Abstract
A simple and efficient method for the synthesis of 1,1-diarylalkanes via the Friedel–Crafts-type alkylation reaction of electron-rich arenes with cinnamic acid ester derivatives or chalcones is reported. Iron triflate has been found to be the best catalyst for the Friedel–Crafts-type alkylation reaction with α,β-unsaturated carbonyl compounds. This reaction afforded β,β-diaryl carbonyl compounds in good yields (65–93%) and with excellent regioselectivities. Remarkably, this method is also compatible with a variety of indoles to provide 3-indolyl-aryl carbonyl compounds in excellent yields. Great efforts have been made to deduce a plausible reaction mechanism based on isotopic labelling experiments.
Keywords: Friedel–Crafts alkylation; 1,1-diarylalkane; iron triflate; β,β-diaryl carbonyl compounds
1. Introduction
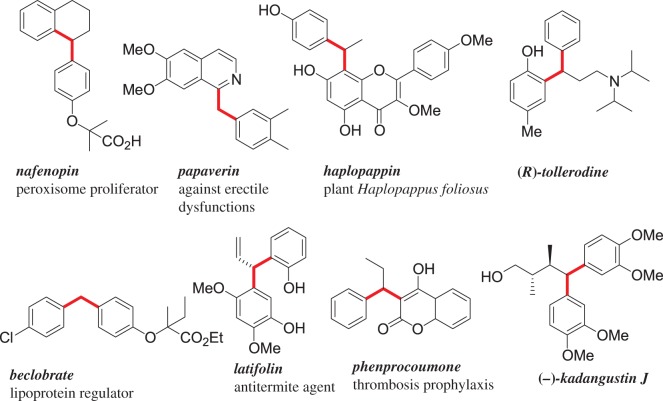
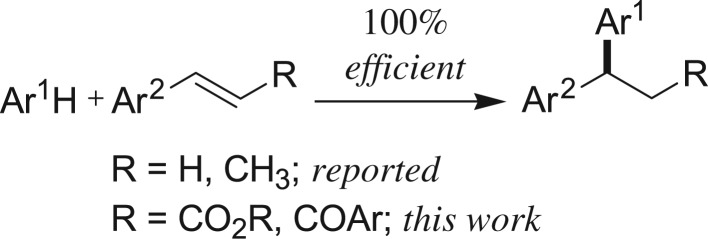
Friedel–Crafts alkylated compounds are of huge importance for the chemical industry as pharmaceuticals, agrochemicals and fine chemicals [1–13]. Especially, the synthesis of 1,1-diarylalkane is highly significant as the core structure of 1,1-diarylalkane is found in many important molecules having biological activities (figure 1). For the preparation of 1,1-diarylalkanes, commonly used alkylating agents are π-activated alcohols, acetates, phosphates and halides in the presence of catalytic amount of Lewis acids or Brönsted acids [14–23]. A significant drawback of large-scale chemical processes is that a vast amount of waste by-products is produced, and it is still a challenging task in standard protocols to free the by-products from arylated products. Three-member cyclic and reactive intermediates, such as epoxides [24–32], aziridines [33–47] and halonium ions [48–53], or other unstable intermediates [54–58] derived in situ from alkenes have also been found as potential electrophiles to afford functionalized Friedel–Crafts alkylated products. Another important strategy to afford 1,1-diarylalkanes is Friedel–Crafts-type hydroarylation reaction with olefins. The greatest advantage of these hydroarylation methods is 100% atom-efficient product formations. The Friedel–Crafts-type hydroarylation reaction with olefins, particularly with styrenes has been realized with AlCl3 [59,60], BiCl3 [61], Bi(OTf)3 [62], Ca(NTf2)2/Bu4NPF6 [63], FeCl3 [64], Au(I) or Au(III)-complexes [65,66], TMSCl/ZnBr2 [67], Ru(III) salt [68], Pt complexes [69,70], graphene oxide [71], resins [72], zeolites [73], I2 [74] and Bronsted acids [75,76]. Hydroarylation reaction with alkynes is also well investigated [77–82]. The Friedel–Crafts-type alkylation reaction with a variety of Michael acceptors is also extensively investigated, although these reactions are mostly limited to heteroarenes such as indoles, pyrroles and thiophenes [83–92]. By contrast, the intermolecular Friedel–Crafts-type reactions of benzenoid arenes with electron-deficient alkenes such as α,β-unsaturated cinnamic acid ester derivatives and chalcones as Michael acceptors are not recognized as substrates. Therefore, keeping in mind the importance of diarylalkanes (figure 1), it is obvious that there is an urgent need for the development of a sustainable, atom-economical and operationally simple approach.
Figure 1.
Representative examples of 1,1-diarylalkanes with biological activities.
2. Model
Recently, it has been well accepted that iron metal salts (Fe is one of the most abundant metals in the earth's crust, approx. 4.7 wt %) could fulfil a few points of the principles of green chemistry, as iron salt is cheap, easily available, non-toxic and environmentally benign. As a result, a series of novel organic transformations including oxidations, reductions, cross-coupling reactions and hydroarylation reactions have also been developed employing iron salts as Lewis acid [93–96]. As a consequence, we envisaged that the iron salt as Lewis acid could be used in Friedel–Crafts-type alkylation to access 1,1-diarylalkanes (scheme 1).
Scheme 1.
1,1-Diarylalkane synthesis.
We, herewith, report an environmentally benign iron triflate-catalysed Friedel–Crafts-type alkylation reaction of electron-rich benzenoid arenes with electron-deficient alkenes such as α,β-unsaturated cinnamic acid ester and chalcone derivatives (scheme 1). Moreover, this reaction provides β,β-diaryl carbonyl compounds; these are interestingly found in many biologically active compounds and pharmaceuticals (figure 1).
3. Result and discussion
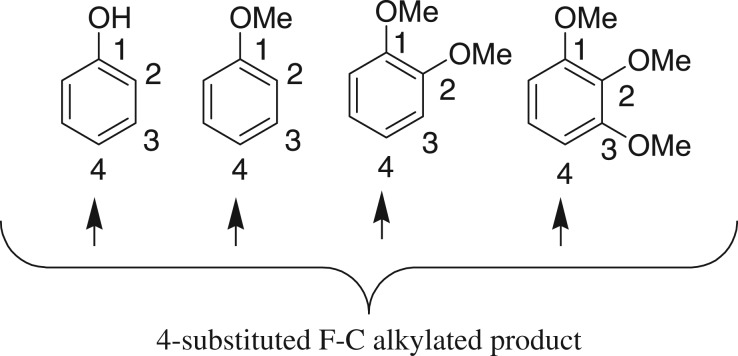
For optimization, 3,4-dimethoxy methylcinnamate and 1,2-dimethoxybenzene were selected as model substrates. Initially, the model reaction was conducted in the presence of various commonly available iron metal salts. The FeCl3 (anhydrous) in 10 mol%-catalysed hydroarylation reaction afforded the desired compound 3a in 43% yield along with some uncharacterized by-products when the reaction was carried out in 1,2-dichloroethane solvent at 85°C (table 1, entry 1). As with FeCl3 (anhydrous), a similar yield (41%) was obtained when FeCl3·6H2O was used for the same set of reaction (entry 2). FeBr3 (10 mol%) was also tested for this reaction but no improvement in yield (28%) was observed (entry 3). Other iron salts such as Fe2O3, Fe2(SO4)3, Fe(NO3)2 6H2O and Fe(OTf)2 were completely inactive for this catalytic method (entries 4 and 5). Iron (III) triflate in 10 mol% has been found to be the best for this catalytic process and yielded 78% of 3a (entry 6). Cu(OTf)2 as Lewis acid also furnished the desired product 3a, albeit in a moderate yield, 51% (entry 7). Other metals such as Zn and Co metals as Lewis acid are completely inactive for this reaction (entries 8 and 9). Bi(OTf)3 also furnished the desired compound 3a in 61% yield (entry 10). Other solvents like nitromethane, tert-butanol, dimethoxy ethane and toluene were also examined, but no tested solvent was found suitable for this reaction (entries 11–13). The yield of the Friedel–Crafts alkylated product 3a was reduced to 47%, while the catalyst loading reduced to 5 mol% (entry 17). Thus, when a suspended solution of 3, 4-dimethoxy methylcinnamate (1a) and 1,2-dimethoxybenzene (2a) was added to iron (III) triflate (10 mol%) in 1,2-dichloroethane at 85°C, the hydroarylated product 3a was obtained in 78% yield with excellent regioselectivity. 1H-NMR analysis of the crude reaction mixture revealed that no trace amount of other arene or alkene regioisomer was formed. Identifying Fe(OTf)3 as a suitable Lewis acid to this catalytic reaction, we began to explore the substrates’ scope (table 2). A variety of electron-rich cinnamate esters and chalcone substrates successfully afforded the desired Friedel–Crafts alkylated products in good to excellent yields. A wide range of electron-rich benzenoid arene compounds yielded Friedel–Crafts alkylated compounds, when the reactions were performed with 3,4-dimethoxy cinnamate ester (table 2; entries 1–4). 4-Methoxy-substituted cinnamate ester also gave the Friedel–Crafts alkylated products in good yields on treatment with electron-donating substituents containing arene compounds (table 2; entries 5–7). More importantly, an arene nucleophile such as phenol was also well tolerated in this catalytic method to afford 3f in 66% yield (entry 6). 4-Methyl-substituted cinnamate ester also afforded the desired β,β-diaryl carbonyl compound 3h in 65% yield, when the reactions were performed with electron-rich arene 1,3,5-trimethoxybenzene (entry 8). However, not even a single product was detected from the 1H NMR of the crude reaction mixture when the reaction was conducted between methylcinnamate and 1,3,5-trimethoxybenzene (table 2; entry 14). Next, we have extended the scope of this reaction with other α,β-unsaturated carbonyl compounds. We were pleased to find that the iron triflate-catalysed Friedel–Crafts-type alkylation reaction was also applicable for chalcones, and the results are shown in table 2 (entries 9–13). Electron-donating group-substituted arenes were tested with chalcone derivatives such as 3-(3,4-dimethoxy-phenyl)-1-phenyl-propenone and 3-(4-methoxy-phenyl)-1-phenyl-propenone, and they furnished the desired compounds in good to excellent yields (65–86%) (table 2; entries 8–11). Chalcone substrates with methyl or methoxy substituents at the opposite side of the aryl part also participated in this catalytic reaction and afforded 3l and 3m with good yields of 86% and 71%, respectively (entries 12–13). Iron triflate-catalysed Friedel–Crafts-type alkylation reaction is limited to electron-rich cinnamate and chalcone derivatives, and it exclusively produced the regioisomer β,β-diaryl carbonyl compounds as arenes preferentially attack the benzylic position. No other regioisomers were detected by 1H NMR of the crude reaction mixture. There is also a possibility for the formation of arene regioisomers. For anisole, 1,2-dimethoxybenzene, phenol produced exclusively para-alkylated products. By contrast, 1,2,3-trimethoxybenzene produced exclusively 4-substituted product. It seems the electronic effect predominates over the steric factor, i.e. C4 is activated by the +R effect of C1-OMe and C3-OMe, whereas C5-position is para-only with C2-OMe (figure 2).
Table 1.
Optimization studya

| entry | Lewis acid (x mol%) | solvent | yield (%) of 3ab |
|---|---|---|---|
| 1 | FeCl3 anhydrous (10) | DCE | 43 |
| 2 | FeCl3, 6H2O (10) | DCE | 41 |
| 3 | FeBr3 (10) | DCE | 28 |
| 4 | Fe2O3 or Fe2(SO4)3 or Fe(NO3)2, 6H2O (10) | DCE | 0 |
| 5 | Fe(OTf)2 (10) | DCE | 0 |
| 6 | Fe(OTf)3 (10) | DCE | 78 |
| 7 | Cu(OTf)2 (10) | DCE | 51 |
| 8 | Zn(OTf)2 (10) | DCE | 0 |
| 9 | Co(ClO4)2·6H2O or Co(NO3)2·6H2O (10) | DCE | 0 |
| 10c | Bi(OTf)3 (10) | DCE | 61 |
| 11 | Fe(OTf)3 (10) | CH3NO2 | 0 |
| 12 | Fe(OTf)3 (10) | Toluene | 0 |
| 13 | Fe(OTf)3 (10) | DME | 16 |
| 14d | Fe(OTf)3 (10) | DCE | 21 |
| 15e | Fe(OTf)3 (10) | DCE | 73 |
| 16f | Fe(OTf)3 (10) | DCE | 58 |
| 17 | Fe(OTf)3 (05) | DCE | 47 |
aReaction conditions: alkene 1a (1.0 equiv, 0.2 mmol), arene 2a (1.2 equiv) and iron catalyst (10 mol%) are heated to 85°C in solvent (1.0 ml).
bIsolated yield after column chromatography.
cBi(OTf)3-catalysed same set of reactions in completely anhydrous DCE solvent afforded no product (0% yield).
dReaction was run at 50°C.
e1,2-Dimethoxybenzene was used at 5.0 equiv.
fReaction was run at 100°C. DCE = 1,2-dichloroethane.
Table 2.
Fe(OTf)3-catalysed hydroarylation reaction with α,β-unsaturated carbonyl compoundsa
| ester or chalcone 1 | ||||
|---|---|---|---|---|
| entry | Ar1 | R | ArH 2 | product 3, yield (%)b |
| 1 | 3,4-OMeC6H3 | OMe | 2a | 3a, 78 |
| 2 | 3,4-OMeC6H3 | OMe | 2b | 3b, 73 |
| 3 | 3,4-OMeC6H3 | OMe | 2d | 3c, 68 |
| 4 | 3,4-OMeC6H3 | OMe | 2f | 3d, 83 |
| 5 | 4-OMeC6H4 | OMe | 2b | 3e, 71 |
| 6 | 4-OMeC6H4 | OMe | 2e | 3f, 66 |
| 7 | 4-OMeC6H4 | OMe | 2f | 3g, 77 |
| 8 | 4-MeC6H4 | OMe | 2c | 3h, 65 |
| 9 | 3,4-OMeC6H3 | Ph | 2c | 3i, 86 |
| 10 | 4-OMeC6H4 | Ph | 2c | 3j, 75 |
| 11 | 4-OMeC6H4 | Ph | 2b | 3k, 74 |
| 12 | 4-OMeC6H4 | 4-MeC6H4 | 2c | 3l, 86 |
| 13 | Ph | 4-OMeC6H4 | 2c | 3m, 71 |
| 14 | Ph | OMe | 2c | 3n, 0 |
aReaction conditions: alkene (1.0 equiv), arene (1.2 equiv) and catalyst Fe(OTf)3 (10 mol%) are heated at 85°C in DCE.
bIsolated yield after column chromatography.
Figure 2.
Regioisomers of arene.
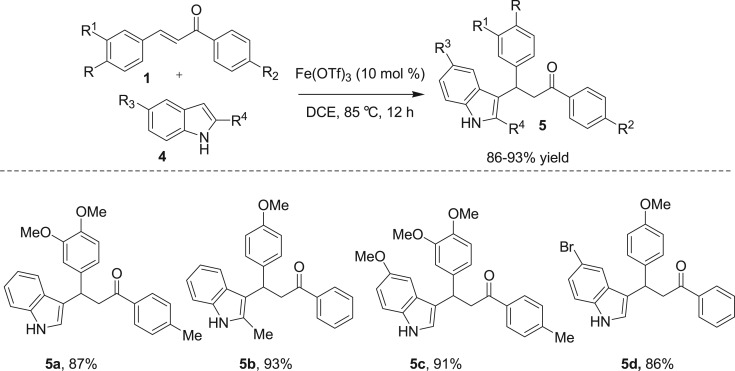
Iron (III) triflate was also tested for Friedel–Crafts alkylation reactions between heteroarenes such as a variety of indoles 4 and chalcone derivatives 1 (scheme 2). Successfully, all the reactions yielded the desired 3-indolyl-aryl-substituted carbonyl compounds 5 in good to excellent yields (86–93%) and with excellent regioselectivities.
Scheme 2.
Reaction condition of Friedel–Crafts alkylation reaction of indoles with chalcones: chalcone (1.0 equiv), indole 4 (1.2 equiv) and catalyst Fe(OTf)3 (10 mol%) are heated to 85°C in DCE for 12 h. Isolated yield after column chromatography.
3.1. Mechanism investigation
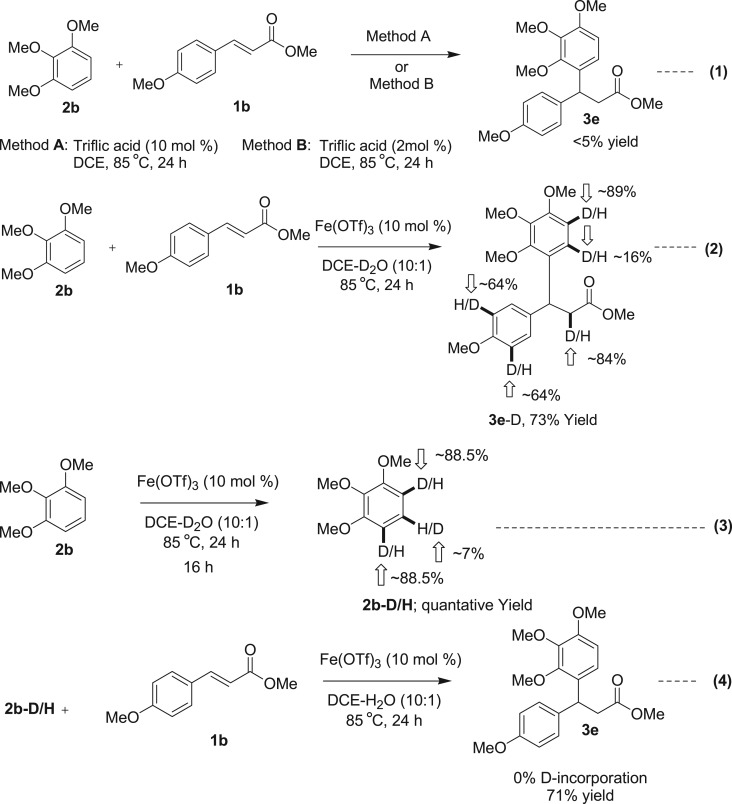
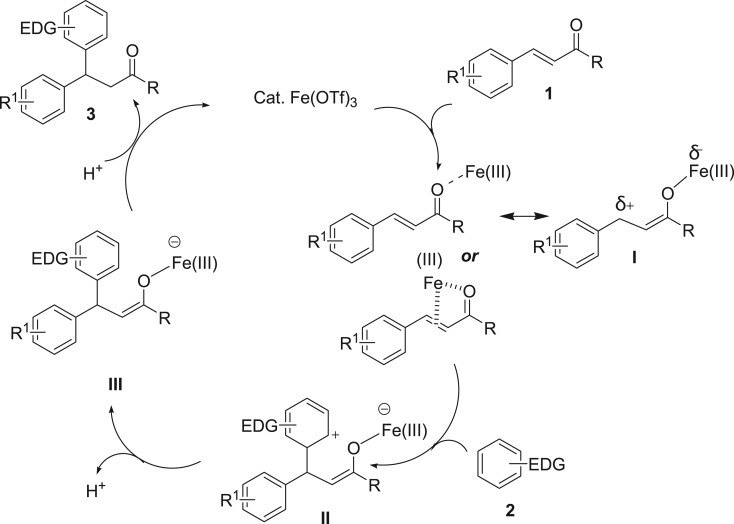
To gain insight into the reaction mechanism, a series of control reactions including isotopic labelling experiments were performed. First, 4-methoxy methylcinnamate ester was reacted with 1,2,3-trimethoxybenzene in super dry 1,2-dichloroethane (in the presence of molecular sieves 4 Å); the desired compound 3e was formed in 11% yield under the optimized condition after quenching with H2O. Removal of molecular sieves and introduction of wet dichloroethane solvents (without drying the solvent) provided the desired Friedel–Crafts alkylated product 3e in 72% yield. Therefore, it can be concluded that the concentration of water in the reaction medium is the detrimental factor to obtaining good yields of the product. Next, control experiments using triflic acid, at both catalyst loading (10 mol%) and significantly lower loading (2 mol%) separately, were conducted to examine whether the complex acid forms or not during progress of the reaction (scheme 3; equation (1)). A trace amount of the desired compound 3e was observed in both the experiments, and the rest of the starting materials were recovered in each case. This experiment clearly suggests the significance of iron (III) triflate as Lewis acid in this catalytic method. Furthermore, to understand a clear mechanism, deuterium labelling experiments were performed in the presence of D2O and using deuterated arene separately under the optimized reaction condition. The reaction of 1,2,3-trimethoxybenzene 2b with 4-methoxy methylcinnamate 1b, in the presence of D2O as co-solvent to ClCH2CH2Cl, provided 3e-D in 73% yield with exclusive (approx. 84%) d-incorporation at α-carbon of the carbonyl centre. In addition, a significant amount of d-incorporations were observed at the arene part of the desired product 3e (scheme 3; equation (2)). This observation may be attributed to d-incorporation taking place at the starting materials first, prior to attack of the alkene or after product formation. Further to verify, arene 2b was reacted in the presence of D2O under the optimized reaction condition and, delightfully, exclusive d-incorporation (88.5%) at the C4-H and C6-H of arene 2b-D/H and 7% d-incorporation at the C5-H (scheme 3; equation (3)) were observed. Next, another experiment was carried out with arene 2b-D/H and alkene 1b in the presence of ClCH2CH2Cl–H2O (10 : 1). Hydroarylated product 3e was isolated in 71% yield. Interestingly, no deuterium scrambled either at the α-carbon of the carbonyl centre or at the arene part in 3e (scheme 3, equation (4)). The isotopic labelling experiment clearly indicates that water plays a significant role in this catalytic reaction and a rapid D/H exchange reaction predominantly occurs in the presence of iron (III) triflate as Lewis acid [97]. Although the mechanism of the present reaction has not been fully established, on the basis of control reactions and isotopic labelling experiments, a Friedel–Crafts-type alkylation reaction is proposed (scheme 4) [98,99]. The reaction would be initiated by activation of the carbonyl group of the starting alkene by iron catalyst and, hence, a partial positive charge at the benzylic centre is developed as intermediate I. Alternatively, the iron catalyst may also coordinate with both C=C double bond and the carbonyl group of ester or chalcone derivatives and enhance the reactivity of the benzylic carbon centre. Next, activated arene π-nucleophile attacks the benzylic centre to generate the intermediate II. Finally, aromatization of the intermediate II and subsequent protonation would provide the desired Friedel–Crafts alkylated product 3.
Scheme 3.
Control experiments to give insight on the reaction mechanism.
Scheme 4.
A plausible reaction mechanism.
4. Conclusion
In conclusion, the present methodology demonstrates the iron triflate-catalysed Friedel–Crafts-type alkylation reaction between α,β-unsaturated carbonyl compounds and electron-rich arene components. This reaction provides exclusively β,β-diaryl carbonyl compounds in good to excellent yields. Usually, electro-rich activated arenes gave the desired Friedel–Crafts alkylated products in good to excellent yields in this catalytic method. Heteroarenes such as a variety of indoles also gave the desired 3-indolyl-aryl carbonyl compounds in excellent yields and with excellent regioselectivities. A series of control reactions including isotopic labelling experiments helps to deduce a plausible reaction mechanism. This successful catalytic method encouraged us to accomplish the formal synthesis of a 1,1-diaryl motif containing molecules such as nafenopin, latifolin and tollerodine, which is ongoing in our laboratory.
Supplementary Material
Acknowledgement
The authors are grateful to the anonymous referees for their insightful comments and suggestions.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
B.M. and A.B. contributed to the method design and wrote the manuscript. A.B. and P.M.S. performed the optimization study, substrate scope experiments and data interpretation of all the compounds. All the authors gave their final approval for the publication.
Competing interests
The authors declare no competing interests.
Funding
Financial support from IGNTU and DST-INSPIRE (IF-CH12-79), India is gratefully acknowledged. A.B. thanks DST-INSPIRE for providing the Fellowship.
References
- 1.Rueping M, Nachtsheim BJ. 2010. A review of new developments in the Friedel–Crafts alkylation—from green chemistry to asymmetric catalysis. Beilstein J. Org. Chem. 6, 1–31. (doi:10.3762/bjoc.6.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar R, Van der Eycken EV. 2013. Recent approaches for C–C bond formation via direct dehydrative coupling strategies. Chem. Soc. Rev. 42, 1121–1146. (doi:10.1039/C2CS35397K) [DOI] [PubMed] [Google Scholar]
- 3.Bandini M, Melloni A, Umani-Ronchi A. 2004. New catalytic approaches in the stereoselective Friedel–Crafts alkylation reaction . Angew. Chem. Int. Ed. 43, 550–556. (doi:10.1002/anie.200301679) [DOI] [PubMed] [Google Scholar]
- 4.Naredla RR, Klumpp DA. 2013. Contemporary carbocation chemistry: applications in organic synthesis. Chem. Rev. 113, 6905–6948. (doi:10.1021/cr4001385) [DOI] [PubMed] [Google Scholar]
- 5.You S-L, Cai Q, Zeng M. 2009. Chiral Brønsted acid catalyzed Friedel–Crafts alkylation reactions. Chem. Soc. Rev. 38, 2190–2201. (doi:10.1039/b817310a) [DOI] [PubMed] [Google Scholar]
- 6.Mondal S, Panda G. 2014. Synthetic methodologies of achiral diarylmethanols, diaryl and triarylmethanes (TRAMs) and medicinal properties of diaryl and triarylmethanes-an overview. RSC Adv. 4, 28 317–28 358. (doi:10.1039/C4RA01341G) [Google Scholar]
- 7.Long YQ, Jiang XH, Dayam R, Sanchez R, Shoemaker R, Sei S, Neamati NJ. 2004. Rational design and synthesis of novel dimeric diketoacid-containing inhibitors of HIV-1 integrase: implication for binding to two metal ions on the active site of integrase. J. Med. Chem. 46, 2561–2573. (doi:10.1021/jm030559k) [DOI] [PubMed] [Google Scholar]
- 8.Panda G, et al. 2007. Effect of substituents on diarylmethanes for antitubercular activity. Eur. J. Med. Chem. 42, 410–419. (doi:10.1016/j.ejmech.2006.09.020) [DOI] [PubMed] [Google Scholar]
- 9.Pathak TP, Gligorich KM, Welm BE, Sigman MS. 2010. Synthesis and preliminary biological studies of 3-substituted indoles accessed by a palladium-catalyzed enantioselective alkene difunctionalization reaction. J. Am. Chem. Soc. 132, 7870–7871. (doi:10.1021/ja103472a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair V, Thomas S, Mathew K, Abhilash KG. 2006. Recent advances in the chemistry of triaryl- and triheteroarylmethanes. Tetrahedron 62, 6731–6747. (doi:10.1016/j.tet.2006.04.081) [Google Scholar]
- 11.Shchepinov MS, Korshun VS. 2003. Recent applications of bifunctional trityl groups. Chem. Soc. Rev. 32, 170–180. (doi:10.1039/b008900l) [DOI] [PubMed] [Google Scholar]
- 12.Ma JC, Dougherty DA. 1997. The cation−π interaction. Chem. Rev. 97, 1303–1324. (doi:10.1021/cr9603744) [DOI] [PubMed] [Google Scholar]
- 13.Duxbury DF. 1993. The photochemistry and photophysics of triphenylmethane dyes in solid and liquid media. Chem. Rev. 93, 381–433. (doi:10.1021/cr00017a018) [Google Scholar]
- 14.Bandini M, Tragni M. 2009. Π-Activated alcohols: an emerging class of alkylating agents for catalytic Friedel–Crafts reactions. Org. Biomol. Chem. 7, 1501–1507. (doi:10.1039/b823217b) [DOI] [PubMed] [Google Scholar]
- 15.Tsuchimoto T, Tobita K, Hiyama T, Fukuzawa S. 1997. Scandium(III) triflate-catalyzed Friedel–Crafts alkylation reactions. J. Org. Chem. 62, 6997–7005. (doi:10.1021/jo970599u) [Google Scholar]
- 16.Mo X, Yakiwchuk J, Dansereau J, McCubbin JA, Hall DJ. 2015. Unsymmetrical diarylmethanes by ferroceniumboronic acid catalyzed direct Friedel–Crafts reactions with deactivated benzylic alcohols: enhanced reactivity due to ion-pairing effects. J. Am. Chem. Soc. 137, 9694–9703. (doi:10.1021/jacs.5b05076) [DOI] [PubMed] [Google Scholar]
- 17.Pallikonda J, Chakravarty M. 2016. Benzylic phosphates in Friedel–Crafts reactions with activated and unactivated arenes: access to polyarylated alkanes. J. Org. Chem. 81, 2135–2142. (doi:10.1021/acs.joc.5b02441) [DOI] [PubMed] [Google Scholar]
- 18.Smith AG, Johnson JS. 2010. Lewis acid-promoted Friedel–Crafts alkylation reactions with α-ketophosphate electrophiles. Org. Lett. 12, 1784–1787. (doi:10.1021/ol100410k) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iovel I, Mertins K, Kischel J, Zapf A, Beller M. 2005. An efficient and general iron-catalyzed arylation of benzyl alcohols and benzyl carboxylates. Angew. Chem. Int. Ed. 44, 3913–3917. (doi:10.1002/anie.200462522) [DOI] [PubMed] [Google Scholar]
- 20.Rueping M, Nachtsheim BJ, Ieawsuwan W. 2006. An effective bismuth-catalyzed benzylation of arenes and heteroarenes. Adv. Synth. Catal. 348, 1033–1037. (doi:10.1002/adsc.200606068) [Google Scholar]
- 21.Mertins K, Iovel I, Kischel J, Zapf A, Beller M. 2005. Transition-metal-catalyzed benzylation of arenes and heteroarenes. Angew. Chem. Int. Ed. 44, 238–242. (doi:10.1002/anie.200460666) [DOI] [PubMed] [Google Scholar]
- 22.Niggemann M, Meel MJ. 2010. Calcium-catalyzed Friedel–Crafts alkylation at room temperature. Angew. Chem. Int. Ed. 49, 3684–3687. (doi:10.1002/anie.200907227) [DOI] [PubMed] [Google Scholar]
- 23.Choudhury J, Podder S, Roy S. 2005. Cooperative Friedel–Crafts catalysis in heterobimetallic regime: alkylation of aromatics by π-activated alcohols. J. Am. Chem. Soc. 127, 6162–6163. (doi:10.1021/ja0506004) [DOI] [PubMed] [Google Scholar]
- 24.Luo M, Yuan R, Liu X, Yu L, Wei W. 2016. Iron(III)-catalyzed arylation of spiro-epoxyoxindoles with phenols/naphthols towards the synthesis of spirocyclic oxindoles. Chem. Eur. J. 22, 9797–9803. (doi:10.1002/chem.201601185) [DOI] [PubMed] [Google Scholar]
- 25.Taylor SK, Clark DL, Heinz KL, Schramm SB, Westermann CD, Barnell KK. 1983. Friedel-Crafts reactions of some conjugated epoxides. J. Org. Chem. 48, 592–596. (doi:10.1021/jo00152a036) [Google Scholar]
- 26.Taylor SK, May SA, Stansby ES. 1996. Substituent effects on intramolecular epoxide cyclizations that can competitively occur at aromatic or double bond positions. J. Org. Chem. 61, 2075–2080. (doi:10.1021/jo951945f) [Google Scholar]
- 27.Reddy R, Jaquith JB, Neelagiri VR, Saleh-Hanna S, Durst R. 2002. Asymmetric synthesis of the highly methylated tryptophan portion of the hemiasterlin tripeptides. Org. Lett. 4, 695–697. (doi:10.1021/ol016982+) [DOI] [PubMed] [Google Scholar]
- 28.Shi Z, He C. 2004. An Au-catalyzed cyclialkylation of electron-rich arenes with epoxides to prepare 3-chromanols. J. Am. Chem. Soc. 126, 5964–5965. (doi:10.1021/ja031953a) [DOI] [PubMed] [Google Scholar]
- 29.Nagumo S, Miyoshi I, Akita H, Kawahara N. 2002. 7-Endo selective Friedel–Crafts type cyclization of vinyloxiranes linked to an ester group. Tetrahedron Lett. 43, 2223–2226. (doi:10.1016/S0040-4039(02)00211-3) [Google Scholar]
- 30.Marcos R, Rodríguez-Escrich C, Herrerías CI, Pericàs MA. 2008. Metal-mediated cyclization of aryl and benzyl glycidyl ethers: a complete scenario. J. Am. Chem. Soc. 130, 16 838–16 839. (doi:10.1021/ja8062887) [DOI] [PubMed] [Google Scholar]
- 31.Li G-X, Qu J. 2010. Friedel–Crafts alkylation of arenes with epoxides promoted by fluorinated alcohols or water. Chem. Commun. 46, 2653–2655. (doi:10.1039/b926684d) [DOI] [PubMed] [Google Scholar]
- 32.Hajra S, Maity S, Roy S. 2016. Regioselective Friedel–Crafts reaction of electron-rich benzenoid arenes and spiroepoxyoxindole at the spiro-centre: efficient synthesis of benzofuroindolines and 2H- spiro[benzofuran]-3,3′-oxindoles. Adv. Synth. Catal. 358, 2300–2306. (doi:10.1002/adsc.201600312) [Google Scholar]
- 33.Onistschenko A, Buchholz B, Mall T. 1989. Reactions with aziridines. 48. Friedel-Crafts reactions with N-sulfonated aziridines and with open-chain sulfonamides: sulfonamides as leaving groups in open-chain structures. J. Org. Chem. 54, 193–199. (doi:10.1021/jo00262a042) [Google Scholar]
- 34.Bennani YL, Zhu G-D, Freeman JC. 1998. Scandium-mediated opening of aziridine carboxylates: a facile synthesis of aryl substituted tryptophans. Synlett 7, 754–756. (doi:10.1055/s-1998-1773) [Google Scholar]
- 35.Yadav JS, Reddy BVS, Parimala G. 2002. Indium tribromide catalyzed highly regioselective ring opening of epoxides and aziridines with pyrrole. Synlett 7, 1143–1145. (doi:10.1055/s-2002-32575) [Google Scholar]
- 36.Bergmeier SC, Katz SJ, Huang J, McPherson H, Donoghue PJ, Reed DD. 2004. Intramolecular cyclization reactions of aziridines with π-nucleophiles. Tetrahedron Lett. 45, 5011–5014. (doi:10.1016/j.tetlet.2004.05.009) [Google Scholar]
- 37.Hudlicky T, Uwe R, Finn KJ, Ghiviriga I. 2005. Reactions of indole derivatives with oxiranes and aziridines on silica: synthesis of β-carbolin-1-one mimic of pancratistatin. J. Org. Chem. 70, 3490–3499. (doi:10.1021/jo040292c) [DOI] [PubMed] [Google Scholar]
- 38.Bera M, Roy S. 2007. A facile C-arylation of N-tosylaziridines via Ag(I) catalysis. Tetrahedron Lett. 48, 7144–7146. (doi:10.1016/j.tetlet.2007.07.200) [Google Scholar]
- 39.Sun X, Sun W, Fan R, Wu J. 2007. Gold(III) chloride/silver triflate: a highly efficient catalyst for ring-opening reaction of aziridines with electron-rich arenes. Adv. Synth. Catal. 349, 2151–2155. (doi:10.1002/adsc.200700101) [Google Scholar]
- 40.Wang Z, Sun X, Wu X. 2008. FeCl3: an efficient catalyst for reactions of electron-rich arenes with imines or aziridines. Tetrahedron 64, 5013–5018. (doi:10.1016/j.tet.2008.03.081) [Google Scholar]
- 41.Hajra S, Maji B, Sinha D, Bar S. 2008. A one-pot stereoselective synthesis of trans-1-aryl-2-aminotetralins from 2-arylethyl styrenes. Tetrahedron Lett. 49, 4057–4059. (doi:10.1016/j.tetlet.2008.04.056) [Google Scholar]
- 42.Pulipaka AB, Bergmeier SC. 2008. Synthesis of hexahydro-1H-benzo[c]chromen-1-amines via the intramolecular ring-opening reactions of aziridines by π-nucleophiles. Synthesis 9, 1420–1430. [Google Scholar]
- 43.Hajra S, Maji B, Mal D. 2008. A catalytic and enantioselective synthesis of trans-2-amino-1- aryltetralins. Adv. Synth. Catal. 351, 859–864. (doi:10.1002/adsc.200800603) [Google Scholar]
- 44.Bera M, Roy S. 2010. Silver(I)−diene complexes as versatile catalysts for the C-arylation of N-tosylaziridines: mechanistic insight from in situ diagnostics. J. Org. Chem. 75, 4402–4412. (doi:10.1021/jo100260k) [DOI] [PubMed] [Google Scholar]
- 45.Hajra S, Sinha D. 2011. Catalytic enantioselective aziridoarylation of aryl cinnamyl ethers toward synthesis of trans-3-amino-4-arylchromans. J. Org. Chem. 76, 7334–7340. (doi:10.1021/jo200711s) [DOI] [PubMed] [Google Scholar]
- 46.Ghorai M, Tiwari DP, Jain N. 2013. Lewis acid catalyzed SN2-type ring opening of N-activated aziridines with electron-rich arenes/heteroarenes. J. Org. Chem. 78, 7121–7130. (doi:10.1021/jo401028j) [DOI] [PubMed] [Google Scholar]
- 47.Yin J, Hyland CTT. 2016. Palladium(II)-catalyzed C3-selective Friedel–Crafts reaction of indoles with aziridines. Asian J. Org. Chem. 5, 1368–1377. (doi:10.1002/ajoc.201600280) [Google Scholar]
- 48.Barluenga J, González JM, Campos PJ, Asensio J. 1988. Iodine-induced stereoselective carbocyclizations: a new method for the synthesis of cyclohexane and cyclohexene derivatives . Angew. Chem. Int. Ed. 27, 1546–1547. (doi:10.1002/anie.198815461) [Google Scholar]
- 49.Barluenga J, Trincado M, Rubio E, González JM. 2004. Intramolecular arylation reaction of alkenes: a flexible approach to chromans and tetrahydroquinolines. J. Am. Chem. Soc. 126, 3416–3417. (doi:10.1021/ja0397299) [DOI] [PubMed] [Google Scholar]
- 50.Hajra S, Maji B, Bar S. 2007. Samarium triflate-catalyzed halogen-promoted Friedel–Crafts alkylation with alkenes. Org. Lett. 9, 2783–2786. (doi:10.1021/ol070813t) [DOI] [PubMed] [Google Scholar]
- 51.Sakakura A, Ukai A, Ishihara K. 2007. Enantioselective halocyclization of polyprenoids induced by nucleophilic phosphoramidites . Nature 445, 900–903. (doi:10.1038/nature05553) [DOI] [PubMed] [Google Scholar]
- 52.Wei H-L, Piou T, Dufour J, Neuville L, Zhu J. 2011. Iodo-carbocyclization of electron-deficient alkenes: synthesis of oxindoles and spirooxindoles. Org. Lett. 13, 2244–2247. (doi:10.1021/ol2005243) [DOI] [PubMed] [Google Scholar]
- 53.Wolstenhulme JR, et al. 2013. Asymmetric electrophilic fluorocyclization with carbon nucleophiles. Angew. Chem. Int. Ed. 52, 9796–9800. (doi:10.1002/anie.201304845) [DOI] [PubMed] [Google Scholar]
- 54.Deziel R, Malenfant E, Thibault C. 1998. Asymmetric arene-alkene cyclizations mediated by a chiral organoselenium reagent. Tetrahedron Lett. 39, 5493–5496. (doi:10.1016/S0040-4039(98)01141-1) [Google Scholar]
- 55.Edstrom ED, Livinghouse T. 1986. New methods for the generation of episulfonium ions. An application to the synthesis of carbocycles via sulfenium ion promoted arene-alkene cyclizations. J. Am. Chem. Soc. 108, 1334–1336. (doi:10.1021/ja00266a055) [Google Scholar]
- 56.Lim HJ, Rajanbabu TV. 2009. Seleniranium ion-triggered reactions: new aspects of Friedel–Crafts and N-detosylative cyclizations . Org. Lett. 11, 2924–2927. (doi:10.1021/ol900961m) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denmark SE, Jaunet A. 2014. Catalytic, enantioselective, intramolecular carbosulfenylation of olefins. Preparative and stereochemical aspects. J. Org. Chem. 79, 140–171. (doi:10.1021/jo4023765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang E, Zhao Y, Li W, Wang W, Zhang M, Dai X. 2016. Catalytic selenium-promoted intermolecular Friedel–Crafts alkylation with simple alkenes. Org. Lett. 18, 912–915. (doi:10.1021/acs.orglett.5b03579) [DOI] [PubMed] [Google Scholar]
- 59.Perego C, Ingallino P. 2002. Recent advances in the industrial alkylation of aromatics: new catalysts and new processes. Catal. Today 73, 3–22. (doi:10.1016/S0920-5861(01)00511-9) [Google Scholar]
- 60.Perego C, Ingallina P. 2004. Combining alkylation and transalkylation of for alkylaromatic production. Green Chem. 6, 274–279. (doi:10.1039/b403277m) [Google Scholar]
- 61.Sun H-B, Li B, Hua R, Yin Y. 2006. An efficient and selective hydroarylation of styrenes with electron-rich arenes, catalyzed by bismuth(III) chloride and affording Markovnikov adducts. Eur. J. Org. Chem. 2006, 4231–4236. (doi:10.1002/ejoc.200600390) [Google Scholar]
- 62.Rueping M, Nachtsheim BJ, Scheidt T. 2006. Efficient metal-catalyzed hydroarylation of styrenes. Org. Lett. 8, 3717–3719. (doi:10.1021/ol0612962) [DOI] [PubMed] [Google Scholar]
- 63.Niggemann M, Bisek M. 2010. Calcium-catalyzed hydroarylation of alkenes at room temperature. Chem. Eur. J. 16, 11 246–11 249. (doi:10.1002/chem.201001375) [DOI] [PubMed] [Google Scholar]
- 64.Kischel J, Jovel I, Mertins K, Zapf A, Beller M. 2006. A convenient FeCl3-catalyzed hydroarylation of styrenes. Org. Lett. 8, 19–22. (doi:10.1021/ol0523143) [DOI] [PubMed] [Google Scholar]
- 65.Wang M-Z, Wong M-K, Che C-M. 2008. Gold(I)-catalyzed intermolecular hydroarylation of alkenes with indoles under thermal and microwave-assisted conditions. Chem. Eur. J. 14, 8353–8364. (doi:10.1002/chem.200800040) [DOI] [PubMed] [Google Scholar]
- 66.Jean M, Weghe PV. 2011. Gold-catalyzed intramolecular hydroarylation of olefins: scope evaluation and preliminary mechanistic studies. Tetrahedron Lett. 52, 3509–3513. (doi:10.1016/j.tetlet.2011.04.122) [Google Scholar]
- 67.Lee SY, Villani-Gale A, Eichman CC. 2016. Room temperature catalyst system for the hydroarylation of olefins. Org. Lett. 18, 5034–5037. (doi:10.1021/acs.orglett.6b02492) [DOI] [PubMed] [Google Scholar]
- 68.Youn SW, Pastine SJ, Sames D. 2004. Ru(III)-catalyzed cyclization of arene-alkene substrates via intramolecular electrophilic hydroarylation. Org. Lett. 6, 581–584. (doi:10.1021/ol036385i) [DOI] [PubMed] [Google Scholar]
- 69.Han X, Widenhoefer RA. 2006. Platinum-catalyzed intramolecular asymmetric hydroarylation of unactivated alkenes with indoles. Org. Lett. 8, 3801–3804. (doi:10.1021/ol061441b) [DOI] [PubMed] [Google Scholar]
- 70.McKewon BA, Foley NA, Lee JP, Gunnoe TB. 2008. Hydroarylation of unactivated olefins catalyzed by platinum(II) complexes. Organometallics 27, 4031–4033. (doi:10.1021/om8006008) [Google Scholar]
- 71.Hu F, Patel M, Luo F, Flach C, Mendelsohn R, Garfunkel R, He H, Szostak M. 2015. Graphene-catalyzed direct Friedel–Crafts alkylation reactions: mechanism, selectivity, and synthetic utility. J. Am. Chem. Soc. 137, 14 473–14 480. (doi:10.1021/jacs.5b09636) [DOI] [PubMed] [Google Scholar]
- 72.Wen JY, Qi HF, Kong XJ, Chen LG, Yan XL. 2014. Hydroarylation of styrenes with electron-rich arenes over acidic ion-exchange resins. Synth. Commun. 44, 1893–1903. (doi:10.1080/00397911.2013.876547) [Google Scholar]
- 73.Mohan DC, Patil RD, Adimurthy S. 2012. H-β-Zeolite-catalysed hydroarylation of styrenes. Eur. J. Org. Chem. 2012, 3520–3525. (doi:10.1002/ejoc.201200283) [Google Scholar]
- 74.Chu C-M, Huang W-J, Liu J-T, Yao C-F. 2007. Highly efficient iodine catalyzed hydroarylation of arenes with styrenes. Tetrahedron Lett. 48, 6881–6885. (doi:10.1016/j.tetlet.2007.07.178) [Google Scholar]
- 75.Fleischer I, Pospech J. 2015. Brønsted acid-catalyzed hydroarylation of activated olefins. RSC Adv. 5, 493–496. (doi:10.1039/C4RA13647K) [Google Scholar]
- 76.Liu M, Zhang J, Zhou H, Yang H, Xia C, Jiang G. 2016. Efficient hydroarylation and hydroalkenylation of vinylarenes by Brønsted acid catalysis. RSC Adv. 6, 76 780–76 784. (doi:10.1039/C6RA16627J) [Google Scholar]
- 77.Nevado C, Echavarren AM. 2005. Transition metal-catalyzed hydroarylation of alkynes. Synthesis 2, 167–182. [Google Scholar]
- 78.Kitamura T. 2009. Transition-metal-catalyzed hydroarylation reactions of alkynes through direct functionalization of C–H bonds: a convenient tool for organic synthesis. Eur. J. Org. Chem. 2009, 1111–1125. (doi:10.1002/ejoc.200801054) [Google Scholar]
- 79.Wang X, Zhou L, Lu W. 2010. Hydroarylation of alkynes via aryl C-H bond cleavage. Curr. Org. Chem. 14, 289–307. (doi:10.2174/138527210790231964) [Google Scholar]
- 80.Tsuchimoto T, Maeda T, Shirakawa E, Kawakami Y. 2000. Friedel- Crafts alkenylation of arenes using alkyne catalysed by metal trifluoromethanesulfonates. Chem. Commun. 36, 1573–1574. (doi:10.1039/b003702h) [Google Scholar]
- 81.Nakhi A, Archana S, Seerapu GPK, Chennubhotla KS, Kumar KL, Kulkarni K, Haldar D, Pal M. 2013. AlCl3-mediated hydroarylation–heteroarylation in a single pot: a direct access to densely functionalized olefins of pharmacological interest. Chem. Commun. 49, 6268–6270. (doi:10.1039/c3cc42840k) [DOI] [PubMed] [Google Scholar]
- 82.Song CE, Jung D, Choung SY, Roh EJ, Lee S. 2004. Dramatic enhancement of catalytic activity in an ionic liquid: highly practical Friedel–Crafts alkenylation of arenes with alkynes catalyzed by metal triflates. Angew. Chem. Int. Ed. 43, 6183–6185. (doi:10.1002/anie.200460292) [DOI] [PubMed] [Google Scholar]
- 83.Harrington PE, Kerr MA. 1996. Reaction of indoles with electron deficient olefins catalyzed by Yb(Otf)3.3H2O. Synlett 1996, 1047–1048. (doi:10.1055/s-1996-5685) [Google Scholar]
- 84.Hashmi ASK, Schwarz L, Choi J-H, Frost TM. 2000. A new gold-catalyzed C−C bond formation. Angew. Chem. Int. Ed. 39, 2285–2288. (doi:10.1002/1521-3773(20000703)39:13<2285::AID-ANIE2285>3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 85.Manabe K, Aoyama N, Kobayashi S. 2001. Friedel–Crafts-type conjugate addition of indoles using a Lewis acid–surfactant-combined catalyst in water. Adv. Synth. Catal. 343, 174–176. (doi:10.1002/1615-4169(20010226)343:2<174::AID-ADSC174>3.0.CO;2-S) [Google Scholar]
- 86.Bandani M, Cozzi PG, Giacomini M, Melchiorre P, Selva S, Umani-Ronchi A. 2002. Sequential one-pot InBr3-catalyzed 1,4- then 1,2-nucleophilic addition to enones. J. Org. Chem. 67, 3700–3704. (doi:10.1021/jo0163243) [DOI] [PubMed] [Google Scholar]
- 87.Bandani M, Melchiorre P, Melloni A, Umani-Ronchi A. 2002. A practical indium (III) tribromide catalyzed addition of indoles to nitroalkenes in aqueous media. Synthesis 2002, 1110–1114. (doi:10.1055/s-2002-31970) [Google Scholar]
- 88.Alam MM, Varala R, Adapa SR. 2003. Conjugate addition of indoles and thiols with electron-deficient olefins catalyzed by Bi(OTf)3. Tetrahedron Lett. 44, 5115–5119. (doi:10.1016/S0040-4039(03)01089-X) [Google Scholar]
- 89.Dessole G, Herrera RP, Ricci A. 2004. H-bonding organocatalysed Friedel-Crafts alkylation of aromatic and heteroaromatic systems with nitroolefins. Synlett 13, 2374–2378. (doi:10.1055/s-2004-832844) [Google Scholar]
- 90.Bartoli G, Bosco M, Giuli S, Giuliani A, Lucarelli L, Marcantoni E, Sambri L, Torregiani E. 2005. Efficient preparation of 2-indolyl-1-nitroalkane derivatives employing nitroalkenes as versatile Michael acceptors: new practical linear approach to alkyl 9H-β-carboline-4-carboxylate. J. Org. Chem. 70, 1941–1944. (doi:10.1021/jo048776w) [DOI] [PubMed] [Google Scholar]
- 91.Murugan R, Karthikeyn M, Perumal PT, Reddy BSR. 2005. A mild, efficient and improved protocol for the synthesis of novel indolyl crown ethers, di(indolyl)pyrazolylmethanes and 3-alkylated indoles using H4[Si(W3O10)3]. Tetrahedron 61, 12 275–12 281. (doi:10.1016/j.tet.2005.09.108) [Google Scholar]
- 92.Liang D, Li X, Zhang W, Li Y, Zhang M, Cheng P. 2016. Br2 as a novel Lewis acid catalyst for Friedel–Crafts alkylation of indoles with α,β-unsaturated ketones. Tetrahedron Lett. 57, 1027–1030. (doi:10.1016/j.tetlet.2016.01.078) [Google Scholar]
- 93.Correa A, Mancheño OG, Bolm C. 2008. Iron-catalysed carbon-heteroatom and heteroatom-heteroatom bond forming processes. Chem. Soc. Rev. 37, 1108–1117. (doi:10.1039/b801794h) [DOI] [PubMed] [Google Scholar]
- 94.Bauer EB. 2008. Current advances in iron catalysis in organic synthesis. Curr. Org. Chem. 12, 1341–1369. (doi:10.2174/138527208786241556) [Google Scholar]
- 95.Czaplik WM, Mayer M, Cvengroš J, Jacobi A, Wangelin V. 2009. Coming of age: sustainable iron catalysed cross-coupling reactions. ChemSusChem 2, 396–417. (doi:10.1002/cssc.200900055) [DOI] [PubMed] [Google Scholar]
- 96.Gopalaiah K. 2013. Chiral iron catalysts for asymmetric synthesis. Chem. Rev. 113, 3248–3296. (doi:10.1021/cr300236r) [DOI] [PubMed] [Google Scholar]
- 97.Munz D, Webster-Gardiner M, Fu R, Strassner T, Goddard WA III, Gunnoe TB. 2015. Proton or metal? The H/D exchange of arenes in acidic solvents. ACS Catal. 5, 769–775. (doi:10.1021/cs501620f) [Google Scholar]
- 98.Mo J, Lee PH. 2010. Platinum-catalyzed intramolecular hydroarylation of allenyl arenes: efficient synthesis of 1,4-dihydronaphthalenes. Org. Lett. 12, 2570–2573. (doi:10.1021/ol1007857) [DOI] [PubMed] [Google Scholar]
- 99.Kang D, Kim J, Oh S, Lee PH. 2012. Synthesis of Naphthalenes via platinum-catalyzed hydroarylation of aryl enynes. Org. Lett. 14, 5636–5639. (doi:10.1021/ol302437v) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.