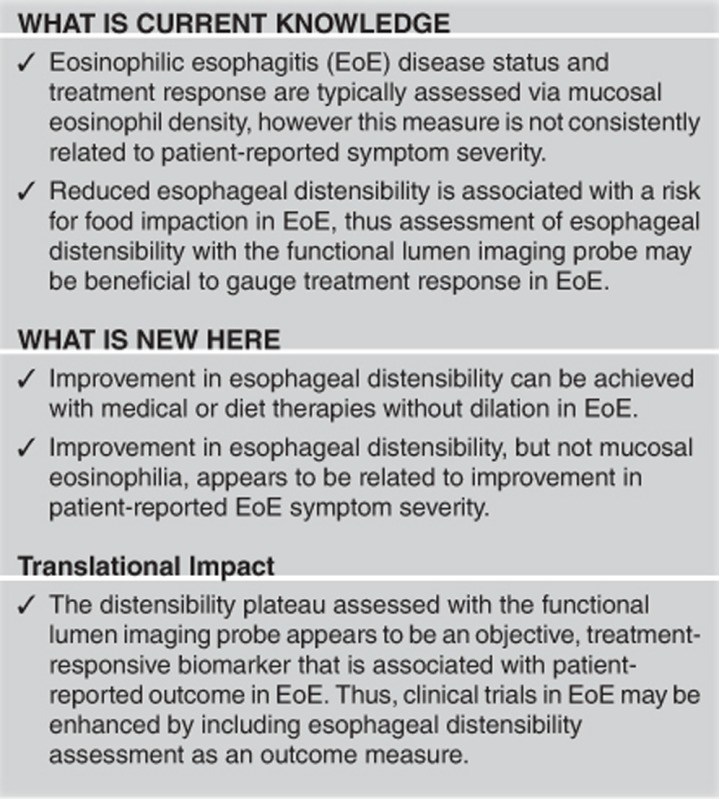
Abstract
Objectives:
We aimed to evaluate the effect of medical and diet therapies on esophageal distensibility assessed using the functional lumen imaging probe (FLIP) and the association of changes in esophageal distensibility with clinical outcomes in eosinophilic esophagitis (EoE).
Methods:
Patients with EoE were evaluated with FLIP during endoscopy at baseline and following therapy without interval dilatation. Evaluation also included a validated patient-reported outcome (PRO; a positive PRO was considered at a 30% score improvement), mucosal biopsies, and scoring of endoscopic features of EoE. FLIP data were analyzed to calculate the distensibility plateau (DP).
Results:
In all, 18 patients (ages 19–54 years; 4 female) treated with topical steroid (8), elimination diet (6), and/or proton-pump inhibitor (4 only treated with proton-pump inhibitor) were included. Follow-up testing occurred at a mean (range) of 14.6 (8–28) weeks. Improvement was observed in DP (13.9 (12.2–19.2) to 16.8 mm (15.8–19.2), P=0.007) and peak eosinophil count (45 (29–65) to 23 per high-power field (h.p.f.) (5–53), P=0.042). Nine patients had a positive symptomatic outcome. Six of 8 (75%) patients with a DP increase ≥2 mm had a positive PRO (P=0.077), while 2 of 7 (29%) patients that achieved an eosinophil count <15/h.p.f. had a positive PRO (P=0.167).
Conclusions:
Improvement in esophageal body distensibility can be achieved with medical and diet therapies without dilation in EoE. Improved DP appeared to be better indicator of symptomatic improvement than eosinophil count, supporting FLIP as a valuable outcome measure in EoE.
Introduction
Eosinophilic esophagitis (EoE) is clinicopathologic disorder characterized by esophageal symptoms and histologic evidence of eosinophilic inflammation.1, 2 Management of EoE typically includes medical or dietary interventions that target reduction in esophageal eosinophil density, which is currently the primary outcome measure in clinical trials.3, 4, 5, 6, 7 The degree of or improvement in eosinophilic mucosal infiltration, however, is not consistently related to patient-reported symptom severity.3, 4, 5, 6, 7, 8, 9
The chronic inflammation of EoE is thought to result in progressive, subepithelial, fibrostenotic remodeling of the esophagus, which endoscopically and radiologically manifest as the characteristic ringed esophagus, focal strictures, and diffuse narrow-caliber esophagus.10, 11, 12 Given that the symptomatic benefit generated from therapeutic esophageal dilation is independent of improvement in mucosal eosinophil density, the biomechanical properties of the esophageal wall appear to be a strong contributor to esophageal symptoms in EoE.13 Endoscopic evaluation may not reliably detect esophageal narrowing;14 and although an endoscopic reference score was developed and validated to semiquantify the endoscopic changes associated with EoE, there remains inconsistency between the relationship of endoscopic changes with symptom severity.8, 15, 16 Thus, the disconnect between symptomatic, histologic, and endoscopic outcome measures creates challenges in measuring treatment effectiveness in therapeutic trials and application of management strategies into clinicial practice.
The functional lumen imaging probe (FLIP) provides a unique evaluation of esophageal function that may augment the assessment of disease activity in EoE. Through simultaneous measurement of esophageal luminal dimensions and distensive pressures during volume-controlled esophageal distension, the biomechanical properties of the esophageal wall, i.e., esophageal distensibility, can be objectively assessed.17 A reduction in esophageal distensibility was initially demonstrated in EoE compared with asymptomatic controls when applying a metric termed the distensibility plateau (DP).17 Further, the clinical significance of a reduction in DP was demonstrated by an association with the risk for food impaction and/or requirement for therapeutic dilation in patients with EoE.18 Therefore, FLIP-measured esophageal distensibility is a potential therapeutic target and outcome measure for clinical trials, and ultimately, clinical practice. Thus, we aimed to assess the response of medical and dietary therapies on esophageal distensibility and the association of changes in esophageal distensibility with clinical outcomes in EoE.
Methods
Subjects
Adult patients with EoE that completed FLIP evaluation during endoscopy at baseline and following initiation of medical or dietary therapy without interval dilation between October 2013 and December 2016 were retrospectively identified for study inclusion from a prospectively maintained registry of patients with suspected EoE that underwent FLIP during endoscopy. Patients were recruited for inclusion in the EoE-FLIP registry from the Esophageal Center of the Northwestern Digestive Health Center during evaluation of esophageal symptoms of dysphagia, food impaction, and/or chest pain. EoE was suspected based on the presence of endoscopic features of EoE (i.e., fissures, rings, or strictures) and patients were diagnosed with EoE per consensus guidelines with ≥15 eosinophils/high-power field (h.p.f.) on esophageal biopsies after at least 8 weeks of proton-pump inhibitor (PPI) therapy.1 Exclusion criteria were performance of esophageal dilation at time of baseline endoscopy, absence of the EoE Symptom Activity Index (EEsAI) patient-reported outcome (PRO) data, presence of erosive esophagitis (Los Angeles grade C or greater), known primary esophageal motility disorder, or identified secondary cause of esophageal eosinophilia. Management included PPI, topical steroid, or elimination diet, and was determined by the treating physician and individual patient preference. The decision to not perform therapeutic dilation at the initial endoscopy, which was often related to the appearance of active inflammation, and the follow-up interval were also dictated by the treating physician. On the basis of recent data and consensus statements indicating that PPI may be an effective therapy for EoE, and not just gastroesophageal reflux disease, we elected to include a subset of patients treated with PPI in the analyses.19, 20, 21 The study protocol was approved by the Northwestern University Institutional Review Board.
Symptom assessment
Patients’ symptoms at the time of endoscopy with FLIP were assessed using the EEsAI score, a validated PRO measure.22 The EEsAI assesses dysphagia, chest pain, and diet-related behavioral modification. The score ranges from 0 to 100 with greater values indicating more severe symptoms. The EEsAI was updated by the EEsAI study group over the course of the study, thus two versions of the EEsAI (version 2 or version 3.1) were utilized: the recall timeframe for the avoidance, modification, and slow eating scores was changed from 30 (version 2) to 7 days (version 3.1). As all patients completed the same EEsAI version at both baseline and treatment follow-up, the intra-subject change in symptom scores was the primary symptom-associated outcome. For the purposes of this study, an EEsAI score improvement of ≥30% was defined as a positive PRO; not meeting this score improvement threshold was defined as a negative PRO.
Endoscopic and histologic assessment
Subjects underwent upper endoscopy in the left lateral decubitus position. Moderate sedation with 2–12 mg midazolam and 0-250 μg fentanyl was administered during the procedure; propofol and ketamine (in addition to midazolam and fentanyl) were used with anesthesiologist assistance at the discretion of the performing endoscopist in one patient. During endoscopy, four-quardant esophageal mucosal biopsies were obtained from the distal and proximal esophagus, obtained at 5 and 15 cm above the squamocolumnar junction, respectively.23 Histologic evaluation of biopsy specimens was performed by local pathologists with expertise in gastrointestinal pathology. The peak number of eosinophils/h.p.f. (0.196 mm2) was recorded for each patient. A positive histologic response was defined as achieving an eosinophil count <15/h.p.f. at follow-up.
Endoscopic features of EoE (edema, rings, exudate, fissures, and stricture) were graded during the upper endoscopy according to a validated endoscopic assessment instrument, Endoscopic EoE Reference Score.15 Edema (score 0–1), furrows (score 0–1), and exudates (score 0–2) were considered inflammatory endoscopic changes and their scores were summed to generate an inflammatory endoscopic score. Improvement in endoscopic inflammatory score was considered if there was a decrease in the cumulative inflammatory score at follow-up. Rings (score 0–3) and stricture (score 0–1) presence were considered endoscopic changes of remodeling. An improved endoscopic ring score was considered when the ring score was downgraded at follow-up endoscopy.
FLIP system and study protocol
The FLIP assembly consisted of a 240 cm long, 3 mm outer diameter catheter with an infinitely compliant balloon (up to a distension volume of 60 ml) mounted on the distal 18 cm of the catheter (EndoFLIP; Crospon, Galway, Ireland). The balloon tapered at both ends to assume a 16 cm long cylindrical shape in the center that housed 17 impedance planimetry ring electrodes spaced at 1 cm intervals, and a solid-state pressure transducer positioned at the distal end to provide simultaneous measurement of 16 channels of luminal diameters (based on the assumption of circular lumen cross-sections) and intra-balloon pressure. Measurements from the impedance planimetry electrode pairs and the pressure transducer were sampled at 10 Hz with the data acquisition system and transmitted to the recording unit.
The endoscope was withdrawn before initiation of the FLIP study protocol. The FLIP was pressure-zeroed to atmospheric pressure before trans-oral probe placement. The FLIP was positioned within the esophagus such that 1–3 impedance sensors were beyond the esophagogastric junction (EGJ) as confirmed by demonstration of a waist in the impedance planimetry segment at a balloon distension volume of 20–30 ml. The FLIP assembly position was adjusted by the endoscopist during the study to maintain placement relative to the EGJ as visualized on real-time output; thus, the EGJ waist provided an anatomic landmark to ensure FLIP probe within the distal esophageal body during the FLIP study. Simultaneous diameters and intra-balloon pressures were measured during 5 ml stepwise distensions beginning with 5 ml and increasing to target volume of 60 ml. The recording unit was set to stop infusing and display an alarm message if the intra-balloon pressure exceeded 60 mm Hg, which sometimes limited the extent of balloon distension; in these cases, the maximal distension volume achieved was recorded. Each stepwise distension volume was maintained for 20–30 s during a single distension protocol for each patient.
FLIP data analysis
Data, including distension volume, intra-balloon pressure, and 16 channels of diameter measurements for the entire study for each subject were exported to MATLAB (The Math Works, Natick, MA, USA) for analysis using a customized MATLAB program to calculate the DP. The MATLAB program identified the EGJ midline by searching for minimal diameter values below an investigator-designated proximal border of the EGJ, which was based on review of the FLIP topography plot. The data array was reconfigured from the EGJ landmark to include an 8 cm esophageal body measurement segment spanning from 3 to 10 cm above the EGJ (Figure 1). To account for the effects of that distension-induced contractility can have on assessment of esophageal narrowing, the maximally achieved diameters among each esophageal body sensor and the nadir pressure that occurred during each 5 ml incremental distension volume were identified.24 The program then identified and plotted the narrowest of these esophageal body diameters by nadir intra-balloon pressure for each incremental distension volume (Figure 2). Finally, the esophageal body diameter–pressure relationship was modeled with a polynomial regression technique to derive the DP.
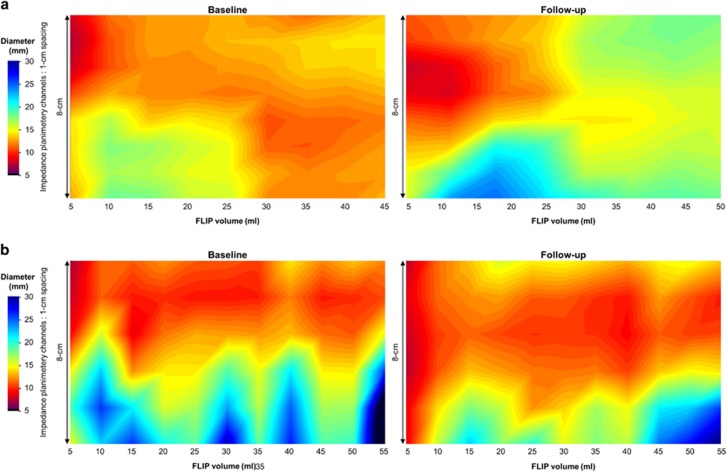
Figure 1.
Functional lumen imaging probe (FLIP) topography plots. Baseline and follow-up FLIP topography plots for a patient treated with topical steroid (a) and another patient treated with dietary therapy (b) are displayed. The topographic plots represent color-coded diameter plots generated from interpolation of the impedance planimetry data by spatial orientation (y-axis) by time (x-axis). The 8 cm of the topographic plot above the white lines, which represents 3 cm above the EGJ midline (thus the analysis accounted for catheter movement during the study by using the narrowing at the esophagogastric junction as an anatomic landmark), was subjected to analysis of esophageal body distensibility. The patient in a achieved a positive patient-reported outcome (i.e., improvement of 30% in the EoE Symptom Activity Index score), while the patient in b did not. Figure used with permission from the Esophageal Center at Northwestern.
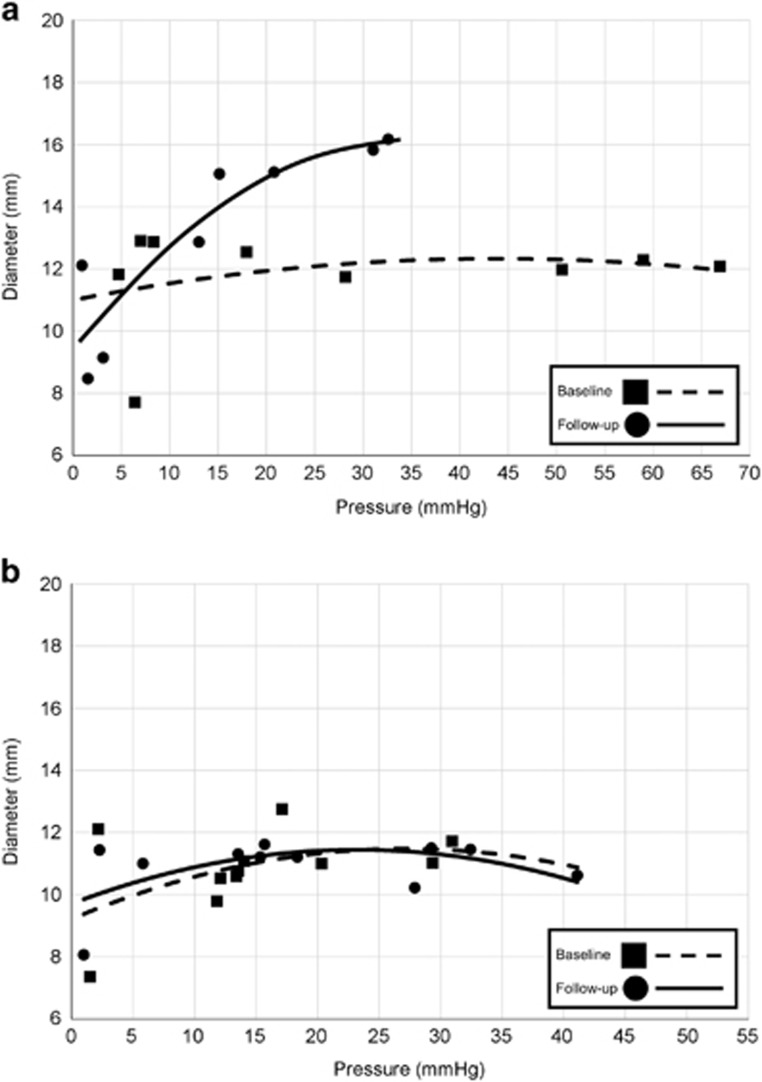
Figure 2.
Distensibility plateau (DP). Baseline and follow-up DP plots for the same two patients as in Figure 1 ((a) treated with topical steroid, positive patient-reported outcome; (b) treated with dietary therapy, negative patient-reported outcome) are displayed. Points represent the narrowest luminal diameter by nadir pressure at each incremental distension volume. The DP was calculated from the polynomial trendlines. Figure used with permission from the Esophageal Center at Northwestern.
Statistical analysis
Results were expressed as median (interquartile range) unless otherwise stated. Intra-subject comparisons of continuous data were compared using Wilcoxon signed rank tests. Groups were compared utilizing the Mann–Whitney U-test. Relationships between dichotomous outcomes were evaluated using the χ2-test. Statistical significance was considered at a P value <0.05.
Results
Patient characteristics
A total of 18 patients (mean age 30 years, range 19–54 years; 4 females) were identified for inclusion (Table 1). Over the course of the study, a total of 114 unique patients with EoE were evaluated with FLIP: 79 did not have a repeat FLIP completed during the defined study period or within 6 months of baseline examination. Of the remainder, 9 had dilation performed on the initial endoscopy, 3 had baseline FLIP while on established therapy with topical steroid or elimination diet, 3 patients had a technical limitation in their baseline or follow-up FLIP, and 2 did not complete the PRO. In all, 13 included patients (72%) had a history of atopic disease (i.e., asthma, eczema, allergic rhinitis, and/or food allergy). Three patients had a symptom duration (presumed disease duration) of ≤1 year; 12 had a symptom duration of >10 years. At the time of the baseline evaluation, 12 patients were actively being treated with PPI; none of the patients were being treated with topical steroid or elimination diet at the time of baseline evaluation. Four patients were initiated on treatment with only PPI, 8 were treated with topical steroid (5 of whom also remained on PPI therapy), and 6 were treated with elimination diet (3 of whom remained on PPI therapy). Follow-up evaluation occurred at a mean (range) interval of 14.68, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 weeks from baseline.
Table 1. Patient characteristics.
| Baseline | Follow-up | |
|---|---|---|
| Symptom duration, years, median (IQR) | 15.5 (3–22) | 16.4 (4–23) |
| Treatment | ||
| PPI | 12 (75) | 11 (61) |
| Topical steroid | 0 | 8 (44) |
| Elimination diet | 0 | 6 (33) |
| Symptom score, median (IQR) | ||
| EEsAI v2.0 (n=9) | 42 (19–60) | 25 (14–42) |
| EEsAI v3.1 (n=9) | 27 (21–34) | 15 (0–31) |
| Endoscopic features | ||
| Hiatal hernia | 3 (17) | 3 (17) |
| Erosive esophagitis | 2 (11)a | 0 |
| EREFS | ||
| Edema | 15 (83) | 8 (44) |
| Rings | ||
| Grade 1 | 5 (28) | 10 (55) |
| Grade 2 | 10 (56) | 8 (44) |
| Grade 3 | 3 (17) | 0 |
| Exudate | ||
| Grade 1 | 8 (44) | 4 (22) |
| Grade 2 | 4 (22) | 0 |
| Furrows | 17 (94) | 7 (39) |
| Stricture | 16 (89) | 17 (94) |
| Stricture location | ||
| EGJ | 15 (83) | 17 (94) |
| Distal esophagus | 10 (56) | 11 (61) |
| Mid-proximal esophagus | 9 (50) | 10 (56) |
| Narrow-caliber esophagus | 12 (75) | 8 (44) |
| Therapeutic dilation performed | 0 | 11 (61) |
EEsAI, Eosinophilic Esophagitis Symptom Activity Index; EGJ, esophagogastric junction; EREFS, Endoscopic Eosinophilic Esophagitis Reference Score; IQR, interquartile range; PPI, proton-pump inhibitor.
Both patients had Los Angeles classification B esophagitis.
Values are n (%) unless otherwise noted.
Clinical outcomes
Nine patients completed the EEsAI version 2.0 and nine complete the EEsAI version 3.1. Paired comparison of PROs demonstrated improvement from baseline to follow-up (Table 1), P value=0.042, with a median (interquartile range) change in PRO score of −10 (−16 to 0). Nine patients (50%) had a positive PRO (i.e., EEsAI score decrease of ≥30%), two of the remaining nine patients with a negative PRO, two patients had worsening (i.e., increased EEsAI score ≥30%) PRO (Table 2).
Table 2. Characteristics related to PRO.
| Positive PRO | Negative PRO | |
|---|---|---|
| n | 9 | 9 |
| Age, years, mean (range)a | 33 (22 to 43) | 37 (19 to 54) |
| Gender (F:M) | 2:7 | 3:6 |
| Symptom duration, yearsa | 16 (2 to 26) | 15 (4 to 19) |
| Treatment, n (%) | ||
| PPI | 6 (67) | 6 (67) |
| Topical steroid | 3 (33) | 5 (57) |
| Elimination diet | 3 (33) | 3 (33) |
| Follow-up interval, weeks | 14 (11 to 15) | 14 (12 to 19) |
| Baseline symptom score | 27 (24 to 53) | 34 (23 to 38) |
| Follow-up symptom score | 15 (0 to 23) | 27 (23 to 47)b |
| Histology | ||
| Baseline eosinophil count, eos/h.p.f. | 40 (35 to 61) | 50 (25 to 88) |
| Follow-up eosinophil count, eos/h.p.f. | 25 (15 to 55) | 10 (0 to 60) |
| Change in eosinophil count, eos/h.p.f. | −15 (−47 to 11) | −20 (−50 to 10) |
| Achieved eosinophil count <15/h.p.f., n (%) | 2 (22) | 5 (57) |
| Endoscopy | ||
| Baseline EREFS, n (%) | ||
| Edema | 7 (78) | 8 (89) |
| Rings | ||
| Grade 1 | 3 (33) | 2 (22) |
| Grade 2 | 4 (44) | 6 (67) |
| Grade 3 | 2 (22) | 1 (11) |
| Exudate | ||
| Grade 1 | 5 (57) | 3 (33) |
| Grade 2 | 1 (11) | 3 (33) |
| Furrows | 8 (89) | 9 (100) |
| Stricture | 8 (89) | 8 (89) |
| Follow-up EREFS, n (%) | ||
| Edema | 5 (56) | 3 (33) |
| Rings | ||
| Grade 1 | 6 (67) | 4 (44) |
| Grade 2 | 3 (33) | 5 (56) |
| Grade 3 | 0 | 0 |
| Exudate | ||
| Grade 1 | 1 (11) | 3 (33) |
| Grade 2 | 0 | 0 |
| Furrows | 4 (44) | 3 (33) |
| Stricture | 8 (89) | 9 (100) |
| Improved EREFS inflammatory score, n (%) | 7 (78) | 7 (78) |
| Improved EREFS ring score, n (%) | 4 (44) | 3 (33) |
| FLIP | ||
| Baseline distensibility plateau, mm | 12.9 (12.2 to 20.8) | 16.8 (11.9 to 18.5) |
| Follow-up distensibility plateau, mm | 16.1 (15.5 to 20.2) | 16.9 (14.3 to 19.6) |
| Change in distensibility plateau, mm | 2.6 (0.4 to 4.5) | 0.9 (0 to 2.3) |
| Improved distensibility plateau ≥2 mm, n (%) | 6 (67) | 2 (22) |
eos, eosinophils; EREFS, Endoscopic Eosinophilic Esophagitis Reference Score; FLIP, functional lumen imaging probe; h.p.f., high-power field; PPI, proton-pump inhibitor; PRO, patient-reported outcome.
Data are presented as median (interquartile range) unless otherwise specified.
At baseline evaluation.
P value <0.05 when compared with positive PRO group.
Peak eosinophil counts were 45 eosinophils/h.p.f. (29 to 65) at baseline with improvement to 23 (5 to 53) at follow-up, P value=0.042. The median (interquartile range) change in eosinophil count was −18 eosinophils/h.p.f. (−50 to 11). In all, 7 patients (39%) achieved a positive histologic response (i.e., follow-up peak eosinophil count <15/h.p.f.).
Rings were the most commonly observed endoscopic feature of EoE and were present in all patients at both baseline and follow-up. Multiple endoscopic features were observed in all but one patient at both baseline and follow-up. Seven (39%) patients had improvement in ring score from baseline to follow-up, but none had resolution of rings. Resolution of stricture was also not observed between testing periods in any patient. Furrows were the most commonly inflammatory endoscopic feature at baseline (94%), while edema was the most common inflammatory feature at follow-up (44%). The endoscopic inflammatory score (edema+exudates+furrows) was 3 (2–4) at baseline and 0 (0–2) at follow-up (P value=0.003). Improvement in inflammatory score was observed in 14 (78%) patients; 10 patients (56%) had resolution of inflammatory features (i.e., no edema, furrows, or exudates) at follow-up.
Esophageal distensibility and relationships with clinical outcomes
The median (interquartile range) DP was 13.9 mm (12.2 to 19.2) at baseline with improvement to 16.8 mm (15.8 to 19.2) observed at follow-up, P value=0.007. The median (range) change in DP was 1.7 mm (−2.2 to 5.2). Improvement in DP ≥2 mm was achieved in eight patients (44%); improvement in DP ≥3 mm was achieved in five patients (28% Figure 3). Only one patient (6%) had worsening in DP ≥2 mm from baseline DP of 20.9 mm to follow-up of 18.7 mm.
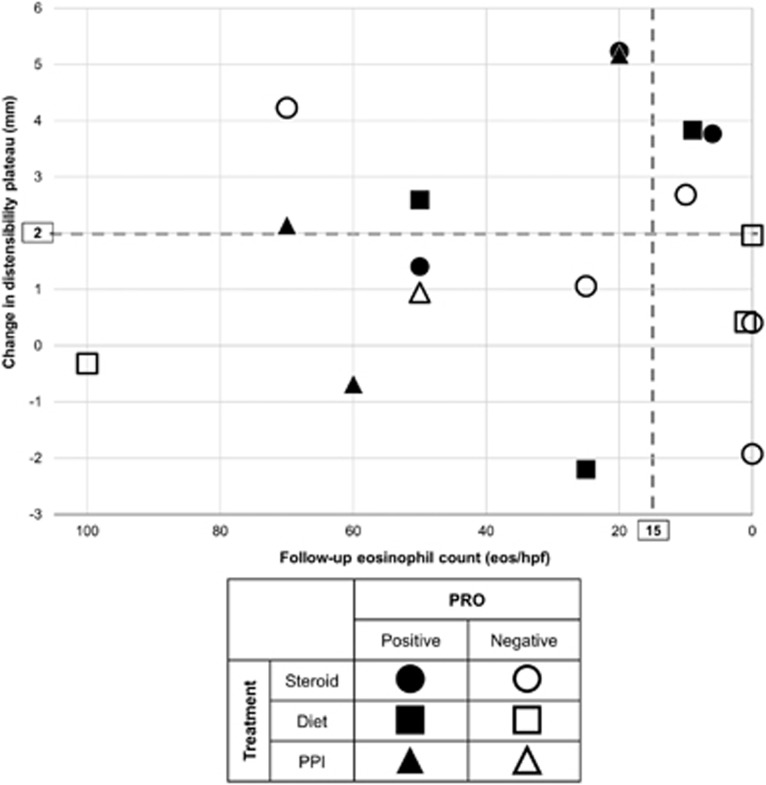
Figure 3.
Relationships between patient-reported outcome (PRO), histologic response, and change in distensibility plateau. A positive PRO was defined as an improvement of 30% in the Eosinophilic Esophagitis Symptom Activity Index.
Baseline, follow-up, and changes in histologic and endoscopic features did not differ between PROs when compared as continuous measures (Table 2). However, there was a trend toward an association between achieving a positive PRO and achieving an improvement in DP ≥2 mm (P=0.077): 6/8 patients with a DP improvement ≥2 mm had a positive PRO (Figure 3); the relationship between positive PRO and DP improvement ≥3 mm was not significant (P value=0.147), though 4/5 patients with a DP improvement ≥3 mm had a positive PRO.
Achieving an eosinophil count <15/h.p.f. was not significantly related to improvement in DP (Figure 3): 5/8 patients with an improvement in DP ≥2 mm also had a histologic response (P value=0.648); 3/5 with an improvement in DP ≥3 mm had a histologic response (P value=0.676).
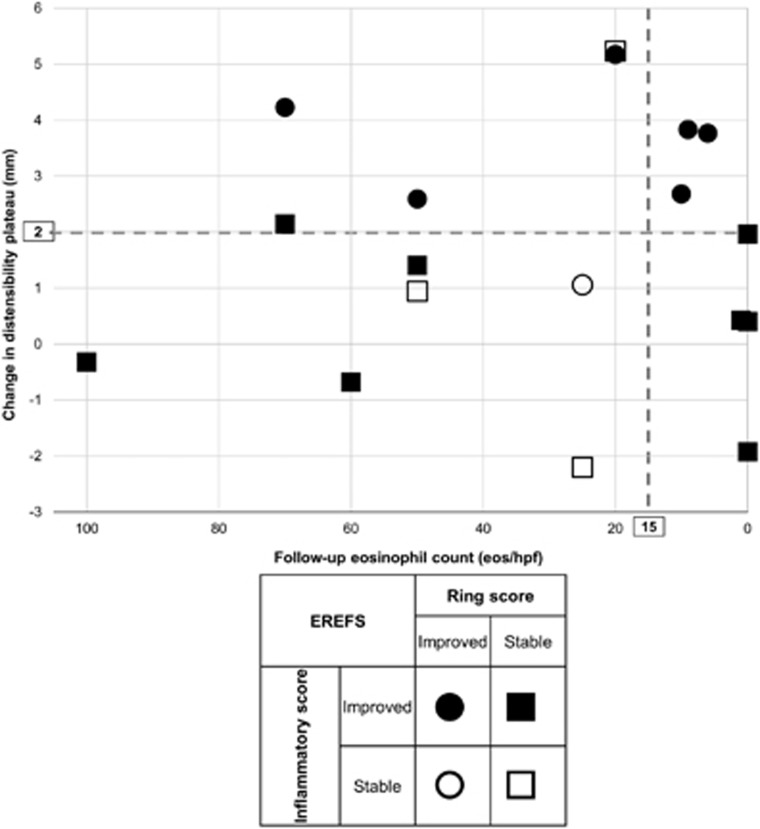
Achieving improvement in endoscopic ring score was associated with an improvement in DP: 6/8 with a DP increase ≥2 mm had an improved ring score (P value=0.009) and 4/5 patients with a DP increase ≥3 mm had an improved ring score (P value=0.047; Figure 4). Improvement in endoscopic inflammatory score was not associated with DP improvement ≥2 mm (P value=0.382) or ≥3 mm (P value=0.701). Neither improvement in endoscopic ring score (4/7 patients, P value=0.583) nor improvement in endoscopic inflammatory score (7/14 patients, P value=0.108) was associated with achieving an eosinophil count <15/h.p.f.
Figure 4.
Relationships between changes in endoscopic features, histologic response, and change in distensibility plateau. The inflammatory score was considered as the sum of edema+exudates+furrows. EREFS, Endoscopic Eosinophilic Esophagitis Reference Score.
Discussion
The main findings of our study are that improvement in esophageal distensibility can be achieved with medical or diet therapies without dilation and that improvement in esophageal distensibility appears to be related to improvement in EoE symptom severity. Clinical responses of mucosal eosinophilia and endoscopic features appeared to have weaker relationships with improvement in PRO than FLIP-quantified distensibility. Therefore, assessment of esophageal distensibility with FLIP may offer a valuable outcome measure to assess treatment response in EoE.
Previous studies evaluating esophageal distensibility with FLIP in EoE have demonstrated reduced DP in EoE (compared with controls) and an association of reduced DP with food impaction and performance of therapeutic dilation.17, 18, 24 This is the first study to evaluate the changes in esophageal body distensibility associated with therapeutic intervention in EoE. Increases in esophageal caliber as assessed with endoscopy or barium radiography were reported following therapy with topical steroids.16, 25 However the accuracy of esophageal caliber or mechanical properties with either endoscopy or radiology is questionable given the inability to control for intraluminal distension forces by air insufflation or swallowed barium, respectively.14 FLIP provides a method to objectively measure the fixed luminal diameter despite increasing intraluminal pressures (i.e., the DP), which therefore represents the luminal diameter that would restrict esophageal bolus transit. Luminal caliber is a well-described feature of symptom generation in esophageal mechanical obstruction, however, an objective measure of this important feature is typically unaccounted for in therapeutic trials in EoE.26, 27 FLIP, however, provides a method to fill this void by assessing an important feature in EoE.12
In our study, the improvement in DP appears to occur independently of improvement in mucosal eosinophilia or inflammatory endoscopic changes. Instead, improvement in DP was related to an improvement in endoscopic ring score, suggests its association with esophageal wall remodeling, similar to a previous study.28 As mucosal eosinophilia is a primary end point is therapeutic trials in EoE, presently recommended therapies are generally based on their efficacy to reduce±normalize mucosal eosinophilia.1 However, the inconsistency between inflammatory improvement and symptomatic improvement seen in this and previous studies, including randomized controlled trials, suggests that the symptomatic improvement in EoE is related to factors other than inflammation, including improvement in the biomechanics of the esophageal wall.3, 4, 5, 6, 7, 8, 9, 18 This is the first study to assess the relationship between the recently validated PRO, the EEsAI, and esophageal distensibility. While the association did not reach statistical significance (possibly related to the small sample size), there appeared to be an association between improvement in esophageal distensibility (but not mucosal eosinophil density) and improvement in PRO.22 Although histologic esophageal fibrosis is inconsistently evaluated in clinical trials of EoE, some evidence exists to suggest that esophageal fibrosis may improve following therapy with topical steroids and diet.29, 30, 31, 32 In addition, improvements in the fibrotic endoscopic changes (rings) are also reported following EoE therapies.4, 16 Biomechanical remodeling in EoE is complex and the potential for improvement may be related to numerous factors, including patient age, duration and activity of disease, neuromuscular effects, and the degree of fibrosis, thus future studies incorporating distensibility assessment and histologic fibrosis will be of great importance.12
The significance of our study may extend beyond the utility of FLIP as an outcome measure in clinical trials and has implications for clinical practice. The 2–3 mm improvement in luminal diameter reflected by the improvement in DP identified in our study mirrors the improvement achieved in esophageal dilation in EoE.13 Although therapeutic dilation is safe and often effective in improving symptoms, it is commonly (70%) associated with post-procedural chest pain with an, albeit low, risk of esophageal perforation.13, 33 Thus, demonstrating that esophageal distensibility can be improved with medical or dietary therapies alone supports the practice of medical or dietary therapy as an initial EoE treatment strategy.1, 34
Although this study suggests utility of esophageal distensibility assessment in EoE, it does have several limitations. The relatively small sample size limits a more robust analysis of the interactions between symptoms, histology, endoscopic features, and esophageal distensibility, as well as subgroup analyses, such as by treatment modality or by baseline characteristics. The small sample size and exclusion criteria also limit the generalizability of our observations. The improvement in esophageal distensibility we observed may represent selection bias related to the initial decision to not perform therapeutic dilation, exclusion of patients without endoscopically apparent remodeling features, or other unaccounted-for factors. Severity of esophageal strictures in adults with EoE has been associated with a significant reduction in histologic response to steroids, which may account for the lower than expected histologic response in our cohort.35 Finally, although DP appears to be a useful measurement, it only accounts for the distal 8 cm of the esophageal body; thus, narrowing in the proximal esophagus or at the EGJ that could contribute to symptoms is not accounted for with this metric. While we believe that quantification of esophageal distensibility has promise with regards to an outcome measure in EoE, ongoing investigation to optimize assessment is warranted.
In conclusion, we report that esophageal distensibility can improve with medical or dietary therapy and is related to symptomatic improvement in EoE. While future study is clearly needed to identify the predictors and mechanisms as well as substantiate the generalizability of this finding, this study demonstrates the potential utility of including esophageal distensibility assessment as an objective outcome measure in therapeutic trials.
Study Highlights
Acknowledgments
This work was supported by R01 DK079902 (J.E.P.) from the Public Health Service, and American Society of Gastrointestinal Endoscopy Research Award (I.H.)
Footnotes
Guarantor of the article: Dustin A. Carlson, MD, MS.
Specific author contributions: D.A.C. contributed to study concept and design, data analysis, data interpretation, drafting of the manuscript, and approval of the final version. I.H. contributed to study concept and design, recruitment of patients, data analysis, revising the manuscript critically, and approval of the final version. A.Z. and N.G. contributed to recruitment of patients, data acquisition, and approval of the final version. Z.L. contributed to data analysis and approval of the final version. J.E.P. contributed to study concept and design, revising the manuscript critically, and approval of the final version.
Financial support: None.
Potential competing interests: John E. Pandolfino: Given Imaging (consultant, grant, speaking), Sandhill Scientific (consulting, speaking), Takeda (speaking), Astra Zeneca (speaking); Ikuo Hirano: Receptos (consulting), Regeneron Pharmaceutical (consulting), Shire (consulting); Nirmala Gonsalves: Nutricia (speaking). The remaining authors declare no conflict of interest.
References
- Dellon ES, Gonsalves N, Hirano I et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013; 108: 679–692, quiz 93. [DOI] [PubMed] [Google Scholar]
- Liacouras CA, Furuta GT, Hirano I et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128: 3–20.e6, quiz 1–2. [DOI] [PubMed] [Google Scholar]
- Alexander JA, Jung KW, Arora AS et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2012; 10: 742–749.e1. [DOI] [PubMed] [Google Scholar]
- Gonsalves N, Yang GY, Doerfler B et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142: 1451–1459.e1, quiz e14–5. [DOI] [PubMed] [Google Scholar]
- Molina-Infante J, Arias A, Barrio J et al. Four-food group elimination diet for adult eosinophilic esophagitis: a prospective multicenter study. J Allergy Clin Immunol 2014; 134: 1093–1099.e1. [DOI] [PubMed] [Google Scholar]
- Miehlke S, Hruz P, Vieth M et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut 2016; 65: 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellon ES, Katzka DA, Collins MH et al. Budesonide oral suspension improves symptomatic, endoscopic, and histologic parameters compared with placebo in patients with eosinophilic esophagitis. Gastroenterology 2016; 152: 776–786.e5. [DOI] [PubMed] [Google Scholar]
- Safroneeva E, Straumann A, Coslovsky M et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology 2016; 150: 581–590.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf WA, Cotton CC, Green DJ et al. Evaluation of histologic cutpoints for treatment response in eosinophilic esophagitis. J Gastroenterol Hepatol Res 2015; 4: 1780–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer AM, Safroneeva E, Bussmann C et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013; 145: 1230–1236.e1-2. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Kim HP, Sperry SL et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014; 79: 577–585.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano I, Aceves SS. Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterol Clin North Am 2014; 43: 297–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer AM, Gonsalves N, Bussmann C et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol 2010; 105: 1062–1070. [DOI] [PubMed] [Google Scholar]
- Gentile N, Katzka D, Ravi K et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment Pharmacol Ther 2014; 40: 1333–1340. [DOI] [PubMed] [Google Scholar]
- Hirano I, Moy N, Heckman MG et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 2013; 62: 489–495. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Cotton CC, Gebhart JH et al. Accuracy of the eosinophilic esophagitis endoscopic reference score in diagnosis and determining response to treatment. Clin Gastroenterol Hepatol 2016; 14: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatek MA, Hirano I, Kahrilas PJ et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology 2011; 140: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodeme F, Hirano I, Chen J et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2013; 11: 1101–1107.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 2009; 54: 2312–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Infante J, Bredenoord AJ, Cheng E et al. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut 2016; 65: 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia L, Hirano I. Distinguishing GERD from eosinophilic oesophagitis: concepts and controversies. Nat Rev Gastroenterol Hepatol 2015; 12: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer AM, Straumann A, Panczak R et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology 2014; 147: 1255–1266.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves N, Policarpio-Nicolas M, Zhang Q et al. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc 2006; 64: 313–319. [DOI] [PubMed] [Google Scholar]
- Carlson DA, Lin Z, Hirano I et al. Evaluation of esophageal distensibility in eosinophilic esophagitis: an update and comparison of functional lumen imaging probe analytic methods. Neurogastroenterol Motil 2016; 28: 1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Huprich J, Kujath C et al. Esophageal diameter is decreased in some patients with eosinophilic esophagitis and might increase with topical corticosteroid therapy. Clin Gastroenterol Hepatol 2012; 10: 481–486. [DOI] [PubMed] [Google Scholar]
- Schatzki R. The lower esophageal ring. Long term follow-up of symptomatic and asymptomatic rings. Am J Roentgenol Radium Ther Nucl Med 1963; 90: 805–810. [PubMed] [Google Scholar]
- Hirano I, Pandolfino JE, Boeckxstaens GE. Functional lumen imaging probe for the management of esophageal disorders: expert review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol 2017; 15: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JW, Pandolfino JE, Lin Z et al. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy 2016; 48: 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straumann A, Conus S, Degen L et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139: 1526–1537, 37.e1. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Morotti RA, Konstantinou GN et al. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy 2012; 67: 1299–1307. [DOI] [PubMed] [Google Scholar]
- Kagalwalla AF, Akhtar N, Woodruff SA et al. Eosinophilic esophagitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol 2012; 129: 1387–1396.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng E, Souza RF, Spechler SJ. Tissue remodeling in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 2012; 303: G1175–G1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge TM, Eluri S, Cotton CC et al. Outcomes of esophageal dilation in eosinophilic esophagitis: safety, efficacy, and persistence of the fibrostenotic phenotype. Am J Gastroenterol 2016; 111: 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavitt RT, Ates F, Slaughter JC et al. Randomized controlled trial comparing esophageal dilation to no dilation among adults with esophageal eosinophilia and dysphagia. Dis Esophagus 2016; 29: 983–991. [DOI] [PubMed] [Google Scholar]
- Eluri S, Runge TM, Cotton CC et al. The extremely narrow-caliber esophagus is a treatment-resistant subphenotype of eosinophilic esophagitis. Gastrointest Endosc 2016; 83: 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]