Abstract
Fibronectin 1 (FN1) is a member of the glycoprotein family located on chromosome 2q35. It has been reported that FN1 is upregulated in many tumors, and its expression is negatively related to the prognosis and survival of cancer patients. Through data analysis, we found that FN1 is upregulated in nasopharyngeal carcinoma (NPC). This study aimed to investigate how FN1 expression affects NPC cell behavior. In this study, we downregulated FN1 in two NPC cell lines, 5-8F (EBV-) and C666-1 (EBV+), and evaluated invasion, migration and apoptosis. FN1 promoted migration and invasion by upregulating MMP9 and MMP2 expression; the NF-κB/P65 signaling pathway was also affected by FN1. FN1 suppressed apoptosis in NPC cells by upregulating BCL2 and increasing the nuclear localization of P65, both by inducing cytosolic accumulation and nuclear translocation, but FN1 expression was not reduced when the NF-κB/P65 pathway was inhibited in the negative control (NC) group. Compared with NC cells, shFN1 cells showed little change in apoptosis when the NF-κB/P65 pathway was activated by LPS. These results suggest that FN1 regulates apoptosis though P65 in the NF-κB pathway. Our results show that FN1 plays an important role in NPC cells and is a potential target for NPC treatment.
Keywords: Nasopharyngeal carcinoma, Fibronectin 1, apoptosis, NF-κB/P65 pathway
Introduction
Nasopharyngeal carcinoma (NPC), a type of head and neck cancer, has a low prevalence throughout the world but a high incidence in southern China, especially in Guangdong, Hong Kong and Taiwan [1]. In southern China, NPC has a high incidence rate, ranging from 20 to 30 cases per 100,000 people and constituting approximately 18% of all cancers in this area [2]. Although NPC can be cured by radiotherapy at early stages, many patients experience local recurrence and distant metastasis, key contributors to NPC mortality.
Fibronectin 1 (FN1) is a member of the ligand glycoprotein family, which is widely expressed in various cell types and is involved in cell adhesion and migration [3]. FN1 overexpression is an indicator of poor prognosis in head and neck cancer [4,5]. It has been reported that FN1 is a potential biomarker for radiotherapy resistance in head and neck squamous cell carcinoma, and FN1 is differentially expressed in ovarian cancer with platinum resistance [6]. The molecular mechanism of cancer progression has been determined to involve FN-induced changes in gene expression. For example, FN1 can enhance the expression of matrix metalloproteinases (MMPs), which are key factors in promoting cancer invasion and metastasis [7]. In colorectal cancer, FN1 can downregulate P53 and inhibit apoptosis [8]. FN1 is also regarded as a prognosticator in NPC. FN1 overexpression in NPC is reportedly associated with advanced disease stage and short survival [9]. However, the exact mechanism by which FN1 evokes these poor outcomes has not been investigated.
In our study, we found that silencing FN1 in NPC cell lines simultaneously increased apoptosis by downregulating BCL2 via the NF-κB/P65 pathway and inhibited migration and invasion by downregulating MMP9 and MMP2; therefore, FN1 is a potential treatment target in NPC.
Materials and methods
Cell culture
The human NPC cell lines 5-8F (EBV-) and C666-1 (EBV+), provided by Southern Medical University, were cultured in RPMI 1640 (Life Technologies, CA, USA) supplemented with 10% fetal bovine serum (FBS; Life Technologies) and maintained at 37°C in an incubator containing 5% CO2.
shRNA transfection and qPCR
Cells were transfected with FN1-specific shRNA (Ribobio, Guangzhou, China). A nonsense shRNA was provided as a negative control (NC). After 36 h of transfection, the efficacy of the FN1 shRNA was ascertained by real-time qPCR and Western blot. Total RNA was extracted using TRIzol (Life Technologies), and cDNA was generated using the Prime ScriptTM RT Reagent Kit with gDNA Eraser (Takara Bio Inc, Japan). Real-time qPCR was performed using SYBR Premix Ex Taq (Takara Bio Inc., Japan). The qPCR primer sequences are as follows: GAPDH forward 5’-GGCTCATGACCACAGTCCATG-3’ and reverse 5’-TCAGCTCTGGGATGACCTTG-3’; FN1 forward 5’-GTTCGGGAGGAGGTTGTTACC-3’ and reverse 5’-GAGTCATCTGTAGGCTGGTTTAGG-3’. qPCR assays were performed using a LightCycler 480 (Roche, Basel, Switzerland).
Scratch, invasion and migration assays
In the scratch assays, cells were seeded in a 6-well plate at a density of 3.5 × 105/ml. After reaching confluence, the cells were starved for 12 h in FBS-free medium. Then, a wound was produced by scraping a 200-µl micropipette tip across the confluent cell layer. The migration area was observed at different times, and photos were taken using a microscope with a 20 × objective lens (Olympus, BX53; Melville, NY, USA). The wound area was evaluated with ImageJ software.
Cell invasion was evaluated with transwell cell culture chambers (24-well plate, 8-μm pore size) that were coated with matrigel (BD, Milan, Italy). Cells (5 × 104 in 100 μl) were transfected or not with FN1 shRNA and placed in the upper chamber without FBS, and 500 μl of culture medium with FBS was added to the lower chamber. After a 12-h incubation at 37°C, the cells in the upper chambers were washed with PBS, fixed in ice-cold methanol for 30 min and stained with crystal violet (Sigma, USA). The cells in five fields on the lower side of the membrane were counted with an upright metallurgical microscope with a 20 × objective lens (Olympus, BX53; Melville, NY, USA). Migration assays were performed similarly to the invasion assays, except the transwell chamber did not contain matrigel.
Analysis of cell viability and colony formation
Cell viability was evaluated with the Cell Counting Kit-8 (CCK8). C666-1 and 5-8F cells transfected with FN1 shRNA and or NC were seeded in 96-well plates at a density of 2 × 103 cells/ml. Cell viability at days 1, 2, 3, 4, and 5 was evaluated with the CCK8 and a microplate reader at 450 nm.
In the colony formation assays, cells (1000 in 2 ml) were transfected or not with FN1 shRNA, placed in 6-well plates with 20% FBS, and incubated (5% CO2, 37°C) for 14 days. Every 5 days, the old medium was replaced with new medium. On the 14th day, the medium was removed, and the cells were washed 3 times with PBS, fixed in ice-cold methanol for 30 min and stained with crystal violet (Sigma, USA). The number of colonies was calculated.
Apoptosis assay
TUNEL assays were performed using an in situ cell death detection kit from Roche according to the manufacturer’s instructions, and the cells were fixed on microslides with DAPI-Fluoromount-G (Southern Biotech, USA). Fluorescence images of five random fields of view were obtained using an upright metallurgical microscope with a 20 × objective lens (Olympus, BX53; Melville, NY, USA).
Annexin V/propidium iodide (PI) (Annexin V-APC Apoptosis Detection Kit, KeyGEN BioTECH, Jiangsu, China) staining was performed as follows: the treated cells were collected and washed as instructed by the manufacturer, followed by staining with Annexin V/PI for 20 min at room temperature. A flow cytometer was used to analyze the percentage of apoptotic cells.
Cell immunofluorescence
Cell immunofluorescence assays were performed to assess the cytosolic and nuclear localization of the NF-κB/P65 protein. Cells were plated on slides, incubated for 12 h, fixed in ice-cold methanol for 15 min at room temperature, washed 3 times with PBS, and incubated in 5% BSA for 1 h. Then, the cells were incubated with a primary antibody against NF-κB/P65 (diluted 1:200, Cell Signal Technology, USA) for 2 h at 37°C, washed 3 times with TBST and incubated with the secondary antibody (goat anti-rabbit immunoglobulin conjugated to FITC, diluted 1:100, Proteintech, Wuhan, China) for 1 h. The cells were washed with PBS and counterstained with DAPI. Photos were taken with a microscope (Olympus, BX53; Melville, NY, USA).
Western blot assay
Proteins were extracted using lysis buffer containing PMSF and a phosphatase inhibitor (both from Beyotime, Shanghai, China). The proteins were separated by SDS-PAGE, transferred to PVDF membranes (Merck Millipore, MA, USA) and incubated in 5% BSA for 1 h. Membranes were incubated overnight at 4°C with primary antibody (Cell Signal Technology, USA), washed 3 times with TBST and incubated with goat anti-rabbit secondary antibody (Proteintech, Wuhan, China) for 1 h. Proteins were visualized by electrochemiluminescence using Western blotting detection reagents (Thermo Scientific, UK).
Subcutaneous transplantation tumor model study
Two groups of 3 male nude mice (4 to 6 weeks old weighing 20 to 25 g) were bred under SPF conditions. Animal experiments were performed according to protocols approved by the National Institutes of Heath Guide for the Care and Use of Laboratory animals (NIH Publication No. 8023, revised 1978). Cells (2 × 106 in 100 µl) were subcutaneously injected into the mice. The tumor was measured every 7 days with calipers, and the tumors were harvested after 6 weeks.
Statistical analysis
All the measurements were analyzed using Student’s t-test. The data are presented as the mean ± SD calculated using GraphPad Prism 5.0 (GraphPad Software, Inc.). The statistical significance was set at P<0.05. The statistical results are shown as *P<0.05 and **P<0.01. All the experiments were performed at least 3 times.
Results
FN1 was upregulated in NPC
We downloaded data from GEO using the accession number GSE12452; this dataset was generated using an Affymetrix Human Genome U133 Plus 2.0 Array (GPL570). To make the data easier to analyze and to obtain high quality genes, we used the median method for data standardization. The gene filters were set as follows: (1) the expression of a probe was no less than 3-fold the median, and changes were more than 20% of the that for the total sample; (2) there were fewer than 50% missing gene expression values; and (3) if a gene corresponded to several probes, only the highest IQR was retained. Finally, we found that FN1 was obviously upregulated, with a fold change of 9.12.
Knocking down FN1 inhibited migration and invasion by regulating MMP9 and MMP2
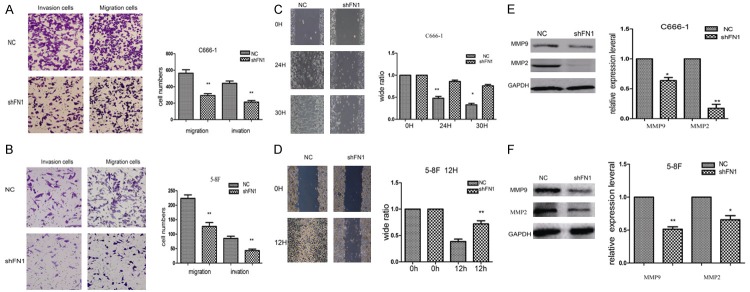
To confirm that downregulating FN1 inhibits the proliferation, migration and invasion of NPC cells, we silenced FN1 with shRNA. qPCR and Western blot confirmed that FN1 was significantly knocked down (Figure 1A-D). Compared with the NC group, cells in the shFN1 group were less aggressive, with reduced migration and invasion (Figure 2A and 2B). In addition, the wound healed more slowly (Figure 2C and 2D).
Figure 1.
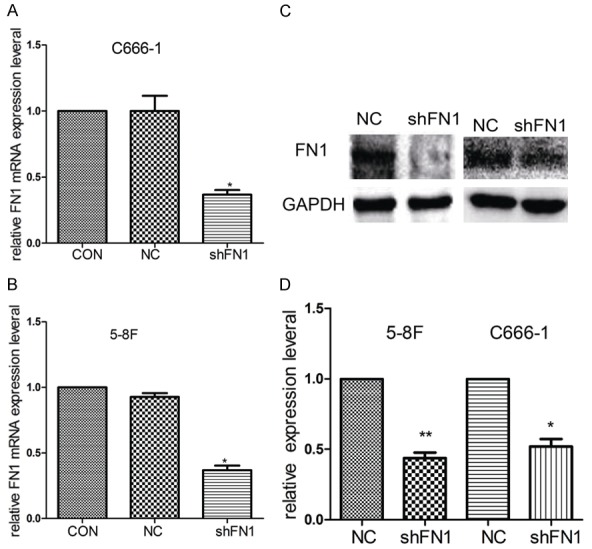
The efficiency of FN1 silencing. After transfecting with shFN1 36 h later, silencing was obvious by qPCR in C666-1 (A) and 5-8F cells (B). The Western blot results showed significant FN1 silencing (C and D). GAPDH was used as a loading control. *P<0.05, **P<0.01.
Figure 2.
Knocking down FN1 inhibited NPC cell migration and invasion. Transwell assays showed that downregulating FN1 suppressed the migration and invasion of C666-1 and 5-8F cells (A and B). In the shFN1 group, the wound healed more slowly than that in the control group (C and D). At the molecular level, the expression of MMP9 and MMP2, which play an important role in cancer migration and invasion, was decreased when FN1 was silenced (E and F). *P<0.05, **P<0.01.
MMP9 and MMP2 have been regarded as invasion biomarkers in many cancer cells. Therefore, we evaluated MMP9 and MMP2 expression in shFN1 and control cells. MMP9 and MMP2 were downregulated in shFN1 cells compared with control cells, as shown by Western blot (Figure 2E and 2F). MMPs are secreted proteins and key factors in degrading collagen type IV [10,11]. Altogether, these results suggest that FN1 can increase NPC cell migration and invasion by upregulating MMP9 and MMP2.
Knocking down FN1 inhibited proliferation and induced NPC cell apoptosis via NF-κB P65
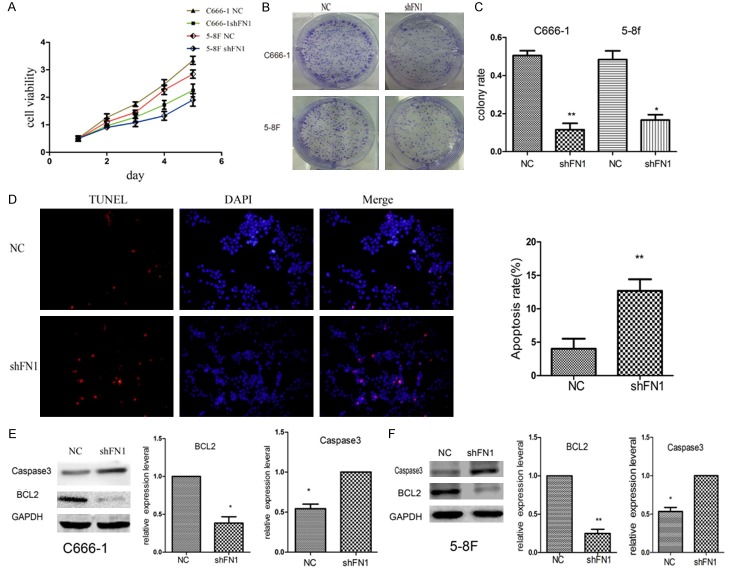
The proliferative ability of C666-1 and 5-8F cells was evaluated. Cells transfected with shFN1 proliferated more slowly (Figure 3A-C).
Figure 3.
Transfection of shFN1 inhibited the proliferation and colony formation ability of NPC cells and induced the apoptosis of C666-1 and 5-8F cells. Silencing FN1 led to the downregulation of proliferation (A) and colony formation (B and C) (*P<0.05, **P<0.01). TUNEL assays were performed to evaluate apoptosis, and TUNEL-positive (red fluorescence) cells were observed (D); there were more positive cells in the shFN1 group than in the control group. Western blot analysis of caspase 3 and BCL2 was performed, and the expression ratios indicated that knocking down FN1 decreased BCL2 levels and increased caspase 3 levels (E and F). *P<0.05, **P<0.01.
TUNEL assays showed that FN1 knockdown resulted in large apoptotic cells with high-intensity red fluorescence (Figure 3D). Western blot assays suggested that caspase 3 was upregulated and that levels of the anti-apoptotic protein BCL2 were significantly decreased (Figure 3E and 3F). Therefore, we hypothesized that FN1 inhibits NPC cell death.
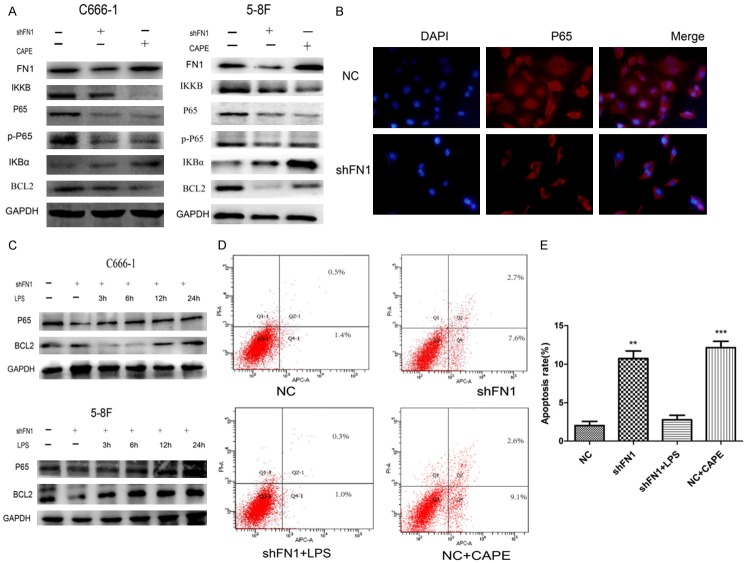
An accumulating body of evidence suggests that the NF-κB pathway plays a crucial role in cancer development. In addition, prominent crosstalk nodes are mediated by other factors, such as STAT3 and P65. To determine whether the NF-κB pathway is associated with the regulation of apoptosis in shFN1 cells, Western blot analysis and immunocytochemistry were employed. Western blots showed that FN1 increased IKKB, P65, and p-P65 expression and decreased IκB-α expression (Figure 4A). To determine whether NF-κB/P65 activation is related to FN1, we suppressed NF-κB by treating NC cells for 12 h with caffeic acid phenethyl ester (CAPE, 20 µg/ml) and activated NF-κB by treating shFN1 cells with lipopolysaccharide (LPS, 1000 ng/ml). CAPE and shFN1 had the same effect on the NF-κB pathway (Figure 4A); both inhibited NF-κB pathway activation. In CAPE-treated cells, BCL2 expression was reduced, but FN1 expression did not change (Figure 4A). Immunocytochemistry showed that in NC cells, FN1 increased the nuclear localization of P65 by inducing cytosolic accumulation and increasing nuclear binding (Figure 4B). shFN1 cells treated with LPS, the NF-κB pathway activator, showed increased P65 and BCL2 expression (Figure 4C) over time. However, the apoptosis rate did not change considerably compared with that of NC cells (Figure 4D and 4E); perhaps FN1 inhibits apoptosis by activating P65, and the same effect was achieved by the NF-κB pathway activator in the shFN1 group.
Figure 4.
Knocking down FN1 induced NPC cell apoptosis via NF-κB P65. Western blots showed that knocking down FN1 induced molecular changes in the NF-κB pathway and that the effect of shFN1 was the same as that of the pathway inhibitor (CAPE, 20 µg/ml, 12 h) (A). Immunofluorescence showed that FN1 caused cytosolic accumulation and increased nuclear binding of P65 in C666-1 cells (B). In the pathway activator (LPS) group, P65 and BCL2 expression increased over time in shFN1 cells (1000 ng/ml LPS; 3 h, 6 h, 12 h, or 24 h) (C). However, flow cytometer showed little difference in the apoptosis rate of NC cells and shFN1 cells (D and E, *P<0.05, **P<0.01).
Tumor growth in the subcutaneous transplantation tumor model
The in vitro experiments showed the effect of FN1 on tumor apoptosis and progression. Next, in vivo experiments were performed to determine whether these effects could be observed in an animal model. The subcutaneous transplantation tumor model assay showed that tumor volume was smaller in the shFN1 group (0.73±0.07 cm3) compared with the control group (1.2±0.147 cm3) (Figure 5A and 5B). In the shFN1 group, the tumor growth rate was significantly reduced (p<0.05; Figure 5C).
Figure 5.
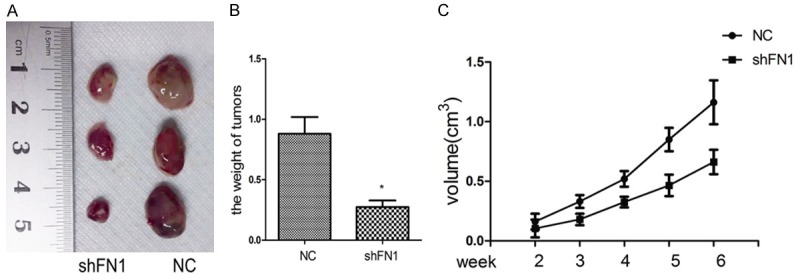
Knocking down FN1 inhibited tumor growth in a subcutaneous transplantation tumor model. Representative images of tumor-bearing mice and tumors from mice in each group (A). The final tumor weight was significantly different between the two groups (B). Tumor volume was measured every 7 days (C). *P<0.05.
Discussion
In this study, we showed that FN1 is anti-apoptotic and promotes the invasion and migration of NPC cells. FN1 dysregulation is often observed in NPC and many other types of cancer. Yoshihiro Morita and colleagues reported that FN1 promotes VEGF-C expression and the epithelial-mesenchymal transition in human oral squamous cell carcinoma cells [12]. Marialuisa Sponziello and colleagues found that FN1 was highly expressed and favored migration and invasion in aggressive thyroid cancer [4]. Gao and his coworkers reported that FN1 plays an important role in the development of cisplatin resistance in non-small cell lung cancer through the β-catenin signaling pathway [5]. Recently, Zhang and colleagues reported that microRNA-200c binds to FN1, suppressing the proliferation, migration and invasion of gastric cancer cells [13]. These data suggest that FN1 has complex roles in tumor formation and development, which prompted us to investigate whether FN1 regulates NPC.
The pathological features of NPC, including the fact that lesions always occur on the top and sidewalls of the nasopharyngeal cavity, result in the lack of specific symptoms at early stages. Many cases of NPC are diagnosed at advanced stages (III or IV). Though NPC can be cured by radiotherapy at the early stage, patients often experience local recurrence and distance metastasis, which are key contributors to mortality. In addition, a study showed that FN1 is a biomarker of radiotherapy resistance in head and neck squamous cell carcinoma [6]. It is therefore necessary to determine the role of FN1 in NPC.
The proliferation, migration and invasion of tumor cells are important processes in tumor metastasis [14]. MMPs play a crucial role in cancer migration and invasion [10]. MMP9 and MMP2, known as type IV collagenases, can degrade the extracellular matrix, which allows cancer cells to invade local tissue, increase the permeability of blood vessels and lymphatic vessels, and form distant metastases [15]. The metastatic ability of NPC seems to be related to the expression of MMP2 and MMP9 [16,17]. In our study, we found that both MMP9 and MMP2 were downregulated after silencing FN1 in NPC cells. This might suggest that FN1 can inhibit NPC invasion and migration by downregulating MMP9 and MMP2.
NF-κB can be expressed in almost all cells and can regulate many target genes involved in multiple processes, such as innate immunity, inflammation, cell proliferation and survival, and tumorigenesis [18,19]. The prototypical form of NF-κB is the P65/P50 heterodimer, which is associated with inhibitor of NF-κB alpha (IκBα) under quiescent conditions. Once cells are stimulated, signal transduction activates the IKK complex, which phosphorylates IκBα, leading to its polyubiquitination and subsequent proteasomal degradation. Once liberated, the P65/P50 heterodimer translocates to the nucleus and induces the aberrant regulation of NF-κB activity that leads to many human diseases, such as cancer and autoimmune disorders.
The abnormal activation of NF-κB may be one of the main anti-apoptosis pathways harnessed by cancer cells during tumor development [20]. Our results showed that knocking down FN1 restrained the NF-κB/P65 pathway. FN1 increased P65 translocation to the nucleus, potentially because high FN1 expression stimulates the expression of IKKB, which degrades IκBα, releases NF-κB, and phosphorylates P65, leading to nuclear translocation of P65. NF-κB activation can result in the abnormal expression of various genes that regulate apoptosis, proliferation, invasion, migration, inflammation and radiotherapy resistance [21].
Our study showed that cell viability, cloning ability and subcutaneous tumor volume were reduced in shFN1 cells and that the apoptosis percentage was increased compared with control cells. These results may be explained by the regulatory role of NF-κB in apoptosis [22]. NF-κB inhibits apoptosis [23,24] by inducing the expression of inhibitors of apoptosis, such as caspase inhibitors and members of the BCL2 family [22]. Elevated NF-κB activity provides a survival mechanism for tumor cells by upregulating anti-apoptotic genes, such as BCL2 [25,26]. Mohamed Salah I Abaza et al. reported that methyl ferulate inhibits colorectal cancer growth, upregulates the caspase family (caspase 2, 3, 6), and downregulates anti-apoptotic gene expression (BCL2, FLIP) via the NF-κB pathway [27]. Our study showed that when FN1 was knocked down, genes in the NF-κB pathway changed, and expression of the anti-apoptotic BCL2 gene was downregulated. In addition, BCL2, but not FN1, was suppressed in the CAPE (NF-κB/P65 inhibitor) group (without shFN1). In the LPS (NF-κB/P65 activator) group (with shFN1), the apoptosis rate was similar to that in the NC group, and the expression of P65 and BCL2 increased. These data might be explained by the NF-κB/P65 pathway being downstream of FN1, so that suppression of the NF-κB/P65 pathway suppresses the apoptosis of NPC cells.
In summary, our study found that FN1 was upregulated in human NPC and was a cancer-promoting gene. FN1 promoted migration and invasion and inhibited apoptosis by activating the NF-κB/P65 pathway. FN1 may serve as a predictor of NPC invasion and migration. Certainly, there are some limitations to our research; for example, we knocked down FN1 but did not overexpress it. Further studies should be conducted to determine whether there are other points of crosstalk between FN1 and NF-κB and to identify specific mechanisms.
Acknowledgements
This work was supported by a grant from the Natural Science Foundation of Guangdong Province (Grant No. 2015A 030313273 to Yanfang Zheng).
Disclosure of conflict of interest
None.
References
- 1.Jia WH, Huang QH, Liao J, Ye W, Shugart YY, Liu Q, Chen LZ, Li YH, Lin X, Wen FL, Adami HO, Zeng Y, Zeng YX. Trends in incidence and mortality of nasopharyngeal carcinoma over a 20-25 year period (1978/1983-2002) in Sihui and Cangwu counties in souther China. BMC Cancer. 2006;6:178. doi: 10.1186/1471-2407-6-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistis. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Wilson CB, Leopard J, Cheresh DA, Nakamura RM. Extracellular matrix and integrin composition of the normal bladde wall. World J Urol. 1996;14(Suppl 1):S30–S37. doi: 10.1007/BF00182062. [DOI] [PubMed] [Google Scholar]
- 4.Sponziello M, Rosignolo F, Celano M, Maggisano V, Pecce V, De Rose RF, Lombardo GE, Durante C, Filetti S, Damante G, Russo D, Bulotta S. Fibronectin-1 expression is increased in aggressive thyroid cancer and favors the migration and invasion of cancer cells 2016. Mol Cell Endocrinol. 2016;431:123–132. doi: 10.1016/j.mce.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Gao W, Liu Y, Qin R, Liu D, Feng Q. Silence of fibronectin 1 increases cisplatin sensitivity of non-small cell lung cancer cell line. Biochm Biophys Res Commun. 2016;476:35–41. doi: 10.1016/j.bbrc.2016.05.081. [DOI] [PubMed] [Google Scholar]
- 6.Jerhammar F, Ceder R, Garvin S, Grenman R, Grafstrom RC, Roberg K. Fibronectin 1 is a potential biomarker for radioresistance in head and neck squamous cell carcioma. Cancer Biol Ther. 2010;10:1244–1251. doi: 10.4161/cbt.10.12.13432. [DOI] [PubMed] [Google Scholar]
- 7.Stanton H, Gavrilovic J, Atkinson SJ, D’Ortho MP, Yamada KM, Zardi L, Murphy G. The activation of ProMMP-2 (gelatinase A) by HT1080 fibrosarcoma cells is promoted by culture on a fibronectin substrate and is concomitant with an increase in processing of MT1-MMP (MMP-14) to a 4 kDa form. J Cell Sci. 1998;111:2789–2798. doi: 10.1242/jcs.111.18.2789. [DOI] [PubMed] [Google Scholar]
- 8.Yi W, Xiao E, Ding R, Luo P, Yang Y. High expression of fibronectin is associated with poor prognosis, cell proliferation and malignancy via the NF-kappaB/p53-apoptosis signaling pathway in colorctal cancer. Oncol Rep. 2016;36:3145–3153. doi: 10.3892/or.2016.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma LJ, Lee SW, Lin LC, Chen TJ, Chang IW, Hsu HP, Chang KY, Huang HY, Li CF. Fibronectin overexpression is associated with latent membrane protein 1 expression and has independent prognostic value for nasopharyngealcarcinoma. Tumour Bio. 2014;35:1703–1712. doi: 10.1007/s13277-013-1235-8. [DOI] [PubMed] [Google Scholar]
- 10.Kohrmann A, Kammerer U, Kapp M, Dietl J, Anacker J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: new findings and review of th literature. BMC Cancer. 2009;9:188. doi: 10.1186/1471-2407-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato Y, Yamashita T, Ishikawa M. Relationship between expression of matrix metalloproteinase-2 and matrix metalloproteinase-9 and invasion ability of cervicalcancer cells. Oncol Rep. 2002;9:565–569. [PubMed] [Google Scholar]
- 12.Morita Y, Hata K, Nakanishi M, Omata T, Morita N, Yura Y, Nishimura R, Yoneda T. Cellular fibronectin 1 promotes VEGF-C expression, lymphangiogenesis and lymph node metastasis associated with human oral squamous cell carcioma. Clin Exp Metastasis. 2015;32:739–753. doi: 10.1007/s10585-015-9741-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Sun Z, Li Y, Fan D, Jiang H. MicroRNA-200c binding to FN1 suppresses the proliferation, migration and invasion of gastric cancer ells. Biomed Pharmacother. 2017;88:285–292. doi: 10.1016/j.biopha.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Zhang Y, Zhang H, Liu X, Gong T, Li M, Sun L, Ji G, Shi Y, Han Z, Han S, Nie Y, Chen X, Zhao Q, Ding J, Wu K, Daiming F. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressr EPB41L3. Mol Cancer Res. 2011;9:824–833. doi: 10.1158/1541-7786.MCR-10-0529. [DOI] [PubMed] [Google Scholar]
- 15.Vizoso FJ, Gonzalez LO, Corte MD, Rodriguez JC, Vazquez J, Lamelas ML, Junquera S, Merino AM, Garcia-Muniz JL. Study of matrix metalloproteinases and their inhibitors in reast cancer. Br J Cancer. 2007;96:903–911. doi: 10.1038/sj.bjc.6603666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang T, Chen MH, Wu MY, Wu XY. Correlation between expression of extracellular matrix metalloproteinase inducer and matrix metalloproteinase-2 and cervical lymph node metastasis of nasopharyngeal carcinom. Ann Otol Rhinol Laryngol. 2013;122:210–215. doi: 10.1177/000348941312200311. [DOI] [PubMed] [Google Scholar]
- 17.Li XY, Lin YC, Huang WL, Hong CQ, Chen JY, You YJ, Li WB. Zoledronic acid inhibits proliferation and impairs migration and invasion through downregulating VEGF and MMPs expression in human nasopharyngealcarcinoma cells. Med Oncol. 2012;29:714–720. doi: 10.1007/s12032-011-9904-1. [DOI] [PubMed] [Google Scholar]
- 18.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and rogression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 19.Karin M. Nuclear factor-kappaB in cancer developent and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. Hallmarks of cancr: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and strss signals. Ann N Y Acad Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- 22.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander tomajor culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 23.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpa-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 24.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activtion prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 25.Katsman A, Umezawa K, Bonavida B. Chemosensitization and immunosensitization of resistant cancer cells to apoptosis and inhibition of metastasis by the specific NF-kappaB nhibitor DHMEQ. Curr Pharm Des. 2009;15:792–808. doi: 10.2174/138161209787582156. [DOI] [PubMed] [Google Scholar]
- 26.McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistace in cancer. Drug Resist Updat. 2008;11:164–179. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CC, Huang SF, Wang JS, Chu WK, Nien JE, Chen WS, Chow SE. Interplay of N-Cadherin and matrix metalloproteinase 9 enhances human nasopharyngeal carinoma cell invasion. BMC Cancer. 2016;16:800. doi: 10.1186/s12885-016-2846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]