ABSTRACT
RNA stable isotope probing and high-throughput sequencing were used to characterize the active microbiomes of bacteria and fungi colonizing the roots and rhizosphere soil of oilseed rape to identify taxa assimilating plant-derived carbon following 13CO2 labeling. Root- and rhizosphere soil-associated communities of both bacteria and fungi differed from each other, and there were highly significant differences between their DNA- and RNA-based community profiles. Verrucomicrobia, Proteobacteria, Planctomycetes, Acidobacteria, Gemmatimonadetes, Actinobacteria, and Chloroflexi were the most active bacterial phyla in the rhizosphere soil. Bacteroidetes were more active in roots. The most abundant bacterial genera were well represented in both the 13C- and 12C-RNA fractions, while the fungal taxa were more differentiated. Streptomyces, Rhizobium, and Flavobacterium were dominant in roots, whereas Rhodoplanes and Sphingomonas (Kaistobacter) were dominant in rhizosphere soil. “Candidatus Nitrososphaera” was enriched in 13C in rhizosphere soil. Olpidium and Dendryphion were abundant in the 12C-RNA fraction of roots; Clonostachys was abundant in both roots and rhizosphere soil and heavily 13C enriched. Cryptococcus was dominant in rhizosphere soil and less abundant, but was 13C enriched in roots. The patterns of colonization and C acquisition revealed in this study assist in identifying microbial taxa that may be superior competitors for plant-derived carbon in the rhizosphere of Brassica napus.
IMPORTANCE This microbiome study characterizes the active bacteria and fungi colonizing the roots and rhizosphere soil of Brassica napus using high-throughput sequencing and RNA-stable isotope probing. It identifies taxa assimilating plant-derived carbon following 13CO2 labeling and compares these with other less active groups not incorporating a plant assimilate. Brassica napus is an economically and globally important oilseed crop, cultivated for edible oil, biofuel production, and phytoextraction of heavy metals; however, it is susceptible to several diseases. The identification of the fungal and bacterial species successfully competing for plant-derived carbon, enabling them to colonize the roots and rhizosphere soil of this plant, should enable the identification of microorganisms that can be evaluated in more detailed functional studies and ultimately be used to improve plant health and productivity in sustainable agriculture.
KEYWORDS: Brassica napus, bacteria, carbon allocation, fungi, high-throughput sequencing, rhizosphere microbiome, root microbiome
INTRODUCTION
The rhizosphere is an active interface in which plants and microorganisms establish a complex and varied molecular dialogue, involving nutrient transfer as well as specific interactions mediated by the release of signaling molecules from plant roots (1, 2) and resulting in enhanced plant productivity (3). Between 20% and 50% of photoassimilated carbon is transferred to the roots, and half of this is subsequently released into the soil (4). These exudates affect soil microbial community structure and activity, resulting in the “rhizosphere effect,” i.e., elevated numbers of microorganisms (5, 6). These microbes can either help the plant to acquire nutrients from the soil or provide indirect pathogen protection. Therefore, rhizosphere competence implies that plant growth-promoting bacteria are well adapted to utilize carbon resources (7).
Numerous studies performed using the model plant Arabidopsis thaliana, a member of the Brassicaceae family, have revealed that both soil type and, to a lesser extent, host genotype shape the profiles of root microbiota and that communities associated with the rhizosphere differ significantly from those of the endophytic root compartment (8–10) as well as from those present in the surrounding bulk soil (11). Additionally, the structure of the root microbiomes in Arabidopsis spp. and other related species appear to be highly conserved (12) and similar between the monocotyledonous crop barley and the dicotyledonous Arabidopsis, despite the existence of some host-specific microbiota (11).
One of the most promising techniques of identifying microorganisms that consume recently fixed plant carbon is stable isotope probing (SIP) (13–16). The technique relies on the incorporation of a stable isotope into nucleic acids from a labeled substrate; thus, microbes that incorporate plant carbon into their biomass become enriched (17). One of the limitations of SIP is the requirement of adding 13C-labeled substrate in large amounts, resulting in an elevated in situ availability of carbon and thus a potential divergence between experimental and actual conditions. Long incubation times also potentially lead to nonspecific labeling. One way to minimize such side effects is to apply highly sensitive RNA-SIP and reduce the labeling to a rather short period, depending on the experimental system and the plant species used (14). On the other hand, short incubation times may introduce bias against microorganisms with low growth rates, thus leading to incomplete labeling of the community (18).
Studies tracking metabolically active rhizospheric populations have been published for both bacteria and fungi and in a variety of plant species (19–23). The incorporation of photosynthesized 13C into the biomass of soil microbes occurs rather rapidly (<24 h), with maximum incorporation into microbial RNA after 4 to 8 days (24). SIP-based experiments indicate that fungi are important organotrophic organisms in the rhizosphere, receiving considerable amounts of plant-derived carbon (25), and that they can respond rapidly to easily degradable substrates in soil (26, 27).
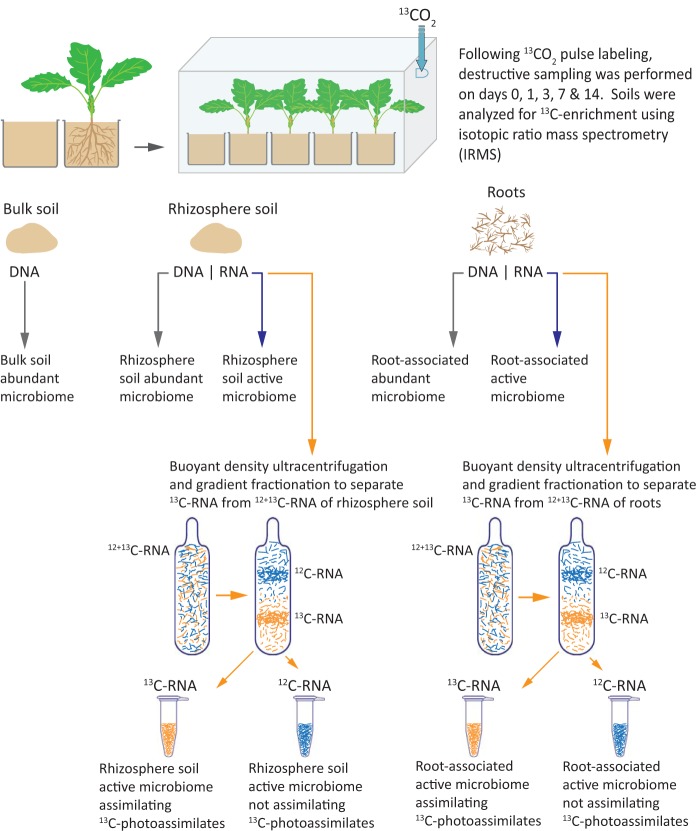
Oilseed rape is a globally important oil crop cultivated for edible oil, biofuel production, and phytoextraction of heavy metals; however, it is susceptible to numerous diseases (28). The rhizospheric environment of this crop has been studied for its potential to harbor biocontrol bacteria (such as Serratia proteamaculans, S. plymuthica, Pseudomonas chlororaphis, P. acidovorans, and P. putida) that can protect the plant against fungal pathogens (29, 30). The aims of the present study were to characterize and compare the structure and composition of the root and rhizosphere bacterial and fungal communities of oilseed rape plants and to identify the microbial groups capable of competing for recently fixed carbon and compare these with other less active groups not incorporating a plant assimilate. This was done by labeling the plants with 13CO2 and applying RNA-SIP. The experimental approach is summarized in Fig. 1.
FIG 1.
Schematic representation of the experimental approach used for identifying the active microbiome associated with roots and rhizosphere soil of oilseed rape. Brassica napus seedlings were grown in pots containing organically managed soil and subjected to 13CO2 pulse labeling after 4 weeks growth. Systems were harvested destructively on days 0, 1, 3, 7, and 14, and soils were analyzed for 13C enrichment to determine the stage at which the maximum labeled carbon was allocated to soil through rhizodeposition. Subsequently, the rhizosphere soil and root samples from that time point were used for coextraction of DNA and RNA for analyses of abundant and active bacterial and fungal microbiomes using high-throughput sequencing. 12 + 13C-RNA was subjected to density gradient ultracentrifugation to separate 13C-RNA and 12C-RNA fractions that were used to characterize the active bacterial and fungal microbiomes assimilating recent 13C-labeled photoassimilates of plants.
RESULTS
13C enrichment in rhizosphere soil.
The overall isotopic signatures of δ13C demonstrated that the rhizosphere soil was significantly (P < 0.05) enriched in 13C from day 1 postlabeling (see Fig. S1 in the supplemental material). The maximum enrichment was observed at days 3 and 7 postlabeling; but, to focus on the primary consumers of current photosynthates, all further analyses were based on samples from day 3 postlabeling.
Overall structures of bacterial and fungal communities associated with rhizosphere soil and roots.
In total, 325,992 bacterial and 350,798 fungal reads were obtained from 454 pyrosequencing of all samples. Following denoising and removal of chimeric sequences, 139,074 bacterial sequences remained. For the fungal data, after demultiplexing and implementing the quality filtering steps in the split_libraries.py command in QIIME, 123,804 sequences remained.
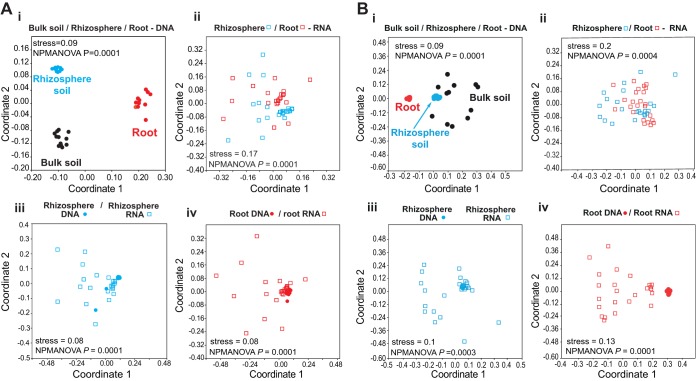
Nonmetric multidimensional scaling (NMDS) ordinations and nonparametric multivariate analysis of variance (NPMANOVA) for bacteria (Fig. 2A) and for fungi (Fig. 2B) revealed significant differences between DNA-based communities in bulk (plant-free) soil, rhizosphere soil, and roots (Fig. 2Ai and Bi). The active communities colonizing rhizosphere soil and roots (Fig. 2Aii and Bii) were also significantly different. The DNA- and RNA-based communities in rhizosphere soil (Fig. 2Aiii and Biii) and in roots (Fig. 2Aiv and Biv) also differed from each other significantly. For bacteria, both the 13C-RNA and the 12C-RNA fractions from rhizosphere soil and roots exhibited similar diversity patterns (data not shown). In contrast, for fungi, the 13C-RNA rhizosphere soil and root fractions exhibited unexpectedly greater diversity compared to the respective 12C-RNA fractions, probably due to the fact that there was high competition for recently fixed carbon from the plant (data not shown). In the 12C-RNA fractions, some dominant taxa were observed, implying that they do not depend highly on recently fixed C, but most probably live on dead cells or cell walls.
FIG 2.
Nonmetric multidimensional scaling ordinations (NMDS) of changes in bacterial (A) and fungal (B) community structures associated with bulk soil DNA, rhizosphere soil DNA, and root DNA (i), rhizosphere soil RNA and root RNA (ii), rhizosphere soil RNA and rhizosphere soil DNA (iii), and root RNA and root DNA (iv).
Abundant and active bacteria in the rhizosphere and root compartments.
The total numbers of operational taxonomic units (OTUs) are shown in Fig. S2A. In general, similar numbers of OTUs were retrieved for all soil samples (rhizosphere DNA, rhizosphere RNA, and bulk soil DNA). In the roots, the number of OTUs from RNA was almost double that retrieved from DNA; however, there were no differences among the major taxa.
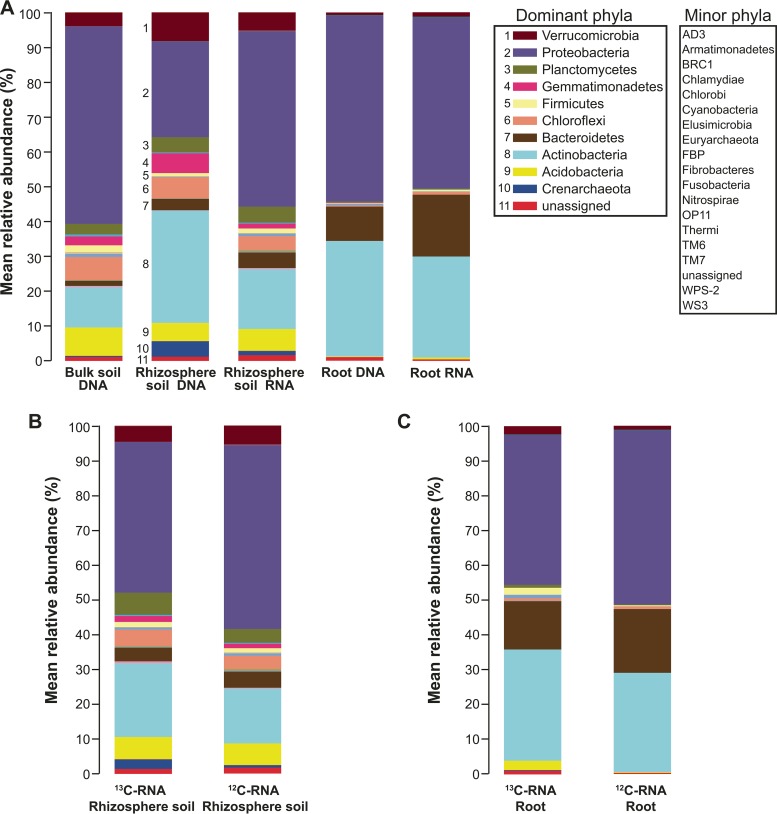
In total, 29 bacterial and two archaeal phyla were identified (Fig. 3A). The relative abundances of Proteobacteria were almost equally high in all communities but not in rhizosphere DNA, implying that Proteobacteria are proportionally more strongly represented among active bacteria in the rhizosphere than those that were simply present. The relative abundance and activity of Verrucomicrobia were much higher in soil samples compared to those in root samples. On the other hand, the relative abundance of Bacteroidetes was higher in root samples compared to that in soil samples, and they were proportionally more abundant in the active community in roots. Actinobacteria DNA was highly abundant in both the rhizosphere soil and the roots, but the relative activity was much greater in the roots than in the rhizosphere. The abundance of Actinobacteria was lowest in the bulk soil DNA-based bacterial community. Acidobacteria were more abundant in plant-free (bulk) and rhizosphere soil samples but more infrequent in root samples (<1%). Chloroflexi and Planctomycetes followed the same trend, being both more abundant and active in rhizosphere soil samples than those associated with roots (Fig. 3A).
FIG 3.
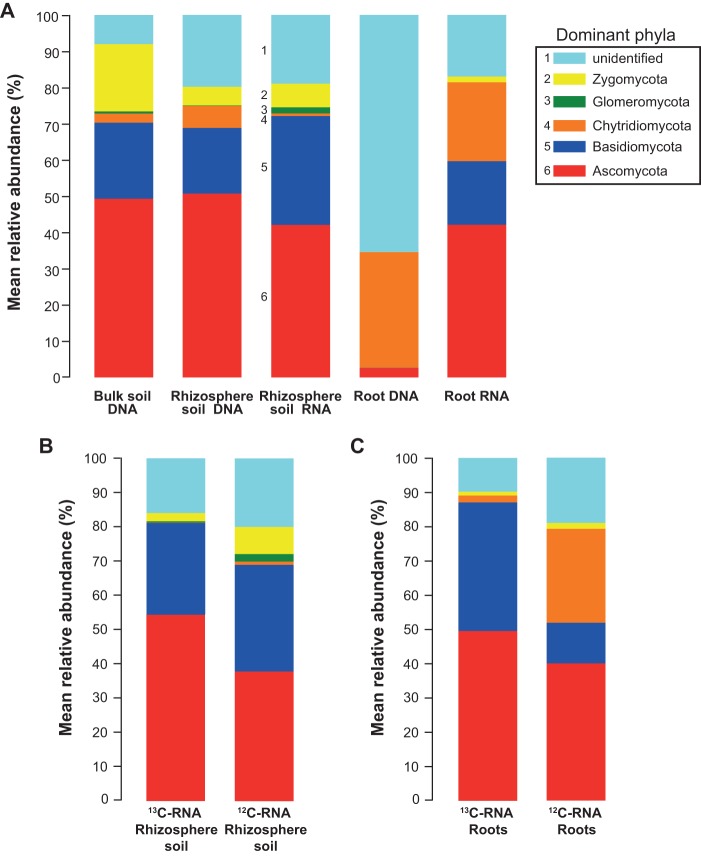
Mean relative abundances of different bacterial phyla in bulk soil DNA, rhizosphere soil DNA and RNA, and root DNA and RNA (A), 13C-RNA- and 12C-RNA in rhizosphere soil (B), and 13C-RNA- and 12C-RNA in roots (C). Taxonomic classification of 16S rRNA gene sequences was performed in QIIME using the Greengenes 16S rRNA reference taxonomy. Colors for dominant phyla that are easily distinguishable are shown in a separate legend, supplemented with a numerical key. Minor phyla are simply listed.
Active bacterial communities assimilating plant-derived carbon in the rhizosphere and root compartments.
In the rhizosphere RNA-SIP based bacterial community, Proteobacteria, Actinobacteria, Planctomycetes, Acidobacteria, Chloroflexi, Verrucomicrobia, and Bacteroidetes were the most active phyla represented in both 13C-RNA and 12C-RNA fractions, but the abundances of Planctomycetes and Actinobacteria sequences were higher in the 13C-RNA based community (Fig. 3B). This difference was also true for the archeal phylum Crenarchaeota (Fig. 3B). In the root-associated RNA-SIP bacterial community, Proteobacteria, Actinobacteria, and Bacteroidetes were the most active phyla, and Verrucomicrobia, Actinobacteria, and Acidobacteria had relatively greater numbers of sequences in the 13C-RNA-based community (Fig. 3C).
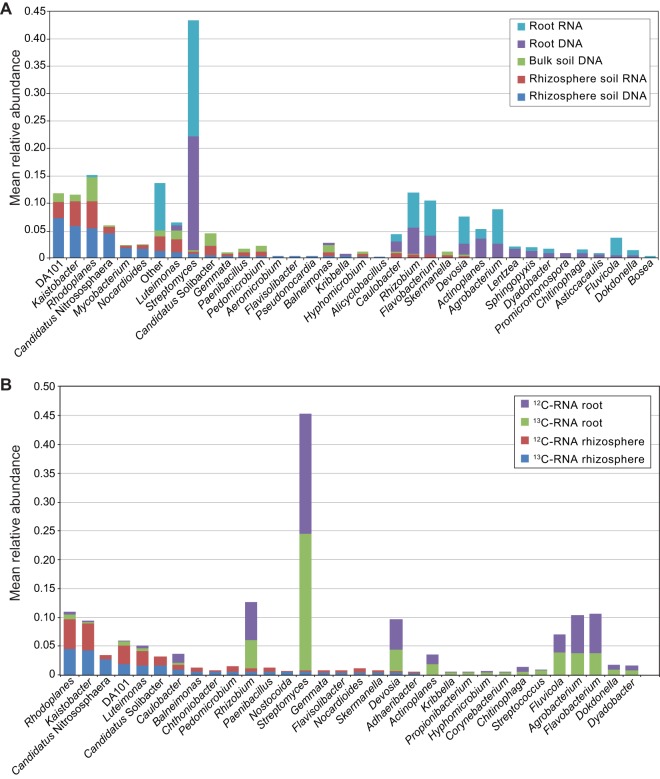
At the genus level in all soil samples, the most abundant genera were Rhodoplanes, Kaistobacter, DA101 (“Candidatus Udaeobacter copiosus”), “Candidatus Nitrososphaera,” Balneimonas, and Luteimonas (Fig. 4A). In the root-derived abundant and active bacterial communities, the dominant genera identified were Streptomyces, Rhizobium, Flavobacterium, Devosia, Actinoplanes, and Agrobacterium (Fig. 4A). Rhodoplanes and Kaistobacter were highly active in both the 13C- and the 12C-RNA rhizosphere fractions, whereas the remaining genera exhibited lower relative activities (Fig. 4B). In the respective root RNA fractions, the highly active genera were Streptomyces, Rhizobium, Flavobacterium, Agrobacterium, Devosia, and Fluviicola (Fig. 4B).
FIG 4.
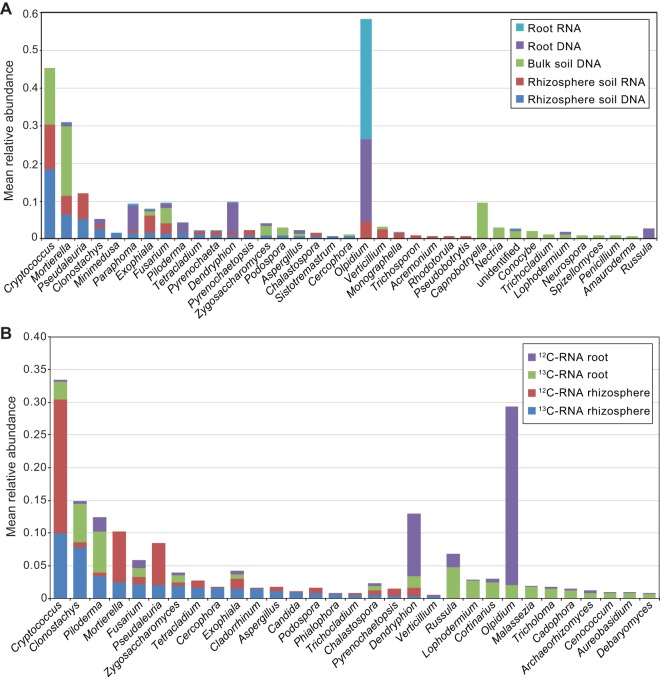
(A) Mean relative abundances of the top 20 most abundant bacterial genera in rhizosphere soil RNA, rhizosphere soil DNA, bulk soil DNA, root RNA, and root DNA. (B) Mean relative abundances of the top 20 bacterial genera found in the 13C-RNA and in the 12C-RNA in the rhizosphere soil and in the root fractions. Taxonomic classification of 16S rRNA gene sequences was performed in QIIME using the Greengenes 16S rRNA reference taxonomy. (Note that more than 20 bars are displayed in each histogram, since the top 20 most abundant genera are not the same in different root/rhizosphere soil DNA/RNA fractions.)
Abundant and active fungi in the rhizosphere and root compartments.
The total numbers of OTUs are shown in Fig. S2B. In the soil samples, the number of OTUs retrieved from DNA was double that retrieved from RNA, whereas the opposite trend was observed for the root-derived samples.
In total, five fungal phyla were identified (Fig. 5A). Basidiomycota were present in all of the communities but not in root DNA samples, and their relative abundance values imply that they were more strongly represented among active fungi in the rhizosphere, whereas in the roots, fungi of this phylum were all active. Ascomycota were present in all of the communities, with greater relative abundances in all of the soil samples as well as in the root RNA-based community. However, their abundance was very low in the root DNA-based community, suggesting that the Ascomycota formed a relatively large proportion of the active fungi in both the rhizosphere soil and the roots, but that they formed a much smaller proportion of the total fungal community that was present in the roots. Fungi of the phylum Chytridiomycota exhibited a higher abundance in root DNA, followed by root RNA, rhizosphere DNA, bulk soil DNA, and rhizosphere RNA. Zygomycota were mostly abundant in bulk and rhizosphere soil communities, whereas in the roots, they were present only in root RNA samples. Glomeromycota were identified only in soil communities with a higher relative abundance in the rhizosphere RNA-based community. However, a large proportion of taxonomic assignments were classified as “unidentified,” especially in the root DNA-based community.
FIG 5.
Mean relative abundances of different fungal phyla in bulk soil DNA, rhizosphere soil DNA and RNA, and root DNA and RNA (A), 13C-RNA- and 12C-RNA in rhizosphere soil (B), and 13C-RNA- and 12C-RNA in roots (C). Taxonomic classification of the ITS region was performed in QIIME using the UNITE reference taxonomy.
At the genus level in all “soil” samples, the most abundant genera were Cryptococcus and Mortierella, whereas in rhizosphere DNA- and RNA-based communities, Pseudaleuria, Clonostachys, Exophiala, and Fusarium were also among the top 20 most abundant/active genera (Fig. 6A). In the roots, Olpidium was the most highly abundant and active genus in both the root DNA- and root RNA-based communities and was followed by Dendryphion and Paraphoma. In the root RNA-derived active community, fungi of the genera Piloderma, Russula, Clonostachys, and Fusarium were also in the list of the top 20 most abundant (Fig. 6A).
FIG 6.
(A) Mean relative abundances of the top 20 most abundant fungal genera in rhizosphere soil RNA, rhizosphere soil DNA, bulk soil DNA, root RNA, and root DNA. (B) Mean relative abundances of the top 20 fungal genera found in the 13C-RNA and 12C-RNA fractions in the rhizosphere soil and in the root fractions. Taxonomic classification of the ITS region was performed in QIIME using the UNITE reference taxonomy. (Note that more than 20 bars are displayed in each histogram, since the top 20 most abundant genera are not the same in different root/rhizosphere soil DNA/RNA fractions.)
Active fungal communities assimilating plant-derived carbon in the rhizosphere and root compartments.
In the rhizosphere RNA-SIP based fungal community, the highly active OTUs identified in the 13C-RNA fraction belonged to Basidiomycota and Ascomycota (Fig. 5B). However, the relative abundances of Zygomycota, Glomeromycota, and Chytridiomycota were higher in the 12C-RNA fraction, suggesting that they were probably less reliant on recently fixed plant-derived 13C. Similarly, in the root RNA fractions, Basidiomycota and Ascomycota became more abundant in the 13C-RNA-based active community, but again, the relative abundances of all other phyla were higher in the 12C-RNA fraction (Fig. 5C).
The rhizosphere 13C- and 12C-RNA-based fungal communities consisted of the genera Cryptococcus, Clonostachys, Mortierella, Fusarium, Pseudaleuria, and Tetracladium (Fig. 6B). Interestingly, the relative activities of most of them were higher in the 12C-RNA fraction, with the exception of Clonostachys, whose activity was much higher in the 13C-RNA-based community. In the root-associated communities, the most active genera were Olpidium, Dendryphion, Piloderma, Russula, Clonostachys, and Cryptococcus. Olpidium and Dendryphion were more active in the 12C-RNA-based root community (Fig. 6B).
DISCUSSION
We are not aware of any other studies using high-throughput sequencing in conjunction with RNA-SIP to study metabolically active microbial communities associated with the roots or rhizosphere of Brassica napus, but interesting differences and similarities exist with older studies. The general predominance of Proteobacteria, Bacteroidetes, Acidobacteria, Actinobacteria, and Chloroflexi that we observed was expected, since these groups have been identified as common inhabitants in the rhizospheres of potato (31), maize (32), desert, and forest soils, the tundra and grasslands (33), and rice (10). The same phyla were also highly abundant in the rhizosphere soil and roots of Arabidopsis spp. (8, 9, 12). Interestingly, Proteobacteria and Actinobacteria have been suggested to be associated with disease suppression in the rhizosphere of sugar beet plants in a study using DNA metagenomics with PhyloChip (34).
As shown previously, both bacterial and fungal DNA communities originating from bulk soil, rhizosphere soil, and roots were structurally distinct from each other (8–10, 35). DNA- and RNA-based communities of bacteria and fungi were also significantly distinct from each other, both in the rhizosphere soil and the root compartment.
The active bacterial community data in our study suggest that Streptomyces were highly active in the root compartment but not in the rhizosphere. Previous studies using denaturing gradient gel electrophoresis (DGGE) and DNA-SIP did not identify Streptomyces in either rhizosphere soil or roots of B. napus (15). Streptomyces were found in the rhizosphere soil of strawberry plants using DGGE, but roots were not examined (36, 37). In our study, the genus Rhizobium was abundant in the 13C-RNA fractions of root samples but not in the rhizosphere soil. Rhizobium spp. were identified as 13C incorporators in the rhizospheres of rape and wheat, and they were also present in the root DNA-based communities of rape, wheat, and maize (15). Rhizobium has also been shown to be abundant in the rhizosphere of the strawberry plant (37). The dominance of Streptomyces and Rhizobium in the roots of B. napus in the present study is supported by earlier studies of Arabidopsis thaliana showing higher abundances of Streptomycetaceae (8, 9) and Rhizobiaceae in the root compartment (8).
Agrobacterium was also abundant in the 13C-RNA root fraction in our study, but it did not incorporate 13C in the rhizosphere and was absent in the root DNA-based community of oilseed rape (15). However, Agrobacterium rhizogenes was identified using BOX-PCR fingerprinting of rhizosphere soil in rape and shown to be antagonistic against the vascular pathogen Verticillium dahliae (38). DGGE and DNA-SIP in A. thaliana revealed that Agrobacterium was abundant in the root DNA-based community and also incorporated 13C in the rhizosphere (21). In the present study, abundant 13C-labeled Flavobacterium was observed in roots but not in the rhizosphere, a finding supported by earlier studies of the root microbiome of A. thaliana demonstrating that bacteria belonging to Flavobacteriaceae constitute a significant component of the root microbiome (8, 12). Devosia was abundant in the 13C-RNA fraction of the roots but not in the rhizosphere soil. In contrast, these bacteria were abundant in the 13C fraction of the A. thaliana rhizosphere but absent in the roots (21). Actinoplanes was found exclusively in association with roots in our study and did incorporate 13C. In an earlier study using RNA-SIP (14), the incorporation of 13C by Actinoplanes in roots of Agrostis stolonifera was demonstrated, but no data were presented concerning the rhizosphere soil. We found abundances of Rhodoplanes in both the 13C and 12C fractions of the RNA-based community in rhizosphere soil but not in the root samples; however, these bacteria were abundant in the root DNA-based community of A. thaliana (21).
Members of Streptomyces are well known for their ability to promote plant growth and for their biocontrol potential (39–41). They are capable of producing several antimicrobial compounds used in both medicine and agriculture (42), as well as a wide range of volatile organic compounds capable of stimulating plant growth both directly and indirectly (43). Studies have been conducted in nonleguminous crops, including canola, lettuce, and Arabidopsis, suggesting the strong potential of Rhizobium to colonize roots of nonlegumes effectively, possibly enhancing plant growth (21) via the involvement of plant growth regulators such as indole-3-acetic acid and cytokinin (44). These results suggest that, even in nonlegumes, the presence of appropriate N-fixing bacteria may enable reduced inputs of synthetic nitrogen fertilizers, a practice commonly used in legume crops. A Flavobacterium sp. was isolated from the rhizosphere of the bell pepper, and its presence was associated with plant growth promotion and an antagonistic potential against pathogens (45). A study which used 15N-DNA-SIP to investigate soil microorganisms responsible for N fixation identified, among others, bacteria of the genus Rhodoplanes as being potential N fixers (46). Kaistobacter was abundant in both the 13C- and 12C-RNA fractions of rhizosphere soil in the present study, and members of this genus have been suggested to be involved in the degradation of aromatic compounds (47). “Candidatus Nitrososphaera” is an ammonia-oxidizing archaeon, playing a central role in global nitrogen cycling, being highly abundant in all environments, including in soils (48).
We found much higher numbers of Clonostachys spp. in the 13C-RNA fraction than in the 12C-RNA fraction of both the rhizosphere soil and root samples, suggesting that this fungus was active in incorporating recently assimilated carbon from B. napus in both compartments. In contrast, Cryptococcus and Mortierella sequences were found primarily in the rhizosphere, and more sequences were associated with the 12C-RNA fraction, suggesting that these fungi primarily assimilated unlabeled carbon, possibly from older structural pools. Abundant Cryptococcus and Mortierella sequences were found predominantly in the rhizosphere soil of the strawberry plant (35). In our study, Fusarium appeared to incorporate recently fixed plant carbon more in the rhizosphere soil than in the roots. High abundances of Fusarium spp. in both rhizosphere soil and roots of the strawberry plant have been observed (35). Fusarium spp. are common soil fungi that have important roles, not only as plant pathogens but also as saprotrophic competitors against other pathogenic fungi (49).
Clonostachys rosea is a common soil saprophyte and an endophyte in some plants (50). It has been shown to be an effective biocontrol agent against Botrytis cinerea, Sclerotinia sclerotiorum, and Plasmodiophora brassicae, with mechanisms including mycoparasitism, competition for space and nutrients, antibiosis, and induction of systemic resistance through root colonization (51, 52). The potential for using Clonostachys in combination with the biocontrol prodigiosin-producing bacterium Serratia rubidaea against the fungal pathogen Fusarium oxysporum in tomato plants has recently been demonstrated (53). In natural soils, Cryptococcus dominates fungal populations (54, 55), and its predominance could be due to the polysaccharide capsules surrounding it (56) and assisting in nutrient assimilation from soil, resulting in a high competitive ability against other fungi and bacteria (54). Olpidium and Dendryphion were abundant in the roots of B. napus and incorporated 13C in the present study, but substantially higher numbers of sequences were associated with the 12C-RNA fraction. Olpidium brassicae is a soilborne obligate parasite that infects plant roots. Its resting spores can remain dormant in the soil for up to 20 years before infecting roots (57). Dendryphion is a pathogen and has been shown to be abundant in organically managed potato fields (58). These labeling patterns suggest that either these fungi are slow growing or that they derive carbon from structural pools that were unlabeled.
The ectomycorrhizal fungal genera Piloderma, Russula, and Cortinarius incorporated 13C in the roots of B. napus despite the fact that rapeseed is normally considered to be a nonmycorrhizal plant (59). Neither glomeromycotan nor ectomycorrhizal fungal sequences were present in the negative controls or the root or rhizosphere DNA samples, suggesting that the samples are unlikely to have been contaminated. The soil in the present study was taken from a field surrounded by forests on two sides, and it is likely to have contained ectomycorrhizal spores. It is possible that the activity of ectomycorrhizal fungal spores adhering to B. napus roots in our study could have been stimulated by plant-derived carbon, as shown for Paxillus involutus (60), explaining the 13C incorporation at an early stage in the present study.
In conclusion, RNA-SIP enabled us to describe the structures and compositions of bacterial and fungal communities associated with the roots and rhizosphere soil of B. napus plants and to identify taxa actively assimilating carbon from different plant-derived pools. The higher relative dominance of certain microbial taxa in the roots compared with those in the rhizosphere soil supports the idea of active selection from a more diverse rhizosphere community demonstrated in other plant species. The identification of specific genera such as Streptomyces, Rhizobium, Clonostachys, and Fusarium as incorporators of recently fixed C suggests that they may be superior competitors in the B. napus rhizosphere and that their potential as inoculants to improve the productivity and health of oilseed crops could be explored. In the 72-h time frame of our SIP analyses, the most abundant taxa were usually well represented in both the 13C- and the 12C-RNA fractions, suggesting that they were active in incorporating both recently fixed carbon and pools of carbon fixed prior to labeling. The spatial and temporal patterns of microbial colonization and acquisition of plant-derived carbon revealed in this study help to identify the microbial genera that can be targeted for more detailed functional studies, including the expression of specific microbial genes involved in plant-microorganism signaling that can be exploited for the sustainable production of oilseed crops such as B. napus.
MATERIALS AND METHODS
Greenhouse experiment.
The experimental approach is outlined in Fig. 1. The winter Brassica napus cultivar “Libraska” was used. Following surface sterilization, seeds were sown on half-strength potato dextrose agar for 10 days to confirm the sterility of the seedlings. Soil was collected from an organically managed field in Ultuna, Sweden (59°49.424′N, 17°39.260′E), in September 2013. The field was plowed prior to collection but was previously planted with Trifolium pratense. The soil was homogenized and sieved and transferred to pots (6 cm by 6 cm by 6 cm; 110 g/pot). Two seedlings of uniform sizes were planted in each pot. Twenty replicate pots were used for each sampling occasion, and 5 replicate pots with soil only (bulk soil) were incubated under the same conditions and used as controls. Plants were grown with a 16-h photoperiod at 18 to 20°C and photon flux density of 250 μmol · m−2 · s−1 and an 8-h dark period at 13 to 15°C for 4 weeks. Four days after planting, the seedlings were thinned to one per pot.
13CO2 pulse labeling.
Plants were labeled with 99 atoms percent (atom%) 13CO2 (Cambridge Isotope Laboratories, Inc., MA, USA) after 4 weeks growth and incubated in a clear Perspex chamber (height by width by length, 30 cm by 48 cm by 98 cm) for a total of 6 h. The total CO2 concentration was maintained at an average value of 420 ppm during this period by monitoring with an infrared gas analyzer (EGM-4; PP Systems, Hertfordshire, UK) and injecting more labeled gas accordingly. After labeling, the pots were returned to the greenhouse.
Sampling.
Three replicate pots were harvested 24 h prior to labeling to monitor the natural abundance of 13C in rhizosphere soil. Following 13CO2 labeling, plants were harvested after 24 h, 72 h, 7 days, and 14 days. Since the sizes of the pots were small and root growth was extensive, we considered the whole soil as rhizosphere soil. However, the soil adhering to the roots was collected separately by gently shaking the roots and carefully mixing it with the remaining soil. “Bulk soil” was collected from plant-free pots. Roots were subsequently immersed in water for 10 min and after thorough, but gentle, washing, they were further washed in a 0.1% Triton X-100 solution followed by repeated rinsing with Milli-Q water. Soil and roots were immediately frozen, freeze-dried, and stored at −20°C prior to coextraction of RNA and DNA.
13C enrichment analysis.
Freeze-dried rhizosphere soil from different time points was milled to a fine powder and weighed using a microbalance. Five milligrams of soil from each sample was transferred to tin capsules (Elemental Microanalysis, Ltd., Devon, UK) and the δ13C signatures of these samples were determined with an elemental analyzer (model EuroEA3024; Eurovector, Milan, Italy) coupled online to a continuous flow Isoprime isotope-ratio mass spectrometer (GV Instruments, Manchester, UK). The resulting δ13C values were expressed in parts per thousand (‰) relative to the international standard of Vienna Pee Dee Belemnite (V-PDB), where δ13C = (Rsample − Rstandard)/Rstandard × 1,000 (‰) and R is the molar ratio of 13C/12C.
RNA and DNA extraction.
Total RNA and DNA were coextracted from 1.0 g of freeze-dried rhizosphere soil and from 50 mg of freeze-dried roots for each sample using the RNA power soil isolation kit (MOBIO Laboratories, CA, USA) and Qiagen plant DNeasy minikit (Qiagen, Germany), respectively (see the supplemental material for further details).
Cesium trifluoroacetate ultracentrifugation.
Seven hundred fifty nanograms of RNA from each replicate was fractionated by cesium trifluoroacetate (CsTFA) equilibrium density gradient ultracentrifugation as described before (17) after some modifications. The gradient mixture per sample consisted of 2641.5 μl of a 2.0-g · ml−1 CsTFA solution (GE Healthcare, Uppsala, Sweden), 112.5 μl of deionized formamide (Sigma-Aldrich), and 516 μl of PCR-grade sterile H2O (Sigma-Aldrich). After adding 750 ng RNA to the CsTFA gradient mixture in a 3.3-ml OptiSeal polyallomer centrifuge tube (Beckman Coulter, USA), the tubes were sealed with plugs and spun in a TLN-100 rotor (Beckman Coulter) in an Optima MAX-XP ultracentrifuge (Beckman Coulter) at 140,000 × g for 48 h at 20°C with maximum acceleration and deceleration.
Following the fractionation of gradients using a fraction collector (model 2110; Bio-Rad, CA, USA), the densities of each fraction were calculated by measuring the refractive indices using an automatic benchtop refractometer (model ATR-F Touch; Schmidt + Haensch & Co., Germany). For each gradient, four fractions (with densities of 1.82 to 1.85 g · ml−1) representing 13C-labeled RNA (heavy) and four fractions (with densities of 1.77 to 1.79 g · ml−1) representing 12C-unlabeled RNA (light) were chosen and pooled. RNA fractions were purified by isopropanol precipitation. RNA pellets were air dried in a laminar hood and resuspended in 10 μl of RNase-free sterile water.
Reverse transcription-PCR.
Reverse transcription of rhizosphere soil and root RNA (heavy and light fractions) was performed using the iScript reverse transcription Supermix (Bio-Rad, CA, USA) in reactions with final volumes of 20 μl. Prior to cDNA synthesis, the four heavy and light fractions were pooled, resulting in one representative heavy and one light fraction (here called 13C-RNA and 12C-RNA, respectively).
PCR amplification.
PCRs were performed using Phusion high-fidelity DNA polymerase (Thermo Fisher Scientific, Germany). The bacterial primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-AACGCACGCTAGGGACTACHVGGGTWTCTAAT-3′) were used to target the variable region V4 (61, 62). The fungal primers fITS7 (5′-GTGARTCATCGAATCTTTG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) were used to target the ITS region (63) (for PCR amplification parameters, see the supplemental material). The primers 806R and ITS4 contained 12-bp and 8-bp barcode sequences, respectively (unique for each sample). The amplification of cDNA samples was performed using 1 μl undiluted cDNA, and for DNA samples, the templates were diluted 10×. Three technical replicates were run for each sample and negative controls were run during all PCRs.
The reactions were run on 1% (wt/vol) agarose gels prestained with Nancy-520 DNA gel stain (Sigma-Aldrich, MO, USA). The triplicate PCR products from each bacterial and fungal amplification were pooled, purified using the Agencourt AMPure kit (Beckman Coulter, USA), and quantified using a Qubit fluorometer (Invitrogen, USA). All bacterial PCR products were pooled in equimolar concentrations and freeze-dried (CoolSafe; ScanLaf A/S, Denmark) for 24 h. The same procedure was followed for fungal PCR products. Pyrosequencing was carried out on 2× one-fourth of a GS FLX titanium Pico titer plate (Macrogen, Seoul, South Korea) according to the manufacturer's recommendations (Roche, Branford, CT, USA).
Microbial community analysis.
The sequences obtained were analyzed using QIIME (64) (MacQiime version 1.9.0). Both bacterial and fungal reads were demultiplexed based on the barcode sequences, and forward and reverse reads were combined. Bacterial data were denoised, and sequences from both bacteria and fungi were clustered into OTUs by UCLUST (65) based on 97% similarity (66). For bacteria, the representative sequences for each OTU were aligned using PyNAST (67), and taxonomic classification was done using the Ribosomal Database Project classifier (68) against the Greengenes 16S rRNA database using default parameters. Chimeric OTUs were identified using ChimeraSlayer (69) and removed. Any reads that were identified in the negative PCR controls were eliminated from the final OTU table. For fungi, the UNITE database version 7 (12_11 alpha release) (70) was used as a reference to assign taxonomy against BLAST results (71) using default parameters. Any reads from organisms other than fungi were eliminated from the final OTU table.
Statistical analyses.
A multivariate analysis of the OTUs was performed using Paleontological Statistics (PAST version 2-17) (72). Beta diversity community dissimilarity calculations were visualized using nonmetric multidimensional scaling (NMDS) with the Bray-Curtis dissimilarity measure. Nonparametric multivariate analysis of variance (NPMANOVA) was used to estimate the significance of the differences in microbial communities. Venn diagrams were generated using the VENNY online program (http://bioinfogp.cnb.csic.es/tools/venny/).
Accession number(s).
The raw sequencing reads were submitted to the NCBI Sequence Read Archive (SRA) under the study number SPR078303, available at http://www.ncbi.nlm.nih.gov/sra/SRP078303.
Supplementary Material
ACKNOWLEDGMENTS
Financial support was provided by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS, grant no. 2011-1211).
The authors declare no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01938-17.
REFERENCES
- 1.Prosser JI, Rangel-Castro JI, Killham K. 2006. Studying plant-microbe interactions using stable isotope technologies. Curr Opin Biotechnol 17:98–102. doi: 10.1016/j.copbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.van Elsas JD, Chiurazzi M, Mallon CA, Elhottova D, Kristufek V, Salles JF. 2012. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci U S A 109:1159–1164. doi: 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Heijden MGA, de Bruin S, Luckerhoff L, van Logtestijn RSP, Schlaeppi K. 2016. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J 10:389–399. doi: 10.1038/ismej.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuzyakov Y, Domanski G. 2000. Carbon input by plants into the soil. Review. J Plant Nutr Soil Sci 163:421–431. doi:. [DOI] [Google Scholar]
- 5.Jones DL, Nguyen C, Finlay RD. 2009. Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant Soil 321:5–33. doi: 10.1007/s11104-009-9925-0. [DOI] [Google Scholar]
- 6.Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- 7.Lugtenberg B, Kamilova F. 2009. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- 8.Bulgarelli D, Rott M, Schlaeppi K, van Themaat EVL, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P. 2012. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488:91–95. doi: 10.1038/nature11336. [DOI] [PubMed] [Google Scholar]
- 9.Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, del Rio TG, Edgar RC, Eickhorst T, Ley RE, Hugenholtz P, Tringe SG, Dangl JL. 2012. Defining the core Arabidopsis thaliana root microbiome. Nature 488:86–90. doi: 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards J, Johnson C, Santos-Medellin C, Lurie E, Podishetty NK, Bhatnagar S, Eisen JA, Sundaresan V. 2015. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci U S A 112:E911–E920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulgarelli D, Garrido-Oter R, Munch PC, Weiman A, Droge J, Pan Y, McHardy AC, Schulze-Lefert P. 2015. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17:392–403. doi: 10.1016/j.chom.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlaeppi K, Dombrowski N, Oter RG, van Themaat EVL, Schulze-Lefert P. 2014. Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives. Proc Natl Acad Sci U S A 111:585–592. doi: 10.1073/pnas.1321597111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumont MG, Murrell JC. 2005. Stable isotope probing—linking microbial identity to function. Nat Rev Microbiol 3:499–504. doi: 10.1038/nrmicro1162. [DOI] [PubMed] [Google Scholar]
- 14.Vandenkoornhuyse P, Mahe S, Ineson P, Staddon P, Ostle N, Cliquet JB, Francez AJ, Fitter AH, Young JPW. 2007. Active root-inhabiting microbes identified by rapid incorporation of plant-derived carbon into RNA. Proc Natl Acad Sci U S A 104:16970–16975. doi: 10.1073/pnas.0705902104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haichar FE, Marol C, Berge O, Rangel-Castro JI, Prosser JI, Balesdent J, Heulin T, Achouak W. 2008. Plant host habitat and root exudates shape soil bacterial community structure. ISME J 2:1221–1230. doi: 10.1038/ismej.2008.80. [DOI] [PubMed] [Google Scholar]
- 16.Bressan M, Roncato MA, Bellvert F, Comte G, Haichar FE, Achouak W, Berge O. 2009. Exogenous glucosinolate produced by Arabidopsis thaliana has an impact on microbes in the rhizosphere and plant roots. ISME J 3:1243–1257. doi: 10.1038/ismej.2009.68. [DOI] [PubMed] [Google Scholar]
- 17.Whiteley AS, Thomson B, Lueders T, Manefield M. 2007. RNA stable-isotope probing. Nat Protoc 2:838–844. doi: 10.1038/nprot.2007.115. [DOI] [PubMed] [Google Scholar]
- 18.Radajewski S, McDonald IR, Murrell JC. 2003. Stable-isotope probing of nucleic acids: a window to the function of uncultured microorganisms. Curr Opin Biotechnol 14:296–302. doi: 10.1016/S0958-1669(03)00064-8. [DOI] [PubMed] [Google Scholar]
- 19.Rasche F, Lueders T, Schloter M, Schaefer S, Buegger F, Gattinger A, Hood-Nowotny RC, Sessitsch A. 2009. DNA-based stable isotope probing enables the identification of active bacterial endophytes in potatoes. New Phytol 181:802–807. doi: 10.1111/j.1469-8137.2008.02744.x. [DOI] [PubMed] [Google Scholar]
- 20.Gschwendtner S, Esperschuetz J, Buegger F, Reichmann M, Muller M, Munch JC, Schloter M. 2011. Effects of genetically modified starch metabolism in potato plants on photosynthate fluxes into the rhizosphere and on microbial degraders of root exudates. FEMS Microbiol Ecol 76:564–575. doi: 10.1111/j.1574-6941.2011.01073.x. [DOI] [PubMed] [Google Scholar]
- 21.Haichar FE, Roncato MA, Achouak W. 2012. Stable isotope probing of bacterial community structure and gene expression in the rhizosphere of Arabidopsis thaliana. FEMS Microbiol Ecol 81:291–302. doi: 10.1111/j.1574-6941.2012.01345.x. [DOI] [PubMed] [Google Scholar]
- 22.Hannula SE, Boschker HTS, de Boer W, van Veen JA. 2012. 13C pulse-labeling assessment of the community structure of active fungi in the rhizosphere of a genetically starch-modified potato (Solanum tuberosum) cultivar and its parental isoline. New Phytol 194:784–799. doi: 10.1111/j.1469-8137.2012.04089.x. [DOI] [PubMed] [Google Scholar]
- 23.Dias ACF, Dini-Andreote F, Hannula SE, Andreote FD, Pereira e Silva MDC, Salles JF, de Boer W, van Veen J, van Elsas JD. 2013. Different selective effects on rhizosphere bacteria exerted by genetically modified versus conventional potato lines. PLoS One 8:e67948. doi: 10.1371/journal.pone.0067948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rangel-Castro JI, Prosser JI, Ostle N, Scrimgeour CM, Killham K, Meharg AA. 2005. Flux and turnover of fixed carbon in soil microbial biomass of limed and unlimed plots of an upland grassland ecosystem. Environ Microbiol 7:544–552. doi: 10.1111/j.1462-2920.2005.00722.x. [DOI] [PubMed] [Google Scholar]
- 25.Wu WX, Liu W, Lu HH, Chen YX, Devare M, Thies J. 2009. Use of 13C labeling to assess carbon partitioning in transgenic and nontransgenic (parental) rice and their rhizosphere soil microbial communities. FEMS Microbiol Ecol 67:93–102. doi: 10.1111/j.1574-6941.2008.00599.x. [DOI] [PubMed] [Google Scholar]
- 26.Broeckling CD, Broz AK, Bergelson J, Manter DK, Vivanco JM. 2008. Root exudates regulate soil fungal community composition and diversity. Appl Environ Microbiol 74:738–744. doi: 10.1128/AEM.02188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Graaff MA, Classen AT, Castro HF, Schadt CW. 2010. Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol 188:1055–1064. doi: 10.1111/j.1469-8137.2010.03427.x. [DOI] [PubMed] [Google Scholar]
- 28.Turan M, Bringu A. 2007. Phytoremediation based on canola (Brassica napus L.) and Indian mustard (Brassica juncea L.) planted on spiked soil by aliquot amount of Cd, Cu, Pb, and Zn. Plant Soil Environ 53:7–15. [Google Scholar]
- 29.Alstrom S. 2001. Characteristics of bacteria from oilseed rape in relation to their biocontrol activity against Verticillium dahliae. J Phytopathol 149:57–64. doi: 10.1046/j.1439-0434.2001.00585.x. [DOI] [Google Scholar]
- 30.Abuamsha R, Salman M, Ehlers RU. 2011. Differential resistance of oilseed rape cultivars (Brassica napus ssp. oleifera) to Verticillium longisporum infection is affected by rhizosphere colonisation with antagonistic bacteria, Serratia plymuthica and Pseudomonas chlororaphis. Biocontrol 56:101–112. doi: 10.1007/s10526-010-9308-8. [DOI] [Google Scholar]
- 31.Inceoğlu O, Abu Al-Soud W, Salles JF, Semenov AV, van Elsas JD. 2011. Comparative analysis of bacterial communities in a potato field as determined by pyrosequencing. PLoS One 6:e23321. doi: 10.1371/journal.pone.0023321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL, Buckler ES, Ley RE. 2013. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci U S A 110:6548–6553. doi: 10.1073/pnas.1302837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA, Knight R. 2012. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J 6:1007–1017. doi: 10.1038/ismej.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JHM, Piceno YM, DeSantis TZ, Andersen GL, Bakker PAHM, Raaijmakers JM. 2011. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100. doi: 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- 35.Nallanchakravarthula S, Mahmood S, Alstrom S, Finlay RD. 2014. Influence of soil type, cultivar and Verticillium dahliae on the structure of the root and rhizosphere soil fungal microbiome of strawberry. PLoS One 9:e111455. doi: 10.1371/journal.pone.0111455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, Roskot N, Heuer H, Berg G. 2001. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl Environ Microbiol 67:4742–4751. doi: 10.1128/AEM.67.10.4742-4751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa R, Gotz M, Mrotzek N, Lottmann J, Berg G, Smalla K. 2006. Effects of site and plant species on rhizosphere community structure as revealed by molecular analysis of microbial guilds. FEMS Microbiol Ecol 56:236–249. doi: 10.1111/j.1574-6941.2005.00026.x. [DOI] [PubMed] [Google Scholar]
- 38.Berg G, Roskot N, Steidle A, Eberl L, Zock A, Smalla K. 2002. Plant-dependent genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different Verticillium host plants. Appl Environ Microbiol 68:3328–3338. doi: 10.1128/AEM.68.7.3328-3338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehr NA, Schrey SD, Hampp R, Tarkka MT. 2008. Root inoculation with a forest soil streptomycete leads to locally and systemically increased resistance against phytopathogens in Norway spruce. New Phytol 177:965–976. doi: 10.1111/j.1469-8137.2007.02322.x. [DOI] [PubMed] [Google Scholar]
- 40.Schrey SD, Tarkka MT. 2008. Friends and foes: streptomycetes as modulators of plant disease and symbiosis. Antonie Van Leeuwenhoek 94:11–19. doi: 10.1007/s10482-008-9241-3. [DOI] [PubMed] [Google Scholar]
- 41.Kanini GS, Katsifas EA, Savvides AL, Karagouni AD. 2013. Streptomyces rochei ACTA1551, an indigenous Greek isolate studied as a potential biocontrol agent against Fusarium oxysporum f.sp lycopersici. Biomed Res Int 2103:pii=387230. doi: 10.1155/2013/387230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Klenk HP, Clement C, Ouhdouch Y, van Wezel GP. 2016. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev 80:1–43. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cordovez V, Carrion VJ, Etalo DW, Mumm R, Zhu H, van Wezel GP, Raaijmakers JM. 2015. Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front Microbiol 6:1081. doi: 10.3389/fmicb.2015.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noel TC, Sheng C, Yost CK, Pharis RP, Hynes MF. 1996. Rhizobium leguminosarum as a plant growth-promoting rhizobacterium: direct growth promotion of canola and lettuce. Can J Microbiol 42:279–283. [DOI] [PubMed] [Google Scholar]
- 45.Kolton M, Green SJ, Harel YM, Sela N, Elad Y, Cytryn E. 2012. Draft genome sequence of Flavobacterium sp. strain F52, isolated from the rhizosphere of bell pepper (Capsicum annuum L. cv. Maccabi). J Bacteriol 194:5462–5463. doi: 10.1128/JB.01249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckley DH, Huangyutitham V, Hsu SF, Nelson TA. 2007. Stable isotope probing with 15N2 reveals novel noncultivated diazotrophs in soil. Appl Environ Microbiol 73:3196–3204. doi: 10.1128/AEM.02610-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kersters K, De Vos P, Gillis M, Swings J, Vandamme P, Stackebrandt E. 2006. Introduction to the Proteobacteria, p 3–37. In Falkow S, Schleifer K-H, Rosenberg E, Stackebrandt E (ed), Prokaryotes: a handbook on the biology of bacteria, vol 6, 3rd ed Springer-Verlag, Berlin, Germany. doi: 10.1007/0-387-30745-1_1. [DOI] [Google Scholar]
- 48.Schleper C, Nicol GW. 2010. Ammonia-oxidising archaea–physiology, ecology and evolution. Adv Microb Physiol 57:1–41. doi: 10.1016/B978-0-12-381045-8.00001-1. [DOI] [PubMed] [Google Scholar]
- 49.Duffy B, Keel C, Defago G. 2004. Potential role of pathogen signaling in multitrophic plant-microbe interactions involved in disease protection. Appl Environ Microbiol 70:1836–1842. doi: 10.1128/AEM.70.3.1836-1842.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Gao H, Ma G, Li S. 2004. Mycoparasitism of Gliocladium roseum 67-1 on Sclerotinia sclerotiorum. Acta Phytopathol Sinica 34:211–214. [Google Scholar]
- 51.Rodríguez MA, Cabrera G, Gozzo FC, Eberlin MN, Godeas A. 2011. Clonostachys rosea BAFC3874 as a Sclerotinia sclerotiorum antagonist: mechanisms involved and potential as a biocontrol agent. J Appl Microbiol 110:1177–1186. doi: 10.1111/j.1365-2672.2011.04970.x. [DOI] [PubMed] [Google Scholar]
- 52.Lahlali R, Peng G. 2014. Suppression of clubroot by Clonostachys rosea via antibiosis and induced host resistance. Plant Pathol 63:447–455. doi: 10.1111/ppa.12112. [DOI] [Google Scholar]
- 53.Kamou NN, Dubey M, Tzelepis G, Menexes G, Papadakis EN, Karlsson M, Lagopodi AL, Jensen DF. 2016. Investigating the compatibility of the biocontrol agent Clonostachys rosea IK726 with prodigiosin-producing Serratia rubidaea S55 and phenazine-producing Pseudomonas chlororaphis ToZa7. Arch Microbiol 198:369–377. doi: 10.1007/s00203-016-1198-4. [DOI] [PubMed] [Google Scholar]
- 54.Vishniac HS. 2006. A multivariate analysis of soil yeasts isolated from a latitudinal gradient. Microb Ecol 52:90–103. doi: 10.1007/s00248-006-9066-4. [DOI] [PubMed] [Google Scholar]
- 55.Connell L, Redman R, Craig S, Scorzetti G, Iszard M, Rodriguez R. 2008. Diversity of soil yeasts isolated from South Victoria Land, Antarctica. Microb Ecol 56:448–459. doi: 10.1007/s00248-008-9363-1. [DOI] [PubMed] [Google Scholar]
- 56.McFadden D, Zaragoza O, Casadevall A. 2006. The capsular dynamics of Cryptococcus neoformans. Trends Microbiol 14:497–505. doi: 10.1016/j.tim.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Campbell RN. 1985. Longevity of Olpidium brassicae in air-dry soil and the persistence of the lettuce big-vein agent. Can J Bot 63:2288–2289. doi: 10.1139/b85-326. [DOI] [Google Scholar]
- 58.Lenc L, Kwasna H, Sadowski C. 2012. Microbial communities in potato roots and soil in organic and integrated production systems compared by the plate culturing method. J Phytopathol 160:337–345. doi: 10.1111/j.1439-0434.2012.01905.x. [DOI] [Google Scholar]
- 59.Vierheilig H, Bennett R, Kiddle G, Kaldorf M, Ludwig-Muller J. 2000. Differences in glucosinolate patterns and arbuscular mycorrhizal status of glucosinolate-containing plant species. New Phytol 146:343–352. doi: 10.1046/j.1469-8137.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- 60.Zeng RS, Mallik AU, Setliff E. 2003. Growth stimulation of ectomycorrhizal fungi by root exudates of Brassicaceae plants: role of degraded compounds of indole glucosinolates. J Chem Ecol 29:1337–1355. doi: 10.1023/A:1024257218558. [DOI] [PubMed] [Google Scholar]
- 61.Bates ST, Cropsey GWG, Caporaso JG, Knight R, Fierer N. 2011. Bacterial communities associated with the lichen symbiosis. Appl Environ Microbiol 77:1309–1314. doi: 10.1128/AEM.02257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ihrmark K, Bodeker ITM, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J, Strid Y, Stenlid J, Brandstrom-Durling M, Clemmensen KE, Lindahl BD. 2012. New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol 82:666–677. doi: 10.1111/j.1574-6941.2012.01437.x. [DOI] [PubMed] [Google Scholar]
- 64.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Tumbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 66.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. 2010. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM. 2009. The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, Ciulla D, Tabbaa D, Highlander SK, Sodergren E, Methe B, DeSantis TZ, Human Microbiome Consortium, Petrosino JF, Knight R, Birren BW. 2011. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abarenkov K, Henrik Nilsson R, Larsson KH, Alexander IJ, Eberhardt U, Erland S, Hoiland K, Kjoller R, Larsson E, Pennanen T, Sen R, Taylor AF, Tedersoo L, Ursing BM, Vralstad T, Liimatainen K, Peintner U, Koljalg U. 2010. The UNITE database for molecular identification of fungi–recent updates and future perspectives. New Phytol 186:281–285. doi: 10.1111/j.1469-8137.2009.03160.x. [DOI] [PubMed] [Google Scholar]
- 71.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 72.Hammer Ø, Harper D, Ryan P. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electronica 4:1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.