Abstract
Global change, like droughts, can destabilize the carbon sink function of peatlands, either directly or indirectly through changes in plant community composition. While the effects of drought and plant community composition on individual carbon (C) related processes are well understood, their effect on multiple C-related processes simultaneously—multifunctionality—is poorly known. We studied the effect of drought on four C-related processes (net and gross CO2 exchange, methane fluxes, and dissolved organic carbon content) in a plant removal experiment. Plant functional type (PFT) removal (graminoids, herbs, Polytrichum spp., incl. combinations) negatively affected multifunctionality; most markedly when all PFTs were removed. Our results corroborate a negative drought effect on C-related multifunctionality. Drought reduced multifunctionality, and this reduction was again largest when all PFTs were removed. Our data further indicate that much of these negative drought effects were carried over and maintained from the initial removal treatment. These results suggest that while a high diversity in plant functional types is associated to high C-related multifunctionality, plant community assembly does not drive the ability of peatlands to withstand the negative impacts of drought on multifunctionality. Hence, to safeguard the carbon cycling function in intact peatlands, the effects of climate change on the functional composition of the peatland plant community needs to be minimized.
Keywords: carbon cycling, ecosystem functions, global change, multiple functions, plant functional types, wetlands
1. Introduction
Global climate change is affecting important ecosystem processes, by altering abiotic and biotic conditions. Climate projections predict, for example, precipitation patterns to become increasingly variable and the frequency of extreme drought to increase for the Northern Hemisphere [1]. Such changes are extremely important for ecosystems that to a certain extent depend on precipitation for their functioning, such as peatlands [2]. As peatlands represent an important sink for atmospheric carbon (C)—they are currently estimated to store about 500 GT of C as peat [3]—the ability of peatlands to act as a carbon sink depends on how the effects of climate change play out on these ecosystems. Much research has been devoted on the role of environmental conditions, including hydrological conditions, on peatland carbon (C) cycling. Overall, consensus exists that the effect of water table drawdown on net peatland C uptake is negative [4,5]. Yet, the responses of individual C-related functions—net ecosystem CO2 uptake, ecosystem respiration, the release of methane, or the production and leaching of dissolved organic carbon—to drought can be complex, often with opposite responses for different processes. A drawdown in the water table, for example, generally leads to enhanced vascular plant productivity in peatlands [6,7], although the effect largely depends on the composition of the plant community [8–10]. Clearer is the effect of water table drawdown on carbon uptake by peat mosses; lower water tables impede peat moss productivity [11–13]. The effects of water table drawdown on peatland ecosystem respiration are less straightforward. In some studies ecosystem respiration has been shown to be independent from the water table [14,15], while others report increases in respiration rates with decreasing water tables [4,5,10]. Oppositely, methane fluxes from peat decrease with water table drawdown [16]—despite a potential peak at early drought [4]—and are probably the result of decreased potential methane production and alteration in the microbial communities [17,18]. Lastly, drought has been linked to increased concentrations in dissolved organic carbon (DOC) leaching, mainly through increased decomposition of organic matter [19,20]. In short, while our understanding on the effects of drought on individual C-related processes in peatlands is strong, the effects of drought on multiple, simultaneously occurring processes are poorly understood.
Climate change affects ecosystem processes directly, but also indirectly through alterations in the plant community composition [21], which then affect ecosystem processes. These indirect effects are rather understudied, yet important as changes in the plant community can modulate the negative impact of climate change [22]. In peatland ecology the latter may be because peatland species composition has long been perceived as remarkably stable [23,24]. Historical records, however, link drought to changes in plant community composition [25]. This has led to increased recognition that projected climate change alters species interactions, especially in the temperate climate zone [26]. Further, alterations in the climate or environmental conditions may reduce biological diversity [27,28], the cornerstone for sustaining a variety of ecosystem processes [29,30]. As species typically differ in their functional traits—a morphological, physiological, or chemical characteristic that strongly influences organismal performance—changes in plant species composition or diversity may alter functional trait composition of the community [31]. Depending on the nature of changes in the plant community, climate change may thus lead to an array of feedbacks on peatland processes, including its carbon sink function. In peatlands, the importance of plant community composition for individual ecosystem processes has recently been put forward [12,21,32–35]. Yet, the role of peatland plant community assembly on the ability of peatlands to maintain multiple carbon-related functions—hereafter referred to a C-related multifunctionality—remains unresolved. Moreover, the role of plant functional types on the robustness of C-related multifunctionality to environmental stress, such as drought, is unknown. We aim to bridge this knowledge-gap, and disentangle the effect of vascular plant community composition (using a plant removal approach) and drought C-related multifunctionality in peatlands. We hypothesize (i) that removing plant functional types (graminoids, herbs, non-Sphagnum mosses) erodes multifunctionality of carbon-related peatland processes, and that this effect is larger with increasing number of removed plant functional types. Additionally, we hypothesize that (ii) the loss of plant functional types reduces the ability of poor fen communities to withstand the negative effects of drought on multifunctionality.
2. Material and methods
2.1. Field sampling
The Molenpolder is situated in the Vechtplassen area, a region rich in plant diversity due to the presence of all successional terrestrialization stages from open peat ponds to ombrotrophic peatlands. A total of 24 samples (diameter 22 cm, depth 26 cm) were collected from a poor fen in the Molenpolder, The Netherlands (52°9′7.07′′ N; 5°5′83.18′′ E), in March 2010. The fen is characteristic for the area and of special interest for conservation. It is characterized by a peat soil (1.5–2 m deep) overlain by homogeneous Sphagnum spp. (mainly S. palustre L., S. squarossum Crome) and Polytrichum commune Hedw. co-dominated bryophyte layer. The vascular plant community is dominated by the graminoids Carex echinata Lam., Juncus effuses L., Juncus acutiflorus Benth., Hierochloë odorata (L.) Britton, Sterns & Poggenb., Carex hirta L., Carex canescens L. and Alopecurus geniculatus Lindh. Ex Scheele, and the herbs Peucedanum palustre (L.) Moench, Lysimachia thyrsiflora L., Drosera rotundifolia L., Potentilla palustris (L.) Scop. and Hydrocotyle vulgaris L. We sampled in a 10 × 10 m area of a 30 × 75 m mesotrophic fen. This approach was assumed to reduce variance in peat structure, and ensure homogeneity in the vegetation. Extracted mesocosms were, however, never extracted closer than 1 m from each other, while taking care that each mesocosm would contain a selection of species from all plant functional types (Sphagnum mosses, Polytrichum spp., graminoids, and herbs). Samples, consisting of fen peat and living vegetation, were placed in PVC containers, and are hereafter referred to as mesocosms.
2.2. Experimental set-up
All mesocosms were subjected to a four-week acclimatization period in a phytotron (20°C/18°C and 12/12 h day/night, 70% relative humidity (RH), 400 ppm CO2, 200 µmol PAR m−2 s−1 light intensity), where we maintained water tables at field condition (1–2 cm below the bryophyte surface), using an artificial rainwater solution [36]. After this acclimatization period, plant communities were experimentally manipulated. All mesocosms were randomly allocated to one of the following treatments (n = 4): controls (C), graminoid removal (–Gram), herb removal (–Herb), Polytrichum removal (–Poly), graminoid + herb removal (–Gram & Herb), and graminoids + herb + Polytrichum removal (–Gram & Herb & Poly). Due to experimental limitations we did not remove Polytrichum mosses in combination with graminoid or herb removal. Further, Sphagnum mosses were never removed as they are considered key to the functioning of the ecosystem. For the vascular plants, removal was realized by clipping the aboveground biomass flush to the moss layer. Polytrichum was removed by pulling all individuals from the mesocosms. This was done cautiously to minimize perturbation in the peat soil. The control mesocosms were left unchanged in species composition, yet to control for potential effects of the treatment we selectively removed 5–10% of the vascular plant and Polytrichum cover (balanced over the present plant functional types (PFTs) and based on visual comparison of the effect of the clipping on the plant cover in removal mesocosms). As a result of these treatments, we removed 0.77 ± 0.1 g in the control mesocosms, 4.55 ± 1.4 g in the graminoids removal mesocosms, 0.24 ± 0.07 g in the herb removal mesocosms, 13.5 ± 4.9 g in the Polytrichum removal mesocosms, 3.53 ± 0.4 g in the graminoids & herb removal mesocosms, and 9.76 ± 2.6 g in the graminoid & herb & Polytrichum removal mesocosms. Throughout the experiment, treatments were reinforced by regular removal of regrowth.
After plant removal, mesocosms were left to acclimatize over a four-week period (post-clipping acclimatization). Previous research with ombrotrophic bog mesocosms has shown this time to be sufficient to minimize the effect of decaying roots on ecosystem respiration [12]. During the post-clipping acclimatization period, mesocosms were watered two times per week to maintain field conditions. Following the post-clipping acclimatization, a 25 day drought period was initiated. This period commenced by draining all water from the mesocosms, and a full stop of the watering regime.
2.3. Ecosystem function measurements
Closed transparent flux chambers (diameter 20 cm, height 29 cm, fitted with a circulating fan) were placed over the mesocosms to measure CO2 and CH4 fluxes, using a photoacoustic multi-gas analyser (Innova Bruel and Kjær BK 1302) connected to a multipoint sampler (CBISS MK2, 4-channel, CBISS Ltd., England). Chamber measurements comprised five succeeding sampling points with an 8 min interval. Net ecosystem exchange (NEE) was measured weekly during the acclimatization and the post-acclimatization period, to enable calculating reliable initial values (see below). To assess the effect of drought, NEE was measured at the first and last day of the drought period. Ecosystem CO2 respiration (RECO) was measured in parallel, using a darkened chamber. Further, pore-water samples were extracted from all mesocosms at the time of gas measurements using Rhizon soil moisture samplers (type MOM, pore size 0.1 µm, Eijkelkamp, Giesbeek, NL). Pore-water samples were stored in glass vials in the dark at 4°C and were analysed for dissolved organic carbon (DOC) within two weeks after collection, using a Skalar SANPLUS segmented flow analyser (Skalar analytical b.v. Breda, NL). As DOC is highly mobile in the peat, we assume this part of the peat carbon pool to represent the labile carbon pool, vulnerable to leaching and able to enhance decomposition of organic matter by stimulating the microbial community.
NEE was calculated from the change in concentration in the chamber headspace with time, using an exponential nonlinear function [37]. CH4 fluxes and RECO were calculated using linear regression of gas concentrations in the chamber headspace over time. Gross ecosystem production (GEP) was calculated as the sum of NEE and RECO. The ecological sign convention was used for the CO2 (NEE and GEP) and CH4 data, so that positive values indicate a sink function, while negative values indicate a source function of the ecosystem. High concentrations of DOC, which is the product of decomposition and plant exudation, were seen as a negative function, as these DOCs can be lost from the ecosystem. To maintain directional change comparable to the other functions we calculated inversed DOC concentrations (i.e. low inversed values = high DOC concentrations).
2.4. Ecosystem multifunctionality
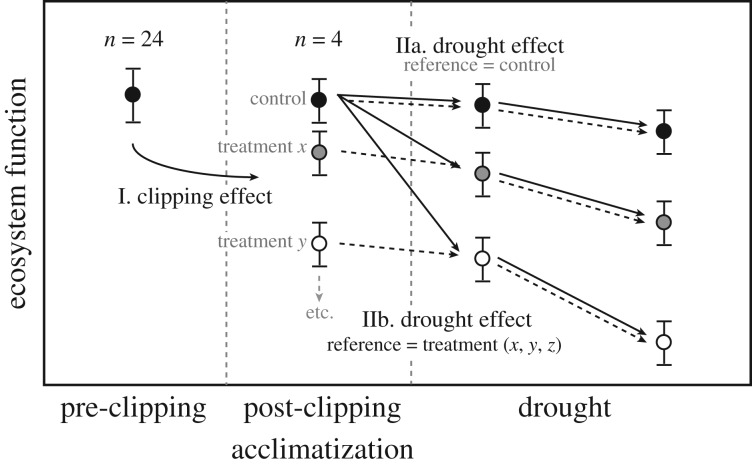
The overall effects of plant removal and drought on C-related processes were tested using a multifunctionality approach [38]. Here, multifunctionality is defined as a single metric (z-score) describing the overall function of net CO2 and CH4 ecosystem exchange, gross ecosystem production, and the production and leaching of dissolved organic carbon. We calculated multifunctionality in a multiple-step approach. First, per mesocosms data for each process were averaged for the four time points during the acclimatization and post-clipping acclimatization period. This step was necessary to reduce the variance in the ecosystem process values, hence provide a robust value for further analyses. Next, to calculate the effect of plant functional type removal on ecosystem processes per treatment, we standardized the process values by the global mean and standard deviation (i.e. the mean and standard deviation of all mesocosms, n = 24) of the pre-clipping ecosystem process (figure 1). To assess the effect of drought on ecosystem processes we used two approaches (figure 1). In the first approach (figure 1, IIa), we standardized ecosystem process during the drought period for each treatment by the mean and standard deviation of the post-clipping acclimatization control values. In the second approach (figure 1, IIb), ecosystem process values during drought for each treatment were standardized by the mean and standard deviation of the post-clipping acclimatization values of the corresponding treatment. These approaches differ in that the first approach takes the non-clipped control values as a reference, while the second approach tests for the effect of drought irrespective of apparent effects of clipping on ecosystem processes. Subsequently, the z-scores were used as an index of ecosystem multifunctionality [39]. To remain close to natural processes and differences in the individual process rates, and hence be relevant to land managers, we did not scale individual process values. Negative z-scores indicate low multifunctionality, while positive z-scores indicate high multifunctionality.
Figure 1.
Schematic set-up of the data analyses. The effect of clipping on each ecosystem function (I. clipping effect) was calculated as the difference in ecosystem function before clipping (mean of all values, n = 24) and after clipping. z-values were calculated after standardization by the pre-clipping mean and standard deviation. The effect of drought was calculated in two different ways. First (IIa), the effect of drought for each plant removal treatment on each ecosystem function was calculated as the difference in the respective function during drought and the post-clipping acclimatization control values. Hence, for each treatment z-values were calculated after standardization by the post-clipping acclimatization control. In the second approach (IIb), instead of using the post-clipping acclimatization control as a reference, the post-clipping acclimatization ecosystem function values for each corresponding treatment were used.
2.5. Data analyses
The effects of the plant functional type removal treatments on individual ecosystem processes and multifunctionality was tested using generalized linear models (GLMs) assuming Gaussian data distribution. As the amount of biomass removed may explain part of the effects, we initially tested different models: one that excluded, and one that included biomass removed (g) as a covarying factor. Using the selMod function in the pgirmess package in R, we selected the model with the smallest corrected Akaike information criterion (AICc). This model was then subjected to analysis of variance, using the ANOVA function and the F statistic in the stats package, followed by a Tukey multicomparison test to highlight differences between treatments. The effect of clipping and drought (for both standardization approaches) on multifunctionality was analysed in a similar manner. All analyses were performed with the software R 3.2.3.
3. Results
3.1. Plant removal effect on individual ecosystems functions
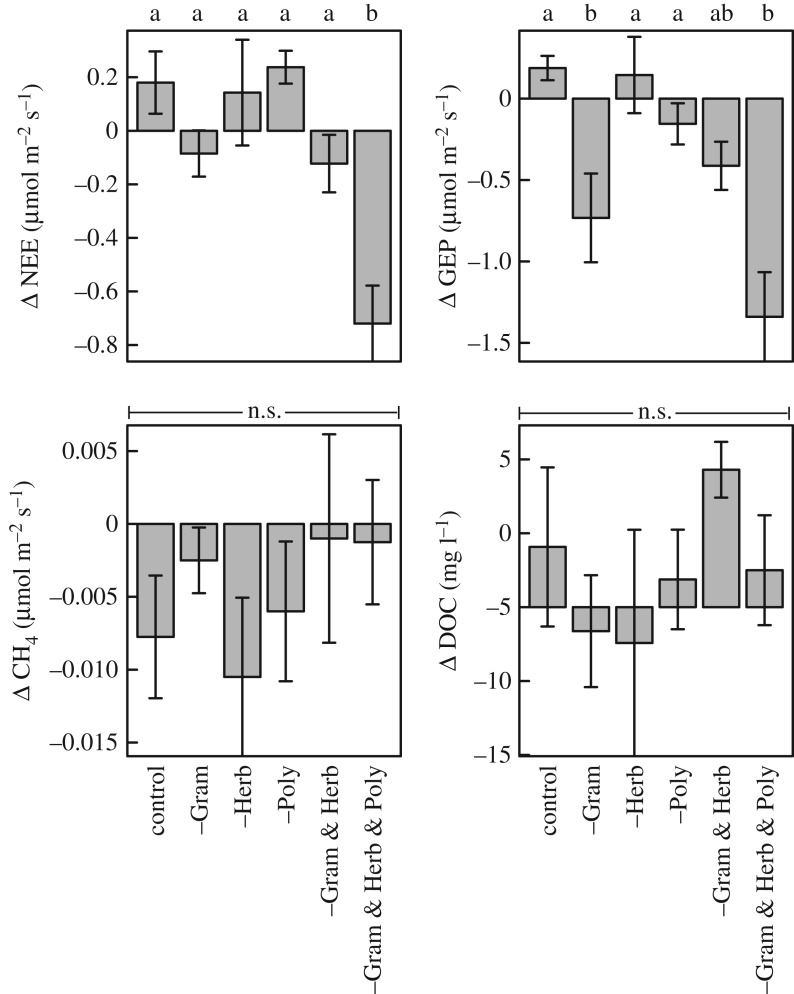
Plant functional type removal affected net ecosystem carbon exchange (NEE) and gross ecosystem production (GEP), but not methane (CH4) fluxes and pore water dissolved organic carbon (DOC) concentrations (table 1 and figure 2). Notably, removing all vascular plant functional types from the mesocosms caused NEE to decrease dramatically. GEP decreased upon plant removal in those treatments that included the removal of graminoids (i.e. –Gram, –Gram & Herb, –Gram & Herb & Poly), and could be partly explained by the loss of biomass (table 1 and figure 2). While not significant (table 1), the removal of more than one plant functional type seemed to lower methane production by the peat (figure 2).
Table 1.
(a) Results of the model testing, comparing the power of two models—one with only the plant removal treatments, one with both the removal treatment and the amount of biomass removed as factors—in explaining the change in ecosystem CO2 net exchange (NEE), gross ecosystem production (GEP), net methane (CH4) flux, and the content of dissolved organic carbon, before and after plant removal. LLmax = maximized log-likelihood of the model, AIC = Akaike Information criterion, AICc = corrected AIC. (b) Results of analysis on variance (ANOVA) on the most significant model, i.e. the model with the lowest AICc.
| (a) | ||||
|---|---|---|---|---|
| ecosystem process | model | LLmax | AIC | AICc |
| NEE | ∼treatment | 2.5 | 9.1 | 16.06 |
| ∼treatment + biomass removed | 3.3 | 9.4 | 18.95 | |
| GEP | ∼treatment + biomass removed | –5.3 | 26.6 | 36.21 |
| ∼treatment | –9.0 | 32.0 | 39.00 | |
| CH4-flux | ∼treatment | 80.3 | –146.7 | –139.69 |
| ∼treatment + biomass removed | 80.3 | –144.7 | –135.10 | |
| DOC | ∼treatment | –84.2 | 182.4 | 189.44 |
| ∼treatment + biomass removed | –83.9 | 183.8 | 193.36 | |
| (b) | ||||
| factor | F-value | p-value | ||
| NEE | treatment | 7.9 | ≤0.001 | |
| GEP | treatment | 10.5 | ≤0.001 | |
| biomass removed | 6.1 | 0.024 | ||
| CH4-flux | treatment | 0.6 | 0.686 | |
| DOC | treatment | 0.8 | 0.547 | |
Figure 2.
The effect of the removal of plant functional types on net ecosytem CO2 exchange, gross ecosystem production, CH4 production, and the dissolved organic carbon (DOC) content in the pore water. Bars represent the change in the four carbon-related processes after plant biomass removal. Different letters indicate significant difference between PFT removal treatments (Tukey's multi-comparison test, p ≤ 0.05). We tested the effect of biomass removal on our model outcomes, see table 1.
3.2. Plant removal effect on carbon-related multifunctionality
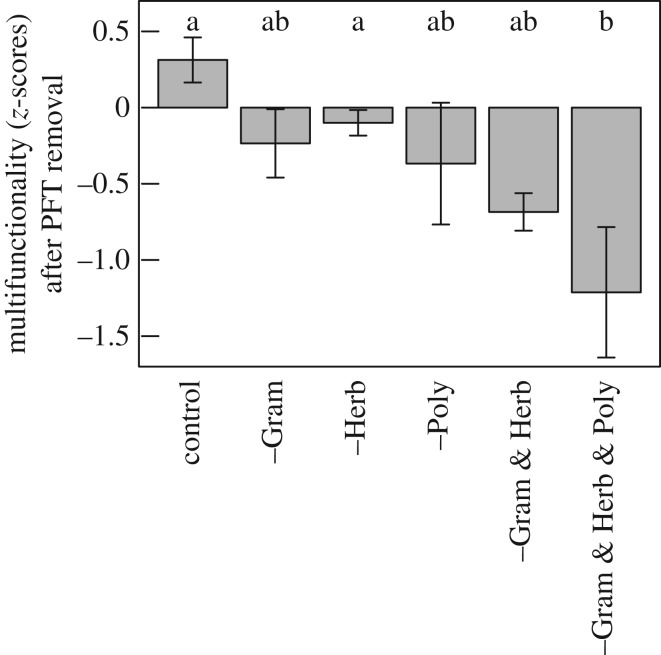
Multifunctionality in carbon-related ecosystem processes was in general eroded when plant functional types were removed (figure 3). The removal of graminoids, herbs, Polytrichum, and graminoids & herbs resulted in a non-significant decrease (175, 132, 217, and 319%, respectively) in multifunctionality. The removal of all vascular plant functional types enhanced this trend (figure 3, 588%), and in line with the aforementioned was mainly due to the decrease (p ≤ 0.05) in NEE and GEP (figure 2). The removal of 5–10% of the plant cover without changing the community composition, i.e. the control plots, showed an increase in multifunctionality, underpinning the negative effect of the loss of PFTs on C-related multifunctionality.
Figure 3.
Ecosystem multifunctionality after biomass removal in relation to plant functional type (PFT) removal treatment. Multifunctionality was calculated as the mean z-value calculated from the standardized (overall pre-clipping mean and standard deviation, figure 1) ecosystem values. Different letters indicate significant difference between PFT removal treatments (Tukey's multi-comparison test, p ≤ 0.05).
3.3. The effect of drought on ecosystem multifunctionality
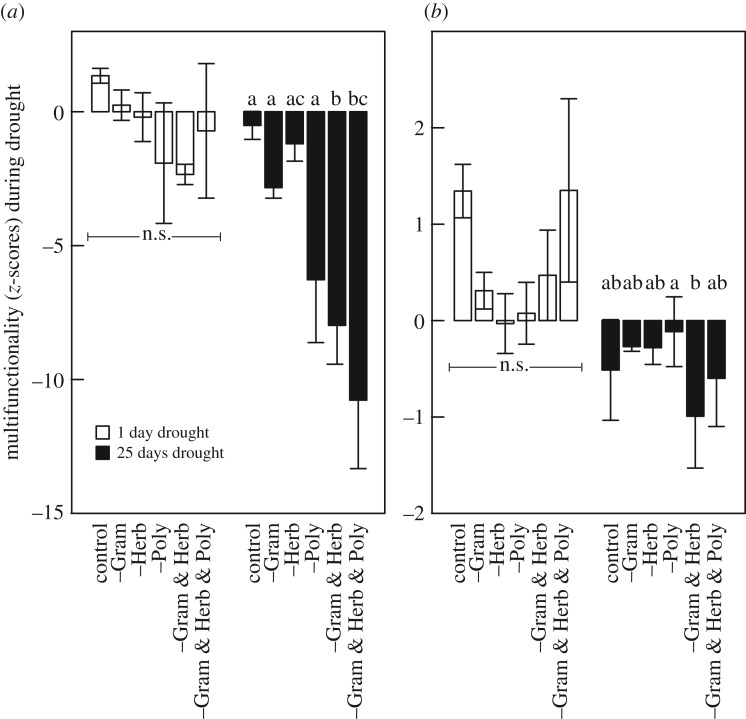
When compared to the post-clipping acclimatization values of the control, i.e. the unchanged communities, C-related multifunctionality was unaffected directly after the initiation of drought, but eroded after the 25 day drought period (figure 4a and table 2). Within-time analysis (t = 1, t = 25) indicates no plant removal effect on multifunctionality directly after the initiation of drought owing to, in part, the loss of biomass (table 2). After the 25 day drought period, multifunctionality was eroded (p ≤ 0.05) when more than one plant functional types were removed (figure 4a and table 2). The negative effects of PFT removal in C-related multifunctionality are likely ‘carried over’ from decreased z-values prior to the initiation of drought (figure 4a; electronic supplementary material, figure S1). To elucidate this, we performed a similar test but now compared multifunctionality values during drought to the post-clipping acclimatization values of the corresponding treatment (intra-treatment comparison, figure 1). Multifunctionality one day after the initiation of drought increased (non-significant) irrespective of plant functional type removal, but decreased after 25 days of drought (figure 4b). These results underpin a strong eroding effect of drought on C-related multifunctionality, but also attest for an absence of a role of plant community assembly thereon (figure 4b and table 2).
Figure 4.
Ecosystem multifunctionality after the initiation of an experimental drought in relation to plant functional type (PFT) removal treatment. C-related multifunctionality was calculated in two ways: First, (a) as the mean z-value calculated from individual ecosystem values standardized by mean and standard deviation values of the post-clipping acclimatization control treatments (figure 1, IIa drought effect); second, (b) as the mean z-value calculated from individual ecosystem values standardized by mean and standard deviation values of the corresponding post-clipping acclimatization PFT treatments (figure 1, IIb drought effect). The relationships between PFT removal and each individual ecosystem function over the experimental period are shown in electronic supplementary material, figure S1. Different letters indicated significant difference between PFT removal treatments (Tukey's multi-comparison test, p ≤ 0.05; n.s., not significant), analysed separately for the two times after initiation of drought.
Table 2.
Results of repeated measures analysis of variance (RM–ANOVA), testing the effect of plant functional type removal, incl. biomass removed (co-variable) on the z-values during drought. The two different ways of testing refer to the method of calculating multifunctionality (see text in Material and methods, figure 1). n.s., non-significant.
| dfnum,dfden | F-value | p-value | |
|---|---|---|---|
| drought effect IIa on multifunctionality | |||
| overall | |||
| time | 1,46 | 29.7 | ≤0.001 |
| treatment | 1,41 | 7.0 | ≤0.001 |
| time × treatment | 1,35 | 2.8 | ≤0.05 |
| biomass removed | 1,40 | 9.0 | ≤0.01 |
| t = 1 | |||
| treatment | 1,18 | 0.9 | 0.530 |
| biomass removed | 1,17 | 0.3 | 0.569 |
| t = 2 | |||
| treatment | 1,18 | 13.9 | ≤0.001 |
| biomass removed | 1,17 | 20.8 | ≤0.001 |
| drought effect IIb on multifunctionality | |||
| overall | |||
| time | 1,46 | 16.4 | ≤0.001 |
| treatment | 1,41 | 0.8 | 0.582 |
| time × treatment | 1,36 | 1.6 | 0.184 |
| biomass removed | n.s. | ||
4. Discussion
Fens have been intensively studied in the context of nitrogen and phosphorus pollution [40–42] and vegetation succession and distribution [43,44]. Additionally, the effects of climate change driven alterations in plant community assembly on poor fen ecosystem processes are well understood [21]. The combined effects of plant functional types on the overall performance of these ecosystems, and on the ecosystems' ability to sustain these functions, to our best of knowledge, however, remain elusive. In grassland ecosystems, functional identity and diversity of the plant community are important drivers for ecosystem multifunctionality [45–47]. Our study does not necessarily reconcile these findings. We suggest that the less pronounced effects of plant functional type (PFT) removal on C-related multifunctionality in our study are explained by opposing responses in individual processes that balance the mean performance over the four carbon-related processes [48]. To illustrate, a decrease in NEE upon PFT removal seems to be counteracted by a reduction in methane fluxes; the latter most likely caused by reduced methanogenic activity through decreased input of labile carbon [18,34,49]. While PFT removal decreases gross C uptake (GEP), depending on the nature of the PFT, the related decrease in biomass results in lower maintenance respiration. This explains, in part, the close resemblance in patterns of NEE and GEP. Nevertheless, and despite the absence of pronounced statistical differences in multifunctionality after PFT removal, the removal of PFTs from the communities always caused multifunctionality to shift from positive to negative. Moreover, the removal of single PFT resulted in a decrease in the simultaneous performance of the four C-related processes. These results reflect general understanding that a high level of diversity is needed to sustain multifunctionality [50–52]. Most likely, plant–microbe interactions play an important role in explaining our results, as changes in the peatland plant community are repeatedly reported to be reflected in the microbial community [34,53,54]. Decreased biodiversity, and in our case the loss of plant functional types, may even reduce the functional diversity of the microbial community [55]. Such reduction of microbial functional diversity may in turn further erode ecosystem multifunctionality [46].
Multifunctionality is an average measure that considers a set of ecosystem processes simultaneously, and should be interpreted with care. If, for example, different functions (i.e. carbon, nitrogen and phosphorus cycling, biodiversity provisioning, etc.) are considered, opposite function may moderate the value of multifunctionality, making ecological interpretation difficult without assessing individual functions. We argue, however, that describing multifunctionality from simultaneous processes that contribute to a single function—C-related multifunctionality—is very powerful as it allows a holistic assessment of the ecosystem function. In other words, while the individual processes that underlie an ecosystem function are important, how these individual processes play out simultaneously—multifunctionality—provides more understanding on the overall status of the particular ecosystem function. Our results highlight that although the influence of plant community assembly on C-related multifunctionality seems to level out due to contrasting effects on individual processes, the loss of individual plant functional types had an overall negative effect on these processes. This then resulted in a slight, though non-significant, decrease in the overall carbon cycling function of the poor fen system.
We calculated the effect of drought on C-related multifunctionality in two ways, one where pre-drought ecosystem function values of the undisturbed control mesocosms served as a reference, and one where the values during drought were compared to values pre-drought from the same treatment. Both results show that drought exacerbates the erosion in poor fen C-related multifunctionality. The first approach shows that, with almost one month of drought, the removal of more than one plant functional types strongly reduces ecosystem multifunctionality. The second approach, where within-treatment effect of drought on multifunctionality was assessed, does not show such effect. This would mean that pre-drought differences in multifunctionality, caused by PFT removal, while exacerbated during drought, persist during drought. In a previous study on mesocosms from a Sphagnum-dominated bog, it has been shown that removing vascular plant functional types decreased net carbon uptake but not the robustness of the ecosystem as a carbon sink to withstand drought [12]. Our results corroborate these findings, and underpin earlier findings on the importance of the peat moss community and the peat matrix for sustaining the functioning of the ecosystem. In other words, non-Sphagnum plant functional types are largely responsible for the magnitude of the peatland carbon sink function, but play less a role in sustaining that function during environmental perturbation like drought.
The results from our study are important, as climate change is known to alter the composition of peatlands, with pronounced shifts between vascular plants and peat mosses, as well as between plant functional types within these larger groups [7,56]. Increased temperature and drought occurrences can increase vascular plant growth leading to a decrease in peat moss growth [21]. Subsequently, shifts in the competitive balance between plant functional types may result in the loss of key plant functional types, weakening the carbon sink function of peatlands [21,57]. In this study, Sphagnum mosses were never removed as they are a crucial part of the ecosystem in Sphagnum-dominated peatland [58], and removing them from the system would result in a non-viable ecosystem [32]. In the light of our results, the loss of single vascular plant functional types from fen ecosystems only marginally affects the ability of these ecosystems to sustain multiple carbon-related functions. Oppositely, our results indicate that a diverse plant functional type composition is most effective in sustaining C-related multifunctionality. Results from a recent study show that the protection of current carbon stocks is important in order to slow down the rate of increases in atmospheric CO2 [59]. Our study reconciles with such statement; protecting the diversity in plant communities in northern peatlands, while not increasing the robustness of these systems to projected drought, increases the overall C-sink function of these systems.
Supplementary Material
Acknowledgements
We thank R. van Rossum for collecting many of the flux data, and J. Kuiper for help during the set-up of the experiment. Eric Allan provided help with the R-codes on multifunctionality. Further, we are indebted to the input of two anonymous reviewers whose contributions improved the quality of our manuscript.
Ethics
Permission for sample collection was granted to B.B. by the Dutch State Forestry Service (Staatsbosbeheer).
Data accessibility
Datasets are deposited at the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.g1pk3) [60].
Authors' contributions
B.J.M.R. and M.M.H. designed the study; B.J.M.R. and B.B. collected the samples. B.J.M.R. and M.M.H. collected the data. B.J.M.R. and V.E.J.J. analysed and interpreted the data. B.J.M.R. and V.E.J.J. wrote the first draft of the manuscript, to which B.B. and M.M.H. contributed. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Financial support for this work has come from the Dutch Foundation for the Conservation of Irish Bogs.
References
- 1.Dai A. 2012. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 3, 52–58. (doi:10.1038/nclimate1633) [Google Scholar]
- 2.Clymo RS. 1987. The ecology of peatlands. Sci. Prog. 71, 593–615. [Google Scholar]
- 3.Yu ZC. 2012. Northern peatland carbon stocks and dynamics: a review. Biogeosciences 9, 4071–4085. (doi:10.5194/bg-9-4071-2012) [Google Scholar]
- 4.Estop-Aragonés C, Zając K, Blodau C. 2016. Effects of extreme experimental drought and rewetting on CO2 and CH4 exchange in mesocosms of 14 European peatlands with different nitrogen and sulfur deposition. Glob. Chang. Biol. 22, 2285–2300. (doi:10.1111/gcb.13228) [DOI] [PubMed] [Google Scholar]
- 5.Lund M, Christensen TR, Lindroth A, Schubert P. 2012. Effects of drought conditions on the carbon dioxide dynamics in a temperate peatland. Environ. Res. Lett. 7, 045704 (doi:10.1088/1748-9326/7/4/045704) [Google Scholar]
- 6.Weltzin JF, Pastor J, Harth C, Bridgham SD, Updegraff K, Chapin CT. 2000. Response of bog and fen plant communities to warming and water-table manipulations. Ecology 81, 3464–3478. (doi:10.1890/0012-9658(2000)081[3464:ROBAFP]2.0.CO;2) [Google Scholar]
- 7.Buttler A, et al. 2015. Experimental warming interacts with soil moisture to discriminate plant responses in an ombrotrophic peatland. J. Veg. Sci. 26, 964–974. (doi:10.1111/jvs.12296) [Google Scholar]
- 8.Korrensalo A, Alekseychik P, Hájek T, Rinne J, Vesala T, Mehtätalo L, Mammarella I, Tuittila E-S. 2017. Species-specific temporal variation in photosynthesis as a moderator of peatland carbon sequestration. Biogeosciences 14, 257–269. (doi:10.5194/bg-14-257-2017) [Google Scholar]
- 9.Breeuwer A, Robroek BJM, Limpens J, Heijmans MMPD, Schouten MGC, Berendse F. 2009. Decreased summer water table depth affects peatland vegetation. Basic Appl. Ecol. 10, 330–339. (doi:10.1016/j.baae.2008.05.005) [Google Scholar]
- 10.Strack M, Waddington JM, Rochefort L, Tuittila E-S. 2006. Response of vegetation and net ecosystem carbon dioxide exchange at different peatland microforms following water table drawdown. J. Geophys. Res. 111, G02006 (doi:10.1029/2005JG000145) [Google Scholar]
- 11.Adkinson AC, Humphreys ER. 2011. The response of carbon dioxide exchange to manipulations of Sphagnum water content in an ombrotrophic bog. Ecohydrol. 4, 733–743. (doi:10.1002/eco.171) [Google Scholar]
- 12.Kuiper JJ, Mooij WM, Bragazza L, Robroek BJM. 2014. Plant functional types define magnitude of drought response in peatland CO2 exchange. Ecology 95, 123–131. (doi:10.1890/13-0270.1) [DOI] [PubMed] [Google Scholar]
- 13.Malmer N, Svensson BM, Wallén B. 1994. Interactions between Sphagnum mosses and field layer vascular plants in the development of peat-forming systems. Folia Geobot. Phytotax. 29, 483–496. (doi:10.1007/BF02883146) [Google Scholar]
- 14.Lafleur PM, Moore TR, Roulet NT, Frolking S. 2005. Ecosystem respiration in a cool temperate bog depends on peat temperature but not water table. Ecosystems 8, 619–629. (doi:10.1007/s10021-003-0131-2) [Google Scholar]
- 15.Updegraff K, Bridgham SD, Pastor J, Weishampel P, Harth C. 2001. Response of CO2 and CH4 emissions from peatlands to warming and water table manipulation. Ecol. Appl. 11, 311–326. (doi:10.1890/1051-0761(2001)011[0311:ROCACE]2.0.CO;2) [Google Scholar]
- 16.Strack M, Waddington JM. 2007. Response of peatland carbon dioxide and methane fluxes to a water table drawdown experiment. Global Biogeochem. Cycles 21, GB1007 (doi:10.1029/2006GB002715) [Google Scholar]
- 17.Yrjälä K, et al. 2011. CH4 production and oxidation processes in a boreal fen ecosystem after long-term water table drawdown. Glob. Chang. Biol. 17, 1311–1320. (doi:10.1111/j.1365-2486.2010.02290.x) [Google Scholar]
- 18.Galand PE, Fritze H, Conrad R, Yrjälä K. 2005. Pathways for methanogenesis and diversity of methanogenic archaea in three boreal peatland ecosystems. Appl. Environ. Microb. 71, 2195–2198. (doi:10.1128/AEM.71.4.2195-2198.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenner N, Freeman C. 2011. Drought-induced carbon loss in peatlands. Nat. Geosci. 4, 895–900. (doi:10.1038/ngeo1323) [Google Scholar]
- 20.Kalbitz K, Geyer S. 2002. Different effects of peat degradation on dissolved organic carbon and nitrogen. Org. Geochem. 33, 319–326. (doi:10.1016/S0146-6380(01)00163-2) [Google Scholar]
- 21.Dieleman CM, Branfireun BA, McLaughlin JW, Lindo Z. 2014. Climate change drives a shift in peatland ecosystem plant community: implications for ecosystem function and stability. Glob. Chang. Biol. 21, 388–395. (doi:10.1111/gcb.12643) [DOI] [PubMed] [Google Scholar]
- 22.Ward SE, Ostle NJ, Oakley S, Quirk H, Henrys PA, Bardgett RD. 2013. Warming effects on greenhouse gas fluxes in peatlands are modulated by vegetation composition. Ecol. Lett. 16, 1285–1293. (doi:10.1111/ele.12167) [DOI] [PubMed] [Google Scholar]
- 23.Rydin H, Barber KE. 2001. Long-term and fine-scale coexistence of closely related species. Folia Geobot. 36, 53–61. (doi:10.1007/BF02803138) [Google Scholar]
- 24.Backéus I. 1972. Bog vegetation re-mapped after sixty years: studies on Skagershultamossen, central Sweden. Oikos 23, 384–393. (doi:10.2307/3543178) [Google Scholar]
- 25.Słowiński M, et al. 2016. Drought as a stress driver of ecological changes in peatland—a palaeoecological study of peatland development between 3500BCE and 200BCE in central Poland. Palaeogeogr. Palaeoclimatol. Palaeoecol 461, 272–291. (doi:10.1016/j.palaeo.2016.08.038) [Google Scholar]
- 26.Schwarzer C, Heinken T, Luthardt V, Joshi J. 2013. Latitudinal shifts in species interactions interfere with resistance of southern but not of northern bog-plant communities to experimental climate change. J. Ecol. 101, 1484–1497. (doi:10.1111/1365-2745.12158) [Google Scholar]
- 27.Field CD, et al. 2014. The role of nitrogen deposition in widespread plant community change across semi-natural habitats. Ecosystems 17, 864–877. (doi:10.1007/s10021-014-9765-5) [Google Scholar]
- 28.Stevens CJ, Dise NB, Mountford JO, Gowing DJ. 2004. Impact of nitrogen deposition on the species richness of grasslands. Science 303, 1876–1879. (doi:10.1126/science.1094678) [DOI] [PubMed] [Google Scholar]
- 29.Hector A. et alet al. 1999. Plant diversity and productivity experiments in European grasslands. Science 286, 1123–1127. (doi:10.1126/science.286.5442.1123) [DOI] [PubMed] [Google Scholar]
- 30.Eisenhauer N, et al. 2016. Biodiversity–ecosystem function experiments reveal the mechanisms underlying the consequences of biodiversity change in real world ecosystems. J. Veg. Sci. 27, 1061–1070. (doi:10.1111/jvs.12435) [Google Scholar]
- 31.Lavorel S, Garnier E. 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 16, 545–556. (doi:10.1046/j.1365-2435.2002.00664.x) [Google Scholar]
- 32.Ward SE, Bardgett RD, McNamara NP, Ostle NJ. 2009. Plant functional group identity influences short-term peatland ecosystem carbon flux: evidence from a plant removal experiment. Funct. Ecol. 23, 454–462. (doi:10.1111/j.1365-2435.2008.01521.x) [Google Scholar]
- 33.Ward SE, Orwin KH, Ostle NJ, Briones MJI, Thomson BC, Griffiths RI, Oakley S, Quirk H, Bardgett RD. 2015. Vegetation exerts a greater control on litter decomposition than climate warming in peatlands. Ecology 96, 113–123. (doi:10.1890/14-0292.1) [DOI] [PubMed] [Google Scholar]
- 34.Robroek BJM, et al. 2015. Peatland vascular plant functional types affect methane dynamics by altering microbial community structure. J. Ecol. 103, 925–934. (doi:10.1111/1365-2745.12413) [Google Scholar]
- 35.Potvin LR, Kane ES, Chimner RA, Kolka RK, Lilleskov EA. 2015. Effects of water table position and plant functional group on plant community, aboveground production, and peat properties in a peatland mesocosm experiment (PEATcosm). Plant Soil 387, 277–294. (doi:10.1007/s11104-014-2301-8) [Google Scholar]
- 36.Garrels RM, Christ CL. 1965. Solutions, minerals and equilibria. Boston, MA: Jones and Bertlett Publishers. [Google Scholar]
- 37.Kutzbach L, Schneider J, Sachs T, Giebels M, Nykänen H, Shurpali NJ, Martikainen PJ, Alm J, Wilmking M. 2007. CO2 flux determination by closed-chamber methods can be seriously biased by inappropriate application of linear regression. Biogeosciences 4, 1005–1025. (doi:10.5194/bg-4-1005-2007) [Google Scholar]
- 38.Allan E, et al. 2015. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 18, 834–843. (doi:10.1111/ele.12469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maestre FT, et al. 2012. Plant species richness and ecosystem multifunctionality in global drylands. Science 335, 214–218. (doi:10.1126/science.1215442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulissen MPCP, Van Der Ven PJM, Dees AJ, Bobbink R. 2004. Differential effects of nitrate and ammonium on three fen bryophyte species in relation to pollutant nitrogen input. New Phytol. 164, 451–458. (doi:10.1111/j.1469-8137.2004.01196.x) [Google Scholar]
- 41.Kooijman AM. 2012. ‘Poor rich fen mosses’: atmospheric N-deposition and P-eutrophication in base-rich fens. Lindbergia 35, 42–52. [Google Scholar]
- 42.Kooijman AM. 1992. The decrease of rich fen bryophytes in The Netherlands. Biol. Conserv. 59, 139–143. (doi:10.1016/0006-3207(92)90573-6) [Google Scholar]
- 43.Faber AH, Kooijman AM, Brinkkemper O, van der Plicht J, van Geel B. 2016. Palaeoecological reconstructions of vegetation successions in two contrasting former turbaries in the Netherlands and implications for conservation. Rev. Palaeobot. Palynol. 233, 77–92. (doi:10.1016/j.revpalbo.2016.07.007) [Google Scholar]
- 44.Sarneel JM, Soons MB, Geurts JJM, Beltman B, Verhoeven JTA. 2011. Multiple effects of land-use changes impede the colonization of open water in fen ponds. J. Veg. Sci. 22, 551–563. (doi:10.1111/j.1654-1103.2011.01281.x) [Google Scholar]
- 45.Mouillot D, Villéger S, Scherer-Lorenzen M, Mason NWH. 2011. Functional structure of biological communities predicts ecosystem multifunctionality. PLoS ONE 6, e17476 (doi:10.1371/journal.pone.0017476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagg C, Bender SF, Widmer F, van der Heijden MGA. 2014. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl Acad. Sci. USA 111, 5266–5270. (doi:10.1073/pnas.1320054111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González Macé O, Steinauer K, Jousset A, Eisenhauer N, Scheu S. 2016. Flood-induced changes in soil microbial functions as modified by plant diversity. PLoS ONE 11, e0166349 (doi:10.1371/journal.pone.0166349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradford MA, et al. 2014. Discontinuity in the responses of ecosystem processes and multifunctionality to altered soil community composition. Proc. Natl Acad. Sci. USA 111, 14 478–14 483. (doi:10.1073/pnas.1413707111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ström L, Tagesson T, Mastepanov M, Christensen TR. 2012. Presence of Eriophorum scheuchzeri enhances substrate availability and methane emission in an Arctic wetland. Soil Biol. Biochem. 45, 61–70. (doi:10.1016/j.soilbio.2011.09.005) [Google Scholar]
- 50.Gamfeldt L, Hillebrand H, Jonsson PR. 2008. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology 89, 1223–1231. (doi:10.1890/06-2091.1) [DOI] [PubMed] [Google Scholar]
- 51.Isbell F, et al. 2011. High plant diversity is needed to maintain ecosystem services. Nature 477, 199–202. (doi:10.1038/nature10282) [DOI] [PubMed] [Google Scholar]
- 52.Gamfeldt L, Roger F. 2017. Revisiting the biodiversity–ecosystem multifunctionality relationship. Nat. Ecol. Evol. 1, 0168 (doi:10.1038/s41559-017-0168) [DOI] [PubMed] [Google Scholar]
- 53.Martí M, Juottonen H, Robroek BJM, Yrjala K, Danielsson Å, Lindgren P-E, Svensson BH. 2015. Nitrogen and methanogen community composition within and among three Sphagnum dominated peatlands in Scandinavia. Soil Biol. Biochem. 81, 204–211. (doi:10.1016/j.soilbio.2014.11.016) [Google Scholar]
- 54.Opelt K, Berg C, Schönmann S, Eberl L, Berg G. 2007. High specificity but contrasting biodiversity of Sphagnum-associated bacterial and plant communities in bog ecosystems independent of the geographical region. ISME J. 1, 502–516. (doi:10.1038/ismej.2007.58) [DOI] [PubMed] [Google Scholar]
- 55.Lefcheck JS, et al. 2015. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 6, 6936 (doi:10.1038/ncomms7936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker TN, Ward SE, Ostle NJ, Bardgett RD. 2015. Contrasting growth responses of dominant peatland plants to warming and vegetation composition. Oecologia 178, 141–151. (doi:10.1007/s00442-015-3254-1) [DOI] [PubMed] [Google Scholar]
- 57.Bragazza L, Buttler A, Robroek BJM, Albrecht R, Zaccone C, Jassey VEJ, Signarbieux C. 2016. Persistent high temperature and low precipitation reduce peat carbon accumulation. Glob. Change Biol. 22, 4114–4123. (doi:10.1111/gcb.13319) [DOI] [PubMed] [Google Scholar]
- 58.Turetsky MR, Bond-Lamberty B, Euskirchen E, Talbot J, Frolking S, McGuire AD, Tuittila E-S. 2012. The resilience and functional role of moss in boreal and arctic ecosystems. New Phytol. 196, 49–67. (doi:10.1111/j.1469-8137.2012.04254.x) [DOI] [PubMed] [Google Scholar]
- 59.Keenan TF, Prentice IC, Canadell JG, Williams CA, Wang H, Raupach M, Collatz GJ. 2016. Recent pause in the growth rate of atmospheric CO2 due to enhanced terrestrial carbon uptake. Nat. Commun. 7, 13428 (doi:10.1038/ncomms13428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robroek BJM, Jassey VEJ, Beltman B, Hefting MM. 2017. Data from: Diverse fen plant communities enhance carbon-related multifunctionality, but do not mitigate negative effects of drought Dryad Digital Repository. (doi:10.5061/dryad.g1pk3) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Robroek BJM, Jassey VEJ, Beltman B, Hefting MM. 2017. Data from: Diverse fen plant communities enhance carbon-related multifunctionality, but do not mitigate negative effects of drought Dryad Digital Repository. (doi:10.5061/dryad.g1pk3) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Datasets are deposited at the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.g1pk3) [60].