Abstract
Ocean acidification (OA) studies typically use stable open-ocean pH or CO2 values. However, species living within dynamic coastal environments can naturally experience wide fluctuations in abiotic factors, suggesting their responses to stable pH conditions may not be reflective of either present or near-future conditions. Here we investigate the physiological responses of the mussel Mytilus edulis to variable seawater pH conditions over short- (6 h) and medium-term (2 weeks) exposures under both current and near-future OA scenarios. Mussel haemolymph pH closely mirrored that of seawater pH over short-term changes of 1 pH unit with acidosis or recovery accordingly, highlighting a limited capacity for acid–base regulation. After 2 weeks, mussels under variable pH conditions had significantly higher metabolic rates, antioxidant enzyme activities and lipid peroxidation than those exposed to static pH under both current and near-future OA scenarios. Static near-future pH conditions induced significant acid–base disturbances and lipid peroxidation compared with the static present-day conditions but did not affect the metabolic rate. These results clearly demonstrate that living in naturally variable environments is energetically more expensive than living in static seawater conditions, which has consequences for how we extrapolate future OA responses in coastal species.
Keywords: ocean acidification, natural variability, acid–base balance, metabolism, oxidative stress
1. Introduction
Atmospheric carbon dioxide (CO2) levels reached 400 ppm in 2016, a rise of more than 100 ppm since the industrial revolution, and continues to rise at a current rate of 2.73 ppm yr−1. Projections from the representative concentration pathways (RCP) 8.5 business as usual scenario predict atmospheric CO2 levels will exceed 1000 ppm early into the next century [1]. The accompanying absorption of atmospheric CO2 by the oceans has led to a 30% increase in average global ocean pH (reduction of pH by 0.1 units) and an alteration in carbonate chemistry [2], termed ocean acidification (OA). There is now substantial evidence that OA can negatively impact the health and physiology of a wide range of marine invertebrates, with experimental OA negatively affecting over 50% of all molluscs tested to date [3,4].
While calcifying species are generally considered to be more susceptible to OA owing to the impact on calcium carbonate formation and dissolution [4,5], focus has now shifted to the impacts of OA on other physiological processes, in particular acid–base physiology and the energetic cost of ion regulation. Marine animals acutely exposed to elevated pCO2 can experience an extracellular acidosis [6]. The ability to compensate extracellular pH changes and maintain cellular homeostasis is thought to play a key role in the future survival and distribution of a species [6], and hence is considered to be a key determinant of an organism's susceptibility to future OA [7]. Marine mussels, such as Mytilus edulis, provide a range of ecosystem services such as habitat structure and water purification, as well as contributing an estimated $19 billion to the global economy in 2014. Previous studies in M. edulis revealed that hypercapnia induced extracellular acidosis [8–10] with no compensatory increase in 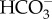 , suggesting a poor ability to acid–base regulate. If uncompensated, reductions in extracellular pH have the potential to influence protein stability and enzyme function [11], leading to concerns over the future impacts of OA on this commercially important species.
, suggesting a poor ability to acid–base regulate. If uncompensated, reductions in extracellular pH have the potential to influence protein stability and enzyme function [11], leading to concerns over the future impacts of OA on this commercially important species.
The majority of experimental OA studies to date for M. edulis and other coastal species measure responses at the end of medium-term exposures to static seawater pH levels, using predictions based on global open-ocean averages, where pH and other carbonate chemistry parameters vary minimally both temporally and spatially. This relative uniformity is uncharacteristic of coastal habitats where natural fluctuations of pH, temperature, salinity and oxygen can occur on daily, tidal, seasonal and annual time scales [12–17]. Hence static seawater pH exposures do not accurately reflect the conditions that most mussel populations are likely to experience in situ either presently or under future OA scenarios. Recent field observations have shown this environmental variability can result in present-day situations in which seawater pH exceeds OA predictions for the end of the century [18,19], with pH deviations of up to one unit [9,16,17]. The magnitude of coastal fluctuations in seawater pH is predominately influenced by freshwater inputs (salinity), upwelling events, temperature, tidal cycles, photosynthesis and respiration [20,21]. These pH fluctuations are expected to intensify in the future, along with other global climatic changes [22], hence as OA progresses, seawater pH in coastal habitats may regularly drop to well below 7.6 by the year 2100 [23].
Unfortunately, our current understanding of how near-shore and intertidal marine species will respond to the natural variability of seawater pH in either present-day or near-future pH conditions, or how close these species are to their physiological limits, is very limited. Where fluctuating seawater pH/pCO2 conditions have been included in OA exposures, it has generally been found that responses are considerably different compared with those under static conditions. For example, Dufault et al. [24] found that recruits of the brooding reef coral, Seriatopora caliendrum, exposed to diurnally oscillating pCO2 grew 6–19% larger than those in static ambient or high pCO2 conditions. Frieder et al. [25] found the impact of low pH (pH 7.5) on early development of mussel larvae was reduced when pH fluctuated by 0.15 pH units around this low average pH value. Other studies, however, have found fluctuating conditions appear to enhance the impacts of OA [26,27]. While contrasting, these early results across a range of organisms all demonstrate that variable pH/pCO2 conditions can alter responses to OA conditions, highlighting the need to consider variability when conducting OA experiments for coastal species [28].
Here we test the hypothesis that a variable seawater pH regime will pose a greater physiological challenge compared with static conditions in the common mussel M. edulis. We first investigate the immediate acid–base and metabolic response of mussels to short-term changes in seawater pH to determine the rate of responses to external changes in seawater pH. We then investigate the consequences of a variable compared with a static pH regime on physiological responses over 2 weeks. We chose to use a semidiurnal pattern of fluctuations characteristic of tidally driven estuarine environments [13], where submerged mussels will experience a range of conditions. Indeed, Baumann et al. [16] show pH to be significantly correlated with tidal height in a temperate salt marsh, with ΔpH ≥ 1 unit on several occasions. Tidally driven variability in carbonate systems is likely to be important for many estuarine species and particularly for those comprising natural shellfish aquaculture globally, yet it has not been studied in detail to date. This approach is also relevant for submerged mussels living in tide pools, where the pH will vary primarily as a result of biological activity through each low-tide period when the pool is isolated, but will return to a nominal seawater pH condition when the tide is high [17].
2. Material and methods
(a). Collection of animals
Adult M. edulis (55–73 mm shell length) were collected from a muddy estuary at Starcross, Devon, UK (50°37′03′ N 3°26′56′ W). Animals were first cleaned of barnacles then maintained in a recirculating system of artificial seawater (Tropic Marine, pHNBS 8.10, salinity 33, at temperature 15 ± 0.5°C) with a photoperiod of 12 L : 12 D cycle. Mussels were held for a minimum of 7 d prior to experimentation and fed 5000 cells ml−1 of dried Isochrysis Instant Phyto (ZM Systems) daily.
(b). Seawater manipulation
Artificial seawater was acidified to the desired pH level via the addition of CO2 gas using a computerized pHNBS control system (Aqua Medic, Germany). Seawater pH (NBS scale) was additionally monitored with a Metrohm 826 pHNBS mobile electrode and meter. Gentle aeration maintained oxygen levels close to 100% air-saturation. Seawater samples were collected at each sample time point (details below) for assessment of pH and dissolved inorganic carbon (DIC) (full methods in electronic supplementary material). The seawater carbonate chemistry data for the short-term changes in seawater pH are provided in the electronic supplementary material, tables S1 and S2, and for the medium-term exposure in table 1.
Table 1.
Seawater carbonate chemistry from the 14-day experiment, showing mean ± s.d. for the stable treatments on day 0 and day 14. Temperature (temp.), salinity, pH and DIC were measured, while other carbonate parameters were calculated using CO2sys.
| treatment | day | temp. (°C) | salinity | pHNBS | DIC (µmol kg−1) | TA (µmol kg−1) | pCO2 (µatm) |
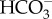 (µmol kg−1) (µmol kg−1) |
CO32−(µmol kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| pH 8.10 static | 0 | 13.2 ± 0.1 | 31.7 ± 0.1 | 8.14 ± 0.01 | 2145 ± 9 | 2322 ± 10 | 439 ± 1 | 1992 ± 9 | 135 ± 1 |
| pH 8.10 static | 14 | 13.2 ± 0.1 | 30.1 ± 0.1 | 8.11 ± 0.00 | 1975 ± 34 | 2122 ± 36 | 441 ± 8 | 1845 ± 32 | 112 ± 2 |
| pH 7.70 static | 0 | 13.2 ± 0.0 | 31.6 ± 0.1 | 7.69 ± 0.01 | 2341 ± 92 | 2360 ± 90 | 1404 ± 79 | 2231 ± 88 | 53 ± 1 |
| pH 7.70 static | 14 | 13.2 ± 0.0 | 30.4 ± 0.1 | 7.69 ± 0.01 | 2081 ± 121 | 2100 ± 121 | 1276 ± 72 | 1985 ± 115 | 46 ± 3 |
(c). Responses to short-term changes in seawater pH
Mussels were placed into tanks at a starting pHNBS of 8.12 (pCO2 = 355 µatm, salinity 32.3, temperature 13.5 ± 0.5°C). The manipulation of gaseous CO2 gradually reduced seawater pH by one unit over a period of 6 h (to measure mussel response to acidification). CO2 manipulation was then stopped and the seawater pH was allowed to increase again by one unit over the following 6 h (to measure mussel recovery response). Seawater samples for DIC analysis and acid–base measurements of exposed mussels were taken every 30 min over each of the two 6 h periods (acidification and recovery). Six mussels were used for determination of acid–base response at each of the 26 sampling time points (i.e. 13 acidification and 13 recovery), with all mussels only being sampled once (to avoid any stress responses from the sampling process). Haemolymph (approx. 1 ml) from the posterior abductor muscle was extracted using a 21 g needle connected to a 2 ml syringe and immediately measured for pHNBS (Metrohm 826 pH mobile electrode and meter). Haemolymph samples were then transferred to 100 µl glass micro-haematocrit capillary tubes sealed with paraffin oil and Hemato-Seal (Fisher) and placed on ice until subsequent analysis of total CO2 using a Corning 965 CO2 analyser (Corning Ltd., UK) calibrated with 10 mM NaHCO3. Acid–base parameters were calculated using a modified version of the Henderson–Hasselbalch equation using the first dissociation constant (pK) for carbonic acid and solubility constant (αCO2) for CO2 derived from Truchot [29] for invertebrate haemolymph.
Mussel metabolic responses to gradual reductions in seawater pH from pHNBS 8.15 to 7.15 were measured during a separate exposure using mussels that had been starved for 3 days prior to the experiment (to avoid postprandial metabolism) and left to acclimate to the respirometry chambers overnight (to avoid handling stress). Mussels (n = 12) were placed in individual glass beakers (200 ml) initially containing seawater at pHNBS 8.15. Oxygen consumption was measured for each mussel over 30 min before this seawater was replaced (flushed) with new seawater pre-gassed to the next experimental pH level (taking approx. 5 min for each water change). A total of nine measurements were taken over the gradual reduction before mussels were then immediately returned to ambient seawater (pHNBS 8.20) to assess recovery of metabolic rate. Full details of the method are provided in the electronic supplementary material.
(d). Physiological responses to fluctuating pH
To investigate the role of seawater pH variability in determining OA response, mussels (n = 16) were placed into one of four treatments: (1) static pHNBS 8.1; (2) static pHNBS 7.7; (3) fluctuating pHNBS 8.1; and (4) fluctuating pHNBS 7.7. An experimental pHNBS value pf 7.7 was targeted to represent near-future OA under IPCC RCP 8.5 [22,30]. Each exposure system consisted of a 150 l header tank and 16 individual 1 l replicate tanks (i.e. one mussel per tank) in self-contained re-circulation. For the fluctuating treatments, dial compact 24 h timers were connected to the pH computers and set to replicate a semidiurnal tidal cycle (6 h on, 6 h off) to attain a fluctuating pH regime that first gradually reduced seawater over 6 h then allowed it to recover via aeration for the following 6 h (electronic supplementary material, table S3) (i.e. a 12 h cycle). Mussels were exposed to a total of 28 cycles of pH over the two-week exposure. Seawater was aerated and pHNBS, measured as previously described, was additionally recorded in each tank every 10 min for 24 h on a rotation, such that each tank was monitored for 24 h every 4 days (see electronic supplementary material, figure S1 and table S3). Mussels were fed as before, on days 0 and 7.
Oxygen consumption rates were measured after 14 days, as described above (and in electronic supplementary material). Briefly, each mussel was kept in its individual tank (to avoid handling stress) but the water supply was removed. After a 5 min settling period, pO2 was determined for a total of 2.5 h. Acid–base parameters were then measured as described above (after 14 days). The remaining haemolymph was immediately frozen in liquid nitrogen for further analysis (below). For the variable treatments, measurements were taken on the downwards phase at the mean pH value (either pHNBS 8.10 or 7.70) to allow for direct comparisons with the static pH treatments.
(e). Lysosomal stability and oxidative stress assays
The neutral red retention (NRR) assay was used as a measure of lysosomal stability of mussel haemocytes. This assay has been demonstrated to provide a useful indicator of general health status in M. edulis, correlating well with growth and reproduction [31]. Methodology followed that of Cajaraville et al. [32] (detailed in electronic supplementary material). Superoxide dismutase (SOD) is an important antioxidant enzyme catalysing the dismutation of superoxide anions and is often measured as an indicator of oxidative stress. SOD activity was quantified using the methodology of van der Oost et al. [33]. Lipid peroxidation was determined using the thiobarbituric acid reactive substances (TBARS) assay which quantifies malondialdehyde, a secondary product of lipid peroxidation, via its reaction with thiobarbituric acid following the methodology of Camejo et al. [34]. Full details of these assays are provided in the electronic supplementary material.
(f). Statistical analysis
All data are presented as mean ± standard error (s.e.) and tested for normality using the Shapiro–Wilk Test. Normally distributed data for the 6 h exposure were analysed using a one-way ANOVA followed by the Holm–Sidak post hoc test. Where repeated measurements were taken from one mussel (6 h metabolic rate) a repeated-measures ANOVA was used. For data that failed the normality test (6 h haemolymph 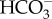 concentration) a Kruskal–Wallis test followed by Dunn's method post hoc test was used. A general linear model testing for effects of ‘pH’ (i.e. seawater pHNBS of 8.1 or 7.7), ‘variability’ (static or fluctuating regime) and ‘pH × variability’ (to test for an interaction term) was used for the 14-day exposure endpoints. Data were analysed using Sigma Plot v. 12 and SPSS v. 24.
concentration) a Kruskal–Wallis test followed by Dunn's method post hoc test was used. A general linear model testing for effects of ‘pH’ (i.e. seawater pHNBS of 8.1 or 7.7), ‘variability’ (static or fluctuating regime) and ‘pH × variability’ (to test for an interaction term) was used for the 14-day exposure endpoints. Data were analysed using Sigma Plot v. 12 and SPSS v. 24.
3. Results
(a). Short-term responses to changes in seawater pH
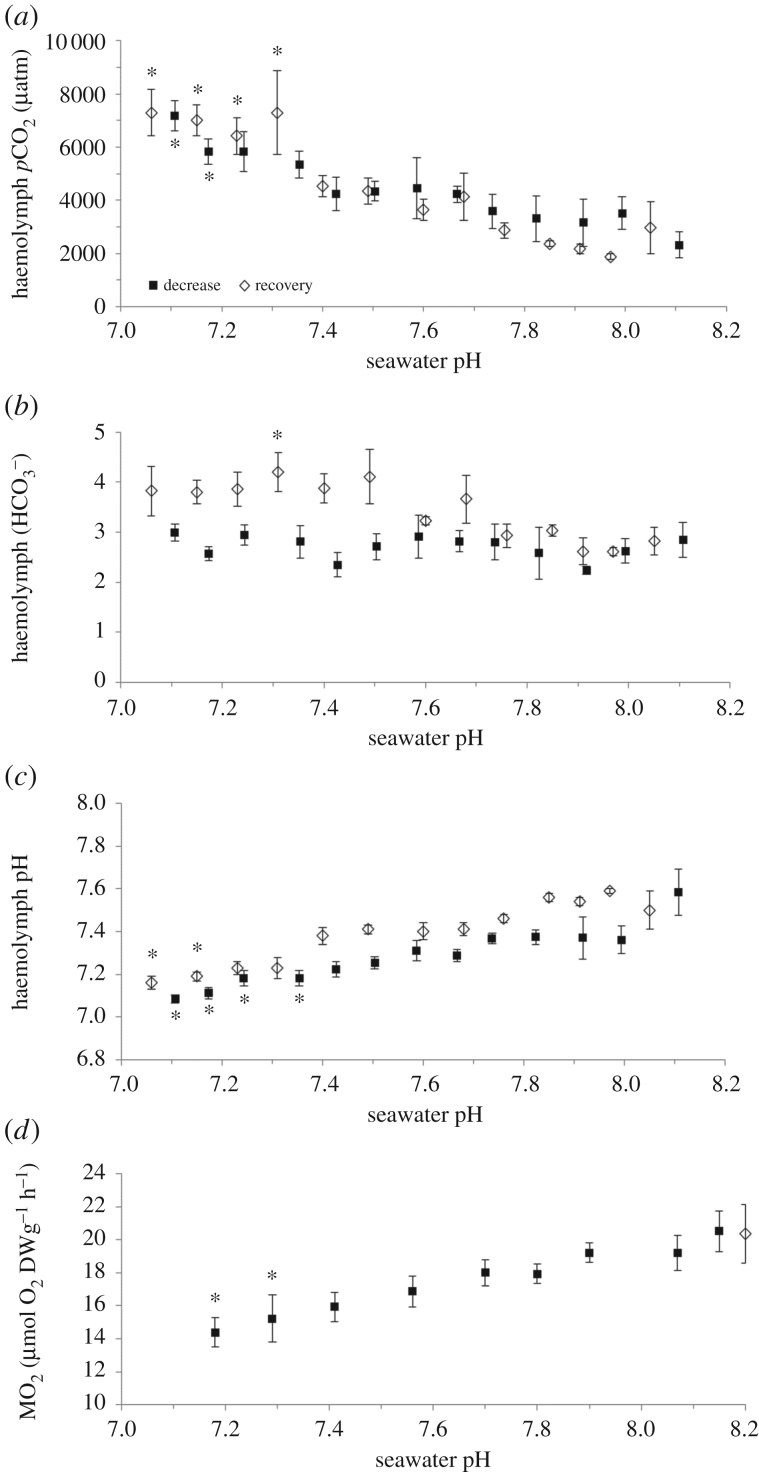
Acidification from pHNBS 8.11 to 7.11 resulted in a threefold increase in haemolymph pCO2 (figure 1a; F = 3.894, p < 0.001). No accompanying change in haemolymph bicarbonate levels was observed (figure 1b; F = 0.588, p = 0.844), leading to a significant decrease in haemolymph pH from pHNBS 7.58 to 7.09 (figure 1c; F = 0.699, p < 0.001). Mussels steadily recovered from this acidosis during the following 6 h increase in seawater pH, with haemolymph pCO2 significantly decreasing (figure 1a; F = 8.207, p = < 0.001) and pH increasing back to levels similar to those measured at ambient seawater pH (pHNBS 8.10) (figure 1c; F = 14.679, p < 0.001). Haemolymph bicarbonate (figure 1b) started the 6 h ‘recovery’ phase significantly higher (F = 3.054, p = 0.02) but reached values comparable to those in ambient pH animals halfway through the recovery of seawater pH. Metabolic rate was also significantly affected by acidification (figure 1d; F = 3.21, p < 0.001), decreasing by 30% over a one-unit decrease in seawater pH (6 h) and recovering immediately when exposed to ambient seawater (approx. 15 min).
Figure 1.
Acid–base parameters in the haemolymph (a–c) and metabolic rate (d) of M. edulis over a 6 h gradual exposure to decreasing seawater pH (dark grey square symbols) and recovery (light grey diamond symbols). Data shown as mean ± s.e. Asterisk represents a significant difference from that measured at seawater pH 8.11.
(b). Physiological responses to fluctuating pH
The pH fluctuating regimes are shown in electronic supplementary material, figure S1. We were unable to raise the pH of the control in the fluctuating conditions greater than to about 8.2 solely by aeration, which resulted in an uneven fluctuation above and below the average value of 8.1, as we had aimed to achieve. To further increase the pH in this system would require carbonate chemistry to be manipulated in ways unrepresentative of CO2-induced changes. Details of the fluctuating regimes are therefore as follows: pHNBS 8.1 fluctuating had a mean ± s.d. of 7.92 ± 0.20, max = 8.23, min = 7.66; pHNBS 7.7 fluctuating had a mean ± s.d. of 7.70 ± 0.32, max = 8.13, min = 7.26 (electronic supplementary material, table S3).
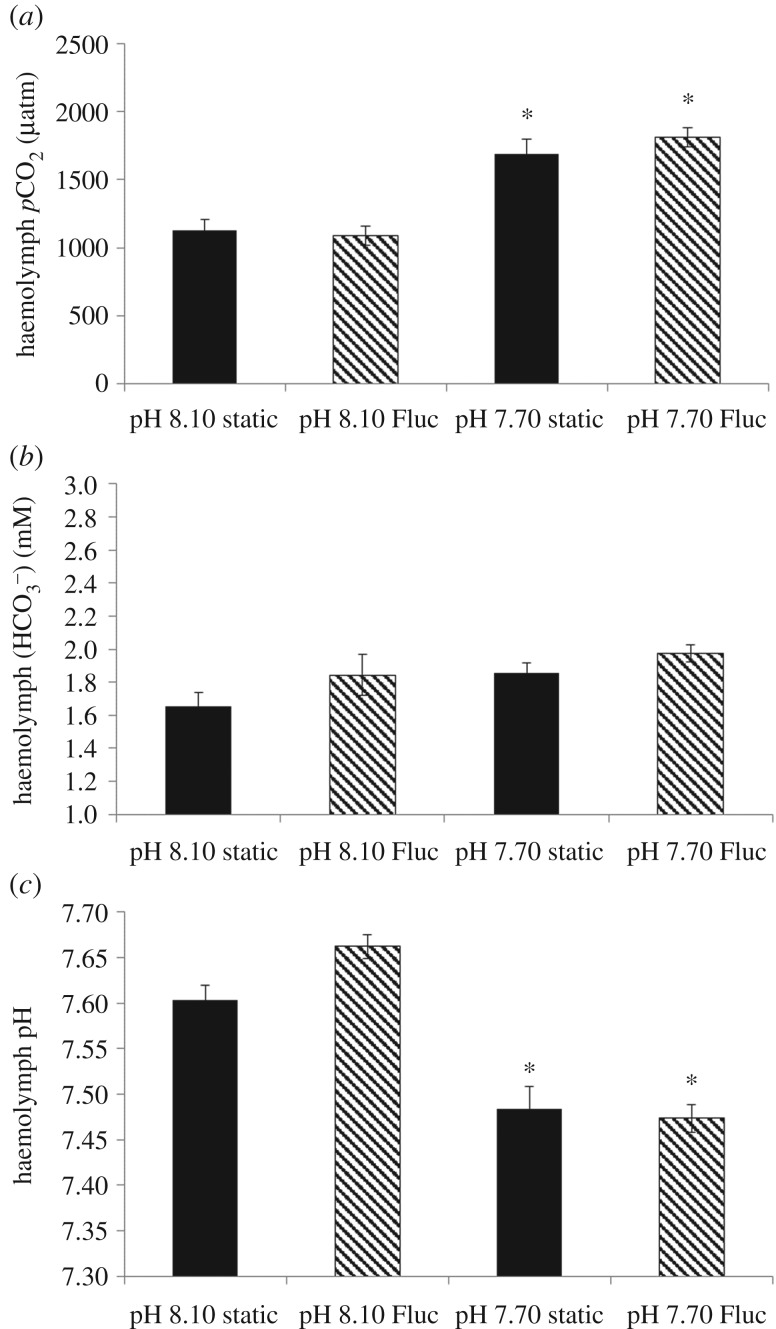
Similar to the response observed during the short-term experiment, haemolymph pCO2 levels were significantly affected by seawater pH, and were 43% and 48% higher in the pHNBS 7.70 static and fluctuating treatments, respectively, compared with the pHNBS 8.10 static and fluctuating treatments (figure 2a; GLM for ‘pH’; F = 64.487, p < 0.001). A fluctuating regime had no effect on haemolymph pCO2 (GLM for ‘variability’: F = 0.241, p = 0.625; for ‘pH × variability’: F = 1.076, p = 0.304). There was a small but significant increase in haemolymph bicarbonate levels driven by seawater pH (figure 2b; GLM for ‘pH’: F = 4.032, p = 0.049), with no effect of a fluctuating regime (GLM for ‘variability’: F = 3.374, p = 0.71; for ‘pH × variability’: F = 0.178, p = 0.675). Haemolymph pH was significantly decreased by seawater pH (figure 2c; GLM for ‘pH’: F = 70.882, p < 0.001), where pH decreased by up to 0.19 units. There was no effect of a fluctuating regime on haemolymph pH (GLM for ‘variability’: F = 1.737, p = 0.193; for ‘pH × variability’ interaction: F = 3.56, p = 0.064).
Figure 2.
Acid–base parameters in the haemolymph of M. edulis following a 14-day exposure to control and lowered pH in static and fluctuating (Fluc) pH regimes: (a) haemolymph pCO2; (b) haemolymph bicarbonate concentration (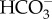 ); and (c) haemolymph pH. Data shown as mean ± s.e. Asterisk represents significant differences from the static pH 8.10 treatment.
); and (c) haemolymph pH. Data shown as mean ± s.e. Asterisk represents significant differences from the static pH 8.10 treatment.
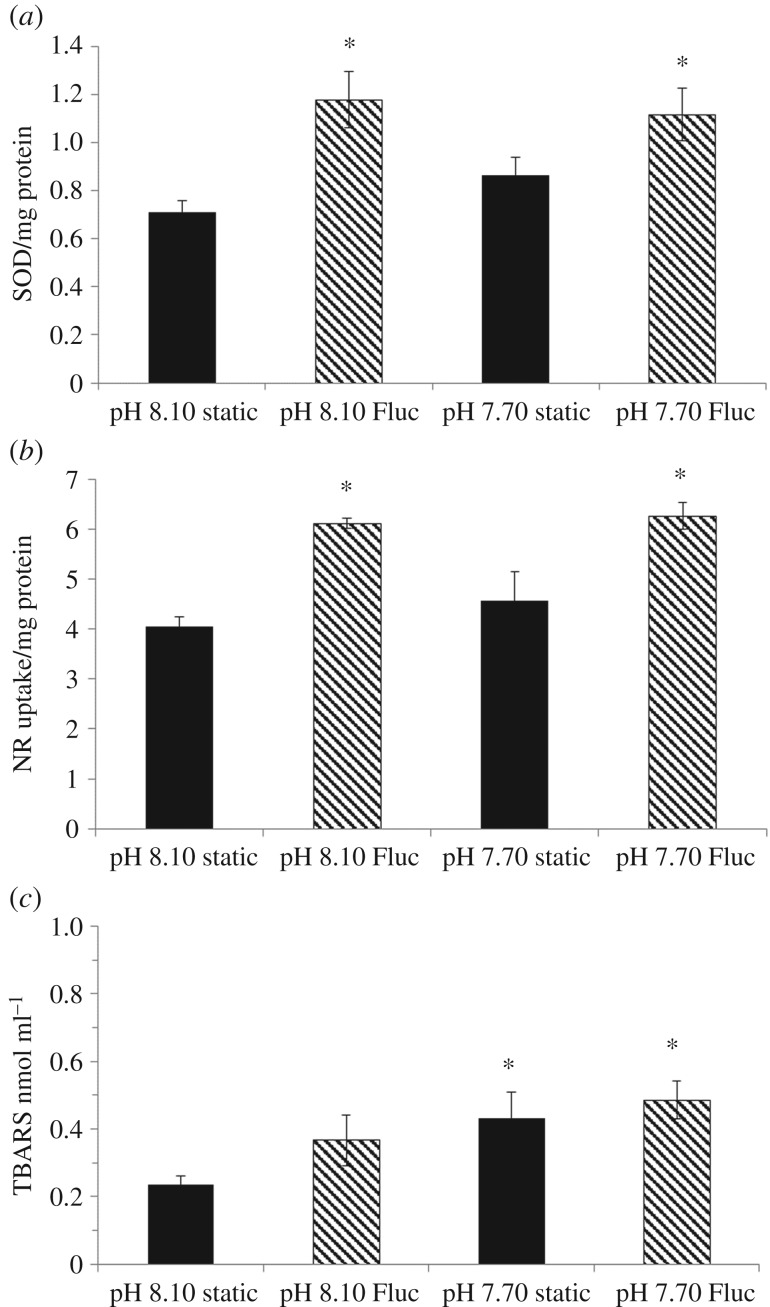
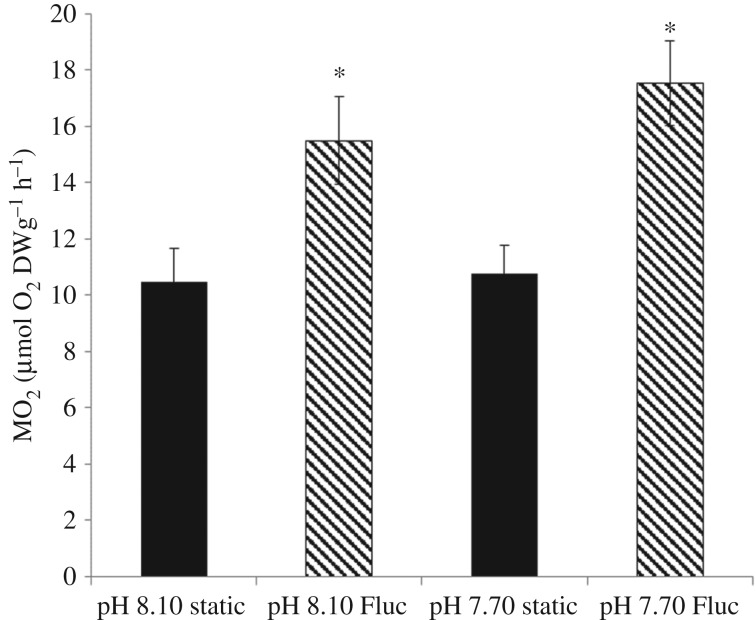
Haemolymph SOD activity was significantly increased by a fluctuating pH/pCO2 regime (figure 3a; GLM for ‘variability’: F = 14.11, p < 0.001), showing an increase of 49% and 44% in the fluctuating pHNBS 8.10 and pHNBS 7.70 treatments, respectively, compared with the pHNBS 8.10 static treatment. There was no effect of pH treatment on SOD activity. Similarly, NRR was significantly influenced by a fluctuating regime (GLM for ‘variability’: F = 26.66, p < 0.001) and was higher by 41% and 43% in the pHNBS 8.10 and 7.70 fluctuating treatments, respectively, compared with pHNBS 8.10 static treatment (figure 3b). NRR was not significantly influenced by pH treatment (GLM for ‘pH’: F = 0.628, p = 0.431; no interaction ‘pH × variability’: F = 0.683, p = 0.412). TBARS was significantly elevated by pH treatment (figure 3c; GLM for ‘pH’: F = 6.916, p = 0.011), with no significant influence of a fluctuating regime on lipid peroxidation and no interaction term (GLM for ‘variability’: F = 2.350, p = 0.131; for ‘pH × variability’: F = 0.429, p = 0.515). In contrast to the shorter (6 h), experiment, there was no significant effect of pH treatment on mussel MO2 after 2 weeks (figure 4; GLM for ‘pH’; F = 0.76, p = 0.388). However, there was a significant effect of a fluctuating pH regime, with increased MO2 of 39% and 50% in the fluctuating pHNBS 8.10 and pHNBS 7.70 treatments, respectively, compared with pHNBS 8.10 static (figure 4; GLM for ‘variability’: F = 19.68, p < 0.001; for ‘pH × variability’ interaction: F = 0.444, p = 0.509).
Figure 3.
Health indicators measured in the haemolymph of M. edulis following a 14-day exposure to control and lowered pH conditions in static and fluctuating (Fluc) pH regimes: (a) activity of the antioxidant enzyme superoxide dismutase (SOD); (b) cell viability measured as neutral red retention; and (c) levels of thiobarbituric acid reactive substances (TBARS). Data shown as mean ± s.e. Asterisk represents significant differences from the static pHNBS 8.10 treatment.
Figure 4.
The metabolic rate of M. edulis following a 14-day exposure to control and lowered pH conditions in static and fluctuating (Fluc) pH regimes (data as mean ± s.e.). Asterisk represents significant differences from the static pHNBS 8.10 treatment.
4. Discussion
Our data clearly show that exposure to fluctuating seawater carbonate chemistry elicits a very different set of physiological responses than exposures to static conditions for both ambient and lowered pH exposures in Mytilus edulis, collected from an estuarine habitat. Importantly, the variable treatments elicited greater oxidative stress and a greater effect on metabolic rate than the static seawater conditions for both ambient and near-future pCO2 levels, suggesting variable environments are more energetically costly for individuals than static ones.
Consistent with other physiological studies, our results show that M. edulis have a limited ability to acid–base regulate during both short- (6 h) and medium-term (14 day) changes in seawater pH. An increase in seawater pCO2 caused a direct and immediate increase in mussel haemolymph pCO2, with levels reaching up to 1816 µatm (at the mid-point of the pH cycle) in the 2-week exposure and up to 7200 µatm at the highest seawater pCO2 value (pHNBS 7.06) in the 6 h experiment. An increase in extracellular pCO2 under OA conditions has been observed in a number of marine organisms [35,36], including M. edulis, where extracellular pCO2 increased linearly with seawater pCO2 [8]. Mussel haemolymph pCO2 values as high as 5724 µatm have previously been recorded in M. galloprovincialis exposed to seawater pH of 7.3 [37]. A corresponding reduction in haemolymph pH was observed resulting from the elevated pCO2 levels, which was then immediately reversed when seawater conditions were gradually returned to their starting conditions. These observations are in agreement with previous studies reporting that M. edulis cannot actively avoid acid–base disturbances [8–10,38], but conforms to changes in pCO2 of the external medium. The short-term extracellular accumulation of bicarbonate ions is often reported as an efficient mechanism in stabilizing extracellular pH in active marine ectotherms [39]. Although we saw a trend of increasing haemolymph 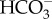 concentrations after 2 weeks of acclimation, this was not enough to compensate extracellular pH. The inability to compensate extracellular acid–base disturbances potentially has negative impacts on growth and calcification under extended exposures to OA.
concentrations after 2 weeks of acclimation, this was not enough to compensate extracellular pH. The inability to compensate extracellular acid–base disturbances potentially has negative impacts on growth and calcification under extended exposures to OA.
In addition to influencing the acid–base status of mussels over short-term exposures, increasing seawater pCO2 also led to a reduction in metabolic rate, with an immediate recovery of oxygen consumption when returned to ambient seawater conditions (pHNBS 8.20). This could be driven by a number of mechanisms, such as metabolic suppression or a reduction in enzyme efficiency under the acidic conditions. Similar responses have been recorded in a number of invertebrates; for example, a reduction in metabolic rate in response to reduced extracellular pH in M. galloprovincialis was suggested to result from an inhibition of net proton transport across the cell membrane [37,40]. Metabolism may also be influenced by reductions in intracellular pH by inhibiting enzymatic activity [41].
Interestingly, the metabolic response of M. edulis to OA treatments over the 2-week exposure differed considerably when compared with their short-term responses. We found no significant effect of OA on mussel metabolic rate after 2 weeks of static exposure. However, there was a significant increase in metabolic rate by 39% and 50% under both fluctuating regimes of pHNBS 8.10 and 7.70, respectively, compared with those exposed to static pHNBS 8.10. Hence, the metabolic suppression in response to short-term changes in seawater pH was replaced with no overall effect of static OA but a clear energetic cost of fluctuating pH conditions over the longer exposure. Other studies have shown food availability can influence individuals' OA response because of these changes in metabolic cost [9] and that increased food, when available, can provide the additional energy required. Our findings align with recent work on the influence of tidal biological rhythms on metabolic rates of the ghost shrimp, Neotrypaea uncinata, where metabolic rates were higher under simulated fluctuating tidal conditions compared with static conditions in the laboratory [42]. The difference in metabolic rate between static and fluctuating OA conditions may partly be explained via changes in energy demand associated with intracellular acid–base regulation. As shown in M. galloprovincialis, intracellular pH can take a few days to be fully compensated [37], which could explain the difference between the immediate reduction in metabolic rate compared with increases in metabolic rate over the longer exposure.
Elevated stress responses under the fluctuating seawater pH regimes were also observed for a number of the stress-related biomarkers within the haemolymph. While static OA conditions had no effect on the activity levels of the antioxidant enzyme superoxide dismutase (SOD), both ambient and OA fluctuating conditions induced a significant increase in SOD activity, of 49% and 44%, respectively. This is in agreement with previous studies where no change in SOD activity was observed in M. edulis and M. galloprovincialis exposed to static OA conditions [43,44]. Despite the increase in SOD activity measured in mussels from both of the fluctuating treatments, an increase in lipid peroxidation was seen in the OA static and both fluctuating treatments. This strongly suggests that fluctuating pH seawater conditions exert a much greater oxidative stress on individuals than static conditions, both triggering an increase in SOD activity and overwhelming this response to accumulate lipid peroxidation. Our data suggest that the static OA conditions did not exert enough oxidative stress to trigger an antioxidant response in the mussels, resulting in low levels of lipid peroxidation accumulating over the 14 days.
We also found that while static OA conditions had no significant effect on NRR, a significant increase in NRR was measured in response to both fluctuating treatments. This contrasts with the previous work of Beesley et al. [45], who found M. edulis exposed to static seawater pHNBS treatments of 7.80, 7.60 and 6.80 showed a significant reduction in NRR; however, this study used a much longer exposure time of 60 days, which may account for this difference. The increased NRR in fluctuating treatments may again be explained by the upregulation of SOD production translating into an intensification of the immune system which decreases the permeability of the lysosome membrane. However, this does appear to come at an energetic cost as shown via the significant increase in metabolic rate under both fluctuating regimes.
Running experiments using a fluctuating system posed two significant challenges: First, in order to create replicate fluctuating systems we used just one header tank per treatment to create the conditions before the manipulated water was then fed into individual mussel experimental tanks. While we acknowledge the limitation of this design, we believe that by careful continual monitoring of all the tanks, we did not see any tank effects and all results presented here are in response to the various pH treatments. The second challenge was that the two fluctuating conditions were achieved by bubbling with CO2 to reduce the pH and aerating the tanks to allow them to recover back up to higher levels. This method was successful in the pH 7.7 treatment, because the mean pH was already lower than ambient. However, it was difficult to raise the pH above the ambient 8.1 just using bubbling. Thus the 8.1 fluctuating treatment actually had a smaller overall pH range compared with the 7.1 fluctuating treatment. Nevertheless, we still achieved fluctuating regimes that were significantly different from the stable regimes, and therefore have confidence in our results.
Overall, our data clearly show significantly different physiological responses of organisms exposed to a fluctuating compared with a static seawater pH regime. Analysis of several health parameters suggests exposure to a fluctuating pH regime is more energetically demanding for coastal organisms than static pH conditions. The few studies to date using a fluctuating pH cycle all found that the responses of their test organisms differed between static and fluctuating conditions. In some cases fluctuating conditions appeared to alleviate the effects of elevated pCO2 [25] while others found that fluctuating conditions exacerbated a negative OA effect [26,27]. With seawater pH fluctuations expected to increase in intensity and frequency over the coming decades [22], it is vital to understand how coastal organisms respond to current variability in order to predict their responses to additional OA. Our results highlight the difficulties in extrapolating outcomes of static pH exposures for species which experience large fluctuations in their physiochemical environment, with static pH experiments potentially incorrectly estimating coastal species responses to future OA. The precise nature of the fluctuations experienced currently by natural populations remain poorly understood, but are likely to vary considerably with habitat, location and time of year. Collecting good-quality data on the conditions currently experienced by intertidal and subtidal populations is urgent to better understand the range and periodicity of these fluctuations and enhance future experimental designs if we are to suitably predict coastal organism responses to future OA.
Supplementary Material
Supplementary Material
Acknowledgements
Thanks to Rob Ellis, Steve Cooper, Darren Rowe and John Dowdle for their excellent technical support.
Ethics
All work was approved by the University of Exeter Ethics Committee and conducted under their guidelines according to the 3Rs principles.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
S.M. conducted the experimental work, performed the data analysis and drafted the manuscript. M.A.U., H.S.F., R.W.W. and C.L. contributed to the design and implementation of the experimental work, and advised on interpretation. C.L. also co-ordinated the study and drafted the manuscript. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
S.M. was funded by an Exeter University—Plymouth Marine Laboratory scholarship fund. C.L. and R.W.W. were supported by a NERC UK-OARP NERC consortium grant no. NE/H017496/1. M.A.U. was supported by CONICYT FONDECYT grant no. 11160019.
References
- 1.Bopp L, et al. 2013. Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10, 6225–6245. ( 10.5194/bg-10-6225-2013) [DOI] [Google Scholar]
- 2.Orr JC, et al. 2005. Anthropogenic ocean acidification over the 21st century and its impact on calcifying organisms. Nature 437, 681–686. ( 10.1038/nature04095) [DOI] [PubMed] [Google Scholar]
- 3.Doney SC, Fabry VJ, Feely RA, Kleypas JA. 2009. Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192. ( 10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 4.Kroeker KJ, Kordas RL, Crim RN, Singh GG. 2010. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434. ( 10.1111/j.1461-0248.2010.01518.x) [DOI] [PubMed] [Google Scholar]
- 5.Gattuso JP, Buddemeier RW. 2000. Ocean biogeochemistry - calcification and CO2. Nature 407, 311–313. ( 10.1038/35030280) [DOI] [PubMed] [Google Scholar]
- 6.Poertner HO, Farrell AP. 2008. Ecology physiology and climate change. Science 322, 690–692. ( 10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 7.Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Portner HO. 2009. Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6, 2313–2331. ( 10.1080/10236244.2012.727235) [DOI] [Google Scholar]
- 8.Heinemann A, Fietzke J, Melzner F, Boehm F, Thomsen J, Garbe-Schoenberg D, Eisenhauer A. 2012. Conditions of Mytilus edulis extracellular body fluids and shell composition in a pH-treatment experiment: acid-base status, trace elements and delta B-11. Geochem. Geophys. Geosyst. 13, Q01005 ( 10.1029/2011gc003790) [DOI] [Google Scholar]
- 9.Thomsen J, Casties I, Pansch C, Koertzinger A, Melzner F. 2013. Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments. Glob. Change Biol. 19, 1017–1027. ( 10.1111/gcb.12109) [DOI] [PubMed] [Google Scholar]
- 10.Thomsen J, Melzner F. 2010. Moderate seawater acidification does not elicit long-term metabolic depression in the blue mussel Mytilus edulis. Mar. Biol. 157, 2667–2676. ( 10.1007/s00227-010-1527-0) [DOI] [Google Scholar]
- 11.Somero GN. 1986. Protons, osmolytes, and fitness of internal milieu for protein function. Am. J. Physiol. 251, R197–R213. [DOI] [PubMed] [Google Scholar]
- 12.Shim J, Kim D, Kang YC, Lee JH, Jang ST. 2007. Seasonal variations in pCO2 and its controlling factors in surface seawater of the northern East China Sea. Cont. Shelf Res. 27, 2623–2636. ( 10.1016/j.csr.2007.07.005) [DOI] [Google Scholar]
- 13.Hofmann GE, et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 ( 10.1371/journal.pone.0028983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saderne V, Fietzek P, Herman PMJ. 2013. Extreme variations of pCO2 and pH in a macrophyte meadow of the Baltic Sea in summer: evidence of the effect of photosynthesis and local upwelling. PLoS ONE 8, e62689 ( 10.1371/journal.pone.0062689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frieder CA, Nam SH, Martz TR, Levin LA. 2012. High temporal and spatial variability of dissolved oxygen and pH in a nearshore California kelp forest. Biogeosciences 9, 3917–3930. ( 10.5194/bg-9-3917-2012) [DOI] [Google Scholar]
- 16.Baumann H, Wallace RB, Tagliaferri T, Gobler CJ. 2015. Large natural pH, CO2 and O2 fluctuations in a temperate tidal salt marsh on diel, seasonal, and interannual time scales. Estuaries Coasts 38, 220–231. ( 10.1007/s12237-014-9800-y) [DOI] [Google Scholar]
- 17.Kwiatkowski L, et al. 2016. Nighttime dissolution in a temperate coastal ocean ecosystem increases under acidification. Sci. Rep. 6, 22984 ( 10.1038/srep22984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson MD, Moriarty VW, Carpenter RC. 2014. Acclimatization of the crustose coralline alga Porolithon onkodes to variable pCO2. PLoS ONE 9, e87678 ( 10.1371/journal.pone.0087678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price NN, Martz TR, Brainard RE, Smith JE. 2012. Diel variability in seawater pH relates to calcification and benthic community structure on coral reefs. PLoS ONE 7, e43843 ( 10.1371/journal.pone.0043843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poertner H.-O. 2008. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist's view. Mar. Ecol. Prog. Ser. 373, 203–217. ( 10.3354/meps07768) [DOI] [Google Scholar]
- 21.Kapsenberg L, Hofmann GE. 2016. Ocean pH time-series and drivers of variability along the northern Channel Islands, California, USA. Limnol. Oceanogr. 61, 953–968. ( 10.1002/lno.10264) [DOI] [Google Scholar]
- 22.IPCC. 2014. Impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC.
- 23.Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Hansen HP, Koertzinger A. 2013. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 160, 1875–1888. ( 10.1007/s00227-012-1954-1) [DOI] [Google Scholar]
- 24.Dufault AM, Cumbo VR, Fan TY, Edmunds PJ. 2012. Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proc. R. Soc. B 279, 2951–2958. ( 10.1098/rspb.2011.2545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frieder CA, Gonzalez JP, Bockmon EE, Navarro MO, Levin LA. 2014. Can variable pH and low oxygen moderate ocean acidification outcomes for mussel larvae? Glob. Change Biol. 20, 754–764. ( 10.1111/gcb.12485) [DOI] [PubMed] [Google Scholar]
- 26.Alenius B, Munguia P. 2012. Effects of pH variability on the intertidal isopod, Paradella dianae. Mar. Freshw. Behav. Physiol. 45, 245–259. ( 10.1080/10236244.2012.727235) [DOI] [Google Scholar]
- 27.Eriander L, Wrange AL, Havenhand JN. 2016. Simulated diurnal pH fluctuations radically increase variance in-but not the mean of-growth in the barnacle Balanus improvisus. ICES J. Mar. Sci. 73, 596–603. ( 10.1093/icesjms/fsv214) [DOI] [Google Scholar]
- 28.Small DP, Milazzo M, Bertolini C, Graham H, Hauton C, Hall-Spencer JM, Rastrick SPS. 2016. Temporal fluctuations in seawater pCO2 may be as important as mean differences when determining physiological sensitivity in natural systems. ICES J. Mar. Sci. 73, 604–612. ( 10.1093/icesjms/fsv232) [DOI] [Google Scholar]
- 29.Truchot JP. 1976. Carbon dioxide combining properties of blood of shore crab Carcinus maenas-l - carbon dioxide solubility coefficient and carbonic acid dissociation constants. J. Exp. Biol. 64, 45–57. [DOI] [PubMed] [Google Scholar]
- 30.Meinshausen M, et al. 2011. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Change 109, 213–241. ( 10.1007/s10584-011-0156-z) [DOI] [Google Scholar]
- 31.Lowe DM, Soverchia C, Moore MN. 1995. Lysosomal membrane responses in the blood and digestive cells of mussels experimentally exposed to fluoranthene. Aquat. Toxicol. 33, 105–112. ( 10.1016/0166-445x(95)00015-v) [DOI] [Google Scholar]
- 32.Cajaraville MP, Olabarrieta I, Marigomez I. 1996. In vitro activities in mussel hemocytes as biomarkers of environmental quality: a case study in the Abra Estuary (Biscay Bay). Ecotoxicol. Environ. Saf 35, 253–260. ( 10.1006/eesa.1996.0108) [DOI] [PubMed] [Google Scholar]
- 33.van der Oost R, Porte Visa C, van den Brink N. 2005. Biomarkers in environmental assessment. In Exotoxicological testing of marine and freshwater ecosystems: emerging techniques, trends, and strategies (eds den Besten P, Munawar M), pp. 87–152. Boca Raton, FL: Taylor and Francis Group. [Google Scholar]
- 34.Camejo G, Wallin B, Enojärvi M. 1999. Analysis of oxidation and 610 antioxidants using microtiter plate. In Free radical and antioxidant protocols methods in molecular biology (eds RM Uppu, SN Murthy, WA Pryor, NL Parinandi), pp. 377–387. Totowa, NJ: Humana. [DOI] [PubMed] [Google Scholar]
- 35.Portner HO, Langenbuch M, Reipschlager A. 2004. Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J. Oceanogr. 60, 705–718. ( 10.1007/s10872-004-5763-0) [DOI] [Google Scholar]
- 36.Spicer JI, Raffo A, Widdicombe S. 2007. Influence of CO2-related seawater acidification on extracellular acid-base balance in the velvet swimming crab Necora puber. Mar. Biol. 151, 1117–1125. ( 10.1007/s00227-006-0551-6) [DOI] [Google Scholar]
- 37.Michaelidis B, Ouzounis C, Paleras A, Portner HO. 2005. Effects of long-term moderate hypercapnia on acid-base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 293, 109–118. ( 10.3354/meps293109) [DOI] [Google Scholar]
- 38.Booth CE, McDonald DG, Walsh PJ. 1984. Acid–base balance in the sea mussel, Mytilus edulis. 1. Effects of hypoxia and air-exposure on hemolymph acid-base status. Mar. Biol. Lett. 5, 347–358. [Google Scholar]
- 39.Gutowska MA, Melzner F, Langenbuch M, Bock C, Claireaux G, Poertner HO. 2010. Acid–base regulatory ability of the cephalopod (Sepia officinalis) in response to environmental hypercapnia. J. Comp. Physiol. B 180, 323–335. ( 10.1007/s00360-009-0412-y) [DOI] [PubMed] [Google Scholar]
- 40.Portner HO, Bock C, Reipschlager A. 2000. Modulation of the cost of pHi regulation during metabolic depression: a P-31-NMR study in invertebrate (Sipunculus nudus) isolated muscle. J. Exp. Biol. 203, 2417–2428. [DOI] [PubMed] [Google Scholar]
- 41.Somero GN. 1985. Intracellular pH, buffering substances and proteins: imidazole protonation and the conservation of protein structure and function. In Transport processes, iono- and osmoregulation, (eds Gilles R, Gilles-Baillien M), pp. 454–468. Berlin, Germany: Springer. [Google Scholar]
- 42.Leiva FP, Niklitschek EJ, Paschke K, Gebauer P, Urbina MA. 2016. Tide-related biological rhythm in the oxygen consumption rate of ghost shrimp (Neotrypaea uncinata). J. Exp. Biol. 219, 1957–1960. ( 10.1242/jeb.133785) [DOI] [PubMed] [Google Scholar]
- 43.Bibby R, Widdicombe S, Parry H, Spicer J, Pipe R. 2008. Effects of ocean acidification on the immune response of the blue mussel Mytilus edulis. Aquat. Biol. 2, 67–74. ( 10.3354/ab00037) [DOI] [Google Scholar]
- 44.Matozzo V, Chinellato A, Munari M, Bressan M, Marin MG. 2013. Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chamelea gallina and the mussel Mytilus galloprovincialis? Mar. Pollut. Bull. 72, 34–40. ( 10.1016/j.marpolbul.2013.05.004) [DOI] [PubMed] [Google Scholar]
- 45.Beesley A, Lowe DM, Pascoe CK, Widdicombe S. 2008. Effects of CO2-induced seawater acidification on the health of Mytilus edulis. Clim. Res. 37, 215–225. ( 10.3354/cr00765) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.