ABSTRACT
Carpets have been implicated in prolonged and reoccurring outbreaks of human noroviruses (HuNoV), the leading cause of acute gastroenteritis worldwide. Viral recovery from environmental surfaces, such as carpet, remains undeveloped. Our aim was to determine survival of HuNoV surrogates on an understudied environmental surface, carpet. First, we measured the zeta potential and absorption capacity of wool and nylon carpet fibers, we then developed a minispin column elution (MSC) method, and lastly we characterized the survival of HuNoV surrogates, feline calicivirus (FCV) and murine norovirus (MNV), over 60 days under 30 and 70% relative humidity (RH) on two types of carpet and one glass surface. Carpet surface charge was negative between relevant pH values (i.e., pH 7 to 9). In addition, wool could absorb approximately two times more liquid than nylon. The percent recovery efficiency obtained by the MSC method ranged from 4.34 to 20.89% and from 30.71 to 54.14% for FCV and MNV on carpet fibers, respectively, after desiccation. Overall, elution buffer type did not significantly affect recovery. Infectious FCV or MNV survived between <1 and 15 or between 3 and 15 days, respectively. However, MNV survived longer under some conditions and at significantly (P < 0.05) higher titers compared to FCV. Albeit, surrogates followed similar survival trends, i.e., both survived longest on wool then nylon and glass, while 30% RH provided a more hospitable environment compared to 70% RH. Reverse transcription-quantitative PCR signals for both surrogates were detectable for the entire study, but FCV genomic copies experienced significantly higher reductions (<3.80 log10 copies) on all surfaces compared to MNV (<1.10 log10 copies).
IMPORTANCE Human noroviruses (HuNoV) are the leading cause of acute gastroenteritis worldwide. Classical symptoms of illness include vomiting and diarrhea which could lead to severe dehydration and death. HuNoV are transmitted by the fecal-oral or vomitus-oral route via person-to-person contact, food, water, and/or environmental surfaces. Published laboratory-controlled studies have documented the environmental stability of HuNoV on hard surfaces, but there is limited laboratory-based evidence available about survival on soft surfaces, e.g., carpet and upholstered furniture. Several epidemiological reports have suggested soft surfaces may be HuNoV fomites illustrating the importance of conducting a survival study. The three objectives of our research were to demonstrate techniques to characterize soft surfaces, develop a viral elution method for carpet, and characterize the survival of HuNoV surrogates on carpet. These results can be used to improve microbial risk assessments, the development of much-needed soft surface disinfectant, and standardizing protocols for future soft surface studies.
KEYWORDS: human norovirus, feline calicivirus, murine norovirus, survival, carpet, recovery
INTRODUCTION
Human noroviruses (HuNoV) are recognized as the leading cause of acute gastroenteritis worldwide, as well as the most common cause of foodborne disease in the United States (1). Symptoms may include both diarrhea and vomit, which can contain up to 1011 viruses/g and 107 viruses/30 ml, respectively (2, 3). This, coupled with their environmental stability and low infectious dose, makes HuNoV highly contagious. The primary modes of transmission are person to person or spread via food, water, and environmental surfaces (4). Although environmental transmission of HuNoV is estimated to be low, environmental surfaces may act as a temporary reservoir serving as a secondary source of transmission (5). Temporary reservoirs allow one to become exposed without direct contact with the primary source of infection, leading to prolonged and reoccurring outbreaks. This thinking is supported by epidemiological investigations that have attributed prolonged and reoccurring HuNoV outbreaks (6, 7) to soft surfaces and a laboratory-controlled study that documented transfer of a surrogate virus between soft surfaces, such as cotton and polyester, and hands (8).
Understanding survival profiles under various conditions could improve epidemiologic investigations and microbial risk assessment in addition to answering key questions surrounding environmental stability, decontamination strategies, and the seasonality of HuNoV. Most studies investigating enteric virus survival examined hard surfaces, whereas few used soft surfaces (9, 10). These hard surface studies demonstrated the resiliency of HuNoV, especially under low-temperature conditions, i.e., 4°C. For example, Escudero et al. (11) detected HuNoV via reverse transcription-quantitative PCR (RT-qPCR) on three hard surfaces for up to 42 days, whereas infectious murine norovirus (MNV), a surrogate for HuNoV, was detectable for at least 14 days. Likewise, Lamhoujeb et al. (12) demonstrated HuNoV genome was detectable for up to 56 days on polyvinyl chloride and stainless steel. In the absence of laboratory-based evidence, ample epidemiological evidence suggests nonlaunderable, soft surfaces, such as carpet, may also be a HuNoV fomite (6, 7). Currently, only two laboratory-based studies have documented HuNoV and their surrogate survival on soft surfaces (13, 14). Moreover, no U.S. Environmental Protection Agency-registered disinfectants rated for soft surfaces are available to disinfect these fomites in the United States (15). Taken together, these findings illustrate a significant public health concern, especially in settings where HuNoV outbreaks and soft surfaces are common, such as long-term-care facilities and child care facilities.
Given these public health concerns, it is important to estimate the survival of pathogens under simulated field conditions and account for surface characteristics. Some key differences between hard and soft surfaces, overlooked in previous studies, are a soft surface's ability to retain liquid, i.e., absorption capacity, regain liquid, wettability, and longer times to desiccation compared to hard surfaces. Perhaps the most important feature of soft surfaces, as it relates to virus survival, is absorption capacity. These factors should be incorporated to evaluate virus behavior and to simulate field conditions. Inherently, this would change the fundamental designs of survival studies.
To our knowledge, no published studies have reported the survival of HuNoV, or their surrogates, on carpet. Therefore, our three specific objectives were (i) to study the carpet characteristics, i.e., zeta potential and absorption capacity, of wool and nylon carpet fibers, (ii) to develop and assess the recovery efficiency of a minispin column based virus elution method, and (iii) to—under simulated field conditions—determine the survival of HuNoV surrogates, i.e., feline calicivirus (FCV) and MNV, on wool and nylon carpet fibers and a glass surface (as a hard surface control) over 60 days under 2 relative humidities (RH), 30 and 70%, at 25°C. As a first-generation study, the intent was to develop an experimental model to produce infectious estimates for microbial risk assessments while providing an analysis of these complex surfaces.
RESULTS
Electrokinetic potential.
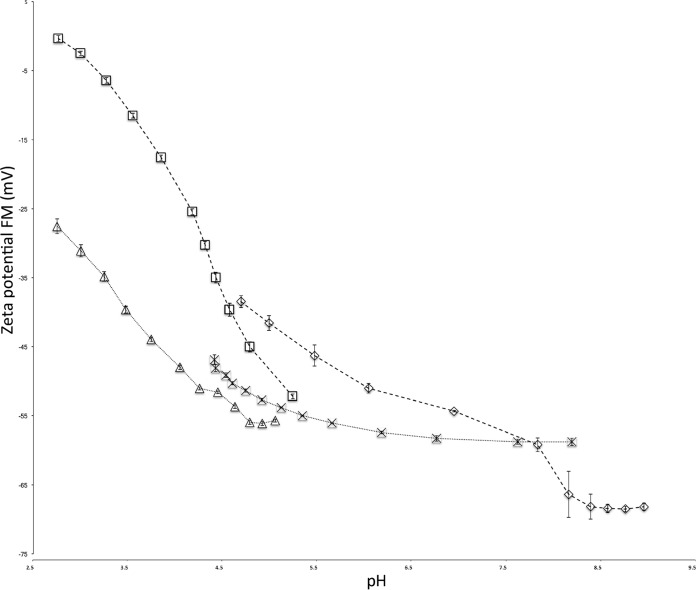
Both wool and nylon fibers were negatively charged and the values inversely proportional to pH between pH 2.7 and 9 based on their electrokinetic potential analysis (Fig. 1). Between pH 2.7 and 9, nylon fiber's ζ values ranged from −0.33 to −68.20 mV, whereas wool fiber's ζ value ranged from −27.55 to −58.78 between pH 2.7 and 8.3. Comparatively, nylon fibers maintained a higher ζ values than wool fibers until pH 8.5 when the nylon fiber's ζ value decreased to ca. −68 mV. During the HCl titration, wool and nylon fibers differed by ca. 25 mV between pH 2.7 and 4 and then progressively grew closer. However, between pH 7.5 and 8.2, both fiber types showed little difference in ζ value.
FIG 1.
Electrokinetic potential analysis of wool and nylon via SurPASS titration. One gram of each fiber, wool and nylon, was packed into a cylindrical cell to estimate the electrokinetic potential of each fiber. Hydrochloric acid (□) titrations for nylon were followed by a NaOH (◇) titration. Similarly, HCl (△) titrations for wool were followed by a NaOH (×) titration. Parameters were set to assess electrokinetic potential between pH 2 and 9. Error bars represent the indicated standard deviations among 12 replicates from three independent experiments. FM, Fairbrother-Mastin.
Carpet absorption capacity.
Wool absorbed up to 0.75 to 0.8 ml/0.1 g of safranin solution, whereas nylon only absorbed up 0.4 to 0.45 ml/0.1 g of safranin solution. Although not compared statistically, wool fibers were capable of absorbing approximately two times more liquid than nylon fibers (Table 1).
TABLE 1.
Absorptive capacity of carpet fibers
| Samplea | Vol added (ml) | Residual wt (g)b |
|---|---|---|
| Wool | 0.650 | 0.009 ± 0.004A |
| 0.700 | 0.015 ± 0.003AB | |
| 0.750 | 0.018 ± 0.003BC | |
| 0.800 | 0.021 ± 0.003C | |
| 0.850 | 0.051 ± 0.013D | |
| Nylon | 0.300 | 0.004 ± 0.002A |
| 0.350 | 0.006 ± 0.003A | |
| 0.400 | 0.011 ± 0.002B | |
| 0.450 | 0.012 ± 0.005B | |
| 0.500 | 0.017 ± 0.003BC |
Carpet fiber samples were 0.1 g each.
Data are expressed as log means ± the standard deviations. Log means with different superscript capital letters in the same column and surface type are significantly different (P < 0.05).
Recovery efficiency.
Tables 2 and 3 show the recovery efficiency (RE) percentages of FCV and MNV at 0, 6, and 12 h when recovered using the minispin column elution (MSC) method with four different elution buffers. Desiccation of wool took 12 h compared to 6 h for nylon. For FCV, RE percentages from carpet fibers ranged from 82.63 to 100%, 4.34 to 80.92%, and 0 (not detected) to 20.89% after 0, 6, and 12 h of drying, respectively. The recovery of MNV (Table 3) from carpet fibers ranged from 55.22 to 100%, 45.13 to 100%, and 4.05 to 38.34% after 0, 6, and 12 h of drying, respectively. More infectious FCV and MNV were recovered from wool fibers compared to nylon when both surrogates were recovered at each time point. Overall, elution buffer type did not significantly affect the recovery of FCV and MNV when using the MSC method under ambient conditions.
TABLE 2.
RE values for FCV from wool and nylon fibers using a minispin column extraction method with four buffer types
| Time point (h) | Materiala | FCV RE% (log recovery [PFU])b |
|||
|---|---|---|---|---|---|
| Buffer 1 | Buffer 2 | Buffer 3 | Buffer 4 | ||
| 0 | Wool | 100 (6.00 ± 0.04) | 100 (6.01 ± 0.12) | 100 (5.98 ± 0.12) | 100 (6.03 ± 0.11) |
| Nylon | 94.74 (5.87 ± 0.07) | 93.62 (5.87 ± 0.08) | 91.56 (5.86 ± 0.06) | 82.63 (55.81 ± 0.14) | |
| 6 | Wool | 80.92 (5.86 ± 0.04) | 64.79 (5.75 ± 0.15) | 70.94 (5.79 ± 0.11) | 61.55 (5.73 ± 0.15) |
| Nylon | 4.34 (4.52 ± 0.12) | 6.22 (4.67 ± 0.24) | 8.05 (4.80 ± 0.10) | 7.23 (4.75 ± 0.13) | |
| 12 | Wool | 15.38 (5.06 ± 0.31) | 11.44 (4.92 ± 0.32) | 13.47 (5.02 ± 0.23) | 20.89 (5.19 ± 0.28) |
| Nylon | ND | 0.01 (1.90 ± 0.42) | ND | 0.03 (2.05 ± 0.72) | |
Wool desiccated after 12 h. Nylon desiccated after 6 h.
Data are expressed as the percent recovery (log mean ± standard deviation). Statistical analysis was completed for buffer type only. For each surrogate, none of the buffers were significantly different (P < 0.05). ND, not determined.
TABLE 3.
RE of MNV from wool and nylon fibers using a minispin column extraction method with four buffer types
| Time point (h) | Materiala | MNV RE% (log recovery [PFU])b |
|||
|---|---|---|---|---|---|
| Buffer 1 | Buffer 2 | Buffer 3 | Buffer 4 | ||
| 0 | Wool | 100 (5.93 ± 0.14) | 100 (5.86 ± 0.05) | 100 (5.88 ± 0.11) | 100 (5.87 ± 0.09) |
| Nylon | 78.31 (5.73 ± 0.15) | 65.67 (5.65 ± 0.11) | 55.22 (5.57 ± 0.07) | 66.32 (5.65 ± 0.18) | |
| 6 | Wool | 100 (5.95 ± 0.08) | 100 (5.95 ± 0.12) | 100 (6.00 ± 0.08) | 100 (5.97 ± 0.18) |
| Nylon | 45.13 (5.46 ± 0.17) | 40.98 (5.42 ± 0.19) | 50.27 (5.47 ± 0.18) | 54.14 (5.36 ± 0.20) | |
| 12 | Wool | 38.34 (5.35 ± 0.13) | 32.63 (5.28 ± 0.10) | 30.71 (5.24 ± 0.17) | 32.25 (5.27 ± 0.18) |
| Nylon | 4.05 (4.43 ± 0.17) | 5.66 (4.51 ± 0.43) | 4.47 (4.47 ± 0.22) | 6.05 (4.60 ± 0.26) | |
Wool desiccated after 12 h. Nylon desiccated after 6 h.
Data are expressed as the percent recovery (log mean ± standard deviation). Statistical analysis was completed for buffer type only. For each surrogate, none of the buffers were significantly different (P < 0.05).
Survival of surrogate viruses.
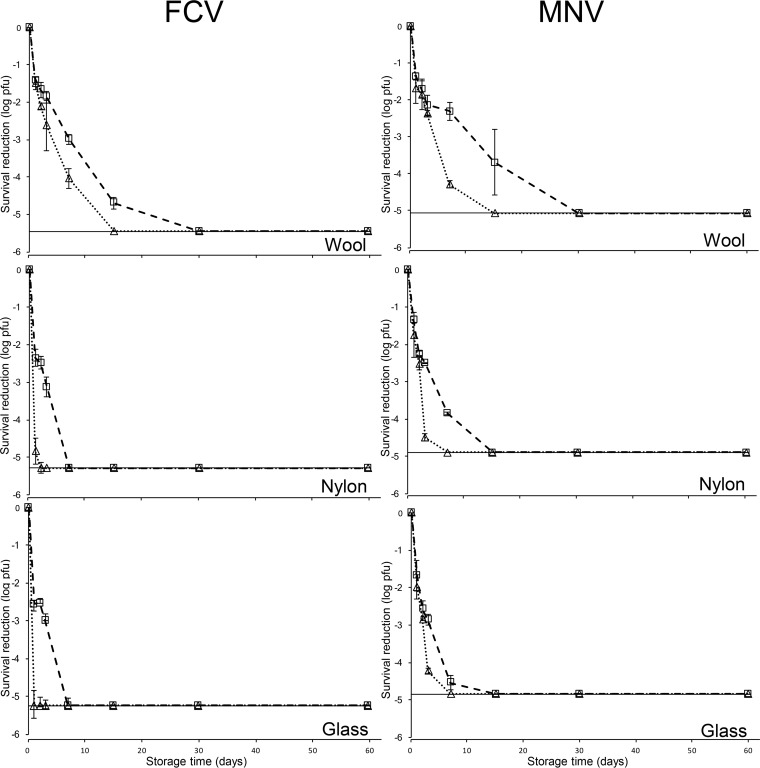
Figure 2 shows the survival characteristics of infectious FCV and MNV inoculated onto carpet fibers and glass under 30 and 70% RH at 25°C over a 60-day period. Infectious FCV survived up to 15, 3, and 3 days at 30% RH, whereas FCV only survived for 7, 1, and <1 days at 70% RH on wool, nylon, and glass, respectively. Infectious MNV survived for up to 15, 7, and 7 days at 30% RH, while MNV held at 70% RH survived for 7, 3, and 3 days on wool, nylon, and glass, respectively. Overall, FCV and MNV survived longer and at significantly higher infectious levels when held at 30% RH compared to 70% RH. In addition, surface type played a significantly role in the survival of both surrogates with wool providing a more hospitable environment. Generally, under each RH condition the survival for both surrogate viruses was wool > nylon > glass. Comparatively, MNV survived longer and at a significantly higher titer on each surface after the first day compared to FCV.
FIG 2.
Survival analysis of FCV and MNV assessed via plaque assay on nylon, wool, and glass surfaces at 30% RH (□) and 70% RH (△). The dotted line indicates detection limits. Surfaces were individually inoculated with ca. 6 log PFU of FCV and MNV and recovered with the MSC method using four washes with 500-μl portions of buffer on days 0, 1, 2, 3, 7, 15, 30, and 60. Data are expressed as means ± the standard deviations of six replicates from two independent experiments.
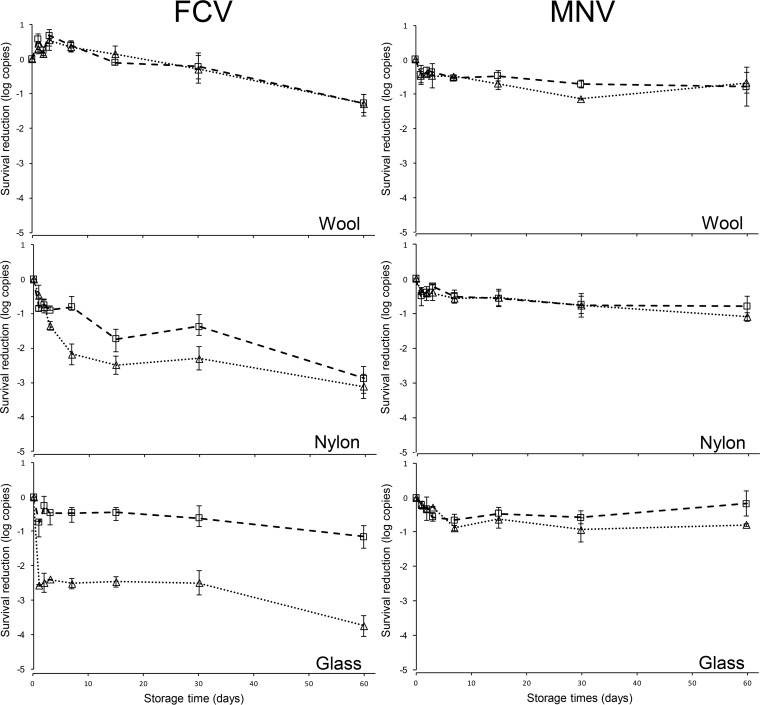
Figure 3 illustrates the reduction of FCV and MNV genomic copies contaminated on wool, nylon, and glass under 30 and 70% RH at 25°C over a 60-day period. FCV and MNV genomic copies were detected for up to 60 days on all surfaces. The maximum log10 copy reductions for FCV after 60 days were <1.30, <3.10, and <3.80 log10 copies, whereas MNV exhibited maximum log10 copy reductions of <0.70, <1.10, and < 0.80 on wool, nylon, and glass, respectively, all at 70% RH. FCV genomic copies recovered from all surfaces were significantly different, whereas no significant difference was observed between MNV genomic copies among surfaces tested. Comparatively, after day 3, significantly more MNV genomic copies were detected compared to FCV.
FIG 3.
Survival analysis of FCV and MNV assessed via RT-qPCR on wool, nylon, and glass surfaces at 30% RH (□) and 70% RH (△). Surfaces were individually inoculated with ca. 6 log PFU of FCV and MNV and recovered with the MSC method using four washes with 500-μl portions of buffer on days 0, 1, 2, 3, 7, 15, 30, and 60. Data are expressed as means ± the standard deviations of six replicates from two independent experiments.
DISCUSSION
Historically, FCV and MNV have been used as surrogates to study HuNoV on both hard and soft surfaces (16). To date, no studies have investigated the survival of HuNoV or their surrogates on carpet despite ample epidemiological evidence suggesting soft surfaces, such as carpet, may be a mode of transmission for HuNoV (6, 7). In this study, we characterized the carpet fiber's ζ value and absorption capacities, developed and assessed a new virus elution method for carpet fibers, and provided evidence that infectious HuNoV surrogates, FCV and MNV, can survive for at least 15 days, depending on environmental conditions and on carpet fiber type. These findings provide laboratory-based evidence to support published epidemiological evidence regarding the prolonged survival of viruses on soft surfaces, e.g., carpet.
It is important to note that soft surface studies can be very challenging, especially when drawing conclusions between studies with limited information regarding surface characteristics (10). ζ is a useful intermediate value for estimating surface charge, and knowing this information may aid in a better understanding of the virus-soft surface interaction. Our ζ results support previous findings that suggest an inverse relationship with pH (17, 18). ζ is considered pH dependent because functional groups at the surface can become ionized under various pH conditions. For example, carboxylate groups, commonly found on wool and nylon fibers, can contribute to an increased negative ζ when the pH of a solution is increased (18). The difference can be attributed to absorption qualities of the fibers. Due to limited absorption, synthetic fibers, such as nylon, have a higher ζ compared to wool. Furthermore, swelling of fibers can affect a surface's ζ (17, 18). Using the SurPASS electrokinetic analyzer, we conducted the acid titration immediately after saturation, whereas the base titration is completed ca. 30 min after saturation. This may further explain the ζ differences between these fibers observed between pH 3 and 5. The carpet ζ values reported here differ in the range of ca. 5 and 30 mV between pH 3 and 7 from previous findings with wool and nylon surfaces (18). Although values were distinct between studies, the wool and nylon trends reported were similar. Differences are expected because ζ measurements can be affected by many experimental factors, such as surface type, fiber aging and processing, porosity, dyes, electrolyte solution, surface treatments, and cleaning procedures (17, 18).
The fibers used in this study were autoclaved prior to use. Common laboratory procedures for cleaning fibers prior to ζ measurements include scouring by washing with detergents, petroleum ethers, or via Soxhlet extraction (17). Our intent was to measure the behavior of these fibers under their natural conditions and how they interact with FCV and MNV, not to assess the ζ of pure wool or nylon. Taken together, these results suggest that buffers, intended for the elution of HuNoV or their surrogates from wool and nylon fibers, should be greater than pH 7.25 based on reported isoelectric points of FCV (4.9) and MNV (5.0 to 6.0) and both fibers' zeta potential profiles. At a pH of >7.25, both viruses and fibers would be negatively charged, leading to increased repulsion and better virus recovery.
As expected, wool and nylon fibers are capable of absorbing different amounts of liquid. Safranin was selected over traditional inocula to enable direct observation of wetting, in addition to weighing residual liquid. Unfortunately, these characteristics were not reported in studies investigating the enteric virus-soft surface relationship (10). Like all condensed-phase material, wool and nylon are hydrophilic in nature, but the magnitude of hydrophilicity can vary between surfaces (19). By the same token, each surface's magnitude of hydrophilicity is directly related to its absorption capacity. Our results are supported by observed and calculated sorption isotherms previously investigated (20): Hailwood and Horrobin demonstrated in 1946 that wool's percent regain could be more than four times times that of nylon, depending on the RH. The absorption capacity of a soft surface is a critical factor to consider. Higher absorption capacities may allow for higher adsorption of viruses, and, as stated previously, viruses in the environment are less susceptible to desiccation and inactivation when adsorbed to a surface (21). These relationships may explain the longer virus survival observed on wool compared to nylon fibers and glass surface. Previous work has demonstrated that natural fibers provide a more protective environment for enteric viruses, such as poliovirus, when inoculated at the same volume (22, 23). Because these fibers absorb different amounts of liquid, we chose to inoculate the carpet fibers based on their absorptive capacity to mimic a natural contamination event but maintained the same level of each virus. Therefore, natural surfaces with high absorption capacity may facilitate longer survival times compared to synthetic, low-absorbing surfaces.
These observations highlight the importance of assessing the characteristics of a soft surface, such as ζ and absorption capacity. Because of these surfaces' absorptive natures, it is not plausible to inoculate surfaces with the same volume and expect to adequately predict the fate of a virus under real world conditions and improve microbial risk assessments. Furthermore, as previously stated, the lack of consistency between soft surface studies and limited descriptions of surfaces leaves little room for adequate comparisons (10). Moreover, studies should consider characterizing soft surfaces prior to analysis or use the same materials from previous studies to broaden our knowledge regarding virus-soft surface interactions.
Previous studies with hard surfaces typically allowed the surface to desiccate prior to evaluating the recovery efficiency of an elution method. However, to adequately evaluate the survival of viruses on soft surfaces under simulated field conditions, where drying times can vary greatly, two recovery efficiencies should be calculated: immediately after inoculation, i.e., time zero, and after desiccation. Desiccation times vary between different types of saturated soft surfaces under the same condition. In addition, the same surface type will desiccate at a different rate when placed in disparate environments. Because of this it is impossible to standardize drying time. Alternatively, by using time zero, i.e., point of contamination, with soft surfaces investigators can more realistically report a virus's survival profile and provide practical data to improve microbial risk assessments.
To evaluate the survival of a virus inoculated onto carpet, improved recovery methods and buffer optimization were needed. Previous recovery methods designed for carpet are time-consuming and resource intense (24, 25). Some methods, e.g., orbital shaking and bottle extraction, require high buffer volumes that may lower the RE and increase detection limits. The MSC method, developed in this study, allows for a simplistic, volume-adaptable, and resource-light approach to assess the survival of nonenveloped viruses on soft surfaces. Our results indicate that the MSC method is efficient at eluting both FCV and MNV from wool and nylon carpet fibers. In addition, buffer type does not significantly influence % RE when using the MSC approach for both viruses and carpet fiber type, among tested buffers. The mechanism of recovery is likely a result of fiber rehydration that changes the surface charge and assists with resuspending the virus in solution while the centrifugal force pulls the solution into the collect tube. Ultimately, a Tween 80-based solution was selected for follow-up experiments, i.e., virus survival assessment, since it has been used previously and is a safe storage medium for both virus surrogates (26).
Virus survival can be affected by many factors, such as temperature, RH, organic content, deposition method, and adsorption (10). Temperature remains the most important environmental factor affecting virus survival. Typically, a virus's survival is inversely proportional to temperature on both hard and soft surfaces (10, 27). For instance, Lee et al. (14) reported that MNV survived longer and at higher titers on cotton gauze and diapers at low (4°C) temperatures compared to higher (18 and 30°C) temperatures. However, carpet is generally found indoors with climate control where temperature varies little. In contrast, RH can vary indoors (40 to 70%) and has been shown to significantly affect the survival of enteric viruses (10). Equally important are the discrepancies among studies investigating effect of RH on virus survival. As described previously, nonenveloped viruses tend to survive longer under high RH conditions (27). However, other studies countered this trend by demonstrating that enteric viruses and their surrogates, such as rotavirus, poliovirus, MNV, and MS2 phage, favor low RH (10). Reasons behind these conflicting results are unclear but can be attributed to difference in interactions of the following factors: temperature, surface type, virus type, and experimental design. In our study, FCV and MNV favored low RH on all surface types. Ideally, low RH provides a quicker time to desiccation and adsorption. Moreover, an adsorbed nonenveloped virion is more stable compared to a free, unbound virion. Others have hypothesized that longer times to desiccation observed under high RH conditions permit virions to stay free and unbound where they are more vulnerable to environmental conditions, e.g., temperature, and solution characteristics, e.g., ionic strength and pH (28). Furthermore, Robinson et al. (29) suggested that poliovirus might not require all 60 identical capsid binding motifs to gain entry to its host. It is possible that portions of a virion are protected from these factors via aggregation or adsorption to surfaces, although the exact mechanism of increased survival under adsorbed conditions compared to unadsorbed conditions is unknown and warrants further investigation.
The differences between survival profiles of FCV and MNV were not surprising as previous studies have demonstrated that FCV has a higher susceptibility to pH, temperature, and some environmental conditions compared to other HuNoV surrogates, such as MNV (16, 26, 30). For instance, D'Souza et al. (30) found that FCV could survive on three hard surfaces for 7 days but experienced up to 4 and 7 log PFU reduction after 2 and 7 days, respectively. Similarly, when compared under wet conditions, MNV's survival was significantly enhanced compared to FCV inoculated onto hard surfaces (16).
Previous studies have documented the divergence among infectious and molecular data, i.e., RT-qPCR (11, 14). RT-qPCR is necessary for studies that incorporate a HuNoV genotype. Typically, investigators have treated samples with RNase or proteinase K to remove exogenous RNA or lysis unstable capsids (12). However, there is a benefit for not completing this step if an infectious surrogate is used. For instance, RT-qPCR can be useful in determining the mode of inactivation of a virus along with assessing the fidelity of a recovery method after infectious virus falls below the limit of detection. Our results assist with confirming this trend toward acknowledging the possibility that the presence of a viral genome does not necessarily signify the presence of infectious virus. Furthermore, our MNV RT-qPCR results demonstrated a limited reduction of genomic copies suggesting that our MSC recovery method is not affected by surface wetting or study duration. However, the reduction of FCV genomic copies over 60 days suggested that capsid integrity and binding motifs may be more susceptible to environmental factors compared to MNV.
Conclusion.
In summary, the results presented here demonstrate that characterizing a soft surface can improve our understanding of virus-soft surface interactions. Furthermore, when simulating field conditions, infectious HuNoV surrogates, FCV and MNV, can survive for at least 15 days on carpet fibers under certain conditions. This survival can be affected by at least two factors: RH and surface type. Specifically, low RH favors FCV and MNV survival, while natural fibers, such as wool, may provide a more protective environment compared to synthetic fibers and hard surfaces.
MATERIALS AND METHODS
Virus propagation, cells, and plaque assay.
A stock of murine norovirus (MNV) strain CW3 (kindly provided by Herbert Virgin at the University of Washington, St. Louis) was propagated by infecting 60 to 80% confluent monolayers of RAW 264.7 cells (ATCC TIB-71; American Type Culture Collection, Manassas, VA) at a multiplicity of infection (MOI) of 0.05 in complete Dulbecco modified essential medium (Corning, Corning, NY) supplemented with 10% low-endotoxin heat-inactivated fetal bovine serum (FBS), 10 mM HEPES buffer (HyClone/GE Healthcare [HyClone/GE], Boston, MA), 100 U/liter penicillin (HyClone/GE), 100 μg/liter streptomycin (HyClone/GE), 1 mM nonessential amino acids (NEAA) (HyClone/GE), and 2 mM l-glutamine (HyClone/GE) (31). Feline calicivirus (FCV) strain F9 was propagated by infecting 90% confluent monolayers of Crandell Rees kidney cell (CRFK; ATCC CCL-94) at an MOI of 0.01 in complete Eagle modified essential medium (Corning) supplemented with 10% low-endotoxin heat-inactivated FBS (Seradigm; VWR International, Radnor, PA), 100 U/liter penicillin (HyClone/GE), and 100 μg/liter streptomycin (HyClone/GE). Both cell lines were incubated at 37°C and 5% CO2 (Symphony; VWR International) until a complete cytopathic effect was observed (1 to 3 days). Both surrogate viruses were harvested from cell lysates by three cycles of freeze-thawing, followed by centrifugation for 10 min at 5,000 × g and 4°C and then extracted with chloroform as previously described (31). MNV (ca. 7 log PFU/ml) and FCV (ca. 8 log PFU/ml) stocks were divided into aliquots and stored at −80°C.
Infectious MNV and FCV were quantified by standard plaque assays as previously described with modifications (31, 32). Briefly, MNV plaque assays were completed by seeding 6-well dishes with RAW 264.7 cells at 106 viable cells/well and incubated until 60 to 80% confluent (4 to 8 h). MNV experimental samples were diluted, if needed, in MNV infection medium, described elsewhere, containing 5% FBS (CDMEM-5) to improve plaque formation (31). FCV plaque assays were based on previous work with significant modifications (32). CRFK cells were seeded in 6-well dishes at 2.5 × 105 viable cells/well and incubated until ca. 90% confluent (2 days). FCV samples were serially diluted in 1× phosphate-buffered saline (PBS) if needed. After a 1-h absorption phase, 2 ml of 1:1 mixtures of 3% SeaPlaque agarose (Lonza, Switzerland) and 2× Temin's modified Eagle medium (MEM) were added to each well and incubated until visible plaque formation (1 to 3 days). The 2× MEM was supplemented with 10% low-endotoxin heat-inactivated FBS, 100 U/liter penicillin, 100 μg/liter streptomycin, 10 mM HEPES (HyClone/GE), and 1 mM NEAA (HyClone/GE). MNV and FCV plaques were visualized by staining agarose plugs with a 0.03% neutral red solution (Carolina Biological, Burlington, NC) mixed with 1× PBS and enumerated on a light box (Futura light box; Logan Electric, Bartlett, IL). Plaque assays for both MNV and FCV contained a stock suspension of virus and CDMEM-5 or PBS as positive and negative controls, respectively, to test for cell line permissiveness and contamination. Both cell lines were not passaged >25 times.
RNA extraction and RT-qPCR.
Viral extraction was performed as previously described with minor modifications (26). Viral RNA was extracted from 0.15 ml of a sample or virus stock with an ENZA viral RNA kit (Omega Bio-Tek, Norcross, GA) according to the manufacturer's instructions. Viral RNA was extracted on the day of recovery experiments and stored at −80°C prior to use. RT-qPCR for FCV and MNV was completed with a KAPA SYBR Fast Universal one-step RT-qPCR kit (Kapa Biosystems, Wilmington, MA) on a Realplex2 Mastercycler platform (Eppendorf, Germany). The forward and reverse primer sequences for FCV RT-qPCR analysis were GCCATTCAGCATGTGGTAGTAACC and GCACATCATATGCGGCTCTG, respectively, whereas MNV RT-qPCR forward and reverse primer sequences were TGATCGTGCCAGCATCGA and GTTGGGAGGGTCTCTGAGCAT, respectively (33). The standard curves for both viruses were prepared by performing an 8-step 10-fold dilution of virus stocks. Log reductions (equation 1) of virus RNA were performed as previously described (33).
| (1) |
where CT,t is the cycle threshold (CT) for the experiment group, CT,c is the cycle threshold for the control recovered at time zero, and k is the slope obtained from plotting the CT values versus the log10 of the RNA copy number used for presenting the standard curve (33).
Carpet and carpet fiber preparation.
Wool and nylon carpet panels (SDL-ATLAS, Rock Hill, SC) were selected from ASTM standard F655-13 (34). Carpet materials had no finishes, e.g., antimicrobial or stain-resistant finishes. Carpet fibers were prepared by shaving nylon and wool fibers from their polypropylene backings with a scalpel with a no. 22 blade. Carpet fibers were prepared from the same carpet panel for the entire study and autoclaved on a 30-min dry cycle prior to use in all experiments.
Electrokinetic potential.
The zeta potential (ζ) of sterile wool and nylon fibers was measured as previously described with modifications to surface only (35). Wool and nylon fibers (1 g) were packed into a SurPASS electrokinetic analyzer cylinder (Anton Paar GmbH, Graz, Austria). The ζ value was calculated using VisioLab software from streaming potential measurements using the Fairbrother-Mastin equation (see equation 2). Flow of the electrolyte (0.001 M KCl) was directed through the cell by linearly ramping pressure from 0 to 30,000 Pa in both directions. Electrodes on either side measured the streaming current. Two cycles of pressure ramping in each direction were performed and the average ζ reported. HCl (0.1 M) and NaOH (0.1 M) titrations were used to measure the ζ value between pH 2 and 9. The pH conditions were adjusted by increments of 0.2 with an autotitration unit. The system was rinsed with Nanopure water (Thermo Fisher Scientific) between titrations and between trials (n = 3) to reduce ionic strength buildup.
| (2) |
where dU/dp is the slope of streaming potential versus pressure, η is the electrolyte viscosity, ε is the dielectric constant of elect, ε0 is the vacuum permittivity, κB is the electrical conductivity of electrolyte outside the capillary cell, and R is the ohm resistance inside the measuring cell (35).
Carpet absorption capacity.
To test the absorption capacity and visualize the wetting of the carpet fiber, empty 2-ml microcentrifuge tubes (VWR International) were weighed and then packed with either wool and nylon fibers (0.1 g) and autoclaved on a 30-min dry cycle. Wool and nylon fibers were saturated with 0.1% safranin solution in increments of 0.05 ml. After application of the indicator liquid, samples were vortexed for 30 s, carpet fibers were removed, and empty microcentrifuge tubes were weighed (XS64; Mettler-Toledo, Switzerland) for residual liquid. Absorption capacities were tested in triplicate at room temperature in three separate experiments.
Recovery efficiency.
Four elution buffers were assessed for their ability to elute FCV and MNV from wool and nylon carpet fibers using a newly designed MSC method. Virus were recovered from samples at time zero and at 6 and 12 h. It took 6 and 12 h to desiccate 0.1 g of saturated nylon and wool carpet fibers, respectively, at room temperature. The buffers assessed were as follows: deionized (DI) water (buffer 1) at pH 7, Butterfield's buffer (buffer 2) at pH 7.2, DI water plus 0.01 M sodium bicarbonate plus 0.02% Tween 20 (buffer 3) at pH 8.2, and 0.01 M PBS plus 0.02% Tween 80 (buffer 4) at pH 7.4.
Wool and nylon carpet fibers were prepared, packed, and autoclaved as described above. Previously determined concentrations of FCV and MNV were thawed in a 37°C water bath (IR35; New Brunswick Scientific, New Brunswick, NJ) and diluted in CDMEM-5 cell culture medium. Separate virus inocula were prepared for each carpet type based on their absorption capacity, i.e., 0.8 ml/0.1 g wool carpet and 0.4 ml/0.1 g nylon carpet, but the total PFU were the same (ca. 6 log PFU/sample). Samples were vortexed for 30 s, followed by the removal of carpet fibers and placement into 60-mm-diameter dishes (Corning). Residual liquid, if any, was pipetted back onto the carpet fibers. The samples were then placed into a chamber (480 HP; VWR International), with lids ajar to expose samples to ambient conditions.
To recover viruses, carpet fibers were packed into empty minispin columns (USA Scientific, Orlando, FL) with sterile forceps and eluted two times by applying 0.5-ml aliquots of the respective elution buffers and centrifuged (model 5424; Eppendorf, Germany) at 2,000 × g for 1 min at room temperature. Each 0.5-ml fraction was combined into a microcentrifuge tube, vortexed, weighed, and stored at −80°C. All samples were assayed via plaque assay. Recovery efficiency (RE) was tested in duplicate in three separate experiments. RE is defined as the number of PFU recovered and divided by the number of PFU initially seeded (36).
Survival study design.
Prior to characterizing the survival of surrogate viruses artificially contaminated on carpet fibers and a glass surface, we established environmental chambers with 30 and 70% RH chambers (480 HP; VWR International); the relative humidity (RH) was maintained with a saturated MgCl2 solution and a 1:1 mixture of NaCl-KCl solution, respectively (37). Briefly, both saturated salt solutions were prepared by heating 3 liters of DI water to 50°C and mixing the salt until saturated. After saturation at 50°C, the flasks were cooled to form a supersaturated salt solution and water activity (AquaLab Series 3 TE; Decagon Devices, Inc., Pullman, WA) was measured to verify whether the solution could provide the appropriate RH. Solutions were housed in noncorrosive container and placed into the chamber. The RH was monitored with a digital hydrothermometer (EU 620-0915; VWR International) and maintained by observing the RH monitor and periodically adding DI water to maintain the 3-liter level.
Carpet fibers and virus inocula were prepared as described above. Glass coverslips (25 mm2; VWR International), included as a hard surface control, were rinsed in sterile DI water, 100% ethanol, and sterile DI water again prior to autoclaving on a 30-min dry cycle. Sterile carpet fibers and glass samples were stored at room temperature under ambient conditions until used.
Wool and nylon fibers were inoculated with ca. 6 log PFU/sample as described above. Glass coverslips were inoculated with the same inoculum as nylon, i.e., 0.4 ml/25 mm2. Samples were recovered on days 0, 1, 2, 3, 7, 15, 30, and 60. Viruses inoculated on carpet were recovered with the MSC method as described above using 0.01 M PBS plus 0.02% Tween 80 and four separate washes. Using the sample recovery buffer, glass coverslips were recovered as previously described (16). After elution fractions were collected, the microcentrifuge tubes were vortexed, weighed, and stored at −80°C. Prior to storage, an aliquot was removed for RT-qPCR analysis. All samples were assayed via plaque assay and RT-qPCR.
Statistical analysis.
Statistical analysis was performed using a one-way multiple-comparison t test. A power analysis was performed prior to the survival study with a 95% confidence interval. All results are expressed as means ± the standard deviations. Statistical significance was defined as P ≤ 0.05. Statistical analyses were conducted using JMP (JMP 11.2.1; SAS Inc., Cary, NC).
ACKNOWLEDGMENTS
We thank David Lander for his assistance with zeta potential measurements and William Bridges for his help with the statistical analysis and power analysis.
This research was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture (USDA; agreement 2011-68003-30395). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the USDA.
REFERENCES
- 1.Glass RI, Parashar UD, Estes MK. 2009. Norovirus gastroenteritis. N Engl J Med 361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Graham DY. 2008. Norwalk virus shedding after experimental human infection. Emerg Infect Dis 14:1553–1557. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caul EO. 1994. Small round structured viruses: airborne transmission and hospital control. Lancet 343:1240–1242. doi: 10.1016/S0140-6736(94)92146-6. [DOI] [PubMed] [Google Scholar]
- 4.Hall AJ, Vinjé J, Lopman B, Park GW, Yen C, Gregoricus N, Parashar UD, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention . 2011. Updated norovirus outbreak management and disease prevention guidelines. Morb Mortal Wkly Rep 60:1–18. [Google Scholar]
- 5.Kosa KM, Cates SC, Hall AJ, Brophy JE, Fraser A. 2014. Knowledge of norovirus prevention and control among infection preventionists. Am J Infect Control 42:676–678. doi: 10.1016/j.ajic.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheesbrough JS, Green J, Gallimore CI, Wright PA, Brown DW. 2000. Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol Infect 125:93–98. doi: 10.1017/S095026889900432X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Repp KK, Keene WE. 2012. A point-source norovirus outbreak caused by exposure to fomites. J Infect Dis 205:1639–1641. doi: 10.1093/infdis/jis250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez GU, Gerba CP, Tamimi AH, Kitajima M, Maxwell SL, Rose JB. 2013. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl Environ Microbiol 79:5728–5734. doi: 10.1128/AEM.01030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotwal G, Cannon JL. 2014. Environmental persistence and transfer of enteric viruses. Curr Opin Virol 4:37–43. doi: 10.1016/j.coviro.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Yeargin T, Buckley D, Fraser A, Jiang X. 2016. The survival and inactivation of enteric viruses on soft surfaces: a systematic review of the literature. Am J Infect Control 44:1365–1373. doi: 10.1016/j.ajic.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Escudero BI, Rawsthorne H, Gensel C, Jaykus LA. 2012. Persistence and transferability of noroviruses on and between common surfaces and foods. J Food Prot 75:927–935. doi: 10.4315/0362-028X.JFP-11-460. [DOI] [PubMed] [Google Scholar]
- 12.Lamhoujeb S, Fliss I, Ngazoa SE, Jean J. 2008. Evaluation of the persistence of infectious human noroviruses on food surfaces by using real-time nucleic acid sequence-based amplification. Appl Environ Microbiol 74:3349–3355. doi: 10.1128/AEM.02878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher E, Shaffer R. 2010. Survival of bacteriophage MS2 on filtering facepiece respirator coupons. J Am Biol Saf Assoc 15:72–76. [Google Scholar]
- 14.Lee JE, Zoh KD, Ko GP. 2008. Inactivation and UV disinfection of murine norovirus with TiO2 under various environmental conditions. Appl Environ Microbiol 74:2111–2117. doi: 10.1128/AEM.02442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EPA. 2012. Product performance test guidelines OCSPP 810.2400: disinfectants and sanitizer for use on fabrics and textiles. EPA, Washington, DC. [Google Scholar]
- 16.Cannon JL, Papafragkou E, Park GW, Osborne J, Jaykus LA, Vinjé J. 2006. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J Food Prot 69:2761–2765. doi: 10.4315/0362-028X-69.11.2761. [DOI] [PubMed] [Google Scholar]
- 17.Capablanca JS, Watt IC. 1986. Factors affecting the zeta potential at wool fiber surfaces. Text Res J 56:49–55. [Google Scholar]
- 18.Grancaric AM, Tarbuk A, Pusic T. 2005. Electrokinetic properties of textile fabrics. Color Technol 121:221–227. doi: 10.1111/j.1478-4408.2005.tb00277.x. [DOI] [Google Scholar]
- 19.Van Oss CJ, Giese RF. 1995. The hydrophilicity and hydrophobicity of clay minerals. Clays Clay Miner 43:474–477. doi: 10.1346/CCMN.1995.0430411. [DOI] [Google Scholar]
- 20.Hailwood AJ, Horrobin S. 1946. Absorption of water by polymers: analysis in terms of a simple model. Trans Faraday Soc 42:B084–B092. [Google Scholar]
- 21.Gerba CP. 1984. Applied and theoretical aspects of virus adsorption to surfaces. Adv Appl Microbiol 30:133–168. doi: 10.1016/S0065-2164(08)70054-6. [DOI] [PubMed] [Google Scholar]
- 22.Sidwell RW, Dixon GJ, Westbrook L, Forziati FH. 1970. Quantitative studies on fabrics as disseminators of viruses. IV. Virus transmission by dry contact of fabrics. Appl Microbiol 19:950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon GJ, Sidwell RW, Mcneil E. 1966. Quantitative studies on fabrics of viruses disseminators. II. Persistence of poliomyelitis virus on cotton and wool fabrics. Appl Microbiol 14:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ASTM. 2015. Standard test method for quantitative assessment of sanitizing solutions for carpet. American Society for Testing and Materials, West Conshohocken, PA. [Google Scholar]
- 25.Malik YS, Allwood PB, Hedberg CW, Goyal SM. 2006. Disinfection of fabrics and carpets artificially contaminated with calicivirus: relevance in institutional and healthcare centres. J Hosp Infect 63:205–210. doi: 10.1016/j.jhin.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Yeargin T, Fraser A, Guohui H, Jiang X. 2015. Recovery and disinfection of two human norovirus surrogates, feline calicivirus and murine norovirus, from hard nonporous and soft porous surfaces. J Food Prot 78:1842–1850. doi: 10.4315/0362-028X.JFP-14-515. [DOI] [PubMed] [Google Scholar]
- 27.Sobsey MD, Meschke JS. 2003. Virus survival in the environment with special attention to survival in sewage droplets and other environmental media of fecal or respiratory origin. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 28.Gerba CP, Goyal SM, Cech I, Bogdan GF. 1981. Quantitative assessment of the adsorptive behavior of viruses to soils. Environ Sci Technol 15:940–944. doi: 10.1021/es00090a600. [DOI] [PubMed] [Google Scholar]
- 29.Robinson CM, Jesudhasan PR, Pfeiffer JK. 2014. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 15:36–46. doi: 10.1016/j.chom.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Souza DH, Sair A, Williams K, Papafragkou E, Jean J, Moore C, Jaykus LA. 2006. Persistence of caliciviruses on environmental surfaces and their transfer to food. Int J Food Microbiol 108:84–91. doi: 10.1016/j.ijfoodmicro.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Hwang S, Alhatlani B, Arias A, Caddy SL, Christodoulou C, Cunha JB, Emmott E, Gonzalez-Hernandez MB, Kolawole A, Lu J, Rippinger C, Sorgeloos F, Thorne L, Vashist S, Goodfellow IG, Wobus CE. 2014. Murine norovirus: propagation, quantification, and genetic manipulation. Curr Protoc Microbiol 33:15K.2.1-61. doi: 10.1002/9780471729259.mc15k02s33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bidawid S, Malik N, Adegbunrin O, Sattar SA, Farber JM. 2003. A feline kidney cell line-based plaque assay for feline calicivirus, a surrogate for Norwalk virus. J Virol Methods 107:163–167. doi: 10.1016/S0166-0934(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 33.Park GW, Barclay L, Macinga D, Charbonneau D, Pettigrew CA, Vinjé J. 2010. Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII.4 norovirus. J Food Prot 73:2232–2238. doi: 10.4315/0362-028X-73.12.2232. [DOI] [PubMed] [Google Scholar]
- 34.ASTM. 2015. Standard specification for test carpets and pads for vacuum cleaner testing 1–3. American Society for Testing and Materials, West Conshohocken, PA. [Google Scholar]
- 35.Ladner DA, Steele M, Weir A, Hristovski K, Westerhoff P. 2012. Functionalized nanoparticle interactions with polymeric membranes. J Hazard Mater 211–212:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Julian TR, Tamayo FJ, Leckie JO, Boehm AB. 2011. Comparison of surface sampling methods for virus recovery from fomites. Appl Environ Microbiol 77:6918–6925. doi: 10.1128/AEM.05709-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenspan L. 1976. Humidity fixed points of binary saturated aqueous solutions. J Res Natl Bur Stand (1934) 81:89–96. [Google Scholar]