Abstract
Syndecan-binding protein (SDCBP), which is induced by tumor necrosis factor-α and interferon-γ, controls the proliferation and invasion of several different types of cancer cells. Interleukin-6 (IL-6) is known to play an important role in the glioma cell growth and invasion. The present study aimed to investigate the relationship between IL-6 and SDCBP in glioma cells. SDCBP expression was knocked down in two glioma cell lines (T98G and U87) by small interfering RNA (siRNA) transfection. Cell proliferation and invasion were significantly repressed following SDCBP knockdown, and there was a positive correlation between SDCBP and IL-6 expression levels in glioma tissues. IL-6 stimulation dose- and time-dependently induced SDCBP expression at both mRNA and protein levels. Furthermore, pre-treatment with the Janus kinase 2 (JAK2) inhibitor AG490 abolished the IL-6-induced SDCBP expression, suggesting that the effect of IL-6 on SDCBP transcription is dependent on JAK2/signal transducer and activator of transcription 3 (STAT3) signaling. Finally, IL-6 did not stimulate glioma cell growth or invasion when SDCBP expression was suppressed. In summary, our results suggest that IL-6 promotes glioma cell proliferation and invasion by inducing SDCBP expression, which is mediated by JAK2/STAT3 signaling.
Keywords: SDCBP, IL-6, glioma, JAK2/STAT3
Introduction
In the past 10 years, the incidence of primary brain tumors has increased rapidly, and gliomas remain the most common central nervous system malignancy [1]. As a leading cause of cancer-related death, malignant gliomas account for more than 45% of primary intracranial tumors with an annual incidence of greater than 5 per 100,000 population [2]. Despite recent treatment advances, the prognosis of glioma patients is poor [3]. A deeper understanding of the pathophysiological mechanisms of glioma development could help identify novel therapeutic strategies.
Interleukin-6 (IL-6) is a pleiotropic cytokine that orchestrates the inflammatory tumor microenvironment [4]. It can regulate the proliferation, apoptosis, and invasion of tumor cells, thus contributing to malignant progression [5]. IL-6 stimulation can activate Janus kinase 2 (JAK2)/signal transducer and activator of transcription (STAT3), Ras/mitogen-activated protein kinase (MAPK), and phosphoinositide (PI)-3 kinase signaling pathways [6]. Notably, IL-6 promotes glioma cell invasion and migration via JAK/STAT3 signaling [7]. IL-6 expression may therefore be a useful prognostic indicator for patients with glioma [8].
Syndecan-binding protein (SDCBP), also known as MDA-9 (melanoma differentiation associated gene-9) or syntenin, was first discovered in melanoma cells [9]. This scaffold protein contains two PDZ domains and participates in diverse biological processes [9]. Recent studies have shown that SDCBP can control the proliferation and invasion of several different types of cancer cells [10-15]. Its effect on melanoma metastasis has been extensively studied [16-21]. After interacting with c-Src, SDCBP promotes the formation of an active focal adhesion kinase (FAK)/c-Src signaling complex, ultimately activating nuclear factor (NF)-κB and matrix metalloproteinase (MMP) [16,17]. Recent glioma studies have demonstrated that SDCBP is an important mediator of cell invasion. Besides s-Src and NF-κB, SDCBP overexpression can enhance glioma cell migration capacity by activating p38, JNK, and AKT signaling [12,15]. SDCBP expression in umbilical arterial endothelial cells and melanoma cells can be induced by TNF-α (tumor necrosis factor-α) [22] and IFN-γ (interferon-γ) [23], which also augment IL-6 transcription [24]. It is unclear whether IL-6 regulates SDCBP expression.
The present study investigated the effects of SDCBP on glioma cell growth and invasion, revealing a positive correlation between SDCBP and IL-6 expression. More importantly, the effects of IL-6 on SDCBP expression, cell growth, and invasion are mediated by JAK2/STAT3 signaling.
Materials and methods
Patients and glioma tissue collection
The study protocol was approved by the Clinical Research Ethics Committee from the First People’s Hospital of Zunyi and the first affiliated hospital of Zunyi medical college. (Zunyi, Guizhou, China). Written informed consent was obtained from all participants. Glioma (n=45) and non-neoplastic (n=45) brain tissue (sampled during surgical procedures for epilepsy) were obtained from the First People’s Hospital of Zunyi and the first affiliated hospital of Zunyi medical college, immediately frozen in liquid nitrogen, and stored at -80°C until use. All tissues were subjected to quantitative polymerase chain reaction (qPCR) to measure SDCBP and IL-6 mRNA expression. Western blots were performed to measure protein levels of SDCBP, IL-6, p-STAT3, and STAT3 in 10 glioma and 10 normal brain tissue samples.
Cell culture
The human glioblastoma cell lines U251, U87, U373, SHG44, and T98G were obtained from the cell bank of the Shanghai biology institute, Chinese Academy of Science (Shanghai, China).Cells were maintained at 37°C in a humidified atmosphere of 5% CO2. U87, U373, and T98G were cultured in Eagle’s Minimum Essential Medium (MEM; Hyclone, Logan, UT, USA), while other two cell lines were cultured in Dulbecco’s modified Eagle’s medium (Hyclone). All culture media were supplemented with 10% fetal bovine serum (FBS, Hyclone).
RNA isolation, reverse transcription, and qPCR
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed into first-strand cDNA using the First Strand Synthesis System (TOYOBO, Osaka, Japan) according to manufacturers’ protocols. Real-time quantitative reverse-transcription (qRT)-PCR assays were carried out by using SYBR Green PCR kits (Thermo Fisher Scientific, Waltham, MA, USA) on an ABI 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) as previously described [25]. SDCBP mRNA expression levels were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression. Primer sequences were used as follows: SDCBP (NM_001007067.1), forward primer: 5’-TTCTGCAAACCCTGCCAATC-3’; reverse primer: 5’-AGCACCTTGTGCGCTTTATC-3’; GAPDH (NM_001256799.2), forward primer: 5’-CACCCACTCCTCCACCTTTG-3’; reverse primer: 5’-CCACCACCCTGTTGCTGTAG-3’.
Western blot
Tissue samples and cells were homogenized and lysed with radioimmunoprecipitation assay buffer (Beyotime, Shanghai, China). A bicinchoninic acid assay kit (Thermo Fisher Scientific) was then used to quantify the protein concentrations. The obtained protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidenedifluoride (PVDF) membrane in a semi-dry transblot apparatus. Immune-detection of specific proteins was performed with enhanced chemiluminescence (ECL, Millipore, Billerica, MA, USA) following the incubation of primary antibodies and horseradish peroxidase-conjugated secondary antibodies. The sources of primary antibodies were as follows: anti-SDCBP, anti-IL-6, anti-p-STAT3, anti-STAT3, anti-MMP-2, and anti-MMP-9 (Abcam, Cambridge, MA, USA); anti-p-JAK2, anti-JAK2, and anti-GAPDH (Cell Signaling Technology, Danvers, MA, USA). The immunoblot intensities were quantified using with ImageJ software (National Institutes of Health, Bethesda, MD, USA). The protein expression levels were normalized to GAPDH.
Silencing of SDCBP by small interfering RNA (siRNA)
siRNA targeting human SDCBP mRNA (siSDCBP: 5’-GGGACCAAGTACTTCAGATCA) and negative control (siNC, AATTCTCCGAACGTGTCACGT) were synthesized by Genepharma Co., Ltd (Shanghai, China). T98G and U87 cells were transiently transfected with siSDCBP or siNC using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. At 36 h post transfection, cells were harvested, and the RNA interference effect was evaluated by qPCR and western blot. The effects of siSDCBP on the glioma cell proliferation and invasion were determined by Cell Count Kit-8 (CCK-8, Beyotime) and Transwell invasion assays, respectively.
CCK-8 assays
T98G or U87 cells were transfected with siSDCBP or siNC. At 24 h after transfection, cells were seeded in 96-well plates at a density of 1000-1500 cells per well. After incubation for 0, 1, 2, or 3 days, CCK-8 solution (Beyotime) was added to each well and incubated for 1 h. The absorbance was measured at the wavelength of 450 nm with a spectrophotometer.
Transwell invasion assays
At 24 h post transfection, T98G or U87 cells were seeded onto the upper chamber (8-μm pore size, pre-coated with Matrigel; Corning, Lowell, MA, USA) containing MEM. The lower chamber was filled with MEM containing 10% FBS. After incubation for 24 h, the non-migrated cells were removed with a cotton swab, and the migrated cells were fixed and stained using crystal violet solution. Cell numbers were counted under a light microscope in five fields.
Effects of IL-6 on SDCBP expression
After overnight starvation with MEM, T98G or U87 cells were stimulated with serial doses of IL-6 (0-200 ng/mL; Sigma, St Louis, MO, USA) and cultured for an additional 12 or 24 h. For JAK2 inhibitor experiments, starved cells were pre-treated with 30 μM AG490 (Selleck Chemicals, Houston, TX, USA) or dimethyl sulfoxide for 1 h and then treated with IL-6 for another 24 h. SDCBP expression was evaluated at the end of culture.
Effects of IL-6 and siSDCBP transfection on cell proliferation and invasion
T98G or U87 cells were transfected with siSDCBP or siNC. After 24 h of culture, cells were serum-starved overnight, and then CCK-8 and invasion assays were performed in the presence of 100 ng/mL IL-6.
Bioinformatic analysis
Gene expression data of the GBM (glioblastomamultiforme) cohort were downloaded from The Cancer Genome Atlas (TCGA) website (https://tcga-data.nci.nih.gov/tcga/) and analyzed with Gene Set Enrichment Analysis (GSEA) using software version 2.0.1 (http://www.broad.mit.edu/gsea) as previously described [26]. Overall survival analysis was performed on data from the GSE 16011 dataset [27](http://www.ebi.ac.uk/arrayexpress/experiments/E-GEOD-16011/?query=GSE16011) with the Kaplan-Meier method and log-rank tests.
Data analysis
All experiments were independently performed at least three times, and the data are presented as the mean ± S.D. Statistical analyses were conducted with GraphPad Prism software (version 6.0, GraphPad Software, La Jolla, CA, USA). Analysis of variance (ANOVA) was used to analyze the mean values for different groups. The relationships between SDCBP and IL-6 expression were assessed by Pearson correlation analysis. P-values less than 0.05 were considered statistically significant.
Results
Knocking down SDCBP expression inhibits glioma cell growth
To explore the functions of SDCBP in glioma cells, we knocked down its expression with specific siRNA transfection. First, SDCBP expression was estimated in five glioma cell lines. T98G and U87 cells showed higher levels of SDCBP and were used for RNAi assays (Figure 1A). SDCBP-specific siRNA (siSDCBP) significantly reduced SDCBP mRNA and protein levels in both cell lines, but SDCBP expression in cells treated with nonsense siRNA (siNC) was not affected compared to non-treated cells (Mock; Figure 1B, P<0.001). Subsequently, glioma cell proliferation was detected with CCK-8 assays. At 24 h after siRNA transfection, cells were plated onto 96-well plates, defined as Day 0. As illustrated in Figure 1C, the proliferation of glioma cells transfected with siSDCBP was significantly decreased at Days 2 and 3 (P<0.001) compared to Mock and siNC cells. Our results indicate an anti-proliferation role of siSDCBP in glioma cells.
Figure 1.
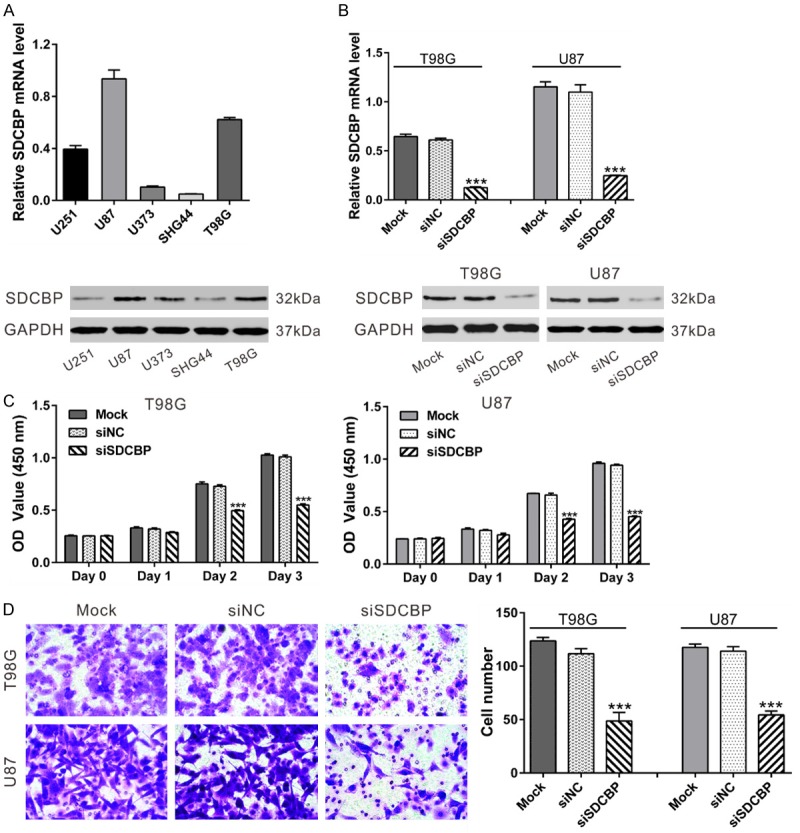
SDCBP knockdown suppressed glioma cell proliferation and invasion. A. SDCBP mRNA (top) and protein expression (bottom) in glioma cell lines. B. T98G and U87 cells were transfected with SDCBP siRNA (siSDCBP) or control siRNA (siNC). At 48 h after transfection, knockdown efficiency was evaluated using qPCR (top) and western blot (bottom). C. Cell proliferation rates were assessed by CCK-8 assay. D. Cell invasion was evaluated in Matrigel-coated Transwell chambers. Representative images are shown at the original magnification: 200 × (left panel). Invasion was quantified using five fields per triplicate well (right panel). ***P<0.001 vs. siNC.
Silencing SDCBP inhibits the invasive ability of glioma cells
We then determined the effect of siSDCBP on glioma cell invasion on Day 1 using a Matrigel-coated Transwell chamber. Cells with silenced SDCBP exhibited 50%-60% decreases in invasive ability compared with Mock and siNC-transfected cells (Figure 1D, P<0.001). Our results suggest an anti-invasive role of siSDCBP in glioma cells.
SDCBP levels positively correlate with IL-6 levels in glioma tissues
Increased expression of SDCBP [12,15] and IL-6 [8] have been reported in glioma. Re-analysis of the GBM cohort of TCGA showed significantly increased SDCBP expression in glioma tissue compared with normal brain tissue (Figure 2A, P<0.0001).Glioma SDCBP expression was significantly correlated with patient survival time in the GSE 16011 dataset [27] (Figure 2B). GSEA identified the Biocarta IL-6 signaling pathway as strongly associated with SDCBP expression in TCGA GBM cohort (Figure 2C).
Figure 2.
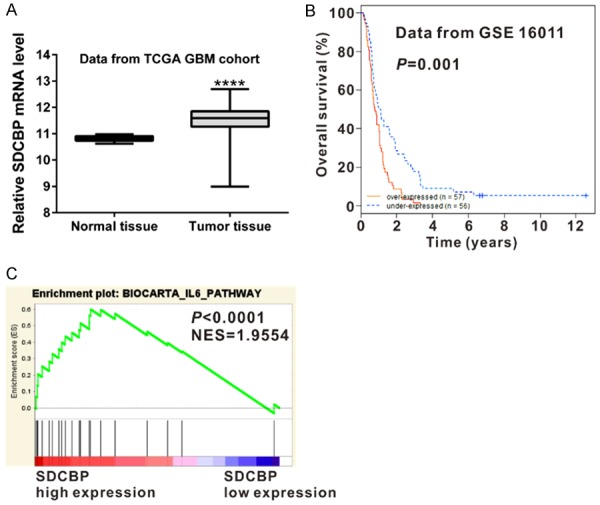
Bioinformatic analysis. A. SDCBP mRNA expression was significantly higher in glioma tissues (n=529) than in normal tissues (n=10) from the GBM cohort of TCGA (P<0.0001). B. Survival analysis of patients from the GSE 16011 dataset (P=0.001). C. TCGA GBM cohort was analyzed by Gene Set Enrichment Analysis (GSEA) as previously described. Enrichment plots of gene expression signatures for Biocarta IL-6 pathways according to SDCBP expression levels.
We then measured mRNA levels of IL-6 and SDCBP in 45 snap-frozen glioma tissues and 45 normal brain tissues by real-time qPCR. mRNA levels of IL-6 and SDCBP were significantly up-regulated in glioma tissues compared with normal brain (Figure 3A, 3B, P<0.0001). Pearson correlation analysis revealed a positive correlation between SDCBP and IL-6 mRNA levels in glioma tissues (Figure 3C, P<0.0001). Similar results were observed for protein levels when western blot analysis was performed on 10 glioma tissues (Figure 3D-F). STAT3 is an important downstream effector of IL-6 [28]. STAT3 phosphorylation was notably enhanced in glioma tissues, but total STAT3 levels were similar in glioma and normal brain tissue. These data implicate SDCBP and IL-6 signaling in glioma tumor genesis.
Figure 3.
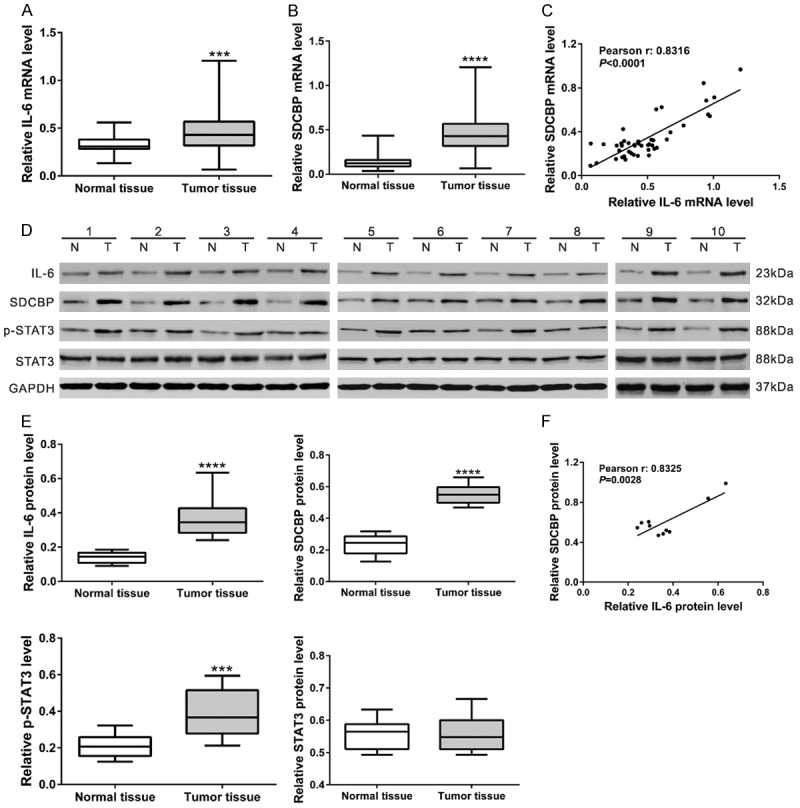
SDCBP and IL-6 expression levels correlated in glioma tissue. (A, B) qPCR analysis of SDCBP and IL-6 in glioma and normal brain tissues (n=45). (C) Pearson correlation scatter plot of SDCBP and IL-6 mRNA levels in glioma tissues (P<0.0001). (D, E) Western blot analysis of SDCBP, IL-6, p-STAT3, and STAT3 in glioma and normal brain tissues (n=10). Representative blots (D) and protein levels relative to GAPDH (E) are shown. (F) Pearson correlation scatter plot of SDCBP and IL-6 protein levels in glioma tissues (P<0.01).
IL-6 up-regulates SDCBP expression via JAK2/STAT3
To determine whether IL-6 is involved in suppressing SDCBP expression, we treated two glioma cell lines with increasing doses of recombinant IL-6 (0, 50, 100, 200 ng/mL) for 12 or 24 h. IL-6 increased mRNA and protein levels of SDCBP in a time- and concentration-dependent manner (Figure 4A, 4B).
Figure 4.
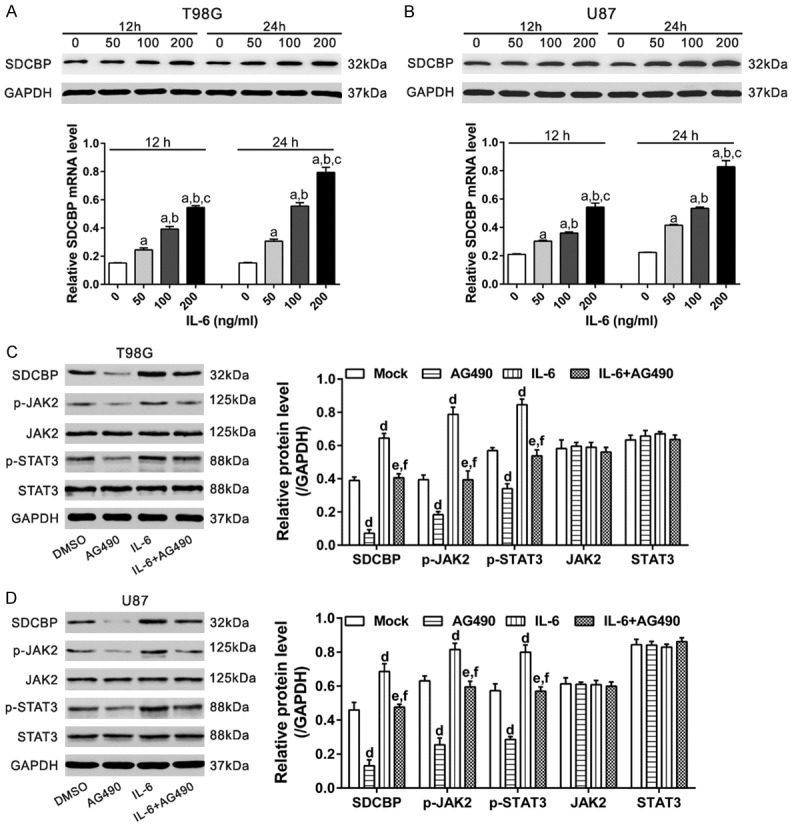
IL-6 increased SDCBP expression by activating JAK2/STAT3 signaling. A, B. T98G and U87 cells were exposed to IL-6 (0, 50, 100, or 200 ng/mL) for 12 or 24 h. SDCBP protein (upper panel) and mRNA (lower panel) levels were analyzed by western blotting and qPCR, respectively. a, P<0.001 vs. 0 ng/mL; b, P<0.001 vs. 50 ng/mL; c, P<0.001 vs. 100 ng/mL. C, D. T98G and U87 cells were divided into four groups: Group 1, no treatment (Mock); Group 2, pre-treatment with 30 μM AG490 (JAK2 inhibitor); Group 3, treatment with 100 ng/mL IL-6; Group 4, pre-treatment with AG490 and then 100 ng/mL IL-6. Western blotting was performed 24 h after treatment. d, P<0.001 vs. Group 1; e, P<0.001 vs. Group 2; f, P<0.001 vs. Group 3.
We then explored whether JAK2/STAT2 was involved in the effects of IL-6 on SDCBP expression. Western blotting was performed in four groups of cells (Figure 4C, 4D): Group 1, no treatment (Mock); Group 2, pre-treated with 30 μM AG490 (JAK2 inhibitor); Group 3, treated with 100 ng/mL IL-6; Group 4, pre-treated with AG490 and then 100 ng/mL IL-6. IL-6 exposure significantly increased JAK2 and STAT3 phosphorylation. Pre-treatment with AG490 significantly reversed the stimulatory effects of IL-6 on SDCBP protein expression (P<0.001). These results indicate that IL-6/JAK2/STAT3 contribute to increased SDCBP expression in glioma cells.
IL-6 promotes glioma cell growth and invasion via SDCBP
Several groups have proposed that IL-6 is involved in glioma development [7,29,30]. To investigate the association between IL-6 and SDCBP in cell growth and invasion, T98G and U87 cells were transfected with siSDCBP or siNC and then treated with IL-6. Glioma cell growth and invasion were remarkably promoted by IL-6 exposure and weakened by siSDCBP transfection (Figure 5A, 5B). These results indicate that IL-6 is an upstream regulator of SDCBP during glioma cell growth and invasion.
Figure 5.
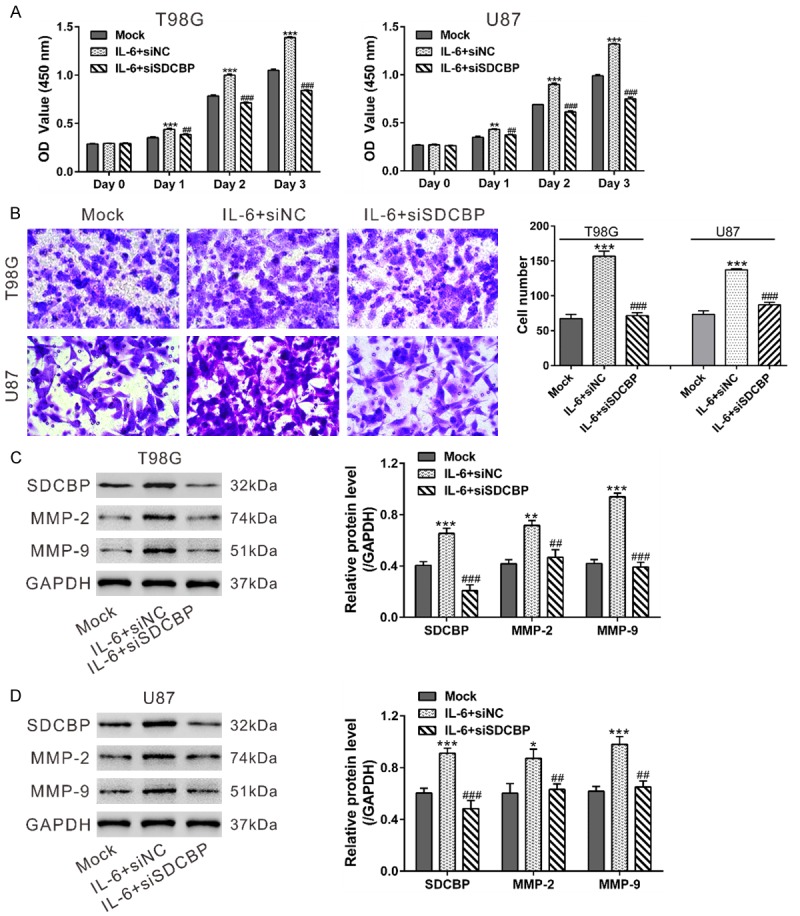
IL-6 promoted glioma cell growth and invasion via SDCBP. T98G and U87 cells were transfected with siSDCBP or siNC. At 24 h after transfection, cells were serum-starved overnight. A. Cells were seeded onto 96-well plates for CCK-8 assays to measure cell proliferation in the presence of IL-6 (100 ng/mL). Untreated cells (Mock) was used as a control. B. Cells were seeded onto the upper chamber containing MEM, and the lower chamber was filled with MEM+100 ng/mL IL-6. Transwell assays were used to assess the invasive potential of glioma cells after 24 h. C, D. Expression of SDCBP, MMP-2, and MMP-9 were determined in cells treated in the same manner as in the Transwell assays. *P<0.05, **P<0.01, and ***P<0.001 vs. Mock; ##P<0.01 and ###P<0.001 vs. IL-6+siNC.
MMPs are important enzymes for the proteolyzing extracellular matrix (ECM) components, thus playing crucial roles in cell invasion. IL-6 treatment of glioma cells elevates MMP-9 levels [7], and forcing overexpression SDCBP in glioma cells increases MMP-2 expression [12]. We analyzed MMP-2 and -9 expression by western blot. IL-6 exposure significantly increased levels of SDCBP, MMP-2, and MMP-9, whereas SDCBP knockdown attenuated IL-6-induced MMP expression (Figure 5C, 5D).
Discussion
Recent studies have demonstrated a critical role of SDCBP in glioma cell invasion, and the underlying mechanisms have also been explored [12,15]. In this study, we performed RNAi experiments to confirm the roles of SDCBP in T98G and U87 cell proliferation and invasion. Previous studies have identified SDCBP as a TNF-α- and IFN-γ-responsive gene [22,23]. Both cytokines induce IL-6 transcription [24], so we investigated the effects of IL-6 exposure on SDCBP expression. Elevated SDCBP and IL-6 expression were observed in glioma tissues compared with paired non-tumorous brain tissues at both the mRNA and protein levels, which was consistent with previous findings [8,12]. More importantly, SDCBP and IL-6 expression were positively correlated in glioma tissues. In vitro experiments demonstrated that IL-6 exposure led to notable decreases in SDCBP mRNA and protein expression in a time- and dose-dependent manner in two glioma cell lines. IL-6 stimulation can activate STAT3, which is implicated in tumor formation and metastatic progression [31]. We found that STAT3 phosphorylation was increased in glioma tissues. We hypothesized that the effects of IL-6 on SDCBP expression are dependent on JAK2/STAT3 activity, which is supported by the observation that pre-treatment with the JAK2 inhibitor AG490 significantly suppressed IL-6-induced SDCBP expression.
We also investigated the involvement of SDCBP in IL-6-stimulated glioma cell proliferation and invasion. CCK-8 assays revealed that IL-6 stimulation significantly promoted glioma cell proliferation, and Transwell assays showed that it remarkably induced cell invasion. Knocking down SDCBP significantly suppressed the effects of IL-6 on cell proliferation and invasion, indicating that IL-6 may be an upstream regulator of SDCBP. Enhanced ECM lysis during the in vivo invasion process is associated with greater invasive ability. MMP-2 and -9 are the main enzymes responsible for ECM lysis. Here, the expression changes in MMP-2 and MMP-9 were consistent with the Transwell results. A previous study showed that IL-6 (10-40 ng/mL) increased MMP-9 expression but had no effect on MMP-2 expression in glioma cells [7]. The inconsistency between the results may due to the different doses of IL-6 used.
In summary, IL-6 exposure significantly enhanced SDCBP expression by inducing transcription through a JAK2/STAT3-dependent pathway. SDCBP knockdown partially suppressed IL-6-induced glioma cell proliferation and invasion.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81660421), the High-level Personnel Funded Projects, the Science and Technology benefits of Zunyi and the first people’s hospital of Zunyi Joint research and development of science and technology projects (grant number [2011] 24).
Disclosure of conflict of interest
None.
References
- 1.Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM. Editor’s choice: the epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;17:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15:1–56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107:359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 4.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 5.Grivennikov S, Karin M. Autocrine IL-6 Signaling: A Key Event in Tumorigenesis? Cancer Cell. 2008;13:7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Li G, Li R, Shen J, He Q, Deng L, Zhang C, Zhang J. IL-6 promotion of glioblastoma cell invasion and angiogenesis in U251 and T98G cell lines. J Neurooncol. 2010;100:165–176. doi: 10.1007/s11060-010-0158-0. [DOI] [PubMed] [Google Scholar]
- 8.Chang CY, Li MC, Liao SL, Huang YL, Shen CC, Pan HC. Prognostic and clinical implication of IL-6 expression in glioblastoma multiforme. J Clin Neurosci. 2005;12:930–933. doi: 10.1016/j.jocn.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Jiao JL, Jiang H, Fisher PB. Melanoma differentiation associated gene-9, mda -9, is a human gamma interferon responsive gene. Gene. 1998;207:105–110. doi: 10.1016/s0378-1119(97)00562-3. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta S, Menezes ME, Das SK, Emdad L, Janjic A, Bhatia S, Mukhopadhyay ND, Shao C, Sarkar D, Fisher PB. Novel role of MDA-9/syntenin in regulating urothelial cell proliferation by modulating EGFR signaling. Clin Cancer Res. 2013;19:4621–4633. doi: 10.1158/1078-0432.CCR-13-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashyap R, Roucourt B, Lembo F, Fares J, Carcavilla AM, Restouin A, Zimmermann P, Ghossoub R. Syntenin controls migration, growth, proliferation, and cell cycle progression in cancer cells. Front Pharmacol. 2015;6:241. doi: 10.3389/fphar.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kegelman TP, Das SK, Hu B, Bacolod MD, Fuller CE, Menezes ME, Emdad L, Dasgupta S, Baldwin AS, Bruce JN, Dent P, Pellecchia M, Sarkar D, Fisher PB. MDA-9/syntenin is a key regulator of glioma pathogenesis. Neuro Oncol. 2014;16:50–61. doi: 10.1093/neuonc/not157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Zhang X, Lv Y, Xiang J, Shi J. Overexpression of syntenin enhances hepatoma cell proliferation and invasion: potential roles in human hepatoma. Oncol Rep. 2014;32:2810–2816. doi: 10.3892/or.2014.3498. [DOI] [PubMed] [Google Scholar]
- 14.Qian XL, Li YQ, Yu B, Gu F, Liu FF, Li WD, Zhang XM, Fu L. Syndecan binding protein (SDCBP) is overexpressed in estrogen receptor negative breast cancers, and is a potential promoter for tumor proliferation. PLoS One. 2013;8:e60046. doi: 10.1371/journal.pone.0060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong D, Ran JH, Tang WY, Zhang XD, Tan Y, Chen GJ, Li XS, Yan Y. Mda-9/syntenin promotes human brain glioma migration through focal adhesion kinase (FAK)-JNK and FAK-AKT signaling. Asian Pac J Cancer Prev. 2012;13:2897–2901. doi: 10.7314/apjcp.2012.13.6.2897. [DOI] [PubMed] [Google Scholar]
- 16.Boukerche H, Su ZZ, Emdad L, Sarkar D, Fisher PB. mda-9/Syntenin regulates the metastatic phenotype in human melanoma cells by activating nuclear factor-kappaB. Cancer Res. 2007;67:1812–1822. doi: 10.1158/0008-5472.CAN-06-3875. [DOI] [PubMed] [Google Scholar]
- 17.Boukerche H, Su ZZ, Prevot C, Sarkar D, Fisher PB. mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src. Proc Natl Acad Sci U S A. 2008;105:15914–15919. doi: 10.1073/pnas.0808171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das SK, Bhutia SK, Kegelman TP, Peachy L, Oyesanya RA, Dasgupta S, Sokhi UK, Azab B, Dash R, Quinn BA, Kim K, Barral PM, Su ZZ, Boukerche H, Sarkar D, Fisher PB. MDA-9/syntenin: a positive gatekeeper of melanoma metastasis. Front Biosci (Landmark Ed) 2012;17:1–15. doi: 10.2741/3911. [DOI] [PubMed] [Google Scholar]
- 19.Das SK, Bhutia SK, Sokhi UK, Azab B, Su ZZ, Boukerche H, Anwar T, Moen EL, Chatterjee D, Pellecchia M, Sarkar D, Fisher PB. Raf kinase inhibitor RKIP inhibits MDA-9/syntenin-mediated metastasis in melanoma. Cancer Res. 2012;72:6217–6226. doi: 10.1158/0008-5472.CAN-12-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gangemi R, Mirisola V, Barisione G, Fabbi M, Brizzolara A, Lanza F, Mosci C, Salvi S, Gualco M, Truini M, Angelini G, Boccardo S, Cilli M, Airoldi I, Queirolo P, Jager MJ, Daga A, Pfeffer U, Ferrini S. Mda-9/syntenin is expressed in uveal melanoma and correlates with metastatic progression. PLoS One. 2012;7:e29989. doi: 10.1371/journal.pone.0029989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan M, Chen X, Ma Y, Tang L, Guan L, Ren X, Yu B, Zhang W, Su B. MDA-9 and GRP78 as potential diagnostic biomarkers for early detection of melanoma metastasis. Tumour Biol. 2015;36:2973–2982. doi: 10.1007/s13277-014-2930-9. [DOI] [PubMed] [Google Scholar]
- 22.Stier S, Totzke G, Grunewald E, Neuhaus T, Fronhoffs S, Sachinidis A, Vetter H, Schulze-Osthoff K, Ko Y. Identification of syntenin and other TNF-inducible genes in human umbilical arterial endothelial cells by suppression subtractive hybridization. FEBS Lett. 2000;467:299–304. doi: 10.1016/s0014-5793(00)01177-7. [DOI] [PubMed] [Google Scholar]
- 23.Lin JJ, Jiang H, Fisher PB. Melanoma differentiation associated gene-9, mda-9, is a human gamma interferon responsive gene. Gene. 1998;207:105–110. doi: 10.1016/s0378-1119(97)00562-3. [DOI] [PubMed] [Google Scholar]
- 24.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 25.Saha SK, Roy S, Khuda-Bukhsh AR. Ultra-highly diluted plant extracts of Hydrastis canadensis and Marsdenia condurango induce epigenetic modifications and alter gene expression profiles in HeLa cells in vitro. J Integr Med. 2015;13:400–411. doi: 10.1016/S2095-4964(15)60201-1. [DOI] [PubMed] [Google Scholar]
- 26.Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, Chang Q, Chu GC, Al-Khalil R, Jiang S, Xia H, Fletcher-Sananikone E, Lim C, Horwitz GI, Viale A, Pettazzoni P, Sanchez N, Wang H, Protopopov A, Zhang J, Heffernan T, Johnson RL, Chin L, Wang YA, Draetta G, DePinho RA. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gravendeel LA, Kouwenhoven MC, Gevaert O, de Rooi JJ, Stubbs AP, Duijm JE, Daemen A, Bleeker FE, Bralten LB, Kloosterhof NK, De Moor B, Eilers PH, van der Spek PJ, Kros JM, Sillevis Smitt PA, van den Bent MJ, French PJ. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 2009;69:9065–9072. doi: 10.1158/0008-5472.CAN-09-2307. [DOI] [PubMed] [Google Scholar]
- 28.Senft C, Priester M, Polacin M, Schroder K, Seifert V, Kogel D, Weissenberger J. Inhibition of the JAK-2/STAT3 signaling pathway impedes the migratory and invasive potential of human glioblastoma cells. J Neurooncol. 2011;101:393–403. doi: 10.1007/s11060-010-0273-y. [DOI] [PubMed] [Google Scholar]
- 29.Okumura F, Yoshida K, Liang F, Hatakeyama S. MDA-9/syntenin interacts with ubiquitin via a novel ubiquitin-binding motif. Mol Cell Biochem. 2011;352:163–172. doi: 10.1007/s11010-011-0750-4. [DOI] [PubMed] [Google Scholar]
- 30.Weissenberger J, Loeffler S, Kappeler A, Kopf M, Lukes A, Afanasieva TA, Aguzzi A, Weis J. IL-6 is required for glioma development in a mouse model. Oncogene. 2004;23:3308–3316. doi: 10.1038/sj.onc.1207455. [DOI] [PubMed] [Google Scholar]
- 31.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. Journal of Clinical Oncology. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]


