ABSTRACT
Complete utilization of carbohydrate fractions is one of the prerequisites for obtaining economically favorable lignocellulosic biomass conversion. This study shows that xylan in untreated rice straw was saccharified to xylose in one step without chemical pretreatment, yielding 58.2% of the theoretically maximum value by Paenibacillus curdlanolyticus B-6 PcAxy43A, a weak lignin-binding trifunctional xylanolytic enzyme, endoxylanase/β-xylosidase/arabinoxylan arabinofuranohydrolase. Moreover, xylose yield from untreated rice straw was enhanced to 78.9% by adding endoxylanases PcXyn10C and PcXyn11A from the same bacterium, resulting in improvement of cellulose accessibility to cellulolytic enzyme. After autoclaving the xylanolytic enzyme-treated rice straw, it was subjected to subsequent saccharification by a combination of the Clostridium thermocellum endoglucanase CtCel9R and Thermoanaerobacter brockii β-glucosidase TbCglT, yielding 88.5% of the maximum glucose yield, which was higher than the glucose yield obtained from ammonia-treated rice straw saccharification (59.6%). Moreover, this work presents a new environment-friendly xylanolytic enzyme pretreatment for beneficial hydrolysis of xylan in various agricultural residues, such as rice straw and corn hull. It not only could improve cellulose saccharification but also produced xylose, leading to an improvement of the overall fermentable sugar yields without chemical pretreatment.
IMPORTANCE Ongoing research is focused on improving “green” pretreatment technologies in order to reduce energy demands and environmental impact and to develop an economically feasible biorefinery. The present study showed that PcAxy43A, a weak lignin-binding trifunctional xylanolytic enzyme, endoxylanase/β-xylosidase/arabinoxylan arabinofuranohydrolase from P. curdlanolyticus B-6, was capable of conversion of xylan in lignocellulosic biomass such as untreated rice straw to xylose in one step without chemical pretreatment. It demonstrates efficient synergism with endoxylanases PcXyn10C and PcXyn11A to depolymerize xylan in untreated rice straw and enhanced the xylose production and improved cellulose hydrolysis. Therefore, it can be considered an enzymatic pretreatment. Furthermore, the studies here show that glucose yield released from steam- and xylanolytic enzyme-treated rice straw by the combination of CtCel9R and TbCglT was higher than the glucose yield obtained from ammonia-treated rice straw saccharification. This work presents a novel environment-friendly xylanolytic enzyme pretreatment not only as a green pretreatment but also as an economically feasible biorefinery method.
KEYWORDS: cellulolytic enzyme, glucose, pretreatment, saccharification, xylanolytic enzyme, xylose, lignocellulosic biomass, rice straw
INTRODUCTION
Lignocellulosic biomass is an attractive alternative for second-generation biofuel and chemical production because it does not compete with food and animal feed production. This material is cheaper than first-generation biomass, which are food crops such as rice, wheat, sugar cane, corn, cassava, and sugar beets (1). It is the largest source of hexose and pentose sugars, which can be used for the production of biofuels and value-added products (2). Rice straw is one of the most abundant lignocellulosic waste materials in the world, with approximately 731 million tons being produced annually. The options for the disposal of rice straw are limited by its low bulk density, high mineral content, low protein content, and slow degradation in the soil (3). Presently, field burning is the main practice for disposing of rice straw; however, it causes air pollution and consequently affects human health (4). Hence, rice straw disposal needs to be improved to reduce the impact on the environment and human health. Since rice straw predominantly contains carbohydrates that account for about 40% (wt/wt) of the whole, including cellulose (30%) and xylan (10%) (3), they can be converted to fermentable sugars by enzymatic saccharification. Therefore, the use of rice straw is considered an important option for energy application and chemical production. However, the high cost of producing biofuels and chemicals still remains the largest obstacle to emerging biorefineries.
Usually, bioconversion of lignocellulosic biomass to ethanol and biomolecules involves three major steps: (i) pretreatment to solubilize and separate xylan and lignin from lignocellulose to allow the cellulolytic enzymes to access the cellulose; (ii) enzymatic hydrolysis to hydrolyze polysaccharides (mainly cellulose) into fermentable sugars; and (iii) fermentation to convert sugars (mainly glucose) into ethanol and biomolecules (5, 6). Pretreatment is an essential step in the cellulose conversion process, especially for lignocellulosic biomass saccharification by commercial cellulases, which are produced by fungi such as Aspergillus spp. and Trichoderma spp. (7). These cellulase preparations require a pretreatment step to remove lignin, which adsorbs enzymes and prevents them from attacking cellulose (8). However, the pretreatments have been viewed as one of the most expensive steps in the conversion of lignocellulosic biomass to fermentable sugars, with costs as high as 30 cents per gallon of ethanol produced, representing 33% of the total cost (7, 9), which is a major barrier in bioethanol conversion. Moreover, the typical pretreatment methods, such as chemical and physicochemical pretreatments, have serious disadvantages, for example, environmental impacts caused by toxic, hazardous, and corrosive chemicals, the necessity of downstream posttreatment for the disposal of unwanted inhibitory compounds, hemicellulose loss during pretreatments, high energy consumption, expensive special equipment requirements, and costly wastewater treatment (7, 10, 11). Therefore, a number of different technologies have been developed for the pretreatment of lignocellulose. Microbial pretreatment, as an environment-friendly and low-cost method, has been attracting increasing attention in recent years. To date, microbial pretreatment has focused mainly on white-rot fungi, which have the ability to break down and mineralize lignin. However, fungal pretreatment has major disadvantages, including relatively low efficiency, a considerable loss of carbohydrates, and long residence periods (12).
To overcome obstacles to chemical, physicochemical, and microbial pretreatments, it is necessary to develop a new pretreatment strategy for improving rice straw conversion. The economy of biofuel and chemical production from lignocellulosic biomass requires the use of not only cellulose but also hemicellulose and lignin in order to obtain an economically feasible biomass conversion (4). A new pretreatment strategy should recover xylose at a high rate from lignocellulosic biomass, since genetically engineered microorganisms, including Saccharomyces cerevisiae and Zymobacter palmae, can efficiently produce ethanol from xylose (13, 14). In addition, xylose itself can be used as the substrate for the production of biomolecules other than ethanol in energy, food, beverage, pharmaceutical, and cosmetic industries (15).
Recently, we reported that PcAxy43A from Paenibacillus curdlanolyticus B-6, a single protein consisting of a catalytic domain of family 43 of the glycoside hydrolases and a family 6 carbohydrate-binding module, exhibited three distinct activities, endoxylanase, β-d-xylosidase, and arabinoxylan arabinofuranohydrolase activities, and functioned cooperatively with the endoxylanase PcXyn10C (16) from the same bacterium in arabinoxylan degradation to increase pentose sugar yields (17). This observation has given us an idea for enzymatic pretreatment of rice straw, resulting in xylose production and subsequent improvement of cellulose accessibility to cellulolytic enzymes.
In this study, we determined the saccharification of xylan in rice straw by PcAxy43A alone and a combination of PcAxy43A, PcXyn10C, and/or PcXyn11A (18), and we found that the combination of these three enzymes was capable of efficiently removing xylan from untreated rice straw (URS) and releasing xylose but not arabinose or any xylooligosaccharides (XOSs). To gain insight into its potential as an enzymatic pretreatment using PcAxy43A, the structural change of the rice straw after the saccharification was observed by scanning electron microscopy (SEM). Nonspecific adsorption onto lignin was also tested for PcAxy43A, PcXyn10C, and PcXyn11A, two cellulolytic enzymes, including Clostridium thermocellum endoglucanase Cel9R (CtCel9R) and Thermoanaerobacter brockii β-glucosidase CglT (TbCglT) (19), and the commercial cellulase Celluclast 1.5 L (Novozymes, Copenhagen, Denmark), prepared from Trichoderma reesei ATCC 26921. Furthermore, we evaluated the saccharification of cellulose in xylanolytic enzyme-treated rice straw (XRS), steam-xylanolytic enzyme-treated rice straw (SXRS), and ammonia-treated rice straw (ARS) by CtCel9R, TbCglT, combinations of these, or the commercial cellulase Celluclast 1.5 L. This work may be very beneficial in producing xylose from xylan in untreated rice straw in one step without chemical pretreatment by a single protein, PcAxy43A from P. curdlanolyticus B-6, and offers a new environment-friendly pretreatment that not only removes xylan from rice straw to improve the cellulose saccharification process but also produces xylose for many applications.
RESULTS AND DISCUSSION
Saccharification of rice straw by PcAxy43A.
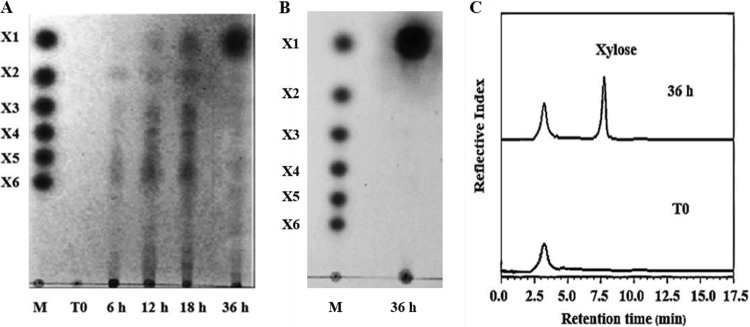
It has long been known that xylan is one of the physical barriers to cellulose hydrolysis in lignocellulosic biomass degradation (7). Recently, we found that PcAxy43A, a novel trifunctional endoxylanase/β-xylosidase/arabinoxylan arabinofuranohydrolase from P. curdlanolyticus B-6, was highly capable of hydrolyzing xylan from various sources, including arabinoxylan (17). Since xylan in rice straw is a kind of arabinoxylan, saccharification of arabinoxylan in rice straw by PcAxy43A was performed. PcAxy43A was incubated with URS, and the hydrolysis products were analyzed by thin-layer chromatography (TLC) (Fig. 1A) and high-performance liquid chromatography (HPLC) analyses (Fig. 1C). TLC patterns of rice straw hydrolysis products showed release of XOSs but not X1 at 6 h, indicating that PcAxy43A had endolytic activity that randomly cleaved the substrate in the early phase of the reaction. Xylose was detected after 12 h of incubation, which implied the presence of β-xylosidase activity, while XOSs had accumulated significantly. Between 12 and 18 h, the amount of xylose increased and became the main product at 36 h, whereas arabinose was not observed (Fig. 1A), suggesting that PcAxy43A is able to hydrolyze arabinoxylan in rice straw at the linear backbone by the actions of endoxylanase and β-xylosidase activities, while arabinoxylan arabinofuranohydrolase activity could not release arabinose from internal β-d-Xylp replaced with arabinose. This result was consistent with the previous observation of the hydrolysis of rye arabinoxylan by PcAxy43A (17). HPLC profiles clearly indicated that xylose was the main hydrolysis product of xylan in rice straw at 36 h of incubation with PcAxy43A (Fig. 1C), accounting for 58.21% of theoretical xylan conversion (Table 1). However, the long incubation (36 h) is not suitable to use in practice due to the stability of enzymes. Thus, in an effort to make the process more economically feasible, we plan to optimize the process conditions to reduce the hydrolysis time of PcAxy43A in the future. In addition to being capable of conversion of xylan in URS to xylose, PcAxy43A also could hydrolyze other lignocellulosic biomass, such as corn hull, to generate xylose (Fig. 1B). This result indicated that PcAxy43A can be used as an enzymatic pretreatment for the hydrolysis of a wide variety of cereal biomass. The theoretical xylose yield of 67.34%, obtained from the hydrolysis of untreated corn hull by PcAxy43A, was higher than that of xylan in URS conversion to xylose by PcAxy43A (58.21%). The reason may be due to corn hull having more xylan content than rice straw (20). However, rice straw is a more abundant agricultural waste in Asian countries, especially Thailand, than corn hull (3). Therefore, rice straw was selected as a potential candidate for glucose production. After PcAxy43A saccharification, the surface area of rice straw was expected to be loosened and swollen, resulting in improved cellulose accessibility to cellulases. The incomplete hydrolysis of xylan in the rice straw might be due to partial adsorption of the enzyme onto lignin, thereby preventing its action on xylan, and the complexity of the rice straw structure, in which xylan is densely packed with layers of lignin through ether and ester linkages and interacts with cellulose microfibrils through hydrogen bonds, protecting xylan against enzymatic hydrolysis (10). However, the xylose yield brought by PcAxy43A was higher than pentose yields (44.00%) in saccharification of 1% NaOH-treated rice straw by Acremozyme, a commercially available enzyme preparation from Acremonium cellulolyticus (21). Recently, biological pretreatment as an environment-friendly approach has received attention for enhancing enzymatic saccharification of lignocellulosic biomass. This method employs microorganisms, including white- and soft-rot fungi, actinomycetes, and bacteria that produce lignin-degrading enzymes (10, 22). Although microbial pretreatments offer the advantages of a low energy requirement and environmentally mild conditions, the rate of lignin decomposition of fungal pretreatment processes is very slow for industrial purposes. Moreover, some of the carbohydrate fractions are consumed by the microorganisms, resulting in a loss of carbohydrates during pretreatment (12). On the other hand, the chemically pretreated hydrolysates typically contain aromatic aldehydes that severely inhibit microbial growth and ethanol formation and the catalytic action of cellulolytic enzymes (23). Treatment of rice straw with PcAxy43A for 36 h showed a high xylose yield (58.21%) and rapid saccharification rate compared to microbial pretreatments reported so far. For example, wheat straw pretreated by Pleurotus ostreatus for 5 weeks showed that only 35% of the original straw was convertible to reducing sugars by a Trichoderma reesei cellulase preparation, and wheat straw pretreated with Pycnoporus cinnabarinus 115 for 4 weeks showed that 54.6% of the residue was converted to reducing sugars (24). Recently, the conversion of wheat straw to sugars by a thermostable Thermobacillus xylanilyticus hemicellulase cocktail consisting of endoxylanase, xylosidase, arabinofuranosidase, and esterase activities was reported (25). Although the enzyme cocktail revealed resistance to alkaline pH and high temperatures, the hydrolysis products contained mixed sugars which differed from hydrolysis products of xylan in rice straw by PcAxy43A that released only xylose. Also, the yield of xylose liberated from wheat straw by the mixed enzymes represented 35%, which was lower than that for xylose obtained from rice straw saccharification by PcAxy43A (58.21%).
FIG 1.
Analysis of hydrolysis products of rice straw and corn hull saccharifications. (A) TLC patterns of rice straw. (B) TLC patterns of corn hull. (C) HPLC profiles of rice straw hydrolysates. Rice straw or corn hull (2%, wt/vol) was incubated with 3 U of PcAxy43A endoxylanase in 50 mM sodium phosphate buffer at pH 7.0, 50°C, and shaken at 200 rpm for 36 h.
TABLE 1.
Conversion of xylan to xylose from untreated rice straw saccharification by xylanolytic enzyme system of PcAxy43A, PcXyn10C, and PcXyn11Af
| Enzyme | Conversion by rice straw type |
||||
|---|---|---|---|---|---|
| Untreated |
Ammonia treated |
||||
| Reducing sugara (g · liter−1) | Xyloseb (g · liter−1) | % Xylose yieldc | Xyloseb (g · liter−1) | % Xylose yieldc | |
| PcAxy43A | —e | 1.21 ± 0.05 | 58.21 ± 1.74 | 1.50 ± 0.05 | 64.11 ± 1.80 |
| PcXyn10C | 0.68 ± 0.02 | NDd | ND | — | ND |
| PcXyn11A | 0.42 ± 0.01 | ND | ND | — | ND |
| PcAxy43A + PcXyn10C | — | 1.51 ± 0.07 | 72.63 ± 2.66 | — | — |
| PcAxy43A + PcXyn11A | — | 1.37 ± 0.06 | 65.94 ± 1.41 | — | — |
| PcAxy43A + PcXyn10C + PcXyn11A | — | 1.64 ± 0.09 | 78.91 ± 2.58 | 2.02 ± 0.07 | 87.53 ± 2.30 |
Reducing sugar was determined by the Somogyi-Nelson method as described in Materials and Methods.
Xylose yield in the reaction mixture was measured by HPLC as described in Materials and Methods.
Percent xylose yield in the hydrolysis of untreated and ammonia-treated rice straw was calculated as described in the equations in Materials and Methods.
ND, could not be detected.
—, not determined.
Saccharification of untreated rice straw by xylanolytic enzyme was carried out using 2% (wt/vol) untreated rice straw, which was incubated independently or in combination with 3.0 U of each of PcAxy43A xylanase, PcXyn10C, and PcXyn11A in 50 mM sodium phosphate buffer, pH 7.0, at 50°C and shaken at 200 rpm for 36 h. The values are the means from triplicate experiments.
The aim of the pretreatment of lignocellulose, namely, rice straw, by xylanolytic enzyme from P. curdlanolyticus B-6 in this study is to utilize xylan and retain cellulose. Ammonia is a well-known chemical pretreatment to remove lignin from lignocellulosic biomass under low operating temperatures while preserving carbohydrates, hemicellulose, and cellulose (3). Thus, the ammonia pretreatment was selected for comparison to our work. It is worth noting that xylose yield in the saccharification of URS by PcAxy43A (58.21%) was comparable to that of ARS by PcAxy43A (64.11%) (Table 1). As shown in Table 2, ammonia pretreatment exclusively removed lignin from URS, i.e., about a 42% reduction in lignin content was observed (3), indicating that the removal of lignin did not strongly affect xylan accessibility to PcAxy43A. Lignin is well known to reduce the effectiveness of enzymatic hydrolysis of lignocellulosic biomass by nonspecifically adsorbing the lignocellulolytic enzymes (26). Thus, the selection of enzymes with a low affinity for lignin, so-called weak lignin-binding enzymes, is a potential strategy for obtaining enzymes suitable for hydrolysis of lignocellulosic substrates (8). It is surprising that PcAxy43A exhibited effective saccharification of xylan in URS to produce xylose as the main product in one step (Fig. 1) in spite of the presence of a large amount of lignin.
TABLE 2.
Chemical compositions of untreated and pretreated rice straws
| Sample | Composition (% dry wt) |
||
|---|---|---|---|
| Cellulose | Xylan | Lignin | |
| URSa | 30.05 ± 1.21 | 10.44 ± 0.36 | 23.32 ± 0.42 |
| XRS | 36.26 ± 1.50 | 4.35 ± 0.18 | 24.08 ± 0.53 |
| ARSa | 32.62 ± 1.06 | 12.51 ± 0.63 | 13.43 ± 0.31 |
| SXRS | 44.03 ± 1.19 | 2.85 ± 0.08 | 10.42 ± 0.27 |
The compositions of URS and ARS have been reported by Phitsuwan et al. (3).
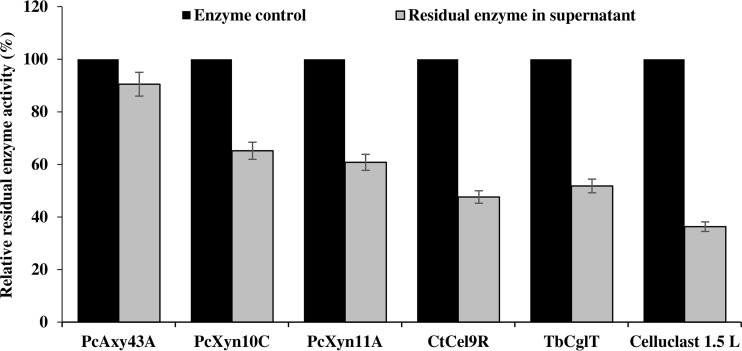
The affinity of PcAxy43A and other enzymes used in this study for lignin was examined using a commercially available lignin preparation. As shown in Fig. 2, 90.5% of PcAxy43A activity existed in the supernatant after the mixture of enzyme and lignin was centrifuged, indicating that PcAxy43A was slightly adsorbed to lignin. On the other hand, residual activities of PcXyn10C, PcXyn11A, CtCel9R, TbCglT, and Celluclast 1.5 L in the supernatant were 65.2%, 60.8%, 47.6%, 51.8%, and 36.3% of their initial activities, respectively. These results suggest that PcAxy43A is a weak lignin-binding enzyme and is less affected by the presence of lignin than other enzymes. Thus, the application of PcAxy43A would be beneficial to the saccharification of other rigid-structure lignocellulosic biomass with high lignin content, such as sawdust, empty fruit bunches from oil palm, and coconut palm residue. The tendency of adsorption of bacterial xylanases, cellulases, and β-glucosidase to lignin was similar to the previous observation that fungal cellulases were more strongly inhibited by lignin than fungal xylanases or β-glucosidase (8). Since hydrophobic interactions appear to play an important role in the adsorption of cellulases and related enzymes to lignin (8, 27), the tendency of the adsorption of enzymes to lignin may be attributed to the difference in protein structure, especially hydrophobic sites exposed to the outside enzyme surfaces (8). This approach would increase the efficiency of enzyme recycling as well, because binding of enzymes to residual lignin is implicated by their low recovery following extensive hydrolysis of lignocellulosic biomass (8).
FIG 2.
Adsorption of 5.0 U of each of PcAxy43A endoxylanase, PcXyn10C, and PcXyn11A from P. curdlanolyticus B-6, C. thermocellum CtCel9R, T. reesei ATCC 26921 Celluclast 1.5 L endoglucanase, and 1.0 U of T. brockii TbCglT on 10 mg · ml−1 of lignin residuals at 50°C for 1 h. Adsorption was evaluated by measurement of the remaining enzyme activity in supernatant relative to initial activity (control). Error bars represent ± standard deviations (n = 3).
Enhancement of xylan saccharification in rice straw by a combination of PcAxy43A with PcXyn10C and PcXyn11A.
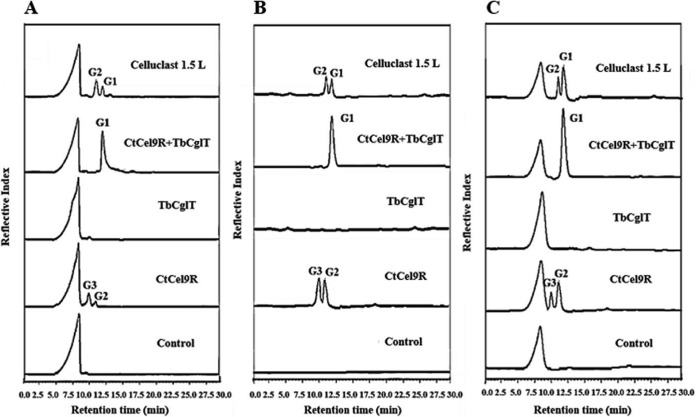
PcAxy43A alone was able to release xylose from URS, and a moderate amount of xylose was recovered (Table 1). The endoxylanase PcXyn10C was reported to have the ability to support PcAxy43A in arabinoxylan degradation to increase xylose and arabinose yields (17). The endoxylanase PcXyn11A is a major xylanase subunit in the extracellular multienzyme complex of P. curdlanolyticus B-6 and has a high specific activity for arabinoxylan (18). Hence, in order to support PcAxy43A in the saccharification of arabinoxylan in URS and improve cellulose accessibility to cellulases, PcXyn10C and/or PcXyn11A was added to the reaction mixture containing PcAxy43A and URS. ARS was also used as the substrate. When URS was incubated with PcXyn10C or PcXyn11A for 36 h, small amounts of reducing sugars (0.68 and 0.42 g · liter−1, respectively) were produced (Table 1), and the former produced xylobiose and xylotriose while the latter released only xylotriose (Fig. 3) and neither of them could produce xylose, unlike PcAxy43A. When URS was incubated with PcAxy43A along with PcXyn10C or PcXyn11A, xylose concentrations increased from 1.21 g · liter−1 to 1.51 g · liter−1 and 1.37 g · liter−1, which accounted for 72.63% and 65.94% of theoretical xylose yield, respectively. These values were higher than that of xylose yield by PcAxy43A alone (58.21%) (Table 1). These results clearly indicated that both PcXyn10C and PcXyn11A helped PcAxy43A to saccharify xylan in untreated rice straw to xylose. Although PcAxy43A had arabinoxylan arabinofuranohydrolase activity (17), neither arabinoxylooligosaccharides nor arabinose were detected in the hydrolysate of URS by PcAxy43A alone or in combination with PcXyn10C or PcXyn11A (Fig. 3), probably because arabinose residues of arabinoxylan in rice straw were attached to lignin (28).
FIG 3.
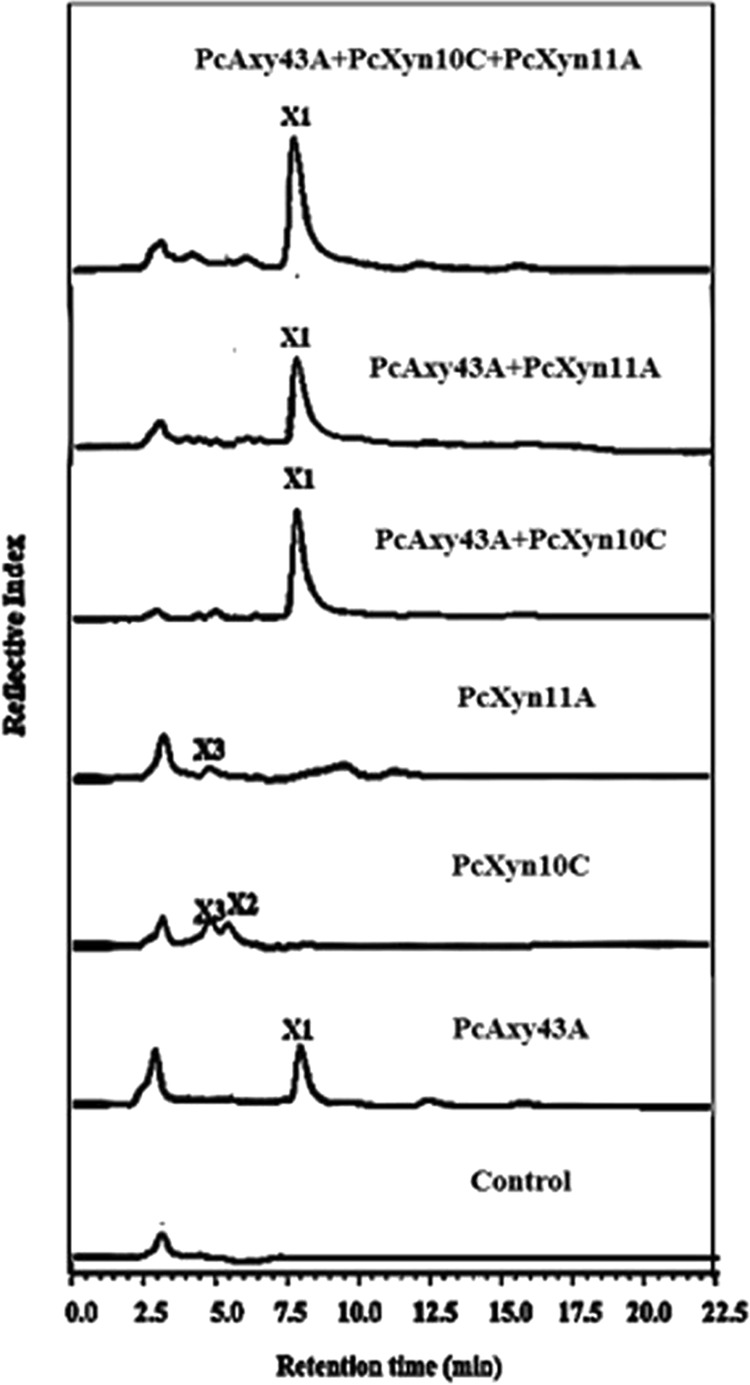
HPLC profiles of saccharification of rice straw. This experiment was carried out with 3.0 U of each of PcAxy43A endoxylanase, PcXyn10C, and PcXyn11A, which was the optimal ratio for enzymatic hydrolysis, and they were incubated independently or in combination with 2% (wt/vol) rice straw in 50 mM sodium phosphate buffer, pH 7.0, at 50°C and shaken at 200 rpm for 36 h.
The cooperative action of PcAxy43A, PcXyn10C, and PcXyn11A toward URS gave the largest amount of xylose (1.64 g · liter−1) and xylose yield (78.91%) compared to PcAxy43A alone or combinations of two enzymes (Table 1 and Fig. 3). These results strongly suggest that PcAxy43A, PcXyn10C, and PcXyn11A have different modes of action toward arabinoxylan in rice straw to degrade it more efficiently and probably loosen the cellulose-xylan matrix, increasing the cellulose surface area and improving cellulose accessibility for cellulases. GH10 xylanases are known to be able to cleave main chains closer to the substituted xylose residues, while GH11 xylanases prefer cleaving main chains in unsubstituted regions (29). On the other hand, PcAxy43A, having endoxylanase and β-xylosidase activities, can cleave main chains far from the substituted xylose residues and further hydrolyze resultant XOSs to xylose (Fig. 1). It should be noted that the xylose yield (78.91%) in the saccharification of URS (containing 23.32% lignin) through the combination of PcAxy43A, PcXyn10C, and PcXyn11A was comparable to the 87.53% xylose yield from ARS (containing 13.43% lignin) (Table 1), indicating that lignin in untreated xylan allowed these bacterial xylanolytic enzymes to access xylan, i.e., these enzymes were less affected by lignin. The lignin resistance of these enzymes is attractive for using them in the saccharification of xylan in lignocellulosic biomass to improve cellulose accessibility to cellulases.
The glucose production from feedstock cellulose by cellulases requires the pretreatment step(s) to make cellulose amenable to cellulase hydrolysis, which is a key step in biochemical processing of lignocellulose based on the sugar platform concept (10). General pretreatments are divided into two approaches, including removing lignin or lignin and xylan, which are considered to have a negative impact on the saccharification of lignocellulosic feedstocks by physically barring cellulolytic enzymes (10, 30). For enzymatic pretreatment, most researchers removed lignin from lignocellulosic biomass by lignin-degrading enzymes, leading to enhancing the impact on further enzymatic hydrolysis of cellulose. However, the lignin degradation by ligninases generates inhibitory compounds which inhibit cellulolytic enzymes and the fermenting microorganisms (23, 31, 32). Moreover, ligninases produced by microorganisms, especially fungi, usually were secreted with xylanolytic and cellulolytic enzymes, causing the loss of xylan and cellulose during the pretreatments. On the other hand, general xylanolytic enzymes were highly adsorbed onto lignin, and this phenomenon was considered to have negative effects on the xylanolytic enzymes for hydrolysis of lignocellulosic biomass that contained high lignin content. For instance, a rare case report of the pretreatment of lignocellulosic biomass using only the xylanolytic enzymes has been described. Rakotoarivonina and coworkers (25) reported that 35% of xylose was liberated from wheat straw by Thermobacillus xylanilyticus xylanolytic enzyme consisting of endoxylanase, β-xylosidase, arabinofuranosidase, and esterase activities (25), whereas the saccharification of rice straw by mixed enzymes containing xylanolytic enzymes had been reported. The theoretical xylose yield derived from saccharification of rice straw for 72 h by each type of xylanolytic enzyme from Trichoderma asperellum KIF125, Aspergillus niger KIF109, or A. aculeatus KIF78 together with Talaromyces cellulolyticus cellulase was 67%, 65%, and 58%, respectively (33), while saccharification of rice straw for 72 h by crude enzyme containing xylanolytic enzyme derived from Trichoderma reesei CDU-11 or Acremonium cellulolyticus CF-2612 exhibited 43% and 40% xylose yields, respectively (34). The yields of xylose obtained from rice straw saccharifications by these fungal xylanolytic enzymes were less than that of the combination of PcAxy43A, PcXyn10C, and PcXyn11A (78.91%). These results indicated that the combination of xylanolytic enzymes from P. curdlanolyticus B-6 had higher hydrolysis efficiency with rice straw xylan than other xylanolytic enzymes. The shorter hydrolysis time of rice straw by the combination of xylanolytic enzyme from P. curdlanolyticus B-6 would give an advantage in the biorefinery industry. In addition, the saccharification of URS by the combination of the xylanolytic enzymes from P. curdlanolyticus B-6 could not only provide cellulases with easily accessible substrate but also produce xylose from rice straw, probably leading to improvement of the overall fermentable sugar yields. Moreover, the pretreatment of rice straw by xylanolytic enzymes offers many advantages over conventional pretreatments in terms of reducing the costs of chemicals, equipment maintenance, and wastewater treatment (12). This study attempts to provide basic information for a novel “green” pretreatment strategy. However, we plan to optimize the process conditions, like the substrate particle size, enzyme and substrate loadings, temperature, pH, and hydrolysis time, to improve economic feasibility. Moreover, modification of enzyme systems to improve hydrolysis efficiency by supplementation of some accessory enzymes, such as lytic polysaccharide monooxygenases, that offer the ability to efficiently boost the activity of biomass-degrading enzymes with xylanolytic enzyme from P. curdlanolyticus B-6 will be interesting alternatives to increase biomass conversion efficiency, supporting an economical biorefinery platform.
Saccharification of XRS by cellulolytic enzymes.
Glucose is the fundamental saccharide for biofuel and value-added biomolecule production. Since a large amount of cellulose (36.26%) exists in XRS (Table 2), enzymatic glucose production from XRS has become an interesting and important topic. The processive endo-β-1,4-glucanase CtCel9R, from C. thermocellum, produced cellotetraose as a primary hydrolysis product and slowly but further hydrolyzed it to smaller oligosaccharides, while the β-glucosidase TbCglT, from T. brockii, was capable of rapidly converting cellobiose and short-chain cellooligosaccharides to glucose. These two enzymes demonstrated high thermostability and high glucose tolerance (19). Since the biorefineries generally employ high temperatures (2), these two enzymes were selected for glucose production from the pretreated rice straws, including XRS, ARS, and SXRS. In order to evaluate the potential use of the pretreated rice straws as substrates for glucose production, the hydrolysis of pretreated rice straws (1%) by CtCel9R (5.0 U), TbCglT (0.36 U), their combination, or Celluclast 1.5 L (containing 5.0 U of endoglucanase and 0.36 U of β-glucosidase) was examined at 50°C. As shown in Fig. 4, after saccharification of pretreated rice straws by CtCel9R, cellobiose and cellotriose were detected in all of the hydrolysates, while TbCglT could not produce any hydrolysis products after incubation. The combination of CtCel9R and TbCglT released glucose (1.94 g · liter−1), accounting for a 48.27% glucose yield, from XRS (Table 3). In contrast, URS was absolutely tolerant to cellulolytic actions of CtCel9R and TbCglT, indicating that xylanolytic enzymes from strain B-6 destroyed xylan, a physical barrier of cellulose in URS, and allowed the cellulolytic enzymes to access cellulose for generating glucose. Glucose yields in hydrolysis of XRS (48.27%), ARS (59.63%), and SXRS (88.58%) by the combination of CtCel9R and TbCglT (Table 3) were higher than those by Celluclast 1.5 L, i.e., 27.41%, 32.28%, and 64.85% for XRS, ARS, and SXRS, respectively (Fig. 4A to C), which may be explained by the observation that CtCel9R and TbCglT were less adsorbed to lignin than Celluclast 1.5 L (Fig. 2). Moreover, the saccharification of microbial-treated rice straws by Sphingobacterium sp. strain LD-1 (35) and Pleurotus ostreatus (36), with commercial Trichoderma viride cellulase ONOZUKA 3S and Aspergillus niger cellulase Y-NC, resulted in 18.6% and 32.0% glucose yields, respectively, which were lower than the values obtained by saccharification of all of the treated rice straws by the combination of CtCel9R and TbCglT (Table 3). These results may be ascribed to higher glucose tolerance and thermostability (19) and lower affinity for lignin of bacterial cellulases CtCel9R and TbCglT than fungal cellulases, and these factors together may have shown their best performance in cellulose saccharification. Therefore, the combination of CtCel9R and TbCglT is a great formulation in the saccharification of cellulose to generate glucose.
FIG 4.
HPLC profiles of saccharification of XRS (A), ARS (B), and SXRS (C) by cellulolytic enzymes. The reactions were performed by using 1% (wt/vol) of substrates that were incubated with individual or mixed CtCel9R (5.0 U of endoglucanase) and TbCglT (0.36 U of β-glucosidase) or Celluclast 1.5 L (5.0 U of endoglucanase and containing 0.36 U of β-glucosidase) in 50 mM sodium phosphate buffer, pH 7.0, at 50°C with shaking at 200 rpm for 48 h.
TABLE 3.
Conversion of glucan to glucose from pretreated rice straw saccharification by cellulolytic enzymes from CtCel9R and TbCglTd
| Substrate | Glucose concna (g/liter) | % Glucose yieldb |
|---|---|---|
| URS | NDc | ND |
| XRS | 1.94 ± 0.08 | 48.27 ± 0.93 |
| ARS | 2.14 ± 0.11 | 59.63 ± 1.40 |
| SXRS | 3.85 ± 0.18 | 88.58 ± 2.06 |
Glucose concentration was measured by HPLC as described in Materials and Methods.
Percent glucose yield in the hydrolysis of XRS, ARS, and SXRS was calculated as described in the equations in Materials and Methods.
ND, could not be detected.
Saccharifications of XRS, ARS, and SXRS by cellulolytic enzymes were carried out using 1% (wt/vol) substrates, which were incubated with CtCel9R (5.0 U of endoglucanase) and TbCglT (0.36 U of β-glucosidase) in 50 mM sodium phosphate buffer, pH 7.0, at 50°C with shaking at 200 rpm for 48 h. The values are the means from triplicate experiments.
Saccharification of SXRS by combination of CtCel9R and TbCglT showed a higher glucose yield (88.58%) at 48 h than glucose obtained from rice straw saccharification by crude enzyme containing mixed xylanolytic and cellulolytic enzymes from Acremonium cellulolyticus CF-2612 or Trichoderma reesei CDU-11 at 72 h, yielding approximately 65% and 60%, respectively (34). These results suggest that the xylanolytic and cellulolytic enzyme system in this work is better than that of either fungi. Moreover, the hydrolysis time of SXRS cellulose to glucose by combination of CtCel9R and TbCglT (48 h) was faster than the conversion of rice straw to glucose by crude enzymes from the fungi (72 h). In addition, the hydrolysis products of sequential rice straw saccharification by xylanolytic and cellulolytic enzymes in this work separately released neither xylose nor glucose, unlike the hydrolysis products of rice straw saccharifications by mixed xylanolytic and cellulolytic enzymes of other works that contained the mixture of pentose and hexose sugars (34). One sugar is more convenient to use in industries than the mixed sugars.
When ARS was saccharified by CtCel9R and TbCglT, the glucose yield was 59.63%, which was higher than the value obtained from XRS saccharification (48.27%) (Table 3 and Fig. 4A and B). Ammonia pretreatment is known to simultaneously reduce lignin content, partially remove xylan, and decrystallize cellulose to make more cellulose accessible to cellulases (7). However, the ammonia pretreatment is considered to be a high-cost process in ammonia recovery and also to generate environmental pollution (7). Taking into consideration environmental issues, one approach to increase the glucose yield from XRS is steam pretreatment, which is one of the most practical and versatile methods to have been shown to be effective in the pretreatment of agricultural residues. Furthermore, steam pretreatment is an environmentally friendly pretreatment approach (10), which is one criteria of this study. As shown in Table 2, the chemical composition of steam-treated XRS (SXRS) showed an increase in cellulose (44.03%) and decrease in xylan (2.85%) and lignin (10.42%) compared to XRS and ARS, in which cellulose, xylan, and lignin contents were 36.26 and 32.62%, 4.35 and 12.51%, and 24.08 and 13.43%, respectively, on a dry weight basis. The enrichment of cellulose is due to the removal of lignin and xylan during steam pretreatment. This result agreed with a previous report that steam pretreatment enriched cellulose through depolymerization of lignin and xylan components (37). Moreover, Mes-Hartree et al. reported that ammonia pretreatment affected the solubilization of hemicellulose less than steam pretreatment (38). Thus, it can be concluded that steam pretreatment had a greater effect than ammonia pretreatment, because steam pretreatment was highly efficient at solubilizing xylan, removing lignin, and decreasing the cellulose crystallinity and degree of polymerization (37). Pretreatment of rice straw by successive xylanolytic enzymes and steaming processes is more attractive than ammonia pretreatment because it more effectively enhances cellulose hydrolysis by cellulolytic enzymes and does not require chemical reagents, resulting in low environmental impact and costs. On the other hand, the pretreatment of rice straw by steaming without PcAxy43A and then being saccharified with Celluclast 1.5 L leads to loss of xylan and lignin into the steaming liquid fraction. As a result, it is difficult to recover xylan for further saccharification. Moreover, if the rice straw was pretreated with steam without PcAxy43A and then hydrolyzed with Celluclast 1.5 L supplemented with PcAxy43A, a mixture of pentose and hexose sugars will be produced. In addition, saccharifications by xylanolytic and cellulolytic enzymes demonstrated the efficient hydrolysis of xylan and cellulose in rice straw for xylose and glucose production. It was reported that remaining lignin after saccharifications of xylan and cellulose can be used for value-added compound production, such as vanillin, phenolic resins, phenol-formaldehyde adhesive, carbon fiber, synthetic gasoline, and a binder for fertilizer (2).
Morphological structures of pretreated rice straws.
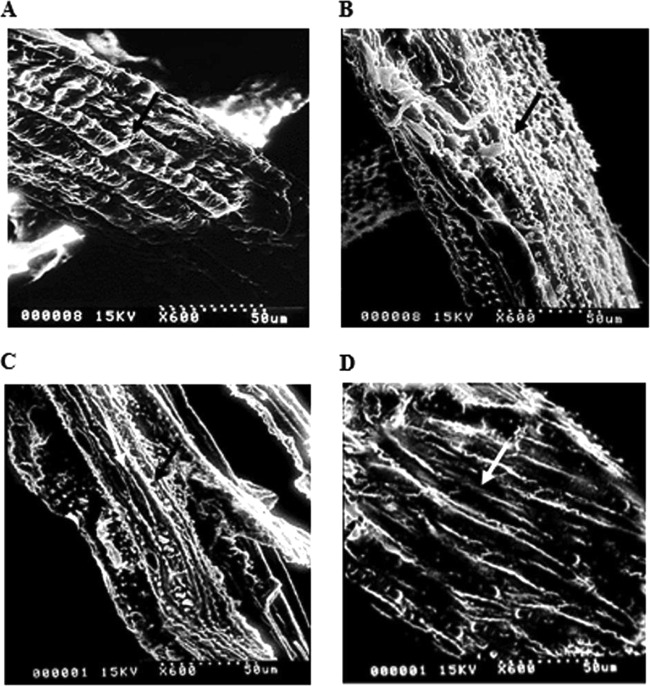
Effective pretreatments enhance hydrolysis of cellulose in lignocellulosic biomass due to structural modifications (7). Thus, SEM was used to investigate structural changes of the enzyme-treated rice straws. URS exhibited a rigid and well-ordered arrangement (Fig. 5A), suggesting that cellulose microfibrils are coated by hemicellulose-lignin matrix. After saccharification of rice straw by PcAxy43A, the fibrous surfaces became loosened and swollen as a consequence of partial xylan removal (Fig. 5B). The partial separation of fibrils is evident, which is attributed to fiber swelling. The disorganization of the rice straw fiber resulted in porosity, which allows cellulolytic enzymes to access the internal surface areas, as observed previously (3). As shown in Fig. 5B and C, XRS had greater changes in the microstructure and surface morphology than PcAxy43A-saccharified rice straw. The fiber surface destroyed by the three xylanolytic enzymes became more porous than that by PcAxy43A alone. Similar observations were made regarding porosity on the surface structure of rice straw after pretreatment using α-l-arabinofuranosidase from Geobacillus thermoleovorans IT-08 to release arabinose side chains linked to lignin (39), while the morphological changes of rice straw were observed after the microbial pretreatment by coculture of Paenibacillus sp. and Aspergillus fumigatus, which was able to remove the external fibers of rice straw (40). Moreover, the removal of xylan by xylanase treatment increased pore size and accessibility of cellulases to the cellulosic regions, resulting in an increase of glucose production (41). As shown in Fig. 5D, after steaming, the SXRS structural surfaces appeared more hollow than those of XRS (Fig. 5C). The larger porous surface of SXRS resulted in more efficient removal of xylan and lignin. Consequently, SXRS fibers seem to be more susceptible to enzymatic attack than XRS. This is in agreement with the finding of DeMartini and coworkers, who observed that steam pretreatment strongly removed xylan and lignin and increased the surface area of cellulose available for cellulolytic enzymes (42).
FIG 5.
SEM of rice straw surface structures at ×600 magnification. (A) Untreated rice straw (URS). (B) PcAxy43A-saccharified rice straw. (C) Xylanolytic enzyme-saccharified rice straw (XRS). (D) Steam- and xylanolytic enzyme-treated rice straw (SXRS). Arrows indicate obviously altered fiber surface areas of pretreated rice straws compared to the untreated rice straw.
In conclusion, the P. curdlanolyticus strain B-6 PcAxy43A, a weak lignin-binding trifunctional xylanolytic enzyme, in association with PcXyn10C and PcXyn11A, was capable of directly producing xylose (78.9% xylose yield) from xylan in rice straw without chemical pretreatment and made fibrous surfaces of rice straw loose and swollen, improving cellulose accessibility to cellulases. Moreover, the saccharification of SXRS by the combination of CtCel9R and TbCglT showed a higher glucose yield (88.5%) than that of ARS (59.6%). This environment-friendly enzymatic pretreatment not only enhanced cellulose saccharification by cellulolytic enzymes but also produced xylose from various cereal biomass, including rice straw and corn hull, without chemical pretreatment.
MATERIALS AND METHODS
Feedstock.
Rice straw was collected from Ayutthaya Province, Thailand, while corn hull was obtained from Lampang Province, Thailand. The samples were cut by scissors to small sizes and ground by an Ultra Centrifugal Mill ZM-100 with a 0.5-cm mesh screen (Retsch, Haan, Germany). The samples were washed several times with warm distilled water to remove the remaining sugar and then oven dried at 60°C until constant weight (∼24 h). The milled rice straw is referred to as untreated rice straw in this study. The ammonia pretreatment was carried out by soaking 10 g of rice straw in 27% (wt/wt) ammonium hydroxide at a solid/liquid ratio of 1:12 (wt/vol) at room temperature (25 ± 3°C) for 14 days (3). The suspension then was filtered through Whatman's no. 1 filter papers under vacuum suction to remove the liquid phase and retrieve the solid. The retrieved solid was washed with water to remove inhibitors/impurities. The solid suspension was neutralized with around 5 ml of 1 N HCl, washed with water (∼400 ml), and dried at 60°C (3). Both URS and ARS were used as substrates for subsequent enzymatic hydrolysis experiments.
Expression and purification of recombinant enzymes.
The recombinant Escherichia coli BL21(DE3) cells harboring plasmids pPcAxy43A (17), pXyn10C (16), pXyn11A (18), pET28Cel9R, and pET19CglT (19) were grown at 37°C in Super Broth medium (3.5% tryptone [Wako, Osaka, Japan], 2.0% yeast extract [Merck, Darmstadt, Germany], and 0.5% NaCl [Merck], pH 7.0) containing 30 μg · ml−1 kanamycin. When the cultures reached an optical density at 600 nm of 0.6, isopropyl-β-d-thiogalactopyranoside was added for a final concentration of 1 mM. After cultivation for 16 h at 16°C, the cells were harvested and resuspended in cell lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8.0) and disrupted by sonication (Vibra-Cell, Connecticut, United States) at a power of 50 W and frequency of 60 MHz on ice with 5 short bursts of 30 s, followed by intervals of 30 s for cooling. The cell debris then was removed by centrifugation at 4°C for 30 min at 10,000 rpm to obtain cell extract. The cell extract was subjected to purification on a HisTrap HP column (GE Healthcare, Tokyo, Japan) according to the manufacturer's protocol. The purity of purified proteins was analyzed by SDS-PAGE (43).
Enzyme and protein assays.
The endoxylanase activity of PcAxy43A, PcXyn10C, and PcXyn11A from P. curdlanolyticus B-6 was measured by determining the amount of reducing sugar released from birchwood xylan (Sigma-Aldrich, St. Louis, MO, USA). The reaction mixture consisted of 0.5 ml of 1% (wt/vol) xylan in 50 mM sodium phosphate buffer (pH 7.0) and 0.1 ml of enzyme (2 to 20 μg protein). After incubation for 10 min at 50°C, the increase in the amount of reducing sugar was determined by the Somogyi-Nelson method (44) with xylose as the standard. One unit of enzyme activity was defined as the amount of enzyme that liberated 1 μmol of reducing sugar per minute under the above-described conditions. The endoglucanase activity of CtCel9R from C. thermocellum and Celluclast 1.5 L was measured by determining the amount of reducing sugar released from 1% (wt/vol) carboxymethyl cellulose (Sigma-Aldrich) with glucose as the standard. The reaction mixture was carried out following the xylanase activity assay. The β-xylosidase activity of PcAxy43A was tested by determining the amount of p-nitrophenol (pNP) released from p-nitrophenyl-β-d-xylopyranoside (pNPX) with pNP as the standard (17). Determination of β-glucosidase activity of TbCglT from T. brockii and Celluclast 1.5 L was based on measurement of the release of p-nitrophenol from p-nitrophenyl β-d-glucoside (pNPG) (Sigma-Aldrich) with pNP as the standard. One unit of enzymatic activity was defined as the amount of enzyme that released 1 μmol of p-nitrophenol per min. The protein concentration was determined with bovine serum albumin as the standard using the Lowry method (45). Each assay was performed in triplicate.
Analysis of saccharification products of xylan in lignocellulosic biomass by PcAxy43A.
URS (2%, wt/vol) or untreated corn hull (2%, wt/vol) was incubated with 3 U of PcAxy43A as the endoxylanase in 50 mM sodium phosphate buffer, pH 7.0, at a final volume of 3 ml and at 50°C with shaking at 200 rpm for 36 h. The hydrolysis products of URS and untreated corn hull were determined by thin-layer chromatography (TLC) on silica gel 60 F245 plates (1.05554; 20 by 20 cm) (Merck, Darmstadt, Germany) with a mixture of n-butanol, acetic acid, and water (2:1:1) as a solvent system (46). The sugar spots were detected by heating the plates to 100°C after spraying them with a reagent composed of 4 g of α-diphenylamine, 4 ml of aniline, 200 ml of acetone, and 30 ml of 80% phosphoric acid. Quantitative analysis of hydrolysis products of URS was carried out by HPLC (Shimadzu, Kyoto, Japan) with a reflective index detector (Shimadzu RID-10A) on a BP-100 Pb++ carbohydrate column (Benson Polymeric, Sparks, NV, USA) operated at 85°C with deionized water at a flow rate of 0.6 ml/min. d-Xylose (Merck), XOSs (xylobiose to xylohexaose) (Megazyme, Wicklow, Ireland), and l-arabinose (Merck) were used as standards.
Adsorption of enzymes to lignin.
Commercially available lignin residue (lignosulfonic acid sodium salt; purchased from Sigma-Aldrich) was used for assays of adsorption of enzymes to lignin. Sulfuric acid lignin was prepared from pine wood sulfite pulp by treatment with 72% sulfuric acid (47). All adsorption experiments were carried out in 1.5 ml of 50 mM sodium phosphate buffer (pH 7.0) containing 10 mg · ml−1 lignin, 5.0 U of each of PcAxy43A, PcXyn10C, PcXyn11A, CtCel9R, and Celluclast 1.5 L, and 1.0 U of TbCglT by continuously mixing the reaction mixtures on a rotating shaker at 50°C for 1 h. The supernatants were then collected and used for the determination of residual activities using their substrates, including birchwood xylan for PcAxy43A, PcXyn10C, and PcXyn11A, carboxymethyl cellulose for CtCel9R and Celluclast 1.5 L, and cellobiose for TbCglT. All experiments were carried out in triplicate. Results were expressed in percentages as residual activities in the supernatant relative to the initial activities.
Saccharification of URS and ARS by xylanolytic enzymes from P. curdlanolyticus B-6.
Two percent (wt/vol) URS or ARS was treated with 3 U each of PcAxy43A, PcXyn10C, and PcXyn11A in 50 mM sodium phosphate buffer (pH 7.0) at 50°C with shaking at 200 rpm for 36 h. Xylose, XOSs, and arabinose in the supernatant were identified by HPLC as described above. Residual solids were collected and washed with water until soluble sugars were not detected, and the dried solid is referred to as xylanolytic enzyme-treated rice straw (XRS). XRS was also used as the substrate for cellulose saccharification.
Steam treatment of XRS.
The steam treatment of XRS was carried out by autoclaving 3 g of XRS in water at 15 lb/in2 and 121°C for 1 h (38). After autoclaving, the sample was cooled to room temperature and filtered to remove the liquid phase, and the retrieved solid was washed with water to remove impurities. The steam-treated solid was oven dried at 60°C until achieving a constant weight. The dried solid fraction (steam-treated XRS, or SXRS) was used as the substrate for cellulose saccharification.
Compositional analysis of XRS, ARS, and SXRS.
The chemical compositions of XRS, ARS, and SXRS were analyzed by following the NREL chemical analysis and testing standard procedure (48). In brief, sulfuric acid (72%) was mixed with each sample at 30°C for 1 h, followed by 3% sulfuric acid hydrolysis at 121°C for 1 h. The sample slurry was neutralized to pH 6.0 with calcium carbonate and vacuum filtered through a filtering crucible. Monosaccharides were quantified by HPLC as described above. Acid-insoluble lignin (Klason lignin) content was defined as the weight of the filter cake after being oven dried at 70°C to a constant weight (49).
Saccharification of XRS, ARS, and SXRS by cellulolytic enzymes.
The reactions were initiated by adding Celluclast 1.5 L (containing 5.0 U of endoglucanase activity and 0.36 U of β-glucosidase activity) or mixing 5.0 U of endoglucanase PcCel9R and 0.36 U of β-glucosidase TbCglT with 3 ml of 50 mM sodium phosphate buffer (pH 7.0) containing 1% (wt/vol) URS, XRS, ARS, or SXRS, followed by incubation at 50°C with shaking at 200 rpm for 48 h. The hydrolysis products in the reaction were identified by HPLC as described above. Glucose and cello-oligosaccharide concentrations were determined by HPLC.
Observation by SEM.
The configurations of URS, PcAxy43A-saccharified untreated rice straw, XRS, and SXRS were observed using a JEOL JSM-6610LV scanning electron microscope (Tokyo, Japan) under low-vacuum or variable-pressure modes at room temperature. The samples were coated with gold prior to imaging.
Calculation of xylose and glucose production yields.
The xylose and glucose production yields in the hydrolysis of untreated and treated rice straw were calculated using the following equations (50): percent xylose yield = [(total xylose release × 0.88)/initial xylan loading] × 100, where 0.88 is the conversion factor of xylose to equivalent xylan, and percent glucose yield = [(total glucose release × 0.90)/initial glucan loading] × 100, where 0.90 is the conversion factor of glucose to equivalent glucan.
ACKNOWLEDGMENTS
We gratefully acknowledge financial support given by the Royal Golden Jubilee Ph.D. program of the Thailand Research Fund (grant 2.J.KT/52/B.1), King Mongkut's University of Technology Thonburi, Thailand, under the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (2016), KMUTT 55th Anniversary Commemorative Fund, and the Energy Policy and Planning Office, Ministry of Energy, Thailand.
REFERENCES
- 1.Bhalla A, Bansal N, Kumar S, Bischoff KM, Sani RK. 2013. Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresour Technol 128:751–759. doi: 10.1016/j.biortech.2012.10.145. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar N, Ghosh SK, Bannerjee S, Aikat K. 2012. Bioethanol production from agricultural wastes: an overview. Renew Energ 37:19–27. doi: 10.1016/j.renene.2011.06.045. [DOI] [Google Scholar]
- 3.Phitsuwan P, Permsriburasuk C, Waeonukul R, Pason P, Tachaapaikoon C, Ratanakhanokchai K. 2016. Evaluation of fuel ethanol production from aqueous ammonia-treated rice straw via simultaneous saccharification and fermentation. Biomass Bioenergy 93:150–157. doi: 10.1016/j.biombioe.2016.07.012. [DOI] [Google Scholar]
- 4.Binod P, Sindhu R, Singhania RR, Vikram S, Devi L, Nagalakshmi S, Kurien N, Sukumaran RK, Pandey A. 2010. Bioethanol production from rice straw: an overview. Bioresour Technol 101:4767–4774. doi: 10.1016/j.biortech.2009.10.079. [DOI] [PubMed] [Google Scholar]
- 5.Van Dyk JS, Pletschke BI. 2012. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes-factors affecting enzymes, conversion and synergy. Biotechnol Adv 30:1458–1480. doi: 10.1016/j.biotechadv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Fan Z, Wagschal K, Chen W, Montross MD, Lee CC, Yuan L. 2009. Multimeric hemicellulases facilitate biomass conversion. Appl Environ Microbiol 75:1754–1757. doi: 10.1128/AEM.02181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M. 2005. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686. doi: 10.1016/j.biortech.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Berlin A, Balakshin M, Gilkes N, Kadla J, Maximenko V, Kubo S, Saddler J. 2006. Inhibition of cellulase, xylanase and β-glucosidase activities by softwood lignin preparations. J Biotechnol 125:198–209. doi: 10.1016/j.jbiotec.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Tomas-Pejo E, Alvira P, Ballesteros M, Negro MJ. 2011. Pretreatment technologies for lignocellulose-to-bioethanol conversion, p 149–176. In Pandey A, Larroche C, Ricke SC, Dussap CG, Gnansounou E (ed), Biofuels: alternative feedstocks and conversion processes, 1st ed Elsevier, Oxford, United Kingdom. [Google Scholar]
- 10.Kumar P, Barrett DM, Delwiche MJ, Stroeve P. 2009. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729. doi: 10.1021/ie801542g. [DOI] [Google Scholar]
- 11.Iwaki A, Kawai T, Yamamoto Y, Izawa S. 2013. Biomass conversion inhibitors furfural and 5-hydroxymethylfurfural induce formation of messenger RNP granules and attenuate translation activity in Saccharomyces cerevisiae. Appl Environ Microbiol 79:1661–1667. doi: 10.1128/AEM.02797-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller FA, Hamilton JE, Nguyen QA. 2003. Microbial pretreatment of biomass: potential for reducing severity of thermochemical biomass pretreatment. Appl Biochem Biotechnol 105:27–41. doi: 10.1385/ABAB:105:1-3:27. [DOI] [PubMed] [Google Scholar]
- 13.Almeida JR, Runquist D, Sànchez Nogué V, Lidén G, Gorwa-Grauslund MF. 2011. Stress-related challenges in pentose fermentation to ethanol by the yeast Saccharomyces cerevisiae. Biotechnol J 6:286–299. doi: 10.1002/biot.201000301. [DOI] [PubMed] [Google Scholar]
- 14.Yanase H, Sato D, Yamamoto K, Matsuda S, Yamamoto S, Okamoto K. 2007. Genetic engineering of Zymobacter palmae for production of ethanol from xylose. Appl Environ Microbiol 73:2592–2599. doi: 10.1128/AEM.02302-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Valdehuesa KNG, Nisola GM, Ramos KRM, Chung WJ. 2012. High yield production of D-xylonic acid from D-xylose using engineered Escherichia coli. Bioresour Technol 115:244–248. doi: 10.1016/j.biortech.2011.08.065. [DOI] [PubMed] [Google Scholar]
- 16.Imjongjairak S, Jommuengbout P, Karpilanondh P, Katsuzaki H, Sakka M, Kimura T, Pason P, Tachaapaikoon C, Romsaiyud J, Ratanakhanokchai K, Sakka K. 2015. Paenibacillus curdlanolyticus B-6 xylanase Xyn10C capable of producing a doubly arabinose-substituted xylose, α-l-Araf-(1→2)-[α-l-Araf-(1→3)]-d-Xylp, from rye arabinoxylan. Enzyme Microb Technol 72:1–9. doi: 10.1016/j.enzmictec.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Teeravivattanakit T, Baramee S, Phitsuwan P, Waeonukul R, Pason P, Tachaapaikoon C, Sakka K, Ratanakhanokchai K. 2016. Novel trifunctional xylanolytic enzyme Axy43A from Paenibacillus curdlanolyticus strain B-6 exhibiting endo-xylanase, β-D-xylosidase, and arabinoxylan arabinofuranohydrolase activities. Appl Environ Microbiol 82:6942–6951. doi: 10.1128/AEM.02256-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sermsathanaswadi J, Pianwanit S, Pason P, Waeonukul R, Tachaapaikoon C, Ratanakhanokchai K, Septiningrum K, Kosugi A. 2014. The C-terminal region of xylanase domain in Xyn11A from Paenibacillus curdlanolyticus B-6 plays an important role in structural stability. Appl Microbiol Biotechnol 98:8223–8233. doi: 10.1007/s00253-014-5748-x. [DOI] [PubMed] [Google Scholar]
- 19.Baramee S, Teeravivattanakit T, Phitsuwan P, Waeonukul R, Pason P, Tachaapaikoon C, Kosugi A, Sakka K, Ratanakhanokchai K. 2016. A novel GH6 cellobiohydrolase from Paenibacillus curdlanolyticus B-6 and its synergistic action on cellulose degradation. Appl Microbiol Biotechnol 101:1175–1188. doi: 10.1007/s00253-016-7895-8. [DOI] [PubMed] [Google Scholar]
- 20.Baramee S, Phitsuwan P, Waeonukul R, Pason P, Tachaapaikoon C, Kosugi A, Ratanakhanokchai K. 2015. Alkaline xylanolytic-cellulolytic multienzyme complex from the novel anaerobic alkalithermophilic bacterium Cellulosibacter alkalithermophilus and its hydrolysis of insoluble polysaccharides under neutral and alkaline conditions. Process Biochem 50:643–650. doi: 10.1016/j.procbio.2015.01.019. [DOI] [Google Scholar]
- 21.Yasuda M, Takeo K, Matsumoto T, Shiragami T, Sugamoto K, Matsushita Y, Ishii Y. 2013. Effectiveness of lignin-removal in simultaneous saccharification and fermentation for ethanol production from napiergrass, rice straw, silvergrass, and bamboo with different lignin-contents. In Chandel AK, Silverio da Silva S (ed), Sustainable degradation of lignocellulosic biomass-techniques, applications and commercialization. InTech, Rijeka, Croatia. doi: 10.5772/54194. [DOI] [Google Scholar]
- 22.Rémond C, Aubry N, Crônier D, Noël S, Martel F, Roge B, Rakotoarivonina H, Debeire P, Chabbert B. 2010. Combination of ammonia and xylanase pretreatments: impact on enzymatic xylan and cellulose recovery from wheat straw. Bioresour Technol 101:6712–6717. doi: 10.1016/j.biortech.2010.03.115. [DOI] [PubMed] [Google Scholar]
- 23.Jönsson LJ, Martín C. 2016. Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Biores Technol 199:103–112. doi: 10.1016/j.biortech.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Hatakka AI. 1983. Pretreatment of wheat straw by white-rot fungi for enzymic saccharification of cellulose. Eur J Appl Microbiol Biotechnol 18:350–357. doi: 10.1007/BF00504744. [DOI] [Google Scholar]
- 25.Rakotoarivonina H, Revol P-V, Aubry N, Rémond C. 2016. The use of thermostable bacterial hemicellulases improves the conversion of lignocellulosic biomass to valuable molecules. Appl Microbiol Biotechnol 100:7577–7590. doi: 10.1007/s00253-016-7562-0. [DOI] [PubMed] [Google Scholar]
- 26.Kumar L, Arantes V, Chandra R, Saddler J. 2012. The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour Technol 103:201–208. doi: 10.1016/j.biortech.2011.09.091. [DOI] [PubMed] [Google Scholar]
- 27.Palonen H, Tjerneld F, Zacchi G, Tenkanen M. 2004. Adsorption of Trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. J Biotechnol 107:65–72. doi: 10.1016/j.jbiotec.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Wallace G, Russell WR, Lomax JA, Jarvis MC, Lapierre C, Chesson A. 1995. Extraction of phenolic-carbohydrate complexes from graminaceous cell walls. Carbohydr Res 272:41–53. doi: 10.1016/0008-6215(95)00036-S. [DOI] [Google Scholar]
- 29.de Gonzalo G, Colpa DI, Habib MHM, Fraaije MW. 2016. Bacterial enzymes involved in lignin degradation. J Biotechnol 236:110–119. doi: 10.1016/j.jbiotec.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Mussatto SI, Fernandes M, Milagres AMF, Roberto NC. 2008. Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer's spent grain. Enzyme Microbial Technol 43:124–129. doi: 10.1016/j.enzmictec.2007.11.006. [DOI] [Google Scholar]
- 31.Jeremic D, Goacher RE, Yan R, Karunakaran C, Master ER. 2014. Direct and up-close views of plant cell walls show a leading role for lignin-modifying enzymes on ensuing xylanases. Biotechnol Biofuels 7:496. doi: 10.1186/s13068-014-0176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins T, Gerday C, Feller G. 2005. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23. doi: 10.1016/j.femsre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Inoue H, Kitao C, Yano S, Sawayama S. 2016. Production of β-xylosidase from Trichoderma asperellum KIF125 and its application in efficient hydrolysis of pretreated rice straw with fungal cellulase. World J Microbiol Biotechnol 32:186. doi: 10.1007/s11274-016-2145-x. [DOI] [PubMed] [Google Scholar]
- 34.Fujii T, Fang X, Inoue H, Murakami K, Sawayama S. 2009. Enzymatic hydrolyzing performance of Acremonium cellulolyticus and Trichoderma reesei against three lignocellulosic materials. Biotechnol Biofuels 2:24. doi: 10.1186/1754-6834-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai Y, Si M, Chen Y, Zhang N, Zhou M, Liao Q, Shi D, Liu Y. 2015. Combination of biological pretreatment with NaOH/urea pretreatment at cold temperature to enhance enzymatic hydrolysis of rice straw. Bioresour Technol 198:725–731. doi: 10.1016/j.biortech.2015.09.091. [DOI] [PubMed] [Google Scholar]
- 36.Taniguchi M, Suzuki H, Watanabe D, Sakai K, Hoshino K, Tanaka T. 2005. Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J Biosci Bioeng 100:637–643. doi: 10.1263/jbb.100.637. [DOI] [PubMed] [Google Scholar]
- 37.Choudhary J, Saritha M, Nain L, Arora A. 2014. Enhanced saccharification of steam-pretreated rice straw by commercial cellulases supplemented with xylanase. J Bioprocess Biotech 4:188–194. doi: 10.4172/2155-9821.1000188. [DOI] [Google Scholar]
- 38.Mes-Hartree M, Dale BE, Craig WK. 1988. Comparison of steam and ammonia pretreatment for enzymatic hydrolysis of cellulose. Appl Microbiol Biotechnol 29:462–468. doi: 10.1007/BF00269069. [DOI] [Google Scholar]
- 39.Kurniati A, Darmokoesoemo H, Puspaningsih NNT. 2016. Scanning electron microscope analysis of rice straw degradation by a treatment with α-L-arabinofuranosidase. Procedia Chem 18:63–68. doi: 10.1016/j.proche.2016.01.011. [DOI] [Google Scholar]
- 40.Matthews S. 2016. Structural changes of rice straw pre-treated with Paenibacillus and Aspergillus fumigatus. Int J Argric Food Res 5:1–8. [Google Scholar]
- 41.Gonçalves GAL, Takasugi Y, Jia L, Mori Y, Noda S, Tanaka T, Ichinose H, Kamiya N. 2015. Synergistic effect and application of xylanases as accessory enzymes to enhance the hydrolysis of pretreated bagasse. Enzyme Microb Technol 72:16–24. doi: 10.1016/j.enzmictec.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 42.DeMartini JD, Foston M, Meng X, Jung S, Kumar R, Ragauskas AJ, Wyman CE. 2015. How chip size impacts steam pretreatment effectiveness for biological conversion of poplar wood into fermentable sugars. Biotechnol Biofuels 8:209. doi: 10.1186/s13068-015-0373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 44.Nelson N. 1944. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380. [Google Scholar]
- 45.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. [PubMed] [Google Scholar]
- 46.Sornyotha S, Kyu KL, Ratanakhanokchai K. 2007. Purification and detection of linamarin from cassava root cortex by high performance liquid chromatography. Food Chem 104:1750–1754. doi: 10.1016/j.foodchem.2006.10.071. [DOI] [Google Scholar]
- 47.Matsushita Y, Yasuda S. 2005. Preparation and evaluation of lignosulfonates as a dispersant for gypsum paste from acid hydrolysis lignin. Bioresour Technol 96:465–470. doi: 10.1016/j.biortech.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 48.Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D. 2008. Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory, Golden, CO: https://purl.access.gpo.gov/GPO/LPS94089. [Google Scholar]
- 49.Waeonukul R, Kosugi A, Tachaapaikoon C, Pason P, Ratanakhanokchai K, Prawitwong P, Deng L, Saito M, Mori Y. 2012. Efficient saccharification of ammonia soaked rice straw by combination of Clostridium thermocellum cellulosome and Thermoanaerobacter brockii β-glucosidase. Bioresour Technol 107:352–357. doi: 10.1016/j.biortech.2011.12.126. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Kim TH. 2011. Low-liquid pretreatment of corn stover with aqueous ammonia. Bioresour Technol 102:4779–4786. doi: 10.1016/j.biortech.2011.01.008. [DOI] [PubMed] [Google Scholar]