Abstract
Wnt signaling is intrinsic to embryonic stem cell self-renewal and mammalian development. However, the effects of wnts on ES cells self-renewal and iPS cells transduction was not clearly understood. In this study, L-Wnt3a cells that secreted activated Wnt3a protein into medium were used to produce Wnt3a condition medium (Wnt3a-CM) or feeder layer for ES cells cultivation and iPS cells transduction. The results showed that L-Wnt3a cells as feeder layer significantly promoted establishment of ES cell lines and generation of iPS cells. The ES cells robustly maintained pluripotency in Wnt3a-CM on feeder free condition. Moreover, we demonstrate that activated Wnt signaling by Wnt3a-CM at the early stage of reprogramming promoted generation of iPS cells by up-regulating Tcf3 and Tcf4, improving mesenchymal-to-epithelial transition (MET), promptly reactivating endogenous pluripotent genes, and regulating epigenetic remodeling. Taken together, L-Wnt3a cells and their condition medium could be a novel culture system to robustly maintained pluripotency of ES cells and accelerated somatic reprogramming by activating Wnt signaling.
Keywords: Wnt signaling, ES cells, iPS cells, somatic reprogramming
Introduction
Embryonic stem cells (ES cells) derive from the inner cell mass of the blastocysts, and have ability to form all tissues in our body [1]. The growth factors and several signaling pathway had pivotal role to ES cells self-renewal or differentiation. Although a core and extended transcriptional networks were clearly critical for maintaining and establishing cellular pluripotency, the role of extracellular signals, such as WNTs, has not been investigated [2]. Previously, medium supplemented with purified Wnt3A protein promoted the proliferation and suppress the differentiation of human hematopoietic stem cells (HSCs) [3]. The canonical Wnt signaling pathway up-regulated expression of HoxB4 and Notch1 genes which played important role in the self-renewal of HSCs [4]. The signal pathway also promoted the proliferation and suppressed the differentiation of human epidermal stem cells by keeping its internal environment stability [5]. Importantly, the canonical Wnt signaling pathway was maintained pluripotency of human and mouse ES cells, and promoted expression of oct4, nanog, rex-1 genes [6]. However, wnt proteins could not maintain ES cell self-renew, alone, they need to synergistically act with LIF [7,8]. During ES cells self-renewal, the activated Wnt signaling pathway inhibits GSK-3β activity and lead to the accumulation of β-catenin. The stable β-catenin interacted with T-cell factors, and the latter transcripted their target genes [9,10]. The member of T-cell factor family, Tcf3 existed in an activating and repressive complex, and the activating complex promoted maintenance of pluripotency. On the contrary, the repressive complex regulated differentiation of ES cells [11].
During somatic nuclear reprogramming, activation of Wnt/β-catenin signaling strikingly enhances the ability of ES cells to reprogram somatic cells after fusion. Fused somatic cells lost their markers, and demethylation of DNA was observed in Oct4 and Nanog promoters [12]. Wnt signaling also improved transcription factors mediating induced pluripotency. Murine fibroblasts could be reprogrammed into induced plruipotent stem cells (iPS) without c-Myc in Wnt3a-conditioned medium [13]. Zhang and their colleagues confirmed Wnt/β-catenin enhanced iPS cells generation at early stage of reprogramming, and it was irrelevant to stimulate cell proliferation or reactivate c-Myc [14]. However, other studies showed that the reprogramming was inhibited by stimulating Wnt signal during early stages [15,16]. Finally, despite the effect of Wnt signaling on generation of iPS cells and pluripotent maintenance of ES cells was established, a strict examination for Wnt3a during ES cells culture and iPS cells transduction were not investigated.
In the study, we employed an immortal cell line, L-Wnt3a cells that were screened from overexpressing mouse Wnt3a gene in L-cell from mouse subcutaneous connective tissue, and secreted activated Wnt3a protein into medium [3]. The cells as feeder layer and its conditioned medium were used to isolate, culture mouse ES cells and induce iPS cells in feeder or feeder-free culture condition. The results showed that inner cell mass outgrowth was significantly promoted on L-Wnt3a cells feeder layer, the feeder or Wnt3a-CM supported ES cells self-renewal and maintained its pluripotency. L-Wnt3a cells that mixed with MEFs at appropriate ratio also promoted iPS cells transduction as feeder layer. When Wnt3a-CM was added from PD3 to PD6 during iPS cells transduction on feeder free condition, the efficiency of generation of iPS cells was significant increase. Analysis showed that Wnt3a-CM treatment up-regulated expression of Tcf3 and Tcf4, improved MET, reactivated endogenous pluripotent genes and regulated epigenetic remodeling.
Materials and methods
Unless otherwise mentioned, all reagents used were purchased from Life Technologies Company (USA). All the procedures involving the care and use of animals were approved by Inner Mongolia University’s Animal Care and Use Committee.
Cell culture
L-Wnt3a cells were purchased from cellbank of China Academy of Science, and maintained in medium: Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 4 mM L-glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin and 400 μg/ml G-418. Mouse embryonic fibroblasts (MEFs) were isolated from 13.5 day post-coitum (DPC) C57BL/6 mouse embryos, and cultured in DMEM supplemented with 10% FBS, L-glutamine, sodium pyruvate, non-essential amino acids (NEAA) and penicillin/streptomycin. The ES or iPS cell medium was Knock-out DMEM supplemented with 20% knock-out Serum Replacement (KSR), L-glutamine, sodium pyruvate, NEAA, β-mercaptoethanol, penicillin/streptomycin and 1000 units/ml leukemia inhibitory factor (LIF). Wnt3a-CM [3] were prepared according to standard protocols (ATCC), and supplemented with 10%, 30%, and 50% (v/v) concentrations of conditioned medium from L-Wnt3a cells in ES or iPS culture medium, named Wnt3a-CM-1, Wnt3a-CM-3 and Wnt3a-CM-5. The blastocysts from 3.5 DPC C57BL/6 mouse embryos were cultured in ES cells or Wnt3a-CM medium for outgrowth. Isolated primary outgrowths, ES cells and iPS cells were expanded on mitomycin C inactivated MEFs, L-Wnt3a feeder layers or 1% gelatin coated dishes in ES cell medium or Wnt3a-CM.
iPS cells transduction
pMXs retroviral vectors containing reprogramming factors, Oct4, Sox2, Klf4 and c-Myc and packaging vectors that expressed gag-pol and VSV-G were transfected into HEK293T cells by the calcium phosphate precipitation method. After 16 h post-transfection, the medium was changed with MEF medium. Two days later, virus supernatant was collected and centrifuge at 1500×g for 10 min at 4°C, store at -80°C. For infecting somatic cells, equally mixed retroviral supernatant, and infected cells two times, each 24 hr together with 8 μg/mL polybrene. The day that retroviral supernatant was replaced recorded post-infection day 0 (PD0). For induction on feeder layer, the infected cells were splited onto MEFs, L-Wnt3a cell feeder layer at PD2, and the MEF medium was changed with iPS cell medium at PD4. iPS colonies were picked up at PD14 to PD18.
Alkaline phosphatase staining and immunofluorescence analysis
Alkaline phosphatase (AKP) staining was performed with BCIP/NBT Alkaline Phosphatase Colour Development Kit (Beyotime) according to manufacturer’s instructions. For immunostaining, cells were fixed with 4% paraformaldehyde for 20 min, and then permeabilized with 1% Triton X-100 for 30 min followed by blocking with 2% BSA (Sigma). Cells were incubated in primary antibody overnight at 4°C and secondary antibody at 37°C for 1 h. Primary antibodies were used that were anti-Oct4 (Santa Cruz), anti-Nanog (Abcam), anti-Sox2 (Cell signaling), anti-SSEA1 (Santa Cruz), anti-SSEA4 (Santa Cruz), anti-E-Cadherin (BD Bioscience).
Quantitative and semi-quantitative RT-PCR
Total RNA was isolated from cells using MiniBEST Universal RNA Extraction Kit (TaKaRa) according to manufacturer’s instructions. RNA quality and quantity were examined using a NanoDrop 2000C Spectrophotometer (Thermo Scientific). Before reverse transcription (RT), concentration of total RNA was adjusted to 100 ng/μl. RT reaction solution was prepared which contained 500 ng RNA, RNase Free H2O and PrimeScript RT Master Mix (TaKaRa) according to manufacturer’s instructions. RT reaction was performed on MasterCycler Nexus (Eppendorf). Quantitative analysis of cDNA was performed by Applied Biosystems® 7500 Real-Time PCR System. PCR reactions were performed by initially denaturing cDNA at 94°C for 3 min, which was followed by 35 cycles of denaturing at 94°C for 30 s, annealing for 30 s at the temperature that was specified above for each primer pair, and extension at 72°C for 20 s, with a final 10 min extension step. For semi-quantitative RT-PCR, equal amount of cDNA was added into the reaction, and PCR products were ran electrophoresis on a 1.5% agarose gel. The primer sequences for quantitative and semi-quantitative RT-PCR are provided in Tables S1, S2.
In vitro and in vivo differentiation assay
ES or iPS cells were harvested by trypsinization and transferred to Petri-dishes in the ES medium without LIF. After 3 days, aggregated cells were plated onto gelatin-coated tissue culture dishes and incubated for another 3 days. The cells were stained with anti-Nestin antibody (Santa Cruz), anti-Brachyury antibody (T, Santa Cruz) and anti-Gata4 antibody (Santa Cruz).
ES of iPS cells were suspended at 3×106 cells/ml in DMEM containing 10% FBS, and injected 330 ul of cell suspension (1×106 cells) subcutaneously into groin and oxter of nude mice. Four weeks after the injection, teratomas were isolated and fixed in 4% paraformaldehyde, embedded in paraffin. Histological sections were stained with hematoxylin/eosin.
Statistical analysis
Statistically significant differences between groups were identified using t-test. Significance was established at p<0.05.
Results
L-Wnt3a cell feeder layers promoted mouse ES cells isolate and culture
Fifty C57BL/6 mouse blastocysts at 3.5 DPC that the zonae pellucidae was removed were seeded on L-Wnt3a cells or MEFs feeder layer, respectively. After 6 days, domed outgrowths appeared from the attached blastocysts. Compared to ICM outgrowths on MEFs feeder layer, no trophectoderm expansion around ICM outgrowths were observed (Figure 1A, arrow). In addition, formation of primary outgrowths on L-Wnt3a cells feeder layer was more effective than that on MEFs feeder layer (Table 1, p<0.05). At last, twelve ES cell lines on L-Wnt3a cells feeder layer and nine ES cell lines on MEFs feeder layer were established, named WF-ES cells and MF-ES cells, respectively. WF-ES cells showed more round and compacted colonies during long-term cultivation (Figure 1A). ES cells from these two feeder layers were Alkaline phosphatase (AKP) positive (Figure 1A), and expressed pluripotent markers, like Oct4, Sox2, Nanog, SSEA1 and E-Cadherin (Figure 1B). Embryoid bodies (EBs) could be derived from these two ES cells. Although immunostaining of three germ layers markers, like Gata4, T and Nestin were no difference in these two ES cells (Figure 1C), Semi-quantitative analysis of differentiation markers (nestin, βIII-tubulin, T, fit1, gata4 and foxa2) revealed that High-level nestin (ectoderm), flt1 and T (mesoderm) were detected in WF-ES cells (Figure 1D). After subcutaneous injection into nude mice, all ES cells differentiated into all three germ layers, including epidermis, cartilage, and columnar epithelium (Figure 1E).
Figure 1.
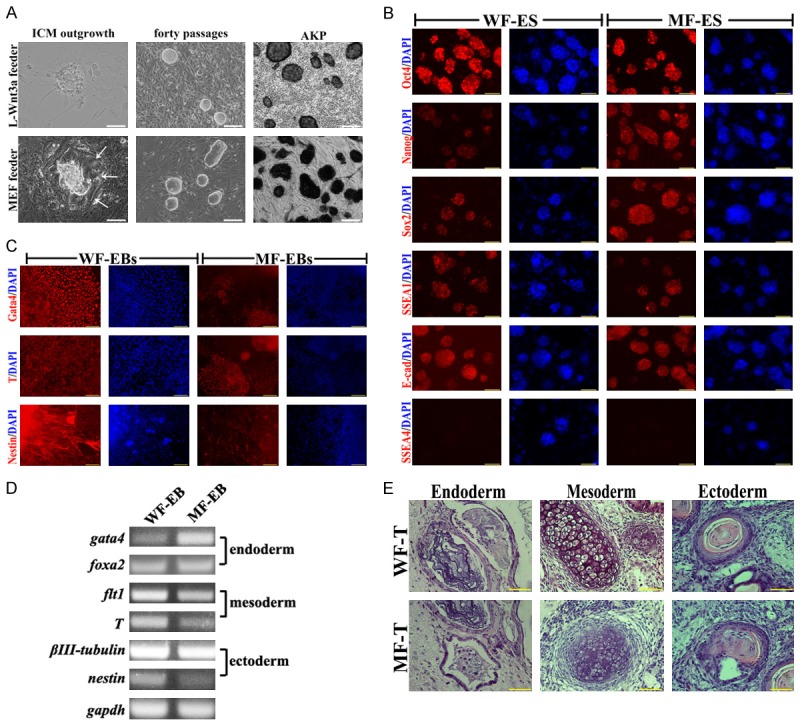
Pluripotent analysis of ES cells on MEFs and L-Wnt3a cells feeder layer. A. Blastocyst outgrowth on L-Wnt 3a cell and MEFs feeder layers, morphology of WF-ES and MF-ES cells, and AKP staining, bar=100 μm; B. Immunostaining of Oct4, Nanog, Sox2, SSEA1, SSEA4 and E-cadherin in WF-ES and MF-ES cells, bar=100 μm. C. Immunostaining of Gata4, T and Nestin in EBs that derived from WF-ES and MF-ES cells, bar=100 μm; D. Expression of three germ layer genes in EBs that derived from WF-ES and MF-ES cells; E. Tertomas from WF-ES and MF-ES cells, bar=50 μm.
Table 1.
Mouse ES cell line derived from L-Wnt3a cell and MEF feeder layer
| Feeder Layer | No. of embryos | No. of attached embryos (%) | No. of primary outgrowths (%) | No. of established ESC lines (%) |
|---|---|---|---|---|
| L-Wnt3a feeder | 22 | 21 (95.5)a | 19 (86.4)a | 12 (54.5)a |
| MEFs feeder | 28 | 25 (89.3)a | 16 (57.1)b | 9 (32.1)b |
Value in columns with different superscripts differ significantly (P<0.05).
Value in columns with different superscripts differ significantly (P<0.05).
In summary, L-Wnt3a cells could be used to isolate and culture mouse ES cells as a novel feeder layer. The WF-ES cells showed similar expression of pluripotent genes with MF-ES cells. However, robust differentiated ability was observed in WF-ES cells, suggested that pluripotency of ES cells could be effective maintained on L-Wnt3a cells feeder layer.
L-Wnt3a cells conditioned medium promoted maintenance of pluripotency of ES cells on feeder-free cultivation
Mouse ES cells that expressed GFP in Oct4 gene cassette (OG-ES cells) were derived from MEF or L-Wnt3a cell feeder layer, and then transferred on 1% gelatin coated dishes with MEF medium, ES medium and Wnt3a-CM. To characterize the concentration-dependent effects of conditioned medium on the formation of compact and GFP positive colonies during long-termed cultivation, OG-ES cells were exposed to Wnt3a-CM-1, Wnt3a-CM-3 and Wnt3a-CM-5 for long-termed cultivation. Formation of the colonies was also estimated in MEF and ES medium. The results showed that ES cells could form more compact colonies in Wnt3a-CM-5 than that in Wnt3a-CM-1, Wnt3a-CM-3 and ES medium (Figure S1). ES cells had exposed to Wnt3a-CM-5 formed 85.3% compact colonies for 150 days compared with Wnt3a-CM-1, Wnt3a-CM-3, MEF medium and ES medium (Table 2). During long-termed passages, number of compact and GFP positive colonies was lost in Wnt3a-CM-1, Wnt3a-CM-3, and ES medium, gradually (Table 2).
Table 2.
Percent compact and GFP positive ES cell colonies in Wnt3a-CM, ES and MEF medium
| Days | Wnt3a-CM (% conditioned medium v/v) | ES medium | MEF medium | ||
|---|---|---|---|---|---|
|
| |||||
| 10% (%) | 30% (%) | 50% (%) | |||
| 3 | 90.8±1.9a | 93.5±0.7a | 93.9±2.2a | 94.6±0.8a | 63.8±10.3b |
| 5 | 92.7±4.0a | 97±0.8a | 95.2±2.3a | 95.9±2.1a | 30.1±7.1b |
| 10 | 90.5±2.2a | 85.5±2.9b | 93.5±3.0a | 94.1±2.1a | - |
| 20 | 87.5±3.5a | 87.2±4.8a | 94.2±3.1a | 84.5±3.1b | - |
| 30 | 76.1±9.5a | 84.9±4.2a | 92.3±4.0b | 65.2±7.3c | - |
| 60 | 27.9±5.4a | 65.3±9.0b | 91.0±2.9c | 25.5±11.5a | - |
| 150 | - | - | 85.5±3.0 | - | - |
Value in columns with different superscripts differ significantly (P<0.05).
Value in columns with different superscripts differ significantly (P<0.05).
Value in columns with different superscripts differ significantly (P<0.05).
Wnt3a-CM-5 cultured ES cells (W-CM-ES cells) kept more intensive GFP expression and compacted colonies than that in ES medium (EM-ES cells) (Figure 2A). However, ES cells that were cultured in MEF medium (MM-ES cells) quickly formed flat colonies and lost GFP expression (Figure 2A). Analysis of pluripotency showed that AKP stainings rapidly dissolved in MM-ES cells, but kept intensively in W-CM-ES cells (Figure 2B). Intensive signals of Oct4, Sox2, Nanog, SSEA1 and E-cadherin were observed in W-CM-ES cells by immunostaining and qPCR (Figure 2C and 2D). Only ES cells that were cultured in Wnt3a-CM and ES medium could form EBs. Although these EBs derived from W-CM-ES and EM-ES cells emerged positive staining in three germ layer marker (Nestin, T and Gata4), analysis of expression of three germ layer genes showed that high-level mesoderm markers flt1, T and endoderm marker foxa2 were detected in W-CM-EBs (Figure 2E and 2F). Histological examination revealed that the teratomas from W-CM-ES and EM-ES cells contained tissues from three germ layers, including epidermis, cartilage and columnar epithelium (Figure 2G). However, chimeras were only derived from W-CM-ES cells, suggested that Wnt3a-CM cultured ES cells on feeder free condition showed intact pluripotency (Figure 2H).
Figure 2.
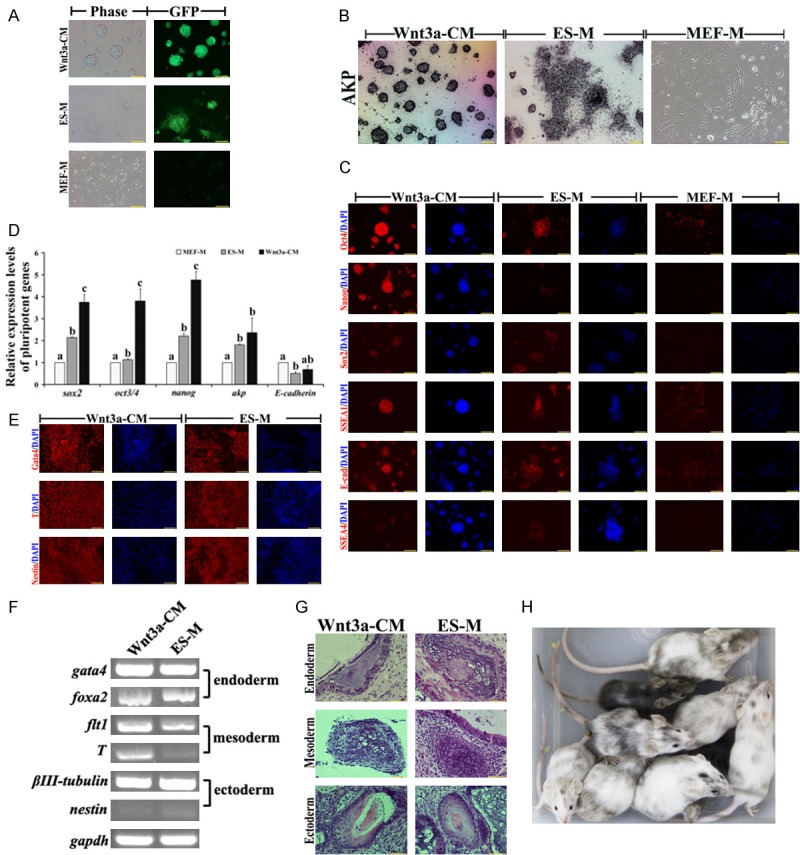
Pluripotent analysis of ES cells in Wnt3a-CM, ES medium (ES-M) and MEF medium (MEF-M) on feeder-free condition. A. Morphology of ES cells on Wnt3a-CM, ES-M and MEF-M; B. AKP staining of W-CM-ES, EM-ES and MM-ES cells, bar=100 μm; C. Immunostaining of Oct4, Nanog, Sox2, SSEA1, SSEA4 and E-cadherin in W-CM-ES, EM-ES and MM-ES cells, bar=100 μm; D. Expression of pluripotent genes in W-CM-ES, EM-ES and MM-ES cells; E. Immunostaining of Gata4, T and Nestin in EBs that derived from W-CM-ES and EM-ES cells, bar=100 μm; F: expression of three germ layer genes in EBs that derived from W-CM-ES and EM-ES cells; G. Tertomas from W-CM-ES and EM-ES cells, bar=50 μm; H. Chimeras generated from W-CM-ES cells.
In summary, Wnt3a-CM could significantly maintain pluripotency of mouse ES cells on feeder free condition during long-term cultivation. The W-CM-ES cells kept domed and compact colonies, expressed high-level pluripotent genes, differentiated into three germ layers in vitro and in vivo, and generated chimeric offspring.
L-Wnt3a cells as feeder layers promoted somatic reprogramming
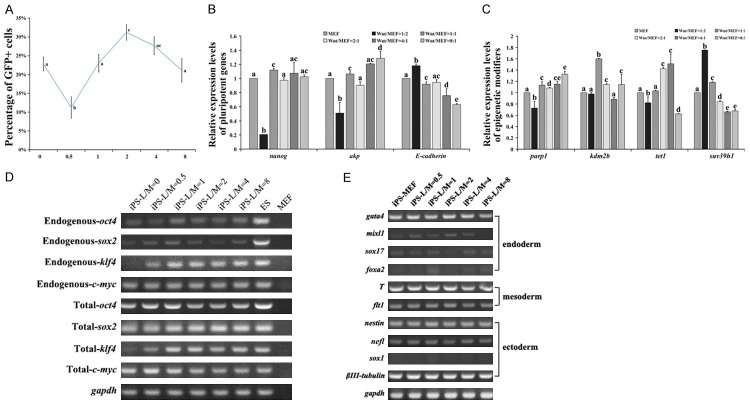
During somatic reprogramming by transcriptional factors, feeder layer was necessary to generate iPS cells. In our study, L-Wnt3a cells could be used to feeder layer during ES cells cultivation in vitro and maintain their pluripotency. However, it is unclear if the feeder layer also could be used to generate iPS cells, or not. When transferring infected OG-MEFs on L-Wnt3a cell feeder layer, generation of iPS cells was significantly inhibited. So, mixture of MEFs and L-Wnt3a cells at different ratio was prepared feeder layer. When the ratio was 2:1 (L-Wnt3a cells: MEFs), the Oct4-GFP positive iPS cells were significant increasing, compared with MEFs feeder layer or other ratio of these two cells (1:2, 1:1, 4:1 and 8:1) (Figure 3A, p<0.05). Interestingly, When the ratio was 1:2 (L-Wnt3a cells:MEFs), the Oct4-GFP positive iPS cells were significant decreasing (Figure 3A, p<0.01). The iPS cells derived from L-Wnt3a cell feeder layer (LF-iPS cells) maintained a comparable expression of pluripotent factors (Figures 3B, S2). Parp1, kdm2b and tet1 were significant up-regulation in LF-iPS cells (2:1), and suv39h1 was significant down-regulation, compared with iPS cells that derived from MEFs feeder layer (MF-iPS cells) (Figure 3C). In LF-iPS cells, endogenous transcriptional factors were reactivated (Figure 3D). There was no significant difference in expression of three germ layer markers in EBs that derived from LF-iPS and MF-iPS cells (Figure 3E).
Figure 3.
Generation of iPS cells on L-Wnt3a cell feeder layer. A. Efficiency of Oct4-GFP positive cells on L-Wnt3a cell feeder layer; B and C. Expression of pluripotent genes and epigenetic modifiers; D. Expression of transcriptional factors in iPS cells derived from L-Wnt3a cell feeder layer; E. Expression of three germ layer genes in EBs that derived from iPS cells.
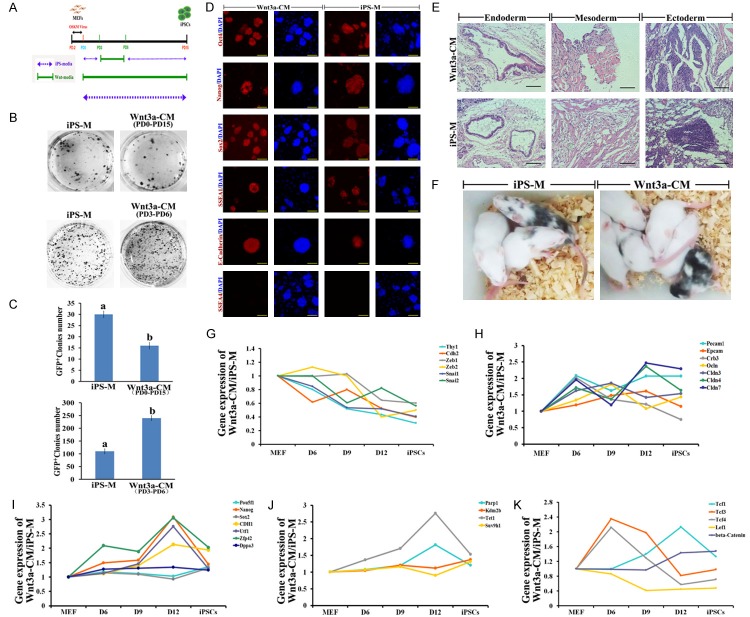
L-Wnt3a cells conditioned medium promoted somatic reprogramming by stage-specific regulation on feeder-free condition
OG-MEFs were transduced by Yamanaka factors, and cultured in Wnt3a-CM from PD0 to PD15 for generating iPS cells on 1% gelatin coated dishes (Figure 4A). However, few Oct4-GFP positive colonies formed (Figure 4B, 4C). Further, by optimizing usage of Wnt3a-CM during reprogramming, we found that the efficiency of iPS cells transduction was higher when Wnt3a-CM was added from PD3 to PD6 (Figure 4B, 4C). Expression of pluripotent markers in the W-iPS cells was comparable with iPS cells derived from iPS medium (I-iPS cells) (Figure 4D). W-iPS cells also differentiated into three germ layers, and generated chimeras (Figure 4E, 4F). During reprogramming in Wnt3a-CM, infected MEFs undergo mesenchymal-to-epithelial transition (MET) more quick than that in iPS medium around day 4 to 6. To examine the phenotypic progression of reprogramming in Wnt3a-CM, we collected reprogramming intermediates after 6, 9, and 12 days of induction. Compared to reprogram somatic cells in iPS medium, mesenchymal markers, Thy1, N-cadherin (Cdh2), Snai1, Snai2, Zeb1 and Zeb2 were significantly reduced (Figure 4G), and mRNA for epithelial markers, Pecam1, Ep-CAM, Crb1, Ocln and Cldn3, 4, 7 were significantly up-regulated when the cells were reprogrammed in Wnt3a-CM (Figure 4H). Furthermore, except for Oct4, Sox2 and Dppa3, pluripotent genes, Nanog, Zfp42, Utf1 and CDH1 in somatic cells were significantly up-regulated when treating with Wnt3a-CM (Figure 4I). Reprogramming somatic cells in Wnt3a-CM, the epigenetic modifiers, Parp1 and Tet1 were up-regulation, but there is no difference in expression of Kdm2b and Suv39h1 (Figure 4J). After treatment with Wnt3a-CM, expression of β-catenin and Tcf1 was significantly upregulated after day 9 (Figure 4K). Expression of Wnt/β-catenin signaling effectors, Tcf3 and Tcf4 was up-regulation at PD6, but down-regulation from PD9 to PD12 (Figure 4K). Lef1 was quickly down-regulated from PD6 to PD12 (Figure 4K).
Figure 4.
Wnt3a-CM promoted production of iPS cells on feeder free condition. A. Experimental design for tranducing iPS cells in wnt3a-CM; B. AKP staining of iPS cells; C. Number of Oct4-GFP colonies; D. Immunostaining of Oct4, Nanog, Sox2, SSEA1, SSEA4 and E-cadherin in W-iPS and I-iPS cells, bar=100 μm; E. Tertomas from W-iPS and I-iPS cells, bar=50 μm; F. Chimeras generated from W-iPS and I-iPS cells; G. The relative abundance of transcripts for mesenchymal genes during reprogramming in wnt3a-CM. H. The relative abundance of transcripts for epithelial genes during reprogramming in wnt3a-CM. I. The relative abundance of transcripts for pluripotent genes during reprogramming in wnt3a-CM. J. The relative abundance of transcripts for epigenetic modifiers during reprogramming in wnt3a-CM. K. The relative abundance of transcripts for wnt target genes during reprogramming in wnt3a-CM. The mRNA levels for quantitative RT-PCR were normalized by GAPDH mRNA level.
Discussion
For mouse ES cells cultivation or iPS cells transduction, feeder cell layer and LIF are usually necessary to maintain their generation and pluripotency in vitro. Recently, several gene-engineered feeder systems, like feeder cells that expressing E-cadherin or Wip1 were robustly maintained pluripotency of ES cells [17,18]. Wnt signaling plays major roles in various developmental events [19]. In pluripotent stem cells, the canonical Wnt pathway have pivotal role in the maintenance of ES cell self-renewal. The pathway is also one of the few common pathways involved in self-renewal in both mouse and human ES cells [20]. However, it is unclear if feeder cells that expressed Wnt3a protein can maintain pluripotency of ES cells and iPS cells transduction. L-Wnt3a cells from mouse subcutaneous connective tissue that expressed mouse Wnt3a gene, and secreted activated Wnt3a protein into medium [3]. In present study, L-Wnt3a cells as feeder cells and their conditioned medium were used to culture ES cells and induce iPS cells. The results showed that formation of primary outgrowths on L-Wnt3a cells feeder layer was more effective than that on MEFs feeder layer (p<0.05). The L-Wnt3a cells feeder layer could maintain ES cells self-renew, stably, and showed comparable pluriptency with ME-ES cells. These results suggested that L-Wnt3a cells could be a noval feeder cells for using to isolate and culture mouse ES cells. Furthermore, the feeder layers were used when transducing iPS cells, but no iPS colonies were achieved. When the feeder layer were prepared by mixing L-Wnt3a cells with MEF at ratio of 2:1, iPS cells generation were significantly improved. These cells had comparable pluriptency with iPS cells that derived from MEF feeder layer. These results suggested that the L-Wnt3a cells could be a novel feeder cells for ES cultivation and iPS cells transduction.
Previous studies revealed that Wnt3a-CM acted synergistically with LIF to inhibit ES cell differentiation in feeder-free culture [7,21]. Present study also confirmed that 50% (v/v) Wnt3a-CM could maintain domed colonies of ES cells, long-termed self-renew, and pluripotency of ES cells was dose-dependent without feeder layer. Furthermore, we tried to induce iPS cells in Wnt3a-CM for 15 days in feeder-free condition. However, generation of iPS cells was inhibited. The results were contrary to previous study that Wnt3a-CM improved iPS cells generation, even without c-Myc [13]. In our observations, although MEFs in Wnt3a-CM underwent more quick epithelial-like morphological changes than that in traditional iPS medium early in the reprogramming process, many colonies exhibiting rough surfaces were achieved late in the reprogramming process. Finally, our results revealed generation of iPS cells was significantly improved when MEFs were exposed in Wnt3a-CM from post-infection day 3 to day 6. Previous reports revealed that active Wnt signaling was required the late stage of reprogramming to iPS cells, and during the early stage, the signal pathway need to be inhibited [15,16]. Active canonical Wnt/β-catenin pathway activated target genes by β-catenin association with the TCF factors which include Tcf1, Lef1, Tcf3, and Tcf4 [9,22,23]. In iPS cells transduction and pluripotent regulation of ES cells, TCF factors usually have opposing effects [16,24]. Early in reprogramming, Tcf1 or Lef1 were inhibitors, whereas Tcf3 and Tcf4 are enhancers. Late in reprogramming, depletion of Tcf3 or Tcf4 regulated the activity of the Wnt signaling pathway, and improved iPS cells generation [16]. Our data also revealed that Tcf4 and Tcf3 was up-regulation, thereafter was down-regulation in Wnt3a-CM during somatic reprogramming.
In present study, Wnt3a-CM treatment did not directly activate Tcf1 and β-catenin, but induced MET and up-regulated expression of pluripotent genes like Nanog, Zfp42, Utf1 and CDH1. It was similar to previous results that Tcf1 and low-level β-catenin in MEFs that expressed Yamanaka factors down-regulated senescence genes, and improve MET during early reprogramming [15]. Generation of iPS cells by somatic cell reprogramming involves global epigenetic remodeling [25]. Therefore, the reprogramming enhancers, Parp1, Tet1 and Kdm2b, and barrier, Suv39h1 were detected during reprogramming [26-29]. Expression of Parp1, Tet1 and Kdm2b were significant up-regulation, whereas Suv39h1 was down-regulation in Wnt3a-CM. The results suggested that Wnt3a-CM treatment during iPS transduction improved epigenetic remodeling.
In summary, L-Wnt3a cells as a novel feeder cells significantly improved mouse ES cells isolation and self-renew, and promoted iPS cells transduction. On feeder-free condition, Wnt3a-CM robustly maintained pluripotency and self-renew of ES cells during long-term cultivation. Furthermore, when treating infected MEFs with Wnt3a-CM early in reprogramming, synergic factors of Wnt signal pathway, Tcf3 and Tcf4 was up-regulation, and mesenchymal-to-epithelial transition, reactivation of pluripotent genes and epigenetic remodeling was accelerated. Taken together, L-Wnt3a cells and their condition medium robustly maintained pluripotency of ES cells and accelerated somatic reprogramming by activating Wnt signaling.
Acknowledgements
We appreciate these persons whose efforts make the project and present manuscript possible (Zhuying Wei in Inner Mongolia University for technical assistance). This work was supported by the following funds: National Natural Sciences Foundation of China (31301197, 31660343), Natural Sciences Foundation of Inner Mongolia (2016MS0304), Natural Sciences Major Program of Inner Mongolia (2016ZD01), Governmental Major Program of Inner Mongolia, China, Opening major programs for grass-feeding livestock stem cells, Inner Mongolia, China (20130902) and Program for scientific talents of Inner Mongolia, China (NJYT-13-B03).
Supporting Information
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 5.Jia L, Zhou J, Peng S, Li J, Cao Y, Duan E. Effects of Wnt3a on proliferation and differentiation of human epidermal stem cells. Biochem Biophys Res Commun. 2008;368:483–488. doi: 10.1016/j.bbrc.2008.01.097. [DOI] [PubMed] [Google Scholar]
- 6.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- 8.Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- 9.Hoppler S, Kavanagh CL. Wnt signalling: variety at the core. J Cell Sci. 2007;120:385–393. doi: 10.1242/jcs.03363. [DOI] [PubMed] [Google Scholar]
- 10.Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 11.Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lluis F, Pedone E, Pepe S, Cosma MP. Periodic activation of Wnt/beta-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell. 2008;3:493–507. doi: 10.1016/j.stem.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young RA, Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P, Chang WH, Fong B, Gao F, Liu C, Al Alam D, Bellusci S, Lu W. Regulation of induced pluripotent stem (iPS) cell induction by Wnt/beta-catenin signaling. J Biol Chem. 2014;289:9221–9232. doi: 10.1074/jbc.M113.542845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aulicino F, Theka I, Ombrato L, Lluis F, Cosma MP. Temporal perturbation of the Wnt signaling pathway in the control of cell reprogramming is modulated by TCF1. Stem Cell Reports. 2014;2:707–720. doi: 10.1016/j.stemcr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho R, Papp B, Hoffman JA, Merrill BJ, Plath K. Stage-specific regulation of reprogramming to induced pluripotent stem cells by Wnt signaling and T cell factor proteins. Cell Rep. 2013;3:2113–2126. doi: 10.1016/j.celrep.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horie M, Ito A, Kiyohara T, Kawabe Y, Kamihira M. E-cadherin gene-engineered feeder systems for supporting undifferentiated growth of mouse embryonic stem cells. J Biosci Bioeng. 2010;110:582–587. doi: 10.1016/j.jbiosc.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Lee JS, Moon BH, Lee MO, Song SH, Li H, Fornace AJ, Cha HJ. Wip1-expressing feeder cells retain pluripotency of co-cultured mouse embryonic stem cells under leukemia inhibitory factor-deprivated condition. Arch Pharm Res. 2010;33:1253–1260. doi: 10.1007/s12272-010-0816-y. [DOI] [PubMed] [Google Scholar]
- 19.Sonderegger S, Pollheimer J, Knofler M. Wnt signalling in implantation, decidualisation and placental differentiation--review. Placenta. 2010;31:839–847. doi: 10.1016/j.placenta.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okita K, Yamanaka S. Intracellular signaling pathways regulating pluripotency of embryonic stem cells. Curr Stem Cell Res Ther. 2006;1:103–111. doi: 10.2174/157488806775269061. [DOI] [PubMed] [Google Scholar]
- 21.Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 23.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 24.Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol. 2011;13:762–770. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papp B, Plath K. Epigenetics of reprogramming to induced pluripotency. Cell. 2013;152:1324–1343. doi: 10.1016/j.cell.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y, Chen J, Li K, Wu T, Huang B, Liu W, Kou X, Zhang Y, Huang H, Jiang Y, Yao C, Liu X, Lu Z, Xu Z, Kang L, Chen J, Wang H, Cai T, Gao S. Replacement of Oct4 by Tet1 during iPSC Induction Reveals an Important Role of DNA Methylation and Hydroxymethylation in Reprogramming. Cell Stem Cell. 2013;12:453–469. doi: 10.1016/j.stem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Doege CA, Inoue K, Yamashita T, Rhee DB, Travis S, Fujita R, Guarnieri P, Bhagat G, Vanti WB, Shih A, Levine RL, Nik S, Chen EI, Abeliovich A. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang G, He J, Zhang Y. Kdm2b promotes induced pluripotent stem cell generation by facilitating gene activation early in reprogramming. Nat Cell Biol. 2012;14:457–466. doi: 10.1038/ncb2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onder TT, Kara N, Cherry A, Sinha AU, Zhu N, Bernt KM, Cahan P, Marcarci BO, Unternaehrer J, Gupta PB, Lander ES, Armstrong SA, Daley GQ. Chromatin-modifying enzymes as modulators of reprogramming. Nature. 2012;483:598–602. doi: 10.1038/nature10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.