Abstract
Regulating maternal immunity is necessary for successful human pregnancy. Whether this is needed in mammals with less invasive placentation is subject to debate. Indeed, the short gestation times in marsupials have been hypothesized to be due to a lack of immune regulation during pregnancy. Alternatively, the maternal marsupial immune system may be unstimulated in the absence of a highly invasive placenta. Transcripts encoding pro-inflammatory cytokines were found to be overrepresented in the whole uterine transcriptome at terminal pregnancy in the opossum, Monodelphis domestica. To investigate this further, immune gene transcripts were quantified throughout opossum gestation. Transcripts encoding pro-inflammatory cytokines remained relatively low during pre- and peri-attachment pregnancy stages. Levels dramatically increased late in gestation, peaking within 12 h prior to parturition. These results mirror the spike of inflammation seen at eutherian parturition but not at attachment or implantation. Our results are consistent with the role of pro-inflammatory cytokines at parturition being an ancient and conserved birth mechanism in therian mammals.
Keywords: marsupial, pregnancy, inflammation, parturition, viviparity
1. Introduction
Over 60 years ago, Peter Medawar outlined the challenge that a viviparous organism faces when simultaneously nourishing a fetus through intimate contact with allogeneic membranes (the placenta) while maintaining an immune system exquisitely sensitive to foreign allografts [1]. Understanding how this balance between allogeneic response and viviparous reproduction is regulated or maintained has been a topic of investigation, particularly given its importance to successful human pregnancy. It is apparent that the transition from egg-laying to live birth has occurred independently multiple times during vertebrate evolution, including cartilaginous fishes, teleosts, amphibians, reptiles, as well as the mammals [2]. In squamates alone viviparity has evolved independently over one hundred times [2,3,4]. By looking for common regulatory features among different vertebrates, insights may be gained into which evolutionary adaptations are common and ancient and which represent lineage-specific innovations in the transition from oviparity to viviparity.
Currently there are three living mammalian lineages: the monotremes (platypuses and echidnas), the marsupials (e.g. opossums and kangaroos), and the eutherians (e.g. humans, mice, and cattle). For biomedical and economic reasons, most reproductive immunology has focused on eutherians. Marsupials and eutherians, collectively known as the therians, are both live-bearing and are believed to share a common viviparous ancestor [5,6]. Comparative analyses between marsupials and eutherians, therefore, may reveal both common characteristics that evolved early in the therians as well as unique adaptations associated with placental evolution among the lineages.
In eutherians, the placenta is in close contact with maternal tissues, and in some cases such as humans, in direct contact with maternal blood circulation [7]. Analyses of pregnancy in eutherians, particularly humans and mice, have revealed numerous mechanisms regulating the maternal immune system that appear to be necessary for successful gestation [8–10]. Examples include regulation of inflammation and the complement system during pregnancy [11,12]. Failure to regulate can lead to complications or pregnancy loss [13–15]. In spite of this, some aspects of immune recognition and response appear to be part of normal pregnancy [16–18]. One in particular is the role that inflammation plays both early during implantation and late during parturition in human pregnancy.
Studies of marsupial reproductive immunology have been limited. Antibodies against paternal antigens were undetectable in maternal wallaby serum after successful pregnancies [19]. By contrast, maternal anti-paternal antibodies are normal during pregnancy in humans and other eutherian species [20,21]. Attempts to immunize female tammar wallabies, Macropus eugenii, with male alloantigens failed to alter fecundity [22]. Investigators have concluded from these collective results that the marsupial maternal immune system may be unaware of the conceptus throughout pregnancy. Indeed, marsupial embryos have a maternally derived mucoid shell coat that potentially masks paternal alloantigens prior to attachment to the maternal endometrium [23,24]. This shell coat is intact for at least the first two-thirds of pregnancy in marsupial species possibly leaving little time to stimulate an allogeneic response [25].
Previously we reported that gene transcripts associated with the immune system were overrepresented among those with increased abundance at terminal pregnancy in the opossum, Monodelphis domestica [26]. However, it was unclear whether the high transcription levels of these genes are only at terminal pregnancy and possibly due to imminent parturition, or if there is a lack of regulation of inflammation throughout pregnancy. In order to address this question, here we examined the transcription of several cytokines at multiple points during pregnancy in opossum, focusing on just prior to attachment through parturition.
2. Material and methods
(a). Opossum husbandry
Opossums used in this study were sourced from a captive-bred research colony housed at the University of New Mexico, Department of Biology Animal Research Facility. Opossum care and euthanasia were as previously described [26].
Time of copulation was determined in all pregnancies by observing a single mating event. A male opossum was introduced into a female's cage for approximately 72 h to induce oestrus in the female. After the third day the cage was divided by a clear plastic barrier with 2-cm interspaced holes 1 cm in diameter around opossum ‘nose-level’ so the male and female could clearly see and smell each other but not physically interact. Once per day the barrier was removed approximately 2–3 h after the start of the opossums' night cycle. The female and male were allowed to directly interact for up to 1 h with an investigator present. If the opossum pair mated then the time of copulation initiation was recorded and the opossums were moved to separate cages. If the pair did not mate the barrier was replaced and the male and female were placed on the opposite sides of the cage of where they were previously. This procedure was repeated for 5 days or until opossums mated. One hundred per cent of trials where a mating event was recorded resulted in a pregnancy of the expected developmental stage at the time of collection.
(b). Tissue collection
Uterine tissues were collected from opossums euthanized by isoflurane overdose followed by decapitation. For both pregnant and non-pregnant (NP) animals, uterine horns were removed, separated from ovaries and lateral vaginal canals, and dissected in shallow Petri dishes filled with RNALater buffer (Ambion). For RNA samples uterine horns were opened laterally and any visible embryos were removed. The exposed endometrial tissue was pulled away from the myometrium with tweezers and snipped with surgical scissors to excise primarily endometrial tissue for collection. Tissues were collected as 100–200 mg samples and were submerged in 1 ml RNALater immediately. Samples were collected from time points on embryonic days 3 (E3), 9 (E9), 10 (E10), 11 (E11), 12 (E12, attachment), 13 (E13), 14 (E14, parturition), and postnatal day 1 (P1). In addition, NP control uterine tissue was collected from females who had given birth three to six months prior to collection. N = 3 for all treatment groups. In pregnant samples from time points E12, E13 and E14 the fetal placenta membranes were attached to the maternal endometrium and could not be readily separated out and, therefore, these samples contained fetal membranes. After a 24-h incubation at 4°C in RNALater, the tissue samples were removed and stored at −80°C until RNA extraction.
(c). RNA extraction and cDNA synthesis
All RNA extractions were performed by first freezing tissues in liquid nitrogen and then homogenizing them using a sterile liquid nitrogen-cooled mortar and pestle. Subsequently, 1 ml TRIzol (Ambion) for every 100 mg of tissue was added to the mortar and homogenized with the powdered tissue until the mixture warmed enough to become liquid again. The homogenized tissue was then phase-separated by adding 0.2 ml chloroform for every 1 ml TRIzol used and shaken vigorously for 15 s. The samples were incubated at room temperature for 3 min and then centrifuged at 12 000g for 15 min at 4°C. The clear aqueous upper phase was transferred to a sterile tube and an equal amount of RNase-free 70% ethanol was added to the tube. Then the RNA was isolated using the PureLink RNA Mini Kit (Ambion) according to manufacturer's instructions for purifying RNA from animal tissues. The resulting total RNA samples were purified of DNA contamination using the TURBO DNA-free Kit (Ambion) according to manufacturers' recommended protocols.
All cDNA libraries were generated by reverse transcriptase PCR (RT-PCR) using the SuperScript III First Strand Synthesis Kit (Invitrogen) according to manufacturer's instructions for generating cDNA from poly(A) RNA. A total of 500 ng of RNA was used for each RT-PCR reaction and reactions were performed in triplicate for each sample. The RT-PCR reactions were pooled by individual tissue sample and 87 µl of PCR-grade water was added to bring the total volume of cDNA to 150 µl. cDNA samples were stored at −20°C until use in qPCR reactions.
(d). Quantitative PCR
All qPCR reactions were performed in triplicate using 18 µl SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) and 2 µl cDNA for each sample. All reactions were performed on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) under the following conditions: 95°C for 3 min, then 40 cycles of 95°C for 10 s followed by 60 s at the appropriate annealing temperature during which data were collected. Primers spanning at least one intron were generated and optimized for each gene examined (electronic supplementary material, table S1). qPCR data were analysed in the CFX Manager Software (Bio-Rad) using tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta polypeptide (YWHAZ) and TATA box-binding protein (TBP) as reference genes for normalization using the Vandesompele method [27]. These reference genes were chosen based on their relatively uniform transcription across pregnant and NP opossum uterine tissues [26].
Differences in transcript abundance were evaluated in two ways. First transcription levels of target genes were tested for significant change between treatment groups using ordinary one-way ANOVA with Tukey's multiple comparisons test. All statistical tests and graphs were generated using Prism 7 (GraphPad) and edited for clarity in Illustrator (Adobe). In addition effect size was calculated using the Cohen's difference (d) of the mean method [28,29].
3. Results
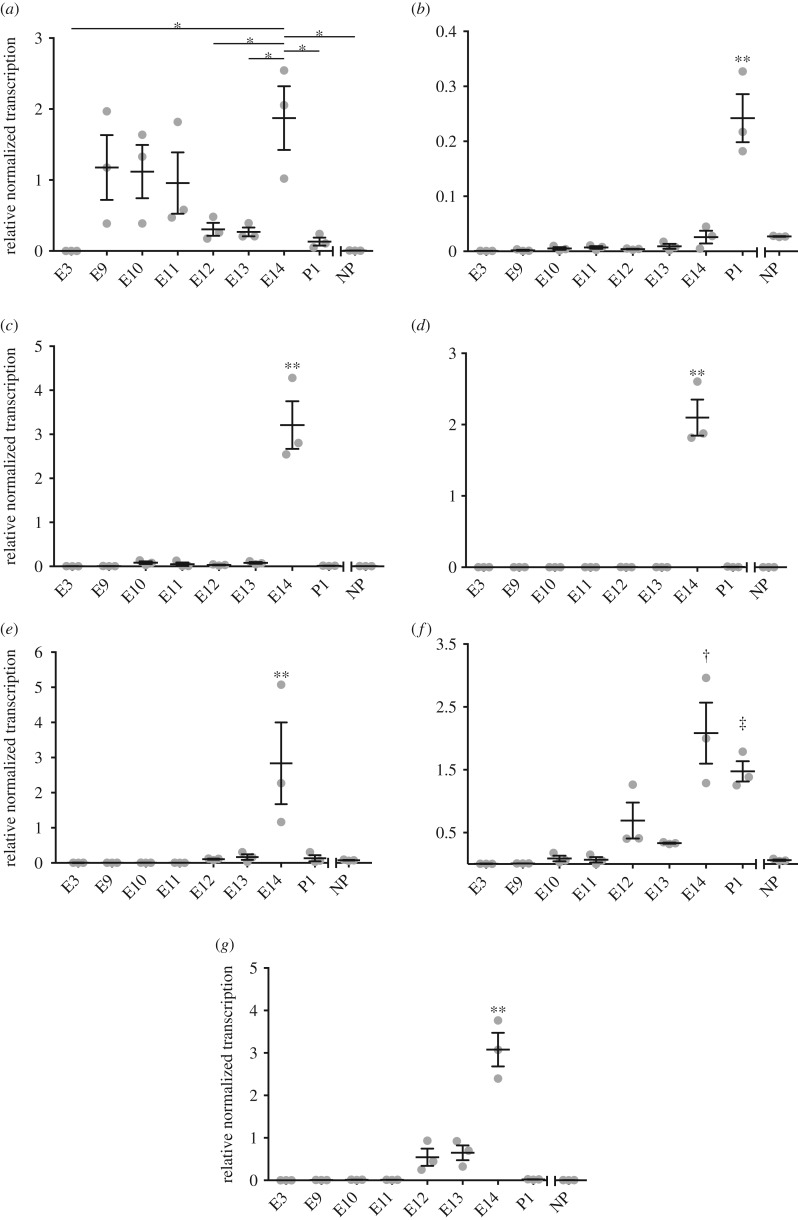
In a previous study of opossum uterine transcriptomes we found that some cytokine gene transcripts were substantially more abundant at terminal pregnancy relative to NP samples [26]. These included pro-inflammatory cytokine genes such as IL1A, IL6, IL10, IL17A and TNF. The pregnancy time point assessed in that study was only the last 24 h of gestation. Therefore, the inflammatory response could not be specifically attributed to parturition or other aspects of pregnancy. Here we have assessed the transcription of these pro-inflammatory cytokine genes by qPCR to quantify cytokine transcript levels at additional pregnancy time points, focusing in particular around attachment (E12) and parturition (E14). We normalized transcription levels of target genes relative to two reference genes, YWHAZ and TBP.
In a study of opossum uterine transcriptomes, IL1A had significantly more transcripts in terminal pregnant than NP samples [26]. IL1A transcripts were low at E3 and in the NP samples, however were measurable at all other pregnancy time points and P1 (figure 1a). The abundance of IL1A transcripts is higher at E9, E10 and E11 than E3. This was not significant, likely due to variance in E9, E10 and E11 samples. The IL1A transcript levels decreased on E12, the day the shell-coat breaks down and the embryo attaches to the maternal endometrium, and remained low on E13 as well (figure 1a). Consistent with our previous report, IL1A transcript levels then increased on E14, the last day of pregnancy (figure 1a). IL1A transcript levels at E14 were higher than E3, E12, E13, P1 and NP. The effect size based on the differences of the means of E13 and E14 was very large (figure 1 legend).
Figure 1.
Cytokine transcript abundance for IL1A (a), IL1B (b), IL6 (c), IL17A (d), TNF (e), IL8 (f), and IL10 (g) as measured by qPCR. The y-axis is the fold change transcription relative to reference genes YWHAZ and TBP. The x-axis represents time points; E12 represents attachment and E14 represents imminent parturition. Lines and bars represent mean and upper and lower SEM. Grey dots represent expression levels of individual samples. p-Values based on ANOVA are indicated as *p < 0.05 for comparisons between specified time points, **p < 0.05 for comparisons for the indicated time point and all other time points, †p < 0.05 for comparisons between specified time point and all other time points with the exception of P1, and ‡p < 0.05 for comparisons between specified time point and all other time points with the exception of E14. The measures of effect size as determined using Cohen's d were: (a) d = 3.14 for E14 versus E13; (b) d = 3.91 for P1 versus E14; (c) d = 4.72 for E14 versus E13; (d) d = 6.77 for E14 versus E13; (e) d = 1.87 for E14 versus E13; (f) d = 2.94 for E14 versus E13; and (g) d = 4.59 for E14 versus E13.
IL1B was not among those genes with significantly differentially abundant transcripts in the opossum uterine transcriptome results [26]. However IL-1β expression is known to change at the fetomaternal interface during pregnancy and labour in humans and, therefore, it was investigated here further [30–32]. IL1B consistently had low transcript levels throughout pregnancy until after parturition in opossum uterine tissue (figure 1b). IL1B transcription was significantly higher at P1, with a very large effect size, when compared with all other time points including NP.
IL6, IL17A and TNF all had significantly more transcripts in pregnant versus NP tissue according to the opossum uterine transcription study [26]. All three cytokine genes had consistently low to undetectable transcription at time points other than E14 (figure 1c–e). Transcription levels for IL6, IL17A and TNF at E14 were significantly higher than at all other time points including NP, and the effect sizes were all very large.
Although IL8 was not one of the original cytokines identified as upregulated during opossum pregnancy, it was examined here because it is associated with parturition in eutherian pregnancy [33]. IL8 transcripts were low from E3 through E13 with a slight, but not significant, increase on E12 (figure 1f). Transcript levels significantly increased with a very large effect size, on E14 and P1 just before and after parturition relative to other stages of pregnancy and NP controls (figure 1f).
IL10 transcripts were identified as more abundant in pregnant than NP opossum tissue in the transcriptome study [26]. Moreover, IL-10 is a cytokine that is thought to play an important part in regulating the maternal immune system during eutherian pregnancy [34]. In pregnant opossum uterine tissues IL10 transcripts remained low with a slight, but not significant, increase on E12 and E13 (figure 1g). Transcription of IL10 did significantly increase with a large effect size at terminal pregnancy on E14 and significantly decreased after parturition on P1. IL10 transcription levels were significantly higher at E14 than all other pregnant time points and controls (figure 1g).
4. Discussion
Inflammation at the fetomaternal interface is one of the major causes of pregnancy complications [12]. In humans it can lead to spontaneous abortion, pre-term birth, pre-eclampsia, and is even associated with lifelong conditions affecting the child such as autism and schizophrenia [35,36]. However, at two points during eutherian pregnancy pro-inflammatory cytokines appear to be beneficial and, perhaps, necessary. The first is during the peri-implantation period when the embryo implants into the maternal endometrium [37]. Studies have shown that human in vitro fertilization can benefit from a minor wound at the implantation site to induce an inflammation response [16]. The second point when inflammation is beneficial is at parturition. At parturition, pro-inflammatory cytokines play a role in the ripening of the cervix and initiating the onset of labour in humans [38–41]. Uncontrolled inflammation at other times during pregnancy is associated with pre-eclampsia and miscarriage [42–45].
Since eutherians benefit from pro-inflammatory cytokines at both implantation and parturition, marsupials, which share a common viviparous ancestor with eutherians, might be expected to share this reproductive trait or provide insights into its origins. Here we have shown that the transcription of pro-inflammatory cytokines during parturition at the fetomaternal interface in a model marsupial, M. domestica, is similar to the pattern of cytokine expression seen in eutherian parturition. The results presented are consistent with an ancient role of these cytokines in mammalian birth.
Of the pro-inflammatory cytokine genes tested, only IL1A exhibited moderate levels of transcription prior to attachment (figure 1a). Embryonic day 12 is when the opossum embryo loses its shell coat and attaches to the maternal endometrium and nourishment shifts from being provided by uterine secretions to haemotrophe from maternal circulation [24,46]. In mice IL-1α is expressed by endometrial cells during the pre-implantation period [47,48]. In murine pregnancy embryos implant around day 5 of gestation, which is also when IL-1α expression decreases [47]. This is similar to the pattern we observed in M. domestica uterine tissues where IL1A transcription decreased on E12 (figure 1a). The IL1A transcription levels did not significantly increase until E14 at parturition before dropping again on P1 (figure 1a). IL-1α also has increased expression at parturition in humans, though not as much as IL-1β [49].
In humans IL1B mRNA transcription in the choriodecidua increases in the third trimester when compared with the first and second [30]. However, IL1B is also upregulated in post-labour choriodecidual tissues. This is similar to our observations in the opossum where the only significant increase or IL1B transcripts are on P1 (figure 1b). Human choriodecidua also has higher IL-1β protein content in the third trimester compared with earlier pregnancy, but the IL-1β concentration did not significantly increase before labour compared with after [30]. Expression of IL-1 is observed not only in the mammalian term uterus, but also in those of squamate reptiles and cartilaginous fishes [49–52]. Therefore, the IL-1 system is likely an important component of parturition in even the earliest viviparous vertebrates, which has been preserved in many extant lineages [52]. Since human blastocysts express IL-1, IL-1 receptors and IL-1 antagonists, there is speculation that the IL-1 system is used as a means of communication and regulation between embryo and mother [18].
IL6 transcription at the opossum fetomaternal interface was low during pregnancy, peaked at parturition and dropped afterwards (figure 1c). Similarly, during human labour, IL-6 levels are also elevated at the fetomaternal interface [53–55]. IL6 mRNA is significantly more abundant in human myometrium and choriodecidua after the onset of labour than prior to labour [38]. It is possible that this is analogous to a state of labour in the opossum. However in other marsupials labour is brief; it is only a matter of minutes in macropods [56].
The patterns of transcription over the course of pregnancy for TNF and IL17A were virtually identical to that of IL6 in the opossum (figure 1c–e). TNFα is a cytokine that has been shown to be elevated in human parturition [53]. However in other eutherians, such as cows, TNFα expression actually decreases at parturition [57], indicating that elevated TNFα levels at this time point is not a strongly conserved mammal characteristic. Pongcharoen et al. [58] demonstrated that IL-17 is expressed in human terminal pregnancy explants as well. Unfortunately, their examination did not include earlier pregnancy time points for comparison.
Increased IL-8 expression is associated with parturition in eutherians [33,57,59]. Therefore, we expected to see IL8 transcription peak on E14 (figure 1f). It is somewhat surprising that IL8 transcription was not identified as significantly increased in our previous transcriptome study [26]. A possible explanation is that the parameters used to identify differential transcription were too stringent to include IL8 in the list of significantly differentially abundant transcripts. Human choriodecidual and myometrial tissues have significantly greater IL8 transcription after the initiation of labour than before [38].
IL10 gene transcripts were significantly increased at parturition in the opossum as well (figure 1g). In human placenta IL-10 expression has been reported as normally downregulated at term labour compared with first and second trimester expression levels [60]. There are conflicting reports on IL-10 expression at the fetomaternal interface at term pregnancy in humans. One report did not find a significant change in IL-10 expression between human laboured and non-laboured decidual cells [61]. Another found that choriodecidual tissue had significantly less IL-10 expression after labour onset compared to term pre-labour tissue [62]. Regardless of which report is most representative of human cytokine production at labour, our observations of increased IL10 transcription in opossum placental tissues appears to diverge from the human norm.
Recently, Griffith et al. [63] demonstrated increased transcription of pro-inflammatory cytokines following attachment and prior to parturition in the opossum. Their results are entirely consistent with what is presented here. These investigators however attribute the increased presence of pro-inflammatory transcripts to be an inflammatory response to the attachment, analogous to implantation in eutherians. This is certainly a viable hypothesis. Indeed, in some species, inflammation appears to be dependent upon an allogeneic immune response. In cows, for example, if the mother and fetus are too genetically similar the allogeneic response is insufficiently strong to expel the fetal tissues from the womb [64]. Furthermore, opossums have been shown to lack many of the mechanisms important to controlling allogeneic responses against the fetus. For example, marsupials lack the FoxP3 enhancer element that enables peripheral regulatory T cells (pTregs) specific to paternal alloantigens to be generated at the fetomaternal interface [65].
Nonetheless, we believe our results are consistent with the transcription of pro-inflammatory cytokines being associated more closely with parturition at day 14 and not a response to attachment at day 12. The profile of pro-inflammatory cytokines at terminal pregnancy in the opossum overlap with those found associated with parturition in eutherians and other distantly related viviparous vertebrates (table 1).
Table 1.
Similarity of pro-inflammatory cytokine profiles among vertebrates at the fetomaternal interface near parturition. Y, present; N, not present; Y/N, species dependent; U, not conclusive, or contradictory in the literature; n.d., not determined.
An alternative mechanism to attachment for the trigger of pro-inflammatory cytokine production and parturition in the opossum is needed. One hypothesis would be developmental triggers of parturition similar to those seen in eutherians [73]. These could be based on fetal developmental stage, senescence of fetal membranes, maternal hormones or perhaps some combination. This could account for the pronounced pro-inflammatory cytokine transcription only just prior to parturition in M. domestica. While the upstream trigger of parturition remains unknown, it may be more analogous to the cervical ripening and uterine contractions seen in eutherians. In other words, it is the pleiotropic nature of pro-inflammatory cytokines performing their role in parturition, not an inflammatory reaction per se.
In conclusion, we propose that pro-inflammatory cytokine expression at the fetomaternal interface is an ancient characteristic of the therian lineage and is possibly a key factor in the evolution of mammalian parturition.
Supplementary Material
Acknowledgements
The authors wish to thank Dr Thomas Turner for advice on statistical analyses.
Ethics
This study was approved under protocol numbers 13-100920-MCC and 15-200334-B-MC from the University of New Mexico Institutional Animal Care and Use Committee.
Data accessibility
This article has no additional data.
Authors' contributions
V.L.H. collected tissues, designed and performed experiments, and conducted the formal analysis. L.S.F. and A.A.S. performed experiments. R.D.M. supervised the experimental design and analysis. V.L.H. and R.D.M. wrote the original draft of the manuscript. All authors reviewed and edited the manuscript.
Competing interests
We have no competing interests.
Funding
We wish to thank the National Science Foundation (IOS-1353123) and the National Institute of General Medical Sciences (P30GM110907) for funding this research.
References
- 1.Medawar PB. 1953. Some immunological and endorinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 7, 320–338. [Google Scholar]
- 2.Blackburn DG. 2015. Evolution of vertebrate viviparity and specializations for fetal nutrition: a quantitative and qualitative analysis. J. Morphol. 276, 961–990. ( 10.1002/jmor.20272) [DOI] [PubMed] [Google Scholar]
- 3.Blackburn DG. 1999. Are viviparity and egg-guarding evolutionarily labile in squamates? Herpetologica 55, 556–573. [Google Scholar]
- 4.Blackburn DG. 1999. Viviparity and oviparity: evolution and reproductive strategies. In Encyclopedia of reproduction (eds Knobil E, Neill JD), pp. 994–1003. London, UK: Academic Press. [Google Scholar]
- 5.Baker ML, Wares JP, Harrison GA, Miller RD. 2004. Relationships among the families and orders of marsupials and the major mammalian lineages based on recombination activating gene-1. J. Mamm. Evol. 11, 1–16. ( 10.1023/B:JOMM.0000029143.39776.ec) [DOI] [Google Scholar]
- 6.Meredith RW, et al. 2011. Impacts of the cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334, 521–524. ( 10.1126/science.1211028) [DOI] [PubMed] [Google Scholar]
- 7.Moffett A, Loke C. 2006. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 6, 584–594. ( 10.1038/nri1897) [DOI] [PubMed] [Google Scholar]
- 8.Erlebacher A. 2012. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat. Rev. Immunol. 13, 23–33. ( 10.1038/nri3361) [DOI] [PubMed] [Google Scholar]
- 9.Erlebacher A. 2013. Immunology of the maternal–fetal interface. Annu. Rev. Immunol. 31, 387–411. ( 10.1146/annurev-immunol-032712-100003) [DOI] [PubMed] [Google Scholar]
- 10.Racicot K, Kwon J-A, Aldo P, Sailasi M, Mor G. 2014. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 72, 107–116. ( 10.1111/aji.12289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denny KJ, Woodruff TM, Taylor SM, Callaway LK. 2013. Complement in pregnancy: a delicate balance. Am. J. Reprod. Immunol. 69, 3–11. ( 10.1111/aji.12000) [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Gotsch F, Pineles B, Kusanovic JP. 2007. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr. Rev. 65, S194–S202. ( 10.1111/j.1753-4887.2007.tb00362.x) [DOI] [PubMed] [Google Scholar]
- 13.Matthiesen L, Kalkunte S, Sharma S. 2012. Multiple pregnancy failures: an immunological paradigm. Am. J. Reprod. Immunol. 67, 334–340. ( 10.1111/j.1600-0897.2012.01121.x) [DOI] [PubMed] [Google Scholar]
- 14.Roberts JM, Bell MJ. 2013. If we know so much about preeclampsia, why haven't we cured the disease? J. Reprod. Immunol. 99, 1–9. ( 10.1016/j.jri.2013.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. 2006. Inflammation in preterm and term labour and delivery. Semin. Fetal Neonatal Med. 11, 317–326. ( 10.1016/j.siny.2006.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekel N, Gnainsky Y, Granot I, Mor G. 2010. Inflammation and implantation. Am. J. Reprod. Immunol. 63, 17–21. ( 10.1111/j.1600-0897.2009.00792.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keelan JA, Blumenstein M, Helliwell RJA, Sato TA, Marvin KW, Mitchell MD. 2003. Cytokines, prostaglandins and parturition—a review. Placenta 24, S33–S46. ( 10.1053/plac.2002.0948) [DOI] [PubMed] [Google Scholar]
- 18.Van Mourik MSM, Macklon NS, Heijnen CJ. 2009. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J. Leukoc. Biol. 85, 4–19. ( 10.1189/jlb.0708395) [DOI] [PubMed] [Google Scholar]
- 19.Van Oorschot RAH, Cooper DW. 1988. Lack of evidence for complement-dependent cytotoxic antibodies to fetal paternally derived antigens in the marsupial Macropus eugenii (tammar wallaby). Am. J. Reprod. Immunol. Microbiol. 17, 145–148. ( 10.1111/j.1600-0897.1988.tb00219.x) [DOI] [PubMed] [Google Scholar]
- 20.Mowbray JF, Liddell H, Underwood J, Gibbings C, Reginald PW, Beard RW. 1985. Controlled trial of treatment of recurrent spontaneous abortion by immunisation with paternal cells. Lancet 325, 941–943. ( 10.1016/S0140-6736(85)91723-4) [DOI] [PubMed] [Google Scholar]
- 21.Cauchi MN, Lim D, Young DE, Kloss M, Pepperell RJ. 1991. Treatment of recurrent aborters by immunization with paternal cells—controlled trial. Am. J. Reprod. Immunol. 25, 16–17. ( 10.1111/j.1600-0897.1991.tb01057.x) [DOI] [PubMed] [Google Scholar]
- 22.Rodger JC, Fletcher TP, Tyndale-Biscoe CH. 1985. Active anti-paternal immunization does not affect the success of marsupial pregnancy. J. Reprod. Immunol. 8, 249–256. ( 10.1016/0165-0378(85)90044-0) [DOI] [PubMed] [Google Scholar]
- 23.Freyer C, Zeller U, Renfree MB. 2003. The marsupial placenta: a phylogenetic analysis. J. Exp. Zool. A Ecol. Genet. Physiol. 299, 59–77. ( 10.1002/jez.a.10291) [DOI] [PubMed] [Google Scholar]
- 24.Zeller U, Freyer C. 2001. Early ontogeny and placentation of the grey short-tailed opossum, Monodelphis domestica (Didelphidae: Marsupialia): contribution to the reconstruction of the marsupial monotype. J. Zool. Syst. Evol. Res. 39, 137–158. ( 10.1046/j.1439-0469.2001.00167.x) [DOI] [Google Scholar]
- 25.Tyndale-Biscoe H, Renfree M. 1987. Reproductive physiology of marsupials. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 26.Hansen VL, Schilkey FD, Miller RD. 2016. Transcriptomic changes associated with pregnancy in a marsupial, the gray short-tailed opossum Monodelphis domestica. PLoS ONE 11, e0161608 ( 10.1371/journal.pone.0161608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, research0034 1. ( 10.1186/gb-2002-3-7-research0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen J. 1992. Statistical power analysis. Curr. Dir. Psychol. Sci. 1, 98–101. ( 10.1111/1467-8721.ep10768783) [DOI] [Google Scholar]
- 29.Sawilowsky SS. 2009. New effect size rules of thumb. J. Mod. Appl. Stat. Methods. 8, 597–599. ( 10.22237/jmasm/1257035100) [DOI] [Google Scholar]
- 30.Elliott CL, Loudon JAZ, Brown N, Slater DM, Bennett PR, Sullivan MHF. 2001. IL-1β and IL-8 in human fetal membranes: changes with gestational age, labor, and culture conditions. Am. J. Reprod. Immunol. 46, 260–267. ( 10.1034/j.1600-0897.2001.d01-11.x) [DOI] [PubMed] [Google Scholar]
- 31.Hu X-L, Yang Y, Hunt JS. 1992. Differential distribution of interleukin-1α and interleukin-1β proteins in human placentas. J. Reprod. Immunol. 22, 257–268. ( 10.1016/0165-0378(92)90047-8) [DOI] [PubMed] [Google Scholar]
- 32.Steinborn A, Kuhnert M, Halberstadt E. 1996. Immunomodulating cytokines induce term and preterm parturition. J. Perinat. Med. 24, 381–390. ( 10.1515/jpme.1996.24.4.381) [DOI] [PubMed] [Google Scholar]
- 33.Osmers RGW, Blaser J, Kuhn W, Tschesche H. 1995. Interleukin-8 synthesis and the onset of labor. Obstet. Gynecol. 86, 223–229. ( 10.1016/0029-7844(95)93704-4) [DOI] [PubMed] [Google Scholar]
- 34.Thaxton JE, Sharma S. 2010. Interleukin-10: a multi-faceted agent of pregnancy. Am. J. Reprod. Immunol. 63, 482–491. ( 10.1111/j.1600-0897.2010.00810.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer U, Feldon J, Yee BK. 2009. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr. Bull. 35, 959–972. ( 10.1093/schbul/sbn022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson PH. 2009. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 204, 313–321. ( 10.1016/j.bbr.2008.12.016) [DOI] [PubMed] [Google Scholar]
- 37.Mor G, Cardenas I, Abrahams V, Guller S. 2011. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 1221, 80–87. ( 10.1111/j.1749-6632.2010.05938.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. 2003. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 9, 41–45. ( 10.1093/molehr/gag001) [DOI] [PubMed] [Google Scholar]
- 39.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. 2008. Inflammatory processes in preterm and term parturition. J. Reprod. Immunol. 79, 50–57. ( 10.1016/j.jri.2008.04.002) [DOI] [PubMed] [Google Scholar]
- 40.Nagamatsu T, Schust DJ. 2010. The contribution of macrophages to normal and pathological pregnancies. Am. J. Reprod. Immunol. 63, 460–471. ( 10.1111/j.1600-0897.2010.00813.x) [DOI] [PubMed] [Google Scholar]
- 41.Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. 2002. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol. Reprod. 66, 445–449. ( 10.1095/biolreprod66.2.445) [DOI] [PubMed] [Google Scholar]
- 42.Christiansen OB, Nielsen HS, Kolte AM. 2006. Inflammation and miscarriage. Semin. Fetal Neonatal Med. 11, 302–308. ( 10.1016/j.siny.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 43.Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Martin JN, Bennett WA. 1999. Expression of the placental cytokines tumor necrosis factor α, interleukin 1β, and interleukin 10 is increased in preeclampsia. Am. J. Obstet. Gynecol. 181, 915–920. ( 10.1016/S0002-9378(99)70325-X) [DOI] [PubMed] [Google Scholar]
- 44.Rusterholz C, Hahn S, Holzgreve W. 2007. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin. Immunopathol. 29, 151–162. ( 10.1007/s00281-007-0071-6) [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Walsh SW. 1996. TNFα concentrations and mRNA expression are increased in preeclamptic placentas. J. Reprod. Immunol. 32, 157–169. ( 10.1016/S0165-0378(96)00998-9) [DOI] [PubMed] [Google Scholar]
- 46.Freyer C, Zeller U, Renfree MB. 2002. Ultrastructure of the placenta of the tammar wallaby, Macropus eugenii: comparison with the grey short-tailed opossum, Monodelphis domestica. J. Anat. 201, 101–119. ( 10.1046/j.1469-7580.2002.00084.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noda N, Minoura H, Nishiura R, Toyoda N, Imanaka-Yoshida K, Sakakura T, Yoshida T. 2000. Expression of tenascin-C in stromal cells of the murine uterus during early pregnancy: induction by interleukin-1α, prostaglandin E2, and prostaglandin F2α. Biol. Reprod. 63, 1713–1720. ( 10.1095/biolreprod63.6.1713) [DOI] [PubMed] [Google Scholar]
- 48.Takacs P, Kauma S. 1996. The expression of interleukin-1α, interleukin-1β, and interleukin-1 receptor type I mRNA during preimplantation mouse development. J. Reprod. Immunol. 32, 27–35. ( 10.1016/S0165-0378(96)00987-4) [DOI] [PubMed] [Google Scholar]
- 49.Taniguchi T, Matsuzaki N, Kameda T, Shimoya K, Jo T, Saji F, Tanizawa O. 1991. The enhanced production of placental interleukin-1 during labor and intrauterine infection. Am. J. Obstet. Gynecol. 165, 131–137. ( 10.1016/0002-9378(91)90241-I) [DOI] [PubMed] [Google Scholar]
- 50.Cateni C, Paulesu L, Bigliardi E, Hamlett WC. 2003. The interleukin 1 (IL-1) system in the uteroplacental complex of a cartilaginous fish, the smoothhound shark, Mustelus canis. Reprod. Biol. Endocrinol. 1, 1–9. ( 10.1186/1477-7827-1-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulesu L, Romagnoli R, Marchetti M, Cintorino M, Ghiara P, Guarino FM, Ghiara G. 1995. Cytokines in the viviparous reproduction of squamate reptiles: interleukin-1α (IL-1α) and IL-1β in placental structures of a skink. Placenta 16, 193–205. ( 10.1016/0143-4004(95)90008-X) [DOI] [PubMed] [Google Scholar]
- 52.Paulesu L, Romagnoli R, Bigliardi E. 2005. Materno–fetal immunotolerance: is Interleukin-1 a fundamental mediator in placental viviparity? Dev. Comp. Immunol. 29, 409–415. ( 10.1016/j.dci.2004.09.007) [DOI] [PubMed] [Google Scholar]
- 53.Dudley DJ, Collmer D, Mitchell MD, Trautman MS. 1996. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J. Soc. Gynecol. Investig. 3, 328–335. ( 10.1177/107155769600300606) [DOI] [PubMed] [Google Scholar]
- 54.Steinborn A, Niederhut A, Solbach C, Hildenbrand R, Sohn C, Kaufmann M. 1999. Cytokine release from placental endothelial cells, a process associated with preterm labour in the absence of intrauterine infection. Cytokine 11, 66–73. ( 10.1006/cyto.1998.0399) [DOI] [PubMed] [Google Scholar]
- 55.Steinborn A, Von Gall C, Hildenbrand R, Stutte HJ, Kaufmann M. 1998. Identification of placental cytokine-producing cells in term and preterm labor. Obstet. Gynecol. 91, 329–335. ( 10.1016/S0029-7844(97)00680-7) [DOI] [PubMed] [Google Scholar]
- 56.Shaw G, Renfree MB. 2006. Parturition and perfect prematurity: birth in marsupials. Aust. J. Zool. 54, 139–149. ( 10.1071/ZO05070) [DOI] [Google Scholar]
- 57.Van Engelen E, De Groot MW, Breeveld-Dwarkasing VNA, Everts ME, Van Der Weyden GC, Taverne MAM, Rutten VPMG. 2009. Cervical ripening and parturition in cows are driven by a cascade of pro-inflammatory cytokines. Reprod. Domest. Anim. 44, 834–841. ( 10.1111/j.1439-0531.2008.01096.x) [DOI] [PubMed] [Google Scholar]
- 58.Pongcharoen S, Somran J, Sritippayawan S, Niumsup P, Chanchan P, Butkhamchot P, Tatiwat P, Kunngurn S, Searle RF. 2007. Interleukin-17 expression in the human placenta. Placenta 28, 59–63. ( 10.1016/j.placenta.2006.01.016) [DOI] [PubMed] [Google Scholar]
- 59.Sennström MK, Brauner A, Lu Y, Granström LM, Malmström AL, Ekman GE. 1997. Interleukin-8 is a mediator of the final cervical ripening in humans. Eur. J. Obstet. Gynecol. Reprod. Biol. 74, 89–92. ( 10.1016/S0301-2115(97)02757-7) [DOI] [PubMed] [Google Scholar]
- 60.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. 2000. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J. Immunol. 164, 5721–5728. ( 10.4049/jimmunol.164.11.5721) [DOI] [PubMed] [Google Scholar]
- 61.Jones CA, Finlay-Jones JJ, Hart PH. 1997. Type-1 and type-2 cytokines in human late-gestation decidual tissue. Biol. Reprod. 57, 303–311. ( 10.1095/biolreprod57.2.303) [DOI] [PubMed] [Google Scholar]
- 62.Simpson KL, Keelan JA, Mitchell MD. 1998. Labor-associated changes in interleukin-10 production and its regulation by immunomodulators in human choriodecidua. J. Clin. Endocrinol. Metab. 83, 4332–4337. ( 10.1210/jcem.83.12.5335) [DOI] [PubMed] [Google Scholar]
- 63.Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, Wagner GP. 2017. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl Acad. Sci. 114, E6566–E6575. ( 10.1073/pnas.1701129114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davies CJ, Hill JR, Edwards JL, Schrick FN, Fisher PJ, Eldridge JA, Schlafer DH. 2004. Major histocompatibility antigen expression on the bovine placenta: its relationship to abnormal pregnancies and retained placenta. Anim. Reprod. Sci. 82, 267–280. ( 10.1016/j.anireprosci.2004.05.016) [DOI] [PubMed] [Google Scholar]
- 65.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. 2012. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal–fetal conflict. Cell 150, 29–38. ( 10.1016/j.cell.2012.05.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gunn L, Hardiman P, Tharmaratnam S, Lowe D, Chard T. 1996. Measurement of interleukin-1 α and interleukin-6 in pregnancy-associated tissues. Reprod. Fertil. Dev. 8, 1069–1073. ( 10.1071/RD9961069) [DOI] [PubMed] [Google Scholar]
- 67.Ito M, et al. 2010. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J. Reprod. Immunol. 84, 75–85. ( 10.1016/j.jri.2009.09.005) [DOI] [PubMed] [Google Scholar]
- 68.Hirsch E, Blanchard R, Mehta SP. 1999. Differential fetal and maternal contributions to the cytokine milieu in a murine model of infection-induced preterm birth. Am. J. Obstet. Gynecol. 180, 429–434. ( 10.1016/S0002-9378(99)70227-9) [DOI] [PubMed] [Google Scholar]
- 69.Robertson SA, Christiaens I, Dorian CL, Zaragoza DB, Care AS, Banks AM, Olson DM. 2010. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology 151, 3996–4006. ( 10.1210/en.2010-0063) [DOI] [PubMed] [Google Scholar]
- 70.Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Lye SJ. 2013. Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. J. Cell. Mol. Med. 17, 90–102. ( 10.1111/j.1582-4934.2012.01650.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brandley MC, Young RL, Warren DL, Thompson MB, Wagner GP. 2012. Uterine gene expression in the live-bearing lizard, Chalcides ocellatus, reveals convergence of squamate reptile and mammalian pregnancy mechanisms. Genome Biol. Evol. 4, 394–411. ( 10.1093/gbe/evs013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paulesu L, Bigliardi E, Paccagnini E, Ietta F, Cateni C, Guillaume CP, Heulin B. 2005. Cytokines in the oviparity/viviparity transition: evidence of the interleukin-1 system in a species with reproductive bimodality, the lizard Lacerta vivipara. Evol. Dev. 7, 282–288. ( 10.1111/j.1525-142X.2005.05034.x) [DOI] [PubMed] [Google Scholar]
- 73.Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. 2016. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum. Reprod. Update. 22, 535–560. ( 10.1093/humupd/dmw022) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.