Abstract
Resveratrol (Res), a natural phenolic compound, has been proven to have a wide variety of beneficial health effects. For example, resveratrol has neuroprotective effects in different central nervous system diseases. However, the mechanisms underlying resveratrol neuroprotection in spinal cord injury (SCI) remain unclear. In this study, we showed that resveratrol treatment improved the restoration of locomotor function, and decreased the degeneration of neurons in SCI mice, which was paralleled by a reduction of apoptosis. We further examined autophagy markers via western blot and immunofluorescence. Results showed that the beneficial effects of resveratrol were related to the promotion LC3II and beclin-1 expression. In addition, autophagy suppression with chloroquine (CQ) partially abolished apoptosis inhibition and locomotor functional improvement of Res on SCI, which indicated that the beneficial effect of resveratrol on SCI was through autophagy enhancement. In conclusion, these results illustrated that the neuroprotective effects of resveratrol in SCI are partially through autophagy stimulation, and implied that Res is a promising drug for SCI therapy.
Keywords: Spinal cord injury, resveratrol, apoptosis, autophagy, chloroquine
Introduction
Spinal cord injury (SCI) is a highly debilitating pathology that includes primary and secondary injury mechanisms [1]. Impact injury to the spinal cord causes damage to neuronal and vascular tissue that cannot be recovered and regenerated. This is referred to as primary damage [2]. Studies showed that neurons continue to die for hours after traumatic SCI and many pathological changes seen following SCI include edema, electrolyte changes, biochemical changes, loss of energy metabolism, and changes in microvascular permeability, which could amplify irreversible neural tissue injury [3]. These events that characterize this successive phase to primary injury are identified as “secondary injury”, and may lead to excessive apoptosis of neurons and oligodendrocytes, progressive degeneration of the spinal cord, and finally neurological dysfunction [2,4]. Although the exact molecular pathway of secondary injury is still controversial, attenuating the death of secondary neurons may contribute to locomotor functional improvement.
By maintaining cellular homeostasis, autophagy is a significant process in the pathology and physiology of the central nervous system [5,6]. The Atg8 protein, known as microtubule-associated protein 1 light chain 3 (LC3), is crucial for autophagy, and is thus identified as a marker protein to monitor autophagy [7]. Previous studies suggested that the conversion into LC3-II expression is up-regulated at lesion sites in damaged neural brain tissue. These studies revealed that autophagic activity increased after traumatic brain injury (TBI) and cerebral ischemia. However, the function of autophagy in the secondary injury after TBI and SCI has long been a controversial topic, with both beneficial [8,9] and harmful roles proposed [10,11]. More recent data supported the idea that the increased autophagy flux after injury is identified as a protective function, and a recent report from the focal cerebellar lesion model provided strong evidence about this viewpoint [12]. In this study, researchers confirmed that, compared with wildtype controls, stimulated autophagy flux declined and behavioral outcomes were worsened in autophagy-deficient beclin-1 +/- mice [12]. Furthermore, many investigations have also indicated that increased autophagy flux has a neuroprotective effect on other injury models, including SCI and contusive TBI.
Resveratrol (3,4’,5-trihydroxystilbene, Res), which occurs naturally in grapes, is synthesized by various medicinal plants in response to injurious substances [13]. It has broad pharmacological applications, and produces anti-anaphylactic and anti-inflammatory effects by influencing arachidonic acid metabolism, possesses anti-cancer, anti-bacterial, and anti-mutation effects, and inhibits protein kinase activities [14,15]. Resveratrol also has therapeutic effects on neurodegenerative disease, TBI, and cerebral ischemia [16]. In different SCI models, autonomic dysfunction and motor function were improved by resveratrol treatment [16,17]. Furthermore, resveratrol protects the spinal cord from ischemia-reperfusion injury by anti-oxidation, anti-inflammation, and anti-apoptosis effects [15]. Thus, it is necessary to further investigate the neuroprotective mechanism of resveratrol against SCI.
The objective of the current study was to investigate the neuroprotective effect of Res on acute SCI in vivo. Using a mice model of SCI, we found improved functional recovery paralleled by restored neural morphology after Res treatment. Additionally, Res administration inhibited apoptosis by enhancing autophagy. Our study supported the application of Res as a therapeutic strategy drug for SCI and its potential role for recovery from central nervous system injury.
Materials and methods
Procedure for investigating animal SCI model and drug treatment
Adult male C57BL/6 mice (2-3 months of age) were used in our SCI studies. Animals were housed in a 12 h light/dark cycle environment and provided with standard rodent food and water. The study procedures strictly complied with the recommended National Institute of Health Guidelines for Laboratory Animals, which were approved by the Ethics Committee of the Second Military Medical University, Shanghai, China (no. 13071002114).
We used the SCI model as reported previously [18]. Briefly, mice were anesthetized under pentobarbital sodium (60 mg/kg of body weight). A longitudinal skin incision was made on the midline of the back to expose the spine between T7-T9 vertebral levels. Muscles surrounded by the spine were dissected, which was followed by a laminectomy. The wound was closed in the sham-operated group. A lateral compression of the SCI model was established by a 10 s crush using a pair of forceps (0.4 mm spacer). After the injury, animals were kept on a highly absorbent bedding. Room temperature was set at 25±3°C and received manual bladder expression twice daily.
Mice were randomly assigned into four groups: the sham group, the SCI group, the Res group and the Res+CQ group. Mice in the sham group received a laminectomy with no trauma hit. Mice in other three groups all experienced lateral compression of the spinal cord. After the injury, mice in the SCI group experienced saline injection only. As the method reported previously [16], mice in the Res group were treated with resveratrol (Sigma, 200 mg/kg, i.p.) and mice in the Res+CQ group were treated with Res and CQ (Sigma, 60 mg/kg, i.p.). During these experiments, the therapeutic treatment were sustained three times per day after the injury until death, and no significant side effects were found in any animals following drug treatment.
Grading of motor disturbance
The motor function of mice was evaluated by the open-field Basso Mouse Scale (BMS), which has been identified as a valid locomotor rating scale for mice [19]. Mice were evaluated by 2 observers and the final score was given by 2 consensuses to assess the functional recovery after SCI.
Tissue preparation and histology
Spinal cord tissues were taken 3 days post-surgery. Then the mice were transcardially perfused with normal saline and 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4). Tissue segments containing the lesion (1 cm on each side of the lesion) were removed and post-fixed by immersion in 4% paraformaldehyde overnight. Then the spinal cord tissue was dehydrated by graded ethanol, and embedded in Paraplast (Sherwood Medical, Mahwah, NJ). Sections 5 μm thick were cut from the paraffin-embedded tissue and stained with Hematoxylin/Eosin (H&E), then studied using light microscopy (Dialux 22 Leitz) by an experienced histopathologist. Damaged neurons were counted and histopathology changes of gray matter were scored on a 6-point scale [20]: 0, no lesion detected; 1, gray matter contained 1 to 5 eosinophilic neurons; 2, gray matter contained 5 to 10 eosinophilic neurons; 3, gray matter contained more than 10 eosinophilic neurons; 4, small infarction (less than one-third of the gray matter area); 5, moderate infarction (one-third to one-half of the gray matter area); 6, large infarction (more than half of the gray matter area). Scores from all sections from each spinal cord tissue were averaged to give a final score for the individual mice. All histological analyses were performed in a blinded fashion.
Immunofluorescence staining
The spinal cord tissues were cut into 6 μm thick sections coronally from the anterior to the posterior using a frozen slicer. The frozen sections were treated with 0.4% Triton X100 for 30 min, and then blocked in donkey serum for 1 h. The sections were incubated with a mixture of rabbit anti-LC3 monoclonal antibodies (ab48494, abcam, USA; 1:400 dilution) in PBS overnight at 4°C. The following day, the sections were incubated with a mixture of anti-rabbit alexa fluor 594 which is red (diluted 1:200; Santa Cruz Biotechnology, Inc.) for 1 h at 37°C in the dark. All cell nuclei were counterstained with 4’,6-diamidino-2-phenylindole. Images were observed using fluorescein microscopy (Olympus FluoView™ FV1000; Olympus, Tokyo, Japan).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Three days following SCI, sections were prepared as mention above, were permeabilized for 30 min with 0.1% (v/v) PBS-Triton X-100, and stained for TUNEL to identify DNA fragmentation. According to the manufacturer’s instructions, slides were washed 3 times for 10 min and the apoptotic cells were assayed using an In Situ Cell Death Detection Kit (Roche Molecular Biochemicals). Images were observed by a fluorescence microscope (Olympus, Tokyo, Japan). The total number of TUNEL-positive cells in the entire transverse section was counted by a blind researcher.
Western blot analysis
Spinal cord tissue samples were removed 3 days following injury, and spinal cord segments (0.5 cm length) from the T7-T10 level at the injury site were dissected. As previously reported [21], all spinal cord samples were homogenized on ice-cold lysis buffer containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 10 mM Na2P2O7, 10 mM NaF, 1 mg/ml aprotinin, 10 mg/ml leupeptin, 1 mM sodium vanadate, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Tissue homogenates were centrifuged at 15000 rpm at 4°C for 20 minutes. Then, proteins were loaded onto sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (Bio-Rad). According to the manufacturer’s recommendations, the polyvinylidene difluoride membrane was blocked with 5% non-fat milk in tris-buffered saline (TBS) with 0.1% Tween 20 for 90 min, and then incubated overnight at 4°C with the primary antibodies. The following primary antibodies were used for Western blot: anti-LC-3B (ab51520, abcam, USA; 1:3000 dilution), anti-beclin-1 (ab62557, abcam, USA; 1:500 dilution), anti-BAX (ab32503, abcam, USA; 1:1000 dilution), anti-Bcl-2 (ab59348, abcam, USA; 1:800 dilution), anti-β-action (ab8227, abcam, USA; 1:1000 dilution). After washing, the membranes were incubated with secondary peroxidase-conjugated anti-rabbit antibodies diluted (1:2000) in TBS with 0.01% Tween 20. Immunoreactive proteins were visualized using an enhanced chemiluminescence (Millipore, Billerica, MA, USA) kit and quantitatively analyzed by densitometry using Image J software.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM) from 3 independent experiments. To compare the results between 2 groups, statistical significance was detected using Student’s t test. To compare the results of more than 2 groups, statistical evaluation of the data was implemented using Dunnett’s post hoc test and one-way analysis of variance (ANOVA). P values <0.05 were considered statistically significant.
Results
Res reverses tissue structure damage, and improves functional recovery after acute SCI
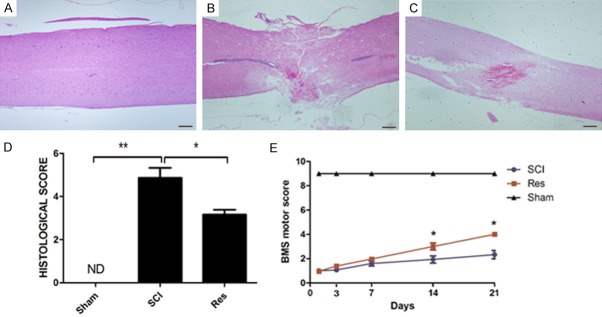
H&E staining was performed to observe the beneficial effects of Res 3 days after SCI. Compared with mice in the sham group, there were significant morphological changes in the spinal cord of SCI mice (Figure 1A, 1B). And the severity of injury decreased in mice treated with Res (Figure 1C, 1D).
Figure 1.
Res reduces tissue structure damage and improves functional recovery after experimental acute SCI. A, B. Extensive damage to the spinal cord was observed in SCI mice group compared to sham-operated mice. C, D. Improvement and the relative quantification showed a decrease in trauma severity in mice treated with resveratrol. E. The motor function of mice subjected to compression trauma assessed at 3, 7, 14 and 21 days after injury. Recovery from motor disturbance was graded using BMS. In the Res-treated mice group, the neurological score improved more than in the SCI group, and persisted up to 21 days after SCI. Figures are representative of at least 3 experiments performed on different experimental days. Scale bars are 0.2 mm. *P<0.05; **P<0.01.
To detect whether histological changes to the spinal cord were related to a loss of motor function, the BMS open-field score was performed. Our results suggested that motor function in the sham group was not impaired, and the hind limb movement in SCI mice after had significant deficits. Seven days after SCI, there were no clear differences in BMS scores between the SCI group and the Res-treated group. However, 14 days following SCI, BMS scores increased with Res-treatment compared to the SCI group (Figure 1E). Our data further strengthened the hypothesis that Res may improve locomotor activity after SCI.
Res attenuates apoptosis caused by acute SCI
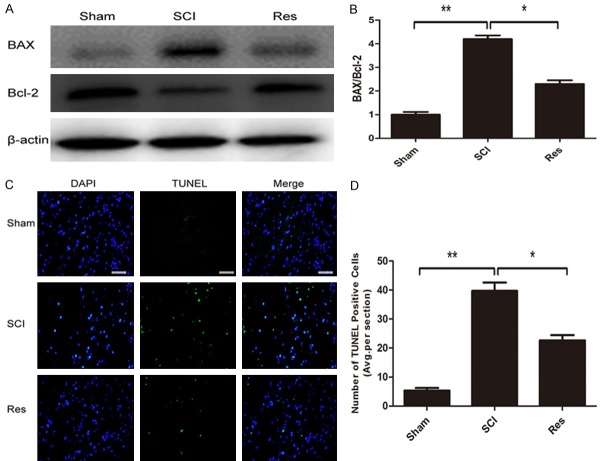
To test whether Res modulated cellular apoptosis, the TUNEL test was performed and levels of Bax and Bcl-2 were analyzed by western blot. The number of apoptotic cells increased significantly following SCI, but the group treated with Res had lesser apoptotic cells (Figure 2C, 2D). Moreover, western blot analysis of anti-apoptotic protein Bcl-2 was upregulated, while pro-apoptotic protein Bax was downregulated. Furthermore, and the ratio of Bax/Bcl-2 decreased after Res-treatment (Figure 2A, 2B). These data indicated that Res administration effectively reduces apoptosis after acute SCI.
Figure 2.
Res attenuates apoptosis caused by SCI in mice. A. Western blot identified expression level of apoptosis relevant proteins Bax and Bcl-2 in the spinal cord 72 h post-lesion. B. Compared with the sham group, the SCI group showed a significant increase of Bax, but a decrease of Bcl-2. Compared with the SCI group, the Res group displayed a significant decreased of Bax, but an increase of Bcl-2. C. TUNEL staining to assess apoptosis 72 h post-lesion. Scale bars are 50 μm. D. Quantitative estimation of apoptotic and TUNEL cells. *P<0.05; **P<0.01.
Res promotes autophagy after acute SCI
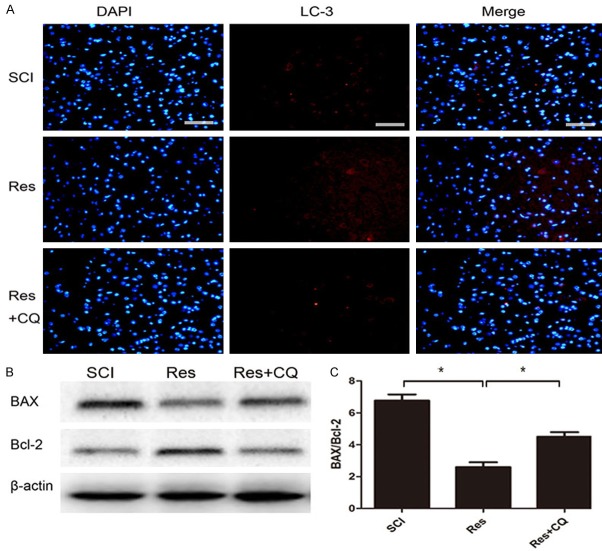
Since previous researchers have reported that secondary damage principally occurs between 24 h and 3 days after the initial SCI [22], we investigated changes in autophagy on day 3 after SCI. Conversion of LC3-I to LC3-II is crucial for the formation of autophagosomes, and LC3-II is an important marker in autophagosome formation. Western blot analysis indicated that the protein expression of autophagy markers LC3-II and beclin-1 in the SCI group was markedly higher than that in the sham group, and compared with the SCI group, the levels of LC3-II and beclin-1 were higher in the Res group (Figture 3A-C). Furthermore, we assessed the fluorescence of LC3B (red) to observe the levels of autophagy in each group. Fluorescence results suggested that LC3 was up-regulated following treatment with Res, which may suggest that Res promoted autophagy (Figure 3D). Our data certified that autophagic activities were activated after acute SCI, and were higher in the Res-treated group.
Figure 3.
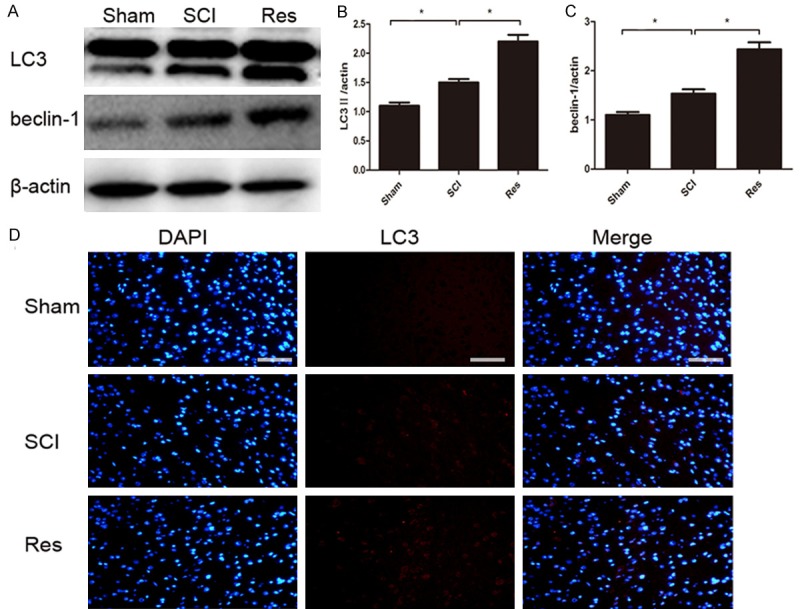
Res treatment promotes autophagy after SCI. A-C. Representative western blots and quantification data of LC3II, beclin-1, and β-actin in each group; D. Immunohistochemical staining of LC3 on the injured side in sections at each group. The LC3-positive cells (red) increased in the SCI group in comparison to the sham group, and the population of LC3-positive cells was relatively higher after Res treatment. Scale bars are 50 μm. *P<0.05; **P<0.01.
Anti-apoptosis effect of res is related to autophagy activity after acute SCI
Among the potential protective mechanisms of Res treatment, we hypothesized that autophagy activation could have a role in anti-apoptosis effects. To identify whether autophagy flux was involved in the anti-apoptosis effect of Res, CQ was utilized to reduce the autophagic flux. Fluorescence results suggested that LC3 was down-regulated following the administration of Res+CQ, which showed that CQ treatment succeeded in suppressing acute SCI-induced autophagic flux (Figure 4A). Then, Bax and Bcl-2 expression were detected by western blot to investigate the degree of apoptosis. Compared with Res treatment, combination therapy of Res and CQ increased the ratio of Bax/Bcl-2 (Figure 4B, 4C). These relevant data indicated that after acute SCI, the anti-apoptosis effect of Res is partly involved with autophagy activity.
Figure 4.
Inhibition of autophagy reverses anti-apoptosis effect of Res therapy after experimental acute SCI. (A) Immunohistochemical staining of LC3 on the injured side in sections at each group. The LC3-positive cells (red) increased in the Res group in comparison to the SCI group, and the population of LC3-positive cells was significant suppressed by CQ treatment. (B, C) Western blot identified expression level of apoptosis relevant proteins Bax and Bcl-2 in the spinal cord 72 h post-lesion. Compared with the SCI group, the Res group showed a significant decrease of Bax, but an increase of Bcl-2. Compared with the Res group, the Res+CQ group displayed a significant increase of Bax, but a decrease of Bax (C). Scale bars are 50 μm, *P<0.05; **P<0.01.
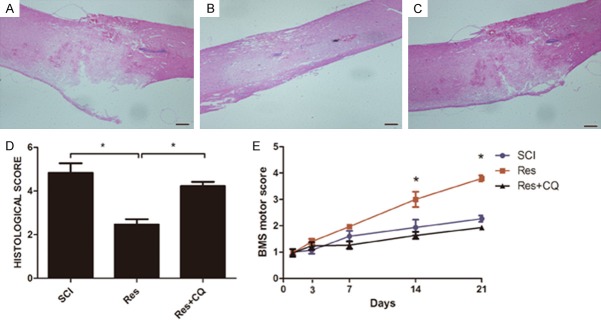
Inhibiting autophagy reversed histological and functional recovery
Behavioral tests and histological examination were used to determine whether the beneficial effects of Res on functional recovery were related to the autophagy pathway. H&E staining revealed that, compared with single Res treatment, CQ treatment increased injury severity (Figure 5A-C). BMS scores indicated that the effect of Res treatment on functional recovery was reversed by CQ (Figure 5D). Overall, the results suggested that Res regulated autophagy to promote locomotor activity recovery and decreased histological damage following acute SCI.
Figure 5.
Inhibition of autophagy reverses functional recovery and tissue preservation of Res therapy after experimental acute SCI. A-D. A decrease in the severity of damage to the spinal cord was observed in the Res mice group compared to SCI mice and the relative quantification in showed that the beneficial effects are reversed when autophagy is inhibited. E. The motor function of mice subjected to compression trauma was assessed at 3, 7, 14 and 21 days after injury. Recovery from motor disturbance was graded using BMS. In the Res+CQ mice group, suppression of autophagy remarkably enlarged injury of single Res group, and persisted up to 21 days after SCI. Figures are representative of at least 3 experiments performed on different experimental days. Scale bars are 0.2 mm. *P<0.05; **P<0.01.
Discussion
Resveratrol treatment is a viable option to reverse nerve injury, and its beneficial effects are mediated by many synergistic pathways that control oxidative stress, apoptosis, and inflammation [15]. In this study, we reported that Res promotes autophagy by inhibiting apoptosis, and improved the recovery of locomotor function and neuronal structure after SCI.
Since its discovery, resveratrol has been widely known for its many physiological properties that could be useful in human medicine. Extensive studies have shown that resveratrol has pleiotropic health benefits, including anti-inflammatory, anti-oxidant, anti-aging, and neuroprotective effects [15]. Subsequently, resveratrol has been proven to ameliorate histopathological and locomotive function after various types of acute central nervous system injuries including stroke, subarachnoid hemorrhage, TBI, and SCI [15,16].
First, we assessed damage severity at the level of the perilesional area using H&E staining. In the control group, we observed severe edema and reactive gliosis, hyperemia, and neuronal degeneration with condensed nuclei. However, compared with the SCI group, we found many neuroprotective effects in above abnormal ultrastructures in the Res group, suggesting that resveratrol improved neurological recovery through attenuating tissue structure injuries.
Moreover, neurological function assessment is a viable method for analogizing the degree of neurological recovery and the outcome of resveratrol treatment [21]. Therefore, the BMS locomotor score test was performed 21 days after SCI. In SCI mice, we detected an increased improvement of locomotion in the Res-treated group than in the standard SCI group, suggesting that resveratrol treatment can ameliorate behavioral outcomes in SCI animals.
It is known that apoptosis plays a significant part in secondary injury in the pathological process of SCI [3]. Studies proved that many apoptosis-related genes participate in the regulation of cell apoptosis, including Bcl-2, Bax, c-myc, p53, and Fas [13]. Many researchers suggested that Res promotes the recovery of rat neurological functions after SCI, and these beneficial effects are related to its anti-apoptosis characteristics [16]. Likewise, in our experiment, TUNEL staining and western blot analysis revealed that Res remarkably reduced neuronal apoptosis in the spinal cord following SCI, which impeded the pathological process of apoptosis, and thus enhanced neurological function recovery.
Autophagy plays an important role in injured neuronal tissue, which is characterized by the degradation of damaged organelles and the formation of autophagosomes [6,23]. Increased autophagic hallmarks have been assessed after SCI, and activated autophagy following SCI is a self-protective mechanism responding to neuronal injury [23]. However, the effects of Res on autophagic activity in injured neuronal tissue has rarely been studied in the literature. A recent study focused on the protective effects of resveratrol against cerebral ischemia-induced neuronal injury, revealing that resveratrol could alleviate neurocyte apoptosis by increasing the Bcl-2/Bax ratio and LC3-II expression [24]. However, another investigation reported that the neuroprotective effects of resveratrol on traumatic brain injury are related to Res-mediated down-regulation of neuronal autophagy and impediment of the TLR4/NF-κB signaling pathway [25].
In our study, we firstly hypothesized that resveratrol could facilitate neurological recovery through promoting autophagic activity. Western blot analysis was utilized to show that the expression of LC3-II and beclin-1 increased significantly after SCI, and this up-regulation was promoted by Res treatment. In addition, immunofluorescence staining determined that the number of LC3-positive cells were significantly induced at the lesion site after SCI, and increased following treatment with Res. These results indicated that Res may promote autophagic activity. However, the in-depth mechanism is still unknown and requires further study.
In several models of acute nervous system injury, autophagy has different functions and different presentations. Recent studies have focused on the relationship between apoptosis and autophagy following acute SCI, and certified that autophagy activation resulted in decreased neuronal loss by inhibiting apoptosis after acute SCI [26]. We hypothesized that inhibiting autophagy could be a target to improve functional recovery in Res-treatment therapy. Our study revealed that CQ, which was utilized to inhibit the autophagic flux, significantly attenuated the anti-apoptosis effect in the Res-treated group [19]. In addition, we further studied the functional recovery and neural morphology after CQ treatment. BMS scores were significantly lower for CQ mice compared with SCI-only mice and Res-treated mice. These data indicated that autophagy protected neuronal locomotor function and enhanced the behavioral recovery of mice after acute SCI. One possible mechanism of the anti-apoptosis effect of Res is that Res-stimulated and unobstructed autophagy flux improves the clearance of hazardous substances that may attribute to apoptosis in the spinal cord. However, the in-depth mechanism remains unknown. The limitation of our study is that the relative signal pathway was not involved in Res-stimulated autophagy in SCI. To ascertain the mechanism of Res therapy, further exploration is required.
Overall, this study firstly assessed the effects of Res on functional recovery, apoptosis, and autophagy following acute SCI. We revealed that resveratrol preserves tissue structure and promotes the recovery of neurological function at the impacted lesion after SCI. We also reported that the neuroprotective effects of resveratrol on SCI are related to Res-mediated increases of neuronal autophagy and decreases of anti-apoptosis effects, which provides new insight into the therapeutic capacity of resveratrol.
Acknowledgements
The study was supported by the international cooperation program of shanghai science and technology committee (13430721000).
Disclosure of conflict of interest
None.
References
- 1.Hall ED, Yonkers PA, Andrus PK, Cox JW, Anderson DK. Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J Neurotrauma. 1992;9(Suppl 2):S425–442. [PubMed] [Google Scholar]
- 2.Zhu SP, Wang ZG, Zhao YZ, Wu J, Shi HX, Ye LB, Wu FZ, Cheng Y, Zhang HY, He S, Wei X, Fu XB, Li XK, Xu HZ, Xiao J. Gelatin nanostructured lipid carriers incorporating nerve growth factor inhibit endoplasmic reticulum stress-induced apoptosis and improve recovery in spinal cord injury. Mol Neurobiol. 2016;53:4375–4386. doi: 10.1007/s12035-015-9372-2. [DOI] [PubMed] [Google Scholar]
- 3.Warden P, Bamber NI, Li H, Esposito A, Ahmad KA, Hsu CY, Xu XM. Delayed glial cell death following wallerian degeneration in white matter tracts after spinal cord dorsal column cordotomy in adult rats. Exp Neurol. 2001;168:213–224. doi: 10.1006/exnr.2000.7622. [DOI] [PubMed] [Google Scholar]
- 4.Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol. 2002;15:355–360. doi: 10.1097/00019052-200206000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Kanno H, Ozawa H, Sekiguchi A, Itoi E. Spinal cord injury induces upregulation of beclin 1 and promotes autophagic cell death. Neurobiol Dis. 2009;33:143–148. doi: 10.1016/j.nbd.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976) 2011;36:E1427–1434. doi: 10.1097/BRS.0b013e3182028c3a. [DOI] [PubMed] [Google Scholar]
- 7.Chen HC, Fong TH, Lee AW, Chiu WT. Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine (Phila Pa 1976) 2012;37:470–475. doi: 10.1097/BRS.0b013e318221e859. [DOI] [PubMed] [Google Scholar]
- 8.Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Guo D, Zeng L, Brody DL, Wong M. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS One. 2013;8:e64078. doi: 10.1371/journal.pone.0064078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai Y, Hickey RW, Chen Y, Bayir H, Sullivan ML, Chu CT, Kochanek PM, Dixon CE, Jenkins LW, Graham SH, Watkins SC, Clark RS. Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant gamma-glutamylcysteinyl ethyl ester. J Cereb Blood Flow Metab. 2008;28:540–550. doi: 10.1038/sj.jcbfm.9600551. [DOI] [PubMed] [Google Scholar]
- 11.Lee DH, Luo X, Yungher BJ, Bray E, Lee JK, Park KK. Mammalian target of rapamycin’s distinct roles and effectiveness in promoting compensatory axonal sprouting in the injured CNS. J Neurosci. 2014;34:15347–15355. doi: 10.1523/JNEUROSCI.1935-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viscomi MT, D’Amelio M, Cavallucci V, Latini L, Bisicchia E, Nazio F, Fanelli F, Maccarrone M, Moreno S, Cecconi F, Molinari M. Stimulation of autophagy by rapamycin protects neurons from remote degeneration after acute focal brain damage. Autophagy. 2012;8:222–235. doi: 10.4161/auto.8.2.18599. [DOI] [PubMed] [Google Scholar]
- 13.Engelhard K, Werner C, Eberspacher E, Pape M, Stegemann U, Kellermann K, Hollweck R, Hutzler P, Kochs E. Influence of propofol on neuronal damage and apoptotic factors after incomplete cerebral ischemia and reperfusion in rats: a long-term observation. Anesthesiology. 2004;101:912–917. doi: 10.1097/00000542-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Hong YB, Kang HJ, Kim HJ, Rosen EM, Dakshanamurthy S, Rondanin R, Baruchello R, Grisolia G, Daniele S, Bae I. Inhibition of cell proliferation by a resveratrol analog in human pancreatic and breast cancer cells. Exp Mol Med. 2009;41:151–160. doi: 10.3858/emm.2009.41.3.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez MS, Dempsey RJ, Vemuganti R. Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem Int. 2015;89:75–82. doi: 10.1016/j.neuint.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, Shi Z, Fan L, Zhang C, Wang K, Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res. 2011;1374:100–109. doi: 10.1016/j.brainres.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 17.Kesherwani V, Atif F, Yousuf S, Agrawal SK. Resveratrol protects spinal cord dorsal column from hypoxic injury by activating Nrf-2. Neuroscience. 2013;241:80–88. doi: 10.1016/j.neuroscience.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Terayama R, Bando Y, Murakami K, Kato K, Kishibe M, Yoshida S. Neuropsin promotes oligodendrocyte death, demyelination and axonal degeneration after spinal cord injury. Neuroscience. 2007;148:175–187. doi: 10.1016/j.neuroscience.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Xuan J, Zheng BB, Zhou YL, Lin Y, Wu YS, Zhou YF, Huang YX, Wang Q, Shen LY, Mao C, Wu Y, Wang XY, Tian NF, Xu HZ, Zhang XL. Metformin improves functional recovery after spinal cord injury via autophagy flux stimulation. Mol Neurobiol. 2017;54:3327–3341. doi: 10.1007/s12035-016-9895-1. [DOI] [PubMed] [Google Scholar]
- 20.Sirin BH, Ortac R, Cerrahoglu M, Saribulbul O, Baltalarli A, Celebisoy N, Iskesen I, Rendeci O. Ischaemic preconditioning reduces spinal cord injury in transient ischaemia. Acta Cardiol. 2002;57:279–285. doi: 10.2143/AC.57.4.2005427. [DOI] [PubMed] [Google Scholar]
- 21.Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Bramanti P, Morittu VM, Procopio A, Perri E, Britti D, Cuzzocrea S. The effects of a polyphenol present in olive oil, oleuropein aglycone, in an experimental model of spinal cord injury in mice. Biochem Pharmacol. 2012;83:1413–1426. doi: 10.1016/j.bcp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Lin CM, Tsai JT, Chang CK, Cheng JT, Lin JW. Development of telmisartan in the therapy of spinal cord injury: pre-clinical study in rats. Drug Des Devel Ther. 2015;9:4709–4717. doi: 10.2147/DDDT.S86616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou H, Zhang L, Zhang L, Tang P. Acute spinal cord injury in rats should target activated autophagy. J Neurosurg Spine. 2014;20:568–577. doi: 10.3171/2014.1.SPINE13237. [DOI] [PubMed] [Google Scholar]
- 24.Wang R, Liu YY, Liu XY, Jia SW, Zhao J, Cui D, Wang L. Resveratrol protects neurons and the myocardium by reducing oxidative stress and ameliorating mitochondria damage in a cerebral ischemia rat model. Cell Physiol Biochem. 2014;34:854–864. doi: 10.1159/000366304. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y, Cui Y, Gao JL, Li MH, Li R, Jiang XH, Tian YX, Wang KJ, Cui CM, Cui JZ. Resveratrol attenuates neuronal autophagy and inflammatory injury by inhibiting the TLR4/NF-kappaB signaling pathway in experimental traumatic brain injury. Int J Mol Med. 2016;37:921–930. doi: 10.3892/ijmm.2016.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, He C, Du H, Zhang L. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49:276–287. doi: 10.1007/s12035-013-8518-3. [DOI] [PubMed] [Google Scholar]