Abstract
Remifentanil is one of the most frequently prescribed opioids used in combination with inhalation anesthetics in clinical practice, but the effects of such combinations on the developing rat brain are unknown. In our study, we investigated first the potential neurotoxic effects of remifentanil on the developing brain and then the effects of remifentanil on isoflurane-induced apoptosis in the neonatal rat brain following exposure to a nociceptive stimulus. In the first experiment, postnatal day (P) 7 rats were randomly exposed to 30% oxygen, 1.5% isoflurane alone, 1.5% isoflurane and a plantar incision, normal saline, or remifentanil at a low (5 µg·kg-1·h-1), moderate (20 µg·kg-1·h-1) or high (80 µg·kg-1·h-1) dose for 4 h. In the second 4-h experiment, P7 rats were randomly exposed to 1.5% isoflurane, infused with different doses of remifentanil (5, 10, and 20 µg·kg-1·h-1), and subjected to a plantar incision. In both experiments, the number of apoptotic neurons in the cortex, hippocampus, and thalamus was assessed after two hours by cleaved caspase-3 or TUNEL staining. Our data showed that unlike 1.5% isoflurane, remifentanil at any dose did not cause significant neuronal apoptosis in any brain section. In addition, in response to a nociceptive stimulus, the infusion of 10 µg·kg-1·h-1 remifentanil reduced isoflurane-induced apoptosis in the hippocampus (P = 0.003 in CA1, P = 0.002 in CA3) but not in the cortex or thalamus. Our findings suggest that remifentanil does not induce apoptosis and reduces isoflurane-induced apoptosis in the developing brain.
Keywords: Opioid effect, neurotoxicity, developing brain, remifentanil
Introduction
Commonly used general anesthetics, including inhaled and intravenous anesthetics, are associated with neurotoxic effects on the developing brain. These consequences include neuronal apoptosis [1-4] and impaired neurogenesis [5-7], reduced synapse formation [8-10] and damaged glial cell development [11,12]. Anesthetic-induced neurotoxicity is associated with neuroinflammation, and inhaled anesthetics stimulate the inflammatory response by increasing intracellular calcium ion levels and thereby activating the transcription factor NF-κB, which recognizes DNA sequences in the nucleus and subsequently induces the transcription of the proinflammatory cytokine IL-6 [13].
Recent studies have primarily focused on the effects of a single anesthetic on the developing brain, which is not consistent with clinical practice, where inhalation anesthetics are typically used in combination with opioids under conditions of a nociceptive stimulus. Both a single inhaled anesthetic and the combination of opioids and nociceptive stimuli are associated with neurotoxic effects on the developing brain.
However, unlike general anesthetics, the neurotoxic effects of opioid analgesics on the developing brain are controversial. Different opioids may have different effects on the developing brain. Remifentanil is frequently prescribed due to its fast onset of action, rapid metabolism and controllable profile. In addition, the use of remifentanil in children and pregnant women has increased. However, few studies have investigated the effects of remifentanil on the developing brain. Remifentanil exerts its anti-inflammatory action by down-regulating the NF-κB pathway in lung injury models [14] and liver ischemia-reperfusion injury models [15]. Thus, we hypothesized that remifentanil reduces isoflurane-induced apoptosis in the brain of neonatal rats subjected to a nociceptive stimulus.
Therefore, in the first experiment, isoflurane was used as a positive control to investigate the potential neurotoxic effects of remifentanil on the developing brain, and we subsequently investigated the effects of remifentanil on isoflurane-induced apoptosis in the neonatal rat brain following exposure to a nociceptive stimulus.
Materials and methods
Animals
The animal experimental protocol was approved by the Institutional Animal Care and Use Committee at Fudan University. Pregnant Sprague-Dawley rats (from the Shanghai Laboratory Animal Centre, Chinese Academy of Sciences, permission number: SCXK 2012-0002) at gestational days 16-18 were housed in individual cages and maintained under temperature-controlled environmental conditions on a 12-h light-dark cycle. Animals had free access to food and water. Pups that had been delivered spontaneously were maintained with their mothers until postnatal day (P) 7. The body weight at P7 ranged from 12-14 g.
Experimental protocol
Two experiments were performed. In the first experiment, sixty P7 rats were randomly exposed to 30% oxygen (Sham group), 1.5% isoflurane (Iso group), or isoflurane and a plantar incision (Iso+I group) or received a continuous subcutaneous infusion of normal saline or remifentanil at a low (5 µg·kg-1·h-1), moderate (20 µg·kg-1·h-1) or high (80 µg·kg-1·h-1) dose for 4 h (n = 10 per group). The loss of the righting reflex was observed in neonatal rats exposed to isoflurane or remifentanil.
In the second 4-h experiment, P7 rats were randomly exposed to 1.5% isoflurane, infused with different doses of remifentanil (5, 10, and 20 µg·kg-1·h-1), and subjected to a plantar incision (Iso+Rf5+I , Iso+Rf10+I, and Iso+Rf20+I; n = 14 per group).
Based on a previous model of hyperpathia induced by high-dose remifentanil in rats [16,17], we administered remifentanil (Humanwe-ll Pharmaceutical Co., Ltd., Yichang, China) by continuous subcutaneous infusion using a microinjection pump (Agilia, Fresenius Kabi, France) at a rate of 0.4 mL/h. An intravenous catheter (24 gauge, B. Braun, Germany) was inserted approximately 1 cm into the posterior region of the rat’s neck and fixed with a transparent dressing. Remifentanil was dissolved in saline (0.9% NaCl) to a volume of 1.6 mL. In both experiments, animals received the interventions in a temperature (37-38°C)-controlled box continuously supplied with 30% oxygen at 2-2.5 L/min. The concentrations of isoflurane and CO2 in the chamber were measured using a gas analyser (Vamos, Drager Medical Co., Germany) with a sensing device placed in the chamber immediately adjacent to the animals. The fresh gas flow was regulated to ensure that the CO2 concentration was zero. Within each group, four animals were used for the analyses of blood glucose and blood gas levels, whereas the remaining animals were used to obtain samples for immunofluorescence assays. Righting reflex was also assessed in the first experiment. The animals’ tails were clipped to obtain blood for the blood glucose analysis, which was performed at 0, 2 and 4 h. The blood gas analysis (i-STAT, Abbott) was performed at the end of the 4-h treatment session using arterial blood collected via percutaneous puncture of the left ventricle. After treatment, the remaining pups in each group were placed in a heat-preserving experiment box and allowed to gradually regain consciousness.
Tissue preparation
Two hours after treatment, the pups (n = 6) were sacrificed via an intraperitoneal injection of 0.34 mL·kg-1 chloral hydrate and perfused transcardially with normal saline and then 4% paraformaldehyde in 0.1 M phosphate buffer. After the skulls were removed, the brain tissues were post-fixed for 24 h at 4°C in paraformaldehyde and then transferred to a 20% sucrose solution containing 4% paraformaldehyde. The brains were sectioned at 30-µm thickness on a cryostat microtome (CM1900, Leica, Germany) after precipitation in a 30% sucrose solution. At least four sections (300 µm apart) corresponding to Figures 55-58 in the Atlas of the NEONATAL RAT BRAIN [18] from each animal were immunohistochemically stained for cleaved caspase-3 or terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL).
Immunofluorescence staining
Sections were double-labelled with antibodies against cleaved caspase-3 and the neuron-specific marker NeuN to determine whether the apoptotic cells were neuronal cells. Briefly, the sections were incubated with a rabbit anti-cleaved caspase-3 antibody (1:400, Cell Signaling Technology, USA) overnight at 4°C. After incubation with a secondary antibody (Alexa Fluor 594-conjugated goat anti-rabbit, Jackson ImmunoResearch Laboratories, USA) for 1-2 h at room temperature in the dark, the sections were incubated with a mouse anti-NeuN clone A60 antibody (1:500, Merck-Millipore, USA) overnight at 4°C and then a secondary antibody (Alexa Fluor 488-conjugated goat anti-mouse, Jackson Immuno Research Laboratories) for 1 h. The sections were coverslipped with 4’,6-diamidino-2-phenylindole (DAPI) reagent (Southern Biotech, Birmingham, USA).
Fluorescent terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay
TUNEL staining was performed using the In Situ Cell Death Detection Kit (Roche, USA). Briefly, after fixation and permeabilization, 50 µL of TUNEL reaction mixture was added to the slides. The slides were incubated for 60 min at 37°C in a humidified atmosphere in the dark, mounted in DAPI reagent and coverslipped.
Statistical analysis
An experienced observer who was blinded to the experimental protocol counted the number of cleaved caspase-3 and NeuN double-labelled neurons and TUNEL-positive cells in the cortex (10 × magnification), hippocampus and thalamus (20 × magnification) under a microscope (Olympus, BX53F, Japan). In addition, the entire cortex area in each section was imaged at 1.25 × magnification, and the hippocampus and thalamus areas in each section were imaged at 20 × magnification. Positive cells and the corresponding areas in the cortex, hippocampus and thalamus within each section were quantified using ImageJ software (NIH, Bethesda, USA). The density was calculated as the number of positive cells divided by the area of the particular brain region. Four sections per animal were examined.
All parameters, including the apoptotic cell density, blood glucose levels, and blood gas levels, are presented as the mean ± standard deviation (SD). Histograms were generated using GraphPad Prism 5 (GraphPad Software, USA). The statistical analysis was performed using SPSS version 21 (SPSS, Inc., Chicago, USA). The mean density of cleaved caspase-3 and NeuN double-labelled neurons and TUNEL-positive cells are compared among the different treatment groups using one-way ANOVA. Significant F-tests were followed by post hoc Tukey’s HSD comparisons for pairwise tests. This test was selected to protect against false-positive results (type I error). P < 0.05 with Tukey’s correction indicated statistical significance. Blood glucose and arterial blood gas levels were also compared among the different treatment groups using one-way ANOVA. Repeated measures ANOVA was performed to analyse blood glucose levels at different time points within the same group.
Results
Remifentanil did not induce apoptosis in the developing brain
In the first experiment, no deaths were observed in any of the groups, except the Iso+I group (mortality rate 10%), following the 4-h drug treatment. All rats exposed to drugs lost their righting reflex within 45 min. No pups experienced hypoglycaemia or hypoxaemia. Four hours after drug treatment, the isoflurane and Rf80 groups showed slightly increased partial carbon dioxide pressure (PaCO2) and decreased pH compared with the Sham group (P < 0.05); however, the difference between these two drug-treated groups was not significant. There was no statistically significant difference in partial oxygen pressure (PaO2) across all groups, but the pH and PaCO2 levels were better in the Iso+I group than in the Iso group (Table 1).
Table 1.
Blood glucose (mmol/l) and arterial blood gas levels
| Sham group | Iso group§ | Iso+I group¶ | NS groupα | Rf5 groupβ | Rf20 groupδ | Rf80 groupθ | |
|---|---|---|---|---|---|---|---|
| Glucose 0 h | 5.37±0.34 | 5.47±0.66 | 6.07±0.65 | 6.90±0.53 | 6.70±0.45 | 6.75±0.75 | 5.85±0.58 |
| Glucose 2 h | 5.60±0.33 | 7.10±1.59 | 7.20±0.50 | 6.40±0.69 | 5.95±0.40 | 5.35±0.58 | 6.60±0.81 |
| Glucose 4 h | 5.5±0.24 | 6.30±1.72 | 6.40±0.70 | 7.10±0.92 | 5.60±0.81 | 5.25±0.64 | 5.65±0.29 |
| pH | 7.38±0.06 | 7.24±0.04* | 7.36±0.04# | 7.40±0.06 | 7.37±0.07 | 7.35±0.08 | 7.28±0.06* |
| PO2 (mmHg) | 97±16 | 102±11 | 97±3 | 98±12 | 99±13 | 113±10 | 103±12 |
| PCO2 (mmHg) | 42±7 | 51±6* | 35±3# | 41±3 | 40±6 | 43±8 | 52±6* |
The data are presented as the mean ± SD.
P < 0.05 compared with the Sham group.
P < 0.05 compared with the Iso group.
Sham = untreated in the presence of 30% oxygen;
Iso, inhaled 1.5% isoflurane;
Iso+I, inhaled 1.5% isoflurane with a planter incision;
NS, normal saline;
Rf5, remifentanil infused at 5 µg·kg-1·h-1;
Rf20, remifentanil infused at 20 µg·kg-1·h-1;
Rf80, remifentanil infused at 80 µg·kg-1·h-1.
The inhalation of 1.5% isoflurane significantly increased the number of apoptotic cells in the cortex (cleaved caspase-3 staining: P = 0.008; TUNEL staining: P = 0.004), hippocampus (in CA1, cleaved caspase-3 staining: P < 0.0001; TUNEL staining: P < 0.0001; in CA3, cleaved caspase-3 staining: P < 0.0001; TUNEL staining: P < 0.0001), and thalamus (cleaved caspase-3 staining: P = 0.005; TUNEL staining: P < 0.001) compared with Sham treatment. However, significant differences were not observed between the Iso and Iso+I groups in these three brain areas (P > 0.05). Thus, regardless of the nociceptive stimulus, inhalation anesthetics are associated with neurotoxic effects on the developing brain.
The number of apoptotic cells was not significantly different between any of the remifentanil groups (0, 5, 20, and 80 µg·kg-1·h-1) and the Sham group in the cortex (cleaved caspase-3 staining: P = 0.970; TUNEL staining: P = 0.885), hippocampus CA1 (cleaved caspase-3 staining: P = 0.262; TUNEL staining: P = 0.907), hippocampus CA3 (cleaved caspase-3 staining: P = 0.231; TUNEL staining: not observed) or thalamus (cleaved caspase-3 staining: P = 0.864; TUNEL staining: P = 0.898; Tables 2, 3).
Table 2.
Numbers of cleaved caspase-3 positive cells (cells/mm2)
| Sham group (n = 6) | Iso group§ (n = 6) | Iso+I group¶ (n = 6) | NS groupα (n = 6) | Rf5 groupβ (n = 6) | Rf20 groupδ (n = 6) | Rf80 groupθ (n = 6) | |
|---|---|---|---|---|---|---|---|
| Cortex | 0.06±0.1 | 1.1±0.6* | 1.0±0.7* | 0.03±0.1 | 0.06±0.1 | 0.06±0.1 | 0.05±0.1 |
| Hip CA1 | 7.29±7.0 | 192.4±72.5* | 200.8±89.1* | 6.81±5.1 | 5.47±4.6 | 12.49±7.1 | 10.42±10.4 |
| Hip CA3 | 40.72±36.4 | 869.2±598.8* | 727.9±497.3* | 79.22±41.9 | 92.17±44.0 | 93.07±70.8 | 72.72±32.8 |
| Thalamus | 0.63±1.7 | 45.6±30.3* | 49.6±33.8* | 1.19±2.1 | 1.95±3.1 | 2.14±3.9 | 2.00±4.5 |
The data are presented as the mean ± SD.
P < 0.05 compared with the Sham group.
Sham = untreated in the presence of 30% oxygen;
Iso, inhaled 1.5% isoflurane;
Iso+I, inhaled 1.5% isoflurane with a planter incision;
NS, normal saline;
Rf5, remifentanil infused at 5 μg·kg-1·h-1;
Rf20, remifentanil infused at 20 μg·kg-1·h-1;
Rf80, remifentanil infused at 80 μg·kg-1·h-1.
Table 3.
Numbers of TUNEL positive cells (cells/mm2)
| Sham group (n = 6) | Iso group§ (n = 6) | Iso+I group¶ (n = 6) | NS groupα (n = 6) | Rf5 groupβ (n = 6) | Rf20 groupδ (n = 6) | Rf80 groupθ (n = 6) | |
|---|---|---|---|---|---|---|---|
| Cortex | 0.05±0.05 | 0.29±0.1* | 0.25±0.1* | 0.04±0.03 | 0.04±0.05 | 0.05±0.06 | 0.05±0.05 |
| Hip CA1 | 5.56±6.8 | 111.0±54.4* | 116.6±73.3* | 5.17±6.07 | 3.96±4.55 | 7.11±8.24 | 7.29±7.88 |
| Hip CA3 | 0±0.03 | 70.7±24.7* | 55.6±22.8* | 0±0 | 0±0 | 0±0 | 0±0 |
| Thalamus | 1.00±2.2 | 44.8±17.7* | 46.0±28.0* | 2.00±2.7 | 2.00±2.7 | 3.00±4.5 | 2.00±2.7 |
The data are presented as the mean ± SD.
P < 0.05 compared with the Sham group.
Sham = untreated in the presence of 30% oxygen;
Iso, inhaled 1.5% isoflurane;
Iso+I, inhaled 1.5% isoflurane with a planter incision;
NS, normal saline;
Rf5, remifentanil infused at 5 μg·kg-1·h-1;
Rf20, remifentanil infused at 20 μg·kg-1·h-1;
Rf80, remifentanil infused at 80 μg·kg-1·h-1.
Remifentanil exerted anti-apoptotic effects on the developing brain
In the second experiment, we explored whether remifentanil reduced isoflurane-induced apoptosis in the neonatal rat brain following exposure to a nociceptive stimulus. No deaths occurred in the Iso+Rf5+I group, one death occurred in the Iso+Rf10+I group (mortality 10%), and four deaths occurred in the Iso+Rf20+I group after the 4-h drug treatment. No pups experienced hypoglycaemia, and no significant differences in blood glucose levels at each time point were observed in each group (P > 0.05). The blood glucose level was significantly increased in the Iso+Rf20+I group compared with the Sham group at 2 h (P = 0.003), but no significant differences were observed in blood glucose levels at other time points (P > 0.05). Four hours after drug treatment, the Iso+Rf20+I group exhibited a significant increase in PaCO2 (P < 0.001) and decreases in pH (P = 0.002) and PaO2 (P = 0.009) compared with the Sham group (Table 4).
Table 4.
Blood glucose (mmol/l) and arterial blood gas levels
| Sham group | Iso+I group¶ | Iso+Rf5+I group†† | Iso+Rf10+I group‡ | Iso+Rf20+I group$ | |
|---|---|---|---|---|---|
| Glucose 0 h | 5.37±0.24 | 6.07±0.65 | 6.77±0.41 | 6.37±2.23 | 7.30±1.1 |
| Glucose 2 h | 5.60±0.33 | 7.20±0.50 | 7.6±0.95 | 6.37±2.35 | 8.87±1.15* |
| Glucose 4 h | 5.50±0.25 | 6.40±0.70 | 6.43±0.17 | 6.33±2.45 | 6.67±0.06 |
| pH | 7.38±0.06 | 7.36±0.04 | 6.43±0.17 | 7.30±0.09 | 7.12±0.07* |
| PO2 (mmHg) | 97±13 | 97±3 | 110±10 | 111±16 | 50±11* |
| PCO2 (mmHg) | 42±5 | 35±3 | 45±12 | 42±4 | 72±5* |
The data are presented as the mean ± SD.
P < 0.05 compared with the Sham group.
Sham = untreated in the presence of 30% oxygen;
Iso+I, inhaled 1.5% isoflurane with a planter incision;
Iso+Rf5+I , inhaled 1.5% isoflurane with a plantar incision and an infusion of 5 µg·kg-1·h-1 remifentanil;
Iso+Rf10+I, inhaled 1.5% isoflurane with a plantar incision and an infusion with 10 µg·kg-1·h-1 remifentanil;
Iso+Rf20+I, inhaled 1.5% isoflurane with a plantar incision and an infusion of 20 µg·kg-1·h-1 remifentanil.
Remifentanil had no significant impact on isoflurane-induced cellular apoptosis in the cortex
The inhalation of 1.5% isoflurane in the presence of a nociceptive stimulus (Iso+I group) significantly increased the number of cleaved caspase-3-positive cells and TUNEL-positive cells in the cortex compared with Sham treatment (P = 0.002 and P = 0.002, respectively). The combination of 5 µg·kg-1·h-1 remifentanil infusion and isoflurane did not significantly reduce apoptosis in the cortex (cleaved caspase-3 staining: P = 0.331; TUNEL staining: P = 0.350). The combination of 10 µg·kg-1·h-1 remifentanil infusion and isoflurane reduced apoptosis in the cortex, but this effect was not statistically significant (cleaved caspase-3 staining: P = 0.142; TUNEL staining: P = 0.331). The combination of 20 µg·kg-1·h-1 remifentanil infusion and isoflurane did not significantly reduce apoptosis in the cortex (cleaved caspase-3 staining: P = 0.328; TUNEL staining: P = 0.470) (Figure 1).
Figure 1.
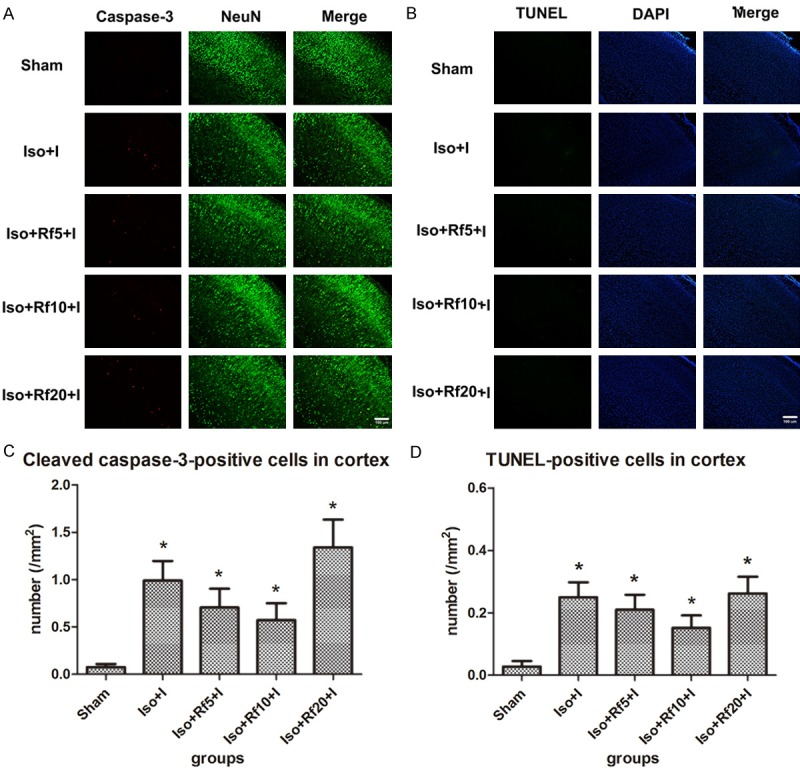
Remifentanil has no significant effect on isoflurane-induced apoptosis in the cortex. (A) Representative images of immunofluorescence staining for cleaved caspase-3 in the cortex. Red staining indicates apoptotic (cleaved caspase-3-positive) cells, and green staining indicates neurons in the cortex. Merge: cleaved caspase-3 and NeuN double-labelled neurons (apoptotic neurons). Scale bar = 100 µm. (B) Representative images of TUNEL staining in the cortex. Green staining indicates apoptotic (TUNEL-positive) cells, and blue staining indicates DAPI-stained nuclei. Merge: TUNEL and DAPI double-labelled cells (apoptotic cells). Scale bar = 100 µm. (C, D) Quantification of the number of (C) cleaved caspase-3- and (D) TUNEL-positive cells in the cortex. Sham (n = 10): control group; Iso+I (n = 10): inhaled 1.5% isoflurane with a plantar incision; Iso+Rf5+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 5 µg·kg-1·h-1 remifentanil; Iso+Rf10+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 10 µg·kg-1·h-1 remifentanil; Iso+Rf20+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 20 µg·kg-1·h-1 remifentanil. * P < 0.05 compared with the Sham group.
Although the combination of inhaled isoflurane with infusion of low and moderate doses of remifentanil (5 and 10 µg·kg-1·h-1) reduced the isoflurane-induced increase in apoptosis in the cortex, significant differences in the ability of different doses of remifentanil (5, 10, and 20 µg·kg-1·h-1) to reduce isoflurane-induced apoptosis were not observed.
A specific remifentanil dose reduced isoflurane-induced apoptosis in the hippocampus
Compared with the Iso group, combined treatment with 10 µg·kg-1·h-1 remifentanil significantly reduced apoptosis in the CA1 region in the presence of a nociceptive stimulus (cleaved caspase-3 staining: P = 0.003; TUNEL staining: P = 0.037). However, when isoflurane was combined with low-dose remifentanil (5 µg·kg-1·h-1), the number of apoptotic cells showed a decreasing trend that was not statistically significant (cleaved caspase-3 staining: P = 0.077; TUNEL staining: P = 0.455). When isoflurane was combined with high-dose remifentanil (20 µg·kg-1·h-1), the number of apoptotic cells showed an increasing trend (cleaved caspase-3 staining: P = 0.077; TUNEL staining: P = 0.789) (Figure 2).
Figure 2.
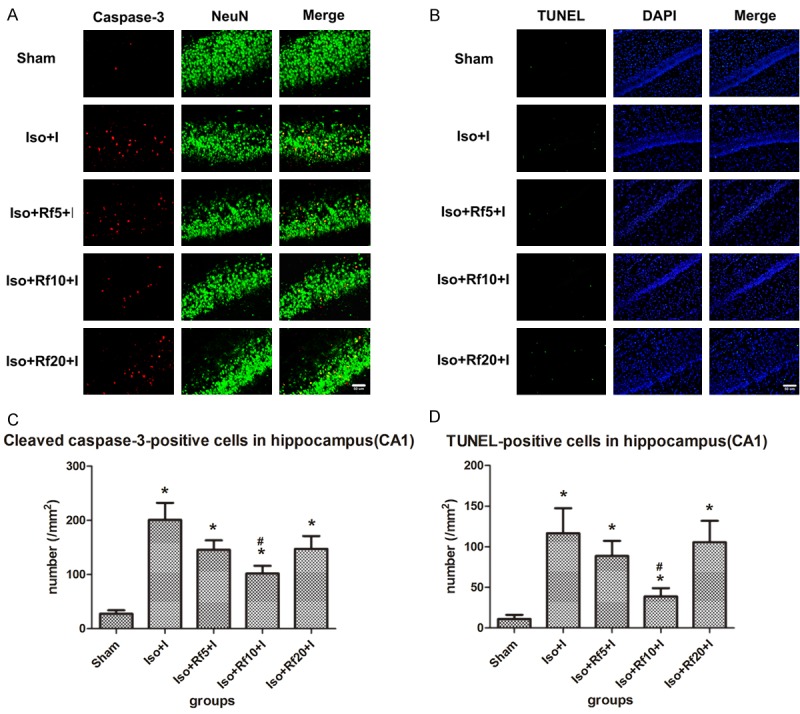
A specific remifentanil dose reduced isoflurane-induced apoptosis in the hippocampus CA1 region. (A) Representative images of immunofluorescence staining for cleaved caspase-3 in the CA1. Red staining indicates apoptotic (cleaved caspase-3-positive) cells, and green staining indicates neurons in the CA1. Merge: cleaved caspase-3 and NeuN double-labelled neurons (apoptotic neurons). Scale bar = 50 µm. (B) Representative images of TUNEL staining in the CA1. Green staining indicates apoptotic (TUNEL-positive) cells, and blue staining indicates DAPI-stained nuclei. Merge: TUNEL and DAPI double-labelled cells (apoptotic cells). Scale bar = 50 µm. (C, D) Quantification of the number of (C) cleaved caspase-3- and (D) TUNEL-positive cells in the CA1. Sham (n = 10): control group; Iso+I (n = 10): inhaled 1.5% isoflurane with a plantar incision; Iso+Rf5+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 5 µg·kg-1·h-1 remifentanil; Iso+Rf10+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 10 µg·kg-1·h-1 remifentanil; Iso+Rf20+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 20 µg·kg-1·h-1 remifentanil. * P < 0.05 compared with the Sham group. # P < 0.05 compared with the Iso+I group.
In addition, the combination of isoflurane and 10 µg·kg-1·h-1 remifentanil significantly reduced apoptosis in the CA3 region in the presence of a nociceptive stimulus (cleaved caspase-3 staining: P = 0.002; TUNEL staining: P = 0.017). However, neither low (cleaved caspase-3 staining: P = 0.594; TUNEL staining: P = 0.721) nor high (cleaved caspase-3 staining: P = 0.788; TUNEL staining: P = 0.461) doses of remifentanil reduced isoflurane-induced apoptosis (Figure 3).
Figure 3.
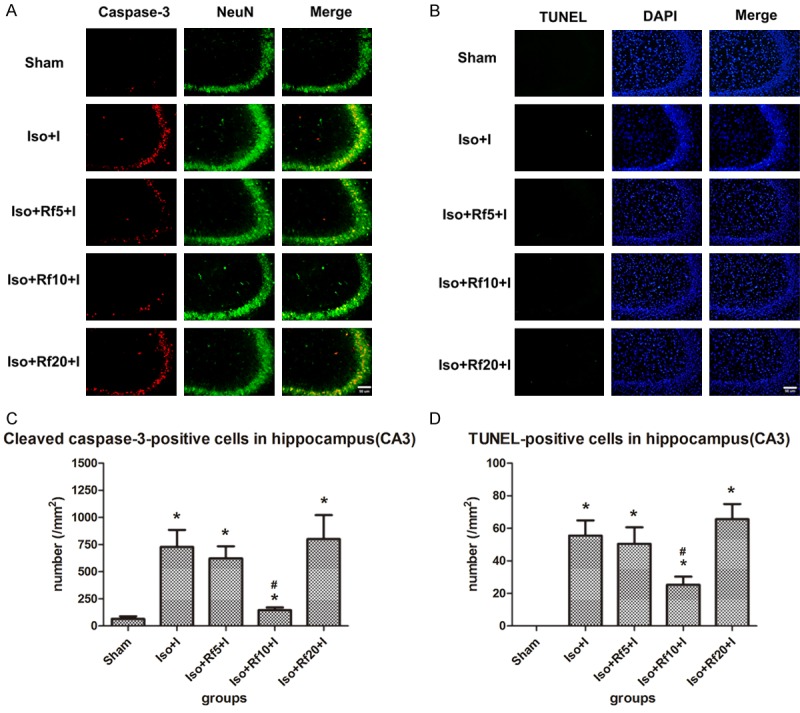
A specific remifentanil dose reduced isoflurane-induced apoptosis in the hippocampus CA3 region. (A) Representative images of immunofluorescence staining for cleaved caspase-3 in the CA3. Red staining indicates apoptotic (cleaved caspase-3-positive) cells, and green staining indicates neurons in the CA3. Merge: cleaved caspase-3 and NeuN double-labelled neurons (apoptotic neurons). Scale bar = 50 µm. (B) Representative images of TUNEL staining in the CA3. Green staining indicates apoptotic (TUNEL-positive) cells, and blue staining indicates DAPI-stained nuclei. Merge: TUNEL and DAPI double-labelled cells (apoptotic cells). Scale bar = 50 µm. (C, D) Quantification of the number of (C) cleaved caspase-3- and (D) TUNEL-positive cells in the CA3. Sham (n = 10): control group; Iso+I (n = 10): inhaled 1.5% isoflurane with a plantar incision; Iso+Rf5+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 5 µg·kg-1·h-1 remifentanil; Iso+Rf10+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 10 µg·kg-1·h-1 remifentanil; Iso+Rf20+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 20 µg·kg-1·h-1 remifentanil. * P < 0.05 compared with the Sham group. # P < 0.05 compared with the Iso+I group.
Remifentanil had no effect on isoflurane-induced apoptosis in the thalamus
The number of apoptopic cells in the thalamus increased similarly in the Iso group and the group that received 1.5% isoflurane and an infusion of 5, 10, or 20 µg·kg-1·h-1 remifentanil in the presence of a nociceptive stimulus (Figure 4).
Figure 4.
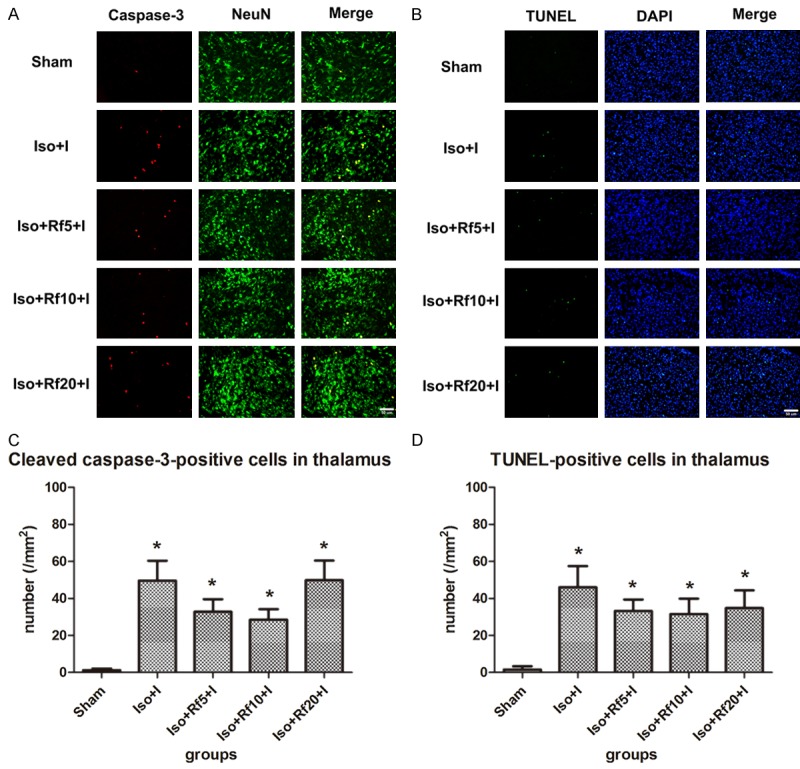
Remifentanil has no significant effect on isoflurane-induced apoptosis in the thalamus. (A) Representative images of immunofluorescence staining for cleaved caspase-3 in the thalamus. Red staining indicates apoptotic (cleaved caspase-3-positive) cells, and green staining indicates neurons in the thalamus. Merge: cleaved caspase-3 and NeuN double-labelled neurons (apoptotic neurons). Scale bar = 50 µm. (B) Representative images of TUNEL staining in the thalamus. Green staining indicates apoptotic (TUNEL-positive) cells, and blue staining indicates DAPI-stained nuclei. Merge: TUNEL and DAPI double-labelled cells (apoptotic cells). Scale bar = 50 µm. (C, D) Quantification of the number of (C) cleaved caspase-3- and (D) TUNEL-positive cells in the thalamus. Sham (n = 10): control group; Iso+I (n = 10): inhaled 1.5% isoflurane with a plantar incision; Iso+Rf5+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 5 µg·kg-1·h-1 remifentanil; Iso+Rf10+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 10 µg·kg-1·h-1 remifentanil; Iso+Rf20+I (n = 10): inhaled 1.5% isoflurane with a plantar incision and infusion of 20 µg·kg-1·h-1 remifentanil. * P < 0.05 compared with the Sham group.
Discussion
Commonly used general anesthetics, including inhaled and intravenous anesthetics, inhibit the N-methyl-D-aspartate (NMDA) receptor and potentiate the gamma-aminobutyric acid (GABA) receptor. Many infants and pregnant/parturient women undergo surgery each year. Based on accumulating evidence from recent basic science studies, inhibition of the NMDA receptor and potentiation of the GABA receptor are associated with neurotoxic effects on the developing brain, including neuronal apoptosis [1-4], impaired synapse formation [5-7], decreased dendrite density [8-10], and damaged glial cell development and proliferation and neural precursor differentiation [11,12]. Pups exposed to general anesthetics may experience learning impairments and cognitive dysfunction during development [19,20]. Although convincing clinical studies are not available [21-23], the effects of anesthetics on the developing brain have become a public health concern.
Unlike many animal studies, which frequently use a single inhaled anesthetic, physicians typically combine inhaled anesthetics and opioids in clinical practice when patients are exposed to nociceptive stimuli. Both opioids and nociceptive stimuli may be associated with neurotoxic consequences on the developing brain. For example, the effects of morphine on the developing brain may vary depending on the presence of pain and trauma. High-dose or long-term use of morphine may induce neuronal and glial apoptosis [24]. Severe trauma or pain per se may also cause neurotoxicity in the brain [25]; however, in the presence of pain, morphine usage is associated with protective effects on the immature brain [26].
As a frequently used opioid analgesic, remifentanil has an impressive pharmacokinetic profile and is more frequently used in young children and parturient/pregnant women exposed to surgical anesthesia. In addition, intravenous remifentanil infusion is an alternative for labour analgesia [27-29] when neuraxial analgesia is contraindicated. Similar to most anesthetics, remifentanil may affect the foetus by easily crossing the placenta [30]; therefore, the effects of remifentanil on the immature brain must be considered. However, few studies have investigated the neurotoxic effects of remifentanil on the developing brain. The in vitro study by Tourrel et al [31], who examined brain slices from newborn mice, showed that remifentanil did not induce nerve cell necrosis but exerted anti-apoptotic effects. Researchers have not yet determined whether remifentanil has potential neurotoxic effects in vivo. Studies investigating whether remifentanil increases or decreases apoptosis when used in combination with inhaled isoflurane will be very important.
In terms of neurodevelopment, a 7-day-old rat pup is at the peak of the synaptogenic period, which is equivalent to the neonatal period in humans [32]. The inhalation of 1 MAC for 2 or 4 h induced apoptosis in many brain regions, whereas it only induced cognitive dysfunction after 4 h [19]. Thus, we used a 4-h isoflurane inhalation as a positive control.
In the first experiment, the selection of a high dose of remifentanil (80 µg·kg-1·h-1) was based on a previous animal model in which 40 µg/kg remifentanil was subcutaneously infused into rats over a period of 30 min to induce hyperpathia [16]. Based on numerous studies, a high dose of remifentanil inevitably induces hyperpathia; consequently, 40 µg/kg remifentanil (for 30 min) is considered an acceptably high dose [17]. Because the duration of drug exposure in the present study was 4 h, the total dose administered to the high-dose remifentanil group (320 µg/kg) was much higher than that administered to the animal model of hyperpathia, but the rate was the same. The infusion rate of the moderate dose (20 µg·kg-1·h-1) was based on a clinically used dose of 3 µg·kg-1·h-1, which was converted based on the 6-fold decrease in the remifentanil dosage in experimental rats.
In the second experiment, we used three different doses of remifentanil in combination with isoflurane. The same doses in the first experiment were first used in a preliminary experiment, but most of the pups died from respiratory depression when isoflurane was administered with 80 µg·kg-1·h-1 remifentanil without tracheal intubation (mortality: 3/4) or with 40 µg·kg-1·h-1 remifentanil (2/3). Thus, one of the limitations of the present study is that we did not intubate each pup. Finally, we used 20 µg·kg-1·h-1, a moderate dose, as the highest dose in the second experiment; this dose induced slight respiratory depression, increased PaCO2, decreased pH and increased mortality (20%).
Both hypoglycaemia and hypoxaemia may induce neurodegeneration and apoptosis in neonatal rodents [33]; thus, blood gas and glucose levels were monitored in the present study. No pups developed hypoglycaemia. In the first experiment, the isoflurane and Rf80 groups showed slightly increased PaCO2 and decreased pH, suggesting mild respiratory depression, but showed no significant differences in PaCO2 or pH values. According to Stratmann and colleagues [19], hypercapnia might cause widespread neuronal apoptosis in neonatal rats, although the magnitude of this previously ob-served neuronal apoptosis was less than that observed in the isoflurane group in our study. The concentration of inhaled isoflurane (1.5%) used in our study was significantly less than the concentration (1 MAC) used by Stratmann et al. The PaCO2 (51 ± 6 mmHg) in the isoflurane group was significantly lower than the level reported by Stratmann (approximately 80 mmHg). Mild hypercapnia might be one factor contributing to isoflurane-induced neuronal apoptosis. In the high-dose remifentanil group, which exhibited a similar increase in PaCO2, significant neuronal apoptosis was not observed in the cortex, hippocampus, or thalamus compared with the Sham group, suggesting that neurotoxicity was not associated with mild hypercapnia. In the second experiment, slight hypercapnia was evident in all groups except that treated with isoflurane and 20 µg·kg-1·h-1 remifentanil, which exhibited a significant increase in PaCO2 and decreases in pH and PaO2. The pups in the Iso+Rf20+I group experienced severe respiratory depression (PaO2: 50 ± 11 mmHg; PaCO2: 72 ± 5 mmHg). As shown in the study by Machaalani [34], hypoxaemia (PaCO2: 40.9 ± 1.9 mmHg) and hypercapnia (PaCO2: 61.2 ± 4.2 mmHg) induce apoptosis in the brain. Thus, in the present study, treatment with isoflurane and 20 µg·kg-1·h-1 remifentanil increased the number of apoptotic cells compared with treatment with isoflurane and 5 or 10 µg·kg-1·h-1 remifentanil; this effect may be associated with hypercapnia.
The cortex, hippocampus and thalamus were assessed in the present study. The cerebral cortex is the highest sensorium and motorium, and we observed that isoflurane is associated with neurotoxic effects, consistent with previous studies. In addition, remifentanil had no effect on cortical neurons and did not reduce isoflurane-induced apoptosis. Isoflurane does not damage the short-term memory associated with cortical functions, although this drug induced apoptosis [20,35]. The hippocampus is associated with learning, cognition and memory. Pups may show abnormal patterns in behavioural experiments as they age if they sustain hippocampal neuron injury [35-38]. Regardless of the presence of a nociceptive stimulus, inhalation of a 1.5% anesthetic was associated with hippocampal neuron apoptosis, and a 10 µg·kg-1·h-1 remifentanil infusion reduced apoptosis, suggesting that remifentanil exerted neuroprotective effects. Isoflurane also induced thalamic neuron apoptosis. The thalamus is the most important sensory conduction station, and various regions, excepting the olfactory sensory pathway, project neurons into the thalamus, which subsequently projects them into the cerebral cortex. The thalamus is also associated with emotion [39], Wernicke-Korsakoff syndrome and amnesia, suggesting that this brain region is involved in memory and attention [40,41]. However, remifentanil did not reduce isoflurane-induced apoptosis in the thalamus in the present study. Briefly, remifentanil exerted a neuroprotective effect on the hippocampus but not on the cortex or thalamus, likely for three reasons. 1: The neurotoxic effect of isoflurane varies among different brain regions. The number of apoptotic cells in each brain region was highest in the hippocampus (200.8 cells/mm2 in CA1 and 727.9 cells/mm2 in CA3), followed by the thalamus (49.6 cells/mm2) and cortex (1.0 cells/mm2). Thus, the neuroprotective effect of remifentanil may be more apparent in more important regions. 2: The sample size was small (n = 10). 3: Because of the limitations of the present study, a higher dose of remifentanil, which may have exerted an anti-apoptotic effect on the thalamus or cortex, could not be used.
Based on our observation that the combination of isoflurane with 10 µg·kg-1·h-1 remifentanil reduced isoflurane-induced apoptosis in the hippocampus, the neurotoxic effect of a single dose of remifentanil in the presence of a nociceptive stimulus was observed. However, remifentanil did not exert a neurotoxic effect on the developing brain.
A plantar incision (Brennan’s model) was used as the pain stimulus in the present study. Brennan’s model is typically used to simulate the effect of pain stimuli on the neonatal nervous system [42] and to examine remifentanil-induced hyperalgesia [16,17].
Our study has some limitations. First, we did not measure vital signs, anesthesia depth and injury severity in the pups in all groups to determine whether the anesthesia depth was consistent with the injury severity. Second, without tracheal intubation, respiratory depression and hypercapnia readily occurred in pups treated with inhaled isoflurane combined with the infusion of moderate-dose remifentanil (Iso+Rf20+I group), and the mice subjected to isoflurane inhalation and 20 µg·kg-1·h-1 remifentanil infusion had more apoptotic cells. Thus, we cannot be certain whether the increased apoptosis induced by hypercapnia or the 20 µg·kg-1·h-1 remifentanil infusion represents a neuroprotective effect. Further studies are required to determine whether the effects in the high-dose group would be different if the pups had received tracheal intubation.
In conclusion, regardless of the presence of a nociceptive stimulus, inhaled anesthetics were associated with neuronal apoptosis in some brain regions, particularly in the hippocampus. However, remifentanil did not exert neurotoxic effects on the developing brain, and it reduced isoflurane-induced apoptosis. Thus, the anti-apoptosis mechanism of remifentanil requires further investigation.
Acknowledgements
Funded by the Municipal Natural Science Foundation of Shanghai (15ZR1404600). Yanqing Gao, PhD, State key Laboratory of Medical Neurobiology, Fudan University, Shanghai, China; Mingyue Xu, PhD, State key Laboratory of Medical Neurobiology, Fudan University, Shanghai, China; Hongfeng Mu, MD, State key Laboratory of Medical Neurobiology, Fudan University, Shanghai, China; Mengfei Cai, MD, State key Laboratory of Medical Neurobiology, Fudan University, Shanghai, China.
Disclosure of conflict of interest
None.
References
- 1.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizzi S, Carter LB, Ori C, Jevtovic-Todorovic V. Clinical anesthesia causes permanent damage to the fetal guinea pig brain. Brain Pathol. 2008;18:198–210. doi: 10.1111/j.1750-3639.2007.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, Dissen GA, Creeley CE, Olney JW. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–384. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nie H, Peng Z, Lao N, Dong H, Xiong L. Effects of sevoflurane on self-renewal capacity and differentiation of cultured neural stem cells. Neurochem Res. 2013;38:1758–1767. doi: 10.1007/s11064-013-1074-4. [DOI] [PubMed] [Google Scholar]
- 6.Dong C, Rovnaghi CR, Anand KJ. Ketamine alters the neurogenesis of rat cortical neural stem progenitor cells. Crit Care Med. 2012;40:2407–2416. doi: 10.1097/CCM.0b013e318253563c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao X, Yang Z, Liang G, Wu Z, Peng Y, Joseph DJ, Inan S, Wei H. Dual effects of isoflurane on proliferation, differentiation, and survival in human neuroprogenitor cells. Anesthesiology. 2013;118:537–549. doi: 10.1097/ALN.0b013e3182833fae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mintz CD, Smith SC, Barrett KM, Benson DL. Anesthetics interfere with the polarization of developing cortical neurons. J Neurosurg Anesthesiol. 2012;24:368–375. doi: 10.1097/ANA.0b013e31826a03a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L. Developmental stagedependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology. 2011;115:282–293. doi: 10.1097/ALN.0b013e318221fbbd. [DOI] [PubMed] [Google Scholar]
- 10.Mintz CD, Barrett KM, Smith SC, Benson DL, Harrison NL. Anesthetics interfere with axon guidance in developing mouse neocortical neurons in vitro via a gamma-aminobutyric acid type A receptor mechanism. Anesthesiology. 2013;118:825–833. doi: 10.1097/ALN.0b013e318287b850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brambrink AM, Back SA, Riddle A, Gong X, Moravec MD, Dissen GA, Creeley CE, Dikranian KT, Olney JW. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525–535. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013;110(Suppl 1):i29–i38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Zhang J, Yang L, Dong Y, Zhang Y, Xie Z. Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factorkappa B pathway in neuroglioma cells. Br J Anaesth. 2013;110(Suppl 1):i82–i91. doi: 10.1093/bja/aet115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Du Z, Zhou Q, Wang Y, Li J. Remifentanil attenuates lipopolysaccharide-induced acute lung injury by downregulating the NF-kappaB signaling pathway. Inflammation. 2014;37:1654–1660. doi: 10.1007/s10753-014-9893-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhao G, Shen X, Nan H, Yan L, Zhao H, Yu J, Lv Y. Remifentanil protects liver against ischemia/reperfusion injury through activation of anti-apoptotic pathways. J Surg Res. 2013;183:827–834. doi: 10.1016/j.jss.2013.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, Cui S, Liu Y, Zhang J, Zhang W, Zhang J, Gu X, Ma Z. Dexmedetomidine prevents remifentanil-induced postoperative hyperalgesia and decreases spinal tyrosine phosphorylation of N-methyl-d-aspartate receptor 2B subunit. Brain Res Bull. 2012;87:427–431. doi: 10.1016/j.brainresbull.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Cabanero D, Campillo A, Celerier E, Romero A, Puig MM. Pronociceptive effects of remifentanil in a mouse model of postsurgical pain: effect of a second surgery. Anesthesiology. 2009;111:1334–1345. doi: 10.1097/ALN.0b013e3181bfab61. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandra R, Subramanian T. Atlas of the NEONATAL RAT BRAIN. Boca Raton: CRC Press; 2011. [Google Scholar]
- 19.Stratmann G, May LD, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–861. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 20.Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, Maze M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 21.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–1707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 22.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen TG, Pedersen JK, Henneberg SW, Pedersen DA, Murray JC, Morton NS, Christensen K. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–1085. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 24.Bajic D, Commons KG, Soriano SG. Morphine-enhanced apoptosis in selective brain regions of neonatal rats. Int J Dev Neurosci. 2013;31:258–266. doi: 10.1016/j.ijdevneu.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, Rogers M, Mackay M, Hubber-Richard P, Johannesen D. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143:138–146. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duhrsen L, Simons SH, Dzietko M, Genz K, Bendix I, Boos V, Sifringer M, Tibboel D, Felderhoff-Mueser U. Effects of repetitive exposure to pain and morphine treatment on the neonatal rat brain. Neonatology. 2013;103:35–43. doi: 10.1159/000341769. [DOI] [PubMed] [Google Scholar]
- 27.Stocki D, Matot I, Einav S, Eventov-Friedman S, Ginosar Y, Weiniger CF. A randomized controlled trial of the efficacy and respiratory effects of patient-controlled intravenous remifentanil analgesia and patient-controlled epidural analgesia in laboring women. Anesth Analg. 2014;118:589–597. doi: 10.1213/ANE.0b013e3182a7cd1b. [DOI] [PubMed] [Google Scholar]
- 28.Shen MK, Wu ZF, Zhu AB, He LL, Shen XF, Yang JJ, Feng SW. Remifentanil for labour analgesia: a double-blinded, randomised controlled trial of maternal and neonatal effects of patient-controlled analgesia versus continuous infusion. Anaesthesia. 2013;68:236–244. doi: 10.1111/anae.12098. [DOI] [PubMed] [Google Scholar]
- 29.Rehberg B, Wickboldt N, Juillet C, Savoldelli G. Can remifentanil use in obstetrics be improved by optimal patient-controlled analgesia bolus timing? Br J Anaesth. 2015;114:281–9. doi: 10.1093/bja/aeu368. [DOI] [PubMed] [Google Scholar]
- 30.Ngan KW, Khaw KS, Ma KC, Wong AS, Lee BB, Ng FF. Maternal and neonatal effects of remifentanil at induction of general anesthesia for cesarean delivery: a randomized, doubleblind, controlled trial. Anesthesiology. 2006;104:14–20. doi: 10.1097/00000542-200601000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Tourrel F, de Lendeu PK, Abily-Donval L, Chollat C, Marret S, Dufrasne F, Compagnon P, Ramdani Y, Dureuil B, Laudenbach V, Gonzalez BJ, Jegou S. The antiapoptotic effect of remifentanil on the immature mouse brain: an ex vivo study. Anesth Analg. 2014;118:1041–1051. doi: 10.1213/ANE.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 32.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 33.Loepke AW, McCann JC, Kurth CD, McAuliffe JJ. The physiologic effects of isoflurane anesthesia in neonatal mice. Anesth Analg. 2006;102:75–80. doi: 10.1213/01.ANE.0000181102.92729.B8. [DOI] [PubMed] [Google Scholar]
- 34.Machaalani R, Waters KA. Correlations between brainstem NMDA receptor changes and active neuronal cell death after intermittent hypercapnic hypoxia in the developing piglet. Brain Res. 2003;975:141–148. doi: 10.1016/s0006-8993(03)02603-9. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Zeng M, Chen W, Liu C, Wang F, Han X, Zuo Z, Peng S. Dexmedetomidine reduces isoflurane-induced neuroapoptosis partly by preserving PI3K/Akt pathway in the hippocampus of neonatal rats. PLoS One. 2014;9:e93639. doi: 10.1371/journal.pone.0093639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meister ML, Buffalo EA. Getting directions from the hippocampus: the neural connection between looking and memory. Neurobiol Learn Mem. 2016;134:135–144. doi: 10.1016/j.nlm.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- 38.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 39.Hirayama K. [Thalamus and Emotion] . Brain Nerve. 2015;67:1499–1508. doi: 10.11477/mf.1416200328. [DOI] [PubMed] [Google Scholar]
- 40.Nishio Y, Mori E. [Neuroanatomical networks supporting memory and their relation to amnesia; a view from the thalamus] . Brain Nerve. 2015;67:1481–1494. doi: 10.11477/mf.1416200326. [DOI] [PubMed] [Google Scholar]
- 41.Tokoro K, Sato H, Yamamoto M, Nagai Y. Thalamus and attention. Brain Nerve. 2015;67:1471–1480. doi: 10.11477/mf.1416200324. [DOI] [PubMed] [Google Scholar]
- 42.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]


