Abstract
Objectives:
In this study, we aimed at investigating the preoperatively available prognostic factors for intrahepatic cholangiocarcinoma (ICC) patients and proposing a new preoperative prognostic scoring system for ICC.
Methods:
A total of 246 consecutive ICC patients who underwent curative hepatectomy were enrolled retrospectively and were randomly divided into training (n=164) and validation cohorts (n=82) at a ratio of 2:1. The prognostic factors were investigated in both cohorts using multivariate Cox’s proportional hazards regression model.
Results:
Multivariate analyses identified that two preoperative factors (serum C-reactive protein (CRP) levels >4.1 mg/l (hazard ratio (HR): 2.75, 95% CI: 1.65–4.73, P<0.001) and carbohydrate antigen 19-9 (CA19-9) levels >300 mg/ml (HR: 3.76, 95% CI: 2.18–6.49)) were independent prognostic factors for postoperative survival in the training cohort. The results were further confirmed in the validation cohort. On the basis of these data, a preoperative prognostic score (PPS) was established by allocating 0 or 1 point to the two factors, respectively. Then, both in the training and validation cohorts, the PPS showed the power to stratify patients into three distinct groups (groups with scores 2, 1, and 0) with significant difference in the risk of postoperative death.
Conclusions:
A new preoperative scoring system consisting of preoperative CRP and CA19-9 levels could effectively predict postoperative survival of ICC patients.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer following hepatocellular carcinoma, accounting ~10–15% of primary liver cancer.1, 2 It differs from hepatocellular carcinoma in pathogenesis and biological behaviors and also differs from hilar and distal bile duct cholangiocarcinoma in clinical features and therapeutic strategies.3, 4 The incidence of ICC is increasing worldwide recently, especially in Asia, where its incidence is far above the average worldwide level.5, 6, 7 Currently, surgery is the only potentially curative treatment option for ICC. Five-year overall survival (OS) rates after surgical resection are in the range of 22–40%.8, 9, 10 For unresectable patients, the median OS time is only about 9 months.11 Thus, identification of novel prognostic factors for ICC patients is essential to select patients who would possibly benefit from surgery. Although several studies have investigated prognostic factors for ICC, the majority of these factors are only available post operation. For instance, lymph node metastasis has been suggested as a strong prognostic factor for ICC.12 However, it is not easy to define lymph node metastasis preoperatively. Recently, a prognostic model that consisted of preoperative platelet–lymphocyte ratio (PLR), C-reactive protein (CRP), albumin, and carcinoembryonic antigen (CEA) levels has been reported for prehilar cholangiocarcinoma.13 However, a preoperative risk model using factors available preoperatively for selecting proper candidates for surgery in ICC is still lacking.
As is well known, inflammation has been suggested as the seventh hallmark of cancer.14 Likewise, chronic inflammation is a common feature underlying the pathogenesis of ICC.15 Recently, several integrative analyses investigating the molecular mechanism of ICC had proposed the inflammation class ICC,16, 17, 18, 19 which was characterized by activation of inflammatory signaling pathway, like dendritic cells and cytokine pathways.18 Functionally, the activation IL-6/STAT3 signaling could significantly promote growth and metastasis of ICC.20 All these reports indicated that inflammation could promote ICC initiation and progression.
In addition, growing evidence has suggested that the status of preoperative systemic inflammation is a key factor affecting the prognosis of various cancers. The neutrophil–lymphocyte ratio (NLR) and CRP, which reflect system inflammation response (SIR), has been reported as a strong prognostic factor in various kinds of cancers, including esophageal cancer, colorectal cancer, hepatocellular carcinoma, and ICC.21, 22, 23, 24, 25, 26, 27 PLR, another indicator of SIR, has also been described as a prognostic factor for pancreatic cancer and prehilar cholangiocarcinoma.13, 28 Therefore, biomarkers of system inflammation could be a useful and preoperatively available prognostic factor to stratify and select candidates who are likely to benefit from surgery in ICC.
On the basis of the above information, we assumed that a prognostic score system using factors available preoperatively could be identified for ICC patients. After comprehensively investigating the prognostic significance of various preoperative factors, we established a preoperative prognostic scoring system, combining CRP and carbohydrate antigen 19-9 (CA19-9), for ICC patients. Compared with risk models based on gene signatures, mutational profiles, and epigenetic features, this prognostic model is more economic and convenient that can effectively stratify patients according to low or high risk of postoperative death.
Methods
Patient selection
A total of 246 consecutive cases with an initial diagnosis of ICC receiving surgical treatment between 2011 and 2014 at the Liver Cancer Institution of Zhongshan Hospital (Fudan University, Shanghai, China) were enrolled in this retrospective study. Inclusion criteria were as follows: (i) patients without history of previous anticancer therapy; (ii) patients without history of other malignancies; (iii) patients with complete resection of macroscopic liver tumors; (iv) patients with pathologically proven ICC. Patients with any kind of pretreatment (e.g., chemotherapy, transcatheter arterial chemoembolization) were excluded. Patients who had clinical evidence of infection and autoimmune disease before operation were excluded. Previously, several papers suggested that high serum CRP level was associated with high risk of myocardial infarction, angina pectoris, heart failure, coronary death, and stroke.29, 30, 31, 32 In order to avoid the influence of cardiovascular disease on preoperative serum CRP level, patients with a history of myocardial infarction (recent, ≤12 months or distant, >12 months), angina pectoris (stable or unstable), or stroke (recent, ≤12 months or distant, >12 months) before operation were excluded from our study. The criteria of the curative operation were as follows: (i) absence of retropancreatic and paraceliac nodal metastases or distant liver metastases; (ii) absence of invasion of the main portal vein or main hepatic artery; (iii) absence of extrahepatic adjacent organ invasion; (iv) absence of disseminated disease.33, 34 These patients were randomly divided into training cohort and validation cohort at a ratio of 2:1. Patients were staged according to the 7th edition of the American Joint Committee on Cancer staging criteria. This study was approved by the institutional review board of Zhongshan Hospital and carried out in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient.
Follow-up strategy
The collection of clinical data and postoperative follow-up procedures were performed according to an established guideline as described previously.35 All patients were monitored prospectively by serum alpha-fetoprotein, CEA, CA19-9, abdomen ultrasonography, and chest X-ray every 2–3 months in the first 2 years after surgery. Thereafter, these examinations were performed every 3–6 months. If a patient was suspected for recurrence, enhanced computed tomography and/or magnetic resonance imaging were performed to figure out whether recurrence had occurred. The diagnosis of recurrence was based on typical imaging appearance in computed tomography/magnetic resonance imaging with an elevated CA19-9 level. After recurrence being confirmed, a second hepatectomy, radiofrequency ablation, chemotherapy, transcatheter arterial chemoembolization, or external radiotherapy were carried out according to the characteristics of tumor, like number, size, and location of the recurrent tumor. OS was defined as the interval between surgery and death or between surgery and the last observation for living patients. Data were censored at the last follow-up for living patients.
Data collection and preoperative blood value test
All blood test values recorded in this study, including NLR, PLR, CEA, CRP, CA19-9, bilirubin, ALB, aspartate aminotransferase, and alanine aminotransferase, were performed within 2–3 days before the operation in the ISO-certified laboratory of Zhongshan Hospital.
Data analysis
X-tile software (Yale University, New Haven, CT, USA) was used to determine the optimal cutoff value of blood test in the training cohort.36 This software is a graphical method that illustrates the presence of substantial tumor subpopulations and shows the robustness of the relationship between a biomarker and outcome by construction of a two-dimensional projection of every possible subpopulation. It has been widely used in oncology research, like colorectal cancer,37 hepatocellular carcinoma,38 and breast cancer.39 Then, based on the optimal cutoff value, all blood tests were divided into low and high groups in both the training and validation cohorts. Then, the prognostic significance of preoperative blood tests was evaluated in the training cohort and verified in the validation cohort.
Statistical analysis
Statistical analysis was conducted by using SPSS software (v19.0; IBM, Armonk, NY, USA).The χ2-test or Fisher’s exact test was used to evaluate the association between CRP level, preoperative prognostic score (PPS), and clinicopathologic features. Variables associated with OS were identified by using univariate Cox’s proportional hazards regression model. Variables with a two-tailed P<0.05 were considered to be significant and were further subjected to a multivariate Cox’s proportional hazards regression model in a backward stepwise manner. Survival curves of CRP and PPS were calculated using Kaplan–Meier method (log-rank test). A two-tailed P<0.05 was considered to be significant. Prism 5 for Windows (v5.01, GraphPad Software, La Jolla, CA, USA) was used to draft the figure of Kaplan–Meier curve.
Results
Patient clinicopathologic profiles
A total of 246 patients were enrolled in this study and were randomly divided into training (n=164) and validation (n=82) cohorts at a ratio of 2:1. Table 1 detailed the characteristics of the training and validation cohorts. In training cohort, 163 (99.4%) patients were mass-forming type and only 1 (0.6%) patient was periductal type. In validation cohort, 76 (92.6%) patients were mass-forming type, 3 (3.7%) patients were periductal type, and 3 (3.7%) patients are intraductal type. There were no significant differences in clinicopathological features between the two cohorts. Currently, a major hepatic resection is defined as resection of three or more liver segments.40, 41, 42, 43 In training cohort, 94 (57.3%) patients underwent minor resection, 70(42.7%) underwent major resection, whereas 53 (64.6%) patients underwent minor resection, and 29 (35.4%) patients underwent major resection in validation cohort (Supplementary Table S1 online). All patients have R0 surgical margin. As for extrahepatic duct resection, we found out that only three patients received extrahepatic duct resection in training cohort and four patients received extrahepatic duct resection in validation cohort. The median follow-up time in training cohort and validation cohort was 19±13 and 18±11 months, respectively. OS rates at 1, 3, and 5 years after operation were 75%, 46%, and 37% for the whole study population, respectively.
Table 1. Clinical characteristics of ICC patients in training cohort (n=164) and validation cohort (n=82).
| Variable | Category |
Training |
Validation |
P | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Gender | Male | 99 | 60.40 | 52 | 63.40 | 0.644 |
| Female | 65 | 39.60 | 30 | 36.60 | ||
| Ages (years; mean±s.d.) | 60±10 | 58±11 | 0.125 | |||
| Tumor size | ≤5 cm | 75 | 48.40 | 44 | 53.70 | 0.242 |
| >5 cm | 89 | 51.60 | 38 | 46.30 | ||
| Tumor number | Single | 137 | 81.40 | 70 | 85.40 | 0.712 |
| Multiple | 27 | 15.60 | 12 | 14.60 | ||
| Vascular invasion | Present | 21 | 14.60 | 15 | 18.30 | 0.252 |
| Absent | 143 | 85.40 | 67 | 81.70 | ||
| Perineural invasion | Present | 23 | 14.60 | 13 | 15.90 | 0.703 |
| Absent | 141 | 85.40 | 69 | 84.10 | ||
| Differentiationa | Poor | 16 | 8.10 | 4 | 4.90 | 0.143 |
| Moderate | 68 | 39 | 28 | 34.10 | ||
| Well | 80 | 52.80 | 50 | 61 | ||
| Lymph node metastasis | Present | 50 | 26.80 | 16 | 19.50 | 0.068 |
| Absence | 114 | 73.20 | 66 | 80.50 | ||
| Morphology of the cancers | Mass-forming | 163 | 99.4 | 76 | 92.7 | 0.554 |
| Intraductal | 0 | 0 | 3 | 3.6 | ||
| Periductal | 1 | 0.6 | 3 | 3.7 | ||
| Cirrhosis | Present | 39 | 25.60 | 24 | 70.70 | 0.354 |
| Absence | 125 | 74.40 | 58 | 29.30 | ||
| TNMb | 0 | 1 | 2 | 3 | 3.70 | 0.158 |
| 1 | 104 | 65 | 56 | 68.30 | ||
| 2 | 22 | 12.60 | 9 | 11 | ||
| 3 | 8 | 4.10 | 2 | 2.40 | ||
| 4A | 28 | 16.30 | 12 | 14.60 | ||
| HBsAg | Positive | 19 | 11.60 | 11 | 86.60 | 0.68 |
| Negative | 145 | 88.40 | 71 | 13.40 | ||
| CRP (mg/l; mean±s.d.) | 9.1±20.4 | 7.4±13.4 | 0.501 | |||
| NLRc | ≤2.1 | 64 | 39.00 | 33 | 41.30 | 0.766 |
| >2.1 | 99 | 60.4 | 47 | 58.70 | ||
| PLRd (mean±s.d.) | 129±62 | 138±79 | 0.346 | |||
| CA19-9e (ng/ml; median) | 32 (0.6–10 000) | 29.9 (0.6–10 000) | 0.619 | |||
| CEAf (ng/ml; median) | 2.46 (0.33–453.20) | 2.51 (0–394.2) | 0.811 | |||
Abbreviations: CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CRP, C-reactive protein; HbsAg, hepatitis B surface antigen; ICC, intrahepatic cholangiocarcinoma; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; TNM, tumor node metastasis.
Tumor differentiation was determined according to the British Society of Gastroenterology guidelines on the management of cholangiocarcinoma.
TNM: American Joint Committee on Cancer 7th edition staging for intrahepatic cholangiocarcinoma.
Missing data: n=3 (due to missing NLR value in training cohort, n=1; due to missing NLR value in validation cohort, n=2).
Missing data: n=1 (due to missing PLR value in training cohort, n=1; due to missing PLR value in validation cohort, n=2).
Missing data: n=11 (due to missing CA19-9 value in training cohort, =10, due to missing Ca19-9 value in validation cohort, n=1).
Missing data: n=12 (due to missing CEA value in training cohort, n=12; due to missing CEA value in validation cohort, n=2).
Prognostic factors for ICC patients
The optimal cutoff value of blood tests in training cohort was presented in Supplementary Table S2 online. The optimal cutoff value of CRP level was determined with X-tile software (Supplementary Figure 1a online), and the prognostic significance of CRP level was presented in Supplementary Figure 1b online (Training cohort (median OS (95% confidence interval (95% CI)) for low CRP (n=105) vs. high CRP (n=59): 38 (25.9–50.8) vs. 11 (5.2–17.4) months; long-rank test, P<0.0001)) and Supplementary Figure 1c online (Validation cohort (median OS (95% CI) for low CRP (n=53) vs. high CRP (n=29): 34 (28.4–39.7) vs. 21 (15.2–27.1) months; long-rank test, P=0.0105)). In univariate analysis of training cohort, patient gender, age, tumor size, number, vascular invasion, perineural invasion, differentiation, lymph node metastasis, cirrhosis, TNM stage, CRP level, NLR level, PLR level, CEA level, and CA19-9 level were evaluated. We found that tumor size (P=0.002), number (P=0.006), vascular invasion (P=0.005), lymph node metastasis (P<0.0001), TNM stage (P=0.002), CRP level (P<0.0001), NLR level (P=0.027), PLR level (P=0.045), CEA level (P<0.0001), and CA19-9 level (P<0.0001) were identified as factors significantly associated with OS (Table 2). Then, these variables were adopted into a multivariable analysis, which showed that CRP level (hazard ratio (HR): 2.62, 95% confidence interval (CI): 1.59–4.31, P<0.0001) and CA19-9 level (HR: 3.62, 95% CI: 2.18–6.01, P<0.0001) remained as independent prognosis factors for ICC (Table 2). Of note, in the validation cohort, multivariable analysis also revealed that CRP level (HR: 2.55, 95% CI: 1.29–5.03) and CA19-9 level (HR: 2.63, 95% CI: 1.24–5.59) were independent prognostic factors (Table 3). These results indicated that preoperative CA19-9 and CRP levels harbored strong prognostic value for postoperative survival in ICC patients.
Table 2. Univariate and multivariate analysis of prognostic factors in ICC patients using the Cox’s proportional hazards model in training cohort (n=164).
| Variable | Category | N |
Univariate analysis; overall survival |
Multivariate analysis; overall survival |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Preoperative available factors | ||||||||
| Gender | Male | 99 | 1.3 | 0.797–2.122 | 0.293 | / | / | / |
| Female | 65 | |||||||
| Age | ≤60 | 77 | 0.866 | 0.542–1.385 | 0.548 | / | / | / |
| >60 | 87 | |||||||
| CRP (mg/l) | ≤4.1 | 105 | 2.74 | 1.707–4.399 | <0.0001 | 2.45 | 1.40–4.30 | P=0.002 |
| >4.1 | 59 | |||||||
| NLRa | ≤2.1 | 64 | 1.788 | 1.067–2.997 | 0.027 | / | / | / |
| >2.1 | 99 | |||||||
| PLRb | ≤190 | 137 | 1.798 | 1.012–3.192 | 0.045 | / | / | / |
| >190 | 26 | |||||||
| CEAc (ng/ml) | ≤4.5 | 111 | 2.747 | 1.652–4.567 | P<0.0001 | / | / | / |
| >4.5 | 41 | |||||||
| CA19-9d (ng/l) | ≤300 | 118 | 3.064 | 1.83–5.13 | P<0.0001 | 2.96 | 1.70–5.15 | P<0.0001 |
| >300 | 36 | |||||||
| ALBe (g/l) | ≤40 | 70 | 1.163 | 0.465–2.904 | P=0.747 | / | / | / |
| >40 | 90 | |||||||
| Pathology factors | ||||||||
| Tumor size | ≤5 | 75 | 2.199 | 1.326–3.648 | 0.002 | / | / | / |
| >5 | 89 | |||||||
| Tumor number | Single | 137 | 2.09 | 1.232–3.544 | 0.006 | 2.37 | 1.31–4.29 | 0.004 |
| Multiple | 27 | |||||||
| Vascular invasion | Present | 21 | 2.349 | 1.3–4.242 | 0.005 | 2.04 | 1.01–4.13 | 0.048 |
| Absent | 143 | |||||||
| Perineural invasion | Present | 23 | 1.645 | 0.880–3.076 | 0.119 | / | / | / |
| Absent | 141 | |||||||
| Differentiationf | Poor | 16 | 1.189 | 0.816–1.732 | 0.367 | / | / | / |
| Moderate | 68 | |||||||
| Well | 80 | |||||||
| Lymph node metastasis | Present | 50 | 3.362 | 2.075–5.450 | <0.001 | 3.06 | 1.76–5.33 | <0.0001 |
| Absent | 114 | |||||||
| Cirrhosis | Present | 39 | 0.885 | 0.506–1.548 | 0.668 | / | / | / |
| Absent | 125 | |||||||
| TNMg | 1 (0.I, II) | 106 | 2.214 | 1.326–3.697 | 0.002 | / | / | / |
| 2 (III, IVa) | 58 | |||||||
Abbreviations: CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CI, confidence interval; CRP, C-reactive protein; HbsAg, hepatitis B surface antigen; HR, hazard ratio; ICC, intrahepatic cholangiocarcinoma; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; TNM, tumor node metastasis.
Missing data: n=1 (due to missing NLR value in training cohort, n=1).
Missing data: n=1 (due to missing PLR value in training cohort, n=1).
Missing data: n=12 (due to missing CEA value in training cohort, n=12).
Missing data: n=10 (due to missing CA19-9 value in training cohort, n=10).
Missing data: n=4(due to missing ALB value in training cohort, n=4).
Tumor differentiation was determined according to the British Society of Gastroenterology guidelines on the management of cholangiocarcinoma.
TNM: American Joint Committee on Cancer 7th edition staging for intrahepatic cholangiocarcinoma.
Table 3. Univariate and multivariate analysis of prognostic factors in ICC patients using the Cox’s proportional hazards model in Validation cohort (n=82).
| Variables | Category | N |
Univariate analysis; overall survival |
Multivariate analysis; overall survival |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Gender | Male | 52 | 1.327 | 0.649–2.712 | 0.438 | / | / | / |
| Female | 30 | |||||||
| Age (years) | ≤60 | 48 | 1.084 | 0.537–2.188 | 0.822 | / | / | / |
| >60 | 34 | |||||||
| Tumor size | ≤5 | 44 | 2.098 | 1.068–4.125 | 0.032 | / | / | / |
| >5 | 38 | |||||||
| Tumor number | Single | 70 | 1.172 | 0.486–2.826 | 0.724 | / | / | / |
| Multiple | 12 | |||||||
| Vascular invasion | Present | 15 | 1.352 | 0.589–3.104 | 0.478 | / | / | / |
| Absent | 67 | |||||||
| Perineural invasion | Present | 13 | 2.085 | 0.893–4.871 | 0.09 | / | / | / |
| Absent | 69 | |||||||
| Differentiationa | Poor | 4 | 1.319 | 0.707–2.461 | 0.384 | / | / | / |
| Moderate | 28 | |||||||
| Well | 50 | |||||||
| Lymph node metastasis | Present | 16 | 1.654 | 0.746–3.668 | 0.216 | / | / | / |
| Absent | 66 | |||||||
| Cirrhosis | Present | 24 | 0.593 | 0.269–1.310 | 0.197 | / | / | / |
| Absent | 58 | |||||||
| TNMb | 1 | 59 | 2.269 | 1.085–4.746 | 0.03 | / | / | / |
| 2 | 23 | |||||||
| CRP (mg/l) | ≤4.1 | 53 | 2.64 | 1.35–5.14 | 0.004 | 2.55 | 1.29–5.03 | 0.007 |
| >4.1 | 29 | |||||||
| CA19-9c (ng/ml) | ≤300 | 62 | 2.59 | 1.22–5.50 | 0.014 | 2.63 | 1.24–5.59 | 0.012 |
| >300 | 19 | |||||||
Abbreviations: CRP, C-reactive protein; CA19-9,carbohydrate antigen 19-9; HbsAg, hepatitis B surface antigen; ICC, intrahepatic cholangiocarcinoma; NLR, neutrophil–lymphocyte ratio; TNM, tumor node metastasis.
Tumor differentiation was determined according to the British Society of Gastroenterology guidelines on the management of cholangiocarcinoma.
TNM: American Joint Committee on Cancer 7th edition staging for intrahepatic cholangiocarcinoma.
Missing data: n=1 (due to missing NLR value in validation cohort, n=1).
The correlation between CRP level and clinical characteristics
Supplementary Table S3 online presented the correlation between CRP level and clinicopathological characteristics in the training and validation cohorts. We observed that the presence of lymph node metastasis (P=0.039), large tumor size (P<0.0001), vascular invasion (P=0.003), perineural invasion (P=0.0009), poor differentiation (P=0.009), and high NLR level (P<0.0001) significantly and positively correlated with elevated CRP level in the training cohort. Then, we observed that larger tumor size (>5 cm), the presence of lymph node metastasis, and high NLR level significantly and positively correlated with elevated CRP level in the validation cohort, which indicated that high CRP level might be associated with advanced stage of ICC. There was no significant correlation between CRP level and the presence of HbsAg neither in the training nor in validation cohorts, which suggested that CRP level was not affected by the presence of hepatitis B.
Subgroup analysis
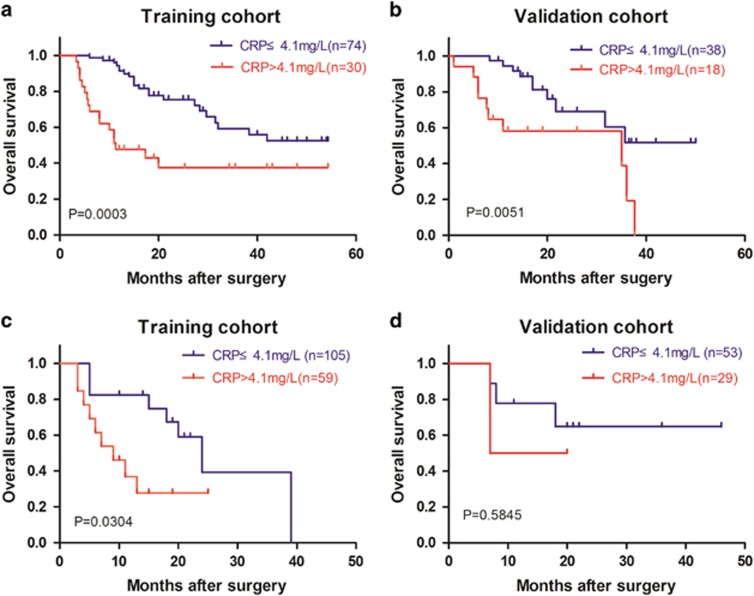
In clinical practice, TNM staging system is the most widely used staging system for ICC patients.44 Therefore, we evaluated the discriminative power of CRP level with respect to the TNM staging system in the training and validation cohorts. For patients with TNM stage I, high CRP level was significantly associated with lower OS in the training cohort (median OS (95% CI) for low CRP (n=74) vs. high CRP (n=30): 39 (34.7–44.4) vs. 25 (16.7–33.6) months; P=0.0003; Figure 1a). Moreover, the same was true in the validation cohort (median OS (95% CI) for low CRP (n=38) vs. high CRP (n=18): 37(30.4–43.3) vs. 22 (14.9–30.0) months; P=0.002; Figure 1b). For patients in TNM II and III stages, high CRP level was significantly associated with lower OS in the training cohort (median OS (95% CI) for low CRP (n=74) vs. high CRP (n=30): 24 (16.7–31.3) vs. 9 (3.5–14.5) months; P=0.0304; Figure 1c). However, there were no significant differences in OS between high and low CRP groups among patients in TNM II and III stages in the validation cohort (P=0.5845, Figure 1d). All these results suggested that system inflammation, as measured by the CRP level, might be an important prognostic factor in early-stage ICC patients. Meanwhile, in advanced-stage ICC patients, the CRP level was a significant prognostic factor in training cohort, but it failed to predict outcome in the validation cohort, which may be attributed to the small sample size in validation cohort.
Figure 1.
Association of preoperative CRP level with OS in ICC patients with respect to TNM stage. (a) Patients with TNM I stage in training cohort (median OS (95% CI) for low CRP (n=74) vs. high CRP (n=30): 39 (34.7–44.4) vs. 25 (16.7–33.6) months; long-rank test, log-rank test P=0.0003). (b) Patients with TNM I stage in validation cohort (median OS (95% CI) for low CRP (n=38) vs. high CRP (n=18): 37 (30.4–43.3) vs. 22 (14.9–30.0) months; log-rank test, P=0.0051). (c) Patients with TNM II and III stages in training cohort (median OS (95% CI) for low CRP (n=74) vs. high CRP (n=30): 24 (16.7–31.3) vs. 9 (3.5–14.5) months, log-rank test: P=0.0304). (d) Patients with TNM II and III stages in validation cohort (log-rank test: P=0.5845). CI, confidence interval; CRP, C-reactive protein; ICC, intrahepatic cholangiocarcinoma; OS, overall survival; TNM, tumor node metastasis.
Tumor vascular invasion is a well-known prognostic factor for ICC patients. Herein, a subgroup analysis was carried out in patients with and without vascular invasion. In the training cohort, patients with high CRP level were significantly associated with lower OS (median OS (95% CI) for low CRP (n=96) vs. high CRP (n=47): 37 (32.4–41.5) vs. 28 (21.4–35.6) months; P=0.0018; Figure 2a) among those patients with vascular invasion. In validation cohort, the same result was observed (median OS (95% CI) for low CRP (n=42) vs. high CRP (n=25): 34 (27.7–40.1) vs. 23 (17.0–30.0) months; P=0.046; Figure 2b). In patients without vascular invasion, patients with high CRP level were also significantly associated with lower OS in both the training cohort (median OS (95% CI) for low CRP (n=9) vs. high CRP (n=12): 24(13.0–35.0) vs. 6 (4.3–7.7) months; P=0.0006; Figure 2c) and the validation cohort (median OS (95% CI) for low CRP (n=11) vs. high CRP (n=4): 33 (22.0–43.9) vs. 7 (5.5–8.0) months; P=0.002; Figure 2d). All these results further supported the prognostic value of CRP level, highlighting that high CRP level indicated a poor prognosis in ICC patients irrespective of the status of vascular invasion.
Figure 2.
Association of preoperative CRP level with OS in ICC patients with vascular invasion. (a) Patients without vascular invasion in training cohort (median OS (95% CI) for low CRP (n=96) vs. high CRP (n=47): 37 (32.4–41.5) vs. 28 (21.4–35.6) months; log-rank test, P=0.0018). (b) Patients without vascular invasion in validation cohort (median OS (95% CI) for low CRP (n=42) vs. high CRP (n=25): 34 (27.7–40.1) vs. 23 (17.0–30.0) months; log-rank test, P=0.046). (c) Patients with vascular invasion in training cohort (median OS (95% CI) for low CRP (n=9) vs. high CRP (n=12): 24 (13.0–35.0) vs. 6 (4.3–7.7) months; log-rank test, P=0.0006). (d) Patients with vascular invasion in validation cohort (median OS (95% CI) for low CRP (n=11) vs. high CRP (n=4): 33 (22.0–43.9) vs. 7 (5.5–8.0) months; log-rank test, P=0.002). CI, confidence interval; CRP, C-reactive protein; ICC, intrahepatic cholangiocarcinoma; OS, overall survival.
We further evaluated the discriminative power of PPS score with respect to the type of resection in both training and validation cohorts. For patients receiving minor hepatic resection, higher PPS score was significantly associated with lower OS in the training cohort (median OS (95% CI) for PPS 0 (n=54): 41 (35.5–46.3); median OS (95% CI) for PPS 1 (n=29): 28 (20.1–35.6); median OS for PPS 2 (n=7): 6 (5.1–6.8); 0 vs. 1, P=0.007; 0 vs. 2, P<0.0001; 1 vs. 2, P=0.009; Supplementary Figure 2a online). Similar result was observed in the validation cohort (median OS (95% CI) for PPS 0 (n=32): 38 (31.3–44.9); median OS (95% CI) for PPS 1 (n=18): 26 (19.8–33.4); median OS for PPS 2 (n=3): 9 (5.6–13.1); 0 vs. 1, P=0.039; 0 vs. 2,P<0.0001; 1 vs. 2, P=0.149; Supplementary Figure 2b online). As for patients receiving major resection, higher PPS score was also associated with lower OS in the training cohort (median OS (95% CI) for PPS 0 (n=23): 39 (34.5–43.9); median OS (95% CI) for PPS 1 (n=27): 25 (5.3–44.6); median OS for PPS 2 (n=14): 7(2.8–11.2); 0 vs. 1, P=0.028; 0 vs. 2,P<0.0001; 1 vs. 2, P=0.037; Supplementary Figure 2c online). However, in validation cohort, survival difference among the three groups was observed, albeit without statistical significance, which may be attributed to the small sample size (median OS (95% CI) for PPS 0 (n=10): 31 (11.6–51.7); median OS (95% CI) for PPS 1 (n=13): 8 (1.5–32.5); median OS for PPS 2 (n=5): 4 (10.1–27.9); 0 vs. 1, P=0.124; 0 vs. 2,P=0.099; 1 vs. 2, P=0.384; Supplementary Figure 2d online).
Preoperative prognostic score
On the basis of the results in multivariable analysis, a PPS that consisted of CRP and CA19-9 for ICC patients was established. One point was allocated to each preoperative factor: serum CRP levels of >4.1 mg/l and CA19-9 levels of >300 ng/ml. The total score was defined as the PPS, generating a tertiary model (0-1-2) for patient stratification (PPS; Supplement Table S4 online).The prognostic performance of PPS in the training and validation cohorts was showed in Figure 3a. In the training cohort, patients with high PPS value were significantly associated with dismal OS (median OS (95% CI) for PPS 0 (n=84): 39 (34.5–43.9); median OS (95% CI) for PPS 1 (n=52): 25 (5.3–44.6); median OS for PPS 2 (n=18): 7(2.8–11.2); 0 vs. 1, P=0.001; 0 vs. 2, P<0.0001; 1 vs. 2, P=0.006; Figure 3a). In the validation cohort, the same result was observed (median OS (95% CI) for PPS 0 (n=42): 36 (30.3–42.6); median OS (95% CI) for PPS 1(n=31): 35 (10.2–59.8); median OS for PPS 2 (n=8): 6 (0–12.5); 0 vs. 1, P=0.006; 0 vs. 2, P<0.0001; 1 vs. 2, P=0.043; Figure 3b). These results indicated that PPS could effectively predict the risk of postoperative death for ICC.
Figure 3.
Association of preoperative scoring system with OS in training cohort and validation cohort. (a) Training cohort (median OS (95% CI) for PPS 0 (n=84): 39 (34.5–43.9); median OS (95% CI) for PPS 1 (n=52): 25 (5.3–44.6); median OS for PPS 2 (n=18): 7 (2.8–11.2); 0 vs. 1, P=0.001; 0 vs. 2, P<0.0001; 1 vs. 2, P=0.006). (b) Validation cohort (median OS (95% CI) for PPS 0 (n=42): 36 (30.3–42.6); median OS (95% CI) for PPS 1 (n=31): 35 (10.2–59.8); median OS for PPS 2 (n=8): 6 (0–12.5); 0 vs. 1, P=0.006; 0 vs. 2, P<0.0001; 1 vs. 2, P=0.043). CI, confidence interval; CRP, C-reactive protein; ICC, intrahepatic cholangiocarcinoma; OS, overall survival; PPS, preoperative prognostic score.
The correlation between PPS and Clinical Characteristics
The correlation between PPS and clinicopathological characteristics of the training and validation cohorts was presented in Supplementary Table S5 online. We observed that the presence of lymph node metastasis (P=0.025), larger tumor size (P<0.0001), vascular invasion (P=0.01), poor differentiation (P=0.001), and advanced TNM stage (P=0.01) significantly and positively correlated with higher PPS in the training cohort. Moreover, we observed that the presence of lymph node metastasis (P=0.004) and advanced TNM stage (P=0.019) was significantly and positively correlated with higher PPS value in the validation cohort. Thus, a higher PPS might indicate an advanced stage of ICC, which may explain the prognostic significance of this preoperative scoring system.
Discussion
In this study, by analysis of two independent cohorts, our results strongly suggested that preoperative CRP level and CA19-9 level were independent prognostic factors for postoperative survival of ICC patients. On the basis of these data, a PPS system was established combining CRP and CA19-9 data that can effectively predict postoperative survival of ICC. Our results provided a convenient and effective way to predict survival of ICC patients, which could influence decision-making on the part of patients and physicians about the possible benefit of surgery.
As an indicator of SIR, numerous studies have observed the association of CRP with prognosis of various kinds of cancer, all of which suggested that an elevated CRP level was associated with a poor outcome.22, 26, 45, 46, 47 Our results were highly consistent with these previous studies, further authenticating the strong prognostic value of SIR in human malignancy. The underlying mechanisms of such association were mainly considered to be related to inflammation in tumor microenvironment and tumor immunoevasion.
Inflammation in the tumor microenvironment creates a protumorigenic and proangiogenic milieu. Notably, there is evidence that the inflammatory field effect, reflected by elevated CRP, may be directly involved in tumor progression. For instance, the gene encoding CRP is located on the long arm of chromosome 1 (1q21–q23) and is under transcription control of various cytokines and transcription factors, among which interleukin-6 (IL-6) is the principal inducer of CRP expression.48, 49, 50 Hence, CRP level may reflect the level of IL-6, while IL-6 is the cytokine that has been reported to be associated with growth and metastasis of ICC.51, 52 If chronic inflammation, as measured by the CRP level, contributes to aggravating cancer, the survival of ICC patients with high CRP level may potentially be improved by intervening against chronic inflammation, such as COX inhibitors. Preclinical study and clinical trials are required to evaluate the potential benefits and harms of such interventions.
Immunoevasion is an emerging hallmark of tumor.53 Solid tumors, including ICC, do appear to have somehow managed to avoid detection by the various arms of the immune system or have been able to limit the extent of immunological killing, thereby evading immune surveillance. Recently, an in vitro study suggested that CRP could have a direct role in promoting cancer progression by downregulating tumor-necrosis factor-related apoptosis-inducing ligand (TRAIL) in immune cells, which is a key molecule mediating cancer immune surveillance.54 However, whether CRP could promote ICC immunoevasion through TRAIL or other pathways need in-depth investigation.
Previously, we have observed that elevated preoperative NLR, another inflammatory marker, predicted poor postoperative prognosis of ICC patients.21, 55 However, our current results indicated that NLR was not an independent prognostic factor. Analyzing the correlation of CRP level with clinicopathological features showed that CRP value was significantly and positively associated with NLR value (r=0.414; P<0.001). As is well known, both CRP and NLR are biomarkers of system inflammation; this may explain why preoperative NLR level was not an independent prognostic factor in this study.
Recently, Lin et al. reported that preoperative CRP level was an independent prognostic factor for ICC patients and proposed that a cutoff value of CRP level was 1.8 mg/l,47 which was different from the one in our study. In the study by Lin et al., receiver operating characteristic curve was used for cutoff value determination, whereas X-tile software was used in our study. This might explain why our cutoff value is different from the previous one. Moreover, we performed Cox’s proportional hazards regression analysis for two different cutoff values, which suggested that our cutoff might be a better choice with larger HR and smaller P-value (HR for 4.1 mg/l vs. HR for 1.8 mg/l: 2.74 vs. 2.56; P<0.0001 vs. P=0.001). However, multicenter, large sample size, and prospective analysis on this topic is needed in the future. In addition, patients with history of cardiovascular disease were excluded from this study, which may affect the generalizability of our conclusions, as cardiovascular diseases were common among older patients.
In summary, this study indicated that preoperative CRP level and CA19-9 level were important prognostic factors for postoperative survival of ICC patients. Together with current tumor-staging methods, measures of the systemic inflammatory response and CA19-9 level before surgery may provide a better prediction of outcome and better treatment allocation in patients with primarily operable ICC. Compared with using expensive genetic analysis to stratify and allocated patients, preoperative blood test is cheaper, reproducible, objective, widely available, and routinely performed in clinical practice.
Study Highlights

Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Guarantor of the article: Qiang Gao, MD, PhD.
Specific author contributions: Each author approved the final draft submitted. Zheng B.H., Fan H.K., and Sun Q.M. designed this retrospective study and contributed equally to this work and should be considered as first authors. Zheng B.H., Duan M., and Shi J.Y. collected data and did statistical analysis. Fan H.K., Yang L.X., and Wang X.Y. drew figures. Zhou J., Fan J., Ma Z.Y., and Gao Q. conceived the study and revised the manuscript. All of the authors discussed the results and commented on the manuscript.
Financial support: This work was supported by the National Natural Science Foundation of China (Nos. 81522036, 81572292, 81372648, 81272725, and 81502028), National Program for Special Support of Eminent Professionals, and Shanghai "Promising Youth Medical Worker" Project (No. 13Y055).
Potential competing interests: None.
Supplementary Material
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- Bridgewater J, Galle PR, Khan SA et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014; 60: 1268–1289. [DOI] [PubMed] [Google Scholar]
- Paik KY, Jung JC, Heo JS et al. What prognostic factors are important for resected intrahepatic cholangiocarcinoma? J Gastroenterol Hepatol 2008; 23: 766–770. [DOI] [PubMed] [Google Scholar]
- Petrowsky H, Hong JC. Current surgical management of hilar and intrahepatic cholangiocarcinoma: the role of resection and orthotopic liver transplantation. Transplant Proc 2009; 41: 4023–4035. [DOI] [PubMed] [Google Scholar]
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001; 33: 1353–1357. [DOI] [PubMed] [Google Scholar]
- Shaib YH, Davila JA, McGlynn K et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004; 40: 472–477. [DOI] [PubMed] [Google Scholar]
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004; 24: 115–125. [DOI] [PubMed] [Google Scholar]
- Maithel SK, Gamblin TC, Kamel I et al. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer 2013; 119: 3929–3942. [DOI] [PubMed] [Google Scholar]
- Mavros MN, Economopoulos KP, Alexiou VG et al. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg 2014; 149: 565–574. [DOI] [PubMed] [Google Scholar]
- Poultsides GA, Zhu AX, Choti MA et al. Intrahepatic cholangiocarcinoma. Surg Clin North Am 2010; 90: 817–837. [DOI] [PubMed] [Google Scholar]
- Berdah SV, Delpero JR, Garcia S et al. A western surgical experience of peripheral cholangiocarcinoma. Br J Surg 1996; 83: 1517–1521. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Xia Y et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013; 31: 1188–1195. [DOI] [PubMed] [Google Scholar]
- Saito H, Noji T, Okamura K et al. A new prognostic scoring system using factors available preoperatively to predict survival after operative resection of perihilar cholangiocarcinoma. Surgery 2016; 159: 842–851. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Cancer: Inflaming metastasis. Nature 2009; 457: 36–37. [DOI] [PubMed] [Google Scholar]
- Kumar M, Zhao X, Wang XW. Molecular carcinogenesis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: one step closer to personalized medicine? Cell Biosci 2011; 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto A, Furuta M, Shiraishi Y et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat Commun 2015; 6: 6120. [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhao YJ, Wang XY et al. Activating mutations in PTPN3 promote cholangiocarcinoma cell proliferation and migration and are associated with tumor recurrence in patients. Gastroenterology 2014; 146: 1397–1407. [DOI] [PubMed] [Google Scholar]
- Sia D, Hoshida Y, Villanueva A et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 2013; 144: 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Li J, Zhou H et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun 2014; 5: 5696. [DOI] [PubMed] [Google Scholar]
- Zheng T, Hong X, Wang J et al. Gankyrin promotes tumor growth and metastasis through activation of IL-6/STAT3 signaling in human cholangiocarcinoma. Hepatology 2014; 59: 935–946. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yang LX, Li XD et al. The elevated preoperative neutrophil-to-lymphocyte ratio predicts poor prognosis in intrahepatic cholangiocarcinoma patients undergoing hepatectomy. Tumour Biol 2015; 36: 5283–5289. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Pinter M, Hucke F et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology 2013; 57: 2224–2234. [DOI] [PubMed] [Google Scholar]
- Graff JN, Beer TM. The role of C-reactive protein in prostate cancer. Cancer 2013; 119: 3262–3264. [DOI] [PubMed] [Google Scholar]
- Roxburgh CS, Salmond JM, Horgan PG et al. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg 2009; 249: 788–793. [DOI] [PubMed] [Google Scholar]
- Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol 2009; 27: 2217–2224. [DOI] [PubMed] [Google Scholar]
- Hefler LA, Concin N, Hofstetter G et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res 2008; 14: 710–714. [DOI] [PubMed] [Google Scholar]
- Crumley AB, McMillan DC, McKernan M et al. An elevated C-reactive protein concentration, prior to surgery, predicts poor cancer-specific survival in patients undergoing resection for gastro-oesophageal cancer. Br J Cancer 2006; 94: 1568–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Bosonnet L, Raraty M et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg 2009; 197: 466–472. [DOI] [PubMed] [Google Scholar]
- Christiansen MK, Larsen SB, Nyegaard M et al. Coronary artery disease-associated genetic variants and biomarkers of inflammation. PLoS ONE 2017; 12: e0180365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek M, Bhatt DL, Vaduganathan M et al. Single and multiple cardiovascular biomarkers in subjects without a previous cardiovascular event. Eur J Prev Cardiol 2017. (doi: 10.1177/2047487317717065). [DOI] [PubMed]
- Ridker PM, Cushman M, Stampfer MJ et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336: 973–979. [DOI] [PubMed] [Google Scholar]
- Singh TP, Morris DR, Smith S et al. Systematic review and meta-analysis of the association between C-reactive protein and major cardiovascular events in patients with peripheral artery disease. Eur J Vasc Endovasc Surg 2017; 54: 220–233. [DOI] [PubMed] [Google Scholar]
- Rajagopalan V, Daines WP, Grossbard ML et al. Gallbladder and biliary tract carcinoma: a comprehensive update, Part 1. Oncology 2004; 18: 889–896. [PubMed] [Google Scholar]
- Tsao JI, Nimura Y, Kamiya J et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg 2000; 232: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Qiu SJ, Fan J et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007; 25: 2586–2593. [DOI] [PubMed] [Google Scholar]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004; 10: 7252–7259. [DOI] [PubMed] [Google Scholar]
- Jiang HH, Li AJ, Tang EJ et al. Prognostic value of the combination of preoperative hemoglobin, lymphocyte, albumin, and neutrophil in patients with locally advanced colorectal cancer. Med Sci Monit 2016; 22: 4986–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, He C, Zhang J et al. Association of high histone H3K4 trimethylation level and prognosis of patients with low-TNM-stage hepatocellular carcinoma. J Clin Oncol 2012; 30: 171. [Google Scholar]
- Agarwal S, Hanna J, Sherman ME et al. Quantitative assessment of miR34a as an independent prognostic marker in breast cancer. Br J Cancer 2015; 112: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H, Kim JH, Ko GY et al. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol 2011; 18: 1251–1257. [DOI] [PubMed] [Google Scholar]
- Palavecino M, Kishi Y, Chun YS et al. Two-surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1,557 consecutive liver resections. Surgery 2010; 147: 40–48. [DOI] [PubMed] [Google Scholar]
- Dahiya D, Wu TJ, Lee CF et al. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery 2010; 147: 676–685. [DOI] [PubMed] [Google Scholar]
- Bismuth H, Chiche L. Surgery of hepatic tumors. Prog Liver Dis 1993; 11: 269–285. [PubMed] [Google Scholar]
- Ronnekleiv-Kelly SM, Pawlik TM. Staging of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017; 6: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep 2002; 4: 250–255. [DOI] [PubMed] [Google Scholar]
- Nozoe T, Matsumata T, Sugimachi K. Preoperative elevation of serum C-reactive protein is related to impaired immunity in patients with colorectal cancer. Am J Clin Oncol 2000; 23: 263–266. [DOI] [PubMed] [Google Scholar]
- Lin ZY, Liang ZX, Zhuang PL et al. Intrahepatic cholangiocarcinoma prognostic determination using pre-operative serum C-reactive protein levels. BMC Cancer 2016; 16: 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathi MK, Rzewnicki D, Samols D et al. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep 3B cells. J Immunol 1991; 147: 1261–1265. [PubMed] [Google Scholar]
- Szalai AJ, van Ginkel FW, Wang Y et al. Complement-dependent acute-phase expression of C-reactive protein and serum amyloid P-component. J Immunol 2000; 165: 1030–1035. [DOI] [PubMed] [Google Scholar]
- Toniatti C, Arcone R, Majello B et al. Regulation of the human C-reactive protein gene, a major marker of inflammation and cancer. Mol Biol Med 1990; 7: 199–212. [PubMed] [Google Scholar]
- Kobayashi S, Werneburg NW, Bronk SF et al. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology 2005; 128: 2054–2065. [DOI] [PubMed] [Google Scholar]
- Park J, Tadlock L, Gores GJ et al. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology 1999; 30: 1128–1133. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Rimondi E, di Iasio MG et al. C-Reactive protein downregulates TRAIL expression in human peripheral monocytes via an Egr-1-dependent pathway. Clin Cancer Res 2013; 19: 1949–1959. [DOI] [PubMed] [Google Scholar]
- Gomez D, Morris-Stiff G, Toogood GJ et al. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2008; 97: 513–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.