Abstract
Objectives:
We aimed to determine the levels of alanine aminotransferase (ALT), hepatitis B virus DNA (HBV DNA), HBsAg, and a novel viral marker (hepatitis B core-related antigen (HBcrAg)); hepatitis B e antigen (HBeAg) seroconversion and drug resistance rates after 7 years of entecavir treatment in chronic hepatitis B (CHB) patients.
Methods:
Two hundred and twenty-two Chinese CHB patients on continuous entecavir treatment were recruited. Serologic, virologic, biochemical outcomes, and the occurrence of entecavir signature mutations were determined.
Results:
The rates of ALT normalization, HBeAg seroconversion, and undetectable HBV DNA were 98.3%, 82.1%, and 98.7%, respectively, after 7 years of entecavir treatment. The genotypic resistance rate was 1.2%. Decline of HBsAg level was modest with a median decline rate of 0.107 log IU/ml/year. Among patients with baseline HBsAg <1,000 IU/ml and annual HBsAg decline rate of ≥0.166 log IU/ml, all have HBsAg of <200 IU/ml (a level highly predictive for HBsAg seroclearance) at year 7. In contrast, in patients with baseline HBsAg ≥1,000 IU/ml and annual HBsAg decline rate of <0.166 log IU/ml, 95.5% had HBsAg of ≥200 IU/ml at year 7. Decline of HBcrAg levels was moderate with a median decline rate of 0.244 log kU/ml/year. Forty-seven patients (32.0%) had undetectable HBcrAg level at year 7.
Conclusions:
Long-term entecavir therapy continued to have good responses with low drug resistance rate. However, the decline of HBsAg with treatment was suboptimal. HBcrAg level declined at a relatively better rate. Baseline HBsAg level of <1,000 IU/ml and annual decline of 0.166 log IU/ml could be used to predict HBsAg response.
Introduction
Chronic hepatitis B (CHB) infection is a disease of major health burden globally, with ~250 million people affected.1 Nucleos(t)ide analogs are the mainstay of therapy. Of these, entecavir is the most widely used agent. Our previous study showed that entecavir is highly effective in terms of potency of viral suppression with low rate of viral resistance up to 5 years of therapy.2 Long-term use of entecavir was also shown to improve liver function, histology in patients with advanced fibrosis/cirrhosis, and, more importantly, reduce the incidence of hepatocellular carcinoma (HCC).3, 4
While most of the CHB patients have undetectable hepatitis B virus DNA (HBV DNA) while on entecavir, a proportion of patients continued to develop complications while on treatment.5 More serological markers other than HBV DNA may help to monitor patients while on nucleos(t)ide analogs to guide treatment and assess prognosis. Hepatitis B core-related antigen (HBcrAg) is a novel marker that can detect hepatitis B core antigen and hepatitis B e antigen (HBeAg) simultaneously.6 HBcrAg was shown to correlate positively with serum HBV DNA, intrahepatic total HBV DNA, and hepatitis B covalently closed circular DNA (cccDNA).7, 8 The correlation between HBcrAg and cccDNA was also observed in patients with negative serum HBV DNA.9 Previous studies showed that HBV DNA decline more rapidly than serum HBcrAg in patients treated with nucleoside analogs.10, 11 As most of the patients on long-term entecavir have undetectable HBV DNA, serum HBcrAg level may be potentially useful to reflect intrahepatic viral activity in this group of patients. According to a recent study, HBcrAg levels are predictive of development of HCC in patients with undetectable HBV DNA under nucleos(t)ide analog therapy.12 High HBcrAg level was also shown to be a predictive factor for reactivation of hepatitis after cessation of lamivudine therapy,13, 14 development of lamivudine resistance,15 and post-treatment recurrence of HCC during anti-viral therapy.16
Quantitative HBsAg level has been recently studied for its usefulness for monitoring disease activity and prognostication.17 For CHB patients with low level of viremia, low HBsAg levels are associated with lower chance of hepatitic flare, cirrhosis, and HCC.18, 19 Lower HBsAg levels, e.g. <200 IU/ml are also associated with higher chance of HBsAg seroclearance,20, 21 which is associated with favorable outcome and improved survival.22, 23 However, the decline of HBsAg level is still slow and HBsAg seroclearance remains a rare event during nucleos(t)ide and interferon therapy.2, 24
We extended our previous 5-year entecavir study2, 25 to 7 years of follow-up in a real-world cohort. The original primary aim was to examine the rate of HBV DNA suppression, HBeAg seroconversion, alanine aminotransferase (ALT) normalization, virological breakthrough, and viral resistance. With the recent increasing interest in the measurement of HBsAg and the new development of novel HBcrAg assay, we added another exploratoy aim to evaluate the kinetics of HBsAg and HBcrAg over an extended period of continuous entecavir therapy.
Methods
Patients
The present study recruited treatment-naïve CHB patients who were started on entecavir 0.5 mg daily in the Department of Medicine, Queen Mary Hospital, the University of Hong Kong from July 2005 to November 2007. All patients were HBsAg positive for at least 6 months before treatment. Patients were started on entecavir based on the following criteria: (i) HBeAg-positive non-cirrhotic patients with elevated ALT (upper limit of normal <50 IU/ml) and HBV DNA >20,000 IU/ml; (ii) HBeAg-negative non-cirrhotic patients with elevated ALT and HBV DNA >2,000 IU/ml; and (iii) HBV DNA >2,000 IU/ml for patients with clinical evidence of cirrhosis.
Patients with the following concomitant conditions were excluded: hepatitis C and D infection, Wilson’s disease, autoimmune hepatitis, primary biliary cirrhosis, and significant intake of alcohol (20 g per day for female; 30 g per day for male).
The present study was approved by the Institutional Review Board of the University of Hong Kong and the Hospital Authority Hong Kong West Cluster, Hong Kong.
Patients were followed up every 3–6 months for clinical assessment and measurement of liver biochemistry and α-fetoprotein. Serum HBV DNA, HBeAg status, and quantitative HBsAg levels were performed at baseline and at every year after initiation of entecavir therapy. HBcrAg levels were measured at baseline, the first, fifth, and seventh year of follow-up.
HBeAg seroconversion was defined by HBeAg negativity with detectable antibody to HBeAg for at least two consecutive follow-up. Virological breakthrough was defined as at least 1 log IU/ml increase of HBV DNA from the nadir for patients with detectable HBV DNA levels, or HBV DNA levels increasing to >20 IU/ml for patients with previously achieved undetectable HBV DNA levels. Viral mutational analysis was performed for all samples with detectable viremia (HBV DNA >20 IU/ml).
For patients who opted to stop entecavir, the date of stopping of treatment was considered as the end of follow-up for the present study. Patients who developed signature entecavir resistance were switched to tenofovir monotherapy. The date of change of anti-viral therapy was considered as the end of follow-up.
Laboratory assays
Serum HBV DNA levels were measured using Cobas Taqman assay (Roche Diagnostics, Branchburg, NJ) with the lower limit of detection of 20 IU/ml. Resistance profile was performed using a line probe assay (Innogenetics NV, Gent, Belgium), with both line probe assay DR version 2 and 3 used to identify the amino acids at codons rt173, rt180, rt240 and rt184, and rt202 and rt250, respectively. Genotypic resistance to entecavir was defined by the presence of three viral mutations: rtL180M, rtM204V/I, and one of the following: rtT184S/C/G/A, rtS202G/C/I, or rtM250V. Serum HBeAg, antibody to HBeAg and antibody to HBsAg were measured by the Architect Immunoassays (Abbott Laboratories, Chicago, IL). Serum HBsAg levels were performed using Elecsys HBsAg II Assay (Roche Diagnostics, Branchburg, NJ), with a linear range of 0.05–52,000 IU/ml. Samples with levels higher than 52,000 IU/ml were tested at a dilution of 1:100 according to the manufacturer’s instruction. The HBcrAg levels were determined by the Lumipulse G HBcrAg Chemiluminescence Enzyme Immunoassay (Fujirebio, Tokyo, Japan). The dynamic range of the assay ranges from 1 to 1 × 104 kU/ml. Samples with HBcrAg>104 kU/ml were retested at dilution of 1:100 according to the manufacturer’s instruction. In the present study, the lower cutoff value of HBcrAg concentration is 1 kU/ml.
Statistical analyses
Serum HBV DNA levels, HBsAg level, and HBcrAg levels were expressed in logarithm. Continuous variables were expressed in median (range). Comparison of continuous variables was performed using Mann–Whitney U-test and Kruskal–Wallis test. Categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test as appropriate. The rate of HBV DNA viral suppression (HBV DNA <20 IU/ml), HBeAg seroconversion, ALT normalization, and virological breakthrough were calculated using Kaplan–Meier analyses. The cumulative rate of development of viral resistance was calculated from the formula: P=1−(1−n1/N1)(1−n2/N2)…(1−nx/Nx).26 The following factors were evaluated for their association with undetectable HBcrAg at the seventh year in univariate analysis: age, sex, baseline ALT, baseline HBeAg status, baseline HBV DNA level, baseline HBsAg level, and baseline HBcrAg level. Logistic regression model was used to estimate odds ratios and 95% confidence interval (95% CI) of parameters to predict undetectable HBcrAg at the seventh year. The multivariable logistic regression model included the following variables: age, baseline HBeAg status, baseline HBV DNA level, baseline HBsAg level, and baseline HBcrAg level. Correlation between clinical parameters was performed using Spearman’s bivariate correlation. All statistical analyses were performed using SPSS version 20 (SPSS, Chicago, IL). A two-sided P value of <0.05 was considered statistically significant.
Results
Baseline
Two hundred and twenty-two treatment-naïve CHB patients were recruited. The baseline demographics were shown in Table 1. In all, 222, 188, 173, 170, 167, 163, and 160 patients were followed up for 1, 2, 3, 4, 5, 6, and 7 years, respectively. Sixty-one patients discontinued entecavir because of various reasons listed in Table 2. One additional patient received additional pegylated interferon prescribed by a private doctor after 6 years of entecavir monotherapy and thus his clinical and laboratory data were censored at 6 years of follow-up.
Table 1. Baseline parameters of all 222 patients.
| All patients (n=222) | HBeAg positive (n=90) | HBeAg negative (n=132) | P value | |
|---|---|---|---|---|
| Age (years) | 47 (21–77) | 41 (21–66) | 50 (24–77) | <0.01 |
| Number of male | 157 (70.7%) | 65 (72.2%) | 92 (70.0%) | 0.69 |
| HBV DNA (log IU/ml) | 6.4 (3.3–>8.1) | 7.3 (3.3–>8.1) | 6.0 (3.5–>8.1) | <0.01 |
| HBV DNA (≥7.3 log IU/ml) | 71 (32.0%) | 48 (53.3%) | 23 (17.4%) | <0.01 |
| HBsAg (log IU/ml) | 3.41 (0.96–5.88) | 3.95 (0.96–5.88) | 3.36 (0.96–5.50) | <0.01 |
| HBcrAg (log kU/ml) | 2.89 (0.00–7.07) | 4.57 (0.00–7.07) | 2.09 (0.00–6.00) | <0.01 |
| Percentage of patients with undetectable HBcrAg | 7.5% (16/214) | 1.1% (1/87) | 11.8% (15/127) | <0.01 |
| Albumin (g/l) | 42 (22–50) | 42 (22–48) | 42 (22–50) | 0.97 |
| Bilirubin (μmol/l) | 13 (2–216) | 13 (2–67) | 13 (4–216) | 0.68 |
| ALT (U/l) | 92 (17–2,168) | 100 (27–2,144) | 81 (17–2,168) | 0.25 |
| Number of patients with elevated ALT level | 181 (81.5%) | 76 (84.4%) | 105 (79.5%) | 0.36 |
Abbreviations: ALT, alanine aminotransferase; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV DNA, hepatitis B virus DNA.
Continuous variables were expressed as median (range).
P values are for the comparisons between HBeAg-positive and -negative patients.
Table 2. Details of patients who had discontinued entecavir.
| Reason | Number of patients |
|---|---|
| Unwilling to commit to long-term treatment or had financial difficulty | 49 |
| Genotypic resistance to entecavir | 2 |
| Virologic breakthrougha | 1 |
| Switch to alternative anti-virals because of pregnancy | 3 |
| HBsAg seroconversionb | 1 |
| Deathc | 5 |
| Total | 61 |
Abbreviations: HBsAg, hepatitis B surface antigen; HBV DNA, hepatitis B virus DNA.
Virologic breakthrough without genotypic resistance.
Patient who had HBsAg seroconversion and undetectable HBV DNA can opt to stop anti-viral after discussion with physician.
Two patients died of hepatocellular carcinoma, 1 died of decompensated cirrhosis, and 2 died of diseases unrelated to chronic hepatitis B infection (carcinoma of lung and subarachnoid hemorrhage, respectively).
At baseline, 90 (40.5%) patients were HBeAg positive and 181 (81.5%) had elevated ALT. Seventy-one (32.0%) patients had high HBV DNA at baseline, which was defined as HBV DNA level of ≥7.3 log IU/ml (≥108 copies/ml) in our previous study.2 Sixteen patients (7.5%) had undetectable HBcrAg levels (≤1 kU/ml) at baseline. Two patients (0.9%) had lamivudine resistance, whereas no patient had entecavir resistance at baseline.
At baseline, there were good correlations between HBcrAg and HBV DNA (r=0.552, P<0.001), HBcrAg and HBsAg (r=0.590, P<0.001), and HBsAg and HBV DNA (r=0.552, P<0.001).
Virological suppression, ALT normalization, and HBeAg seroconversion
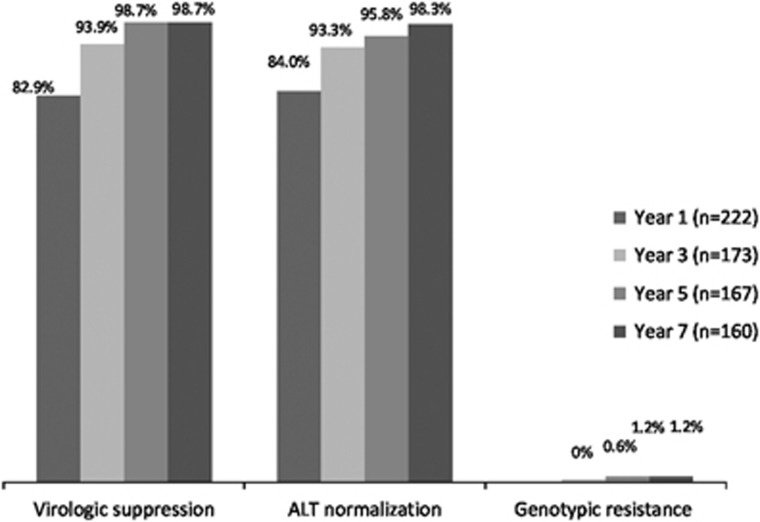
The rates of undetectable HBV DNA and ALT normalization were depicted in Figure 1. The rate of undetectable HBV DNA increased from 82.9% (95% CI: 75.2–85.6) at first year to 98.7% (95% CI: 95.0–99.7) at the seventh year (96.8% for HBeAg-positive patients, 100% for HBeAg-negative patients). There was a more rapid decline of HBV DNA in the group with high baseline HBV DNA, as compared with the group with low baseline HBV DNA (−0.965 vs. −0.646 log IU/ml/year, P<0.001). The 7-year rate of HBeAg seroconversion among HBeAg-positive patients (n=90) was 82.1% (95% CI: 69.1–89.6) and that of ALT normalization among those with elevated ALT at baseline (n=181) was 98.3% (95% CI: 93.7–99.5) (96.7% for HBeAg-positive patients, 100% for HBeAg-negative patients).
Figure 1.
Rates of virologic suppression, alanine aminotransferase (ALT) normalization, and genotypic resistance up to year 7.
Virological breakthrough and resistance profile
A total of seven patients had virological breakthroughs over 7 years of follow-up. The virological breakthrough rate was 4.0% (95% CI: 1.1–6.9) (8.8% for HBeAg-positive patients, 1.0% for HBeAg-negative patients). Among these seven patients, only two patients had genotypic resistance to entecavir at year 3 and year 4, respectively. Both patients were HBeAg-positive at baseline. The characteristics of these two patients were shown in our previous paper.2 The rate of genotypic resistance to entecavir up to the seventh year was 1.2% (95% CI: 0–2.8) (3.0% for HBeAg-positive patients, 0.0% for HBeAg-negative patients). The remaining patients were found to be non-compliant to entecavir treatment.
HBsAg kinetics
Of the 222 patients, 160 patients completed 7 years of follow-up. One hundred and forty-six patients had HBsAg level measured every year. There was a modest decline of HBsAg level over 7 years with an annual rate of −0.107 log IU/ml/year (range: −0.991 to 0.225) (P<0.001).
The median annual decline of HBsAg level was higher in male patients compared with female patients (−0.115 vs. −0.075 log IU/ml/year, P=0.030). The median annual reduction of HBsAg level was higher in those with higher baseline HBV DNA (n=46), compared with those with low baseline HBV DNA (n=100). (−0.132 vs. −0.096 log IU/ml/year, P=0.017). The median annual reduction of HBsAg level was not statistically different among those with baseline-positive HBeAg status and -negative HBeAg status. (−0.116 vs. −0.105 log IU/ml/year, P=0.085).
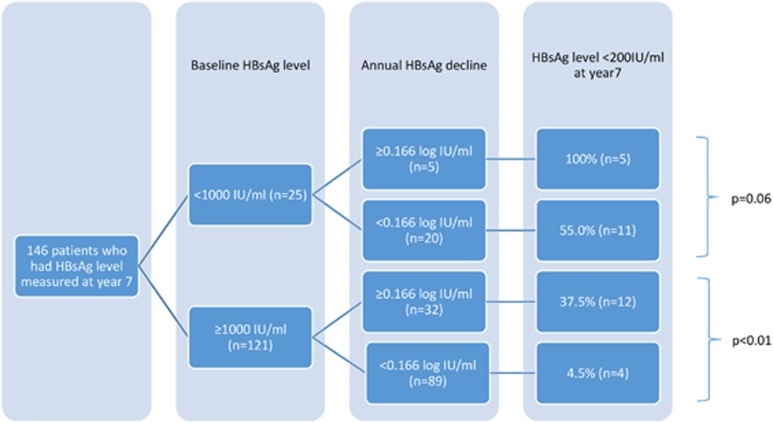
As mentioned in our previous paper,2 one patient had HBsAg seroclearance at the second year of follow-up. For this patient, entecavir was stopped at the fourth year and his data were censored afterwards. Three other patients developed HBsAg seroclearance at the sixth year (n=1) and seventh year (n=2) of follow-up. All four patients did not have cirrhosis at baseline. The cumulative rate of HBsAg seroclearance was 2.5%. Their characteristics were shown in Table 3. Thirty-two patients (21.9%) had HBsAg level of <200 IU/ml (or 2.3 log IU/ml), a level highly predictive of HBsAg seroclearance in subsequent 3 years according to our previous study.27 In addition, another study showed that low baseline HBsAg (<1,000 IU/ml) and a higher rate of on-treatment HBsAg reduction (≥0.166 log IU/ml/year) were predictive of subsequent HBsAg seroclearance.21 The chances of achieving HBsAg level of <200 IU/ml after 7 years with respect to these two parameters are depicted in Figure 2. All patients with baseline HBsAg <1,000 IU/ml and on-treatment HBsAg reduction of ≥0.166 log IU/ml/year had HBsAg of <200 IU/ml at year 7. For patients who had baseline HBsAg ≥1,000 IU/ml and on-treatment HBsAg reduction of <0.166 log IU/ml/year, 95.5% of them had HBsAg ≥200 IU/ml at year 7.
Table 3. Characteristics of the four patients with HBsAg seroclearance.
| Patient | Sex/age | ALT (U/l) |
HBeAg |
HBsAg (log IU/ml) |
HBcrAg (log kU/ml) |
HBV DNA (log IU/ml) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Baseline | Time of HBeAg seroconversion | Baseline | Annual decline | Time of HBsAg seroclearance | Baseline | Time of undetectable HBcrAg | Baseline | Time of undetectable HBV DNA | ||
| 1 | M/46 | 44 | Neg | — | 1.83 | 1.57 | 2nd year | 0.30 | NA | 4.59 | 1st year |
| 2 | M/29 | 622 | Pos | 3rd year | 5.64 | 0.99 | 7th year | 5.93 | 7th year | 8.11 | 1st year |
| 3 | M/51 | 37 | Neg | — | 2.11 | 0.57 | 6th year | 0.51 | 5th year | 4.12 | 1st year |
| 4 | M/45 | 128 | Neg | — | 2.76 | 0.58 | 7th year | NA | NA | 4.90 | 1st year |
Abbreviations: ALT, alanine aminotransferase; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV DNA, hepatitis B virus DNA; NA, not available; Neg, negative; Pos, positive; UD, undetectable.
Figure 2.
Number of patients who had hepatitis B surface antigen (HBsAg) level <200 IU/ml at year 7 as stratified by baseline HBsAg level and on-treatment annual decline of HBsAg.
HBcrAg kinetics
There was a significant decline of HBcrAg level with treatment, with an annual change of −0.244 log kU/ml/year (range: −0.847 to 0.183, P<0.001). The median HBcrAg level was 2.893, 1.914, 0.895, and 0.681 log kU/ml at baseline (n=214), first year (n=186), fifth year (n=156), and seventh year of follow-up (n=147), respectively.
HBcrAg level decreased more rapidly at the first year of follow-up compared with the reduction in subsequent years (−0.815 vs. −0.117 log kU/ml/year, P<0.001).
The median annual decline of HBcrAg level over 7 years was higher for patients positive for HBeAg at baseline compared with HBeAg-negative patients (−0.428 vs. −0.151 log kU/ml/year, P<0.001). The median annual rate of reduction of HBcrAg was higher in the patients with high baseline HBV DNA than those with low level of HBV DNA at baseline (−0.435 vs. −0.165 log kU/ml/year, P<0.001).
Among those who had HBcrAg measured, 11.3% (21 patients), 25.0% (39 patients), and 32.0% (47 patients) of patients had undetectable HBcrAg at the first, fifth, and seventh year of follow-up, respectively. Among those who had undetectable HBcrAg at the seventh year, 43 patients (91.5%) were HBeAg negative at baseline. While most of the patients (97.8%) with undetectable HBcrAg at the seventh year had undetectable HBV DNA, only 31.7% patients (n=46) had undetectable HBcrAg among those who had HBcrAg measured and undetectable HBV DNA at the seventh year (n=145). Negative baseline HBeAg status, low baseline HBcrAg level (<2 log kU/ml), and low baseline HBsAg level (<1,000 IU/ml) were associated with undetectable HBcrAg at year 7 on multivariate analysis (P=0.011, P=0.007 and P=0.013, respectively) (Table 4a). For HBeAg-negative patients, low baseline HBcrAg level (<2 log kU/ml) and low baseline HBsAg level (<1,000 IU/ml) were predictive of undetectable HBcrAg at year 7 (P=0.007 and P=0.022, respectively; Table 4b). Same statistical analyses were not performed in HBeAg-positive patients because of the limited number of patients (n=4) achieving undetectable HBcrAg at year 7.
Table 4a. Factors associated with undetectable HBcrAg at the seventh year in all patients: univariate and mulitvariate analysis.
| Undetectable HBcrAg (n=47) | Detectable HBcrAg (n=100) | P value (univaraite) | OR (95% CI) | P value (multivariate) | |
|---|---|---|---|---|---|
| Age (years (mean, 95% CI)) | 50.9 (28.7–70.8) | 46.9 (21.0–66.8) | 0.004 | 1.004 (0.933–1.081)a | 0.906 |
| Sex (M, %) | 37 (78.7%) | 70 (70.0%) | 0.268 | ||
| Raised ALT at baseline (>50 IU/ml) | 38 (80.9%) | 82 (82.0%) | 0.867 | ||
| Negative HBeAg at baseline | 43 (91.5%) | 50 (50.0%) | <0.001 | 11.47 (1.75–75.29) | 0.011 |
| Baseline HBV DNA <7.8 log IU/ml | 43 (91.5%) | 58 (58.0%) | <0.001 | 0.62 (0.12–3.15) | 0.561 |
| Baseline HBsAg <1,000 IU/ml | 20 (42.6%) | 4 (4.0%) | <0.001 | 10.72 (1.65–69.53) | 0.013 |
| Baseline HBcrAg <2 log KU/ml | 24 (51.1%) | 9 (9.0%) | <0.001 | 5.59 (1.60–19.49) | 0.007 |
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV DNA, hepatitis B virus DNA.
Per unit increase in age.
Table 4b. Factors associated with undetectable HBcrAg at the seventh year in HBeAg-negative patients: univariate and mulitvariate analysis.
| Undetectable HBcrAg (n=43) | Detectable HBcrAg (n=50) | P value (univaraite) | OR (95% CI) | P value (multivariate) | |
|---|---|---|---|---|---|
| Age (years (mean, 95% CI)) | 51.4 (48.8–54.0) | 50.0 (47.6–52.3) | 0.415 | ||
| Sex (M, %) | 33 (76.7%) | 33 (66.0%) | 0.255 | ||
| Raised ALT at baseline (>50 IU/ml) | 34 (79.1%) | 38 (76.0%) | 0.724 | ||
| Baseline HBV DNA <7.8 log IU/ml | 41 (95.3%) | 37 (74.0%) | 0.005 | 1.16 (0.16–8.71) | 0.884 |
| Baseline HBsAg <1,000 IU/ml | 20 (46.5%) | 3 (6.0%) | <0.001 | 13.58 (1.47–125.68) | 0.022 |
| Baseline HBcrAg <2 log KU/ml | 24 (55.8%) | 6 (12.0%) | <0.001 | 6.39 (1.66–24.63) | 0.007 |
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV DNA, hepatitis B virus DNA.
Safety and clinical complications
No patient had entecavir stopped because of side effects. Two patients were switched to tenofovir because of documented genotypic resistance to entecavir. Fourteen patients developed HCC. Among these patients, six of them had cirrhosis at baseline. The median time of development of HCC after initiation of entecavir therapy was 47 months (range: 13–84 months). Three patients underwent liver transplantation, with two because of HCC and one because of decompensated cirrhosis.
Discussion
Our real-life cohort showed that entecavir is a potent nucleoside analog. The frequency of viral suppression (with HBV DNA of ≤20 IU/ml), HBeAg seroconversion and ALT normalization at the seventh year were high: 98.7%, 82.1% and 98.3%, respectively. Genotypic resistance was only 1.2% at year 7. Entecavir is a very effective treatment for CHB with regard to these conventional parameters. Together with the excellent safety profile, entecavir should remain one of the first-line agents for long-term treatment of CHB.
There was a significant decline of HBcrAg level with treatment, and the median rate of decline of HBcrAg was 0.244 log kU/ml/year. In contrast to the excellent suppression of serum HBV DNA, only 32.0% of patients had undetectable HBcrAg at the end of follow-up. Entecavir, a nucleoside analog, inhibits the HBV reverse transcriptase, thus leading to reduction in the production of HBV DNA-containing virion.28 However, it has no direct effect on the transcriptional activity of viral messenger RNA from the cccDNA. Therefore, there would be continued production of viral proteins, e.g., hepatitis B core antigen and HBeAg for a longer period. HBcrAg would decrease only when the transcriptional activity become lower with natural cell death of infected hepatocytes and reduction of infection of new uninfected hepatocytes.
The present study identified several baseline factors, which are predictive of undetectable HBcrAg at 7 years. By multivariate analysis, baseline HBeAg negativity (OR 11.47, P=0.011), HBcrAg level <2 log kU/ml (OR 5.59, P=0.007), and baseline HBsAg <1,000 IU/ml (OR 10.72, P=0.013) were predictive of undetectable HBcrAg at the seventh year. These predictive factors may assist the selection of patients with better outcome and provide guidance for clinical management of CHB. The reasons are twofold. First, it has been shown that serum HBcrAg level has a high correlation with intrahepatic cccDNA level,8 a reservoir for viral replication. Patients achieving undetectable HBcrAg under entecavir treatment may indicate low cccDNA levels in their livers. This is according to the findings of previous studies showing that low serum level of HBcrAg at the time of cessation of lamivudine is associated with a lower chance of reactivation of hepatitis after cessation of lamivudine therapy.13, 14 The 47 patients (32.0%) who had undetectable HBcrAg level at the end of follow-up in the present study could represent a group of patients with a lower chance of reactivation after cessation of entecavir therapy. Second, our group have recently shown that HBcrAg level, but not HBsAg level, was associated with the chance of development of HCC in patients with undetectable serum HBV DNA under nucleos(t)ide analog treatment.12 Therefore, the patients in the present study might have an even lower risk of development of HCC while on anti-viral therapy. It was unfortunate that we could not verify the roles of HBcrAg and HBsAg levels on the development of HCC in the present study because only limited number of patients (n=14) developed HCC. Therefore, it definitely requires future studies to verify this hypothesis.
The HBsAg seroclearance rate was low (2.5%) at the seventh year. This is because entecavir has minimal effect on transcription and translation of HBsAg from cccDNA and secretion of empty HBsAg to the circulation.24 HBsAg may also be produced from integrated HBV DNA in the host. Previous studies showed that HBsAg of <200 IU/ml was predictive of subsequent HBsAg seroclearance in 3 years. For patients on nucleoside analog, baseline HBsAg of <1,000 IU/ml and on-treatment HBsAg decline of ≥0.166 log IU/ml/year were also predictive of subsequent HBsAg seroclearance.21, 27 In our cohort, baseline HBsAg level and the annual decline of HBsAg could predict the chance of having HBsAg of <200 IU/ml at the seventh year (Figure 2). The majority of patients (95.5%) with baseline HBsAg of ≥1,000 IU/ml and decline of HBsAg of <0.166 log IU/ml/year had HBsAg level of ≥200 IU/ml at the seventh year of entecavir treatment. These data should be interpreted with a remark of possible limitation of “reverse analysis” on the outcome, i.e., HBsAg <200 IU/ml at year 7. Nevertheless, additional agents with knockdown effects on HBsAg level, e.g., small interfering RNA (ARC-520)29 could be more indicative for this group of patient to enhance HBsAg seroclearance.
One of the limitations of our study is the lack of HBV genotype data. Most Chinese CHB patients have genotype B or C infection. Previous studies showed that genotype is not a major determinant of HBsAg kinetics21 and that HBcrAg kinetics is similar in genotype B and C patients.30
In conclusion, long-term entecavir therapy achieved effective viral suppression with low drug resistance rate. However, the decline of HBsAg with treatment was still slow. HBcrAg (translational products of the core and e mRNA) level declined at a relatively better rate. Baseline HBsAg level of 1,000 IU/ml and annual decline of 0.166 log IU/ml could be used to predict HBsAg response on entecavir treatment.
Study Highlights
Acknowledgments
We thank John Young and other laboratory staff for technical assistance. We also thank all the patients who participated in the study.
Footnotes
Guarantor of the article: Man-Fung Yuen.
Specific author contributions: Y.F. Lam was involved in study concept and design, acquisition of data, analysis and interpretation of data, and drafting of manuscript. W.-K. Seto and J. Fung were involved in interpretation of data and critical revision of manuscript. D. Wong was involved in study design, performing laboratory tests, and acquisition of data. K.S. Cheung was involved in data analysis. L.Y. Mak was involved in acquisition and interpretation of data. J. Yuen and C.K. Chong were involved in performing laboratory tests. C.L. Lai was involved in study concept and design and critical revision of manuscript. M.-F. Yuen was involved in study concept and design, analysis and interpretation of data, critical revision of manuscript, and overall study supervision.
Financial support: This study was supported by an unrestricted grant from Bristol-Myers Squibb. Serum HBsAg measurements were supported by the S.K. Yee Medical Foundation. Serum HBcrAg measurements were supported by Fujirebio.
Potential competing interests: W.-K. Seto is an advisory board member and received speaker fees from Bristol-Myers Squibb. C.L. Lai received speaker fees from Bristol-Myers Squibb. M.-F. Yuen is an advisory board member and received speaker fees from Bristol-Myers Squibb. He also received research funding from Roche Diagnostics and Bristol-Myers Squibb. The remaining authors have no conflict of interest.
References
- Yuen MF, Ahn SH, Chen DS et al. Chronic hepatitis B virus infection: disease revisit and management recommendations. J Clin Gastroenterol 2016; 50: 286–294. [DOI] [PubMed] [Google Scholar]
- Seto WK, Lam YF, Fung J et al. Changes of HBsAg and HBV DNA levels in Chinese chronic hepatitis B patients after 5 years of entecavir treatment. J Gastroenterol Hepatol 2014; 29: 1028–1034. [DOI] [PubMed] [Google Scholar]
- Chang TT, Liaw YF, Wu SS et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010; 52: 886–893. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Tokumoto Y, Joko K et al. Effects of long-term entecavir treatment on the incidence of hepatocellular carcinoma in chronic hepatitis B patients. Hepatol Int 2016; 10: 320–327. [DOI] [PubMed] [Google Scholar]
- Arends P, Sonneveld MJ, Zoutendijk R et al. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: limited role for risk scores in Caucasians. Gut 2015; 64: 1289–1295. [DOI] [PubMed] [Google Scholar]
- Seto WK, Wong DK, Fung J et al. Linearized hepatitis B surface antigen and hepatitis B core-related antigen in the natural history of chronic hepatitis B. Clin Microbiol Infect 2014; 20: 1173–1180. [DOI] [PubMed] [Google Scholar]
- Kimura T, Rokuhara A, Sakamoto Y et al. Sensitive enzyme immunoassay for hepatitis B virus core-related antigens and their correlation to virus load. J Clin Microbiol 2002; 40: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DK, Tanaka Y, Lai CL et al. Hepatitis B virus core-related antigens as markers for monitoring chronic hepatitis B infection. J Clin Microbiol 2007; 45: 3942–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki F, Miyakoshi H, Kobayashi M et al. Correlation between serum hepatitis B virus core-related antigen and intrahepatic covalently closed circular DNA in chronic hepatitis B patients. J Med Virol 2009; 81: 27–33. [DOI] [PubMed] [Google Scholar]
- Rokuhara A, Tanaka E, Matsumoto A et al. Clinical evaluation of a new enzyme immunoassay for hepatitis B virus core-related antigen; a marker distinct from viral DNA for monitoring lamivudine treatment. J Viral Hepat 2003; 10: 324–330. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Matsumoto A, Yoshizawa K et al. Hepatitis B core-related antigen assay is useful for monitoring the antiviral effects of nucleoside analogue therapy. Intervirology 2008; 51 (Suppl 1): 3–6. [DOI] [PubMed] [Google Scholar]
- Cheung KS, Seto WK, Wong D et al. Relationship between hepatocellular carcinoma development and serum viral markers in chronic hepatitis B patients who achieved undetectable serum HBV DNA while on long-term nucleoside analogue therapy. Hepatology 2015;62 (Suppl 1): 273A AASLD abstract. [Google Scholar]
- Matsumoto A, Tanaka E, Minami M et al. Low serum level of hepatitis B core-related antigen indicates unlikely reactivation of hepatitis after cessation of lamivudine therapy. Hepatol Res 2007; 37: 661–666. [DOI] [PubMed] [Google Scholar]
- Shinkai N, Tanaka Y, Orito E et al. Measurement of hepatitis B virus core-related antigen as predicting factor for relapse after cessation of lamivudine therapy for chronic hepatitis B virus infection. Hepatol Res 2006; 36: 272–276. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Matsumoto A, Suzuki F et al. Measurement of hepatitis B virus core-related antigen is valuable for identifying patients who are at low risk of lamivudine resistance. Liver Int 2006; 26: 90–96. [DOI] [PubMed] [Google Scholar]
- Hosaka T, Suzuki F, Kobayashi M et al. HBcrAg is a predictor of post-treatment recurrence of hepatocellular carcinoma during antiviral therapy. Liver Int 2010; 30: 1461–1470. [DOI] [PubMed] [Google Scholar]
- Chan HL, Thompson A, Martinot-Peignoux M et al. Hepatitis B surface antigen quantification: why and how to use it in 2011—a core group report. J Hepatol 2011 55: 1121–1131. [DOI] [PubMed] [Google Scholar]
- Tseng TC, Liu CJ, Yang HC et al. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology 2013; 57: 441–450. [DOI] [PubMed] [Google Scholar]
- Tseng TC, Liu CJ, Yang HC et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology 2012; 142: 1140–1149.e3; quiz e13–14. [DOI] [PubMed] [Google Scholar]
- Fung J, Wong DK, Seto WK et al. Hepatitis B surface antigen seroclearance: relationship to hepatitis B e-antigen seroclearance and hepatitis B e-antigen-negative hepatitis. Am J Gastroenterol 2014; 109: 1764–1770. [DOI] [PubMed] [Google Scholar]
- Seto WK, Wong DK, Fung J et al. Reduction of hepatitis B surface antigen levels and hepatitis B surface antigen seroclearance in chronic hepatitis B patients receiving 10 years of nucleoside analogue therapy. Hepatology 2013; 58: 923–931. [DOI] [PubMed] [Google Scholar]
- Yuen MF, Wong DK, Fung J et al. HBsAg seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology 2008; 135: 1192–1199. [DOI] [PubMed] [Google Scholar]
- Kim GA, Lim YS, An J et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut 2014; 63: 1325–1332. [DOI] [PubMed] [Google Scholar]
- Wong DK, Seto WK, Fung J et al. Reduction of hepatitis B surface antigen and covalently closed circular DNA by nucleos(t)ide analogues of different potency. Clin Gastroenterol Hepatol 2013; 11: 1004–10 e1. [DOI] [PubMed] [Google Scholar]
- Yuen MF, Seto WK, Fung J et al. Three years of continuous entecavir therapy in treatment-naive chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol 2011; 106: 1264–1271. [DOI] [PubMed] [Google Scholar]
- Pawlotsky JM, Dusheiko G, Hatzakis A et al. Virologic monitoring of hepatitis B virus therapy in clinical trials and practice: recommendations for a standardized approach. Gastroenterology 2008; 134: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto WK, Wong DK, Fung J et al. A large case–control study on the predictability of hepatitis B surface antigen levels three years before hepatitis B surface antigen seroclearance. Hepatology 2012; 56: 812–819. [DOI] [PubMed] [Google Scholar]
- Marion PL, Salazar FH, Winters MA et al. Potent efficacy of entecavir (BMS-200475) in a duck model of hepatitis B virus replication. Antimicrob Agents Chemother 2002; 46: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen MF, Chan HLY, Liu SHK et al. ARC-520 produces deep and durable knockdown of viral antigens and DNA in a phase II study in patients with chronic hepatitis B. Hepatology 2015;62 (Suppl 6): 1385A AASLD abstract. [Google Scholar]
- Rokuhara A, Sun X, Tanaka E et al. Hepatitis B virus core and core-related antigen quantitation in Chinese patients with chronic genotype B and C hepatitis B virus infection. J Gastroenterol Hepatol 2005; 20: 1726–1730. [DOI] [PubMed] [Google Scholar]