Abstract
Berberine (BBR), a Chinese medicine extracted from natural plant, has been demonstrated to improve lipid disorders. Oxidized low-density lipoprotein (oxLDL), a proatherogenic lipoprotein, has been shown to be involved in vascular endothelial cell dysfunction such as excessive or abnormal proliferation. The purpose of the present study was to investigate the impacts of BBR on cell proliferations as well as potential involving signal pathways. HUVECs were stimulated with oxLDL and co-cultured with BBR at a variety of concentrations in different time points. The data showed that oxLDL (10-100 μg/ml) remarkably promoted human umbilical vein endothelial cells (HUVECs) proliferation assessed by Cell Counting Kit-8 (CCK-8) and EdU assay. The effects were found to be involved in up-regulation of proliferating cell nuclear antigen (PCNA), nuclear factor кB (NF-кB) and oxidized low density lipoprotein receptor 1 (LOX-1) and activation of phosphatidylinositol 3 kinase (PI3K)/Akt, ERK1/2 and p38 mitogen-activated protein kinase (MAPK) signaling pathways evaluated by either real time polymerase chain reaction (PCR) or western blot analysis. Interestingly, HUVECs proliferation was significantly inhibited by BBR (5-25 μg/ml), which down-regulated the expression of PCNA, NF-кB and LOX-1 and reduced the phosphorylation of Akt, ERK1/2 and p38MAPK. Furthermore, the anti-proliferative effect of BBR on HUVECs was effectively abrogated by a PI3K inhibitor LY294002, an ERK1/2 inhibitor PD98059 and a p38 inhibitor SB202190 partly through the restoration of phosphorylation of Akt, ERK1/2 and p38MAPK. Taken together, our data suggested that BBR inhibited ox-LDL-induced HUVECs proliferation by decreasing the expression of PCNA, NF-кB and LOX-1 and suppressing the activation of PI3K/Akt, ERK1/2 and p38MAPK pathways, indicating a latent candidate for anti-atherosclerosis clinically.
Keywords: Oxidized low-density lipoprotein, endothelial cell, proliferation, berberine, signal pathway
Introduction
Oxidized low density lipoprotein (oxLDL) within plaques is considered to contribute to the inflammatory state of atherosclerosis (AS) and play a key role in its pathogenesis [1,2]. The mechanisms that oxLDL influences the development of AS are not well understood and may go more than one step beyond the formation of foam cells [3]. Recently, great emphasis has been placed on the ways how oxLDL can influence various steps of endothelial and vascular dysfunction during the development of AS in vitro. Previous study suggested that vascular endothelial cell proliferation are closely linked with the inflammation [4], and excessive proliferation of vascular cells might be important components in the pathobiology of vascular occlusive disease such as in-stent restenosis and vessel bypass graft failure. It has been reported that oxLDL-induced reactive oxygen species (ROS) formation via activation of the NAD(P)H-oxidase, modulation of endothelial Ca2+-activated K+ channels and p27Kip1 expression mediated by RhoA can contribute to the proliferation of endothelial cells involved in some mitogen-activated protein kinases (MAPKs) signal pathways [5,6], suggesting that blockade of oxLDL-induced vascular cell proliferation may represents a potential anti-AS strategy.
Based on the evidence that ROS and inflammation are involved in vascular cell proliferation, more attention has been paid to natural products with established antioxidant or anti-inflammatory activities and low cell toxicities. Berberine (BBR), which is a natural isoquinoline alkaloid with a long history of use in Chinese medicine, has been isolated from a variety of medicinal plants such as Coptis chinensis, Berberis aquifolium and Berberis aristata [7]. It is used to as a strong inhibitory drug in the treatment of inflammation because of its antimicrobial effects and anti-inflammatory activities [8-10]. Interestingly, recent investigations on berberine have revealed several other pharmacological applications such as anti-tumor, anti-oxidation, anti-hyperglycemic and low-density lipoprotein (LDL)-lowering effects [11-13]. However, the data regarding the anti-proliferation and anti-inflammatory activities of berberine in vascular endothelial cells has currently been limited.
In the present study, therefore, we investigated the effects of oxLDL on the proliferation of human umbilical vein endothelial cells (HUVECs) as well as the potential signal pathways. Subsequently, the study has explored the impact of berberine on oxLDL-induced proliferation and putative molecular mechanisms.
Materials and methods
Reagents
Medium 199 (M199) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, USA). Endothelial cell growth factor (ECGF) was from Sciencell (Beijing, China). LY294002, U0126, SB202190, rabbit polyclonal antibodies, anti-phospho-p44/42 (Thr202/tyr204), anti-p44/42, anti-phospho-Akt (ser-473), anti-Akt, anti-caspase-3, anti-phospho-p38MAPK (Thr180/Tyr182), anti-p38MAPK, anti-PCNA, anti-NF-кB, mouse monoclonal antibody, anti-β-Actin, horseradish peroxidase-conjugated secondary antibodies to mouse or rabbit were obtained from were obtained from Cell Signal Technology (Beverly, USA). Rabbit polyclonal antibody, anti-LOX-1 was from Abcam (Cambridge, England).
Isolation and oxidation of LDL
Human native LDL and oxLDL were prepared as described before [14]. Briefly, oxidation of LDL was performed by dialysis against EDTA-free isotonic saline containing 5 μM CuSO4 37°C for 8 h. Oxidation was stopped by the addition of EDTA to a final concentration of 100 μM and the copper ion was removed by extensive dialysis against isotonic saline containing 0.1 μM EDTA at 4°C. LDL was sterilized by passage through a 0.22 μm filter. oxLDL was kept in 10 mM Tris-HCl, 0.14 M NaCl and 0.5 mM EDTA at pH 7.4 at 4°C in the dark and freshly prepared every 2 weeks.
Culture of HUVEC
HUVECs were purchased from Sciencell and grown in M199 with 10% (v/v) FBS, 10 ng/ml ECGF and 100 U/ml penicillin-streptomycin (complete medium) at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. Cells were used at third passage for all experiments. For oxLDL stimulation experiments, HUVECs were grown to 80% confluence and then switched to 2% FBS medium with the absence of ECGF for 6 h resting, followed by incubation with different concentrations of oxLDL (10, 25, 50, 75, 100 μg/ml ) for different time points (3, 6, 12, 24, 48 h). For inhibition experiments, HUVECs were pretreated with berberine (1, 5, 10, 25, 50 μg/ml) for 1 h and continuously for 24 h incubation of 2% FBS medium with the absence of ECGF (basal conditions) after 6 h resting. For inhibitor studies, the cells were pre-incubated with a PI3K inhibitor LY294002 (25 μM) or MEK1/2 inhibitor PD98059 (10 μM) or p38 inhibitor SB202190 (10 μM) before the addition of berberine (25 μg/ml). For CCK-8 and 5-ethynyl-2’-deoxyuridine (EdU) experiments, the cells were seeded in 96-well and 24-well cell culture plates respectively. For real time PCR and western blot analysis, the cells were cultured in 25 m2 cell culture flasks (Corning, USA).
CCK-8 proliferation assay
Cell proliferation was determined by the Cell Counting Kit-8 (CCK-8) (Dojindo, China). This assay is based on the cleavage of the tetrazolium salt WST-8, which is reduced by mitochondrial dehydrogenase in viable cells to produce a yellow-color formazan dye. The total dehydrogenase activity of cells in the medium can be determined by the intensity of the yellow color. Briefly, 5,000 cells in 100 μl medium were seeded into each well of 96-well plates and allowed to adhere overnight. After cells were treated with oxLDL or berberine either alone or in combination for 24 h, 10 μl of the tetrazolium substrate was incubated at 37°C for 2 h. The optical density (OD) at 450 nm was measured with a Microplate Reader (Tecan, Austria).
EdU proliferation assay
HUVECs proliferation was determined by using the EdU labeling/detection kit (Ribobio, China) according to the manufacturer’s protocol. EdU is a nucleoside analog of thymidine that is incorporated into DNA during active DNA synthesis only by proliferating cells. After incorporation, a fluorescent molecule was added that reacted specifically with EdU, making possible fluorescent visualization of proliferating cells. Briefly, cells were incubated with 50 μM EdU for 12 h at 37°C under 5% CO2 before fixation, permeabilization, and EdU staining. Cell nuclei were stained with Hoechst 33342 at a concentration of 5 μg/mL for 30 min. Finally, the cells were observed under a confocal laser scanning microscope (Leica Microsystems, Germany). The percentage of EdU-positive cells was calculated from six random fields in three wells.
Real-time PCR assay
The total cells RNA was extracted with Trizol reagent (Life Technologies, USA) and the RNA quantity was assessed on an Angilent Tecnologies bioanalyzer system. Quantitative real-time polymerase chain reaction (PCR) was performed using Promega’s Access RT-PCR kit (Promega, USA). The sequence of the forward and reverse primers were 5’ CTCCTCCACCTT TGACGCTG 3’ and 5’ TCCTCTTGTGCTCTTGCTGG 3’ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5’ CACCAAACCAGGAGAAAG 3’ and 5’ TCCAATATGGCTGAGATC 3’ for proliferating cell nuclear antigen (PCNA), 5’ TGATAGAAACCCTTGCTCG 3’ and 5’ TTGCTTGCTGGATGAAGTC 3’ for lectin-like ox-LDL receptor (LOX-1), 5’ TGGACTACCTGGTGCCTCT 3’ and 5’ GTGTTTTCCCACCAGGCTG 3’ for nuclear factor kappa B (NF-кB), 5’ CCAGGCTTCATATTCCTA 3’ and 5’ ATGCGACTGGTTTCCTTC 3’ for phosphatidylinositol 3-kinase (PI3K). A 7500 real-time PCR System (Applied Biosystems, USA) was used for Amplification. Relative expression levels of target genes expression were normalized to the GAPDH housekeeping gene as an endogenous reference and then compared to control.
Protein extraction and western blot analysis
The cells were harvested and centrifuged at 500×g for 10 min at 4°C. The pellets were resuspended in lysis buffer containing 1%Triton X-100, 20 mM HEPES [pH 7.5], 5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM phenylmethanesulfonyl fluoride, and 1 mg/ml each of leupeptin, aprotinin, and pepstatin for 30 min. The lysates were centrifuged at 14,000×g for 10 min at 4°C. The supernatants were frozen at -70°C. Protein concentration of the supernatants was quantified with the Bradford Protein assay. For analysis of protein levels, the supernatants were then mixed with 5×SDS sample buffer, boiled for 5 min, and separated through 8% to 15% SDS-PAGE gels. After electrophoresis, the proteins were transferred to nylon membranes by electrophoretic transfer. Nonspecific binding was blocked with 5% skim milk for 2 h, rinsed, and incubated overnight at 4°C with primary antibody in 5% skim milk. The membrane was washed in TBS/0.1% Tween 20, and incubated for 2 h with horseradish peroxidase-conjugated secondary antibody. After washes in TBS/0.1% Tween 20, bands were visualized by enhanced chemoluminescence and acquired by the ChemiDoc XRS systems (Bio-Rad, USA).
Statistical analysis
Data are expressed as mean ± standard deviation. All values were analyzed by using one-way ANOVA and the Newman-Keuls-Student t test. A value of P<0.05 was considered statistically significant.
Results
Proliferation of HUVEC induced by oxLDL
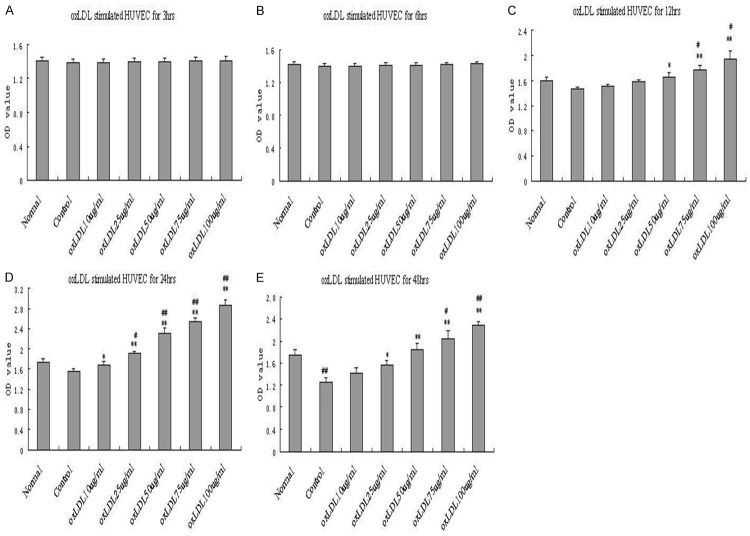
To determine the effect of oxLDL on HUVEC, we treated HUVEC with (10, 25, 50, 75, 100 μg/ml) for different time (3, 6, 12, 24, 48 h) under basal conditions (2% FBS with the absence of ECGF). Proliferation of HUVEC was measured by CCK-8. As shown in Figure 1, the proliferation of HUVEC induced by demonstrated a time-dependent and dose-dependent manner. oxLDL significantly increased the proliferation of HUVEC for both 24 h and 48 h detection. However, for 48 h detection, compared with normal group (complete medium), the proliferation in HUVEC of control group (basal conditions) decreased significantly indicating a poor cell activity result from a long period of low nutrient condition. For 24 h incubation, 50 μg/ml oxLDL significantly enhanced proliferation compared to both normal and control group. Based on these findings, we chose 50 μg/ml oxLDL for 24 h incubation under basal conditions for subsequent experiments.
Figure 1.
Oxidized low-density lipoprotein (oxLDL) induces proliferation in human umbilical vein endothelial cells (HUVECs) by CCK-8 assay. OxLDL at concentrations of 10 μg/ml, 25 μg/ml, 50 μg/ml, 75 μg/ml, 100 μg/ml were applied to HUVECs for 3 h (A), 6 h (B), 12 h (C), 24 h (D), 48 h (E) with basal conditions, and then the optical density (OD) at 450 nm was measured. Each data point represents mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 compared with control. #P<0.05, ##P<0.01 compared with normal.
We also used native LDL as a negative control, and compared the impacts of native LDL and oxLDL on HUVEC. HUVECs were treated with native LDL and oxLDL at the dose of 10, 25, 50, 75 and 100 μg/ml, respectively. Only 100 μg/ml native LDL had obvious proliferative effect on HUVECs, and orther concentrations had no impacts on HUVECs. On the contrary, oxLDL significantly enhanced proliferation at increased concentrations, obviously increased cell number and improved morphology (Figure 2B, 2C).
Figure 2.
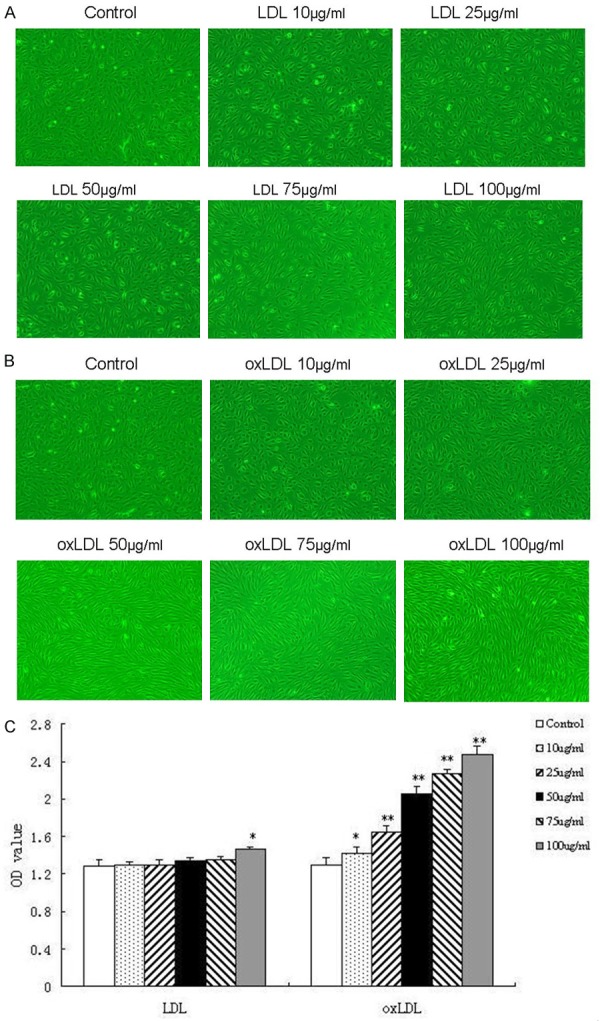
The effects of native LDL and oxLDL on HUVEC. Both native LDL and oxLDL at concentrations of 10 μg/ml, 25 μg/ml, 50 μg/ml, 75 μg/ml, 100 μg/ml for 24 h were applied to HUVECs. The impact of native LDL or oxLDL on the morphological changes in HUVECs using phase-contrast microscope (×100) (A, B, respectively) and on the proliferation using CCK-8 (C). Each data point represents mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 compared with control.
Inhibition of berberine in proliferation of HUVEC induced by oxLDL
To investigate whether berberine has the effect of anti-proliferation in HUVECs induced by oxLDL, we incubation the cells with berberine at concentrations of 1 μg/ml, 5 μg/ml, 10 μg/ml, 25 μg/ml, 50 μg/ml. Berberine at concentrations of 5 μg/ml, 10 μg/ml, 25 μg/ml, 50 μg/ml attenuated the oxLDL-induced proliferation of HUVECs in a dose-dependent manner using CCK-8 assay (Figure 3A). To provide further evidence for the inhibition of berberine on oxLDL-induced HUVECs proliferation, we also used EdU proliferation assay, an established method for measurement of DNA synthesis as indication of proliferation. As shown in Figure 3B and 3C, the ratio of EdU-positive cells to total cells in oxLDL-induced HUVECs was significantly higher than that of control HUVECs, and berberine effectively suppressed the proliferation.
Figure 3.
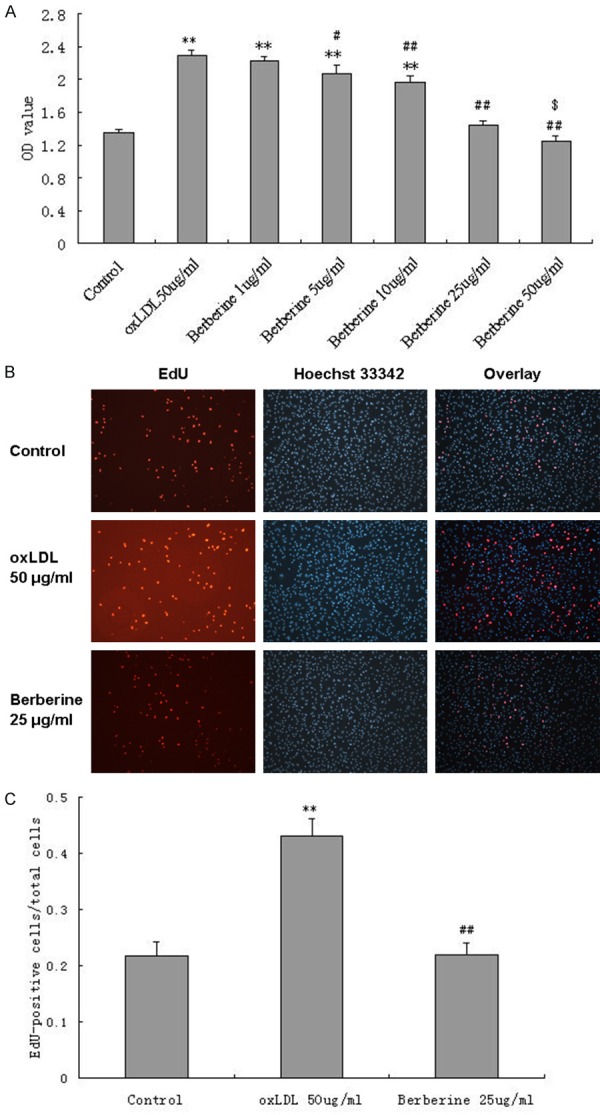
Berberine inhibits proliferation of HUVEC induced by oxLDL. Pretreated with berberine at concentrations of 5 μg/ml, 10 μg/ml, 25 μg/ml, 50 μg/ml, attenuated the oxLDL-induced proliferation of HUVECs in a dose-dependent manner using CCK-8 assay (A). Berberine at concentration of 25 μg/ml significantly inhibited 50 μg/ml oxLDL-induced proliferation of HUVECs using EdU proliferation assay using a confocal laser scanning microscope (×100) (B) and the ratio of EdU-positive cells to total cells was shown (C). Each data point represents mean ± standard deviation of three independent experiments. **P<0.01 compared with control. #P<0.05, ##P<0.01 compared with oxLDL 50 μg/ml. $P<0.05 compared with Control.
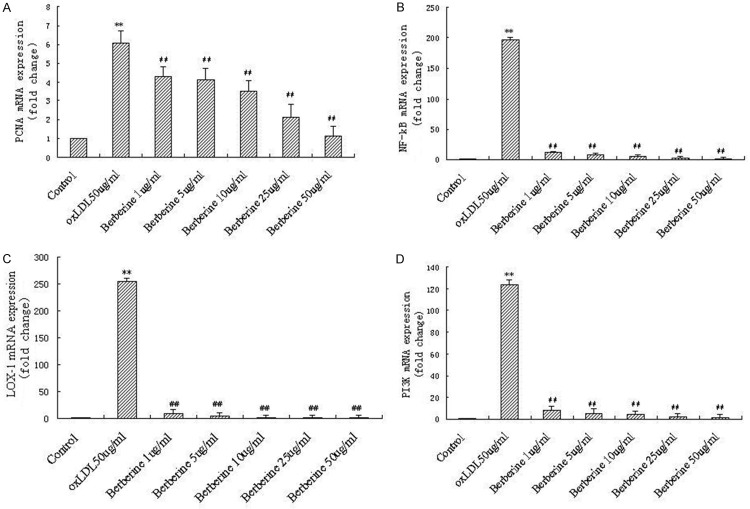
Suppression of berberine in mRNA expression of PCNA, NF-кB, LOX-1 and PI3K in HUVECs induced by oxLDL
In preliminary experiments, we observed increased mRNA expression of some genes related to proliferation in oxLDL-induced HUVECs by Genechip analysis (data not shown), including PCNA, NF-кB, LOX-1 and PI3K. In order to further clarify the mechanism responsible for the inhibition of berberine in HUVECs induced by oxLDL, we next examined the effect of berberine on mRNA expression of PCNA, NF-кB, LOX-1 and PI3K. HUVECs were pretreated with increasing concentrations of berberine for 1 h followed by incubation with oxLDL for 24 h. The expression of PCNA, NF-кB, LOX-1 and PI3K in the HUVECs was evaluated by real-time PCR. As shown in Figure 4, oxLDL significantly increased mRNA expression of PCNA, NF-кB, LOX-1 and PI3K in HUVECs (P<0.01 compared to control), and berberine markedly inhibited oxLDL-induced mRNA expression of PCNA, NF-кB, LOX-1 and PI3K in a concentration dependent fashion.
Figure 4.
Berberine significantly decreased PCNA, NF-кB, LOX-1 and PI3K mRNA expression induced by oxLDL in HUVECs in a dose-dependent manner. Real-time PCR assay for PCNA (A), NF-кB (B), LOX-1 (C) and PI3K (D), respectively. Each data point represents mean ± standard deviation of three independent experiments. **P<0.01 compared with control. ##P<0.01 compared with oxLDL 50 μg/ml.
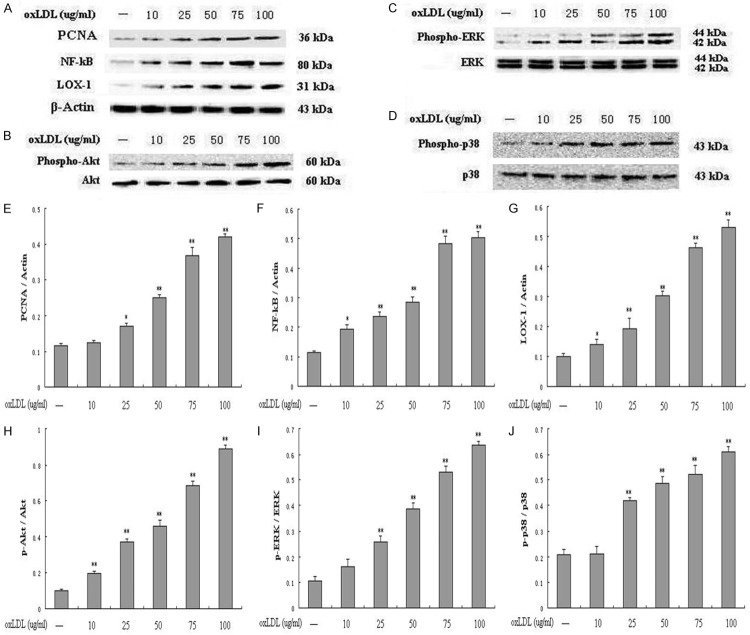
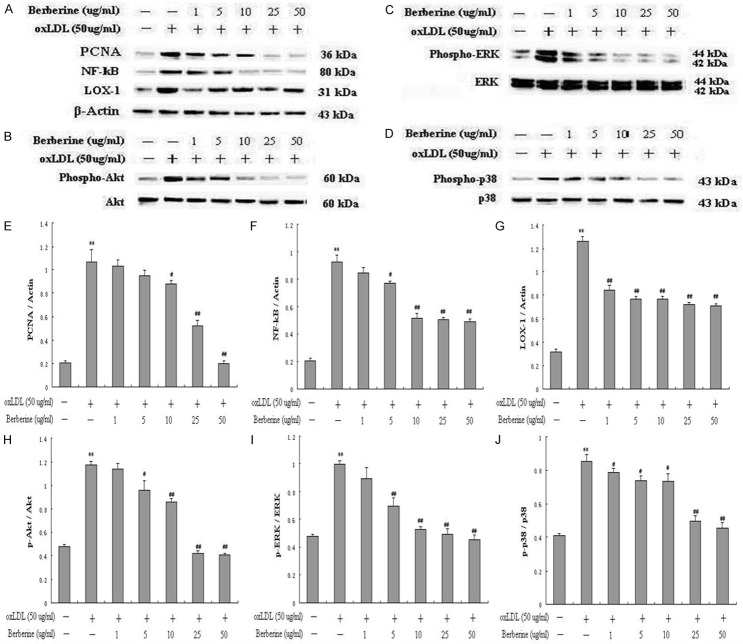
Modification of berberine in the activation of signaling pathways induced by oxLDL
PCNA is identified as an antigen that is expressed in the nuclei of cells during the DNA synthesis phase of the cell cycle. NF-кB and LOX-1 have gained attention for its pro-inflammatory potential in atherogenesis. Both PI3K/Akt and MAPKs pathways, including p38 MAPK and extracellular signal-regulated kinases 1 and 2 (ERK1/2), play important roles in mediating cell proliferation and survival signaling. Therefore, in this study, we explored the effects of oxLDL on PCNA, NF-кB, LOX-1, PI3K, ERK and p38 MAPK signal pathways, and also investigated whether berberine inhibit the above signal pathways. As shown in Figure 5A, 5E-G, oxLDL significantly increased PCNA, NF-κB p65 and LOX-1 expression in a dose-dependent manner compared to control. OxLDL also significantly enhanced the phosphorylation of Akt (Figure 5B and 5H), ERK (Figure 5C and 5I) and p38MAPK (Figure 5D and 5J). However, berberine at concentrations of 1-50 μg/ml markedly inhibited oxLDL-induced PCNA, NF-кB and LOX-1 (Figure 6A, 6E-G), and also apparently decreased the phosphorylation of Akt (Figure 6B and 6H), ERK (Figure 6C and 6I) and p38 (Figure 6D and 6J) in a dose-dependent manner. Among these concentration range of berberine, 25 μg/ml had already exerted significant effects (P<0.01) on all of the above molecular targets. Therefore, in the following experiments, 25 μg/ml of berberine was chosen.
Figure 5.
OxLDL induced the proliferation through up-regulating PCNA, LOX-1 and activating NF-кB, phospho-Akt, phospho-ERK, phospho-p38 signal pathways in a dose-dependent manner. Western blot to PCNA, NF-кB, LOX-1 (A, E, F, G), and phospho-Akt (B, H), phospho-ERK (C, I), phospho-p38 (D, J) in HUVECs. Each data point represents mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 compared with control.
Figure 6.
Berberine inhibited oxLDL-induced HUVECs proliferation by down-regulating the PCNA, LOX-1 and inactivating NF-кB, phospho-Akt, phospho-ERK, phospho-p38 signal pathways. Western blot to PCNA, NF-кB, LOX-1 (A, E, F, G), and phospho-Akt (B, H), phospho-ERK (C, I), phospho-p38 (D, J) in HUVECs. Each data point represents mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 compared with control. #P<0.05, ##P<0.01 compared with oxLDL 50 μg/ml.
To further establish the role for the PI3K/Akt, ERK and p38MAPK signal pathways in the anti-proliferation of berberine in HUVECs induced by oxLDL, a PI3K inhibitor LY294002, an ERK1/2 inhibitor PD98059 and a p38 inhibitor SB202190 were adopted in the next experiments. HUVECs were pretreated for 1 h with LY294002 (25 μM), PD98059 (10 μM) and SB202190 (10 μM) before the addition of berberine (25 μg/ml) followed by incubation with oxLDL (50 μg/ml) induction for 24 h, and then harvested for western blot or prepared for CCK-8 assay. The results revealed that LY294002, PD98059 and SB202190 abolished the inhibition of berberine on oxLDL-induced phosphorylation of Akt (Figure 7A and 7D), ERK (Figure 7B and 7E) and p38 (Figure 7C and 7F), respectively, and also counteracted partly the inhibition of berberine on oxLDL-induced HUVECs proliferation (Figure 7G).
Figure 7.
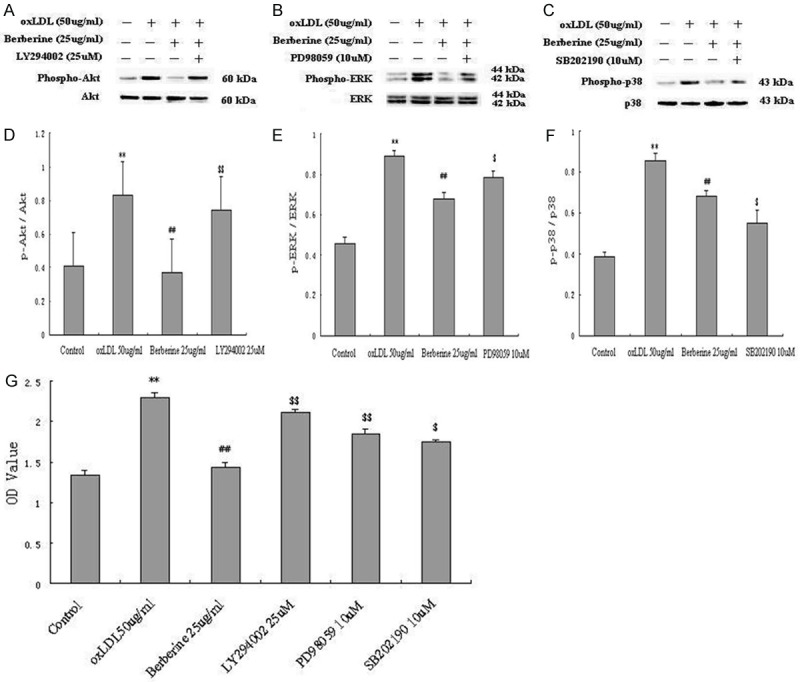
Berberine inhibited oxLDL-induced HUVECs proliferation through PI3k/Akt, ERK and p38MAPK signal pathways. HUVECs were pretreated with or without LY294002 (25 μM), PD98059 (10 μM) and SB202190 (10 μM) for 1 h followed by berberine (25 μg/ml) incubation with oxLDL (50 μg/ml) induction for 24 h. A-F: Western blot analysis showed a clear increase of Akt, ERK and p38 phosphorylation induced by LY294002, PD98059 and SB202190. G: CCK-8 assay showd that LY294002, PD98059 and SB202190 abrogated the inhibition of berberine in oxLDL-induced HUVECs proliferation. Each data point represents mean ± standard deviation of three independent experiments. **P<0.01 compared with control. #P<0.05, ##P<0.01 compared with oxLDL 50 μg/ml. $P<0.05, $$P<0.01, compared with berberine (25 μg/ml).
Discussion
Cell proliferation is one of key steps of a variety of human disease such as AS and cancers. The inhibition of cell proliferation is, therefore, an effective strategy for prevention and treatment of such kinds of diseases. The major novel finding of the present study is that berberine could significantly inhibit proliferation on HUVECs induced by oxLDL in a dose-dependent manner. Moreover, the study clearly showed that effects of berberine on HUVECs proliferation induced by oxLDL were mediated through suppressing the expression of PCNA, NF-кB, LOX-1 and inactivating the phosphorylation of PI3K/Akt, ERK1/2, p38MAPK signal pathways. Apparently, our study confirmed and extended previous studies regarding the role of berberine in inhibition of cell proliferation and potential mechanisms.
It has previously been reported that oxLDL stimulates proliferation in HUVECs mediated by NAD(P)H oxidase-derived O2-formation [14] and activation of the small GTPase RhoA [5]. Recent reviews indicated that oxLDL and LOX-1 played an important role in the pathophysiological process of atherogenesis [15,16]. Our results demonstrated that oxLDL could significantly increase the expression of PCNA, NF-кB and LOX-1 at both transcription and translation levels. In addition, the present study showed a remarkable enhancement in phosphorylation of PI3K/Akt, ERK1/2 and p38MAPK in HUVECs induced by oxLDL. Thereby, the data was in consistent with the results from former studies by characterizing the up-regulation of proliferative and pro-inflammatory signaling pathways, which was presumably linked to the up-regulation of PCNA, NF-кB, LOX-1 and phosphorylation of Akt, ERK1/2, p38MAPK followed by oxLDL stimulation. Especially, the present results were very similar to that of several earlier studies, which also suggesting an involvement of NF-кB, LOX-1 and MAPK components such as ERK1/2 and p38 in either coronary artery endothelial cell or HUVEC proliferation [6,17].
Chinese herb has been used widely and successfully for centuries in preventing and treating different kinds of disease in humans. One of them is berberine, a natural alkaloid that is isolated from a variety of traditional Chinese herbs [7]. According to medical history recordings, berberine has been used in Chinese traditional medicine to treat diarrhea and gastrointestinal disorders for many years in China and other Asian countries [18]. Several previous studies have demonstrated that berberine have several pharmacological properties, including anti-microbial effects, anti-inflammatory activities, and regulating insulin secretion [19,20]. Interestingly, berberine has recently been shown an effective anti-tumor effect. In another word, the growth of several different types of cancers is significantly inhibited by berberine in vitro.
For example, a study indicated that berberine could potentially inhibit proliferation, induce apoptosis, and inhibit the invasion of human skin squamous cell carcinoma A431 cells [21]. Following exposure to different concentrations of berberine, apoptosis was induced in human leukemia HL-60 cells through caspase-3 activation demonstrated by Lin and colleagues [22]. Additionally, berberine combined with galangin had synergistic anti-cancer effects through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells [23]. As an anti-cancer drug, berberine has been demonstrated to act through mechanisms such as the inhibition of DNA topoisomerase II alpha and the induction of topoisomerase II-mediated DNA cleavage [24]. More importantly, several recent studies indicated that berberine had more widely potential clinical implication for future prevention and treatment of different kinds of diseases in humans such as decreasing blood glucose levels [25,26], lowering cholesterol concentration [27] and reducing excess body weight [28]. Finally, berberine has clearly been showed to have a protection against LDL oxidation, subsequently preventing oxLDL-induced cellular dysfunction [29]. Based on these published data, we hypothesized that it might be worthy of further investigation to elucidate the possible molecular mechanism concerning berberine’s anti-disease property. That is the reason that we set out the present study.
It has been well recognized that AS is a complex pathophysiological process, which is strongly associated with inflammation, oxidative stress, and cell proliferations [30-32]. The evidence for the role of berberine in inhibiting proliferation comes from varies of cell types. For example, berberine inhibits the proliferation of epithelial ovarian carcinoma cell line, thyroid cancer cell line, HepG2 cell line and so on, which indicates its anti-proliferation property [33-35]. A recent study revealed that berberine reduced matrix metalloproteinases (MMP) by suppressing the activity of p38MAPK in phorbol myristate acetate (PMA)-induced macrophages, suggesting a potential role as a therapeutic aid for stabilizing AS plaque [36]. Another study from Sarna and colleagues showed that berberine inhibited NADPH oxidase mediated superoxide anion production in lipopolysaccharide-induced macrophages [37]. More recently, the administration of berberine to mice with LPS-induced endotoxemia could increase activation of transcription factor-3 expression and AMPK phosphorylation in spleen and lung tissues and concomitantly reduce the plasma and tissue levels of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), Interleukin-1β (IL-1β), suggesting an anti-inflammatory effect of berberine on macrophages [38]. Those previous studies suggested that berberine might be a potential candidate of anti-atherosclerotic medication. However, little is yet known about the effect of berberine on endothelial cells proliferation. In the present study, we demonstrated, for the first time, that berberine inhibited oxLDL-induced HUVECs proliferation by down-regulation the expression of PCNA, NF-кB and LOX-1 in a dose-dependent manner. More importantly, mechanism investigation of the present study revealed that the impact of berberine on cell proliferation mainly targeted NF-кB and LOX-1 pathways. Additionally, the study indicated that berberine suppressed the phosphorylation of Akt, ERK1/2 and p38MAPK, suggesting that the PI3K/Akt, ERK1/2 and p38MAPK signaling pathways may also participated in the inhibition of berberine on HUVECs proliferation induced by oxLDL.
In summary, AS has been described as a chronic inflammatory disease which is strongly associated with oxLDL. The elucidation of the potential mechanism and exploration of promising strategy for targeting oxLDL is one of the most interesting fields of atherosclerotic disease. In the present study, data suggested that oxLDL could remarkablely induce proliferation of cultured HUVECs and this effect of oxLDL on cell proliferation could significantly be inhibited by berberine, a plant product from Chinese herbs. More importantly, our study indicated that the impacts of berberine on inhibition of proliferation might be involved in its anti-inflammatory and anti-proliferative properties, including down-regulating the expression of PCNA, NF-кB and LOX-1 and inactivating PI3K/Akt, ERK1/2 and p38MAPK signaling pathways. Therefore, our investigation may provide novel findings of berberine for the future prevention and treatment of atherosclerotic diseases, especially regarding the proliferation induced by oxLDL.
Acknowledgements
This article is partly supported by CAMS Major Collaborative Innovation Project (2016-I2M-1-011), Specialized Research Personnel Fund of Fu Wai Hospital (2012-FWXX02), PUMC Youth Fund (2016-XHQN06), Capital Health Development Fund (201614035), Beijing Natural Science Foundation (7131014) and National Natural Scientific Foundation (81100118, 81241121).
Disclosure of conflict of interest
None.
References
- 1.Pedicino D, Giglio AF, Galiffa VA, Cialdella P, Trotta F, Graziani F, Liuzzo G. Infections, immunity and atherosclerosis: pathogenic mechanisms and unsolved questions. Int J Cardiol. 2013;166:572–583. doi: 10.1016/j.ijcard.2012.05.098. [DOI] [PubMed] [Google Scholar]
- 2.Wang R, Zhang Y, Xu L, Lin Y, Yang X, Bai L, Chen Y, Zhao S, Fan J, Cheng X, Liu E. Protein inhibitor of activated STAT3 suppresses oxidized LDL-induced cell responses during atherosclerosis in apolipoprotein e-deficient mice. Sci Rep. 2016;6:36790. doi: 10.1038/srep36790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo C, Lian X, Hong L, Zou J, Li Z, Zhu Y, Huang T, Zhang Y, Hu Y, Yuan H, Wen T, Zhuang W, Cai B, Zhang X, Hisatome I, Yamamoto T, Huang J, Cheng J. High uric acid activates the ROSAMPK pathway, impairs CD68 expression and inhibits OxLDL-induced foam-cell formation in a human monocytic cell line, THP-1. Cell Physiol Biochem. 2016;40:538–548. doi: 10.1159/000452567. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, Zhang X, Yu W, Chen J, Li Q, Jiao Y, He P, Shen C. Effects of chemokine-like factor 1 on vascular smooth muscle cell migration and proliferation in vascular inflammation. Atherosclerosis. 2013;226:49–57. doi: 10.1016/j.atherosclerosis.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Seibold S, Schurle D, Heinloth A, Wolf G, Wagner M, Galle J. Oxidized LDL induces proliferation and hypertrophy in human umbilical vein endothelial cells via regulation of p27Kip1 expression: role of RhoA. J Am Soc Nephrol. 2004;15:3026–3034. doi: 10.1097/01.ASN.0000146425.58046.6A. [DOI] [PubMed] [Google Scholar]
- 6.Kuhlmann CR, Schafer M, Li F, Sawamura T, Tillmanns H, Waldecker B, Wiecha J. Modulation of endothelial Ca(2+)-activated K(+) channels by oxidized LDL and its contribution to endothelial proliferation. Cardiovasc Res. 2003;60:626–634. doi: 10.1016/j.cardiores.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Kuo HP, Chuang TC, Yeh MH, Hsu SC, Way TD, Chen PY, Wang SS, Chang YH, Kao MC, Liu JY. Growth suppression of HER2-overexpressing breast cancer cells by berberine via modulation of the HER2/PI3K/Akt signaling pathway. J Agric Food Chem. 2011;59:8216–8224. doi: 10.1021/jf2012584. [DOI] [PubMed] [Google Scholar]
- 8.Cecil CE, Davis JM, Cech NB, Laster SM. Inhibition of H1N1 influenza A virus growth and induction of inflammatory mediators by the isoquinoline alkaloid berberine and extracts of goldenseal (Hydrastis canadensis) Int Immunopharmacol. 2011;11:1706–1714. doi: 10.1016/j.intimp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Meng S, Wang LS, Huang ZQ, Zhou Q, Sun YG, Cao JT, Li YG, Wang CQ. Berberine ameliorates inflammation in patients with acute coronary syndrome following percutaneous coronary intervention. Clin Exp Pharmacol Physiol. 2012;39:406–411. doi: 10.1111/j.1440-1681.2012.05670.x. [DOI] [PubMed] [Google Scholar]
- 10.Fan X, Wang J, Hou J, Lin C, Bensoussan A, Chang D, Liu J, Wang B. Berberine alleviates ox-LDL induced inflammatory factors by upregulation of autophagy via AMPK/mTOR signaling pathway. J Transl Med. 2015;13:92. doi: 10.1186/s12967-015-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sefidabi R, Mortazavi P, Hosseini S. Antiproliferative effect of berberine on canine mammary gland cancer cell culture. Biomed Rep. 2017;6:95–98. doi: 10.3892/br.2016.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Liu Y, Wang B, Luo Y, Hu N, Chen D, Zhang X, Xiong Y. Protective effect of berberine against oxidative stress-induced apoptosis in rat bone marrow-derived mesenchymal stem cells. Exp Ther Med. 2016;12:4041–4048. doi: 10.3892/etm.2016.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng YX, Zhang XJ, Shi QZ, Chen YS, Qiu XM, Chen B. Anti-hyperglycemic effects and mechanism of traditional Chinese medicine Huanglian Wan in streptozocin-induced diabetic rats. J Ethnopharmacol. 2012;144:425–432. doi: 10.1016/j.jep.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 14.Heinloth A, Heermeier K, Raff U, Wanner C, Galle J. Stimulation of NADPH oxidase by oxidized low-density lipoprotein induces proliferation of human vascular endothelial cells. J Am Soc Nephrol. 2000;11:1819–1825. doi: 10.1681/ASN.V11101819. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Zhang M, Li Y, Liu S, Ping S, Wang J, Ning F, Xie F, Li C. Simvastatin inhibits the additive activation of ERK1/2 and proliferation of rat vascular smooth muscle cells induced by combined mechanical stress and oxLDL through LOX-1 pathway. Cell Signal. 2013;25:332–340. doi: 10.1016/j.cellsig.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Jia YH, Zhao XS, Zhou FH, Pan YY, Wan Q, Cui XB, Sun XG, Chen YY, Zhang Y, Cheng SB. Trichosanatine alleviates oxidized low-density lipoprotein induced endothelial cells injury via inhibiting the LOX-1/p38 MAPK pathway. Am J Transl Res. 2016;8:5455–5464. [PMC free article] [PubMed] [Google Scholar]
- 17.Dandapat A, Hu C, Sun L, Mehta JL. Small concentrations of oxLDL induce capillary tube formation from endothelial cells via LOX-1-dependent redox-sensitive pathway. Arterioscler Thromb Vasc Biol. 2007;27:2435–2442. doi: 10.1161/ATVBAHA.107.152272. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Yu Z, Li Y, Fichna J, Storr M. Effects of berberine in the gastrointestinal tract-a review of actions and therapeutic implications. Am J Chin Med. 2014;42:1053–1070. doi: 10.1142/S0192415X14500669. [DOI] [PubMed] [Google Scholar]
- 19.Huang LH, Pan XP, Gong KR, Shao G. Anti-inflammatory effects of three kinds of traditional Mongolian medicine monomer and its combination on LPS-stimulated RAW264.7 macrophages. Eur Rev Med Pharmacol Sci. 2016;20:950–958. [PubMed] [Google Scholar]
- 20.Pang B, Zhao LH, Zhou Q, Zhao TY, Wang H, Gu CJ, Tong XL. Application of berberine on treating type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:905749. doi: 10.1155/2015/905749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li DX, Zhang J, Zhang Y, Zhao PW, Yang LM. Inhibitory effect of berberine on human skin squamous cell carcinoma A431 cells. Genet Mol Res. 2015;14:10553–10568. doi: 10.4238/2015.September.8.17. [DOI] [PubMed] [Google Scholar]
- 22.Lin CC, Kao ST, Chen GW, Ho HC, Chung JG. Apoptosis of human leukemia HL-60 cells and murine leukemia WEHI-3 cells induced by berberine through the activation of caspase-3. Anticancer Res. 2006;26:227–242. [PubMed] [Google Scholar]
- 23.Ren K, Zhang W, Wu G, Ren J, Lu H, Li Z, Han X. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed Pharmacother. 2016;84:1748–1759. doi: 10.1016/j.biopha.2016.10.111. [DOI] [PubMed] [Google Scholar]
- 24.Kang MR, Chung IK. Down-regulation of DNA topoisomerase IIalpha in human colorectal carcinoma cells resistant to a protoberberine alkaloid, berberrubine. Mol Pharmacol. 2002;61:879–884. doi: 10.1124/mol.61.4.879. [DOI] [PubMed] [Google Scholar]
- 25.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y, Chen YT, Yang YX, Zhou XJ, Dai SJ, Tong JF, Shou D, Li C. Metabolomics study of type 2 diabetes mellitus and the antidiabetic effect of berberine in zucker diabetic fatty rats using Uplc-ESI-Hdms. Phytother Res. 2016;30:823–828. doi: 10.1002/ptr.5587. [DOI] [PubMed] [Google Scholar]
- 27.Pirillo A, Catapano AL. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: from in vitro evidence to clinical studies. Atherosclerosis. 2015;243:449–461. doi: 10.1016/j.atherosclerosis.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Guo T, Woo SL, Guo X, Li H, Zheng J, Botchlett R, Liu M, Pei Y, Xu H, Cai Y, Zeng T, Chen L, Li X, Li Q, Xiao X, Huo Y, Wu C. Berberine ameliorates hepatic steatosis and suppresses liver and adipose tissue inflammation in mice with diet-induced obesity. Sci Rep. 2016;6:22612. doi: 10.1038/srep22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh YS, Kuo WH, Lin TW, Chang HR, Lin TH, Chen PN, Chu SC. Protective effects of berberine against low-density lipoprotein (LDL) oxidation and oxidized LDL-induced cytotoxicity on endothelial cells. J Agric Food Chem. 2007;55:10437–10445. doi: 10.1021/jf071868c. [DOI] [PubMed] [Google Scholar]
- 30.Li JJ. Inflammation in coronary artery diseases. Chin Med J. 2011;124:3568–3575. [PubMed] [Google Scholar]
- 31.Gu L, Bai W, Li S, Zhang Y, Han Y, Gu Y, Meng G, Xie L, Wang J, Xiao Y, Shan L, Zhou S, Wei L, Ferro A, Ji Y. Celastrol prevents atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS One. 2013;8:e65477. doi: 10.1371/journal.pone.0065477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Jia YJ, Li XL, Xu RX, Zhu CG, Guo YL, Wu NQ, Li JJ. RANTES gene G-403A polymorphism and coronary artery disease: a meta analysis of observational studies. PLoS One. 2012;7:e47211. doi: 10.1371/journal.pone.0047211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park KS, Kim JB, Lee SJ, Bae J. Berberine-induced growth inhibition of epithelial ovarian carcinoma cell lines. J Obstet Gynaecol Res. 2012;38:535–540. doi: 10.1111/j.1447-0756.2011.01743.x. [DOI] [PubMed] [Google Scholar]
- 34.Park KS, Kim JB, Bae J, Park SY, Jee HG, Lee KE, Youn YK. Berberine inhibited the growth of thyroid cancer cell lines 8505C and TPC1. Yonsei Med J. 2012;53:346–551. doi: 10.3349/ymj.2012.53.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Wu FX, Luo HL, Liu JJ, Luo T, Bai T, Li LQ, Fan XH. Berberine upregulates miR-22-3p to suppress hepatocellular carcinoma cell proliferation by targeting Sp1. Am J Transl Res. 2016;8:4932–4941. [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Z, Wang L, Meng S, Wang Y, Chen T, Wang C. Berberine reduces both MMP-9 and EMMPRIN expression through prevention of p38 pathway activation in PMA-induced macrophages. Int J Cardiol. 2011;146:153–158. doi: 10.1016/j.ijcard.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Sarna LK, Wu N, Hwang SY, Siow YL, O K. Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophages. Can J Physiol Pharmacol. 2010;88:369–378. doi: 10.1139/Y09-136. [DOI] [PubMed] [Google Scholar]
- 38.Bae YA, Cheon HG. Activating transcription factor-3 induction is involved in the anti-inflammatory action of berberine in RAW264.7 murine macrophages. Korean J Physiol Pharmacol. 2016;20:415–424. doi: 10.4196/kjpp.2016.20.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]