Abstract
Anolis lizards are a model system for the study of adaptive radiation and convergent evolution. Greater Antillean anoles have repeatedly evolved six similar forms or ecomorphs: crown-giant, grass-bush, twig, trunk, trunk-crown and trunk-ground. Members of each ecomorph category possess a specific set of morphological, ecological and behavioural characteristics which have been acquired convergently. Here we test whether the semicircular canal system—the organ of balance during movement—is also convergent among ecomorphs, reflecting the shared sensory requirements of their ecological niches. As semicircular canal shape has been shown to reflect different locomotor strategies, we hypothesized that each Anolis ecomorph would have a unique canal morphology. Using three-dimensional semilandmarks and geometric morphometrics, semicircular canal shape was characterized in 41 Anolis species from the Greater Antilles and the relationship between canal shape and ecomorph grouping, phylogenetic history, size, head dimensions, and perch characteristics was assessed. Further, canal morphology of modern species was used to predict the ecomorph affinity of five fossil anoles from the Miocene of the Dominican Republic. Of the covariates tested, our study recovered ecomorph as the single-most important covariate of canal morphology in modern taxa; although phylogenetic history, size, and head dimensions also showed a small, yet significant correlation with shape. Surprisingly, perch characteristics were not found to be significant covariates of canal shape, even though they are important habitat variables. Using posterior probabilities, we found that the fossil anoles have different semicircular canals shapes to modern ecomorph groupings implying extinct anoles may have been interacting with their Miocene environment in different ways to modern Anolis species.
Keywords: inner ear, bony labyrinth, geometric morphometrics, three-dimensional, anoles, ecomorphology
1. Introduction
The semicircular canals are a functional component of the vestibular system of the inner ear that enable vertebrate animals to coordinate fast and complex movements in three-dimensions (3D). As such, it stands to reason that more agile and mobile animals, such as fast moving arboreal species, would benefit from an enhanced sense of balance, granted through adaptation of canal morphology. Recent theoretical [1,2], physiological [3,4] and comparative [5–9] studies have shown that, in mammals, a relationship exists between canal morphology and vestibular sensitivity [1–4], locomotor activity [8,9], agility [7] and speed [6,10]. Although these studies use different metrics for canal morphology, such as canal size [7,11], torsion [5] and orthogonality [2,6], all agree there is a strong signal between semicircular canal morphology and locomotor ability. Thus, our current understanding of the vestibular system in living mammals has allowed us to investigate and interpret the behaviour and ecology of extinct species [12–22].
The vestibular system has, however, been little explored outside the Mammalia. While the semicircular canals are physiologically and anatomically homologous in all vertebrates, we cannot assume that the relationship between form and function in mammals will hold true for other taxa, especially given considerable morphological differences between mammals and other amniote groups. Extrapolation from mammals is particularly problematic for studies wishing to reconstruct the ecology of non-mammalian fossil species [21,23–26]. Recent studies looking at the morphology of the semicircular canals with respect to ecology in amphibians [27], squamates [28,29] and birds [30,31] have begun to expand our knowledge of the vestibular system beyond mammals, though much work is still needed to fully understand the system from a greater evolutionary and ecological spectrum.
Anolis lizards (Dactyloidae) represent a unique opportunity for furthering such research. Greater Antillean anoles originated ca. 65 Ma, diversifying throughout the Caribbean and neotropical mainland [32]. Within Anolis, six ecomorph types have evolved independently on each of the four Greater Antilles islands (with several exceptions), with each ecomorph encompassing a specific suite of anatomical (e.g. short versus long limbs), ecological (e.g. tree trunk versus branches), and behavioural (e.g. locomotion, territoriality) characteristics (reviewed in [33]): trunk-ground (TG) ecomorphs inhabit lower tree trunks, using infrequent, rapid descents to the ground where they capture prey; trunk-crown (TC) ecomorphs occupy the upper reaches of the tree, navigating the complex 3D canopy with a high rate of movement; trunk (Tr) ecomorphs occupy the trunk area between TG and TC ecomorphs with some overlaps, and are fairly active locomotors; crown-giant (CG) ecomorphs occupy similar habitats to TC ecomorphs, yet are substantially larger, and move more slowly; twig (Tw) ecomorphs occupy the narrowest branches and twigs of the canopy moving regularly, yet slowly; and grass-bush (GB) ecomorphs are also found on narrow vegetation, but close to the ground on grasses, bushes and small trees, navigating their complex 3D environment slowly. The fossil record of anoles is extremely limited, though rich Dominican Miocene amber deposits preserve at least several ecomorph types ca. 23 Ma [34].
The recurrent and consistent adaptive radiations found in Anolis, and close phylogenetic relatedness, make it an excellent starting point for further understanding the morpho-functional relationship of the semicircular canals in squamates and beyond Mammalia in general. Here, we use 3D geometric morphometrics to quantify and investigate whether Anolis semicircular canal morphology is convergent among ecomorph groups. Specifically, we aim to test the hypothesis that anole species adapted to similar ecomorph niches have converged on similar semicircular canal morphologies, reflecting the sensory requirements of their shared ecological and behavioural habits. We also test the influence of phylogenetic relatedness, size, and head proportions on patterns of canal morphology, factors that may have an effect on vestibular system form [7,9,28,35–39], as well as perch height and diameter—the two most frequently reported habitat variables [33]. Further, we reconstruct the vestibular system in five 15–20 Ma fossil anole specimens preserved in Miocene amber [34]. We use our extant dataset to predict the ecomorph affinities and palaeoecology of these extinct Anolis lizards and compare these to predictions based on external morphological traits [34].
2. Material and methods
2.1. Specimens and sample size
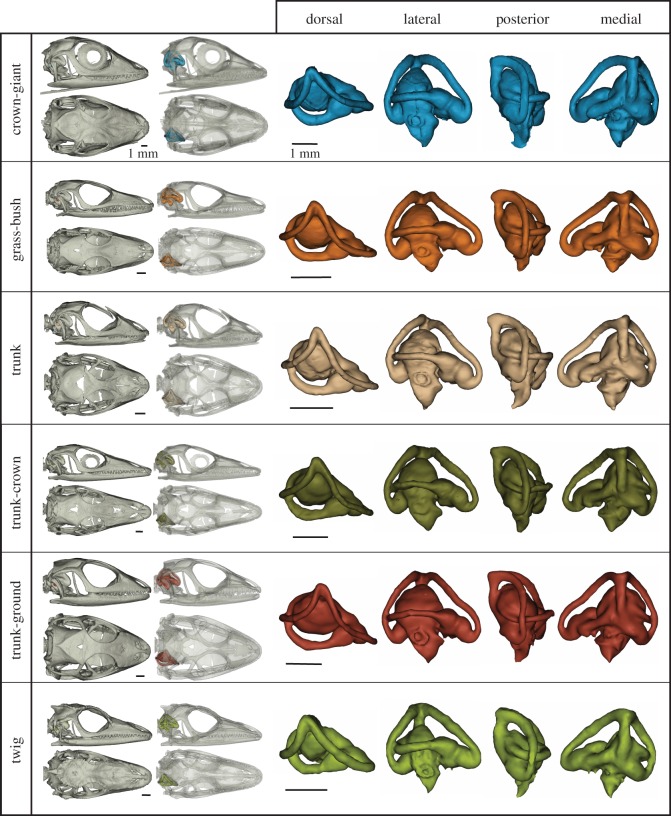
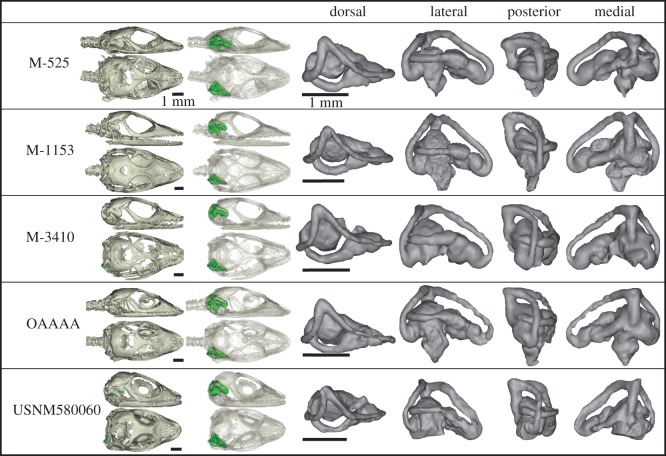
The sample consists of 131 individuals representing 41 species of anoles originating from the four islands of the Greater Antilles: Hispaniola, Cuba, Jamaica and Puerto Rico. Species from Hispaniola are represented by multiple specimens including juvenile individuals (see further below). All six ecomorphs are represented by multiple species: CG (5), GB (6), Tr (3), TC (9) TG (4) and Tw (5) (figure 1), with the addition of eight ‘unique’ species that are endemic to each island but do not form a coherent group, nor conform ecomorphologically to any of the specified ecomorphs [33]. Five fossil anoles of Miocene age (figure 2) were also included from the amber deposits of the Dominican Republic [34,40], details of which can be found in Sherratt et al. [34]. All modern specimens were sourced from the Herpetology collection at the Museum of Comparative Zoology (MCZ), Harvard University. All species and specimen numbers can be found in the electronic supplementary material, table S1.
Figure 1.
3D rendering of the vestibular system from the six modern Anolis ecomorphs. Each ecomorph is represented by the specimen closest to the group shape mean: crown-giant—A. riccordii MCZ R83982; grass-bush—A. hendersoni MCZ R65643; trunk-crown—A. longiceps MCZ R16194; trunk-ground—A. marcanoi MCZ R104402; trunk—A. brevirostris MCZ R155833; twig—A. insolitus MCZ R128310. MCZ, Museum of Comparative Zoology, Harvard. See the electronic supplementary material, table S1 for full specimen list. Scale bar = 1 mm.
Figure 2.
3D rendering of the five Dominican Republic anole fossil specimens, preserved in Miocene amber. The vestibular system of each specimen is shown within the skull, and in four anatomical views. See the electronic supplementary material, table S1 for full specimen identitys. Scale bar = 1 mm.
2.2. Data acquisition and landmarks
Various methods for measuring the complex structure of the three semicircular canals have been used to date. Traditional morphometric approaches, in the form of linear and angular measures of size and orthogonality, have been used extensively in the past [2,6,7,11] with the benefit of being easily comparable across studies. However, these measurements struggle to completely capture the full shape variation in the canals owing to their complex curvature. Geometric morphometrics (GM; [41,42]) has been used increasingly to overcome this shortcoming, with landmark [9,16,28,35] and semilandmark [19,43,44] approaches, particularly the latter, capable of capturing far more morphological variation than standard morphometrics. GM methods do, however, vary between studies and are thus less easily compared across studies and broader taxonomic groups. For morphometric approaches, digital thresholding and segmenting of micro computed tomography (µCT) data can introduce significant variation in canal lumen thickness [45], making measuring and digitizing the canal surface error prone. Instead, using a centreline through the lumen overcomes this potential error as it is not affected by threshold values [43,45]. This is the approach that we took.
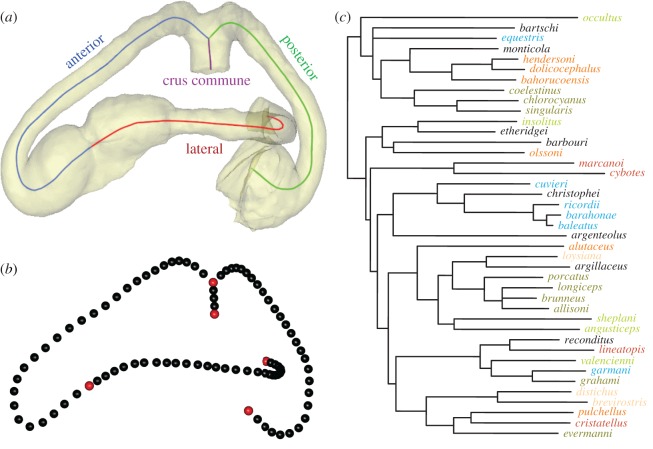
Specimens were µCT scanned using a variety of imaging systems and settings (electronic supplementary material, table S1) and the semicircular canals manually segmented and 3D rendered using Materialise Mimics® software. 3D landmarks were derived from the centreline of the semicircular canals, calculated using the ‘medcad’ module in Mimics®, and then manually adjusted to optimize the position of the centreline through the lumen. This centreline was then split into four segments—the three canals (anterior, posterior and lateral) and the crus commune (figure 3a)—and exported as 3D coordinates describing a curve. The centreline curves were then resampled using the Resample executable [46] so that the three canals were each described by 28 equally-spaced semilandmarks, and the crus commune by three semilandmarks. These semilandmarks were anchored by five landmarks positioned at the junction of the anterior and lateral canals, the posterior and lateral canals with the vestibule, and the junction of the crus commune, resulting in 92 landmarks in total (figure 3b).
Figure 3.
Segmented semicircular canal demonstrating placement of (a) centrelines and (b) semilandmarks; and (c) a time calibrated phylogeny of study taxa coloured by ecomorph. Red landmarks are fixed, while black landmarks are sliding. Species colour coding: blue = crown-giant; orange = grass-bush; pink = trunk; army green = trunk-crown; red = trunk-ground; bright green = twig; black = unique.
2.3. Data analysis
Landmark coordinate data were aligned by Procrustes superimposition, allowing semilandmarks to slide along their tangent directions in order to minimize bending energy [47,48] using the R statistical environment v. 3.2.3 [49] and the package geomorph v. 2.1.4 [50]. The resulting Procrustes residuals were used as shape variables in the subsequent analyses. Alignment was done on both the full shape variable dataset (n = 131) and a phylogenetic subset (n = 41). Since the full dataset represented ontogenetic series of each species, the phylogenetic subset was represented by the largest adult male from each species paired with the phylogeny of Gamble et al. [51] (figure 3c). As a previous study found significant sexual dimorphism in some Anolis species [52], comparing all males avoids a potential source of bias. Partitioning the data was necessary to investigate the role phylogeny might play in determining canal shape as no current methods are available that would account for ontogenetic variation in phylogenetic comparative analyses.
Principal component analysis (PCA) was performed on the phylogenetic subset dataset to visualize semicircular canal shape variation among all species. Eigendecomposition of this PCA was performed using only modern taxa (including unique species); fossil specimens were later projected into this morphospace by matrix multiplication with the PCA eigenvectors. MANOVA was conducted to assess which PC axes significantly separated ecomorph groups. The phylogeny [51] was projected into the phylogenetic subset PC space to visualize the estimated evolutionary trajectory of canal shape change, using the geomorph function ‘plotGMPhyloMorphoSpace’. Phylogenetic signal of canal morphology was calculated using the K statistic [53,54] with geomorph's ‘physignal’ function and tested for significance using 10 000 permutations. To visualize a morphospace independent of the effects of phylogeny and allometry, we plotted the residuals of a phylogenetic regression with log-transformed semicircular canal centroid size, performed on PC scores using the ‘phyl.resid’ function of phytools v. 0.5-10 [55,56]. Centroid size is a measure of size calculated as the square root of the sum of squared distances of a set of landmarks from their centroid [42]. To visualize shape changes throughout the PCA morphospace, partial warps were used to generate maximum and minimum shape warps along each principal component (PC) axis by back-transformation through the eigenvectors.
To determine whether ecomorphs occupy different regions of morphospace (and thus have significantly different canal morphologies), and to test the effect of size and head proportions on canal shape, analysis of covariance (ANCOVA) and phylogenetic generalized least squares (PGLS) were performed on the phylogenetic dataset using the ‘procD.lm’ and ‘procD.pgls’ functions respectively [57] of geomorph, with pairwise comparisons tested using ‘advanced.procD.lm’ [50]. These functions perform statistical assessment of the terms in the model using Procrustes distances among specimens, rather than explained covariance matrices among variables, and are thus suitable for multivariate datasets [57,58]. Three log-transformed size metrics were used: semicircular canal centroid size, skull length (measured between the premaxilla and the dorsal margin of the foramen magnum) and skull width (measured between the paraoccipital processes). The relationship between the size metrics was also explored using linear regressions. Head proportions were determined by taking the ratio of skull length : width. In addition, we investigated the relationship between canal shape and habitat use, represented by perch height and diameter ([50] and J. B. Loses 1988–2005, unpublished)—the two most frequently reported habitat variables—by ANCOVA and PGLS.
Finally, a canonical variate analysis (CVA) with cross-validations was run using the ‘CVA’ function of the package Morpho v. 2.3.0 [59] to explore the morphological shape variables that maximize between-ecomorph-group variance relative to within-group variance, and to predict the potential ecology of the fossil anoles. Prior to running the CVA, a PCA was performed on the full extant dataset (excluding unique species) and the first 40 PC axes representing 99% of the variation were extracted; this reduction in dimensionality was done to ensure that the number of shape variables (n = 40) was less than the number of individual specimens (n = 99) [60] and to remove minor components of shape variance that might be attributable to error. In addition, the full specimen dataset was used to take into consideration both specific and ontogenetic variation to compare against the fossil specimens and to increase the power of the test by incorporating a larger sample size. Within the CVA morphospace, 95% confidence intervals (CI) were generated around each of the modern ecomorph groups. The unique and fossil specimens were then projected into this morphospace using the canonical variates. As CVA is not a rigid ordination and the resulting morphospace may deviate from a Euclidean space, we use Mahalanobis distances in subsequent analyses to correct for any distortions in shape-space [60]. The posterior (typicality) probability of ecomorph-group membership for fossil and unique specimens was assessed by calculating the Mahalanobis distances of each specimen to the mean of each ecomorph. This distance was then compared to within-ecomorph-group distances which had been resampled 10 000 times [61–65]. If the distance between a specimen and group mean was greater than 95% (p < 0.05) of within-group distances, we reject the null-hypothesis that it belongs to that ecomorph group [61,65–68]. Further, log-likelihood estimations were also calculated to allow comparison with previous work [34]. To visualize shape changes throughout the CVA morphospace, partial warps were used to generate maximum and minimum shape warps along each canonical variate (CV) axis [60].
3. Results
3.1. Patterns of shape variation
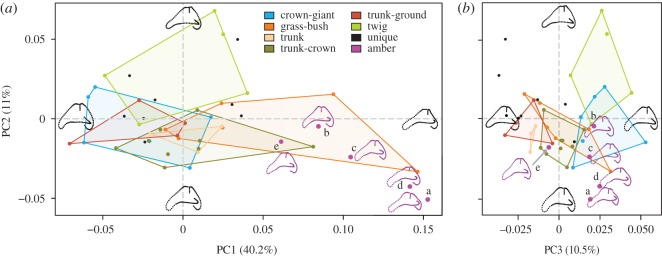
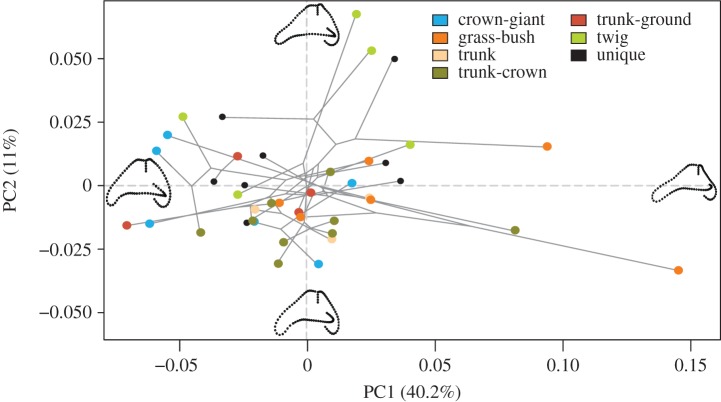
PCA (figure 4a,b) shows that PC1 (40.2% of variation) largely represents changes in anterior and lateral canal morphology. Moving from PC1 positive to PC1 negative, there is a trend for the canals to become more rounded and anterodorsally shortened. PC2 (11.0% of variation) represents moderate changes in all three canals, with a PC2 positive to PC2 negative shift showing rounding of the anterior-most section of the anterior canal, more torsion (out-of-plane curvature) of the lateral canal, and less torsion of the posterior canal. PC3 (10.5% of variation) represents changes in the anterior and posterior canals, with a transition from PC3 positive to PC3 negative showing increased curvature and deepening of the posterior canal and reduction of the lateral aspect of the anterior canal. MANOVA results show that ecomorphs at not distinct on PC1, but they do significantly separate over subsequent PCs (electronic supplementary material, table S2).
Figure 4.
Principal component (PC) analysis of semicircular canal shape showing (a) PC1 versus PC2 and (b) PC2 versus PC3. The first three PCs represent 61.7% of variation in canal shape. Points are specimens, coloured by ecomorph and bounded by convex hulls. Unique specimens are shown in black, and fossil specimens in magenta. Partial warps representing the maxima and minima of each PC are shown on each axis, and partial warps of the fossils are shown in magenta. Anterior canal is to the left. Amber fossils: a = M-525, b = M-1153, c = M-3410, d = OAAAA, e = USNM580060.
Visually, there is significant overlap between ecomorph groupings along PC1 and PC2, with GB anoles occupying most of PC1. PC3 separates the three ‘trunk’ ecomorphs from the Tw and CG ecomorphs. When the shape data were corrected for size and phylogeny, the PC morphospace is minimally altered. The unique species are widely distributed across morphospace, overlapping with most ecomorph grouping (PC2 versus PC1) and falling outside the variation enclosed by the ecomorphs (PC2 versus PC3). All fossil specimens fall along the positive end of PC1, in the GB area of morphospace, which represents flattening and anterodorsal elongation of the anterior canal. Furthermore, three fossil specimens (M-1153, M-3410, USNM580060) overlap with multiple ecomorph groupings along PC3. Fossil specimens M-525 and OAAAA appear to fall beyond the morphologies of all living taxa sampled.
3.2. Predictors of shape
Phylogeny was found to have only a weak influence on semicircular canal morphology (K = 0.58), though permutation found this influence to be greater than expected from random (p = 0.0034). Mapping of the phylogeny onto morphospace (figure 5) shows extensive overlapping of branches through morphospace, indicating convergence towards similar semicircular canal morphologies.
Figure 5.
Time calibrated Anolis phylogeny projected into the morphospace of PC1 versus PC2, representing 51.2% of the total variation. Points are coloured by ecomorph, with unique species in black. Partial warps representing the maxima and minima of each PC are shown in black on each axis.
ANCOVA (table 1) found that ecomorph is a moderate and significant predictor (R2 = 0.36, p < 0.001) of canal shape. Multivariate pairwise post hoc tests found all ecomorphs to be significantly different (p < 0.05) from one another except Tr and TG (p = 0.300, electronic supplementary material, table S3). A weak but significant relationship also exists between canal centroid size and canal shape (R2 = 0.11, p = 0.001) and their interaction (R2 = 0.11, p < 0.001), as well as head proportions and canal shape (R2 = 0.03, p = 0.041) and their interaction (R2 = 0.23, p < 0.001) (table 1). Further, PGLS (table 2) found ecomorph to be a significant predictor of canal shape, though the effect was less strong (R2 = 0.20, p < 0.001). This reduction in the correlation coefficient indicates an interaction between phylogeny and ecomorph and that ecomorph groupings are not entirely independent of phylogeny. PGLS (table 2) also reveals a relationship between canal centroid size and shape (R2 = 0.19, p < 0.001) and an interaction between ecomorph and centroid size (R2 = 0.19, p < 0.001). Head proportions remains weak, yet significant (R2 = 0.08, p = 0.006), though PGLS reveals a much stronger interaction between head proportions and ecomorph (R2 = 0.3, p = 0.002). There was no significant relationship between either perch height or diameter and canal shape, with and without phylogenetic correction (tables 1 and 2).
Table 1.
Analysis of covariance (ANCOVA) of semicircular canal shape against ecomorph, semicircular canal centroid size, head proportion and perch characteristics, with statistical significance assessed through 10 000 permutations. (Significant (p < 0.05) results are indicated in bold.)
| d.f. | SS | MS | R2 | F | Z | p-value | |
|---|---|---|---|---|---|---|---|
| ANCOVA (ecomorph and centroid size) | |||||||
| ecomorph | 5 | 0.042349 | 0.0084698 | 0.36273 | 3.7724 | 4.5749 | 0.0002 |
| centroid size | 1 | 0.013328 | 0.0133283 | 0.11416 | 5.9364 | 4.353 | 0.0001 |
| ecomorph : centroid size | 5 | 0.013925 | 0.002785 | 0.11927 | 1.2404 | 3.16 | 0.0007 |
| residuals | 21 | 0.047149 | 0.0022452 | ||||
| total | 32 | 0.116751 | |||||
| ANCOVA (ecomorph and head proportions) | |||||||
| ecomorph | 5 | 0.042349 | 0.0084698 | 0.36273 | 3.9739 | 4.7372 | 0.0001 |
| head proportion | 1 | 0.00331 | 0.0033099 | 0.02835 | 1.553 | 1.907 | 0.0412 |
| ecomorph : head proportion | 5 | 0.026334 | 0.0052668 | 0.22556 | 2.4711 | 4.5055 | 0.0001 |
| residuals | 21 | 0.044758 | 0.0021313 | ||||
| total | 32 | 0.116751 | |||||
| ANCOVA (perch characteristics) | |||||||
| height | 1 | 1446 | 1446 | 0.000701 | 0.0195 | 0.0143 | 0.9946 |
| diameter | 1 | 39212 | 39212 | 0.019007 | 0.5283 | 0.42849 | 0.60954 |
| height : diameter | 1 | 18447 | 18447 | 0.008942 | 0.2486 | 0.16515 | 0.75882 |
| residuals | 27 | 2003863 | 74217 | ||||
| total | 30 | 2062967 | |||||
Table 2.
Phylogenetic generalized least squares of semicircular canal shape against ecomorph, semicircular canal centroid size, head proportion and perch characteristics, with statistical significance assessed through 10 000 permutations. (Significant (p < 0.05) results are indicated in bold.)
| d.f. | SS | MS | R2 | F | Z | p-value | |
|---|---|---|---|---|---|---|---|
| PGLS (ecomorph and centroid size) | |||||||
| ecomorph | 5 | 0.11454 | 0.022909 | 0.19789 | 1.9571 | 3.3133 | 0.0002 |
| centroid size | 1 | 0.10885 | 0.108846 | 0.18804 | 9.2985 | 4.3487 | 0.0001 |
| ecomorph : centroid size | 5 | 0.10751 | 0.021501 | 0.18572 | 1.8368 | 3.9763 | 0.0002 |
| residuals | 21 | 0.24582 | 0.011706 | ||||
| total | 32 | 0.57884 | |||||
| PGLS (ecomorph and head proportions) | |||||||
| ecomorph | 5 | 0.11454 | 0.022909 | 0.197885 | 1.9964 | 3.2648 | 0.0006 |
| ratio | 1 | 0.04476 | 0.044762 | 0.077331 | 3.9009 | 2.7617 | 0.0064 |
| ecomorph : ratio | 5 | 0.17644 | 0.035287 | 0.304808 | 3.0751 | 3.2949 | 0.0021 |
| residuals | 21 | 0.24097 | 0.011475 | ||||
| total | 32 | 0.57884 | |||||
| PGLS (perch characteristics) | |||||||
| height | 1 | 0.03675 | 0.036751 | 0.086641 | 3.6934 | 0.81864 | 0.45145 |
| diameter | 2 | 0.05866 | 0.029329 | 0.138286 | 2.9475 | 0.4274 | 0.91281 |
| height : diameter | 3 | 0.08996 | 0.029986 | 0.212076 | 3.0135 | 0.37058 | 0.94441 |
| residuals | 24 | 0.23881 | 0.00995 | ||||
| total | 30 | 0.42417 | |||||
3.3. Ecomorph differences
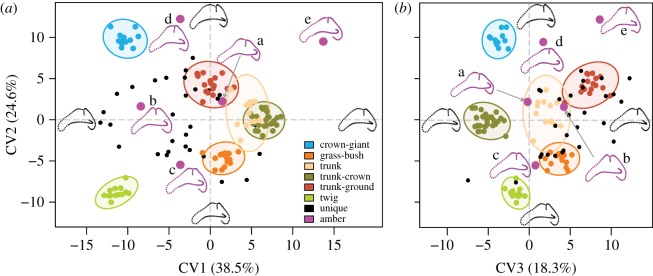
The CVA biplots (figure 6) and posterior probabilities (table 3) show ecomorphs form significantly different groups within the morphospace (p < 0.0001), with a cross-validation accuracy of 87.9% (electronic supplementary material, table S4). On the extreme negative end of canonical function 1 (CV1, figure 6a) are the Tw and CG ecomorphs. This region of morphospace is characterized by greater out-of-plane curvature of the anterior canal. The two groups, however, occupy opposite extremes of CV2 (figure 6a,b). CGs occupy the extreme positive end of CV2 and, when compared with Tw species, display greater dorsoventral curvature and reduced torsion of the posterior canal, and lengthening of the crus-commune. GB and the three ‘trunk’ ecomorphs occupy the central and positive region of CV1, characterized by reduced anterior canal curvature, but they do separate along CV2 and CV3 (figure 6b). TC and GB species occupy a more positive region along CV2, displaying greater dorsoventral curvature of the anterior canal, reduced torsion of the posterior canal, and longer crus-commune. TC and GB species separate along CV3 with the TC ecomorph occupying the positive end of CV3 indicating greater curvature of the lateral canal and reduced curvature of the posterior canal. The remaining ‘trunk’ ecomorphs separate along CV2, with the TG species occupying a more negative region of CV2, representing reduced torsion of the canals in general.
Figure 6.
Canonical variates (CV) analysis showing (a) CV1 versus CV2, and (b) CV2 versus CV3, representing 84.8% of the total variation. Ninety-five per cent confidence ellipses of each ecomorph are plotted, and points are coloured by ecomorph, with unique species plotted in black and fossil specimens in magenta. Partial warps representing the maxima and minima of each CV are shown in black along each axis. Anterior canal is to the left. Amber fossils: a = M-525, b = M-1153, c = M-3410, d = OAAAA, e = USNM580060.
Table 3.
Posterior probabilities of ecomorph groups being significantly different from one another based on Mahalanobis distances using 10 000 permutations. (CG, crown-giant; GB, grass-bush; TC, trunk-crown; TG, trunk-ground; Tr, trunk; Tw, twig.)
| probabilities from Mahalanobis distances | ||||||
|---|---|---|---|---|---|---|
| CG | GB | TC | TG | Tr | Tw | |
| CG | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| GB | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| TC | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| TG | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Tr | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Tw | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
The unique species (those that have not been assigned to any of the ecomorphs in previous studies) are spread throughout morphospace (figure 6), with only some visually falling within the 95% CI of modern ecomorphs in the first few axes (CV1–3). Posterior probabilities of Mahalanobis distances over all CVs find that many unique species fall significantly outside the 95% CIs defined by the ecomorphs, with exceptions (electronic supplementary material, table S5): (i) one specimen of A. argenteolus falls within the 95% CI of TC (p = 0.139); (ii) the sole A. bartschi specimen falls within the 95% CI of TC (p = 0.895); (iii) out of five A. christophei specimens, one falls within the 95% CI of TG (p = 0.223), one in TC (p = 0.151), and one in GB (p = 0.073); (iv) out of five A. etheridgei specimens, two fall within the 95% CI of TG (p = 0.191, p = 0.052); (v) out of five A. monticola specimens, three fall within the 95% CI of TG (p = 0.209, p = 0.075, p = 0.348) and one in Tr (p = 0.103); (vi) the only A. reconditus falls within TG (p = 0.124); and (vii) out of four A. rimarum specimens, one falls within the 95% CI of GB (p = 0.478) and one in TC (p = 0.059). These results indicate that unique species display greater variation in semicircular canal shape than what is encompassed by the ecomorph categories. Our log-likelihood calculations do, however, assign all unique species to the defined ecomorphs, although these assignments are also inconsistent within and between species (electronic supplementary material, table S5).
All the fossil specimens fall either outside or just on the margin of the 95% CI of the modern ecomorphs, much like the unique species (figure 6). Our posterior probabilities support this: all fossil specimens are highly unlikely to belong to any modern ecomorph group (table 4). This contrasts with the log-likelihood tests which assign each fossil to the ‘closest’ ecomorph group regardless of actual morphological distance (table 4). Moreover, log-likelihood tests found only one instance of correspondence with the ecomorphs inferred by Sherratt et al. [34]: OAAAA, which is assigned to TC. Visually, all fossils are broadly distributed around the first three CV axes, with some being in extreme regions of morphospace (figure 6). However, M-525 generally falls close to TG (CV1 vs CV2) and Tr (CV2 vs CV3), even though it statistically falls outside their 95% CIs (table 4).
Table 4.
Probabilities and log-likelihoods of fossil anoles belonging to modern ecomorph groups, compared with the results of Sherratt et al. [34]. (Probabilities below 0.05 indicate the fossil is significantly different from an ecomorph group. Likelihoods are calculated as the most-likely modern group without an alternative hypothesis (i.e. fossils are unique). CG, crown-giant; GB, grass-bush; TC, trunk-crown; TG, trunk-ground; Tr, trunk; Tw, twig.)
| probability |
||||||||
|---|---|---|---|---|---|---|---|---|
| log-likelihood | CG | GB | TC | TG | Tr | Tw | Sherratt et al. [34] log-likelihood | |
| M-1153 | GB (0.99) | <0.0001 | 0.0313 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | TC (1.00) |
| M-3410 | GB (0.99) | <0.0001 | 0.0108 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | TC (0.69) |
| M-525 | TW (0.90) | <0.0001 | <0.0001 | 0.0002 | <0.0001 | <0.0001 | 0.0018 | TC (0.99) |
| OAAAA | TC (0.99) | <0.0001 | <0.0001 | 0.0086 | <0.0001 | <0.0001 | <0.0001 | TC (1.00) |
| USNM580060 | CG (0.21) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | TG (0.99) |
4. Discussion
4.1. Convergence of semicircular canal shape
Our results support the hypothesis that phylogenetically disparate Anolis species have convergent semicircular canal morphologies, allowing them to navigate similar ecological niches. Of the covariates we tested (ecomorph, size, head proportions), we found ecomorph grouping to be the best determinant of canal shape, even when phylogeny is accounted for (25–35%, tables 1 and 2). This is remarkable considering the taxa we examined constitute only a single genus which inhabits a relatively constrained geographical and ecological space. However, given the role semicircular canals play in coordinating fast and complex movements in 3D, and the differences in ecology and locomotor behaviour among the Anolis ecomorphs, this result aligns well with the body of work supporting a relationship between semicircular canal morphology and locomotion [5,7,9,22,27,28].
Though differences among the ecomorphs are subtle, CVA posterior probabilities found that all ecomorphs form significantly different groupings, though our ANCOVA post hoc pairwise comparisons did not find a statistical difference between TG and Tr ecomorphs (electronic supplementary material, table S3). While CV1 and 2 do not discriminate all groups, all axes of variation must be considered to properly establish group separation [67]. Why our CVA results and post hoc tests do not fully align is uncertain—both statistical tests use similar non-parametric methods, though use different distant measures (Procrustes [69] versus Mahalanobis [61]). Of all the groups, Tr and TG ecomorphs are certainly the most similar—demonstrated by their overlap in CVA morphospace (figure 6), which may reflect locomotor/behavioural similarities.
Both CG and Tw ecomorphs display more torsion of the anterior canal than those of the other ecomorphs, separating significantly along CV1 (figure 6a): CG and Tw ecomorphs are the two groups that generally run the least, but also have to negotiate extremely complicated 3D environments. In mammals, increasing out-of-plane torsion of a canal may increase sensitivity to rotations out of the canal's major plane of motion detection [64]; thus such a morphology in CG and Tw ecomorphs may potentially reflect the coordination required to negotiate their complex environment. Further, CV3 groups the three most arboreal ecomorphs: TCs alongside CG and Tw based on increased circularity and length of the lateral canal (figure 6). Both increased canal circularity [5] and length [3] have been associated with greater canal sensitivity and agility in mammals. These potential increases in sensitivity of both anterior and lateral canals may represent adaptations to the specialized arboreal niches of these three ecomorphs—CG, Tw and TC—occupying the complex upper reaches of the canopy, requiring greater sensitivity to movements. For the remaining ecomorphs, out of plane sensitivity may not be as essential to locomotor performance. For Tr and TG ecomorphs the trunk provides a broad, uncomplicated surface on which locomotion is much easier [70–72], requiring less refined balance. The perch diameter for GB ecomorphs is indeed relatively much more narrow [33,73] and complex, though the consequences of falling from grass or a bush are far less severe than falling from the tree canopy as in the higher dwelling ecomorphs. Perhaps these relaxed locomotory constraints result in less drastic semicircular canal specialization among Tr, TG and GB ecomorphs.
Generally, however, the ecomorphological signal we found does not fully explain variation in semicircular canal shape. Analysis of canal shape and perch height and diameter returned non-significant results (tables 1 and 2), despite both being correlated with ecomorph [33]. This finding was unexpected given the importance of balance during locomotion on narrow perches, and the assumed consequences of falling from high perches. The perch data included here are from a different population than our morphological data; perhaps this limitation introduced sufficient error into our analysis to confound the relationship. Further work is needed. Other behavioural characteristics may also be associated with canal shape variation, such as locomotor performance over varied substrates and/or head rotational velocities [19], and we encourage collection of such data. The remaining variation in canal morphology may also be the result of morphological ‘noise’ introduced by the skull. As the morphology of the semicircular canals must be accommodated by the skull, there may be trade-offs with the other functional requirements of the skull—such as the brain, the feeding apparatus and other senses of sight and hearing. Reduced penalties for locomotory performance in less arboreal ecomorphs may release the skull to accommodate these other vital functions. Although our results do not indicate canal shape is strongly influenced by head proportions (tables 1 and 2), recent explorations of Anolis skull morphology using geometric morphometric techniques have established differences in skull shape between ecomorphs [52,74]. Perhaps the semicircular canals are being influenced by covariation of the skull, but our head proportion ratio was not sensitive enough to capture it. Comparisons between semicircular canal shape and multidimensional skull shape should be an interesting route of future inquiry that might reveal additional influences on vestibular anatomy.
4.2. Role of phylogeny and size
We found a small yet significant relationship between phylogenetic relatedness and semicircular canal morphology (figure 5; tables 1 and 2). This weak phylogenetic signal may be the result of repeated convergent evolution for which anoles are famous [33], though similarly weak yet significant phylogenetic signals are consistent across studies dealing with other taxonomic groups [28,35–37]. It is also likely that we are simply dealing with limited divergences as we are working within a single genus—previous studies have generally compared broader taxonomic groups [28,35–37]. Based on these significant phylogenetic signals, some authors have suggested using the inner ear as a source of phylogenetic characters [75,76]. However, the results of our study demonstrate that while semicircular canal morphology is related to phylogenetic history, size and ecology are more important factors (tables 1 and 2) and any phylogenetic analysis based on such characters would be unreliable. Billet et al. [77] concluded similarly in their phylogenetic analysis of litopternan petrosal and inner ear characters, finding a potentially confounding allometric signal.
Skull length and width (and canal size) is correlated with semicircular canal morphology in Anolis and our results also show that it covaries with ecomorph (electronic supplementary material, table S6). This interaction suggests differences among the allometric shape trajectories of the six ecomorph groups. Previous studies have found that canal size appears to scale with negative allometry, such that smaller animals have relatively larger canals [7,9,37,38]. Some have postulated that smaller animals experience relatively greater angular accelerations of the head than do large animals [7,9,78] and that canal sensitivity is tied to canal radius, suggesting that larger canals are more sensitive to rotation [3]. We found that all three size metrics were highly correlated with canal morphology (tables 1 and 2; electronic supplementary material, table S6), though negative (or positive) allometry cannot be determined when the response variable (canal shape) is multivariate. However, regression of log centroid size on skull length in our dataset found evidence of strong negative allometry (slope = 0.63; 95% CI = 0.54–0.71) which is in keeping with prior studies. Further analyses exploring the allometric variation in our data will be the subject of future publications.
4.3. Affinities of fossil anoles
Although further research is needed to determine other factors that may covary with semicircular canal morphology, the significant relationship between ecomorph and canal shape in extant Anolis species enabled us to explore the palaeoecology of fossil taxa. Using posterior (typicality) probabilities, we find that the semicircular canal shapes of all five fossils are significantly different from modern ecomorph groupings (table 4), and that all five also differ from each other (figures 2, 4 and 6). It is not unreasonable for the fossil taxa to differ from modern morphological patterns: Anoles probably first reached Hispaniola in the late Eocene approximately 40 Ma [34], so these 20 Ma Miocene fossils probably represent an intermediate period of diversification between Eocene and modern anoles. Further, the ecological context of Miocene anoles was probably different from the modern Antillean ecosystems. Though little is known about the forest ecosystem structure of the Antilles during the Miocene, Hymenaea protera, the amber forming tree in which the fossils are contained, is more closely related to the African Hymenaea verrucosa than the modern Antillean species [40,79]. Differences in floral composition in the Miocene may have influenced how extinct anoles were navigating their island environment, meaning semicircular canal shape may have been under different selective pressures.
Using a log-likelihood approach (table 4), the fossil anoles are sorted into modern ecomorph groupings, however, only OAAAA matches the predicted groupings of Sherratt et al. [34] who used external morphological features to define ecomorphs. It is possible that the discrepancy between the two studies is a result of quantifying different anatomical structures. Taphonomic distortion may also be an unavoidable factor in fossil specimens. Of the five fossil anoles, two fall into an extreme region of PCA morphospace (figure 4), which could imply taphonomic distortion. However, close inspection of the fossils finds that only M-525 has any noticeable deformation of the basicranium (lateral compression, figure 2). Therefore, we do not expect taphonomic distortion to be causing these differences between studies. Alternatively, the discrepancy between our study and Sherratt et al. [34] may be owing to mosaic evolution: the vestibular system may responded differently to selective pressures than other ecomorphological traits (e.g. limb length, digit length, subdigital lamellae) resulting in varying rates of morphological change [80].
5. Conclusion
Here we demonstrate that the classic ecomorph definitions of Anolis of the Greater Antilles are supported by inner ear morphology, with each ecomorph possessing a distinctive semicircular canal shape. We find that, of the covariates we tested, ecomorph is the single-most important covariate of canal morphology, although phylogenetic history, canal size and head proportions are also significantly correlated with canal shape. Surprisingly, we were unable to find any correlation between canal shape and perch variables; this result may suggest that canal shape is not influenced by where anoles live, but rather how they locomote. Still, much of the morphological variance seen in our sample remains unexplained and further work is required to tease out other ecological, behavioural, and/or anatomical characteristics that may covary with semicircular canal morphology. Using the more conservative metric of posterior (typicality) probabilities, we were unable to assign fossil anoles to modern ecomorph groups based upon semicircular canal shape. Our results indicate that the semicircular canals of these extinct anoles are morphologically different from modern Anolis ecomorphs, suggesting fossil taxa may have been interacting with their Miocene environment in different ways to modern Anolis species.
Supplementary Material
Acknowledgements
We would like to thank the staff of the MCZ Herpetology collection, specifically José Rosado for access to specimens and scan data; George Lauder for the use of his computers and software; the Morphmet and R online communities; and all the members of the MCZ Vertebrate Paleontology laboratory: Katrina Jones, Robert Kambic, Phil Lai, Brianna McHorse and Zachary Morris for their help with R and insights during this project.
Data accessibility
All data and code for analyses are available in the Dryad data repository at the following link: http://dx.doi.org/10.5061/dryad.8s586 [81].
Authors' contributions
Concepts and approach were developed by B.V.D. and S.E.P., in consultation with J.B.L. CT scan data were collected by E.S. and B.V.D. Data analysis and interpretation was performed by B.V.D., E.S. and S.E.P. The manuscript was prepared by B.V.D. and S.E.P., and edited by E.S. and J.B.L. All authors gave final approval for publication.
Competing interests
The authors declare no competing financial interests.
Funding
No external funding sources were used for the conduct of this research.
References
- 1.Ifediba MA, Rajguru SM, Hullar TE, Rabbitt RD. 2007. The role of 3-canal biomechanics in angular motion transduction by the human vestibular labyrinth. Ann. Biomed. Eng. 35, 1247–1263. (doi:10.1007/s10439-007-9277-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlin JC, Kirk EC, Rowe TB.. 2013. Functional implications of ubiquitous semicircular canal non-orthogonality in mammals. PLoS ONE 8, e79585 (doi:10.1371/journal.pone.0079585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang A, Hullar TE. 2007. Relationship of semicircular canal size to vestibular-nerve afferent sensitivity in mammals. J. Neurophysiol. 98, 3197–3205. (doi:10.1152/jn.00798.2007) [DOI] [PubMed] [Google Scholar]
- 4.Hullar TE. 2006. Semicircular canal geometry, afferent sensitivity, and animal behavior. Anat. Rec. - Part A Discov. Mol. Cell. Evol. Biol. 288, 466–472. (doi:10.1002/ar.a.20304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox PG, Jeffery N. 2010. Semicircular canals and agility: the influence of size and shape measures. J. Anat. 216, 37–47. (doi:10.1111/j.1469-7580.2009.01172.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malinzak MD, Kay RF, Hullar TE. 2012. Locomotor head movements and semicircular canal morphology in primates. Proc. Natl Acad. Sci. USA 109, 17 914–17 919. (doi:10.1073/pnas.1206139109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spoor F, Garland T, Krovitz G, Ryan TM, Silcox MT, Walker A. 2007. The primate semicircular canal system and locomotion. Proc. Natl Acad. Sci. USA 104, 10 808–10 812. (doi:10.1073/pnas.0704250104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgi JA. 2008. Semicircular canal morphology as evidence of locomotor environment in amniotes. PhD Dissertation, Stony Brook University, New York, NY, USA. [Google Scholar]
- 9.Schutz H, Jamniczky HA, Hallgrimsson B, Garland T. 2014. Shape-Shift: semicircular canal morphology responds to selective breeding for increased locomotor activity. Evolution 68, 1–39. (doi:10.5061/dryad.3sv4p) [DOI] [PubMed] [Google Scholar]
- 10.Billet G, Hautier L, Asher RJ, Schwarz C, Crumpton N, Martin T, Ruf I. 2012. High morphological variation of vestibular system accompanies slow and infrequent locomotion in three-toed sloths. Proc. R. Soc. B 279, 3932–3939. (doi:10.1098/rspb.2012.1212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandel BM, Hullar TE. 2010. The relationship of head movements to semicircular canal size in cetaceans. J. Exp. Biol. 213, 1175–1181. (doi:10.1242/jeb.040105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billet G, Germain D, Ruf I, de Muizon C, Hautier L. 2013. The inner ear of Megatherium and the evolution of the vestibular system in sloths. J. Anat. 223, 557–567. (doi:10.1111/joa.12114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orliac MJ, Benoit J, O'Leary MA. 2012. The inner ear of Diacodexis, the oldest artiodactyl mammal. J. Anat. 221, 417–426. (doi:10.1111/j.1469-7580.2012.01562.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo ZX, Ruf I, Martin T. 2012. The petrosal and inner ear of the Late Jurassic cladotherian mammal Dryolestes leiriensis and implications for ear evolution in therian mammals. Zool. J. Linn. Soc. 166, 433–463. (doi:10.1111/j.1096-3642.2012.00852.x) [Google Scholar]
- 15.Benoit J, Essid EM, Marzougui W, Khayati Ammar H, Lebrun R, Tabuce R, Marivaux L. 2013. New insights into the ear region anatomy and cranial blood supply of advanced stem Strepsirhini: evidence from three primate petrosals from the Eocene of Chambi, Tunisia. J. Hum. Evol. 65, 551–572. (doi:10.1016/j.jhevol.2013.06.014) [DOI] [PubMed] [Google Scholar]
- 16.Lebrun R, Godinot M, Couette S, Tafforeau P, Zollikofer C. 2012. The labyrinthine morphology of Pronycticebus gaudryi (Primates, Adapiformes). Palaeobiodiver. Palaeoenviron. 92, 527–537. (doi:10.1007/s12549-012-0099-z) [Google Scholar]
- 17.Ryan TM, et al. 2012. Evolution of locomotion in Anthropoidea: the semicircular canal evidence. Proc. R. Soc. B 279, 3467–3475. (doi:10.1098/rspb.2012.0939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker A, Ryan TM, Silcox MT, Simons EL, Spoor F. 2008. The semicircular canal system and locomotion: the case of extinct lemuroids and lorisoids. Evol. Anthropol. 17, 135–145. (doi:10.1002/evan.20165) [Google Scholar]
- 19.David R, Droulez J, Allain R, Berthoz A, Janvier P, Bennequin D. 2010. Motion from the past. A new method to infer vestibular capacities of extinct species. C. R. Palevol. 9, 397–410. (doi:10.1016/j.crpv.2010.07.012) [Google Scholar]
- 20.Ladevèze S, de Muizon C, Colbert M, Smith T. 2010. 3D computational imaging of the petrosal of a new multituberculate mammal from the Late Cretaceous of China and its paleobiologic inferences. C. R. Palevol. 9, 319–330. (doi:10.1016/j.crpv.2010.07.008) [Google Scholar]
- 21.Georgi JA, Sipla JS, Forster CA. 2013. Turning semicircular canal function on its head: dinosaurs and a novel vestibular analysis. PLoS ONE 8, e58517 (doi:10.1371/journal.pone.0058517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spoor F, Bajpai S, Hussain ST, Kumar K, Thewissen JGM. 2002. Vestibular evidence for the evolution of aquatic behaviour in early cetaceans. Nature 417, 163–166. (doi:10.1038/417163a) [DOI] [PubMed] [Google Scholar]
- 23.Coleman MN, Boyer DM. 2012. Inner ear evolution in primates through the Cenozoic: implications for the evolution of hearing. Anat. Rec. 295, 615–631. (doi:10.1002/ar.22422) [DOI] [PubMed] [Google Scholar]
- 24.Knoll F, Witmer LM, Ortega F, Ridgely RC, Schwarz-Wings D.. 2012. The braincase of the basal sauropod dinosaur Spinophorosaurus and 3D reconstructions of the cranial endocast and inner ear. PLoS ONE 7, e30060 (doi:10.1371/journal.pone.0030060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso PD, Milner AC, Ketcham RA, Cookson MJ, Rowe TB. 2004. The avian nature of the brain and inner ear of Archaeopteryx. Nature 430, 666–669. (doi:10.1038/nature02706) [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues PG, Ruf I, Schultz CL. 2013. Digital reconstruction of the Otic region and inner ear of the non-mammalian Cynodont Brasilitherium riograndensis (Late Triassic, Brazil) and its relevance to the evolution of the mammalian ear. J. Mamm. Evol. 20, 291–307. (doi:10.1007/s10914-012-9221-2) [Google Scholar]
- 27.Maddin HC, Sherratt E. 2014. Influence of fossoriality on inner ear morphology: insights from caecilian amphibians. J. Anat. 225, 83–93. (doi:10.1111/joa.12190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boistel R, Herrel A, Lebrun R, Daghfous G, Tafforeau P, Losos JB, Vanhooydonck B. 2011. Shake rattle and roll: the bony labyrinth and aerial descent in squamates. Integr. Comp. Biol. 51, 957–968. (doi:10.1093/icb/icr034) [DOI] [PubMed] [Google Scholar]
- 29.Yi H, Norell MA. 2015. The burrowing origin of modern snakes. Sci. Adv. 1, 1–5. (doi:10.1126/sciadv.1500743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sipla JS. 2007. The semicircular canals of birds and non-avian theropod dinosaurs. Stony Brook, NY: Stony Brook Univ. [Google Scholar]
- 31.Georgi JA, Sipla JS. 2009. Comparative and functional anatomy of balance in aquatic reptiles and birds. In Sensory evolution on the threshold: adaptations in secondarily aquatic vertebrates (eds JGM Thewissen, N Sirpa), p. 98 Berkley, CA: University of California Press. [Google Scholar]
- 32.Nicholson KE, Crother BI, Guyer C, Savage JM. 2012. It is time for a new classification of anoles (Squamata: Dactyloidae). Zootaxa 3477, 1–108. [Google Scholar]
- 33.Losos JB. 2009. Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. Berkley and Los Angle, CA: University of California Press. [Google Scholar]
- 34.Sherratt E, Castañeda MdR, Garwood RJ, Mahler DL, Sanger TJ, Herrel A, de Queiroz K, Losos JB. 2015. Amber fossils demonstrate deep-time stability of Caribbean lizard communities. Proc. Natl Acad. Sci. USA 112, 9961–9966. (doi:10.1073/pnas.1506516112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lebrun R, de León MP, Tafforeau P, Zollikofer C. 2010. Deep evolutionary roots of strepsirrhine primate labyrinthine morphology. J. Anat. 216, 368–380. (doi:10.1111/j.1469-7580.2009.01177.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alloing-Séguier L, Sánchez-Villagra MR, Lee MSY, Lebrun R. 2013. The bony labyrinth in Diprotodontian marsupial mammals: diversity in extant and extinct forms and relationships with size and phylogeny. J. Mamm. Evol. 20, 191–198. (doi:10.1007/s10914-013-9228-3) [Google Scholar]
- 37.Davies KTJ, Bates PJJ, Maryanto I, Cotton JA, Rossiter SJ.. 2013. The evolution of bat vestibular systems in the face of potential antagonistic selection pressures for flight and echolocation. PLoS ONE 8, e61998 (doi:10.1371/journal.pone.0061998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spoor F, Zonneveld F. 1998. Comparative review of the human bony labyrinth. Am. J. Phys. Anthropol. 27, 211–251. (doi:10.1002/(SICI)1096-8644(1998)107:27+<211::AID-AJPA8>3.0.CO;2-V) [DOI] [PubMed] [Google Scholar]
- 39.Ekdale EG. 2016. Morphological variation among the inner ears of extinct and extant baleen whales (Cetacea: Mysticeti). J. Morphol. 227, 1599–1615. (doi:10.1002/jmor.20610) [DOI] [PubMed] [Google Scholar]
- 40.Iturralde-Vinent MA. 2001. Geology of the amber-bearing deposits of the Greater Antilles. Caribb. J. Sci. 37, 141–167. [Google Scholar]
- 41.Bookstein FL. 1991. Morphometric tools for landmark data: geometry and biology. New York, NY: Cambridge University Press. [Google Scholar]
- 42.Dryden L, Mardia K V. 1998. Statistical shape analysis. Chichester, UK: John Wiley & Son. [Google Scholar]
- 43.Gunz P, Ramsier M, Kuhrig M, Hublin JJ, Spoor F. 2012. The mammalian bony labyrinth reconsidered, introducing a comprehensive geometric morphometric approach. J. Anat. 220, 529–543. (doi:10.1111/j.1469-7580.2012.01493.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grohé C, Tseng ZJ, Lebrun R, Boistel R, Flynn JJ. 2016. Bony labyrinth shape variation in extant Carnivora: a case study of Musteloidea. J. Anat. 228, 366–383. (doi:10.1111/joa.12421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coleman MN, Colbert MW. 2007. Technical note: CT thresholding protocols for taking measurements on three-dimensional models. Am. J. Phys. Anthropol. 132, 723–725. (doi:10.1002/ajpa) [DOI] [PubMed] [Google Scholar]
- 46.Reddy D, Kim J, Raaum R. 2006. Resample. See http://www.nycep.org/nmg/downloads/resample.exe .
- 47.Rohlf F, Slice D. 1990. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Biol. 39, 40–59. (doi:10.2307/2992207) [Google Scholar]
- 48.Gunz P, Mitteroecker P, Bookstein FL. 2005. Semilandmarks in three dimensions. In Modern morphometrics in physical anthropology (ed. DE Slice), pp. 73–98. Berlin, Germany: Springer. [Google Scholar]
- 49.R Development Core Team. 2016. R: a language and environment for statistical computing, Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 50.Adams DC, Collyer ML, Sherratt E. 2014. Geomorph: software for geometric morphometric analyses. R package version 2.1.4. See https://cran.r-project.org/package=geomorph. [Google Scholar]
- 51.Gamble T, Geneva AJ, Glor RE, Zarkower D. 2014. Anolis sex chromosomes are derived from a single ancestral pair. Evolution 68, 1027–1041. (doi:10.1111/evo.12328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanger TJ, Sherratt E, Mcglothlin JW, Brodie ED, Losos JB, Abzhanov A. 2013. Convergent evolution of sexual dimorphism in skull shape using distinct developmental strategies. Evolution 67, 2180–2193. (doi:10.1111/evo.12100) [DOI] [PubMed] [Google Scholar]
- 53.Adams DC. 2014. A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Syst. Biol. 63, 685–697. (doi:10.1093/sysbio/syu030) [DOI] [PubMed] [Google Scholar]
- 54.Blomberg SP, Garland TJ, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. (doi:10.1111/j.0014-3820.2003.tb00285.x) [DOI] [PubMed] [Google Scholar]
- 55.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. (doi:10.1111/j.2041-210X.2011.00169.x) [Google Scholar]
- 56.Revell LJ. 2009. Size-correction and principal components for interspecific comparative studies. Evolution 63, 3258–3268. (doi:10.1111/j.1558-5646.2009.00804.x) [DOI] [PubMed] [Google Scholar]
- 57.Adams DC. 2014. A method for assessing phylogenetic least squares models for shape and other high-dimensional multivariate data. Evolution 68, 2675–2688. (doi:10.1111/evo.12463) [DOI] [PubMed] [Google Scholar]
- 58.Goodall C. 1991. Procrustes methods in the statistical analysis of shape. J. R. Stat. Soc. Ser. B 53, 285–339. [Google Scholar]
- 59.Schlager S.2016. Morpho: calculations and visualisations related to geometric morphometrics. R-package version 2.0. See https://cran.r-project.org/package=Morpho .
- 60.Mitteroecker P, Bookstein F. 2011. Linear discrimination, ordination, and the visualization of selection gradients in modern morphometrics. Evol. Biol. 38, 100–114. (doi:10.1007/s11692-011-9109-8) [Google Scholar]
- 61.Albrecht GH. 1992. Assessing the affinities of fossils using canonical variates and generalized distances. Hum. Evol. 7, 49–69. (doi:10.1007/BF02436412) [Google Scholar]
- 62.Manly BFJ. 2006. Randomization, bootstrap and Monte Carlo methods in biology. Boca Raton, FL: CRC Press. [Google Scholar]
- 63.Wilcox RR. 2011. Introduction to robust estimation and hypothesis testing. New York, NY: Academic Press. [Google Scholar]
- 64.Edgington E, Onghena P. 2007. Randomization tests. Boca Raton, FL: CRC Press. [Google Scholar]
- 65.Strauss RE. 2010. Discriminating groups of organisms. In Morphometrics for nonmorphometricians (ed. Elewa Amt.), pp. 73–91. Berlin, Germany: Springer. [Google Scholar]
- 66.Campbell NA. 1984. Some aspects of allocation and discrimination. In Multivariate statistical methods in physical anthropology: a review of recent advances and current developments (eds Van GN, Vark WW, Howells), pp. 177–192. Dordrecht, Netherlands: Springer. [Google Scholar]
- 67.Kovarovic K, Aiello LC, Cardini A, Lockwood CA. 2011. Discriminant function analyses in archaeology: are classification rates too good to be true? J. Archaeol. Sci. 38, 3006–3018. (doi:10.1016/j.jas.2011.06.028) [Google Scholar]
- 68.Liu K, Lam NS-N. 1985. Paleovegetational reconstruction based on modern and fossil pollen data: an application of discriminant analysis. Ann. Assoc. Am. Geogr. 75, 115–130. (doi:10.1111/j.1467-8306.1985.tb00062.x) [Google Scholar]
- 69.Adams DC, Felice RN.. 2014. Assessing trait covariation and morphological integration on phylogenies using evolutionary covariance matrices. PLoS ONE 9, e94335 (doi:10.1371/journal.pone.0094335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Losos JB, Irschick DJ. 1996. The effect of perch diameter on escape behaviour of Anolis lizards: laboratory predictions and field tests. Anim. Behav. 51, 593–602. (doi:10.1006/anbe.1996.0063) [Google Scholar]
- 71.Losos JB, Sinervo B. 1989. The effects of morphology and perch diameter on sprint performance of Anolis lizards. J. Exp. Biol. 145, 23–30. [Google Scholar]
- 72.Sathe EA, Husak JF. 2015. Sprint sensitivity and locomotor trade-offs in green anole (Anolis carolinensis) lizards. J. Exp. Biol. 218, 2174–2179. (doi:10.1242/jeb.116053) [DOI] [PubMed] [Google Scholar]
- 73.Losos JB. 1990. Ecomorphology, performance capacity and scaling of West Indian Anolis lizards: an evolutionary analysis. Ecol. Monogr. 60, 369–388. (doi:10.2307/1943062) [Google Scholar]
- 74.Sanger TJ, Mahler DL, Abzhanov A, Losos JB. 2012. Roles for modularity and constraint in the evolution of cranial diversity among Anolis lizards. Evolution 66, 1525–1542. (doi:10.1111/j.1558-5646.2011.01519.x) [DOI] [PubMed] [Google Scholar]
- 75.Macrini TE, Flynn JJ, Croft DA, Wyss AR. 2010. Inner ear of a notoungulate placental mammal: anatomical description and examination of potentially phylogenetically informative characters. J. Anat. 216, 600–610. (doi:10.1111/j.1469-7580.2010.01224.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macrini TE, Flynn JJ, Ni X, Croft DA, Wyss AR. 2013. Comparative study of notoungulate (Placentalia, Mammalia) bony labyrinths and new phylogenetically informative inner ear characters. J. Anat. 223, 442–461. (doi:10.1111/joa.12108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Billet G, de Muizon C, Schellhorn R, Ruf I, Ladevèze S, Bergqvist L. 2015. Petrosal and inner ear anatomy and allometry amongst specimens referred to Litopterna (Placentalia). Zool. J. Linn. Soc. 173, 956–987. (doi:10.1111/zoj.12219) [Google Scholar]
- 78.Jones GM, Spells KE. 1963. A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc. R. Soc. Lond. B 157, 403–419. (doi:10.1098/rspb.1963.0019) [DOI] [PubMed] [Google Scholar]
- 79.Poinar GO. 1991. Hymenaea protera sp. n. (Leguminosae, Caesalpinioideae) from Dominican amber has African affinities. Experientia 47, 1075–1082. (doi:10.1007/BF01923347) [Google Scholar]
- 80.Hopkins MJ, Lidgard S. 2012. Evolutionary mode routinely varies among morphological traits within fossil species lineages. Proc. Natl Acad. Sci. USA 109, 20 520–20 525. (doi:10.1073/pnas.1209901109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dickson BV, Losos JB, Sherratt E, Pierce SE. 2017. Data from: Semicircular canals in Anolis lizards: ecomorphological convergence and ecomorph affinities of fossil species Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.8s586) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Dickson BV, Losos JB, Sherratt E, Pierce SE. 2017. Data from: Semicircular canals in Anolis lizards: ecomorphological convergence and ecomorph affinities of fossil species Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.8s586) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data and code for analyses are available in the Dryad data repository at the following link: http://dx.doi.org/10.5061/dryad.8s586 [81].