Abstract
NADPH oxidase 4 (NOX4) is a member of the NADPH oxidase (NOX) family of enzymes and has been found abnormally expressed in human cancers. However, its role in gastric cancer (GC) is still unclear. In the current study, we reported that NOX4 expression levels were significantly up-regulated in GC tissues compared to normal tissues (P<0.0001). Higher NOX4 expression was significantly associated with poorer overall survival in GC patients. Silencing NOX4 in two NOX4 high expression GC cell lines, MGC-803 and BGC-823 cells, did not affect cell proliferation, while inhibited cell adhesion and cell invasion of GC cells. Furthermore, Gene set enrichment analysis (GSEA) results indicated that NOX4 expression was strongly associated with cell migration, epithelial-mesenchymal transition (EMT) and Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathways. More interestingly, Interleukin-6 (IL-6) increased the invasion ability and activation of JAK2/STAT3 of MGC-803 and BGC-823 cells. Such effects were attenuated by NOX4 silencing. Overexpression of NOX4 in one NOX4 low expression GC cell line, SGC-7901 cells, significantly promoted cell invasion, which was impaired by treatment of JAK2 inhibitor, AG490. AG490 inhibited STAT3 activation in SW1990 cells. NOX4 may exert its function through JAK2/STAT3 pathway. In summary, the findings of this study indicate that NOX4 may promote the development of GC, potentially representing a novel prognostic marker for overall survival in GC.
Keywords: NOX4, invasion, epithelial-mesenchymal transition, JAK2, STAT3
Introduction
Gastric cancer (GC) is the fourth most common malignant tumor and the second leading cause of cancer-related deaths in the world [1,2]. Despite recent advances in diagnosis and treatment, overall 5-year survival rate for GC is still less than 30% [2]. Because there were no obvious symptoms or only nonspecific symptoms at the early stages of GC, GC has often progressed to advanced stages by the time of diagnosis. At advanced stages, therapy can only treat symptoms, but cannot completely cure GC [3]. Therefore, it is urgent to identify novel biomarkers and therapeutic targets to improve the early diagnosis and treatment outcomes of GC.
NADPH oxidase 4 (NOX4) belongs to the NADPH oxidase (NOX) family of enzymes [4]. NOX family was originally identified as the major source of intracellular reactive oxygen species (ROS), which have emerged as secondary messengers in various signaling pathways [5]. ROS levels have been linked to carcinogenesis because of their involvement in many critical cellular processes including proliferation, DNA damage responses and angiogenesis [6,7]. NOX4 expression is up-regulated in several human tumors, such as pancreatic cancer [8], thyroid carcinomas [9] and breast cancer [10], whereas decreased expression of NOX4 was observed in liver cancer [11]. Recent studies have demonstrated that NOX4 promotes urothelial and melanoma carcinogenesis via regulating cell cycle progression [12,13], but NOX4 exerts inhibitory role in the growth of liver cancer cells and liver cancer progression [11]. NOX4 has also been shown to contribute to cell migration of pancreatic cancer [8], breast cancer cells [14-16] and colon cancer cells [17]. However, the expression and functions of NOX4 in GC have not been revealed before.
In the present study, we characterized the expression of NOX4 in GC patients and investigated its role in GC pathogenesis. We reported the up-regulation of NOX4 expression in human gastric cancer tissues. Elevated NOX4 mRNA expression was strongly correlated with poor overall survival of GC patients. Silencing NOX4 had no effects on GC cell proliferation, but inhibited adhesion and invasive capacities of GC cells. We also studied the involved possible mechanism. Our study suggest that NOX4 may be a valuable therapeutic target for GC.
Materials and methods
Patients and gastric tissue specimens
This study was approved by the Ethics Committee of Fudan University. Written informed consent was obtained from all participants. A total of 120 GC tissues were collected from GC patients who underwent surgery at Cancer Hospital of Fudan University between January 2004 and December 2009. Additionally, 43 matched non-tumorous tissues were taken more than 5 cm distance from tumor tissues. Immediately after resection, all tissue specimens were snap-frozen and stored at -80°C until experimental use. None of the participants received chemotherapy or radiation therapy prior to surgery. Follow-up information was obtained until December 2014. Overall survival (OS) was calculated from the date of initial surgery to the date of death or the date of the last follow-up.
RNA isolation and real-time PCR
Total RNA was isolated from human gastric tissues and gastric cancer cells with TRIzol reagent (Invitrogen, USA) following the manufacturer’s instruction. First strand cDNA was synthesized using Reverse Transcription Reagents (Promega, Madison, WI, USA). Real-time PCR was carried out in ABI Prism 7300 (Applied Biosystems, Foster City, CA, USA) using SYBR Green I Master Mix (Applied Biosystems) with GAPDH as an internal control. The sequences of primers were as follows: NOX4, 5’-GACTTGGCTTTGGATTTCTG-3’ (sense) and 5’-TCTGAGGGATGACTTATGAC-3’ (anti-sense); GAPDH, 5’-CACCCACTCCTCCACCTTTG-3’ (sense) and 5’-CCACCACCCTGTTGCTGTAG-3’ (anti-sense). The relative mRNA expression of NOX4 was estimated by ΔΔCt and normalized to GAPDH [18].
Bioinformatics analysis
We collected gene expression data of 250 stomach adenocarcinoma (STAD) and 32 normal tissues from The Cancer Genome Atlas website (TCGA, https://tcga-data.nci.nih.gov/tcga/). To investigate the biological pathways strongly associated with GC pathogenesis through NOX4, Gene Set Enrichment Analysis (GSEA) [19] was performed using the GSEA desktop software [20]. Enriched gene sets between NOX4 higher expression and lower expression were identified using 1000 permutations of the phenotype labels.
Cell culture
Six human gastric cancer cell lines (MKN-45, SGC-7901, MGC-803, BGC-823, MKN-28 and AGS) obtained from American Type Culture Collection (Rockville, MD, USA) were cultured in Dulbecco’s Modified Eagle’s (DMEM) or RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT, USA) and 100 mg/ml of penicillin-streptomycin. All cells were grown at 37°C under a humidified atmosphere of 5% CO2 and 95% air.
siRNA-mediated gene knock-down and ectopic expression
NOX4 specific siRNAs (siRNA1 and siRNA2) and nonsense siRNA (NC) were prepared by XX (Shanghai, China). The coding strand of human NOX4 siRNA1 was 5’-AGCCAGUCACCAUCAUUUCUU-3’, and that of NOX4 siRNA2 was 5’-GCACUCCAGGCAAAUAUAUUU-3’. siRNAs were introduced into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
Human NOX4 CDNA was cloned into eukaryotic expression vector pcDNA3 (Invitrogen, Carlsbad, CA, USA) by Genewiz Company (Shanghai, China). The construct was confirmed by sequencing. Plasmid transfection in SGC-7901 cells was performed with Lipofectamine 2000 (Invitrogen). pcDNA3 vector was served as a negative control (NC).
Preparation of cell lysates and western blot analysis
Cells were lysed in RIPA lysis buffer (Solarbio, Beijing, China), sonicated and centrifuged at 12,000 rpm for 20 min. The concentration of total protein in cell lysates was assessed by the BCA method (Thermo Fisher Scientific, Rockford, IL, USA). Equal amount of proteins (35 mg/well) were resolved in sodium dodecyl sulfate (SDS)-polyacrylamide gels and electrotransferred onto polyvinylidene difluoride membranes (Millipore, Bredford, MA, USA). After blocked in 5% skim milk at room temperature for 1 h, the membranes were incubated with the indicated primary antibody at 4°C overnight and then with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (Beyotime, Shanghai, China). Signals were detected on X-ray films by using Enhanced Chemiluminescence (ECL) detection system (Millipore). GAPDH was served as a loading control. The primary antibodies were: anti-NOX4, anti-Vimentin, anti-Fibronectin, anti-MMP-2, anti-Twist1, anti-Snail2, anti-p-JAK2, anti-JAK2, anti-p-STAT3 and anti-STAT3 from Abcam (Cambridge, MA, USA); anti-E-cadherin, anti-β-catenin and anti-GAPDH from Cell Signaling (Danvers, MA, USA); anti-ZEB1 from Santa Cruz (Santa Cruz, CA, USA).
Cell proliferation assay
Cell proliferation was assessed by the Cell Counting Kit-8 (CCK-8) according to manufacturer’s instructions (Beyotime). Briefly, MGC-803 and BGC-823 cells plated in 96-well culture plates (3×103 per well) in triplicates. One day post plating, the cells were transfected with NOX4 siRNA1 or control siRNA (NC). At 0 h, 24 h, 48 h and 72 h after siRNA transfection, CCK-8 solution was subsequently added to each well. After 1 h of additional incubation, the absorbance was measured on a microplate reader at a wavelength of 450 nm.
Cell-matrix adhesion assay
MGC-803 and BGC-823 cells were seeded onto 6-well plates and transfected with NOX4 siRNA1 or control siRNA (NC). Two days post transfection, cells were trypsinized and plated in fibronectin-precoated 12-well plates at a density of 1×105 cells per well. After incubated at 37°C for 1 h, cells that did not adherent to the plates were washed off with PBS. Adherent cells were fixed in 4% paraformaldehyde, stained with 0.2% crystal violet, and counted at 5 random fields under an inverted microscope.
Matrigel cell invasion assay
MGC-803 and BGC-823 cells were seeded and transfected as described above. One day after the transfection, cells were trypsinized, suspended in DMEM medium without FBS and added to upper Transwell chambers coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). For interleukin-6 (IL-6) treatment, cells were cultured in medium containing 100 ng/mL IL-6 (Sigma, St Louis, MO, USA). DMEM medium with 10% FBS were added to the lower chamber. After 24 h of incubation, cells remaining in the upper membrane were completely scraped away and invaded cells were stained with 0.2% crystal violet. Invaded cells were counted at 5 random fields in each well under an inverted microscope.
SGC-7901 cells were seeded and transfected with NOX4 expression plasmid or NC. One day after the transfection, cells were collected and seeded to upper Transwell chambers as described above. DMSO or 20 µM AG490 (dissolved in DMSO; Sigma) was added to the upper chamber as indicated, and Matrigel cell invasion assay was performed.
Statistics analysis
All in vitro data were obtained from at least three independent experiments and presented as mean ± standard deviation (SD). Statistical analysis was conducted using GraphPad Prism version 6.0 (GraphPad, San Diego, CA, USA). The overall survival (OS) curve was calculated with the Kaplan-Meier method and analyzed with the log-rank test. Two-tailed student’s t-test was used to compare the difference between two groups. Data were considered significant if P<0.05.
Results
Overexpression of NOX4 in GC tissues
We first examined NOX4 mRNA expression by real-time PCR on GC tissues and non-tumorous tissues. Notably, as shown in Figure 1A, NOX4 mRNA expression was significantly elevated in GC tissues (n=120) compared with that in non-tumorous tissues (n=43, P<0.0001). Western blot analysis also revealed that NOX4 protein expression was elevated in GC tissues (Figure S1). We then re-analyzed high throughput RNA-sequencing data of TCGA STAD dataset and also found a remarkable increase of NOX4 expression in GC tissues (Figure 1B, P<0.0001). Furthermore, Kaplan-Meier analysis was conducted to investigate the correlation of NOX4 expression and prognosis of GC. As shown in Figure 1C, the overall survival time of patients with NOX4 higher expression tumors was notably shorter than those with NOX4 lower expression tumors (P<0.01). Our data demonstrated that NOX4 mRNA was overexpressed in GC tissues, which was strongly associated with poor survival of patients with GC.
Figure 1.
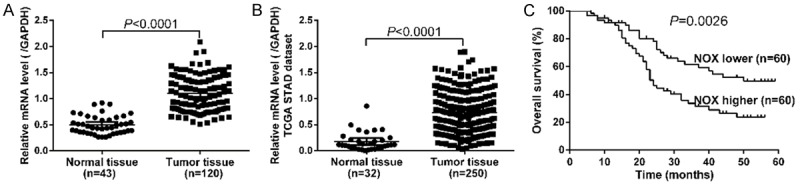
Overexpression of NOX4 in GC. A. The mRNA level of NOX4 in GC (n=120) and normal tissues (n=43) was detected by real-time PCR. NOX4 mRNA was significantly elevated in GC tissues as compared with non-tumorous tissues (P<0.0001). B. NOX4 expression was significantly increased in GC tissues (n=250) when compared with normal tissues (n=32) from TCGA STAD dataset (P<0.0001). C. Overall survival analysis showed that patients with NOX4 higher expression tumors have a shorter overall survival time than those with NOX4 lower expression tumors (P<0.05). (*P<0.05, **P<0.01, ***P<0.001).
Effects of NOX4 knockdown on cell growth, adhesion and invasion of GC cells in vitro
We chose GC cell lines MGC-803 and BGC-823 for NOX4 knockdown because of the high mRNA and protein levels of NOX4 in these two cell lines as indicated in Figure 2A. NOX4 specific siRNAs (siRNA1 and siRNA2) significantly reduced the mRNA and protein expression levels of NOX4 in both cell lines compared with cells transfected with nonsense siRNA (NC), while its expression was comparable in NC cells and cells without transfection (Figure 2B and 2C). siRNA1, which had a higher knockdown efficiency than siRNA2, was then used in the following assays.
Figure 2.
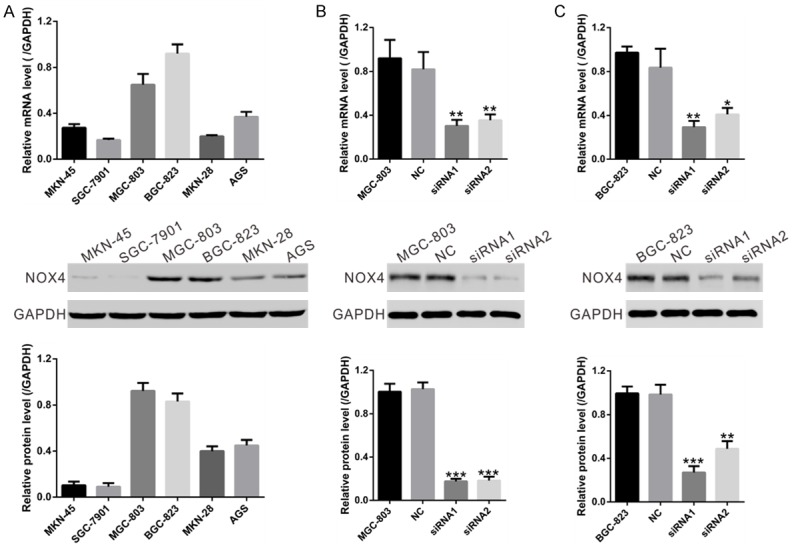
Knockdown of NOX4 in GC cells by siRNA transfection. A. mRNA and protein levels of NOX4 were determined in 6 human GC cell lines using real-time RT-PCR method (upper panel) and Western blot (middle and lower panels), respectively. Representative Western blot (middle panel) and quantitative results (lower panel) were shown. B, C. Expression of NOX4 in MGC-803 and BGC-823 cells was analyzed by real-time RT-PCR method (upper panel) and Western blot (middle and lower panels). NC: nonsense siRNA-transfected cells; siRNA1 and siRNA2: NOX4-siRNA1 or NOX4-siRNA2 transfected cells (*P<0.05, **P<0.01 and ***P<0.001 Vs NC.).
We next determined the effects of NOX4 knockdown on cell growth in MGC-803 and BGC-823 in vitro. The results of the CCK-8 proliferation assay showed that NOX4 knockdown had no effects on the proliferation rate of GC cells (P>0.05; Figure 3A).
Figure 3.
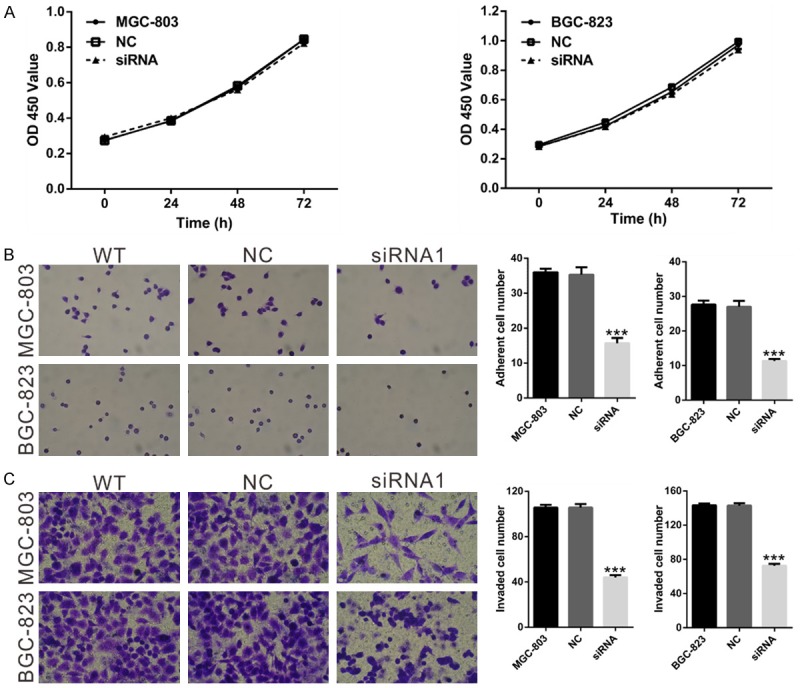
Effects of NOX4 knockdown on the proliferation, adhesion and invasion of GC cells. A. Cell proliferation of MGC-803 and BGC-823 cells was detected by CCK-8 assay as described in Materials and Methods. B. Cell adherent ability of MGC-803 and BGC-823 cells treated with NOX4 siRNA1 or control siRNA (NC) was assessed using cell-matrix adhesion assay. C. Invasive capacity of MGC-803 and BGC-823 cells treated with NOX4 siRNA1 or control siRNA (NC) was checked by using Matrigel-coated transwell chamber. Magnification, ×200. WT: wild type cells; NC: nonsense siRNA-transfected cells; siRNA1: NOX4-siRNA1 transfected cells (***P<0.001 Vs NC).
To investigate the effects of NOX4 on the adhesion and invasion of GC cells, we carried out cell-matrix adhesion and Matrigel invasion assays. As indicated in Figure 3B, MGC-803 and BGC-823 cells adhering to fibronectin slower and had less ability to invade through the Matrigel-coated membranes when NOX4 expression was repressed. Taken together, the results mentioned above suggest that NOX4 may be involved in the metastasis of GC, and more in vivo investigation is still needed.
NOX4 knockdown inhibits multiple signaling pathways associated with tumor progression
To identify NOX4-associated pathways in GC, gene set enrichment analysis (GESA) was conducted with data from TCGA STAD dataset. As illustrated in Figure 4, NOX4 expression was strongly associated with cell migration, epithelial-mesenchymal transition (EMT) and Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathways.
Figure 4.
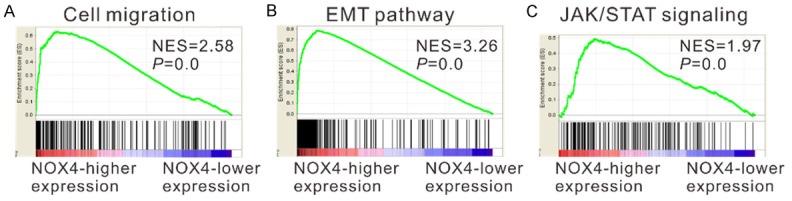
GESA on TCGA STAD dataset identified cell migration (A), EMT (B) and JAK/STAT (C) pathways as regulatory targets of NOX4.
Then, we analyzed the expression of above mentioned signaling pathways-related proteins in MGC-803 and BGC-823 cells at 48 h after siRNA transfection by Western blot. As shown in Figure 5, NOX4 siRNA treatment resulted in a significant decrease in the expression of cell migration-related factors (Fibronectin1, Vimentin, matrix metalloproteinase-2 [MMP-2]), EMT markers (Twist1, Snail2, ZEB1 and β-catenin) and a main activator of JAK/STAT signaling (IL-6 [21]), whereas a notable increase in the expression of the main factor of EMT (E-cadherin). These results further validated the results of GSEA.
Figure 5.
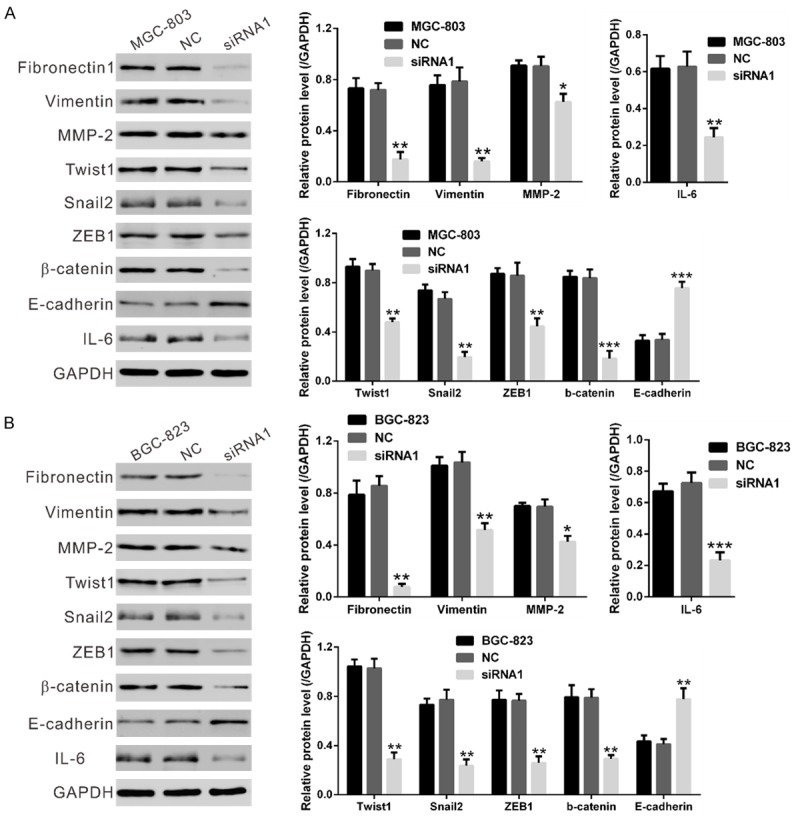
NOX4 expression affected cell migration, EMT and JAK/STAT signaling pathways. Protein levels of the above three pathway related gene were evaluated by Western blotting in MGC-803 (A) and BGC-823 cells (B) at 48 h after transfected with NOX4 siRNA-1 or control siRNA. NC: nonsense siRNA-transfected cells; siRNA1: NOX4-siRNA1 transfected cells (*P<0.05, **P<0.01 and ***P<0.001 Vs NC).
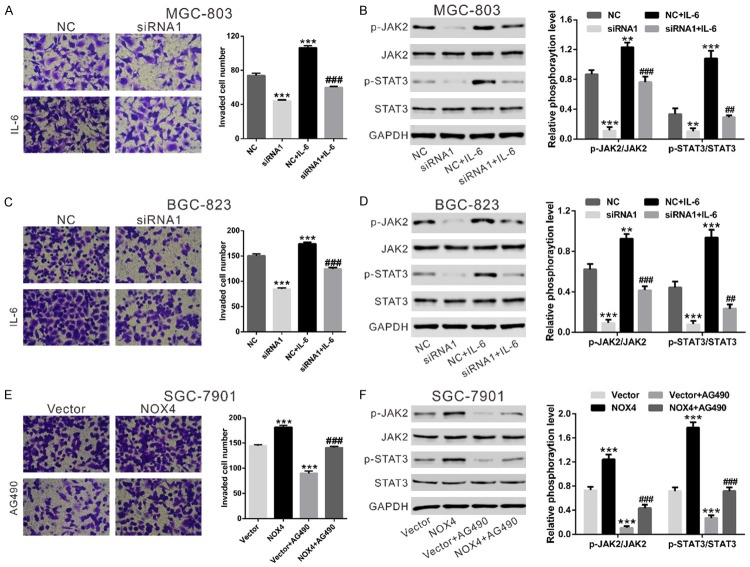
To confirm the involvement of JAK/STAT signaling, MGC-803 and BGC-823 cells transfected with NOX4-siRNA or NC were treated with IL-6 (Figure 6A-D). Cell invasion of GC cells was significantly promoted by IL-6 stimulation. Additionally, the promotion effects of IL-6 on cell invasion of GC cells was impaired by NOX4 knockdown. Meanwhile, although western blot did not suggested an effect on the total JAK2 and STAT3, NOX4 knockdown led to a significant reduction of active phosphorylated proteins in MGC-803 (Figure 6B) and BGC-823 cells (Figure 6D). IL-6 exposure significantly activated JAK2 and STAT3, and such effects were attenuated by NOX4 knockdown. Further, SGC-7901 cells with a lower expression of NOX4 was overexpressed with NOX4 (Figure S2) and treated with JAK2 inhibitor (AG490). As shown in Figure 6E, cell invasion of GC cells was remarkably promoted by overexpression of NOX4, but notably inhibited by treated with JAK2 inhibitor. Additionally, the inhibitory effects of AG490 on cell invasion was weakened by ectopic expression of NOX4. Moreover, AG490 significantly inhibited the levels of p-JAK2 and p-STAT3, and such effects were attenuated by NOX4 overexpression. These data indicated that NOX4 may promote cell invasion partially by activating JAK2/STAT3 pathway.
Figure 6.
Effects of NOX4 on JAK2/STAT3 signaling. Effects of IL-6 (100 ng/mL) on invasive capacity of MGC-803 (A) and BGC-823 cells (C) treated with NOX4 siRNA1 or control siRNA (NC) and IL-6. Phosphorylation of JAK2 and STAT3 was evaluated by immunoblotting (B, D). NC: nonsense siRNA-transfected cells; siRNA1: NOX4-siRNA1 transfected cells; NC+IL-6: nonsense siRNA and IL-6 treated cells; siRNA1+IL-6: NOX4-siRNA1 and IL-6 treated cells (*P<0.05, **P<0.01 and ***P<0.001 Vs NC; ##P<0.01 and ###P<0.001 Vs siRNA1). (E, F) SGC-7901 cells was transfected with control Vector or expression plasmid encoding NOX4. Invasion assay (E) were performed in a Matrigel-coated transwell chamber containing either DMSO or 20 μM JAK2 inhibitor, AG490. Scale bar: 100 mm. Phosphorylation of JAK2 and STAT3 was evaluated by immunoblotting (F). Vector: control plasmid-transfected cells; NOX4: NOX4 expression plasmid transfected cells; Vector+AG490: control plasmid and AG490 treated cells; NOX4+AG490: NOX4 expression plasmid and AG490 treated cells (***P<0.001 Vs Vector; ###P<0.001 Vs Vector+AG490).
Discussion
In our present study, we demonstrated that NOX4 was up-regulated in gastric cancer tissues. The expression level of NOX4 mRNA was significantly correlated with reduced survival time of GC patients. Our study suggests that NOX4 might be a novel marker for the prognosis of GC. Furthermore, silencing expression of NOX4 inhibited adhesion and invasion of GC cells. In addition, we found that NOX4 is involved in the cell invasion by regulating the JAK2/STAT3 signaling pathway.
Previous studies have demonstrated that NOX4 plays an important role in the proliferation of melanoma cells, urothelial carcinoma cells or liver cancer cells via regulating cell cycle progression [11-13]. However, our study showed that silencing of NOX4 did not affect the proliferation of GC cells (Figure 3A). The inconsistency of our results and previous studies may due to different types of cells.
In this study, silencing of NOX4 in GC cells significantly inhibited cell invasion (Figure 3C), which was consistent with the previous findings on breast cancer cells [14-16] and colon cancer cells [17]. Complementary results were observed in GC cells overexpressed NOX4 (Figure 6E). In order to explore the molecular mechanism how NOX4 exerted its functions on GC, we performed GSEA analysis on TCGA STAD dataset. We observed that many cancer-related genesets, including cell migration, EMT and JAK/STAT pathways, were positively correlated with NOX4 expression (Figure 4). The GSEA results were further validated by immunoblotting (Figure 5). Thus, these findings indicate an important role of NOX4 in the development of GC.
JAK2/STAT3 signaling is activated in a variety of humor tumors including GC and implicated in tumor formation and metastatic progression [22]. The pleiotropic cytokine IL-6 is a major activator of JAK2/STAT3 signaling. STAT3 activated by IL-6 contributes to migration and invasion of GC cell lines through regulation of E-cadherin expression [23]. Here, as expected, IL-6 treatment of GC cells significantly promoted cell invasion and JAK2/STAT3 signaling (Figure 6). It is worth noting that the promotion effects of IL-6 on cell invasion and JAK2/STAT3 signaling was impaired by NOX4 knockdown. Further, the inhibitory effects of JAK2 inhibitor, AG490 on cell invasion of GC cells was weakened by NOX4 overexpression (Figure 6), which suggested NOX4 might promote cell invasion partially by activating JAK2/STAT3 pathway. It has been reported that ROS can activate JAK/STAT signaling [24]. NOX4 is a key enzyme for ROS generation and its activity is determined only by its mRNA/protein levels [25]. Further studies will be necessary to explore whether NOX4 regulated JAK/STAT signaling via ROS generation.
In conclusion, this study demonstrated that NOX4 was overexpressed in GC tissues and NOX4 expression influenced the invasive capacity of GC cells via JAK2/STAT3 pathway. Our findings suggest that NOX4-targeting therapies might be a novel treatment strategy for GC patients although further investigation is needed.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Kanat O, O’Neil BH. Metastatic gastric cancer treatment: a little slow but worthy progress. Med Oncol. 2013;30:464. doi: 10.1007/s12032-013-0464-4. [DOI] [PubMed] [Google Scholar]
- 4.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 5.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci. 2012;69:2327–2343. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonner MY, Arbiser JL. Targeting NADPH oxidases for the treatment of cancer and inflammation. Cell Mol Life Sci. 2012;69:2435–2442. doi: 10.1007/s00018-012-1017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weyemi U, Dupuy C. The emerging role of ROS-generating NADPH oxidase NOX4 in DNAdamage responses. Mutat Res. 2012;751:77–81. doi: 10.1016/j.mrrev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Hiraga R, Kato M, Miyagawa S, Kamata T. Nox4-derived ROS signaling contributes to TGF-beta-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2013;33:4431–4438. [PubMed] [Google Scholar]
- 9.Weyemi U, Caillou B, Talbot M, Ameziane-El-Hassani R, Lacroix L, Lagent-Chevallier O, Al Ghuzlan A, Roos D, Bidart JM, Virion A, Schlumberger M, Dupuy C. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr Relat Cancer. 2010;17:27–37. doi: 10.1677/ERC-09-0175. [DOI] [PubMed] [Google Scholar]
- 10.Juhasz A, Ge Y, Markel S, Chiu A, Matsumoto L, van Balgooy J, Roy K, Doroshow JH. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radic Res. 2009;43:523–532. doi: 10.1080/10715760902918683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crosas-Molist E, Bertran E, Sancho P, Lopez-Luque J, Fernando J, Sanchez A, Fernandez M, Navarro E, Fabregat I. The NADPH oxidase NOX4 inhibits hepatocyte proliferation and liver cancer progression. Free Radic Biol Med. 2014;69:338–347. doi: 10.1016/j.freeradbiomed.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Shimada K, Fujii T, Anai S, Fujimoto K, Konishi N. ROS generation via NOX4 and its utility in the cytological diagnosis of urothelial carcinoma of the urinary bladder. BMC Urol. 2011;11:22. doi: 10.1186/1471-2490-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaura M, Mitsushita J, Furuta S, Kiniwa Y, Ashida A, Goto Y, Shang WH, Kubodera M, Kato M, Takata M, Saida T, Kamata T. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009;69:2647–2654. doi: 10.1158/0008-5472.CAN-08-3745. [DOI] [PubMed] [Google Scholar]
- 14.Tobar N, Guerrero J, Smith PC, Martinez J. NOX4-dependent ROS production by stromal mammary cells modulates epithelial MCF-7 cell migration. Br J Cancer. 2010;103:1040–1047. doi: 10.1038/sj.bjc.6605847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Liu Z, Hu X. Inhibiting cancer metastasis via targeting NAPDH oxidase 4. Biochem Pharmacol. 2013;86:253–266. doi: 10.1016/j.bcp.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med. 2012;53:1489–1499. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer KM, Watts TN, Buechler S, Hummon AB. Proteomic and functional investigation of the colon cancer relapse-associated genes NOX4 and ITGA3. J Proteome Res. 2014;13:4910–4918. doi: 10.1021/pr500557n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics. 2007;23:3251–3253. doi: 10.1093/bioinformatics/btm369. [DOI] [PubMed] [Google Scholar]
- 21.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J. Clin. Oncol. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto W, Okamoto I, Arao T, Yanagihara K, Nishio K, Nakagawa K. Differential roles of STAT3 depending on the mechanism of STAT3 activation in gastric cancer cells. Br J Cancer. 2011;105:407–412. doi: 10.1038/bjc.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:C1640–1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 25.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.