528. doi: 10.1093/hmg/ddi160. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Chen N, Miao D. The treatment effects and mechanisms of pyrroloquinoline quinone on defective teeth and mandible in Bmi-1 knockout mice. Zhonghua Kou Qiang Yi Xue Za Zhi. 2015;50:496–502. [PubMed] [Google Scholar]
- 32.Jin J, Lv X, Chen L, Zhang W, Li J, Wang Q, Wang R, Lu X, Miao D. Bmi-1 plays a critical role in protection from renal tubulointerstitial injury by maintaining redox balance. Aging Cell. 2014;13:797–809. doi: 10.1111/acel.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nijweide PJ, Burger EH, Feyen JH. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986;66:855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- 34.Ando K, Mori K, Rédini F, Heymann D. RANKL/RANK/OPG: key therapeutic target in bone oncology. Curr Drug Discov Technol. 2008;5:263–268. doi: 10.2174/157016308785739857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473:201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 36.Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13:66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- 37.Steinbeck MJ, Appel WH Jr, Verhoeven AJ, Karnovsky MJ. NADPH-oxidase expression and in situ production of superoxide by osteoclasts actively resorbing bone. J Cell Biol. 1994;126:765–772. doi: 10.1083/jcb.126.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darden AG, Ries WL, Wolf WC, Rodriguiz RM, Key LL Jr. Osteoclastic superoxide production and bone resorption: stimulation and inhibition by modulators of NADPH oxidase. J Bone Miner Res. 1996;11:671–675. doi: 10.1002/jbmr.5650110515. [DOI] [PubMed] [Google Scholar]
- 39.Yavropoulou MP, Yovos JG. Osteoclastogenesis--current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008;8:204–216. [PubMed] [Google Scholar]
- 40.Fazzalari NL. Bone remodeling: a review of the bone microenviron-ment perspective for fragility fracture (osteoporosis) of the hip. Semin Cell Dev Biol. 2008;19:467–472. doi: 10.1016/j.semcdb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Banfi G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med. 2008;46:1550–1555. doi: 10.1515/CCLM.2008.302. [DOI] [PubMed] [Google Scholar]
- 42.Manolagas SC. De-fense! De-fense! De-fense: scavenging H2O2 while making cholesterol. Endocrinology. 2008;149:3264–3266. doi: 10.1210/en.2008-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mody N, Parhami F, Saraflan TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 44.Shen CL, Wang P, Guerrieri J, Yeh J, Wang JS. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporosis Int. 2008;19:979–990. doi: 10.1007/s00198-007-0527-5. [DOI] [PubMed] [Google Scholar]
- 45.Fatokun AA, Stone TW, Smith RA. Responses of differentiated MC3T3-E1 osteoblast-like cells to reactive oxygen species. Eur J Pharmacol. 2008;587:35–41. doi: 10.1016/j.ejphar.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 46.Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ. Oxidative stress inhibits Osteoblastic differentiation of bone cells by ERK and NF-κB. Biochem Biophys Res Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 47.Dreher I, Schütze N, Baur A, Hesse K, Schneider D, Köhrle J, Jakob F. Selenoproteins are expressed in fetal human osteoblast-like cells. Biochem Biophys Res Commun. 1998;245:101–107. doi: 10.1006/bbrc.1998.8393. [DOI] [PubMed] [Google Scholar]
- 48.Fuller K, Lean JM, Bayley KE, Wani MR, Chambers TJ. A role for TGF-β in osteoclast differentiation and survival. J Cell Sci. 2000;113:2445–2453. doi: 10.1242/jcs.113.13.2445. [DOI] [PubMed] [Google Scholar]
- 49.Yang S, Madyastha P, Bingel S, Ries W, Key L. A new superoxide-generating oxidase in murine osteoclasts. J Biol Chem. 2001;276:5452–5458. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- 50.Sontakke AN, Tare RS. A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin Chim Acta. 2002;318:145–148. doi: 10.1016/s0009-8981(01)00766-5. [DOI] [PubMed] [Google Scholar]
- 51.Garrett JR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]