Abstract
Colorectal cancer (CRC) remains one of the most common cancers worldwide. Increasing evidence indicates that long non-coding RNAs (lncRNAs) regulate diverse cellular processes, including cell growth, differentiation, apoptosis, and cancer progression. However, the function of lncRNAs in the progression of CRC remains largely unknown. Here, we reported that HOXA cluster antisense RNA2 (HOXA-AS2) was upregulated in CRC. Increased HOXA-AS2 expression in CRC was associated with larger tumor size and higher clinical stage. In vitro experiments revealed that HOXA-AS2 knockdown significantly inhibited CRC cell proliferation by causing G1 arrest and promoting apoptosis, whereas HOXA-AS2 overexpression promoted cell proliferation. Further functional assays indicated that HOXA-AS2 overexpression significantly promoted cell migration and invasion by regulating the epithelial-mesenchymal transition (EMT). In conclusion, our study identifies HOXA-AS2C as a potential biomarker in CRC.
Keywords: HOXA-AS2C, LncRNA, CRC, metastasis, EMT
Introduction
Colorectal cancer (CRC) is one of the most common types of cancer worldwide, and it is associated with a high mortality rate due to its rapid progression and advanced tumor presentation at the time of diagnosis [1,2]. Although preoperative treatment with chemoradiotherapy (CRT) in combination with conventional surgery improves local control of CRC and survival, metastasis of cancer is correlated closely with poor prognosis and usually indicates a late stage of cancer [3,4]. Therefore, there is urgent to identify a new therapeutic strategies for patients with CRC.
Long non-coding RNAs (lncRNAs, > 200 nucleotides in length) are important new members of the family of ncRNAs with limited or no protein-coding capacity [5]. Emerging evidence suggests that lncRNAs, may serve as master gene regulators capable of controlling protein-coding and non-coding genes, have important biological functions are closely related to human cancer [6,7]. LncRNAs also function as a competing endogenous RNA and sponge miRNAs, thus regulating the expression of target mRNA [8]. Moreover, lncRNA closely associates with the biological behavior of the tumor, including occurrence, development, invasion and metastasis of tumors.
Recently, increasing evidence has shown that Z HOXA cluster antisense RNA2 (HOXA-AS2), a lincRNA located between and antisense to the human HOXA3 and HOXA4 genes, was shown to be dysregulated in gastric cancer and hepatocellular carcinoma, as well as the potential relationship between HOXA-AS2 and metastasis [9-11]. However, the mechanisms by which HOXA-AS2 promotes cancer progression are not well understood; in particular, the molecular mechanisms by which HOXA-AS2 regulates CRC cell proliferation and invasion remains unknown. It is also necessary to reveal the underlying molecular mechanisms by which HOXA-AS2 is involved in CRC tumorigenesis and cancer progression.
Materials and methods
Patient samples
Sixty CRC tissue samples and matched adjacent normal tissue samples were obtained from surgical resection at Peking University Shenzhen Hospital. All the tissues were diagnosed by two independent pathologists. After resection, all samples were immersed immediately in RNA later solution overnight, then stored at -80°C in order to avoid degradation of RNA. Prior to the use of these clinical materials for research purposes, written consents from all patients and approval of the Hospital Ethic Review Committees were obtained.
Cell lines
Six CRC cell lines (HCT116, LoVo, RKO, SW620, SW480 and HT29) were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). The human colonic epithelial cell line HCoEpiC was obtained from American Type Culture Collection (Manassas, VA). They were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) in humidified air at 37°C with 5% CO2. All media were supplemented with 10% fetal bovine serum (10% FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin (Invitrogen, Shanghai, China).
RNA extraction and qRT-PCR assays
Total RNA was extracted from tissues or cultured cells using TRIZOL reagent (Invitrogen). For qRT-PCR, RNA was reverse transcribed to cDNA by using a Reverse Transcription Kit (Takara, Dalian, China). Real-time PCR analyses were performed with SYBR Premix Ex Taq (Takara, Dalian China). Results were normalized to the expression of GAPDH. The sequence of the primers were as following: HOXA-AS2 (Forward: 5’-CCCGTAGGAAGAACCGATGA-3’, Reverse: 5’-TTTAGGCCTTCGCAGACAGC-3’) and GAPDH (Forward: 5’-GGGAGCCAAAAGGGTCAT-3’, Reverse: 5’-GAGTCCTTCCACGATACCAA-3’). The qRT-PCR assays were conducted on an ABI 7500, and data collected with this instrument. Our qRT-PCR results were analyzed and expressed relative to threshold cycle (CT) values, and then converted to fold changes.
Transfection
The small interfering RNA-HOXA-AS2 (si-HOXA-AS2) or pcDNA-HOXA-AS2 were transfected into cells using Lipofectamine® 2000 Reagent (Thermo Fisher Scientific, USA) in a 6-well cell culture plate following to the manufacturer’s instructions. Plasmid vectors (sh-HOXA-AS2 and empty vector) for transfection were extracted by DNA Midiprep kit (Qiagen, Hilden, Germany). The full-length complementary DNA of HOXA-AS2 was synthesized by Realgene (Nanjing, China) and subcloned into the pcDNA3.1 (+) vector (Invitrogen) according to the manufacturer’s instructions. At 48 h post-transfection, cells were harvested for qRT-PCR or western blot analysis.
Cell counting kit-8 assay
Cell proliferation was monitored by the Cell Counting Kit-8 (CCK8) assay (Promega) every 24 h following the manufacturer’s protocol. In brief, the transfected cells were plated in 96-well plates (3000 cells/well), and then 10 µl of CCK8 solution was added and incubated for 2 h. Each solution was measured spectrophotometrically at 450 nm.
Colony formation assay
Cells (0.5 × 103 cells per well) were seeded in a six-well plate and cultured for 10 days after treatment. Colonies were then fixed with 10% formaldehyde for 10 min and stained for 5 min with 0.5% crystal violet. Then the number of colonies was counted using ImageJ and images were taken under Olympus microscope (Tokyo, Japan).
Flow cytometric analysis
Cells were harvested directly or 48 h after siRNA transient transfection and washed with ice-cold phosphate-buffered saline (PBS). The PI/RNase staining kits (Multisciences, Hangzhou, China) and annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kits (KeyGEN Biotech, Nanjing, China) were used to detect cell cycle and apoptosis in a FACScan instrument (Becton Dickinson, Mountain View, CA, USA), respectively.
Transwell migration/invasion assay
Transwell chamber was used to measure cell migration and invasion abilities. In brief, culture inserts with 8-mm pore size (Transwell; Corning, NY) were placed into 24-well plates. Before the measurement of invasion ability, the plates were pre-coated with matrigel. 2 h before the addition of matrigel, 500 µL of serum-free medium was independently added to the upper and lower chambers, followed by incubation at 37°C for hydration. Cells were digested by typsin, and resuspended in serum-free medium. The cell density was adjusted to 1 × 105/mL. Then, 200 µL of cell suspension was added into the upper chamber, and 500 µL of DMEM containing 10% FBS into the lower chamber. After incubation at 37°C with 5% CO2 for 24 h, the Transwell chamber was removed, cells were washed with 1 × PBS, fixed in paraformaldehyde for 20 min, and then stained with 0.1% crystal violet for 20 min. The cotton swab was used to clean the non-migrated cells in the upper chamber, cells migrating through the membrane were counted in 5 randomly selected fields under a microscope (Nikon) at a magnification of × 100.
Western blot assay and antibodies
Cell protein lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to 0.22-μm nitrocellulose (NC) membranes (Sigma, USA), and incubated with specific antibodies. Autoradiograms were quantified by densitometry (Quantity One software; Bio-Rad). The primary antibodies including anti E-cadherin, anti-N-cadherin, anti-Vimentin (Santa Cruz Bio-technology, Santa Cruz, CA, USA), and anti-β-actin antibody (Cell Signaling Technology) were purchased from Cell Signaling Technology (Boston, MA, USA).
Statistical analysis
All statistical analyses were performed using SPSS 17 software (SPSS, USA). All experiments were performed in triplicate and analyzed for significant differences between two groups were analyzed using Student’s t test; One-way ANOVA was performed to analyze the more than two groups. P < 0.05 was considered statistically significant. The data are showed as means ± SD.
Results
HOXA-AS2 was upregulated in CRC tissues and cell lines
We first investigated expression of HOXA-AS2 by qRT-PCR in a cohort of 60 CRC patients. HOXA-AS2 was upregulated in most CRC tissues (53 of 60 patients) compared with adjacent non-tumor tissues (P < 0.01; Figure 1A). Then we analyzed the association between HOXA-AS2 expression and clinicopathological features of CRC patients. We found that HOXA-AS2 expression was closely related to increased tumor size, advanced TNM stage and lymph node metastasis in the CRC patients.
Figure 1.
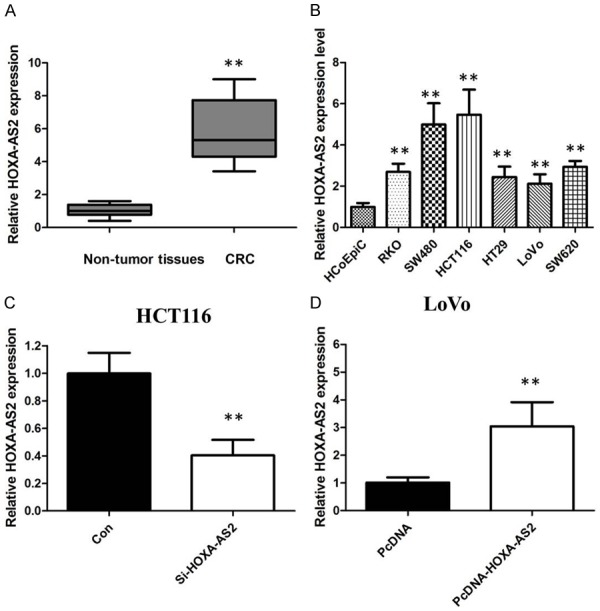
A. HOXA-AS2 was detected in CRC tissues and adjacent noncancerous tissues by qRT-PCR. B. qRT-PCR showing expression level of HOXA-AS2 in CRC cell lines. C. We employed siRNA to enhance efficiency of HOXA-AS2 knockdown in CRC cell lines. D. We employed expressing plasmid to enhance efficiency of HOXA-AS2 overexpression in CRC cell lines.
The qRT-PCR was also conducted to determine the level of HOXA-AS2 in CRC cells (HCT116, LoVo, RKO, SW620, SW480 and HT29) and the control cells HCoEpiC. HOXA-AS2 was significantly up-regulated in these CRC cells compared to that in the control (Figure 1B). In addition, the highest level of HOXA-AS2 was found in HCT116 cells, and LoVo cells expressed the lowest HOXA-AS2.
HOXA-AS2 promotes CRC cell proliferation
HCT116 and LoVo cells were chosen for further mechanistic studies. We transfected LoVo cells with lentivirus to generate cell lines that stably expressed the empty vector and full-length HOXA-AS2 transcript. HOXA-AS2 expression was increased in LoVo cell lines compared with the negative control following transfection with pCDNA-HOXA-AS2. We down-regulated the HOXA-AS2 expression using siRNA or a negative control in HCT116 cells. The qRT-PCR assay was used to confirm the efficacy of over-expression and knockdown (Figure 1C and 1D).
Thereafter, we examined the effect of HOXA-AS2 on the proliferation and colony formation of CRC cell lines. CCK8 and colony formation assays demonstrated that HOXA-AS2 knockdown significantly inhibited cell proliferation and colony formation ability in HCT116 cells. Compared to control cells, overexpression of HOXA-AS2 significantly decreased the cell proliferation and colony formation abilities of LoVo cells (Figure 2A-D).
Figure 2.
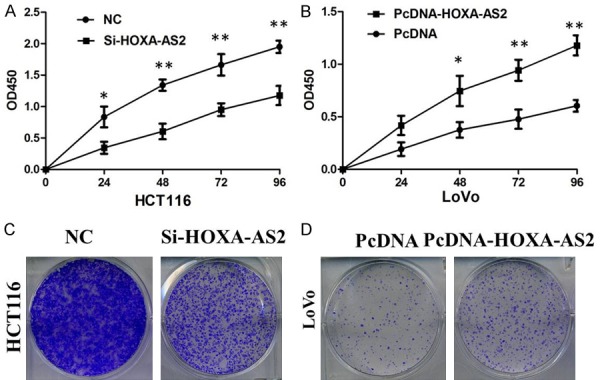
A. CCK8 assay showing knockdown of HOXA-AS2 inhibited cell proliferation of HCT116 cells. B. CCK8 assay showing overexpreesion of HOXA-AS2 promoted cell proliferation of LoVo cells. C. Colony formation assay showing knockdown of HOXA-AS2 inhibited cell proliferation of HCT116 cells. D. Colony formation assay showing overexpreesion of HOXA-AS2 promoted cell proliferation of LoVo cells.
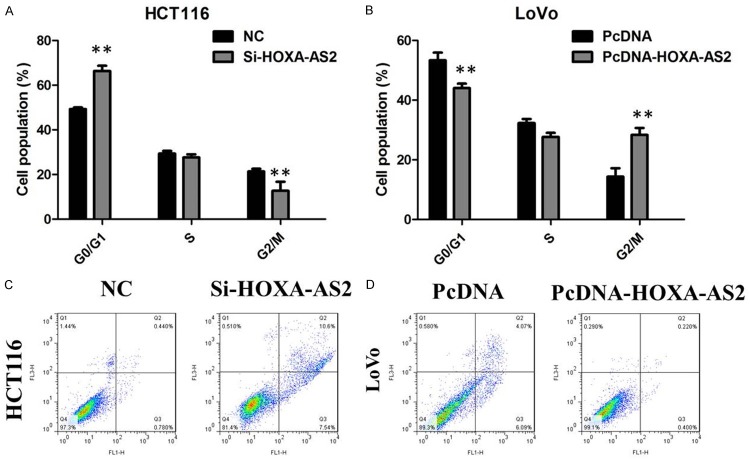
Besides, flow cytometry was used following transfection to assess cell cycle distribution. The data demonstrated that the cell population in the G0/G1 phase was expanded but the S phase population was limited after knockdown of HOXA-AS2 in HCT116 cells compared with the negative group cells (Figure 3A). On the other hand, overexpression of HOXA-AS2 in LoVo cells led to a increase in the number of cells in the S-phase and an decrease in the percentage of cells in the G0/G1 phase, further indicating that inhibition of HOXA-AS2 may restrain cancer cell growth by regulating the cell cycle (Figure 3B).
Figure 3.
A. HCT116 cells transfected with si-HOXA-AS2 all had cell-cycle arrest at the G1-G0 phase compared with cells transfected with si-NC. B. Overexpression of HOXA-AS2 in LoVo cells led to a increase in the number of cells in the S-phase and an decrease in the percentage of cells in the G0/G1 phase. C. Knockdown of HOXA-AS2 induced cell apoptosis of HCT116 cells. D. Overexpression of HOXA-AS2 cell suppressed apoptosis of LoVo cells.
HOXA-AS2 inhibited apoptosis of CRC cells
After transfection, cells were stained with annexin V and propidium iodide (PI), followed by detection using flow cytometry. The results showed that knockdown of HOXA-AS2 can obviously induce cell apoptosis of HCT116 cells (Figure 3C). In addition, overexpression of HOXA-AS2 cell suppressed apoptosis of LoVo cells (Figure 3D).
HOXA-AS2 promoted migration and invasion of CRC cells
To further probe the influence of HOXA-AS2 on CRC cell migration and invasion, we conducted transwell assays in HCT116 and LoVo cells. In the migration and invasion assays, down-regulation of HOXA-AS2 obviously impaired HCT116 cells migration and invasion ability compared with the empty vector group (Figure 4A). Overexpression of HOXA-AS2 promoted migration and invasion of LoVo cells (Figure 4B).
Figure 4.
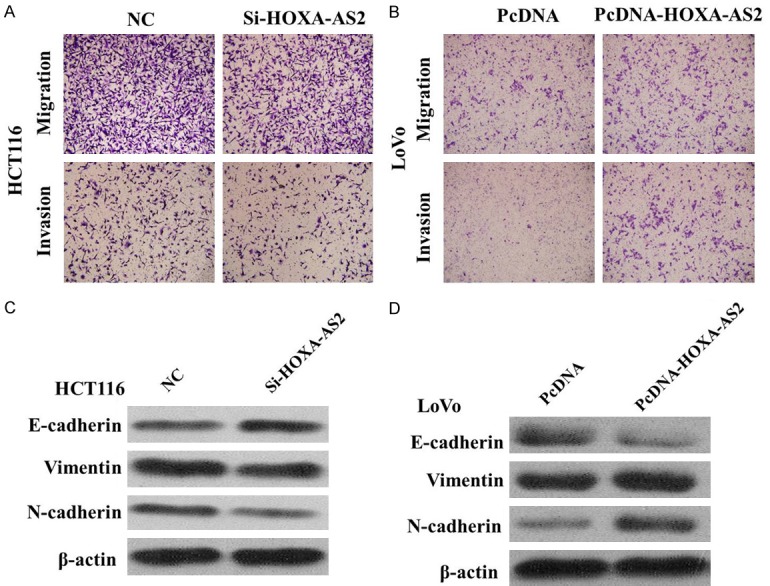
A. Inhibition of Migration and Invasion of HCT116 cells by HOXA-AS2 siRNA. B. Overexpreesion of HOXA-AS2 promoted Migration and Invasion of LoVo cells. C. Knockdown of HOXA-AS2 reverses EMT in HCT116 cells. D. Overexpreesion of HOXA-AS2 induces EMT in LoVo cells.
Silencing HOXA-AS2 attenuates EMT signaling pathway in CRC cells
In order to elucidate the mechanism by which HOXA-AS2 regulates CRC cell proliferation, migration, and invasion, Western blot assays were performed. The results showed that E-Cadherin protein expression was upregulated, and vimentin and N-cadherin protein expression were downregulated in the cells transfected with the HOXA-AS2 siRNA (Figure 4C and 4D). Therefore, inhibition of HOXA-AS2 in CRC cells changed the cell morphology from a mesenchymal to a more epithelial phenotype.
Discussion
In recent years, the role of lncRNAs in tumor development and progression gained substantial attention. Emerging evidence reveal that lncRNAs may play critical roles in tumor formation and progression [12,13]. HOXA-AS2 was previously shown to be an apoptosis repressor in all trans retinoic acid treated NB4 promyelocytic leukemia cells [11]. Xie et al. recently found that the expression of lncRNA HOXA-AS2 were higher in gastric cancer tissues and cell lines. HOXA-AS2 promotes gastric cancer cells proliferation by epigenetically silencing p21, PLK3 and DDIT3 expression [10]. Other researchers also discovered that knockdown of HOXA-AS2 significantly inhibited hepatocellular carcinoma cell proliferation and invasion and resulted in an increase of apoptosis, indicating the promotion mechanism of HOXA-AS2 in hepatocellular carcinoma [9]. However, little is known concerning the potential role of HOXA-AS2 in CRC development and progression.
In the current study, we examined HOXA-AS2 expression in CRC tissues and cell lines, and investigated the role of HOXA-AS2 in the regulation of tumor cell proliferation and invasion in vitro. We found that HOXA-AS2 was overexpressed in tumor tissues and HOXA-AS2 expression was associated increased tumor size and an advanced pathological stage. Knockdown of HOXA-AS2 expression suppressed tumor cell proliferation, migration, and invasion, arrested cells at the G0/G1 phase of cell cycle, and induced apoptosis.
Our results demonstrated that HOXA-AS2 affects the motility of CRC cells. Transwell assays suggested that HOXA-AS2 knockdown inhibits the migratory and invasive ability of CRC cells. To investigate the mechanism underlying the migration process, we measured the protein level of EMT markers following downregulationof HOXA-AS2 expression. EMT is a conserved cellular process in which epithelial tumor cell lacks its polarity and transforms into a mesenchymal phenotype [14,15]. The feature of EMT occurrence is that the epithelial marker E-cadherin is downregulated and mesenchymal marker, like N-cadherin, is upregulated [16]. Our results suggest that HOXA-AS2 knockdown decreased the expression of vimentin and N-cadherin in CRC, increased the expression of E-Cadherin, partly explaining the metastasis-related mechanism in this disease.
In conclusion, our study showed that lncRNA HOXA-AS2 expression is up-regulated in CRC tissues and cells. Knockdown of HOXA-AS2 exerted tumor-suppressive functions through reducing cell proliferation and inducing cell apoptosis. Furthermore, HOXA-AS2 mediated the oncogenic effects is partially through EMT pathway. Our findings further the understanding of CRC pathogenesis, and facilitate the development of lncRNA-directed diagnostics and therapeutics against this disease.
Acknowledgements
This work was supported by the Natural Science Foundation of Guangdong Grant No. 2016A030313381, 2016A030310242); The Development of Strategic Emerging Industry Fund of Shenzhen (Grant No. JCYJ20150529151839281); the Technology Research and Development Fund in Shenzhen (Grant No.JCYJ20150403091443271 and JCYJ20150403091443300); The National Natural Science Foundation project of China (Grant No. 81602489).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 3.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 4.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 5.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu MD, Qi P, Du X. Long non-coding RNAs in colorectal cancer: implications for pathogenesis and clinical application. Mod Pathol. 2014;27:1310–1320. doi: 10.1038/modpathol.2014.33. [DOI] [PubMed] [Google Scholar]
- 7.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Hu X, Feng Y, Zhang D, Zhao SD, Hu Z, Greshock J, Zhang Y, Yang L, Zhong X, Wang LP, Jean S, Li C, Huang Q, Katsaros D, Montone KT, Tanyi JL, Lu Y, Boyd J, Nathanson KL, Li H, Mills GB, Zhang L. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell. 2014;26:344–57. doi: 10.1016/j.ccr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F, Yang H, Deng Z, Su Y, Fang Q, Yin Z. HOX antisense lincRNA HOXA-AS2 promotes tumorigenesis of hepatocellular carcinoma. Cell Physiol Biochem. 2016;40:287–296. doi: 10.1159/000452545. [DOI] [PubMed] [Google Scholar]
- 10.Xie M, Sun M, Zhu YN, Xia R, Liu YW, Ding J, Ma HW, He XZ, Zhang ZH, Liu ZJ, Liu XH, De W. Long noncoding RNA HOXA-AS2 promotes gastric cancer proliferation by epigenetically silencing P21/PLK3/DDIT3 expression. Oncotarget. 2015;6:33587–601. doi: 10.18632/oncotarget.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao H, Zhang X, Frazão JB, Condino-Neto A, Newburger PE. HOX antisense lincRNA HOXAAS2 is an apoptosis repressor in all trans retinoic acid treated NB4 promyelocytic leukemia cells. J Cell Biochem. 2013;114:2375–83. doi: 10.1002/jcb.24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839:1097–1109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Han D, Wang M, Ma N, Xu Y, Jiang Y, Gao X. Long noncoding RNAs: novel players in colorectal cancer. Cancer Lett. 2015;361:13–21. doi: 10.1016/j.canlet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 16.Fang Z, Xu C, Li Y, Cai X, Ren S, Liu H, Wang Y, Wang F, Chen R, Qu M, Wang Y, Zhu Y, Zhang W, Shi X, Yao J, Gao X, Hou J, Xu C, Sun Y. A feed-forward regulatory loop between androgen receptor and PlncRNA-1 promotes prostate cancer progression. Cancer Lett. 2016;374:62–74. doi: 10.1016/j.canlet.2016.01.033. [DOI] [PubMed] [Google Scholar]