Summary
Background
There is a scarcity of evidence about the role of patient choice and hospital competition policies on surgical cancer services. Previous evidence has shown that patients are prepared to bypass their nearest cancer centre to receive surgery at more distant centres that better meet their needs. In this national, population-based study we investigated the effect of patient mobility and hospital competition on service configuration and technology adoption in the National Health Service (NHS) in England, using prostate cancer surgery as a model.
Methods
We mapped all patients in England who underwent radical prostatectomy between Jan 1, 2010, and Dec 31, 2014, according to place of residence and treatment location. For each radical prostatectomy centre we analysed the effect of hospital competition (measured by use of a spatial competition index [SCI], with a score of 0 indicating weakest competition and 1 indicating strongest competition) and the effect of being an established robotic radical prostatectomy centre at the start of 2010 on net gains or losses of patients (difference between number of patients treated in a centre and number expected based on their residence), and the likelihood of closing their radical prostatectomy service.
Findings
Between Jan 1, 2010, and Dec 31, 2014, 19 256 patients underwent radical prostatectomy at an NHS provider in England. Of the 65 radical prostatectomy centres open at the start of the study period, 23 (35%) had a statistically significant net gain of patients during 2010–14. Ten (40%) of these 23 were established robotic centres. 37 (57%) of the 65 centres had a significant net loss of patients, of which two (5%) were established robotic centres and ten (27%) closed their radical prostatectomy service during the study period. Radical prostatectomy centres that closed were more likely to be located in areas with stronger competition (highest SCI quartile [0·87–0·92]; p=0·0081) than in areas with weaker competition. No robotic surgery centre closed irrespective of the size of net losses of patients. The number of centres performing robotic surgery increased from 12 (18%) of the 65 centres at the beginning of 2010 to 39 (71%) of 55 centres open at the end of 2014.
Interpretation
Competitive factors, in addition to policies advocating centralisation and the requirement to do minimum numbers of surgical procedures, have contributed to large-scale investment in equipment for robotic surgery without evidence of superior outcomes and contributed to the closure of cancer surgery units. If quality performance and outcome indicators are not available to guide patient choice, these policies could threaten health services' ability to deliver equitable and affordable cancer care.
Funding
National Institute for Health Research.
Introduction
The centralisation of complex cancer surgery into fewer, high-volume units is occurring across Europe, the USA, and Canada, guided by evidence that centres that carry out a high volume of surgical procedures have better outcomes of care for patients than do centres that carry out a low volume of surgical procedures.1, 2, 3 At the same time, patient choice and hospital competition policies have been introduced in several countries4, 5, 6, 7—and are under consideration in others8—with the aim of improving the responsiveness and efficiency of health services delivered. In health-care systems where hospitals compete on quality and not on price, competition is also expected to incentivise improvements in the quality of hospital services to attract patients.9
Choice and competition, as well as centralisation, attempt to achieve improvements in patient outcomes, but they require different health-system configurations and provider incentives to operate effectively. Finding the right balance between choice and competition on the one hand and centralisation on the other is therefore key, but there is little evidence to guide how best to achieve this.10
The UK National Health Service (NHS) is an example of a health system that remains committed to choice and competition as a health-care reform model since the inception of this model in 2006.11 The cost of providing services is fixed under a national rate tariff scheme12 and hospitals are expected to compete for patients on the basis of quality. Receiving care incurs no additional user charges at the point of access and patients have the right to choose and travel to any hospital that best meets their needs.
Research in context.
Evidence before this study
Several countries have introduced policies that allow patients to choose a specific health-care provider, with the aim of improving the quality of care. We did a systematic review to assess the evidence that patients with cancer are willing to travel beyond (bypass) their nearest hospital for cancer surgery, and to assess the effect of competition on outcomes of surgery. We searched PubMed and Embase for relevant articles published between Jan 1, 1990 and Dec 31, 2015. Search criteria are in the appendix. 5994 titles and abstracts were reviewed. Five studies had empirically assessed the mobility of patients for cancer surgery. Patients were attracted to hospitals that had shorter waiting lists, that offered advanced technology, and that had indicators of better service quality than other hospitals. There was significant heterogeneity in the design of empirical studies, including differences in data quality, the geographical unit of analysis, and limited control for the influence of price competition. No studies had looked at the effect of competition on outcomes of cancer.
Added value of this study
To our knowledge, this is the first national evaluation of the effect of choice and competition policies on the patterns of service configuration and technology adoption for cancer surgery. We studied travel patterns of more than 19 000 patients who had a radical prostatectomy between 2010 and 2014 in the National Health Service (NHS) in England. The mobility of men to alternative, more distant centres resulted in substantial changes in market share for individual surgical centres, which were most marked in areas of highest competition. Centres that lost local patients to other centres were at risk of closure. Patients were attracted to centres offering robotic surgery, and other centres adopted this technology to preserve their market share. We found that, between 2010 and 2017, there has been large-scale adoption of robot-assisted radical prostatectomy, increasing by three times, from 12 centres at the start of 2010 to 42 by 2017. During the same time period, 16 of the 65 NHS radical prostatectomy centres in England closed their prostate cancer surgery unit.
Implications of all the available evidence
Patients with cancer respond to policies that enable them to choose a surgical provider of their choice. In the absence of appropriate information about quality of care, policies based on patient choice and hospital competition could create incentives for adoption of new technologies without evidence of superior outcomes as hospitals look to retain and attract new patients. The resulting changes in market share for individual hospitals could threaten the viability of their surgical services.
Additionally, national policy in the UK continues to advocate centralisation of specialist cancer services such as prostate and oesophagogastric surgery.13, 14, 15, 16 Not only does this serve to reduce the number of hospitals that patients with cancer can choose from, but it is also expected that patients will receive care at their nearest (local) centre on the basis of established secondary care referral pathways for specialist cancer surgery.17
However, our 2017 analysis18 found that not all patients are following the expected referral patterns for specialised cancer surgery. One in three men who had a radical prostatectomy for prostate cancer between 2010 and 2014 in the NHS travelled beyond or bypassed their nearest prostate cancer surgery centre, in many cases across regional boundaries. This observation especially applied to younger, fitter, and more affluent men than to older, less fit, and less affluent counterparts. In the absence of indicators that accurately reflect the quality of prostate cancer surgery, men were attracted to centres offering robot-assisted radical prostatectomy or centres that employed surgeons with a national reputation for prostate cancer surgery.
There is little evidence about what effect patient mobility and hospital competition have had on the configuration of specialist cancer services and the introduction of new surgical technologies into clinical practice. We used patient-level data and geographical information system modelling to analyse the effect of patient mobility for cancer surgery and hospital competition on service configuration and technology adoption within the NHS, using prostate cancer as a model. In light of our findings, we appraised the international evidence exploring the role of choice and competition policies on the delivery of cancer surgery services and considered opportunities for developing the empirical research base in this area.
Methods
Patient population
For this national, population-based study we obtained individual patient-level data from the National Cancer Registration and Analysis Service (NCRAS) for all men who were diagnosed with prostate cancer and underwent a radical prostatectomy in the NHS in England between Jan 1, 2010, and Dec 31, 2014. These data were linked at the individual patient level to Hospital Episode Statistics (HES), the administrative database of all hospital episodes in NHS hospitals in England.19
The study was exempt from NHS Research Ethics Committee approval because it involved analysis of an existing dataset of anonymised data for service evaluation.
Study design
To define each individual patient's residence, we used the population-weighted centroids of small geographical areas termed lower super output areas (LSOAs). These weightings provide location coordinates for the greatest population density in the LSOA. There are 34 753 of these small geographical areas (ie, LSOAs) in England, with an average population of about 1600.20 Both the LSOAs and full postcodes for the hospitals where the surgery was done were inputted into a geographical information system (Esri ArcGIS 10.3) to calculate travel times according to the fastest route by car to all surgical centres in England (calculated by use of the Ordnance Survey MasterMap Integrated Transport Network). Patients receiving surgery at their nearest centre were defined as core users. Those who did not receive care at their nearest surgical centre were classified as bypassers.
For each surgical centre, we identified the number of leavers—patients for whom that centre was nearest but who had their treatment at an NHS centre further away. We also identified the number of arrivers—patients for whom another centre was nearest but who had their surgery at that centre. A centre was identified as being a winner (ie, having a net gain of patients) or loser (ie, having a net loss of patients) if the difference between leavers and arrivers was statistically significant based on the conditional method for testing a difference between two Poisson means.21
For each surgical centre we calculated a spatial competition index (SCI) as a measure of external competition.22, 23 The SCI provides a uniform metric that can be used across all surgical centres and that represents the demand for services and the availability of alternative hospitals. Across England, there is variation in the concentration of available hospitals depending on the degree of urbanisation or rurality. For example, the northeast (one of nine English regions) is a predominantly rural area that is 8592 km2 in size and had three surgical centres at the start of the study period. Conversely, London is 1572 km2 in size (and the largest urbanised region in Europe) and had ten surgical centres at the start of the study period.24
Data analysis
In this analysis, the SCI for a surgical centre was calculated on the basis of both the number of eligible patients within a 60-min drive and the number of surgical centres within a 60-min drive for each eligible patient; in the equation shown, the surgical centre i has n eligible patients within a 60-min drive, and patient j in centre i has k surgical centres within a 60-min drive:
The SCI ranges theoretically from 0 for centres in a monopoly environment to a value close to 1 for centres in the most competitive environment.
At the start of the study period (January, 2010) there were 65 prostate cancer surgical centres in England, of which 12 centres routinely performed robot-assisted radical prostatectomy procedures. These centres were labelled as established robotic centres. An analysis of HES data, in addition to an organisational survey produced as part of the National Prostate Cancer Audit,17 was used to evaluate the change in configuration of prostate cancer surgical units across England and the availability of robotic surgery from 2010 onwards. The χ2 test was used to compare proportions. All analyses were done with Stata, version 14, to assess the effect of competition, as measured by the SCI, on changes in service configuration (expressed as net gains or losses of patients as defined above) and adoption of robotic surgery in the NHS.
Role of the funding source
The funder of the study, National Institute for Health Research, had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication. AA and JvdM had full access to all the data in the study, take responsibility for the integrity of the data and the accuracy of the data analysis, and had final responsibility for the decision to submit for publication.
Results
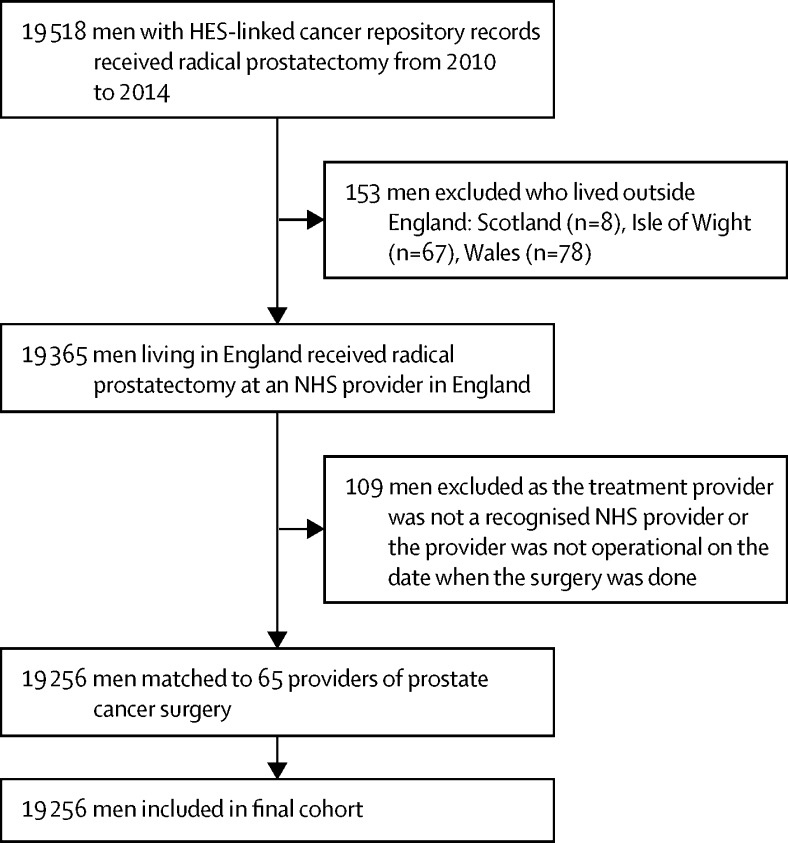
We identified 19 518 men who were diagnosed with prostate cancer and underwent radical prostatectomy in the NHS in England between Jan 1, 2010, and Dec 31, 2014. Of these 19 518 men, 262 (1·3%) were excluded because they either lived outside England or could not be assigned to a particular hospital; 19 256 were eligible for inclusion in the study (figure 1).
Figure 1.
Flowchart of men included in the study
HES=Hospital Episode Statistics. NHS=UK National Health Service.
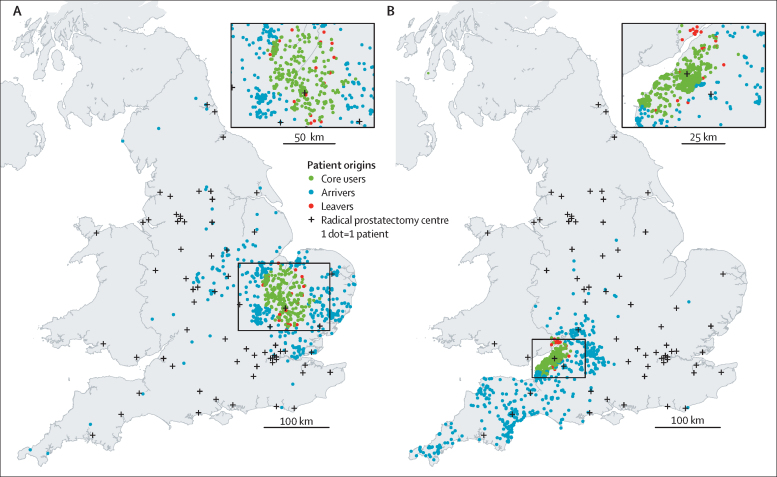
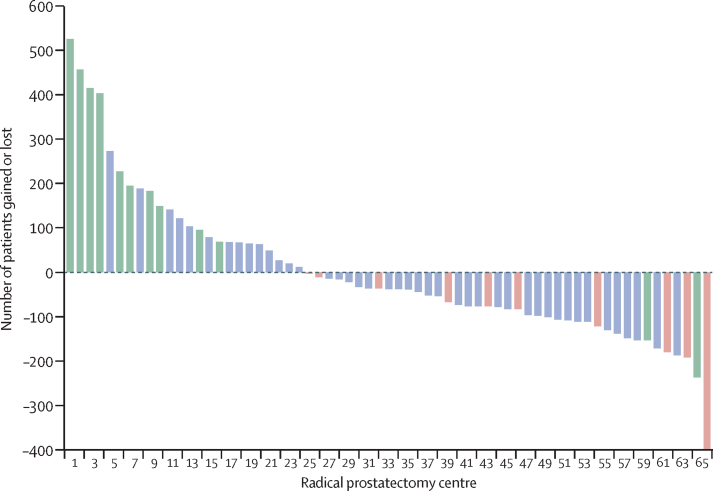
Figure 2 shows the places of residence for patients who had their prostate cancer surgery at two selected surgical centres located in the east of England (figure 2A) and southwest England (figure 2B), both of which were classified as winners. Figure 3 shows the net gains and losses of patients for each radical prostatectomy centre identified during the study period. 23 (35%) of the 65 centres were classified as winners and 37 (57%) of 65 as losers. Five centres did not have a statistically significant net gain or loss of patients. Some of the winners were doing 400 to 500 more procedures than expected if they had only been operating on local men for whom this was the nearest centre. Conversely, some of the losers were doing approximately 200 fewer procedures than expected (and 400 fewer in the case of one centre).
Figure 2.
Mobility patterns of patients receiving radical prostate cancer surgery at two selected NHS cancer centres
Maps of the UK, illustrating the mobility pattern of patients who received radical prostate cancer surgery at two selected National Health Service (NHS) cancer centres (indicated with a + symbol in the area of core users) located in the east of England (A) and southwest England (B) that had a net gain of patients from outside their local area (ie, more arrivers than leavers). Both centres were established robotic centres. The maps include a scaled magnification of the region inset. Contains National Statistics data, © Crown copyright and database right 2017; NHS Research Scotland (NRS) data, © Crown copyright and database right 2017; Ordnance Survey data © Crown copyright and database right 2017; and Northern Ireland Statistics and Research Agency data.
Figure 3.
Net gains and losses of patients for each radical prostatectomy centre (n=65) during the study period
Established robotic radical prostatectomy centres (n=12) shown in green and centres that closed during the 2010–14 study period (n=10) shown in red. Centres in blue are centres that were neither robotic radical prostectomy centres nor centres that closed during the study period.
Figure 3 also shows the relationship between, on the one hand, radical prostatectomy centres having a net gain or net loss of patients and, on the other hand, being an established robotic centre or a centre that closed during the study period. Centres with a net gain were more likely to be established robotic centres (ten [43%] of the 23 winners were robotic centres, compared with two [5%] of the 37 centres with a net loss; p=0·0043). Conversely, ten (27%) of the 37 centres with a net loss of patients closed down during the study period.
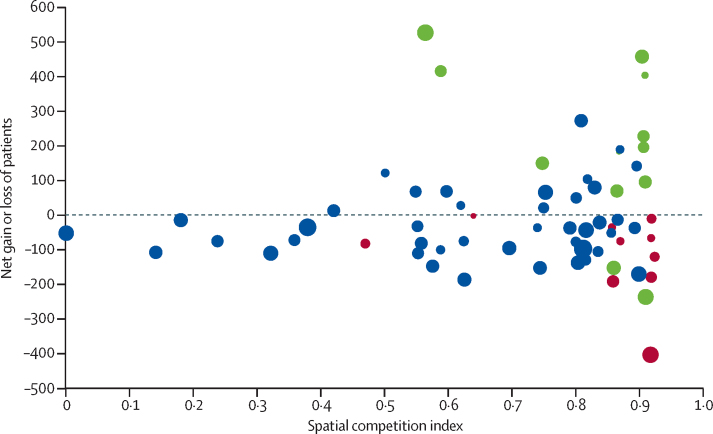
Centres with the largest net gains or losses were predominantly located in the most competitive areas (figure 4). Established robotic centres were most likely to be located in the highest quartile (SCI 0·87–0·92) for hospital competition. Seven (41%) of the 17 centres in the highest SCI quartile were established robotic centres compared with five (10%) of the 48 other centres in the three other quartiles (p=0·0050). Similarly, for centre closures, six (35%) of the 17 centres in the highest SCI quartile closed compared with four (8%) of the 48 other centres (p=0·0081).
Figure 4.
Effect of competition on the net gain or loss of patients for each radical prostatectomy centre during the study period
The size of the circles corresponds to the number of men expected to have surgery at the centre. Red circles correspond to centres that closed during the study period (2010–14). Green circles correspond to established robotic centres. Blue circles correspond to centres that were neither robotic radical prostectomy centres nor centres that closed during the study period. Spatial competition index (SCI) score 0=hospital facing weakest competition. SCI score 1=hospital facing strongest competition.
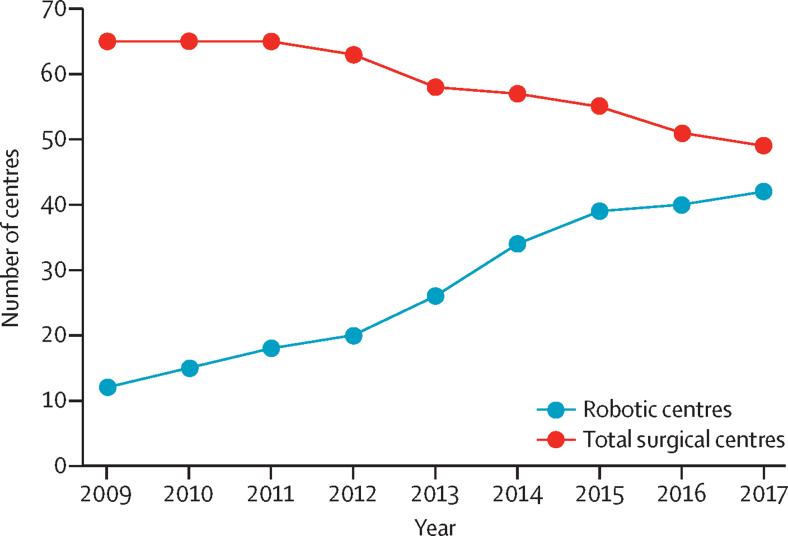
Both the analysis of HES and the results of the national organisational survey showed profound changes in the organisation and practices of prostate cancer surgical care that continued beyond the end of the study period (figure 5). Between 2010 and 2017, there has been large-scale adoption of robotic surgery, increasing by three times, from 12 (18%) of 65 centres open at the start of 2010 to 39 (71%) of 55 centres open in 2014 to 42 (86%) of 49 in 2017. In the same time period, 16 (25%) of the 65 NHS radical prostatectomy centres in England closed. Both the closures and the rapid and widespread adoption of robotic surgery have been unforeseen, effectively rendering commissioning guidelines—published only in 2015 and recommending phased introduction of robotics for prostate cancer surgery within the NHS—obsolete.25
Figure 5.
Changes in the number of robotic centres and total number of centres in the NHS in England (2009–17)
Discussion
Our results suggest that, during the study period analysed, patient choice and hospital competition, rather than a coordinated policy towards centralisation, have been drivers in the changing configuration of surgical cancer services. The proportion of patients who bypassed their nearest hospital to have prostate cancer surgery elsewhere has been far larger than the 5–10% considered to be necessary in the health economics literature to incentivise improvements in hospital quality.26
In the absence of data on outcomes, the mobility of patients has been driven by factors such as availability of advanced surgical technology and the reputation of individual hospitals and clinicians.18 The resulting competition between hospitals has contributed to the closure of radical prostatectomy centres in the NHS in England and widespread adoption of robot-assisted radical prostatectomy as centres have had to respond to potential changes in their market share, which threatened both their income and their ability to meet minimum procedure volume requirements. This finding indicates that patient choice and hospital competition, although rarely considered in redesign of cancer services, are potentially powerful drivers of service change, even within publicly funded systems. It is unlikely that these findings are limited to the NHS in England or to prostate cancer surgery alone.
From a wider system perspective, the geographical layout of cancer services means that not all centres face the same competitive pressures and, in turn, will respond differently to choice and competition policies as mechanisms for quality improvement. For example, ten of the 12 established robotic centres or early adopters of robotics were located in the most competitive areas. However, we found that patients were prepared to travel substantial distances for treatment, in some cases bypassing several surgical units, which means that even centres within less competitive areas face some level of external competition for patients and subsequently become late adopters of technology to retain local patients.27
Attempts to coordinate cancer care services through centralisation and regionalisation have largely ignored the fact that patients are prepared to bypass their local services for treatment. This occurrence is partly due to the paucity of empirical evidence about the extent of patient mobility.28, 29, 30 Additionally, cancer care plans have exerted limited control of the available services and technology at the individual hospital level (eg, introduction of new devices and practices of care), which can serve as proxy measures of quality in the absence of quality indicators.31
Substantial levels of patient mobility mean that centres need to compete with other providers to meet minimum procedure volume thresholds as set down by national policy.16 In England, each prostate cancer surgery centre is expected to do a specified number of operations per year or face the threat of closure.15, 32 Competition policies have therefore stimulated a form of centralisation through natural selection, as centres act to protect their status as a cancer surgery centre, rather than through a coordinated process based on valid indicators of quality. Similar effects have been observed in the US health-care market, where both acute and non-acute care services have closed in response to competition.33, 34 It is unclear whether these effects have improved the quality of care.
None of the centres that closed during the study period did so because of explicit evidence of poor quality. Instead, the closures appear to have been influenced by the decisions of individual patients in selecting their health-care provider. Further research is required to establish what effect the observed pattern of closures has had on travel times, outcomes, and equity in access to surgical services for the most vulnerable groups, given their decreased ability to travel.28, 35
The patterns of patient mobility observed occurred at a time when comparative outcome measures for prostate cancer surgery were not available. This observation highlights that providers of cancer services, just like any other industry, will consider the use of alternative incentives to attract or retain patients.36, 37, 38 Patients will gravitate to places that make themselves attractive and by doing so they will create centres that treat large numbers of patients, which itself will attract further patients.39
Patients with prostate cancer were more likely to travel to centres that were early adopters of robot-assisted radical prostatectomy, showing the powerful effect of advanced technology on perceptions of quality. The result of this travel pattern has been that other centres have invested in costly robotic surgery to avoid losing their patients to other centres and to maintain their market share to preserve their cancer centre status, despite a scarcity of evidence for the superiority of this surgical procedure with respect to functional and oncological outcomes.40, 41 Notably, none of the centres that adopted robotic surgery closed down. Similar patterns have been observed in other health-care markets across the USA and Europe, with cancer centres adopting robotic surgery to increase their market share.36, 42, 43, 44
Our previous systematic review of the literature on patient choice and competition28 identified five empirical studies in high-income settings showing that patients with several tumour types, including breast, bladder, gastric, colorectal, and thoracic cancers, were prepared to bypass their nearest surgical centre.45, 46, 47, 48, 49, 50 The availability of advanced surgical techniques, procedure volume, and both surgeon and hospital reputation were identified as key drivers for patient mobility. Patients of advanced age and from low socioeconomic backgrounds were less likely to consider alternatives than those who were younger and more affluent.
Hospital competition, rather than the pursuit of better quality care by itself, is also cited as a major factor influencing the adoption of new technologies and diversifying individual practices of care for both cancer surgery and radiotherapy.51 There is growing evidence of rapid adoption of technology for cancer surgery across a range of cancer types, beyond prostate cancer, such as renal, colorectal, and gynaecological cancer surgery.36, 52, 53, 54, 55 For radiotherapy, where one would expect potentially less patient mobility than is normally observed for services because of the protracted duration of radiotherapy regimens, the past decade has also seen a substantial increase in the use of an array of high-cost technologies.41 These technologies have included intensity-modulated radiotherapy, stereotactic-beam radiotherapy, and proton-beam therapy, with providers trying to gain a competitive advantage over others.51
The question as to whether competition can stimulate improvements in outcomes of cancer surgery remains unanswered. Two studies have analysed the effect of hospital competition on the pricing of pancreatic cancer56 and colon cancer57 surgery, and one study assessed the effect of such competition on the efficiency of cancer care delivery across tumour types in the US cancer health-care market.58 Studies across other specialties have shown mixed results for the effect of fixed-price markets on improvements in health-care quality.8, 23, 59, 60, 61, 62, 63, 64, 65, 66
The dearth of studies on patient mobility in both high-income and emerging economies is a major limitation for evidenced-based policy making to decide how best to balance patient choice and top-down policy approaches to service coordination in cancer care. We have highlighted potential approaches for management of this health system challenge.
For patients, having choice over their treatment or how a specific treatment is given might be more important than having a choice over the actual service provider.67 Therefore, differences in availability of technology at the local level, even within a system that publishes validated outcome measures, can contribute to shifts in market share.28 Investment in medical devices for cancer care51 seems to be driven predominantly by individual clinicians and clinical departments, possibly because the regulatory hurdles for adoption of new devices are relatively low compared with those of medicines.31, 68
The use of health technology assessment processes or value frameworks for all new technologies across the cancer care spectrum (ie, medicines, radiotherapy, and surgery) would act as a meaningful first step towards providing stronger guidance on which interventions are likely to deliver the greatest value to patients and society.69, 70 Other options for coordination of technology adoption include coverage with evidence development schemes or establishment of nationally designated research centres to trial new technologies before considering reimbursement.71 However, a significant time lag remains before functional and oncological outcomes will be available to inform national implementation, especially for conditions with a lengthy disease course—such as prostate cancer.
Competition between hospitals will continue irrespective of attempts to centralise cancer services. Whether public reporting of performance indicators could help to achieve improvements in care quality through competition is debatable.72 It might never be feasible to develop meaningful indicators for some tumour types. For example, the appropriateness of many available indicators is problematic because they can only be published after a long lag period (eg, side-effects and survival rates at 1 and 5 years), during which clinical practice can change substantially.73 Additionally, there is little evidence to suggest that individuals are more likely to use published performance indicators than proxies for quality, such as a hospital's or clinician's reputation.74, 75
However, in the absence of any indicator, hospitals will try to differentiate themselves to attract new users, and patients will continue to be reliant on lay sources of information, including industry marketing.76 This observation strengthens the need to develop and provide access to performance indicators across different tumour types to inform patients' decision making. Performance indicators are publicly available for oesophageal and bowel cancer surgery in the NHS.77, 78 Additionally, the National Prostate Cancer Audit has completed a national Patient Reported Outcome Measures (PROMs) collection exercise for men following radical surgery or radiotherapy, with the aim of reporting risk-adjusted outcomes at the individual hospital level.79 Public reporting of outcomes would mean that quality improvement could be stimulated through hospitals competing for market share or aiming to avoid reputational losses.80, 81
Finally, the configuration of cancer services needs to account for existing patterns of patient mobility, hospital capacity, catchment areas, and clinical quality. To this end, location-allocation modelling provides a rigorous empirical approach to optimising the configuration of health-care services (including decisions about service centralisation).82, 83 For example, it can guide which centres should close to maximise outcomes, or minimise travel distances for those individuals who face difficulties in accessing services because of financial and physical constraints.82, 84
A limitation of our study is that we used centroids of the LSOAs as the representation of the patients' residence. This will have added noise to the determination of centres' net gain and net loss of patients. It is likely that this noise has attenuated rather than enhanced the observed relationships between spatial competition and technology adoption on the one hand and patient mobility on the other.
In conclusion, we show that patient choice and hospital competition can have a major influence on the configuration of cancer services. The challenge for health systems is to balance choice and competition with service centralisation, but there is a paucity of empirical evidence to inform this decision making. Our study highlights the need to have robust quality performance and outcome measures available to patients and referring health centres, to avoid reliance on often misleading surrogate indicators. Otherwise, choice and competition policies could seriously limit rather than facilitate health services' ability to deliver equitable and affordable improvements in cancer outcomes.
For more on Hospital Episode Statistics see http://content.digital.nhs.uk/hes
Acknowledgments
Acknowledgments
AA is funded by a Doctoral Research Fellowship from the National Institute for Health Research. JvdM is partly supported by the NHS National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care North Thames at Bart's Health NHS Trust. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health. Hospital Episode Statistics were made available by the NHS Health and Social Care Information Centre. (© 2012, Re-used with the permission of NHS Digital. All rights reserved.) Data for this study are based on patient-level information collected by the NHS, as part of the care and support of patients with cancer. The data are collated, maintained, and quality-assured by the National Cancer Registration and Analysis Service, which is part of Public Health England. Access to the data was facilitated by the Public Health England's Office for Data Release. AA and JvdM are members of the Project Team of the National Prostate Cancer Audit funded by the Healthcare Quality Improvement Partnership. We thank Graham Davies for his valuable comments and insights during the drafting of the manuscript.
Contributors
AA conceived the study. AA, JvdM, and DL were involved in the design, analysis, and interpretation. AA wrote the paper, with support from JvdM. All authors were involved in revising the work critically and approved the final version.
Declaration of interests
JvdM reports grants from Healthcare Quality Improvement Partnership during the conduct of the study. All other authors declare no competing interests.
Supplementary Material
References
- 1.Wyld L, Audisio RA, Poston GJ. The evolution of cancer surgery and future perspectives. Nat Rev Clin Oncol. 2015;12:115–124. doi: 10.1038/nrclinonc.2014.191. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EV. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 3.Gruen RL, Pitt V, Green S, Parkhill A, Campbell D, Jolley D. The effect of provider case volume on cancer mortality: systematic review and meta-analysis. CA Cancer J Clin. 2009;59:192–211. doi: 10.3322/caac.20018. [DOI] [PubMed] [Google Scholar]
- 4.Vrangbaek K, Ostergren K, Birk HO, Winblad U. Patient reactions to hospital choice in Norway, Denmark, and Sweden. Health Econ Policy Law. 2007;2:125–152. doi: 10.1017/S174413310700401X. [DOI] [PubMed] [Google Scholar]
- 5.Balia S, Brau R, Marrocu E. What drives patient mobility across Italian regions? Evidence from hospital discharge data. Dev Health Econ Public Policy. 2014;12:133–154. doi: 10.1007/978-88-470-5480-6_6. [DOI] [PubMed] [Google Scholar]
- 6.Siciliani L, Chalkley M, Gravelle H. Policies towards hospital and GP competition in five European countries. Health Policy. 2017;121:103–110. doi: 10.1016/j.healthpol.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Pope DG. Reacting to rankings: evidence from “America's Best Hospitals”. J Health Econ. 2009;28:1154–1165. doi: 10.1016/j.jhealeco.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Gaynor M, Moreno-Serra R, Propper C. Death by market power: reform, competition, and patient outcomes in the National Health Service. Am Econ J Econ Policy. 2013;5:134–166. [Google Scholar]
- 9.White LJ. Quality variation when prices are regulated. Bell J Econ Manag Sci. 1972;3:425–436. [Google Scholar]
- 10.Baicker K, Levy H. Coordination versus competition in health care reform. N Engl J Med. 2013;369:789–791. doi: 10.1056/NEJMp1306268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Department of Health The NHS Choice Framework: what choices are available to me in the NHS? April 29, 2016. https://www.gov.uk/government/publications/the-nhs-choice-framework/the-nhs-choice-framework-what-choices-are-available-to-me-in-the-nhs (accessed Aug 8, 2017).
- 12.Department of Health . Reforming NHS financial flows: introducing payment by results. Department of Health; London: 2002. [Google Scholar]
- 13.Wouters M, Karim-Kos H, le Cessie S. Centralization of esophageal cancer surgery: does it improve clinical outcome? Ann Surg Oncol. 2009;16:1789–1798. doi: 10.1245/s10434-009-0458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trinh QD, Bjartell A, Freedland SJ. A systematic review of the volume-outcome relationship for radical prostatectomy. Eur Urol. 2013;64:786–798. doi: 10.1016/j.eururo.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NICE Improving outcomes in urological cancers. guidance on cancer services. Sept 19, 2002. https://www.nice.org.uk/guidance/csg2 (accessed Aug 8, 2017).
- 16.Association of Upper Gastrointestinal surgeons of Great Britain and Ireland The provision of services for upper gastrointestinal surgery. April, 2016. http://www.augis.org/wp-content/uploads/2016/06/Provision-of-Services-June-2016.pdf (accessed Sept 12, 2017).
- 17.Aggarwal A, Nossiter J, Cathcart P. Organisation of prostate cancer services in the English National Health Service. Clin Oncol (R Coll Radiol) 2016;28:482–489. doi: 10.1016/j.clon.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal A, Lewis D, Charman SC. Determinants of patient mobility for prostate cancer surgery: a population based study of choice and competition. Eur Urol. 2017 doi: 10.1016/j.eururo.2017.07.013. DOI:10.1016/j.eururo.2017.07.013 published online Jul 29. [DOI] [PubMed] [Google Scholar]
- 19.The National Cancer Registration and Analysis Service National Cancer Registration for England. 2016. http://www.ncras.nhs.uk/phe-office-data-release-odr/ (accessed Aug 8, 2017).
- 20.Office for National Statistics. Super Output Area (SOA). http://webarchive.nationalarchives.gov.uk/20160105160709/http://www.ons.gov.uk/ons/guide-method/geography/beginner-s-guide/census/super-output-areas--soas-/index.html (accessed Aug 8, 2017).
- 21.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- 22.Gravelle H, Santos R, Siciliani L, Goudie R. Hospital quality competition under fixed prices. CHE Research Paper 80. Centre for Health Economics (University of York); York: 2012. [Google Scholar]
- 23.Diller G-P, Kempny A, Piorkowski A. Choice and competition between adult congenital heart disease centers: evidence of considerable geographical disparities and association with clinical or academic results. Circ Cardiovasc Qual Outcomes. 2014;7:285–291. doi: 10.1161/CIRCOUTCOMES.113.000555. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal A, Nossiter J, Cathcart P, Rashbass J, Payne H, van der Meulen J. The National Prostate Cancer Audit—results from the organisational survey of NHS Trusts in England. Clin Oncol. 2015;27:e3. [Google Scholar]
- 25.NHS England Clinical commissioning policy: robotic-assisted surgical procedures for prostate cancer. October, 2015. https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/10/b14pa-rbtic-asstd-srgry-prostate-cancer-oct15.pdf (accessed Aug 8, 2017).
- 26.Le Grand J. The other invisible hand: delivering public services through choice and competition. Princeton University Press; Princeton, NJ: 2009. [Google Scholar]
- 27.Rogers EM. Diffusion of innovations. 4th edn. Simon and Schuster; New York, NY: 2010. [Google Scholar]
- 28.Aggarwal A, Lewis D, Mason M, Sullivan R, van der Meulen J. Patient mobility for elective secondary health care services in response to patient choice policies: a systematic review. Med Care Res Rev. 2017;74:379–403. doi: 10.1177/1077558716654631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollock A, Macfarlane A, Kirkwood G. No evidence that patient choice in the NHS saves lives. Lancet. 2011;378:2057–2060. doi: 10.1016/S0140-6736(11)61553-5. [DOI] [PubMed] [Google Scholar]
- 30.Fotaki M, Roland M, Boyd A, McDonald R, Scheaff R, Smith L. What benefits will choice bring to patients? Literature review and assessment of implications. J Health Serv Res Policy. 2008;13:178–184. doi: 10.1258/jhsrp.2008.007163. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal A, Nossiter J, Cathcart P. Organisation of prostate cancer services in the English National Health Service. Clin Oncol. 2016;28:482–489. doi: 10.1016/j.clon.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 32.NICE Prostate cancer: diagnosis and management. January, 2014. https://www.nice.org.uk/guidance/cg175 (accessed Aug 8, 2017).
- 33.Hsia RY, Kellermann AL, Shen Y-C. Factors associated with closures of emergency departments in the United States. JAMA. 2011;305:1978–1985. doi: 10.1001/jama.2011.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Succi MJ, Lee SY, Alexander JA. Effects of market position and competition on rural hospital closures. Health Serv Res. 1997;31:679–699. [PMC free article] [PubMed] [Google Scholar]
- 35.Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009;27:4671–4678. doi: 10.1200/JCO.2008.20.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrishami P, Boer A, Horstman K. Understanding the adoption dynamics of medical innovations: affordances of the da Vinci robot in the Netherlands. Soc Sci Med. 2014;117:125–133. doi: 10.1016/j.socscimed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 37.Holcombe RF. The ethics of marketing cancer. J Cancer Policy. 2015;3:1–2. [Google Scholar]
- 38.Osborne NH, Ghaferi AA, Nicholas LH, Dimick JB, Mph M. Evaluating popular media and internet-based hospital quality ratings for cancer surgery. Arch Surg. 2011;146:600–604. doi: 10.1001/archsurg.2011.119. [DOI] [PubMed] [Google Scholar]
- 39.De Kuijper M. Profit power economics: a new competitive strategy for creating sustainable wealth. Oxford University Press; New York, NY: 2009. [Google Scholar]
- 40.Ramsay C, Pickard R, Robertson C. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess. 2012;16:1–313. doi: 10.3310/hta16410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen PL, Gu X, Lipsitz SR. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaye DR, Mullins JK, Carter HB, Bivalacqua TJ. Robotic surgery in urological oncology: patient care or market share? Nat Rev Urol. 2015;12:55–60. doi: 10.1038/nrurol.2014.339. [DOI] [PubMed] [Google Scholar]
- 43.Neuner JM, See WA, Pezzin LE, Tarima S, Nattinger AB. The association of robotic surgical technology and hospital prostatectomy volumes: increasing market share through the adoption of technology. Cancer. 2012;118:371–377. doi: 10.1002/cncr.26271. [DOI] [PubMed] [Google Scholar]
- 44.Groeben C, Koch R, Baunacke M, Wirth MP, Huber J. Robots drive the German radical prostatectomy market: a total population analysis from 2006 to 2013. Prostate Cancer Prostatic Dis. 2016;19:412–416. doi: 10.1038/pcan.2016.34. [DOI] [PubMed] [Google Scholar]
- 45.Nostedt MC, McKay AM, Hochman DJ. The location of surgical care for rural patients with rectal cancer: patterns of treatment and patient perspectives. Can J Surg. 2014;57:398–404. doi: 10.1503/cjs.002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kronebusch K. Quality information and fragmented markets: patient responses to hospital volume thresholds. J Health Polit Policy Law. 2009;34:777–827. doi: 10.1215/03616878-2009-025. [DOI] [PubMed] [Google Scholar]
- 47.Basu J. Severity of illness, race, and choice of local versus distant hospitals among the elderly. J Health Care Poor Underserved. 2005;16:391–405. doi: 10.1353/hpu.2005.0023. [DOI] [PubMed] [Google Scholar]
- 48.Fabbri D, Robone S. The geography of hospital admission in a national health service with patient choice. Health Econ. 2010;19:1029–1047. doi: 10.1002/hec.1639. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz LM, Woloshin S, Birkmeyer JD. How do elderly patients decide where to go for major surgery? Telephone interview survey. BMJ. 2005;331:821. doi: 10.1136/bmj.38614.449016.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho K. The welfare effects of restricted hospital choice in the US medical care market. J Appl Econom. 2006;21:1039–1079. [Google Scholar]
- 51.Nass SJ, Patlak M. Appropriate use of advanced technologies for radiation therapy and surgery in oncology: workshop summary. National Academies Press; Washington, DC: 2016. [PubMed] [Google Scholar]
- 52.Wright JD, Ananth CV, Lewin SN. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309:689–698. doi: 10.1001/jama.2013.186. [DOI] [PubMed] [Google Scholar]
- 53.Diana M, Marescaux J. Robotic surgery. Br J Surg. 2015;102:e15–e28. doi: 10.1002/bjs.9711. [DOI] [PubMed] [Google Scholar]
- 54.Sivarajan G, Taksler GB, Walter D, Gross CP, Sosa RE, Makarov DV. The effect of the diffusion of the surgical robot on the hospital-level utilization of partial nephrectomy. Med Care. 2015;53:71–78. doi: 10.1097/MLR.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poulsen PB, Adamsen S, Vondeling H, Jorgensen T. Diffusion of laparoscopic technologies in Denmark. Health Policy. 1998;45:149–167. doi: 10.1016/s0168-8510(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 56.Ho V, Town RJ, Heslin MJ. Regionalization versus competition in complex cancer surgery. Health Econ Policy Law. 2007;2:51–71. doi: 10.1017/S1744133106006256. [DOI] [PubMed] [Google Scholar]
- 57.Dor A, Koroukian S, Xu F, Stulberg J, Delaney C, Cooper G. Pricing of surgeries for colon cancer: patient severity and market factors. Cancer. 2012;118:5741–5748. doi: 10.1002/cncr.27573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langabeer JR, Ozcan YA. The economics of cancer care: longitudinal changes in provider efficiency. Health Care Manag Sci. 2009;12:192–200. doi: 10.1007/s10729-008-9079-2. [DOI] [PubMed] [Google Scholar]
- 59.Cooper Z, Gibbons S, Jones S, McGuire A. Does hospital competition save lives? Evidence from the English NHS patient choice reforms. Econ J. 2011;121:F228–F260. doi: 10.1111/j.1468-0297.2011.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng Y, Pistollato M, Charlesworth A, Devlin N, Propper C, Sussex J. Association between market concentration of hospitals and patient health gain following hip replacement surgery. J Health Serv Res Policy. 2015;20:11–17. doi: 10.1177/1355819614546032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dietrichson J, Ellegård LM, Kjellsson G. Effects of increased competition on quality of primary care in Sweden. December, 2016. Department of Economics, Lund University Working Papers. http://project.nek.lu.se/publications/workpap/papers/wp16_36.pdf (accessed Aug 8, 2017).
- 62.Gravelle H, Moscelli G, Santos R, Siciliani L. Patient choice and the effects of hospital market structure on mortality for AMI, hip fracture and stroke patients. CHE Research Paper 106. Centre for Health Economics (University of York); York: 2014. [Google Scholar]
- 63.Propper C, Burgess S, Gossage D. Competition and quality: evidence from the NHS internal market 1991–9. Econ J. 2008;118:138–170. [Google Scholar]
- 64.Chou S-Y, Deily ME, Li S, Lu Y. Competition and the impact of online hospital report cards. J Health Econ. 2014;34:42–58. doi: 10.1016/j.jhealeco.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Kessler DP, McClellan MB. Is hospital competition socially wasteful? Q J Econ. 2000;115:577–615. [Google Scholar]
- 66.Gowrisankaran G, Town RJ. Competition, payers, and hospital quality. Health Serv Res. 2003;38:1403–1422. doi: 10.1111/j.1475-6773.2003.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fotaki M. Is patient choice the future of health care systems? Int J Health Policy Manag. 2013;1:121–123. doi: 10.15171/ijhpm.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potter S, Mills N, Cawthorn S, Wilson S, Blazeby J. Exploring inequalities in access to care and the provision of choice to women seeking breast reconstruction surgery: a qualitative study. Br J Cancer. 2013;109:1181–1191. doi: 10.1038/bjc.2013.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodin D, Aggarwal A, Lievens Y, Sullivan R. Balancing equity and advancement: the role of health technology assessment in radiotherapy resource allocation. Clin Oncol; 29: 93–98. [DOI] [PubMed]
- 70.Young RC. Value-based cancer care. N Engl J Med. 2015;373:2593–2595. doi: 10.1056/NEJMp1508387. [DOI] [PubMed] [Google Scholar]
- 71.van Loon J, Grutters J, Macbeth F. Evaluation of novel radiotherapy technologies: what evidence is needed to assess their clinical and cost effectiveness, and how should we get it? Lancet Oncol. 2012;13:e169–e177. doi: 10.1016/S1470-2045(11)70379-5. [DOI] [PubMed] [Google Scholar]
- 72.Bevan G, Skellern M. Does competition between hospitals improve clinical quality? A review of evidence from two eras of competition in the English NHS. BMJ. 2011;343:d6470. doi: 10.1136/bmj.d6470. [DOI] [PubMed] [Google Scholar]
- 73.Walker K, Neuburger J, Groene O, Cromwell DA, van der Meulen J. Public reporting of surgeon outcomes: low numbers of procedures lead to false complacency. Lancet. 2013;382:1674–1677. doi: 10.1016/S0140-6736(13)61491-9. [DOI] [PubMed] [Google Scholar]
- 74.Victoor A, Delnoij DM, Friele RD, Rademakers JJ. Determinants of patient choice of healthcare providers: a scoping review. BMC Health Serv Res. 2012;12:272. doi: 10.1186/1472-6963-12-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fung CH, Lim Y-W, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Int Med. 2008;148:111–123. doi: 10.7326/0003-4819-148-2-200801150-00006. [DOI] [PubMed] [Google Scholar]
- 76.Mirkin JN, Lowrance WT, Feifer AH, Mulhall JP, Eastham JE, Elkin EB. Direct-to-consumer internet promotion of robotic prostatectomy exhibits varying quality of information. Health Aff. 2012;31:760–769. doi: 10.1377/hlthaff.2011.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Healthcare Quality Improvement Partnership National Bowel Cancer Audit Annual report 2016. Dec 16, 2016. http://www.hqip.org.uk/resources/national-bowel-cancer-audit-annual-report-2016/ (accessed Aug 8, 2017).
- 78.Healthcare Quality Improvement Partnership National Oesophago-Gastric Cancer Audit 2016. Sept 8, 2016. http://www.hqip.org.uk/resources/national-oesophago-gastric-cancer-audit-2016/ (accessed Aug 8, 2017).
- 79.National Prostate Cancer Audit . Third year annual report—results of the NPCA prospective audit and patient survey, 2016. December, 2016. Royal College of Surgeons of England; London: 2016. [Google Scholar]
- 80.Hibbard JH, Stockard J, Tusler M. Hospital performance reports: impact on quality, market share, and reputation. Health Aff. 2005;24:1150–1160. doi: 10.1377/hlthaff.24.4.1150. [DOI] [PubMed] [Google Scholar]
- 81.Jenkins DP, Cooper G. Publicly available outcome data for individual surgeons: lessons from cardiac surgery. Eur Urol. 2017;71:309–310. doi: 10.1016/j.eururo.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 82.Leira EC, Fairchild G, Segre AM, Rushton G, Froehler MT, Polgreen PM. Primary stroke centers should be located using maximal coverage models for optimal access. Stroke. 2012;43:2417–2422. doi: 10.1161/STROKEAHA.112.653394. [DOI] [PubMed] [Google Scholar]
- 83.Santibáñez P, Gaudet M, French J, Liu E, Tyldesley S. Optimal location of radiation therapy centers with respect to geographic access. Int J Radiat Oncol Biol Phys89: 745–55. [DOI] [PubMed]
- 84.Wang F, Onega T. Accessibility of cancer care: disparities, outcomes and mitigation. Ann GIS. 2015;21:119–125. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.