Abstract
Although various kinds of organic molecules are known to occur in fossils and rocks, most soft tissue preservation in animals is attributed to melanin or porphyrins. Lipids are particularly stable over time—as diagenetically altered ‘geolipids’ or as major molecular constituents of kerogen or fossil ‘geopolymers’—and may be expected to be preserved in certain vertebrate tissues. Here we analysed lipid residues from the uropygial gland of an early Eocene bird using pyrolysis gas chromatography mass spectroscopy. We found a pattern of aliphatic molecules in the fossil gland that was distinct from the host oil shale sediment matrix and from feathers of the same fossil. The fossil gland contained abundant n-alkenes, n-alkanes and alkylbenzenes with chain lengths greater than 20, as well as functionalized long-chain aldehydes, ketones, alkylnitriles and alkylthiophenes that were not detected in host sediment or fossil feathers. By comparison with modern bird uropygial gland wax esters, we show that these molecular fossils are likely derived from endogenous wax ester fatty alcohols and fatty acids that survived initial decay and underwent early diagenetic geopolymerization. These data demonstrate the high fidelity preservation of the uropygial gland waxes and showcase the resilience of lipids over geologic time and their potential role in the exceptional preservation of lipid-rich tissues of macrofossils.
Keywords: soft tissue preservation, Messel, molecular fossils, uropygial gland, fossil bird, Eocene
1. Introduction
Compared to biomineralized fossils comprised of calcium carbonate or calcium phosphate, the preservation of animal soft tissue is exceedingly rare over geologic time. Nevertheless, there are documented examples of exceptionally preserved organic tissues going back to the Paleozoic [1–6]. Arguably, the early Eocene (approx. 48 Ma; [7]) sediments of the Messel UNESCO World Heritage Site in Germany yield some of the most outstanding examples of soft tissue preservation in the fossil record, and numerous vertebrate fossils preserve remains of integumentary structures, such as feathers and hair [8]. It has been shown that much of the soft tissue preservation in Messel and other fossil sites is due to the preservation of melanosomes which trace the plumage and fur of birds and mammals, respectively [2,6,9]. However, the Messel site also yields other examples of soft tissue preservation that are unparalleled among other fossil sites (e.g. [10,11]). One of the most unusual examples concerns the preservation of soft tissue structures in fossil birds that correspond in size and morphology with the uropygial gland (also called the preen or oil gland) of birds [11]. This sebaceous gland is situated at the base of the tail and secretes an oil that contains a variety of wax esters (long-chain fatty alcohols esterified to fatty acids) that play an important role in preening, i.e. feather maintenance [12,13]. However, although an identification of uropygial gland remains in some bird fossils from Messel is suggested by the position and shape of the fossil structures in question (figure 1), a characterization of the composition of these structures at the molecular level has yet to be conducted. In this study, and in order to validate the preservation of these structures at the molecular level, a bird specimen preserving a candidate fossil uropygial gland, was explored using pyrolysis gas chromatography-mass spectrometry (Py-GC-MS). We also characterized the fidelity and alteration of these through comparative experiments with modern uropygial glands.
Figure 1.
Photographs of the fossil bird specimen (SMF-ME 11593) showing the location (a) and detail of the preserved uropygial gland (b: before sampling in the unprepared fossil; c: after sampling in the prepared fossil). (Online version in colour.)
2. Material and methods
The fossil specimen is catalogued in the collection of the Senckenberg Research Institute under the number SMF-ME 11593. The fossil is from a very small bird that appears to represent an undescribed new species. Overall, the bones show a resemblance to those of the Messelirrisoridae, but there are some differences in the length proportions of the bones. A reliable determination of this species has to await the discovery of future specimens, in which the skull and legs are preserved, but we consider close affinities to the Messelirrisoridae likely. These upupiform birds are commonly preserved with uropygial gland residues in the Messel lake [11].
Microgram quantities of the fossil uropygial gland, two samples from fossil feathers and two samples from the surrounding oil shale sediment matrix were directly sampled from fresh excavations. The amount of fossil uropygial gland available for analysis was very limited and, since the pyrolysis yield and resulting signal was also uncertain, the fossil uropygial gland analysis could not be duplicated. Modern wax glands were dissected from fresh specimens of the Common Blackbird, Turdus merula (Turdidae, Passeriformes), the Ringed Teal, Callonetta leucophrys (Anatidae, Anseriformes) and the Middle Spotted Woodpecker, Dendrocopos medius (Picidae, Piciformes). Samples were pyrolysed using a CDS analytical 5250T Pyroprobe and pyrolysates were directed via a heated transfer line onto an Agilent 6890N gas chromatograph interfaced to a Waters Micromass AutoSpec Ultima mass spectrometer. Known amounts of commercial and laboratory standards (e.g. tasmanites, melanin, chitin, lignin) were pyrolysed in the same analytical sequence to monitor analytical reproducibility. Internal standards were not added to samples due to concerns over very low analyte yield and potential masking of analyte signals. As such our data are only discussed qualitatively. Wax esters were extracted from modern uropygial glands using a modified Bligh and Dyer extraction [14] and analysed intact and as fatty alcohol and fatty acid constituents by GC-MS. A detailed description of analytical methods can be found in the electronic supplementary material and described elsewhere [15,16].
3. Results
(a). Fossils
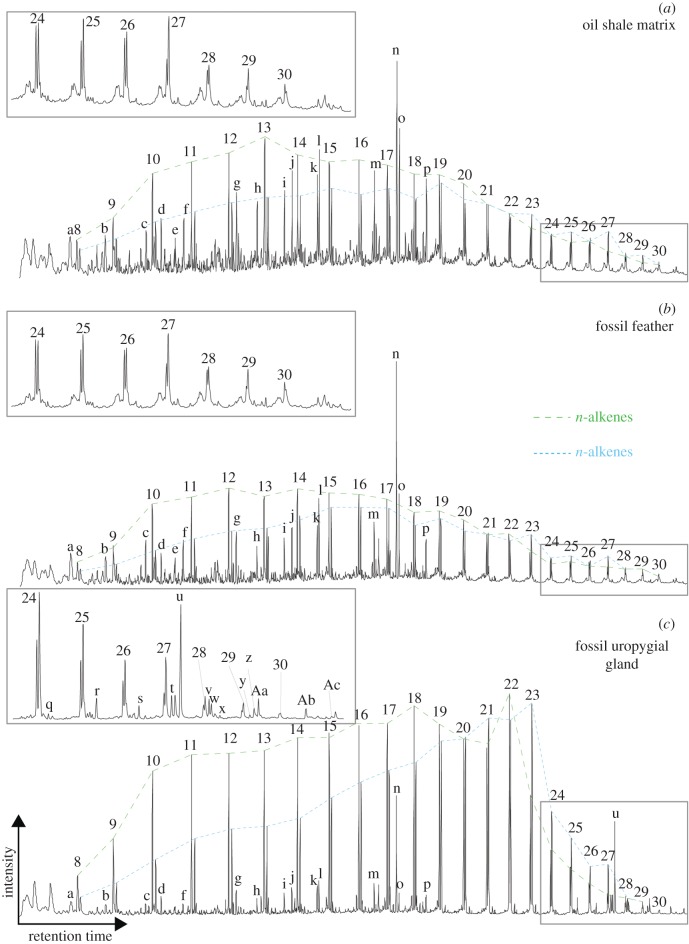
The major compounds in all samples were n-alkenes and n-alkanes ranging from C8 to C30 carbon chain lengths (figure 2). n-alkenes and n-alkanes were more prominent pyrolysates in the fossil uropygial gland (figure 2c) compared to the oil shale samples and fossil feather samples (figure 2a and b, respectively) and the n-alkene/n-alkane chain-length distributions were markedly different. The fossil gland yielded n-alkene Cmax at C22 and a slight even-over-odd carbon number preference between C13 to C23. n-alkane Cmax occurred at C23, with a slight n-alkane odd-over-even carbon number preference between C18 to C24. In contrast, the oil shale matrix and fossil feathers both exhibited an n-alkene Cmax at C13 and n-alkane Cmax at C19 and C16, respectively. The fossil gland also exhibited a distinct series of alkylbenzenes with enhanced C16 and C22 compared to the oil shale and feather samples (electronic supplementary material, figure S1).
Figure 2.
Partial total ion pyrograms of the sediment matrix (a), fossil feather (b) and fossil uropygial gland (c), showing the occurrence of long-chain functionalized lipids in the fossil uropygial gland. Numbers correspond to the total number of carbon atoms for the alkene/alkane doublets. Letters correspond to pyrolysates given in table 1. (Online version in colour.)
Isoprenoid lipids were prominent in the oil shale samples and dominated by prist-1-ene, prist-2-ene, norpristane and 6,10,14-trimethyl-pentadecan-2-one (figure 2, peak m to p). A range of other polymethyl-branched hydrocarbons were also present, and generally in higher relative amounts in the oil shale sediment (table 1). Exact structures could not be assigned in all cases due to significant co-elution on pyrograms and a lack of suitable standards. Steroids and hopanoids were present in low amounts compared to other pyrolysates and yielded similar distributions in all samples. 22,29,30-trisnorhop-17(21)-ene and 22,29,30-trisnorhopane were the major compounds identified (data not shown).
Table 1.
Identity and fragment ions of major pyrolysates reported in this study.
| pyrolysate | M+ | fragment ions | |
|---|---|---|---|
| a | toluene | 92 | 91 |
| b | xylene | 106 | 91 |
| c | ethyltoluene | 120 | 105 |
| d | 4-methyldecene | 154 | 57, 71, 112 |
| e | 2-methylphenol | 108 | 77, 79, 107 |
| f | 3-methylphenol | 108 | 77, 79, 107 |
| g | 5-methyldodecane | 184 | 57, 98, 113 |
| h | dimethyldodecane | 198 | 57, 71, 113 |
| i | dimethyltridecene | 210 | 55, 69, 125, 140 |
| j | dimethyltridecane | 212 | 57, 71, 113, 127 |
| k | dimethyltetradecene | 224 | 57, 71, 113, 127 |
| l | dimethyltetradecane | 226 | 57, 71, 113, 127, 141 |
| m | nor-pristane | 254 | 57, 169, 183 |
| n | prist-1-ene | 266 | 56, 69, 126, 111, 83, 196 |
| o | prist-2-ene | 266 | 69, 57, 126, 111, 83, 196 |
| p | 6,10,14-trimethyl pentadecan-2-one | 268 | 58, 71, 109, 179, 210, 250 |
| q | docosanal | 306 | 57, 82, 96 |
| r | tricosanal | 320 | 57, 82, 96 |
| s | tetracosanal | 334 | 57, 82, 96 |
| t | pentacosan-2-one | 366 | 58, 71 |
| u | pentacosanal | 348 | 57, 82, 96 |
| v | hexacosan-3-one | 380 | 57, 72, 351 |
| w | hexacosan-2-one | 380 | 58, 71 |
| x | hexacosanal | 362 | 57, 82, 96 |
| y | heptacosan-2-one | 394 | 58, 71 |
| z | pentacosanenitrile | 363 | 57, 97, 236, 250 |
| Aa | heptacosanal | 378 | 57, 82, 96 |
| Ab | tricosylthiophene | 406 | 97 |
| Ac | tetracosylthiophene | 420 | 97 |
| Ad | styrene | 104 | 103, 78 |
| Ae | phenol | 94 | 66 |
| Af | indole | 117 | 90, 89 |
| Ag | methylindole | 131 | 130, 103 |
We also detected a number of long-chain functionalized compounds in the fossil gland (figure 2c inset and table 1, compounds q - Ac) that were absent from the oil shale matrix and fossil feather. n-alkanals with carbon chain lengths from C22 to C27 with an odd-over-even carbon number preference were detected. Pentacosanal was the major aldehyde, representing about 65% of total n-alkanals. Alkan-2-ones were detected, with pentacosan-2-one, hexacosan-2-one and heptacosan-2-one dominating. Trace amounts were detected at carbon chain lengths as low as C20. Hexacosan-3-one was also detected. C19 to C24 alkylthiophenes (majorly tricosylthiophene and tetracosylthiophene) and C23 to C27 alkylnitriles (pentacosanenitrile was the major compound) were also detected.
Low molecular weight benzyl and phenolic pyrolysates were detected in all samples. These included toluene, xylene, ethyltoluene, 2- and 3-methylphenol and trace amounts of dimethyl- and trimethylthiophenes (figure 2, table 1). Nitrogen-containing compounds were limited to the long-chain alkylnitriles in the fossil uropygial gland.
(b). Lipid composition and pyrolysis of modern wax glands
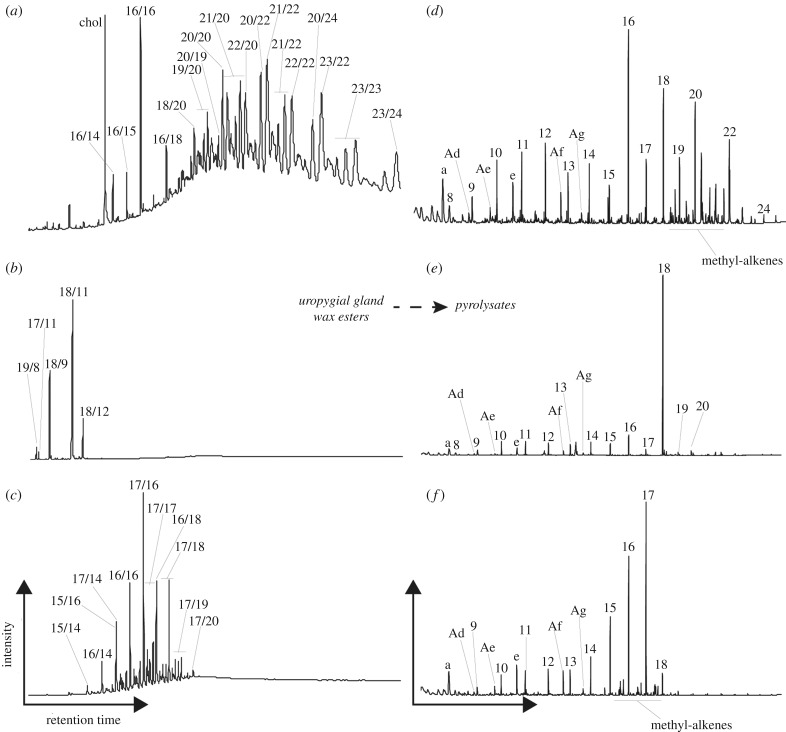
T. merula wax esters ranged from C30 to C47 (figure 3a) with normal fatty alcohols being between C16 to C23 and normal and methyl-branched fatty acids being between C14 to C24. The branched fatty acids contained straight-chain fatty acids between 14 and 22 carbon chain lengths and 2,6-dimethyl fatty acids between 18 and 22 carbon chain lengths (electronic supplementary material, figure S2). Fatty alcohols were dominated by straight-chain and methyl-branched fatty alcohols between C16 and C22. C. leucophrys wax esters contained five wax esters between C27 and C30 (figure 3b), primarily octadecan-1-ol esterified to undecanoic acid, nonanoic acid, dodecanoic acid and octanoic acid. D. medius uropygial gland wax esters ranged between C29 and C37 (figure 2c), with n-heptadecan-1-ol and n-hexadecan-1-ol as major fatty alcohols. Fatty acids consisted of mixtures of C16 and C18 normal and C13 to C15 3-methyl homologues (electronic supplementary material, figure S2). Methyl-branched and 1,2-diol fatty alcohols were also detected in lower amounts.
Figure 3.
Partial total ion chromatograms of wax ester extracts (a–c) and corresponding pyrograms (d–f) of uropygial glands from specimens of the Common Blackbird, Turdus merula (a,d), the Ringed Teal, Callonetta leucophrys (b,e) and the Middle Spotted Woodpecker, Dendrocopos medius (c,f). Numbers correspond to the total number of carbon atoms for n-alkenes, methyl-branched alkenes or wax esters. Wax ester nomenclature corresponds to x/y, where x is the fatty alcohol and y is the fatty acid. Letters correspond to pyrolysates given in table 1. ‘Chol. is cholesterol.
Pyrolysis of the T. merula uropygial gland yielded n-alkenes ranging from C8 to C24 and a number of methyl-branched alkenes (figure 3d). n-alkanes were not detected. Pyrolysis of the C. leucophrys uropygial gland produced n-alkenes from C8 to C20, with n-octadecene being the major pyrolysate (figure 3e). D. medius pyrolysates were dominated by C9 to C18 n-alkenes and lower amounts of C16 to C18 methyl alkenes (figure 3f). n-heptadecene, n-hexadecene and n-pentadecene were the major pyrolysates. Toluene, styrene, phenol, 2-methylphenol, indole and methylindole were also detected (figure 3, table 1).
4. Discussion
While fossilized lipids are common in the plant fossil record and are also known from prokaryotes and eukaryotes as sedimentary biomarkers, endogenous lipids from vertebrate fossils older than the Neogene and Holocene are rare [17]. We report the first chemical characterization of exceptionally preserved bird soft tissues that, based on their shape, size and anatomical position are consistent with being uropygial glands of birds [11]. The high similarity between oil shale and fossil feather pyrograms indicates that there is considerable diagenetic replacement or contamination of the fossil feathers with exogenous organic matter. However, we observed distinct n-alkene, n-alkane and alkylbenzene distributions and long carbon chain functionalized aliphatic ketones, aldehydes, nitriles and thiophenes in the fossil gland compared to the oil shale matrix and fossil feathers. Pyrolysis experiments on modern specimens of the Common Blackbird, Turdus merula (Turdidae, Passeriformes), the Ringed Teal, Callonetta leucophrys (Anatidae, Anseriformes) and the Middle Spotted Woodpecker, Dendrocopos medius (Picidae, Piciformes) allowed us to explore whether the unusual and distinct pyrolysates from the fossil were from intact largely unaltered wax esters or from diagenetic products of the original wax esters. Comparison with solvent-extracted wax esters also allowed us to assess whether or not certain pyrolysates were analytical artefacts created during the pyrolysis process.
Uropygial glands in extant birds primarily contain wax esters composed of saturated and/or branched fatty acids esterified to fatty alcohols and/or hydroxy acids [12,18,19]. Under our experimental conditions, Py-GC-MS of the modern bird uropygial glands showed that constituent wax esters are cleaved and converted to alkenes. n-alkene and n-alkane doublet series are common in most sedimentary kerogen pyrolysates, and are thought to derive from cleavage of insoluble macromolecularly bound aliphatic geopolymers (e.g. [20–22]). We detected clear alkane/alkane doublet series in our fossils but no alkanes were detected when we pyrolysed our modern uropygial glands. This demonstrates that the fossil uropygial gland was preserved as a macromolecular geopolymer rather than being due to preservation of original wax ester biomolecules. No markers for protein [23,24] were detected in the fossils, indicating that protein has not been preserved. Indole and methylindole were the major nitrogen-containing pyrolysates in the modern uropygial glands and are likely derived from protein [23].
Most of the fatty alcohols in our modern uropygial glands were converted to n-alkenes with the same carbon chain length, allowing us to reconstruct the fatty alcohol content of our modern specimens. C. leucophrys wax esters were overwhelmingly dominated by n-octadecan-1-ol and n-octadecene on pyrolysis while D. medius wax esters, containing n-heptadecan-1-ol, hexadecan-1-ol and pentadecan-1-ol, yielded corresponding n-alkene pyrolysates. We propose that the anomalously high amounts of docosene observed in the fossil uropygial gland is derived from n-docosan-1-ol and that this was the original and major fatty alcohol component of the uropygial waxes of the Messel fossil. Methyl and dimethyl alkenes were detected as pyrolysates from T. merula and D. medius and are sourced from the branched fatty acids of the wax esters. Overall the relationship between the fatty acid component of wax esters and their pyrolysates is less clear than the fatty alcohols. For example, undecanoic acid and nonanoic acid were the major acids in C. leucophrys wax esters but did not yield abundant corresponding alkenes or alkanes. Based on the number of short-chain alkenes with a broad distribution that do not match any uropygial gland lipid precursors from our analysis, it is evident that numerous cleavage and rearrangement reactions occur during pyrolysis of the wax esters. Additional experimentation using thermochemolysis with tetramethylammonium hydroxide would have permitted methylation of fatty acids if present and simplification of pyrolysis products [25]. Unfortunately, additional thermochemolysis was not possible for our study due to very limited sample availability.
Previous reports suggest that long-chain alkylbenzenes are artefacts created during pyrolysis of triacylglycerols or unsaturated fatty acids [26]. However, we only detected alkylbenzenes in the fossil samples (not in the modern uropygial glands) and since long-chain alkylbenzenes can be found in solvent extracts of sediments [27], we infer that long-chain alkylbenzenes are produced during early diagenetic reactions of precursor lipids. Our data are also not consistent with long-chain alkylnitriles being produced by reactions of lipids with protein during pyrolysis [20] or from algal biopolymers [28]. We only observed long-chain alkylnitriles in the fossil uropygial gland and did not detect them in our experiments with modern uropygial glands, which contain nitrogen-containing indole and methylindole (figure 3; [23]). Additionally, we did not detect long-chain alkylnitriles in the algal-rich oil shale sediment, suggesting that algal organic matter is not a source. While long-chain alkanones have been reported as pyrolysates of lacustrine kerogens and presumed to be from algal biopolymers [29,30], we did not detect these compounds in the algal-dominated oil shale sediment. The chain length of the functionalized lipids in the fossil uropygial gland are longer than the typical chain lengths of uropygial gland wax esters. It has been shown that pyrolysis of fatty acids salts and fatty acids adsorbed to mineral matrices produces mid-chain ketones by ketonic decarboxylation [31,32]. This does not explain the occurrences of alkan-2-ones, alkan-3-ones or aldehydes here. Numerous studies have suggested that alkan-2-one pyrolysates are decarboxylation products of keto-acids, which are formed during microbial β-oxidation of fatty acids (e.g. [33]). We hypothesize that the long-chain functionalized lipids detected in the fossil uropygial gland are primarily diagenetic products of unusual very long-chain fatty acids from the Messel bird uropygial gland wax esters. Based on the unusual and distinct distributions of hydrocarbon and functionalized aliphatics in the fossil uropygial gland, we propose that not only is the organic matter endogenous to the gland, but that a portion of its original chemical identity has been retained over the past 48 Ma.
The mechanisms by which organic tissues can be preserved over geological time are still actively debated. Proposed models include the ‘selective preservation’ of recalcitrant classes of biomolecules [5,34–36]; in situ ‘geopolymerization’ of organic matter during early diagenesis [20–22,37], as well as incorporation of inorganic sulfur into organic matter (sulfurization) during diagenesis [38–41]. The relative degree to which each of these processes may contribute to organic preservation will depend on the specific environmental and depositional conditions and on the original composition of organisms and tissue types [42]. Alkylthiophenes are common in marine sediments [43,44], and their presence has been given as evidence for the early diagenetic incorporation of inorganic sulfur species into labile functionalized lipids or other susceptible biomolecules [37–40]. Here, we identified a very limited range and low abundance of these compounds (tricosylthiophene and tetracosyltiophene, and trace amounts of dimethyl thiophenes). While others have proposed that sulfurization is an important process responsible for the preservation of vertebrate fossils [41], this mechanism does not appear to have played a significant role here.
Our data support the conclusion that exceptional preservation of the uropygial gland has primarily occurred via geopolymer formation. We have shown that the fossil uropygial gland can still be distinguished at the molecular level, indicating that decay and contamination by endogenous organic matter was restricted. Uropygial oil is known to have an anti-bacterial role in living birds [45] and we hypothesize that the wax esters may also have a role in inhibiting early bacterial colonization and initial decay of uropygial glands upon death. Ester bonds would still be expected to hydrolyse relatively rapidly, and the resulting free fatty acids and fatty alcohols are unlikely to survive over geologic time without being encapsulated in minerals (e.g. [5]). Therefore, we favour rapid geopolymer formation during early diagenesis, i.e. at low temperature and pressure [22]. We propose the following preservation model: (i) limited initial bacterial colonization and decay due to the anti-bacterial properties of uropygial wax, (ii) hydrolysis of uropygial gland wax esters after death and initial deposition of the bird, (iii) limited degradation of recalcitrant and relatively insoluble long-chain fatty acids and fatty alcohols, and (iv) molecular transformation and polymerization of these aliphatic lipids in the early diagenetic window and subsequent preservation as a geopolymer over geologic time. The combined inherent chemical stability of lipids and geopolymerization may be important for other organic-rich preserved soft tissues, such as melanin [4,6,46], and may account for exceptional preservation of lipid-rich arthropod nervous tissues from Cambrian shales [47,48].
5. Conclusion
Our study provides evidence for the preservation of endogenous lipids within 48 Ma fossil bird soft tissues and supports previous reports by Mayr [11] that these structures are the first documented examples of preserved bird uropygial glands. Our data indicate that this soft tissue preserves as a diagenetically altered geopolymer. Despite clear structural rearrangement over time the fossil uropygial gland can be clearly distinguished at the molecular level. We propose that the original lipid composition can be reconstructed using Py-GC-MS and that the original uropygial gland contained, and was perhaps dominated by, long-chain C22 to C28 fatty alcohols and fatty acids. Our data show that molecular signals from endogenous wax lipids survive, probably as a combined result of anti-bacterial properties of uropygial oil, chemical recalcitrance of fatty acid and fatty alcohols and rapid early diagenetic geopolymerization. These combined processes may be important for the preservation of molecular information of other organic tissues, particularly lipid-rich tissues, over geologic timescales and calls for more detailed organic geochemical investigations of other fossils.
Supplementary Material
Acknowledgements
We thank Michael Ackermann (SMF) for sampling the fossil, Stephan Schaal for access to the Messel collection of SMF, Sonja Wedmann (SMF) for providing pictures of the wax gland before preparation, Sven Tränkner (SMF) for taking photos of the prepared fossil specimen, Jens Holtvoeth (UoB) for providing combusted aluminium foil, Brian Kelleher (Dublin City University) for assistance in wax ester analysis, and Frank McDermott (University College Dublin) for advice during manuscript preparation.
Data accessibility
Pyrolysis GC-MS and lipid extraction GC-MS data are available from the Dryad Digital Repository: (http://dx.doi.org/10.5061/dryad.7g1tb) [49].
Authors' contributions
G.M. and J.V. conceived the study. G.M. prepared the modern uropygial glands. S.O.R. performed and interpreted pyrolysis and lipid analysis. R.S. assisted in analysis and data interpretation. All authors wrote the manuscript and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
We gratefully thank the Irish Research Council and the Marie Skłodowska-Curie Actions Programme under the ELEVATE (ELEVATEPD/2014/47) career development research fellowship (S.O.R.). Research at MIT was supported by a grant from the NASA Astrobiology Institute (NNA13AA90A) Foundations of Complex Life, Evolution, Preservation, and Detection on Earth and Beyond.
References
- 1.Butterfield NJ. 1994. Burgess Shale-type fossils from a Lower Cambrian shallow-shelf sequence in northwestern Canada. Nature 369, 477–479. ( 10.1038/369477a0) [DOI] [Google Scholar]
- 2.Vinther J, Briggs DEG, Prum RO, Saranathan V. 2008. The colour of fossil feathers. Biol. Lett. 4, 522–525. ( 10.1098/rsbl.2008.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta NS, Briggs DEG. 2010. Taphonomy of animal organic skeletons through time. In Taphonomy (eds Allison PA, Bottjer DJ) pp. 199–221. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 4.Glass K, et al. 2012. Direct chemical evidence for eumelanin pigment from the Jurassic period. Proc. Natl Acad. Sci. USA 109, 10 218–10 223. ( 10.1073/pnas.1118448109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melendez I, Grice K, Schwark L. 2013. Exceptional preservation of Palaeozoic steroids in a diagenetic continuum. Sci. Rep. 3, 2768 ( 10.1038/srep02768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colleary C, et al. 2015. Chemical, experimental, and morphological evidence for diagenetically altered melanin in exceptionally preserved fossils. Proc. Natl Acad. Sci. USA 112, 12 592–12 597. ( 10.1073/pnas.1509831112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenz OK, Wilde V, Mertz DF, Riegel W. 2015. New palynology-based astronomical and revised 40Ar/39Ar ages for the Eocene maar lake of Messel (Germany). Int. J. Earth Sci. 104, 873–889. ( 10.1007/s00531-014-1126-2) [DOI] [Google Scholar]
- 8.Schaal S, Ziegler W. 1992. Messel: an insight into the history of life and of the earth. Oxford, England: Oxford University Press. [Google Scholar]
- 9.Vinther J, Briggs DEG, Clarke J, Mayr G, Prum RO. 2010. Structural coloration in a fossil feather. Biol. Lett. 6, 128–131. ( 10.1098/rsbl.2009.0524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadena E. 2016. Microscopical and elemental FESEM and Phenom ProX-SEM-EDS analysis of osteocyte- and blood vessel-like microstructures obtained from fossil vertebrates of the Eocene Messel Pit, Germany. PeerJ 4, e1618 ( 10.7717/peerj.1618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayr G. 2006. New specimens of the Eocene Messelirrisoridae (Aves: Bucerotes), with comments on the preservation of uropygial gland waxes in fossil birds from Messel and the phylogenetic affinities of Bucerotes. Palaontol. Z. 80, 390–405. ( 10.1007/BF02990211) [DOI] [Google Scholar]
- 12.Jacob J, Grimmer G. 1975. Composition of uropygial gland waxes in relation to the classification of some passerine birds. Biochem. Syst. Ecol. 3, 267–271. ( 10.1016/0305-1978(75)90013-7) [DOI] [Google Scholar]
- 13.Spearman RIC, Hardy JA. 1985. Integument. In Form and function in birds, vol III (eds King A, McLelland J) pp. 1–56. London, UK: Academic Press. [Google Scholar]
- 14.White DC, Ringelberg DB. 1998. Signature lipid biomarker analysis. In Techniques in microbial ecology (eds Burlage RS, Atlas R, Stahl D, Geesey G, Sayler G), pp. 255–272. New York, USA: Oxford University Press. [Google Scholar]
- 15.O'Reilly SS, et al. 2017. Molecular biosignatures reveal common benthic microbial sources of organic matter in ooids and grapestones from Pigeon Cay, The Bahamas. Geobiology 15, 112–130. ( 10.1111/gbi.12196) [DOI] [PubMed] [Google Scholar]
- 16.Dekker MH, Piersma T, Sinninghe Damsté JS. 2000. Molecular analysis of intact preen waxes of Calidris canutus (Aves: Scolopacidae) by gas chromatography/mass spectrometry. Lipids 35, 533–541. ( 10.1007/s11745-000-553-7) [DOI] [PubMed] [Google Scholar]
- 17.Thiel V, Blumenberg M, Kiel S, Leefman T, Liebenau K, Lindgren J, Sjövall P, Treude T, Zilla T. 2014. Occurrence and fate of fatty acyl biomarkers in an ancient whale bone (Oligocene, El Cien formation, Mexico). Org. Geochem. 68, 71–81. ( 10.1016/j.orggeochem.2013.12.006) [DOI] [Google Scholar]
- 18.Poltz J, Jacob J. 1975. The uropygial gland secretions of birds of the family sylviidae. Biochem. Syst. Ecol. 3, 57–62. ( 10.1016/0305-1978(75)90042-3) [DOI] [Google Scholar]
- 19.Jacob J, Poltz J. 1975. Composition of uropygial gland secretions of birds of prey. Lipids 10, 1–8. ( 10.1007/BF02532185) [DOI] [PubMed] [Google Scholar]
- 20.Gupta NS, Steele A, Fogel M, Griffin P, Adams M, Summons RE, Yang H, Cody GD. 2014. Experimental formation of geomacromolecules from microbial lipids. Org. Geochem. 67, 35–40. ( 10.1016/j.orggeochem.2013.11.006) [DOI] [Google Scholar]
- 21.Gupta NS, Michels R, Briggs DEG, Evershed RP, Pancost RD. 2006. The organic preservation of fossil arthropods: an experimental study. Proc. R. Soc B 273, 2777–2783. ( 10.1098/rspb.2006.3646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta NS, Cody GD, Tetlie OE, Briggs DEG, Summons RE. 2009. Rapid incorporation of lipids into macromolecules during experimental decay of invertebrates: initiation of geopolymer formation. Org. Geochem. 40, 589–594. ( 10.1016/j.orggeochem.2009.02.005) [DOI] [Google Scholar]
- 23.Biller P, Ross AB. 2014. Pyrolysis GC–MS as a novel analysis technique to determine the biochemical composition of microalgae. Algal Res. 6, 91–97. ( 10.1016/j.algal.2014.09.009) [DOI] [Google Scholar]
- 24.Saitta E, et al. 2017. Low fossilisation potential of keratin protein revealed by experimental taphonomy. Palaeontology 60, 547–556. ( 10.1111/pala.12299) [DOI] [Google Scholar]
- 25.Versteegh GJ, Blokker P, Wood GD, Collinson ME, Damsté JS, de Leeuw JW. 2004. An example of oxidative polymerization of unsaturated fatty acids as a preservation pathway for dinoflagellate organic matter. Org. Geochem. 35, 1129–1139. ( 10.1016/j.orggeochem.2004.06.012) [DOI] [Google Scholar]
- 26.Saiz-Jimenez C. 1995. The origin of alkylbenzenes and thiophenes in pyrolysates of geochemical samples. Org. Geochem. 23, 81–85. ( 10.1016/0146-6380(94)00102-7) [DOI] [Google Scholar]
- 27.Sinninghe Damsté JS, Keely BJ, Betts SE, Baas M, Maxwell JR, de Leeuw JW. 1993. Variations in abundances and distributions of isoprenoid chromans and long-chain alkylbenzenes in sediments of the Mulhouse Basin: a molecular sedimentary record of palaeosalinity. Org. Geochem. 20, 1201–1215. ( 10.1016/0146-6380(93)90009-Z) [DOI] [Google Scholar]
- 28.Blumenberg M, Thiel V, Reitner J. 2015. Organic matter preservation in the carbonate matrix of a recent microbial mat—Is there a ‘mat seal effect’? Org. Geochem. 87, 25–34. ( 10.1016/j.orggeochem.2015.07.005) [DOI] [Google Scholar]
- 29.Gelin F, Boogers I, Noordeloos AAM, Damsté JSS, Hatcher PG, de Leeuw JW. 1996. Novel, resistant microalgal polyethers: An important sink of organic carbon in the marine environment? Geochim. Cosmochim. Acta 60, 1275–1280. ( 10.1016/0016-7037(96)00038-5) [DOI] [Google Scholar]
- 30.Gelin F. 1996. Isolation and chemical characterization of resistant macromolecular constituents in microalgae and marine sediments. PhD thesis, Utrecht University. [Google Scholar]
- 31.Raven AM, Van Bergen PF, Stott AW, Dudd SN, Evershed RP. 1997. Formation of long-chain ketones in archaeological pottery vessels by pyrolysis of acyl lipids. J. Anal. Appl. Pyrolysis 40, 267–285. ( 10.1016/S0165-2370(97)00036-3) [DOI] [Google Scholar]
- 32.Zafar R, Watson JS, Weiss DJ, Sephton MA. 2017. Organic compound-mineral interactions: Using flash pyrolysis to monitor the adsorption of fatty acids on calcite. J. Anal. Appl. Pyrolysis 123, 184–193. ( 10.1016/j.jaap.2016.12.007) [DOI] [Google Scholar]
- 33.Teerman SC, Hwang RJ. 1991. Evaluation of the liquid hydrocarbon potential of coal by artificial maturation techniques. Org. Geochem. 17, 749–764. ( 10.1016/0146-6380(91)90019-G) [DOI] [Google Scholar]
- 34.Melendez I, Grice K, Trinajstic K, Ladjavardi M, Greenwood P, Thompson K. 2012. Biomarkers reveal the role of photic zone euxinia in exceptional fossil preservation: An organic geochemical perspective. Geology 41, 123–126. ( 10.1130/G33492.1) [DOI] [Google Scholar]
- 35.Derenne S, Largeau C, Casadevall E, Berkaloff C, Rousseau B. 1991. Chemical evidence of kerogen formation in source rocks and oil shales via selective preservation of thin resistant outer walls of microalgae: origin of ultralaminae. Geochim. Cosmochim. Acta 55, 1041–1050. ( 10.1016/0016-7037(91)90162-X) [DOI] [Google Scholar]
- 36.Largeau C, Derenne S. 1993. Relative efficiency of the selective preservation and degradation recondensation pathways in kerogen formation. Source and environment influence on their contributions to type I and II kerogens. Org. Geochem. 20, 611–615. ( 10.1016/0146-6380(93)90027-9) [DOI] [Google Scholar]
- 37.Stankiewicz BA, Briggs DEG, Michels R, Collinson ME, Flannery MB, Evershed RP. 2000. Alternative origin of aliphatic polymer in kerogen. Geology 28, 559–562. ( 10.1130/0091-7613(2000)28%3C559:AOOAPI%3E2.0.CO;2) [DOI] [Google Scholar]
- 38.Hebting Y, Schaeffer P, Behrens A, Adam P, Schmitt G, Schneckenburger P, Bernasconi SM, Albrecht P. 2006. Biomarker evidence for a major preservation pathway of sedimentary organic carbon. Science 312, 1627–1631. ( 10.1126/science.1126372) [DOI] [PubMed] [Google Scholar]
- 39.Sinninghe Damsté JS, Rijpstra WIC, Kock-van Dalen A, De Leeuw JW, Schenck P. 1989. Quenching of labile functionalised lipids by inorganic sulphur species: evidence for the formation of sedimentary organic sulphur compounds at the early stages of diagenesis. Geochim. Cosmochim. Acta 53, 1343–1355. ( 10.1016/0016-7037(89)90067-7) [DOI] [Google Scholar]
- 40.van Dongen BE, Schouten S, Baas M, Geenevasen JAJ, Sinninghe Damsté JS. 2003. An experimental study of the low-temperature sulfurization of carbohydrates. Org. Geochem. 34, 1129–1144. ( 10.1016/S0146-6380(03)00060-3) [DOI] [Google Scholar]
- 41.McNamara ME, van Dongen BE, Lockyer NP, Bull ID, Orr P. 2016. Fossilization of melanosomes via sulfurization. Paleontology 59, 337–350. ( 10.1111/palaa.12238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tegalaar E, de Leeuw J, Derenne S, Largeau C. 1989. A reappraisal of kerogen formation. Geochim. Cosmochim. Acta 53, 3103–3106. ( 10.1016/0016-7037(89)90191-9) [DOI] [Google Scholar]
- 43.Brassell SC, Lewis CA, de Leeuw JW, de Lange F, Sinninghe Damsté JS. 1986. Isoprenoid thiophenes: novel products of sediment diagenesis? Nature 320, 160–162. ( 10.1038/320160a0) [DOI] [Google Scholar]
- 44.Kohnen MEL, Damsté JSS, Kock-van Dalen AC, Haven HLT, Rullkötter J, De Leeuw JW. 1990. Origin and diagenetic transformations of C25 and C30 highly branched isoprenoid sulphur compounds: further evidence for the formation of organically bound sulphur during early diagenesis. Geochim. Cosmochim. Acta 54, 3053–3063. ( 10.1016/0016-7037(90)90121-Z) [DOI] [Google Scholar]
- 45.Shawkey MD, Pillai SR, Hill GE. 2003. Chemical Warfare? Effects of uropygial oil on feather-degrading bacteria. J. Avian Biol. 34, 345–349. ( 10.1111/j.0908-8857.2003.03193.x) [DOI] [Google Scholar]
- 46.Glass K, et al. 2013. Impact of diagenesis and maturation on the survival of eumelanin in the fossil record. Org. Geochem. 64, 29–37. ( 10.1016/j.orggeochem.2013.09.002) [DOI] [Google Scholar]
- 47.Ma X, Hou X, Edgecombe GD, Strausfeld NJ. 2012. Complex brain and optic lobes in an early Cambrian arthropod. Nature 490, 258–261. ( 10.1038/nature11495) [DOI] [PubMed] [Google Scholar]
- 48.Tanaka G, Hou X, Ma X, Edgecombe GD, Strausfeld NJ. 2013. Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 502, 364–367. ( 10.1038/nature12520) [DOI] [PubMed] [Google Scholar]
- 49.O'Reilly S, Summons R, Mayr G, Vinther J. 2017. Data from: Preservation of uropygial gland lipids in a 48-million-year-old bird Dryad Digital Repository. ( 10.5061/dryad.7g1tb) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- O'Reilly S, Summons R, Mayr G, Vinther J. 2017. Data from: Preservation of uropygial gland lipids in a 48-million-year-old bird Dryad Digital Repository. ( 10.5061/dryad.7g1tb) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Pyrolysis GC-MS and lipid extraction GC-MS data are available from the Dryad Digital Repository: (http://dx.doi.org/10.5061/dryad.7g1tb) [49].