Abstract
Iridescence—change of colour with changes in the angle of view or of illumination—is widespread in the living world, but its functions remain poorly understood. The presence of iridescence has been suggested in flowers where diffraction gratings generate iridescent colours. Such colours have been suggested to serve plant–pollinator communication. Here we tested whether a higher iridescence relative to corolla pigmentation would facilitate discrimination, learning and retention of iridescent visual targets. We conditioned bumblebees (Bombus terrestris) to discriminate iridescent from non-iridescent artificial flowers and we varied iridescence detectability by varying target iridescent relative to pigment optical effect. We show that bees rewarded on targets with higher iridescent relative to pigment effect required fewer choices to complete learning, showed faster generalization to novel targets exhibiting the same iridescence-to-pigment level and had better long-term memory retention. Along with optical measurements, behavioural results thus demonstrate that bees can learn iridescence-related cues as bona fide signals for flower reward. They also suggest that floral advertising may be shaped by competition between iridescence and corolla pigmentation, a fact that has important evolutionary implications for pollinators. Optical measurements narrow down the type of cues that bees may have used for learning. Beyond pollinator–plant communication, our experiments help understanding how receivers influence the evolution of iridescence signals generated by gratings.
Keywords: visual communication, discrimination learning, bumblebees, flower, speed–accuracy trade-off, colour–iridescence trade-off
1. Introduction
Iridescence is defined as the change in dominant wavelength with the viewing/illumination angle [1]. It is widespread in nature and originates from structures with periodicity at wavelength scale. Iridescence produces some of the most saturated and colourful displays in nature, but also very weak colorations. In plants, iridescence is present in leaves [2], fruits [3] and flowers [4], and is produced by multi-layer interference or by diffraction gratings [5]. The presence of iridescence in flowers has been suggested based on the regular striations on the epidermis surface [5] of the flowers in at least 12 different families, spanning from Liliaceae to Asteraceae or Solanaceae [4–7]. While iridescence may protect against destructive UV radiation in some species [7], it may also serve plant–pollinator communication. Among floral displays facilitating flower detection by pollinators, corolla pigment displays operate at large [8,9] or short distances (i.e. at large visual angles [10–12]). They have been shown to improve pollinator orientation and reward finding (references in [9]). Recent studies have shown that bumblebees are able to perceive floral or artificial iridescence irrespective of floral corolla coloration, and to use it to find food rewards [6,13]. Questions regarding the frequency of floral iridescence, its visibility under artificial or natural conditions, and its potential role in plant–pollinator communication [14–18] are still unexplored or lively debated, calling for more experimental research [17].
Efficient pollination requires that pollinators exhibit flower constancy (i.e. the tendency to restrict foraging bouts to one or few species or morphs with a known reward). Flower constancy limits pollen loss and ensures intraspecies fertility [19]. Constancy is particularly important when pollinators are generalists, like hymenopterans [20]. More specifically, constancy requires that pollinators learn and memorize associations between floral cues and reward (nectar or pollen) [21]. As flowers slightly vary in visual appearance (depending on orientation in space, illumination, developmental stage, etc.), constancy requires that pollinators generalize floral choice to novel situations, while preserving the learned cues [22,23]. Hence, any floral feature that would make the signal–reward association more easily learned, generalized or retained in long-term memory would be favoured by selection. If iridescence-related cues are used for foraging, what form of iridescent signals would be selected, especially given that it would interplay and potentially interfere with other flower displays like corolla coloration? Rare recent studies have brought some elements to that question: floral iridescence should be strong enough to provide visually exploitable cues [15], but not too strong, as this would corrupt flower identity and decrease constancy [13].
Here, we experimentally explored how floral iridescence and corolla pigment coloration interact in the context of pollinators' foraging choices. We trained bumblebees to discriminate between punishing non-iridescent targets and rewarding iridescent targets displaying a specific iridescence relative to pigment optical effect [6,13], which could be either high or low. We predicted that increasing the iridescence relative to pigment optical effect would enhance the detectability of rewarding targets, and thus accelerate and/or improve the efficiency of learning an iridescence–reward association, irrespective of flower corolla coloration. Furthermore, we posited that a higher iridescence relative to pigment optical effect would facilitate generalization to novel objects and retention in memory. Beyond documenting the potential communicative value of flower iridescence, studying an iridescence sender–receiver system in controlled conditions helps to understand pollinator–plant relationships and the evolutionary issues related to iridescent signals generated by diffraction gratings, which are widespread in nature [1].
2. Material and methods
(a). Animals and housing conditions
Purchased bumblebee (Bombus terrestris) hives (Biobest) were divided into two compartments, one light-safe with the entire colony and one transparent to allow only specific individuals (selected by the experimenter) entering the experimental arena. Hives were fed with pollen and maintained at 30°C throughout experiments (20 days). The testing room was kept at the same temperature. Age has no great impact on learning and memorization in bumblebee workers (at least for in the olfactory domain [24]). Every day, we collected randomly individuals inside the nesting box and marked them individually using paints (Email Color, Revell GmbH). Selected individuals were naive both with respect to natural flowers and to the experimental set-up. They were starved individually during 48 h before experiments to increase their foraging motivation.
(b). Testing arena
A foraging flight cage 170 × 120 × 200 cm (L × M × H) was connected to the hive by a transparent PVC pipe through which the experimenter controlled a bee's entrance via two shutters. In the flight cage, a circular arena was presented. The arena was painted with a mixture of white and black acrylic paints (70% black and 30% titanium white, Prismo, Dalbe) to achieve a dark grey background that was achromatic for the bees' sensitivity [11]. Twenty-four translucent, 12 cm-high pedestals 4 cm apart from each other were arranged on the arena to evenly fill a circular area (electronic supplementary material, figure S1). This arena was used to train bees and to test their memory retention. For generalization tests, we used a circular arena with 12 pedestals.
We placed artificial flower targets (3 cm diameter resin targets; see below for preparation) at random on pedestals (electronic supplementary material, figure S1). All flower targets were covered with mylar film to prevent bumblebees from using polarization signals (electronic supplementary material) [6]. The artificial flower field had the same number of targets of each colour, and the same number of rewarding and non-rewarding targets. The foraging flight cage was illuminated by two cool-white LED bulbs (7.5 W, 40°, 4000 k; Sylvania) and an ultraviolet (UV) multiple LED bulb (7.5 W, 15°, 370 nm; VioLED) centrally located 1 m above the foraging arena. We measured the emission spectrum of the two LED sources with a spectroradiometer (specbos 1020 UV, JETI), and adjusted the ratio between the two light sources so that it would represent the ratio found in sunny open habitat light (UV corresponding to approximately 12% of the total; irradiance spectrum presented in electronic supplementary material, figure S2). Thus, the illumination provided included the wavelengths to which bumblebees are sensitive (300–650 nm) [25] and constituted a satisfying approximation to daylight conditions.
(c). Visual stimuli, goniospectrometric measurements and analysis
We created iridescent artificial blue, yellow, red and violet artificial iridescent and non-iridescent targets by casting a UV-transparent resin impregnated with pigments on the grated side and the smooth side of compact discs (CDs), respectively, as in [6] (details in electronic supplementary material). Target coloration results from diffraction (iridescent part depending on surface structure only, on CD characteristics) and reflection from pigmented resin (non-iridescent part, flower corolla coloration depending on resin pigment concentration). We created two treatments by manipulating not iridescence itself but the pigment part, hence the iridescent-to-pigment ratio (hereafter called IRP; e.g. figure 1e,f). We used a pigment concentration of 1.6 mg l−1 for the high IRP ratio (figure 1e) and a pigment concentration of 40 mg l−1 for the low IRP ratio (figure 1f). For each treatment, both iridescent and non-iridescent targets had the same pigment concentration. Increasing pigment concentration decreased the IRP ratio, hence iridescence detectability.
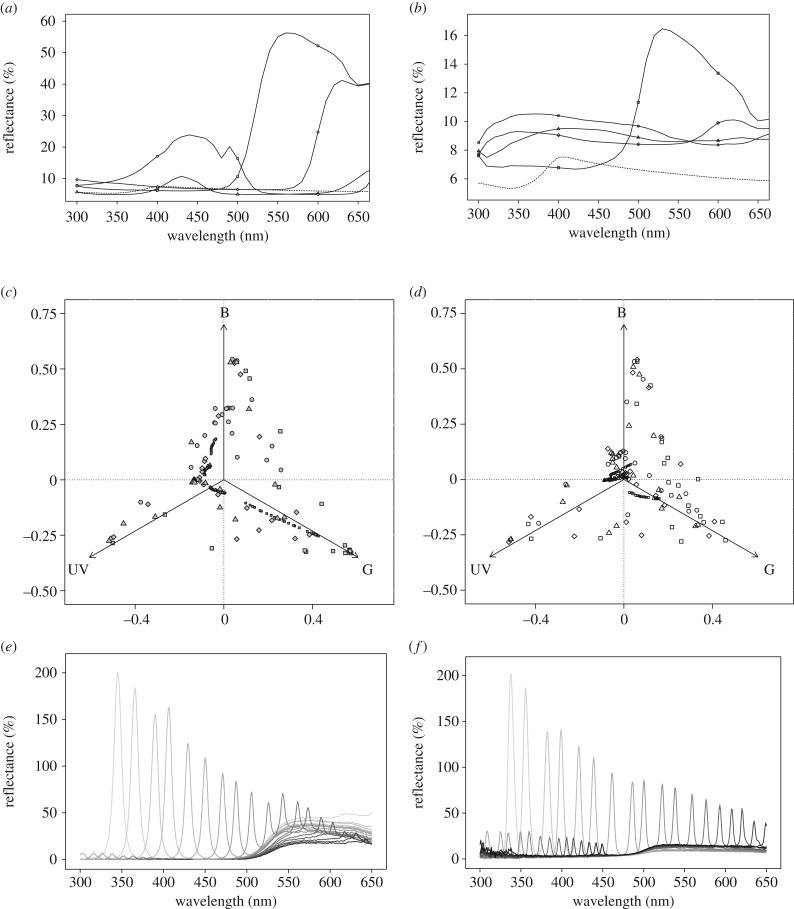
Figure 1.
(a,b) Reflectance spectra obtained in spectrometry with an integrating sphere. Field background (dashed line) and flower targets of (a) low (high pigment concentration) and (b) high (low pigment concentration) level of iridescence. (c,d) Chromatic location of colours in the triangle colour space for targets with (c) low (high pigment concentration) and (d) high (low pigment concentration) level of iridescence detectability (shown in colour in figure S6a,b). The triangle is not entirely presented for clarity reasons. Spectra were measured in goniospectrometry every 2°, between θ = 26° and θ = 70°, between 300 and 650 nm (details in electronic supplementary material). (e,f) Examples of spectra acquired in goniospectrometry for yellow targets of (e) low (high pigment concentration) and (f) high (low pigment concentration) level of iridescence. The peak of the diffraction ray is shifted towards longer wavelengths (darker lines) as the angle of light collection departs from the normal to target surface, as expected in a linear diffraction grating. For the sake of clarity, the intensity of diffracted rays was set to a maximum of 200%. Target colours were violet (triangle), blue (circle), yellow (square) and red (diamond) for high (empty symbols) or low (grey symbols) detectability of iridescence, and for iridescent (large symbols) and non-iridescent (small symbols) targets. Note that diffraction intensity decreases with an increasing collection angle, a fact that is characteristic of blazed gratings (explanation in electronic supplementary material).
We measured targets in goniospectrometry to explore angle-dependent optical properties over the range of bumblebee visual sensitivity (300–650 nm). We set the incident light at 0° as in the arena. For iridescent targets, we rotated the target until finding the highest diffraction peak (defining the φ = 0° orientation). For non-iridescent targets, we chose the φ = 0° orientation at random. We varied light collection angle θ. Unless otherwise stated, we measured one iridescent and one non-iridescent disc per colour and IRP level, and measured one reflectance spectrum per configuration (φ,θ) chosen:
— Fixed target orientation φ = 0°, varying collection angle from θ = 26° to θ = 70° to illustrate iridescence by diffraction. θ range allowed to detect all second-order diffraction peaks. We illustrated how targets were perceived by bumblebees in their colour space (figure 1c,d; electronic supplementary material, figure S6a,b). In that space, we computed the colour volume (minimal volume encompassing a set of points) for iridescent targets of a given pigment concentration, and predicted that it would be larger for high than for low IRP ratio according to the notion that iridescent-related cues would be more detectable when pigmentation was low.
— Varying target orientation φ, fixed collection angle θ = 38°. At θ = 38°, we explored all possible target orientations φ (360 possible orientations) by rotating the target on itself. We tested whether targets displayed recurrent static chromatic and/or achromatic cues at a given viewing angle and at orientations where diffraction emerged. Such cues could be learned by bumblebees instead of iridescence-related cues (see electronic supplementary material for details).
— Fixed target orientation φ = 30°, fixed collection angle θ = 38°. We measured three non-iridescent disks and three iridescent discs per colour and IRP level. Each disc was measured in four randomly chosen locations, each at φ = 30°. We tested whether iridescent targets differed from non-iridescent targets by their resin pigmentation, at orientations where diffraction did not emerge. Such cues could be learned by bumblebees instead of iridescence-related cues (see electronic supplementary material for details).
In all cases, spectra were analysed to extract the dominant wavelength of the diffraction peak and analyse its variations between and within targets (electronic supplementary material, figure S5). We quantified the chromatic contrast between a target and the arena background using the RNQ model, which provides an appropriate representation of bee colour vision (details in the electronic supplementary material). We also quantified relevant visual achromatic parameters such as S (short wave), M (mid wave) and L (long wave) receptor-specific contrasts, defined as a receptor's response to the target divided by its response to the background [11]. From these receptor-specific contrasts, L contrast mediates achromatic detection of visual targets at small subtended angles in bees and other visually driven performances [11,22], while the subtractive contributions of S, M and L contribute to the perception of chromatic contrast via opponent processing.
(d). Conditioning and testing protocol
We used differential conditioning to train two groups of 10 bumblebees to discriminate between iridescent and non-iridescent targets. Targets presented three different colour pigments; all targets had the same IRP level. One group of 10 bumblebees was rewarded with sucrose solution on iridescent targets of high IRP level, and punished with quinine solution on non-iridescent targets of high IRP level. Another group of 10 bumblebees was rewarded with sucrose solution on iridescent targets of low IRP level, and punished with quinine solution on non-iridescent targets of low IRP level. An individual was neither presented with targets with an IRP level different from that experienced during training, nor given the choice between targets of both IRP levels. It only had to choose between iridescent and non-iridescent targets displaying the pigment concentration used during training.
Fifteen microlitres of 50% sucrose solution were used to reward iridescent targets. Fifteen microlitres of a 0.02% quinine hemisulfate solution were used as negative reinforcement. Quinine improves visual discrimination learning in free-flying bees [26,27] and cannot be detected via olfaction at a concentration of 0.12% used in visual conditioning experiments (e.g. [27]). Here, we chose a lower concentration as higher ones tended to decrease foraging motivation (G.d.P. 2013, personal observations).
A focal individual was assigned to a treatment group (high or low IRP level) and to a learning colour trio, the fourth colour being the novel colour used in the generalization test. The time course of an experiment was as follows:
-
—
A learning phase during which the arena presented 24 artificial targets, all of the same IRP level, 12 of which were iridescent (4 for each colour of the trio), and 12 non-iridescent (4 for each colour of the trio).
-
—
A generalization test, which began a few minutes (never more than 10) after the completion of the learning phase, and in which the arena presented 12 artificial targets with a novel colour, 6 of which were iridescent and 6 non-iridescent. All targets had the same IRP level as during learning.
-
—
A memory retention test performed 24 h after the completion of the learning phase in which we presented an arena identical to that used during the learning phase. Given the 24 h (±2 h) delay of this test, the memory addressed corresponds to an early long-term memory in the honeybee [29]. All targets had the same IRP level as during learning.
We could only train and test one marked individual per treatment and day, and we alternated morning and afternoon for a specific treatment (high or low IRP level) to avoid potential experimental biases due to daytime. We trained six individuals with the violet–yellow–red trio, using blue as the novel colour in the generalization test (three in the high IRP treatment and three in the low IRP treatment). Six further individuals were trained with the blue–yellow–red trio, using violet as the novel colour in the generalization test (three in the high IRP treatment and three in the low IRP treatment). Four additional individuals were trained with the violet–blue–yellow trio, using red as the novel colour in the generalization test (two in the high IRP treatment and two in the low IRP treatment). Finally, four individuals were trained with the violet–blue–red trio, using yellow as the novel colour in the generalization test (two in the high IRP treatment and two in the low IRP treatment).
During the experiment, the marked bumblebee was allowed to forage freely in the arena until it decided to stop foraging and returned to the hive. Only one bumblebee was present in the arena at a time. We scored the duration of these foraging bouts and noted each visit to a target. We considered that an individual had visited a target when it made contact with the target and extended its proboscis. Correct choices were visits to iridescent targets (example in electronic supplementary material, figure S7). For each choice, we recorded the colour of the target visited, whether it was iridescent and its rank in the visit sequence. Between foraging bouts, we cleaned all mylar films and pedestals with ethanol 50% and rearranged target positions in the arena (following a randomly chosen mode previously defined).
To analyse the learning phase, we performed a binomial test on a moving window of 15 consecutive visits and we considered that learning was completed when the proportion of correct choices was statistically significant (12/15 correct choices, p-value = 0.03). Since the test concerned the last 15 consecutive visits performed by an individual at any moment, the learning phase stopped at exactly 12 correct choices for all individuals (80% correct choices). The learning phase lasted 45 min on average (range: 18–77 min), depending on bumblebee motivation and performance. Once learning was completed, we immediately changed the arena to perform the generalization test and allowed the focal individual to perform 15 additional visits to targets presenting a novel colour. After 24 h, we tested the same individual for memory retention test, and allowed it to perform 15 visits.
(e). Statistical analyses
All details of statistical analyses are in the electronic supplementary material.
3. Results
(a). Target optical properties
We could not find any statistically significant systematic achromatic or chromatic difference in pigmentation between iridescent and non-iridescent targets that could have been learned by bumblebees (electronic supplementary material, table S1). Likewise, we found that iridescent targets had unstable (thus unpredictable) chromatic and achromatic appearance, hampering any learning and generalization based on such cues (electronic supplementary material, table S2). As expected from targets of various colours with linear gratings, achromatic S, M and L contrasts varied with target colour and faded when target orientation departed from the orientation at which diffraction was highest, both around 0° and around 180°. More interestingly, we revealed that static cues delivered at a given viewing angle showed unpredictable variations between and within targets. (i) The intensity of the diffraction peak varied within a target, depending on whether the target was seen at the 0° or around the 180° orientation. (ii) How quickly the intensity of the diffraction peak faded when the target was turned on itself starting from the peak depended both on the orientation at which the target was seen (0° or approximately 180°) and on target colour (electronic supplementary material, table S2, and figure S5). (iii) The dominant wavelength of the peak diffracted was different at the 0° or at the approximately 180° orientation (electronic supplementary material, figure S5d). Hence, bumblebees were very unlikely to have learned a static information per angle but probably relied on dynamic information of iridescence itself, like the change in colour/intensity with the viewing angle.
We can note that iridescent targets were much more largely dispersed in the colour space than non-iridescent targets, illustrating the potential corruption of flower identity (figure 1c,d; electronic supplementary material, figure S6a,b). Spectra documented iridescence overshadowing by the non-iridescent part of the overall colour signal, illustrating IRP level (figure 1e,f for low and high IRP resp.). Note that within the same order of diffraction, peaks showed a decreased intensity with increasing viewing angle (increasing wavelength), a typical optical feature of blazed (irregular) diffraction gratings. This reinforced the overshadowing effect of iridescent component at long wavelengths (i.e. for yellow and red targets). High IRP targets (with low pigment concentration) occupied in general a larger portion of the colour space than low IRP targets (with high pigment concentration; respectively 0.30/0.23 for blue, 0.32/0.30 for violet, 0.36/0.35 for red but 0.39/0.44 for yellow targets), suggesting a higher detectability for low pigment concentration, except for yellow.
(b). Influence of IRP effect on learning, generalization and memory retention
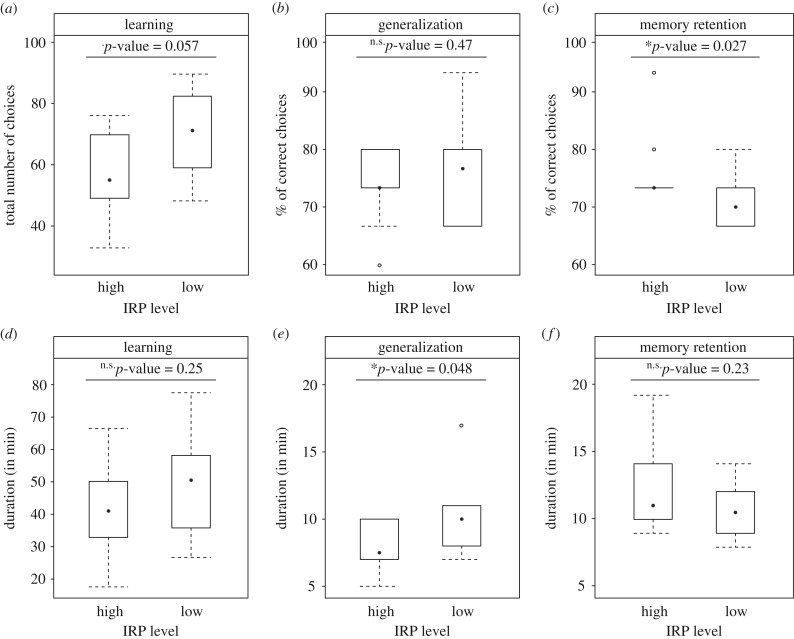
All bumblebees were able to learn the discrimination between iridescent and non-iridescent targets (electronic supplementary material, figure S8). The best model accounting for the bees’ performance (among those including factors without interactions) retained the visit rank as a significant factor, thus suggesting that the probability of visiting a rewarded target increased with visit rank (0.019 ± 0.003, p < 0.0001). Irrespective of the IRP level used for training (low-level or high-level), all bumblebees reached 80% of correct choices upon completion of the learning phase (p > 0.19 for IRP level in a model retaining both visit rank and IRP level). The number of correct choices increased from the first 10 to the last 10 visits of the learning phase, independently of the IRP level (U-test, p < 0,001 both for the high and the low IRP treatment). Individuals marginally required fewer visits to achieve learning when iridescent signals were more detectable (low IRP treatment: mean ± s.e.: 68.7 ± 5.06 visits, high IRP treatment: 54.7 ± 4.70 visits; t-test, p = 0.057; figure 2a). Yet no significant difference was found for learning duration (high IRP treatment: 41 ± 4.70 min, low IRP treatment: 49.1 ± 4.97 min; t-test, p = 0.25; figure 2b) or learning speed (number of visits/duration; t-test, p = 0.84). Moreover, the date and the period had no influence on the total number of visits (t-test, p-value = 0.55 and 0.81, respectively) nor the time required (t-test, p-value = 0.48 and 0.92, respectively) to complete the learning phase. Overall, individuals trained with high and low IRP iridescent targets did not differ in the time necessary to reach the learning criterion but those trained with high IRP needed fewer visits to reach that criterion.
Figure 2.
Effect of the level of iridescence detectability on the duration (a–c), number of visits (d) and proportion of correct choices (e,f) during learning (a,d), generalization (b,e) and memory retention test (c,f).
During the learning phase, individuals rewarded on high or low IRP targets did not differ in their foraging patterns (except for the number of flower visits), the number of bouts required to complete learning (t-test, p-value = 0.13), nor the duration of foraging bouts (linear mixed model, p-value = 0.51). The total number of iridescent targets visited was higher when IRP was low (t-test, p-value = 0.037), confirming that errors occurred in the sequence of visits. Neither the date nor the period of the day (morning/afternoon) affected the total number of visits or the time required to complete the learning phase (t-test, p-values > 0.48).
In the generalization test, bees of the high and low IRP groups did not differ statistically in terms of the errors made when foraging on a novel colour. (M–W test, p = 0.47; figure 2c). Yet they took less time to complete the 15 visits of this test when the IRP level was high (M–W test, p-value = 0.048; figure 2d). In this case, generalization to a novel colour of the learned rule was faster.
In the retention test performed 24 h after the completion of the learning phase, individuals of the high IRP treatment made fewer errors than those of the low IRP treatment (M–W test, p-value = 0.027; figure 2e). Yet no differences between both group were found in the time necessary to complete the 15 visits (t-test, p = 0.23; figure 2f).
(c). Influence of target colour on learning, generalization and memory retention
During the learning phase, bees visited significantly more violet and blue targets than yellow or red targets, regardless of the IRP level trained (linear mixed-effects model, colour effect, p = 0.016). Individuals did not show a significant change in their colour preferences between the first 10 and the last 10 visits of the learning phase (factor not retained in the best model), thus suggesting that colour preferences were unaffected by the on-going learning of iridescence-related cues. Irrespective of the colour trio on which bumblebees were trained, they did not differ statistically in the number of choices to complete the learning criterion (one-way ANOVA, p = 0.55). Yet individuals took marginally less time to complete learning when trained on violet–blue–red or violet–yellow–red trios than on violet–blue–yellow or blue–yellow–red trios (one-way ANOVA, p = 0.011).
In the generalization test, individuals made significantly fewer errors when the novel colour was violet rather than blue (linear mixed-effects model, p = 0.05) and marginally fewer errors when the novel colour was violet compared with red (p = 0.09). The time needed to complete the generalization test did not relate to receptor-specific contrasts (S-receptor contrast, p = 0.89; M-receptor contrast, p = 0.84; L-receptor contrast, p = 0.95; electronic supplementary material, figure S9a), achromatic contrast (L-contrast, p = 0.95) or chromatic contrast (RNQ colour contrast, p = 0.55; electronic supplementary material, figure S9b). The duration of the generalization test tended to be longer for targets with longer dominant wavelengths (p = 0.057; electronic supplementary material, figure S9c). The number of correct choices was not related to any physical or biological descriptor of target colour (p > 0.13). Likewise, the duration of the retention test and the proportion of correct choices were not linked to the category of the novel colour of the generalization test (ANOVA, p > 0.42), nor its chromatic or achromatic features (p > 0.14).
4. Discussion
(a). IRP level affects learning, generalization and memory retention
Our results confirm that bumblebees are able to learn cues associated to iridescent targets for discriminating rewarding from non-rewarding targets, as shown in previous studies [6,13]. Learning performances were similar to those reported in previous bumblebee studies using non-iridescent targets, both for learning rate and variability between individuals [29]. Interestingly, although bees learned both high and low IRP levels, a higher iridescent-relative-to-pigment optical effect improved their cognitive performances. In this case, individuals required fewer visits (but the same amount of time) to learn the visual discrimination between iridescent and non-iridescent targets, generalized faster their response to a novel object with a different colour but displaying the IRP level previously learned, and exhibited better retention in an early long-term memory test performed 24 h after training. Our results thus show that a higher IRP level promotes detectability and better cognitive performances in pollinators in a foraging context.
Which cues are used by bumblebees? This subject has been much debated [6,18,30]. While some studies argued that bees use dynamic cues provided by iridescent targets [6,13], other opinions maintained that bees exploit static cues that appear in a consistent way from target to target at specific viewing angles [30]. Our measurements rule out that bumblebees would discriminate iridescent from non-iridescent targets based on polarization cues (excluded by mylar films that covered all targets) or pigment cues (as we could not detect any systematic achromatic or chromatic difference in reflectance signals coming from pigments of iridescent and non-iridescent targets). Bumblebees had to use signals related to diffraction gratings, and only at orientations where diffraction emerges. There were three possibilities. First, if iridescent targets delivered a static chromatic or achromatic cue constant at a given viewing angle, bumblebees could learn that cue (e.g. if at orientations where diffraction emerged, iridescent targets all diffracted turquoise hue at 38°, bumblebees could learn turquoise was rewarding). Our measurements clearly rule out that possibility because iridescent targets deliver inconstant and unpredictable colour and intensity cues at a fixed viewing angle (variations between and within targets). Second, bumblebees could use the fact that at orientations where diffraction emerges, iridescent targets present a higher intensity or an altered colour compared with non-iridescent targets (figure 1c,d), even if peaks vary from one target to another, and from one viewing angle to the other. In that case, bees would discriminate targets based on iridescence by-products and they would use their ability to see intensity and colour. Third, bumblebees could exploit iridescence per se, either the ‘presence of a changing appearance’ or ‘the quantification of the change in intensity or colour with the viewing angle’ in iridescent targets. As they fly over targets in search of a reward, bees see visual signals changing dynamically, and they can exploit this temporal instability in intensity or colour and learn that this variability is the rewarding cue. In that case, it means bumblebees would need to detect iridescence per se (the change in coloration with angle) and use it. Our measurements clearly rules out the use of static angle-specific cues, but they cannot conclude on the use of the presence of diffraction peaks or their changing appearance. This requires further and specific testing. In all cases, bumblebees exploit iridescence-related cues—either by-products of iridescence or iridescence per se—and from a flower evolutionary perspective, all processes would favour the evolution of iridescence in flowers.
Our results reveal that the IRP level affects either foraging time (duration of a phase or number of visits [29]) or accuracy (proportion of correct choices), documenting the dilemma generally known as the speed–accuracy trade-off [27,31,32]. When foraging on flowers, pollinators need to choose between spending more time to identify highly rewarding flowers or making faster decisions at the cost of visiting non or poorly rewarding flowers [32]. With a higher IRP level, bumblebees gained speed in the generalization test without sacrificing the 80% accuracy they inherited from the learning phase, and gained accuracy without sacrificing speed in the retention test. During learning, the IRP level affected the total number of visits but not the time needed to complete learning. Bumblebees of the high IRP group had a slower foraging speed, probably because they took more time to make foraging decisions and/or fed longer once on iridescent targets. Bumblebees of the low IRP group visited more targets in general, including more rewarding ones, during a similar overall time, which suggests that fast, inaccurate bees can potentially collect nectar more efficiently than slow, accurate bees, as shown in other studies [31]. This behaviour of bees trained on low IRP could be due to a punishment intensity that was not aversive enough to limit incorrect choices. Indeed, in experiments in which quinine was used to improve discrimination via a reduction of foraging speed [26,27,33], higher concentrations were used (e.g. 60 mM in [26,34]).
(b). Target coloration, iridescence and communication efficiency
Target colour affected bumblebees' performances in the generalization test: generalization of the learned rule was faster for a novel violet colour rather than for blue and red, a result that is congruent with the reduced travel time of bees on iridescent blue than on iridescent red disks [13]. During the learning phase (but not during retention), individuals globally visited more violet and blue iridescent targets (reflecting over 373–442 nm) than yellow or red iridescent ones (reflecting over 532–630 nm). This bias can be related to bees’ innate preferences for natural colours maximally reflecting in the short wavelength range [35–37]. Previous studies have shown that generalization is more efficient when novel and learned colours are similar in dominant wavelength [35] and colour contrast [38–40], a fact that could explain why generalization was faster for violet and blue compared with yellow and red, but not why it was faster in violet than blue. We suggest that violet is the least chromatic colour used (electronic supplementary material, figure S4b,c), presenting a higher relative contribution of iridescence to the overall signal, thus facilitating the extraction of iridescence-related cues. An alternative interpretation could be that violet has a higher innate appeal than blue for bumblebees [35,41]. Finally, iridescent targets exhibited irregular (blazed) gratings in which the intensity of diffraction peaks decreased at longer wavelengths. Such gratings overshadowed iridescence at longer wavelengths like yellow or red, thus reducing the range of wavelengths at which iridescence-related cues would be easy to extract. Thus, the preference for colours reflective in shorter wavelengths can be accounted for by the fact that bumblebees searching for iridescence-related cues detect it more easily in such a wavelength range.
In our protocol of iridescence manipulation, with identical diffraction gratings, bumblebees performed worse when targets were more pigmented, hence more detectable in chromatic terms against the background, a fact that may be seen as contradictory of what is known in the literature. At large distances (visual angles smaller than 15°), visual detection is mediated by the achromatic L-receptor-based contrast [14], and higher contrast increases flower attractiveness [10,42]; at closer distances (visual angles larger than 15°), detection is mediated by chromatic contrast [11], and attractiveness is maximal for a centripetally increasing chromatic contrast between background, flower corolla and nectar guides [38,40]. In our experiments, performances were improved when targets offered a low chromatic contrast against the background, and when dominant wavelengths were in the short range of the bees' visual spectrum, in particular in the case of a dark violet coloration. This can result from two mutually non-exclusive processes: a competition process in which iridescence dominates over chromatic contrast if the latter is decreased, and an innate appeal to violet facilitating generalization to that colour. The existence of a trade-off between flower colour and iridescence is supported by the recent finding that discrimination of colour similar in dominant wavelength is impaired when bees exploit iridescence-related cues [13]. Moreover, in our experiments, targets of distinct colours converged to the same loci in the colour space, suggesting a stronger corruption of flower identity. While this can hamper performances when flower constancy is required [13], blurring flower identity may have helped bumblebees to learn faster iridescent-related cues and to generalize it to novel targets.
Several non-mutually exclusive display strategies may allow solving the flower colour–flower iridescence trade-off. (i) Flower patterns with highly chromatic petals (visible from large distance) and diffraction gratings against a dark violet area (maintaining the detectability of iridescence), as in Hibiscus trionum [5,17], Ixia viridiflora [6] or in Tulipa spp. [4,6]. Yet many flowers do not follow this pattern. (ii) Imperfect gratings, which may combine high iridescence but no corruption of flower identity [13]. This strategy is restricted to short wavelengths for which it is easier to extract iridescence-related cues, a fact that may explain the interest of blue and violet colours. Yet the fading of intensity of diffraction peaks at longer wavelengths may help maintaining flower identity for colours like red or yellow. (iii) Gratings with a reduced path length, which reduces angle dispersion, concentrates light (see electronic supplementary material) and shifts accessible wavelengths towards UV. This solution may find a limit given the reduced spatial acuity of bees, which would result in not as many wavelengths being exploitable in shorter-path gratings compared to longer-path gratings.
As a conclusion, we show that pollinators can exploit flower iridescence-related cues predicting appetitive reinforcements and for the first time that increasing the relative contribution of iridescence to that of pigments facilitates learning, generalization and 24 h retention of the learned iridescence cues in bumblebees. A thorough goniospectrometric investigation of target optical properties excluded the use of differences in pigmentation or angle-specific static cues. They confirmed that bumblebees had to rely on iridescence-related cues generated by diffraction gratings, either iridescence by-products (enhanced intensity and/or altered colour at orientations where iridescence emerges) or iridescence per se (the presence of a dynamic change in intensity and/or colour, or its quantification). Flower corolla coloration affects the bees’ ability to extract iridescence information, suggesting a potential competition between both kinds of signals in evolution. Further research is needed to characterize the structural diversity of natural flower gratings, and to test experimentally their efficiency at attracting pollinators. Beyond pollinator–plant communication, these results highlight that exploiting iridescence may occur to the detriment of static colour signals, a fact that should be considered when studying iridescence in animals or plants.
Supplementary Material
Acknowledgements
We thank J.-M. Frigério, S. Berthier and F. Viénot for their useful advice and help with measurements, S. Ferrere for his technical contribution and L. Hotier for her help with bumblebee maintenance.
Data accessibility
Data are accessible on Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.dj1b8 [43].
Authors' contributions
D.G., G.d.P., M.G. and C.A. contributed to study design and manuscript revision. D.G., G.d.P. and C.A. contributed to target design and optical measurements. G.d.P. contributed to behavioural data. G.d.P. and D.G. contributed to data analysis. G.d.P., D.G. and M.G. contributed to manuscript writing.
Competing interests
We declare we have no competing interests.
Funding
M.G. acknowledges the generous support of the Institut Universitaire de France (IUF), and D.G. that of MECADEV lab.
References
- 1.Doucet SM, Meadows MG. 2009. Iridescence: a functional perspective. J. R. Soc. Interface 6, S115–S132. ( 10.1098/rsif.2008.0395.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas KR, Kolle M, Whitney HM, Glover BJ, Steiner U. 2010. Function of blue iridescence in tropical understorey plants. J. R. Soc. Interface 7, 1699–1707. ( 10.1098/rsif.2010.0201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vignolini S, Rudall PJ, Rowland AV, Reed A, Moyroud E, Faden RB, Baumberg JJ, Glover BJ, Steiner U. 2012. Pointillist structural color in Pollia fruit. Proc. Natl Acad. Sci. USA 109, 15 712–15 715. ( 10.1073/pnas.1210105109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitney H, Kolle M, Alvarez-Fernandez R, Steiner U, Glover B. 2009. Contributions of iridescence to floral patterning. Commun. Integr. Biol. 2, 230–232. ( 10.4161/cib.2.3.8084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glover BJ, Whitney HM. 2010. Structural colour and iridescence in plants: the poorly studied relations of pigment colour. Ann. Bot. 105, 505–511. ( 10.1093/aob/mcq007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitney HM, Kolle M, Andrew P, Chittka L, Steiner U, Glover BJ. 2009. Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science 323, 130–133. ( 10.1126/science.1166256) [DOI] [PubMed] [Google Scholar]
- 7.Vigneron JP, Rassart M, Vertesy Z, Kertesz K, Sarrazin ML, Biro LP, Ertz D, Lousse V. 2005. Optical structure and function of the white filamentary hair covering the edelweiss bracts. Phys. Rev. E 71, 011906 ( 10.1103/PhysRevE.71.011906) [DOI] [PubMed] [Google Scholar]
- 8.Song B, Niu Y, Stocklin J, Chen G, Peng D-L, Gao Y-Q, Sun H. 2015. Pollinator attraction in Cornus capitata (Cornaceae): the relative role of visual and olfactory cues. J. Plant Ecol. 8, 173–181. ( 10.1093/jpe/rtv012) [DOI] [Google Scholar]
- 9.Schaefer HM, Schaefer V, Levey DJ. 2004. How plant-animal interactions signal new insights in communication. Trends Ecol. Evol. 19, 577–584. ( 10.1016/j.tree.2004.08.003) [DOI] [Google Scholar]
- 10.Streinzer M, Paulus HF, Spaethe J. 2009. Floral colour signal increases short-range detectability of a sexually deceptive orchid to its bee pollinator. J. Exp. Biol. 212, 1365–1370. ( 10.1242/jeb.027482) [DOI] [PubMed] [Google Scholar]
- 11.Giurfa M, Vorobyev M, Kevan P, Menzel R. 1996. Detection of coloured stimuli by honeybees: minimum visual angles and receptor specific contrasts. J. Comp. Physiol. A 178, 699–709. ( 10.1007/BF00227381) [DOI] [Google Scholar]
- 12.Giurfa M, Menzel R. 1997. Insect visual perception: complex abilities of simple nervous systems. Curr. Opin. Neurobiol. 7, 505–513. ( 10.1016/s0959-4388(97)80030-x) [DOI] [PubMed] [Google Scholar]
- 13.Whitney HM, Reed A, Rands SA, Chittka L, Glover BJ. 2016. Flower iridescence increases object detection in the insect visual system without compromising object identity. Curr. Biol. 26, 802–808. ( 10.1016/j.cub.2016.01.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Kooi CJ, Dyer AG, Stavenga DG. 2015. Is floral iridescence a biologically relevant cue in plant-pollinator signaling? New Phytol. 205, 18–20. ( 10.1111/nph.13066) [DOI] [PubMed] [Google Scholar]
- 15.van der Kooi CJ, Wilts BD, Leertouwer HL, Staal M, Elzenga JTM, Stavenga DG. 2014. Iridescent flowers? contribution of surface structures to optical signaling. New Phytol. 203, 667–673. ( 10.1111/nph.12808) [DOI] [PubMed] [Google Scholar]
- 16.Vignolini S, Moyroud E, Hingant T, Banks H, Rudall PJ, Steiner U, Glover BJ. 2015. The flower of Hibiscus trionum is both visibly and measurably iridescent. New Phytol. 205, 97–101. ( 10.1111/nph.12958) [DOI] [PubMed] [Google Scholar]
- 17.Vignolini S, Moyroud E, Hingant T, Banks H, Rudall PJ, Steiner U, Glover BJ. 2015. Is floral iridescence a biologically relevant cue in plant-pollinator signalling? A response to van der Kooi et al. (2014b). New Phytol. 205, 21–22. ( 10.1111/nph.13178) [DOI] [PubMed] [Google Scholar]
- 18.Whitney HM, Kolle M, Andrew P, Chittka L, Steiner U, Glover BJ. 2009. Response to comment on ‘Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators’. Science 325, 1072 ( 10.1126/science.1173503) [DOI] [PubMed] [Google Scholar]
- 19.Gruter C, Moore H, Firmin N, Helantera H, Ratnieks FLW. 2011. Flower constancy in honey bee workers (Apis mellifera) depends on ecologically realistic rewards. J. Exp. Biol. 214, 1397–1402. ( 10.1242/jeb.050583) [DOI] [PubMed] [Google Scholar]
- 20.Chittka L, Menzel R. 1992. The evolutionary adaptation of flower colours and the insect pollinators' colour vision. J. Comp. Physiol. Ser. A 171, 171–181. ( 10.1007/BF00188925) [DOI] [Google Scholar]
- 21.Chittka L, Thomson JD, Waser NM. 1999. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften 86, 361–377. ( 10.1007/s001140050636) [DOI] [Google Scholar]
- 22.Giurfa M, Vorobyev M. 1997. The detection and recognition of color stimuli by honeybees: performance and mechanisms. Isr. J. Plant Sci. 45, 129–140. ( 10.1080/07929978.1997.10676679) [DOI] [Google Scholar]
- 23.Avargues-Weber A, Deisig N, Giurfa M. 2011. Visual cognition in social insects. In Annual review of entomology, vol. 56 (eds Berenbaum MR, Carde RT, Robinson GE), pp. 423–443. [DOI] [PubMed] [Google Scholar]
- 24.Riveros AJ, Gronenberg W. 2009. Olfactory learning and memory in the bumblebee Bombus occidentalis. Naturwissenschaften 96, 851–856. ( 10.1007/s00114-009-0532-y) [DOI] [PubMed] [Google Scholar]
- 25.Dyer AG, Paulk AC, Reser DH. 2011. Colour processing in complex environments: insights from the visual system of bees. Proc. R. Soc. B 278, 952–959. ( 10.1098/rspb.2010.2412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avargues-Weber A, de Brito Sanchez MG, Giurfa M, Dyer AG. 2010. Aversive reinforcement improves visual discrimination learning in free-flying honeybees. PLoS ONE 5, e15370 ( 10.1371/journal.pone.0015370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chittka L, Dyer AG, Bock F, Dornhaus A. 2003. Bees trade off foraging speed for accuracy. Nature 424, 388 ( 10.1038/424388a) [DOI] [PubMed] [Google Scholar]
- 28.Menzel R. 1999. Memory dynamics in the honeybee. J. Comp. Physiol. Neuroethol. Sens. Neural Behav. Physiol. 185, 323–340. ( 10.1007/s003590050392) [DOI] [Google Scholar]
- 29.Raine NE, Chittka L. 2008. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. B 275, 803–808. ( 10.1098/rspb.2007.1652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morehouse NI, Rutowski RL. 2009. Comment on ‘Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators’. Science 325, 1072 ( 10.1126/science.1173324) [DOI] [PubMed] [Google Scholar]
- 31.Burns JG. 2005. Impulsive bees forage better: the advantage of quick, sometimes inaccurate foraging decisions. Anim. Behav. 70, e1–e5. ( 10.1016/j.anbehav.2005.06.002) [DOI] [Google Scholar]
- 32.Chittka L, Skorupski P, Raine NE. 2009. Speed-accuracy tradeoffs in animal decision making. Trends Ecol. Evol. 24, 400–407. ( 10.1016/j.tree.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 33.Dyer AG, Chittka L. 2004. Fine colour discrimination requires differential conditioning in bumblebees. Naturwissenschaften 91, 224–227. ( 10.1007/s00114-004-0508-x) [DOI] [PubMed] [Google Scholar]
- 34.de Brito Sanchez MG, Serre M, Avargues-Weber A, Dyer AG, Giurfa M. 2015. Learning context modulates aversive taste strength in honey bees. J. Exp. Biol. 218, 949–959. ( 10.1242/jeb.117333) [DOI] [PubMed] [Google Scholar]
- 35.Gumbert A. 2000. Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behav. Ecol. Sociobiol. 48, 36–43. ( 10.1007/s002650000213) [DOI] [Google Scholar]
- 36.Raine NE, Ings TC, Dornhaus A, Saleh N, Chittka L. 2006. Adaptation, genetic drift, pleiotropy, and history in the evolution of bee foraging behavior. In Advances in the study of behavior (eds Brockmann HJ, Slater PJB, Snowdon CT, Roper TJ, Naguib M, Wynne-Edwards KE), pp. 305–354. New York, NY: Academic Press. [Google Scholar]
- 37.Giurfa M, Nunez J, Chittka L, Menzel R. 1995. Colour preferences of flower-naive honeybees. J. Comp. Physiol. Sens. Neural Behav. Physiol. 177, 247–259. ( 10.1007/BF00192415) [DOI] [Google Scholar]
- 38.Lunau K. 1990. Color saturation triggers innate reactions to flower signals - flower dummy experimetns with bumblebees. J. Comp. Physiol. Sens. Neural Behav. Physiol. 166, 827–834. ( 10.1007/BF00187329) [DOI] [Google Scholar]
- 39.Lunau K, Wacht S, Chittka L. 1996. Colour choices of naive bumble bees and their implications for colour perception. J. Comp. Physiol. Sens. Neural Behav. Physiol. 178, 477–489. ( 10.1007/BF00190178) [DOI] [Google Scholar]
- 40.Lunau K, Fieselmann G, Heuschen B, van de Loo A. 2006. Visual targeting of components of floral colour patterns in flower-naive bumblebees (Bombus terrestris Apidae). Naturwissenschaften 93, 325–328. ( 10.1007/s00114-006-0105-2) [DOI] [PubMed] [Google Scholar]
- 41.Raine NE, Chittka L. 2007. The adaptive significance of sensory bias in a foraging context: floral colour preferences in the bumblebee Bombus terrestris. PLoS ONE 2, e556 ( 10.1371/journal.pone.0000556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasas V, Hanley D, Kevan PG, Chittka L. 2017. Multispectral images of flowers reveal the adaptive significance of using long-wavelength-sensitive receptors for edge detection in bees. J. Comp. Physiol. A 203, 301–311. ( 10.1007/s00359-017-1156-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Premorel G, Giurfa M, Andraud C, Gomez D. 2017. Data from: Higher iridescent-to-pigment optical effect in flowers facilitates learning, memory and generalization in foraging bumblebees Dryad Digital Repository. ( 10.5061/dryad.dj1b8) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- de Premorel G, Giurfa M, Andraud C, Gomez D. 2017. Data from: Higher iridescent-to-pigment optical effect in flowers facilitates learning, memory and generalization in foraging bumblebees Dryad Digital Repository. ( 10.5061/dryad.dj1b8) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are accessible on Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.dj1b8 [43].