Abstract
This study tested the hypothesis that combination therapy using extracorporeal shock wave (ECSW)-melatonin (Mel) was superior to either alone at ameliorating neuropathic pain (NP). NP was induced by chronic constriction injury (CCI) to the left sciatic nerve in rats. Animals were categorized into sham control (group 1), CCI only (group 2), CCI-ECSW (group 3), CCI-Mel (group 4) and CCI-ECSW-Mel (group 5). By days 2 and 8 after CCI, the mechanical paw withdrawal threshold (MPWT)/thermal paw withdrawal latency (TPWL) were highest in group 2, lowest in group 1, significantly lower in group 5 than in groups 3 and 4 (all p<0.0001), and not significantly different between groups 3 and 4. The protein expressions of inflammatory (TNF-α/NF-κB/MMP-9/IL-1ß/GFAP/ox42), oxidative-stress (NOX-1/NOX-2/NOX-4/oxidized protein), DNA/mitochondrial-damaged (γ-H2AX/cytosolic mitochondria), apoptotic (cleaved capase-3/PARP), and MAPK family biomarkers (p-P38/p-JNK/p-ERK1/2) in dorsal root ganglia and spinal dorsal horn expressed a similar pattern of MPWT/TPWL among the five groups, except for significantly higher in group 4 than in group 3 (all p<0.0001). The protein expressions of Nav.1.3, Nav.1.8 and Nav.1.9 in sciatic nerve displayed an identical pattern to inflammation among the five groups (all p<0.001). Pain facilitated cellular expressions (p-P38+/peripherin+ cells, P38+/NF200+ cells) displayed an identical pattern to inflammation among the five groups (all p<0.0001). In conclusion, ECSW-Mel combination therapy markedly ameliorated NP induced by CCI.
Keywords: Neuropathic pain, chronic constriction injury, inflammation, oxidative stress, extracorporeal shock wave, melatonin
Introduction
Neuropathic pain (NP) disorders are common and have significant psychological and functional effects on individuals, leading to wider socioeconomic impact [1,2]. NP typically results from lesions or diseases involving the peripheral nerve, dorsal root ganglion, dorsal root, or central nervous system [3]. Despite advanced pharmaceuticals for NP such as tricyclic antidepressants, anticonvulsants, calcium channel ligands and topical lidocaine [4-6], many patients continue to experience refractory pain. There thus remains a need for a new safe and effective treatment modality for this unresolved problem.
The hallmarks of NP are peripheral and central sensitization, which arise through various complex pathophysiological mechanisms making the disorder difficult to treat [3]. Studies have consistently identified that persistent inflammation [7-9], oxidative stress [10-12], inflammatory cell infiltration and cytokine production [7-9] in the damaged/inflammatory tissue and organ, play central roles for the initiation and propagation of NP. A management strategy involving (1) anti-inflammation, (2) suppression of oxidative stress and (3) relief from pain without significant side effects would thus seem ideal.
Extracorporeal shock wave (ECSW) treatments were originally used to fragment painful bodycalculi. However, its application for treating soft tissue pain is gaining interest since significant pain relief has been reported even in circumstances where the symptomatic calcium deposits have not successfully been disintegrated [13]. ECSW therapy is known to suppress musculoskeletal pain and tendinopathyor fasciitis, implicating a role in attenuating certain inflammatory and pain disorders [14-16]. We have additionally shown that ECSW therapy possesses anti-inflammatory, anti-oxidative, anti-apoptotic, and neuroprotective capacities in the settings of diabetic neuropathy [17] and ischemic organ dysfunction [18,19]. Separately, melatonin, an indole hormone secreted mainly by the pineal gland, appears to play important roles in pain relief [20-23], maintenance of cell membrane stability, and enhancing cell survival within stressed environments mainly by reducing cellular susceptibility to oxidative stress and free radical damage, and by suppressing the inflammatory reaction [20,23-28].
Combining therapies with discrete neuroprotective, anti-inflammatory and antioxidant capabilities may have additive benefits to those already provided by current NP treatments [29]. Combination therapies often have synergistic effects, offering greater than expected benefits for patients with intractable medical conditions. Based on the aforementioned reports [14,15,17-21,23,25], this experimental study tested the hypothesis that combined therapy using ECSW and melatonin may be superior to either alone for offering antinociceptive effects in a rat model of NP.
Materials and methods
Ethics
All animal experimental procedures were approved by the Institute of Animal Care and Use Committee at Kaohsiung Chang Gung Memorial Hospital (Affidavit of Approval of Animal Use Protocol No. 2015051303) and performed in accordance with the Guide for the Care and Use of Laboratory Animals [The Eighth Edition of the Guide for the Care and Use of Laboratory Animals (NRC 2011)].
Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-approved animal facility in our hospital with controlled temperature and light cycles (24°C and 12/12 light cycle).
Animal model of neuropathic pain
Left sciatic mononeuropathies in rats were induced using the chronic constriction injury (CCI) procedure as previously described [30]. Under adequate isoflurane anesthesia, the left sciatic nerve was surgically dissected and exposed. Four chromic gut ties (4-0) were used to ligate the nerve at 1 mm intervals loosely enough so that, under microscope inspection, the epineural blood circulation was not obstructed. The incision was then closed in layers and animals recovered from anesthesia. Sham surgery involved dissecting and exposing the sciatic nerve without performing ligations.
Animal grouping and treatment strategy
Pathogen-free, adult male Sprague-Dawley (SD) rats (n = 40) weighing 325-350 g (Charles River Technology, BioLASCO Taiwan Co. Ltd., Taiwan) were randomly divided into five groups: group 1 [sham control (SC), i.e., sciatic nerve exposure without ligatures], group 2 (CCI only), group 3 [CCI + ECSW (0.12 mJ/mm2, 200 impulses/time at post-CCI 3 h, day 3, and 7, skin surface above the femoral areas)], group 4 [CCI + melatonin (50 mg/kg at post-CCI 3 h and 20 mg/kg at post-CCI 18/48 h, intra-peritoneal), and group 5 (CCI + ECSW + melatonin). ECSW energy and melatonin dosages used for treating CCI-induced neuropathic pain were based on our previous reports [17,25,31-33].
Behavioral assessments
To elucidate the impact of ECSW-melatonin therapy on suppressing the neuropathic pain at acute and subacute stages, thermal and mechanical nociceptive thresholds were measured before CCI and on post-CCI days 2 and 8. To assess for thermal hyperalgesia, the animal was placed on a glass plate and radiant heat (Plantar Test Apparatus; UgoBasile, Italy) was applied to the plantar surface of the operated hind paw. The withdrawal latency and duration were recorded, with a minimum value set at 0.1 s and a cut-off latency set at 30 s to avoid paw injury. Each rat was tested three times at an interval of 5 min, and mean values were used in the analysis.
To assess for mechanical allodynia, the animal was placed in a chamber and a servo-controlled mechanical stimulus (Dynamic Plantar Aesthesiometer; UgoBasile, Italy) was applied to the plantar surface of the operated hind paw repeatedly at 5-min intervals with increasing punctate pressure until the rat withdrew its paw.A maximal cut-off value was set at 50 g to prevent paw damage. The threshold was tested thrice for each time point andmean values were used in the analysis.
Western blot analysis
The ipsilateral sciatic nerve, L4-L5 dorsal root ganglia (DRGs) and corresponding dorsal horn of the spinal cord from the rats of the sham control and experimental groups were harvested as previously described [25,33-36]. Equal amounts (50 µg) of protein extracts were loaded and separated by SDS-PAGE using 8-12% acrylamide gradients. After electrophoresis, the separated proteins were transferred electrophoretically to a polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences). Nonspecific sites were blocked by incubation of the membrane in blocking buffer [5% nonfat dry milk in T-TBS (TBS containing 0.05% Tween 20)] overnight. The membranes were incubated with the indicated primary antibodies [cleaved caspase 3 (1:1000, Cell Signaling), cleaved poly (ADP-ribose) polymerase (PARP) (1:1000, Cell Signaling), cytosolic cytochrome C (1:2000, Millipore), NADPH oxidase (NOX)-1 (1:2000, Sigma), NOX-2 (1:750, Sigma), NOX-4 (1:1000, Abcam),interleukin (IL)-1β (1:1000, Cell Signaling), tumor necrosis factor (TNF)-α (1:1000, Cell Signaling), nuclear factor (NF)-κB (1:600, Abcam), matrix metalloproteinase (MMP)-9 (1:3000, Abcam), phosphorylated histone H2AX (γ-H2AX) (1/1000, Abcam), phosphorylated (p)-p38 (1:1000, Cell Signaling), p-JNK (1:1000, Abcam), p-ERK1/2 (1:1000, Abcam), Nav 1.3 (1:200, Alomone Labs), Nav 1.8 (1:2000, Abcam), Nav 1.9 (1:200, Alomone Labs) and actin (1:10000, Millipore)] for 1 hour at room temperature. Horseradish peroxidase-conjugated anti-rabbit immunoglobulin IgG (1:2000, Cell Signaling) was used as the secondary antibody for onehour incubation at room temperature. The washing procedure was repeated eight times within an hour, and immunoreactive bands were visualized by enhanced chemiluminescence (ECL; Amersham Biosciences) after exposure to Biomax L film (Kodak). For quantification, ECL signals were digitized using Labwork software (UVP).
Immunofluorescent (IF) staining
IF staining proceeded as our previously reported [37,38]. Rehydrated paraffin sections were first treated with 3% H2O2 for 30 minutes and incubated with Immuno-Block reagent (BioSB, Santa Barbara, CA, USA) for 30 minutes at room temperature. Sections were then incubated with primary antibodies specifically against p-p38 (1:500, Gene Tex), NF-200 (7.5 µg, Abcam), and peripherin (1:1000, Abcam). Sections incubated with irrelevant antibodies served as controls. Three sections of DRG specimens were analysed in each rat. For quantification, three randomly selected high power fields (HPFs) were analysed per section. The mean number of positively-stained cells per HPF for each animal was determined across all nine HPFs.
Oxidative stress reaction inlung parenchyma
The procedure for assessing protein expression of oxidative stress has previously been described [17,25,39], using the Oxyblot Oxidized Protein Detection Kit (Chemicon S7150, Billerica, MA, USA). For quantification, ECL signals were digitized using Labwork software (UVP, Waltham, MA, USA).
Statistical analysis
Quantitative data are expressed as mean ± SD. Statistical analysis was performed by ANOVA, followed by Bonferroni multiple-comparison post hoc test. Statistical analysis was also performed using SPSS (SPSS for Windows, version 13; SPSS, IL, U.S.A.). The threshold for statistical significance was considered P<0.05.
Results
Mechanical paw withdrawal threshold (MPWT) and thermal paw withdrawal latency (TPWL) at days 2 and 8 after CCI (Figure 1)
Figure 1.
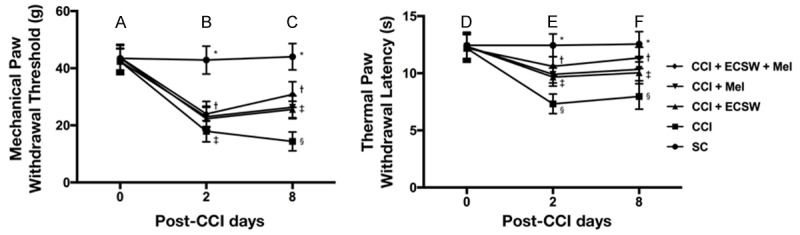
MPWT and TPWL at days 2 and 8 in an experimental model of CCI. Left Panel: A. By day 0, the analytical results of MPWT did not differ among the five groups. B. By day 2, the analytical results of MPWT, * denotes statistical significance vs. other groups with different symbols (†, ‡), p<0.0001. The symbol † indicated CCI + ECSW, CCI + Mel and CCI + ECSW + Mel groups. C. By day 8, the analytical results of MPWT, * denotes statistical significance vs. other groups with different symbols (†, ‡, §), p<0.0001. The symbol ‡ indicated CCI + ECSW and CCI + Mel groups. Right Panel: D. By day 0, the analytical results of MPWT did not differ among the five groups. E. By day 2, the analytical results of TPWL, * denotes statistical significance vs. other groups with different symbols (†, ‡, §), p<0.0001. The symbol ‡ indicated CCI + ECSW and CC I + Mel groups. F. By day 8, the analytical results of TPWL, * denotes statistical significance vs. other groups with different symbols (†, ‡, §), p<0.0001. The symbol ‡ indicated CCI + ECSW and CCI + Mel groups. All statistical analyses were performed by one-way ANOVA and Bonferroni multiple comparison post-hoc test (n = 8 for each group). Symbols (*, †, ‡, §) indicate statistical significance. MPWT = mechanical paw withdrawal threshold; TPWL = thermal paw withdrawal latency; SC = sham control; CCI = chronic constriction injury; ECSW = extracorporeal shock wave; Mel = melatonin.
By day 2 after CCI, MPWT was significantly reduced in group 2 (CCI), group 3 (CCI-ECSW), group 4 (CCI-Mel) and group 5 (CCI-ECSW-Mel) compared to group 1 (SC), significantly reduced in group 2 than in groups 3 to 5, and not significantly different amongst groups 3 to 5. By day 8 after CCI, MPWT was significantly reduced in groups 2 to 5 compared to group 1, significantly reduced in groups 3 and 4 than group 5, more significantly reduced in group 2 than in group 5, but not significantly different between groups 3 and 4. This suggested that combined therapy was superior to either one alone for reducing MPWT.
By day 2 after CCI procedure, TPWL was significantly highest in group 1, lowest in group 2, significantly lower in groups 3 and 4 than in group 5, and not significantly different between groups 3 and 4. By day 8 after CCI procedure, TPWL showed an identical pattern among the five groups.
The protein expressions of sciatic nerve- and L4-5 DRGs-voltage-gated sodium channels and inflammatory biomarkers in L4-5 DRGs by day 8 after CCI (Figure 2)
Figure 2.
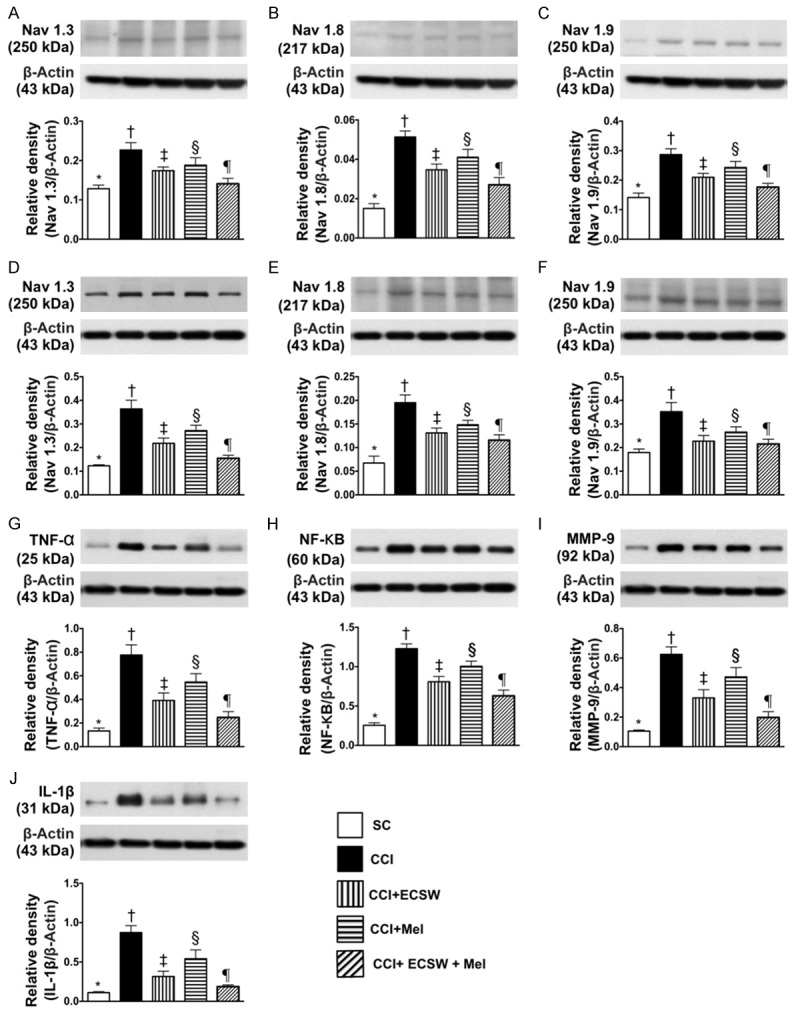
The protein expressions of sciatic nerve- and L4-5 dorsal root ganglions (DRGs)-voltage-gated sodium channels and the protein expressions of inflammatory biomarkers in L4-5 DRGs by day 8 after CCI. A. Protein expression of Nav.1.3 in sciatic nerve, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.001. B. Protein expression of Nav.1.8 in sciatic nerve, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.001. C. Protein expression of Nav.1.9 in sciatic nerve, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.001. D. Protein expression of Nav.1.3 in L4-5 DRGs, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. E. Protein expression of Nav.1.8 in L4-5 DRGs, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.001. F. Protein expression of Nav.1.9 in L4-5 DRGs, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.001. G. Protein expressions of tumor necrosis factor (TNF)-α, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. H. Protein expression of necrosis factor (NF)-κB, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. I. Protein expression of matrix metalloproteinase (MMP)-9, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. J. Protein expression of interleukin (IL)-1ß, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 8 for each group). Symbols (*, †, ‡, §, ¶) indicate significance at the 0.05 level. SC = sham control; CCI = chronic constriction injury; ECSW = extracorporeal shock wave; Mel = melatonin.
The protein expressions of Nav.1.3, Nav.1.8 and Nav.1.9, three indicators of voltage-gated sodium channels for sciatic nerve, were lowest in group 1, highest in group 2, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3. Additionally, the protein expressions of Nav.1.3, Nav.1.8 and Nav.1.9, indicators of voltage-gated sodium channels for L4-5 DRGs, exhibited an identical pattern to sciatic nerve among the five groups.
The protein expressions of TNF-α, NF-κB, MMP-9 and IL-1ß, four inflammatory biomarkers in L4-5 DRGs, were highest in group 2, lowest in group 1, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3.
Protein expressions of apoptotic, mitochondrial-damaged, DNA damaged and oxidative stress biomarkers in L4-5 DRGs by day 8 after CCI (Figure 3)
Figure 3.
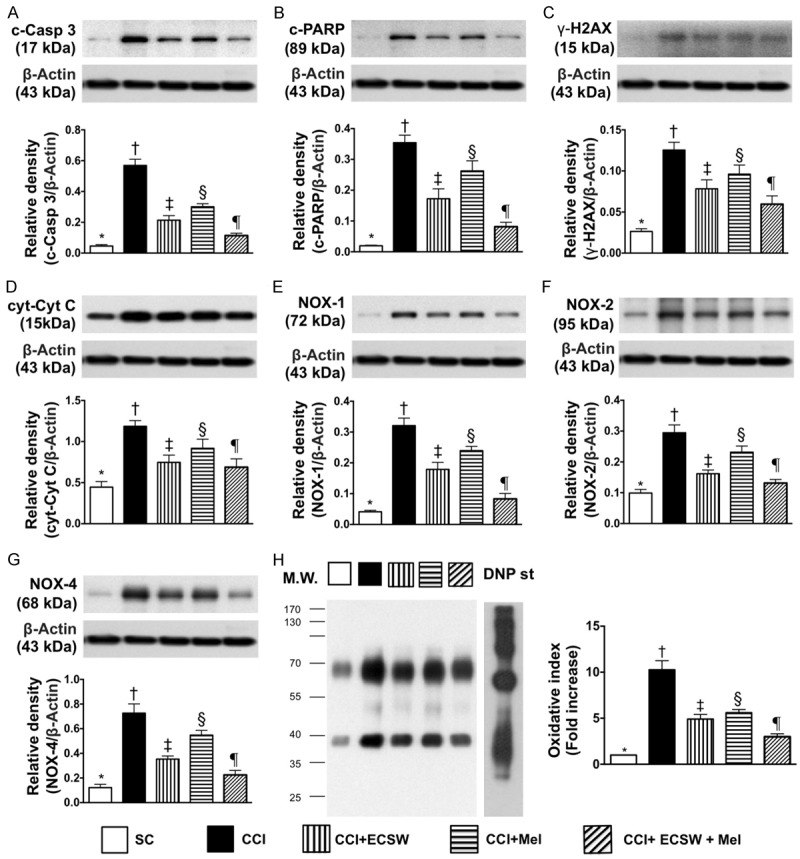
Protein expressions of apoptotic, mitochondrial-damaged, DNA damaged and oxidative-stress biomarkers in L4-5 dorsal root ganglions by day 8 after CCI procedure. A. Protein expression of cleaved caspase 3 (c-Casp 3), * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. B. Protein expression of cleaved poly (ADP-ribose) polymerase (PARP), * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. C. Protein expression of γ-H2AX, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. D. Protein expression of cytosolic cytochrome C (cyt-Cyt C), * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.001. E. Protein expression of NOX-1, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. F. Protein expression of NOX-2, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. G. Protein expression of NOX-4, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. H. Oxidized protein expression, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. (Note: left and right lanes shown on the upper panel represent protein molecular weight marker and control oxidized molecular protein standard, respectively). M.W = molecular weight; DNP = 1-3 dinitrophenylhydrazone. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 8 for each group). Symbols (*, †, ‡, §, ¶) indicate significance at the 0.05 level. SC = sham control; CCI = chronic constriction injury; ECSW = extracorporeal shock wave; Mel = melatonin.
The protein expression of cleaved caspase 3 and cleaved PARP, two indicators of apoptosis, were highest in group 2, lowest in group 1, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3. Additionally, the protein expressions of γ-H2AX, an indicator of DNA damage, and cytosolic cytochrome C, an indicator of mitochondrial-damage, exhibited an identical pattern to apoptosis among the five groups.
The protein expressions of NOX-1, NOX-2, NOX-4 and oxidized protein, four indicators of oxidative stress, were highest in group 2, lowest in group 1, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3.
Protein expressions of signaling transduction molecules in L4-5 DRGs and inflammatory, apoptotic, mitochondrial-damaged and DNA damaged biomarkers in spinal dorsal horn (SDH) by day 8 after CCI (Figure 4)
Figure 4.
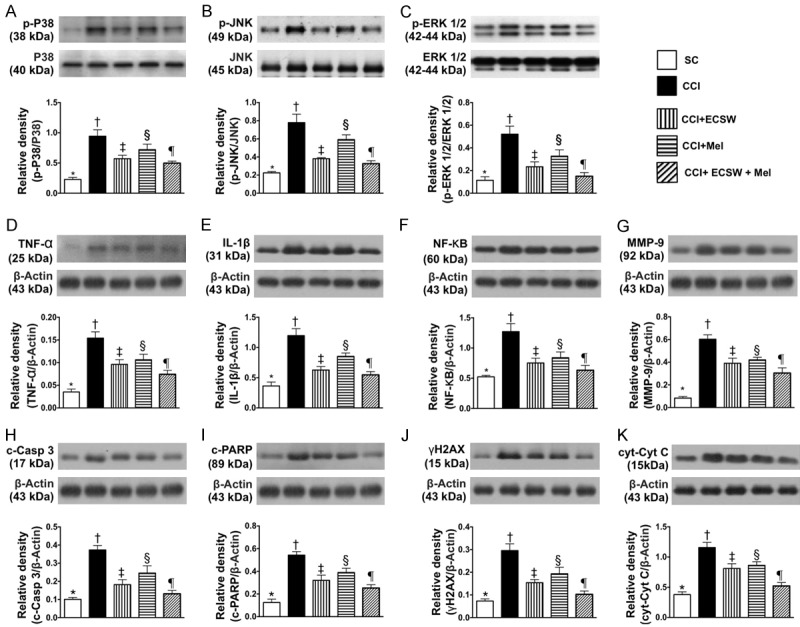
Protein expressions of signaling transduction molecules in L4-5 dorsal root ganglions (DRGs) and inflammatory, apoptotic, mitochondrial-damaged and DNA damaged biomarkers inspinal dorsal horn (SDH) by day 8 after CCI procedure. A. Protein expression of phosphorylated (p)-p38 in L4-5 DRGs, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. B. Protein expression of p-JNK in L4-5 DRGs, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. C. Protein expression of p-ERK1/2 in L4-5 DRGs, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. D. Protein expression of tumor necrosis factor (TNF)-α in SDH, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. E. Protein expression of interleukin (IL)-1ß in SDH, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. F. Protein expression of nuclear factor (NF)-κB in SDH, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. G. Protein expression of matrix metalloproteinase (MMP)-9 in SDH, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. H. Protein expression of cleaved caspase 3 (c-Casp 3) in SDH, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. I. Protein expression of cleaved poly (ADP-ribose) polymerase (c-PARP) in SDH, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. J. Protein expression of γ-H2AX in SDH, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. K. Protein expression of cytosolic cytochrome C (cyt-Cyt C) in SDH, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 8 for each group). Symbols (*, †, ‡, §, ¶) indicate significance at the 0.05 level. SC = sham control; CCI = chronic constriction injury; ECSW = extracorporeal shock wave; Mel = melatonin.
The protein expression of p-p38, p-JNK, p-ERK1/2 in L4-5 DRGs, three indicators of extracellular signal-regulated kinases (i.e., MAPK family) for response to stress stimulations, were lowest in group 1, highest in group 2, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3.
The protein expressions of TNF-α and IL-1ß in SDH, two inflammatory biomarkers, were highest in group 2, lowest in group 1, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3. Additionally, protein expressions of NF-κB and MMP-9 in SDH, another two indicators of inflammation, showed a similar pattern to TNF-α among the five groups.
The protein expression of cleaved caspase 3 and cleaved PARP in SDH, two indicators of apoptosis, were highest in group 2, lowest in group 1, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3. Additionally, the protein expressions of γ-H2AX in SDH, an indicator of DNA damage, exhibited an identical pattern to apoptosis among the five groups. Furthermore, protein expression of cytosolic cytochrome C in SDH, an indicator of mitochondrial-damage, displayed a pattern similar to γ-H2AX among the five groups.
Protein expressions of oxidative stress biomarkers and signaling transduction molecules, microglia and astrocyte activity in SDH by day 8 after CCI (Figure 5)
Figure 5.
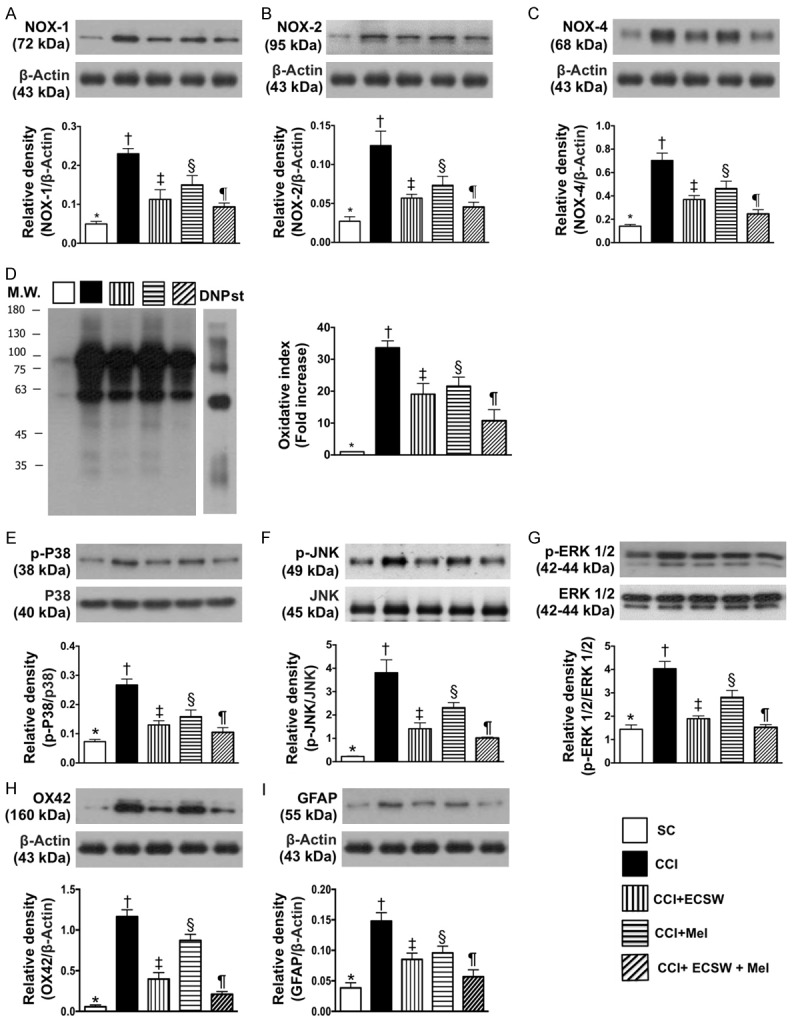
Protein expressions of oxidative stress biomarkers and signaling transduction molecules, microglia and astrocyte activity in spinal dorsal horn (SDH) by day 8 after CCI procedure. A. Protein expression of NOX-1, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. B. Protein expression of NOX-2, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. C. Protein expression of NOX-4, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. D. Oxidized protein expression, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. (Note: left and right lanes shown on the upper panel represent protein molecular weight marker and control oxidized molecular protein standard, respectively). M.W = molecular weight; DNP = 1-3 dinitrophenylhydrazone. E. Protein expression of phosphorylated (p)-p38, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. F. Protein expression of p-JNK, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. G. Protein expression of p-ERK1/2, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. H. Protein expression of ox42, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. I. Protein expression of grialfibrillary acidic protein (GFAP), * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 8 for each group). Symbols (*, †, ‡, §, ¶) indicate significance at the 0.05 level. SC = sham control; CCI = chronic constriction injury; ECSW = extracorporeal shock wave; Mel = melatonin.
The protein expressions of NOX-1, NOX-2, NOX-4 and oxidized protein, four indicators of oxidative stress, were highest in group 2, lowest in group 1, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3.
The protein expressions of p-p38, p-JNK, p-ERK1/2, three indicators of extracellular signal-regulated kinases, were lowest in group 1, highest in group 2, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3.
The neuroinflammatory protein expressions of ox42, an indicator of microglial activation, and GFAP, an indicator of astrocyte activation, displayed an identical pattern to extracellular signal-regulated kinases among the five groups.
IF microscopy for co-localization of p-P38 and peripherin in DRG neurons (Figure 6)
Figure 6.
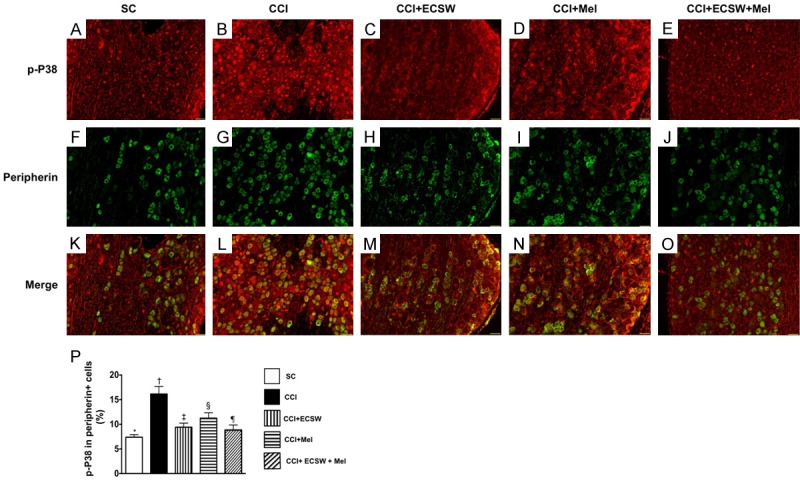
Immunofluorescent (IF) microscopic finding of colocalization of p-P38 and peripherin indorsal root ganglion (DRG) neurons. A-E. Illustrating the IF microscopic finding (200x) positively stained p-P38 in DRG neurons (red color spots). F-J. Illustrating the IF microscopic finding (200x) positively stained peripherincells (green color). K-O. Illustrating the IF microscopic finding (200x) of merged positively-stained p-P38 and peripherin (green-red colocalization). P. Analytical results of number of p-P38+/peripherin+ cells, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 8 for each group). Symbols (*, †, ‡, §, ¶) indicate significance at the 0.05 level. SC = sham control; CCI = chronic constriction injury; ECSW = extracorporeal shock wave; Mel = melatonin.
To elucidate the presence of a peripheral nerve injury,the expression of p38 MAPK activation (i.e., phosphorylated p38) was measured. IF microscopy identified that p-P38 expression in peripherin, an indicator of small unmyelinated C-fiber and thinly myelinated A-δ fiber of DRG neuronsthat transmit signals of thermal and noxious stimuli, were lowest in group 1, highest in group 2, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3.
IF microscopy for co-localization of p-P38 and NF200 in L4-5 DRG neurons (Figure 7)
Figure 7.
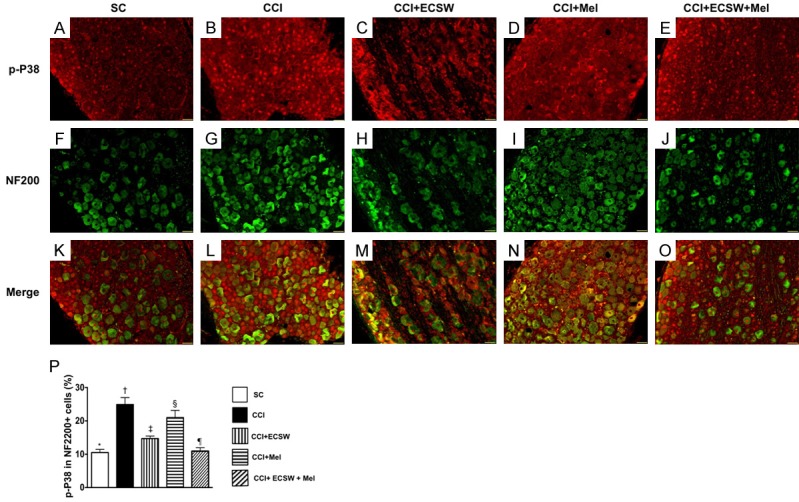
Immunofluorescent (IF) microscopic finding of colocalization of p-P38 and NF200 indorsal root ganglion (DRG) neurons. A-E. Illustrating the IF microscopic finding (200x) positively stained p-P38 in DRG neurons (red color spots). F-J. Illustrating the IF microscopic finding (200x) positively stained NF200 cells (green color). K-O. Illustrating the IF microscopic finding (200x) of merged positively-stained p-P38 and NF200 (green-red colocalization). P. Analytical results of number of p-P38+/NF200+ cells, * denotes statistical significance vs. other groups with different symbols (†, ‡, §, ¶), p<0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 8 for each group). Symbols (*, †, ‡, §, ¶) indicate significance at the 0.05 level. SC = sham control; CCI = chronic constriction injury; ECSW = extracorporeal shock wave; Mel = melatonin.
IF microscopy identified that p-P38 expression in NF200, an indicator of large myelinated A-β fiber of DRG neurons that transmit information of non-noxious mechanical stimuli as well as abnormal mechanical allodynia in the pathologic neuropathic pain, was lowest in group 1, highest in group 2, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3.
Discussion
This study investigated the impact of ECSWMel combination therapy on NP in rat and yielded several striking implications. First, MPWT and TPWL were increased in CCI animals compared to SC animals, suggesting that our experimental model was successfully created for the purpose of this study. Second, both MPWT and TPWL were significantly suppressed in CCI + ECSW-Mel animals compared to CCI only animals, and no ECSW-Mel-related complication was noted, highlighting both the safety and effectiveness of this combination treatment. Third, inflammation, oxidative stress, and MAPK family signaling pathway were found to be involved in NP and were markedly suppressed by ECSW-Mel therapy.
Clinical studies have previously shown that pain derived from the musculoskeletal system (i.e. tendonitis, plantar fasciitis, shoulder pain) were significantly inhibited by ECSW therapy [14-16]. Additionally, experimental studies have shown that Mel treatment inhibited NP [20-23]. The most important finding in the present study was that, as compared with CCI animals, the MPWT and TPWL, two standard tests for identifying the degree of NP, were found to be significantly attenuated in CCI animals treated with ECSW or Mel, and further significantly attenuated in CCI animals after receiving combination ECSW-Mel treatment. Accordingly, our findings extended those of previous studies [25,26,33,35,40,41].
The underlying mechanisms involved in NP have been keenly investigated [3,7,8,42-44] and are known to be complicated, involving persistent inflammation, generation of oxidative stress and elicitation of MAPK family signaling pathways [7-9,12,42,45-51]. A principal finding in the present was that, as compared with SC group, the inflammatory reaction and generation of oxidative stress were markedly higher in the CCI group. Additionally, the MAKP family signaling pathways (i.e., p-p38, p-JNK, p-ERK1/2) were markedly upregulated in CCI animals than in SC animals. Therefore, our findings corroborated those of previous studies [45,48,51-53]. Importantly, the present study found that either ECSW or Mel treatment could significantly suppress the inflammatory reaction and generation of oxidative stress in SDH and DRG neurons of CCI animals. Of particular importance was that combined ECSW-Mel was superior to either therapy alone at suppressing the expressions of inflammation and oxidative stress. In this way, our findings support the reports of synergism from combined therapy in previous studies [25,26,33,35,40,41], and also explain why MPWT and TPWL were substantially ameliorated in CCI animals after receiving ECSW-Mel treatment.
An association between inflammation/oxidative stress and DNA/mitochondrial damage has been well recognized [7-10,12,17,47]. Intriguingly, a link between inflammation/oxidative stress and DNA/mitochondrial damage as well as apoptotic biomarkers in the present study were notably higher in CCI animals than in SC animals. However, these biomarkers were downregulated by ECSW or Mel treatment and further downregulated by combination ECSW-Mel. Our findings, in addition toreinforcing the previous reports [25,26,33,35,40,41], implicated that ECSW-Mel therapy effectively attenuated the DNA/mitochondrial damage through the suppressing the role of inflammation/oxidative stress reaction. Our previous study demonstrated that ECSW therapy protected the sciatic nerve against diabetic-induced neuropathy [17] by inhibiting the inflammatory reaction and oxidative stress and DNA/mitochondrial damage. Accordingly, the findings of our previous study [17] were consistent with those from this current study.
Nav.1.3, Nav.1.8 and Nav.1.9 are three indicators of voltage-gated sodium channels of sciatic nerve/DRG neurons for ectopic discharges or activities in response to mechanical and thermal stimulations [54,55]. Their protein expressions in sciatic nerve/DRG neurons were markedly increased in CCI animals compared to SC animals. Additionally, the co-existing p-P38-peripherin+ and p-P38-NF200+ cells in DRG neurons were substantially higher in the CCI groups than in the SC group. Our findings are thus comparable with those from previous studies [45,46,48,53] and could, at least in part, explain why MPWT and TPWL were significantly higher in CCI animals than in SC animals. However, these two parameters were notably suppressed by ECSW or Mel treatment and further suppressed by ECSW-Mel combined treatment, highlighting that the regulation of voltage-gated sodium channels and the expressions of p-P38+/peripherin+ and p-P38+/NF200+ cells were crucial for stifling NP.
Study limitation
This study has limitations. First, although outcomes observed in the present study were promising, the study period was only eight days in duration. Therefore, long-term outcomes from the present study remain uncertain and invite further study. Second, although extensive work was done in the present study, the exact underlying mechanisms of ECSW-Mel therapy for relieving NP remain unclear.
In conclusion, the present study demonstrated that ECSW-Mel combination therapy effectively ameliorated NP in rat. These findings raise the need for a prospective clinical trial to answer whether this therapeutic option is also effective for patients with NP that is refractory to conventional therapy.
Acknowledgements
This study was supported by a program grant from Chang Gung Memorial Hospital, Chang Gung University (Grant number: CMRPG8E0771).
Disclosure of conflict of interest
None.
References
- 1.Verhaak PF, Kerssens JJ, Dekker J, Sorbi MJ, Bensing JM. Prevalence of chronic benign pain disorder among adults: a review of the literature. Pain. 1998;77:231–239. doi: 10.1016/S0304-3959(98)00117-1. [DOI] [PubMed] [Google Scholar]
- 2.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J Pain. 2004;5:317–328. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:S22–32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118:289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 7.Ellis A, Bennett DL. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111:26–37. doi: 10.1093/bja/aet128. [DOI] [PubMed] [Google Scholar]
- 8.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–814. doi: 10.2147/JPR.S53660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Komirishetty P, Areti A, Gogoi R, Sistla R, Kumar A. Poly(ADP-ribose) polymerase inhibition reveals a potential mechanism to promote neuroprotection and treat neuropathic pain. Neural Regen Res. 2016;11:1545–1548. doi: 10.4103/1673-5374.193222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komirishetty P, Areti A, Yerra VG, Ruby PK, Sharma SS, Gogoi R, Sistla R, Kumar A. PARP inhibition attenuates neuroinflammation and oxidative stress in chronic constriction injury induced peripheral neuropathy. Life Sci. 2016;150:50–60. doi: 10.1016/j.lfs.2016.02.085. [DOI] [PubMed] [Google Scholar]
- 12.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Wess OJ. A neural model for chronic pain and pain relief by extracorporeal shock wave treatment. Urol Res. 2008;36:327–334. doi: 10.1007/s00240-008-0156-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang CJ, Ko JY, Chan YS, Weng LH, Hsu SL. Extracorporeal shockwave for chronic patellar tendinopathy. Am J Sports Med. 2007;35:972–978. doi: 10.1177/0363546506298109. [DOI] [PubMed] [Google Scholar]
- 15.Wang CJ, Wang FS, Yang KD, Weng LH, Ko JY. Long-term results of extracorporeal shockwave treatment for plantar fasciitis. Am J Sports Med. 2006;34:592–596. doi: 10.1177/0363546505281811. [DOI] [PubMed] [Google Scholar]
- 16.Wang CJ. An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med J. 2003;26:220–232. [PubMed] [Google Scholar]
- 17.Chen YL, Chen KH, Yin TC, Huang TH, Yuen CM, Chung SY, Sung PH, Tong MS, Chen CH, Chang HW, Lin KC, Ko SF, Yip HK. Extracorporeal shock wave therapy effectively prevented diabetic neuropathy. Am J Transl Res. 2015;7:2543–2560. [PMC free article] [PubMed] [Google Scholar]
- 18.Yuen CM, Chung SY, Tsai TH, Sung PH, Huang TH, Chen YL, Chen YL, Chai HT, Zhen YY, Chang MW, Wang CJ, Chang HW, Sun CK, Yip HK. Extracorporeal shock wave effectively attenuates brain infarct volume and improves neurological function in rat after acute ischemic stroke. Am J Transl Res. 2015;7:976–994. [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh KH, Sheu JJ, Lin YC, Sun CK, Chang LT, Kao YH, Yen CH, Shao PL, Tsai TH, Chen YL, Chua S, Leu S, Yip HK. Benefit of combined extracorporeal shock wave and bone marrow-derived endothelial progenitor cells in protection against critical limb ischemia in rats. Crit Care Med. 2012;40:169–177. doi: 10.1097/CCM.0b013e31822d74d0. [DOI] [PubMed] [Google Scholar]
- 20.Ambriz-Tututi M, Granados-Soto V. Oral and spinal melatonin reduces tactile allodynia in rats via activation of MT2 and opioid receptors. Pain. 2007;132:273–280. doi: 10.1016/j.pain.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Ambriz-Tututi M, Rocha-Gonzalez HI, Cruz SL, Granados-Soto V. Melatonin: a hormone that modulates pain. Life Sci. 2009;84:489–498. doi: 10.1016/j.lfs.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Cuzzocrea S, Reiter RJ. Pharmacological actions of melatonin in acute and chronic inflammation. Curr Top Med Chem. 2002;2:153–165. doi: 10.2174/1568026023394425. [DOI] [PubMed] [Google Scholar]
- 23.El-Shenawy SM, Abdel-Salam OM, Baiuomy AR, El-Batran S, Arbid MS. Studies on the antiinflammatory and anti-nociceptive effects of melatonin in the rat. Pharmacol Res. 2002;46:235–243. doi: 10.1016/s1043-6618(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 24.Crisafulli C, Mazzon E, Muia C, Bella P, Esposito E, Meli R, Cuzzocrea S. Effects of combination of melatonin and dexamethasone on acute lung injury in a mice model of carrageenan-induced pleurisy. J Pineal Res. 2006;41:228–237. doi: 10.1111/j.1600-079X.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 25.Yip HK, Yang CC, Chen KH, Huang TH, Chen YL, Zhen YY, Sung PH, Chiang HJ, Sheu JJ, Chang CL, Chen CH, Chang HW, Chen YT. Combined melatonin and exendin-4 therapy preserves renal ultrastructural integrity after ischemia-reperfusion injury in the male rat. J Pineal Res. 2015;59:434–447. doi: 10.1111/jpi.12273. [DOI] [PubMed] [Google Scholar]
- 26.Chen HH, Lin KC, Wallace CG, Chen YT, Yang CC, Leu S, Chen YC, Sun CK, Tsai TH, Chen YL, Chung SY, Chang CL, Yip HK. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J Pineal Res. 2014;57:16–32. doi: 10.1111/jpi.12140. [DOI] [PubMed] [Google Scholar]
- 27.Sun CK, Lee FY, Kao YH, Chiang HJ, Sung PH, Tsai TH, Lin YC, Leu S, Wu YC, Lu HI, Chen YL, Chung SY, Su HL, Yip HK. Systemic combined melatonin-mitochondria treatment improves acute respiratory distress syndrome in the rat. J Pineal Res. 2015;58:137–150. doi: 10.1111/jpi.12199. [DOI] [PubMed] [Google Scholar]
- 28.Chang CL, Sung PH, Sun CK, Chen CH, Chiang HJ, Huang TH, Chen YL, Zhen YY, Chai HT, Chung SY, Tong MS, Chang HW, Chen HH, Yip HK. Protective effect of melatonin-supported adipose-derived mesenchymal stem cells against small bowel ischemia-reperfusion injury in rat. J Pineal Res. 2015;59:206–220. doi: 10.1111/jpi.12251. [DOI] [PubMed] [Google Scholar]
- 29.Chanchal SK, Mahajan UB, Siddharth S, Reddy N, Goyal SN, Patil PH, Bommanahalli BP, Kundu CN, Patil CR, Ojha S. In vivo and in vitro protective effects of omeprazole against neuropathic pain. Sci Rep. 2016;6:30007. doi: 10.1038/srep30007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 31.Chen YT, Yang CC, Sun CK, Chiang HJ, Chen YL, Sung PH, Zhen YY, Huang TH, Chang CL, Chen HH, Chang HW, Yip HK. Extracorporeal shock wave therapy ameliorates cyclophosphamide-induced rat acute interstitial cystitis though inhibiting inflammation and oxidative stress-in vitro and in vivo experiment studies. Am J Transl Res. 2014;6:631–648. [PMC free article] [PubMed] [Google Scholar]
- 32.Chen HH, Chen YT, Yang CC, Chen KH, Sung PH, Chiang HJ, Chen CH, Chua S, Chung SY, Chen YL, Huang TH, Kao GS, Chen SY, Lee MS, Yip HK. Melatonin pretreatment enhances the therapeutic effects of exogenous mitochondria against hepatic ischemia-reperfusion injury in rats through suppression of mitochondrial permeability transition. J Pineal Res. 2016;61:52–68. doi: 10.1111/jpi.12326. [DOI] [PubMed] [Google Scholar]
- 33.Chua S, Lee FY, Chiang HJ, Chen KH, Lu HI, Chen YT, Yang CC, Lin KC, Chen YL, Kao GS, Chen CH, Chang HW, Yip HK. The cardioprotective effect of melatonin and exendin-4 treatment in a rat model of cardiorenal syndrome. J Pineal Res. 2016;61:438–456. doi: 10.1111/jpi.12357. [DOI] [PubMed] [Google Scholar]
- 34.Fan CQ, Leu S, Sheu JJ, Zhen YY, Tsai TH, Chen YL, Chung SY, Chai HT, Sun CK, Yang JL, Chang HW, Ko SF, Yip HK. Prompt bone marrowderived mesenchymal stem cell therapy enables early porcine heart function recovery from acute myocardial infarction. Int Heart J. 2014;55:362–371. doi: 10.1536/ihj.14-007. [DOI] [PubMed] [Google Scholar]
- 35.Sheu JJ, Lee FY, Yuen CM, Chen YL, Huang TH, Chua S, Chen YL, Chen CH, Chai HT, Sung PH, Chang HW, Sun CK, Yip HK. Combined therapy with shock wave and autologous bone marrow-derived mesenchymal stem cells alleviates left ventricular dysfunction and remodeling through inhibiting inflammatory stimuli, oxidative stress & enhancing angiogenesis in a swine myocardial infarction model. Int J Cardiol. 2015;193:69–83. doi: 10.1016/j.ijcard.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Yang CH, Sheu JJ, Tsai TH, Chua S, Chang LT, Chang HW, Lee FY, Chen YL, Chung SY, Sun CK, Leu S, Yen CH, Yip HK. Effect of tacrolimus on myocardial infarction is associated with inflammation, ROS, MAP kinase and Akt pathways in mini-pigs. J Atheroscler Thromb. 2013;20:9–22. doi: 10.5551/jat.14316. [DOI] [PubMed] [Google Scholar]
- 37.Chen KH, Lin CR, Cheng JT, Cheng JK, Liao WT, Yang CH. Altered mitochondrial ATP synthase expression in the rat dorsal root ganglion after sciatic nerve injury and analgesic effects of intrathecal ATP. Cell Mol Neurobiol. 2014;34:51–59. doi: 10.1007/s10571-013-9986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen KH, Yang CH, Cheng JT, Wu CH, Sy WD, Lin CR. Altered neuronatin expression in the rat dorsal root ganglion after sciatic nerve transection. J Biomed Sci. 2010;17:41. doi: 10.1186/1423-0127-17-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL, Shao PL, Chen YL, Chai HT, Lin KC, Liu CF, Chang HW, Lee MS, Yip HK. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7:74537–74556. doi: 10.18632/oncotarget.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen KH, Chen CH, Wallace CG, Chen YT, Yang CC, Sung PH, Chiang HJ, Chen YL, Chua S, Yip HK, Cheng JT. Combined therapy with melatonin and exendin-4 effectively attenuated the deterioration of renal function in rat cardiorenal syndrome. Am J Transl Res. 2017;9:214–229. [PMC free article] [PubMed] [Google Scholar]
- 41.Chang YC, Hsu SY, Yang CC, Sung PH, Chen YL, Huang TH, Kao GS, Chen SY, Chen KH, Chiang HJ, Yip HK, Lee FY. Enhanced protection against renal ischemia-reperfusion injury with combined melatonin and exendin-4 in a rodent model. Exp Biol Med (Maywood) 2016;241:1588–1602. doi: 10.1177/1535370216642528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2:259–269. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 44.Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci the U S A. 1999;96:7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci the U S A. 2010;107:14851–14856. doi: 10.1073/pnas.1009926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Noguchi K. Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur J Neurosci. 2004;20:2881–2895. doi: 10.1111/j.1460-9568.2004.03754.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen KH, Yang CH, Juang SE, Huang HW, Cheng JK, Sheen-Chen SM, Cheng JT, Lin CR. Pulsed radiofrequency reduced complete Freund’s adjuvant-induced mechanical hyperalgesia via the spinal c-Jun N-terminal kinase pathway. Cell Mol Neurobiol. 2014;34:195–203. doi: 10.1007/s10571-013-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 53.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 54.Wood JN, Boorman JP, Okuse K, Baker MD. Voltage-gated sodium channels and pain pathways. J Neurobiol. 2004;61:55–71. doi: 10.1002/neu.20094. [DOI] [PubMed] [Google Scholar]
- 55.Lai J, Porreca F, Hunter JC, Gold MS. Voltage-gated sodium channels and hyperalgesia. Annu Rev Pharmacol Toxicol. 2004;44:371–397. doi: 10.1146/annurev.pharmtox.44.101802.121627. [DOI] [PubMed] [Google Scholar]


