Abstract
Individuals at the leading edge of expanding biological invasions often show distinctive phenotypic traits, in ways that enhance their ability to disperse rapidly and to function effectively in novel environments. Cane toads (Rhinella marina) at the invasion front in Australia exhibit shifts in morphology, physiology and behaviour (directionality of dispersal, boldness, risk-taking). We took a common-garden approach, raising toads from range-core and range-edge populations in captivity, to see if the behavioural divergences observed in wild-caught toads are also evident in common-garden offspring. Captive-raised toads from the invasion vanguard population were more exploratory and bolder (more prone to ‘risky’ behaviours) than toads from the range core, which suggests that these are evolved, genetic traits. Our study highlights the importance of behaviour as being potentially adaptive in invasive populations and adds these behavioural traits to the increasing list of phenotypic traits that have evolved rapidly during the toads' 80-year spread through tropical Australia.
Keywords: adaptation, Bufo marinus, evolution, spatial sorting
1. Introduction
Biological invasions impose novel ecological and evolutionary pressures. Individuals at the expanding range edge experience different demographic, physiological and environmental pressures than those faced by conspecifics from the range core, often leading to phenotypic divergence across the invasion range [1]. For example, vanguard individuals often have distinctive phenotypes associated with faster dispersal such as larger size [2–4], longer legs [5] and increased investment in dispersal appendages and mass in plant seeds [6–8]. More generally, successful invaders exhibit a suite of physiological, life-history, morphological and behavioural traits that enhance dispersal rates and facilitate functioning in novel environments (known as an ‘Invasion Syndrome’; [9,10]). Behavioural traits may play an especially critical role (both as direct facilitators of dispersal rate, and via their influence on selection pressures on other traits), and yet are often overlooked as factors contributing to invasion success [10,11].
A propensity to explore, take risks and engage with novel environments (neophilia) is likely to promote range expansion by stimulating dispersal [12,13], and these traits also enhance an individual's ability to find water, food, shelter and mates in novel environments [11,14,15]. In keeping with these predictions, behavioural traits have been linked to range expansion and invasion success in several species. For example, range-edge individuals are more exploratory and bolder (more willing to take risks) than are conspecifics from range-core populations of the round goby (Neogobius melanostomus) [16], dark-eyed junco (Junco hyemalis Thurber) [17,18], common frog (Rana temporaria) [19] and house sparrow (Passer domesticus) [20]. Invasive house sparrows from range-edge populations are also more neophilic than are conspecifics from range-core populations [21,22]. Individuals from range-edge populations also may be more aggressive [23,24] and less social [3,25] compared with those from range-core populations.
Despite the growing body of evidence for distinctive behavioural phenotypes at range edges, the causation for this pattern is ambiguous [1]. The two broad categories of explanation involve (i) behavioural plasticity, induced by the novel environments experienced at the range edge and (ii) the rapid elaboration of heritable traits driven by the unique evolutionary pressures imposed by an invasion.
Many behavioural phenotypes are highly labile, enabling organisms to plastically respond to changes in their physiological, climatic or social environments [26]. Thus, range-edge individuals may shift their behaviour in response to the novel environmental pressures they encounter at the expanding range edge [27,28]. An ability to rapidly shift behaviour in response to novelty may enhance an individual's ability to exploit new ecological niches, habitats or prey items at the range edge [29]. Indeed, innovation has been linked to invasion success in several species [30] including the black rat (Rattus rattus) [31] and bird species worldwide [30,32]. In summary, range-edge individuals may exhibit specific behavioural attributes because those attributes are induced by the novel (range-edge) conditions that an individual experiences during their life.
Alternatively, the distinctive behaviour of invasion-front animals may reflect rapid evolutionary change. Behavioural traits are affected by genes [33], and traits such as exploration and boldness [34–36] and aggression [37] are heritable in some species (reviewed in [38,39]). Variation in behavioural phenotypes between range-core versus range-edge populations is likely to have both ecological and evolutionary consequences through influences on life-history, physiological and morphological traits, and hence be a target for selection [27]. For example, dispersing individuals at the range edge may benefit from reduced competition for resources, a lack of pathogens and parasites, access to the best habitat and a lower risk of predation [40,41]. However, there are also potential costs associated with rapid dispersal, such as the energetic costs of activity, increased risk of injury and a decrease in opportunities to reproduce due to low densities at the range edge [42]. Hence, the benefits of dispersal must outweigh the costs for dispersal-enhancing behavioural traits to be adaptive. Importantly, though, personality-dependent dispersal may evolve even in the absence of natural selection. Bold and neophilic individuals are likely to be the first to disperse out of the range core, where they interbreed. At least some of their progeny inherit dispersal-enhancing traits from both parents, leading to a progressive increase across generations in the dispersal rates of individuals at the range edge (dubbed ‘spatial sorting’ by Shine et al. [43]). These proximate mechanisms (phenotypic plasticity, adaptation, spatial sorting) are not mutually exclusive; for example, an invasion might favour the evolution of specific reaction norms.
To investigate the causal factors driving behavioural divergence across a biological invasion, the first step is to disentangle plastic responses to novel environments from heritable shifts in behaviour. To answer this question, we need to measure behavioural traits not only of wild-caught individuals, but also of individuals raised in standardized (common-garden) conditions. In this study, we gathered these two types of data on the cane toad (Rhinella marina Linnaeus 1758) and its ongoing invasion across Australia.
The cane toad's current invasion range extends from wet tropical Queensland in the northeast to the seasonally dry monsoonal climate of northwestern Western Australia [44]. Hence, toads from the range front versus the range edge experience very different thermal and hydric regimes; environmental factors which can have strong effects on the development of amphibians [45–47]. Previous research has documented rapid evolution of dispersal-enhancing morphological [48], physiological [49] and life-history traits [5,41,48,50–52] across this invasion range. Many of the same traits are seen in offspring raised in ‘common-garden’ experiments, suggesting a heritable component [50,53,54]. Invasion-front toads also are more exploratory and willing to take risks than are toads from long-colonized populations [55], but the heritability of these latter traits has not been investigated using common-garden methods to date. The cane toad's invasion of Australia provides an ideal model system in which to ask our key question: is behavioural divergence across the invasion range due to plastic responses to the different climatic, physical and demographic environments experienced by range-front versus range-core individuals, or are they due to rapid evolutionary (heritable) changes? To answer this question, we conducted standardized laboratory-based behavioural assays to quantify exploratory, risk-taking and neophilic behaviour in both wild-caught and captive-raised cane toads from the range core and range edge of their Australian invasion range.
2. Material and methods
2.1. Collection of wild-caught toads
In 2016, we collected 68 adult cane toads (34 male, 34 female), half from a long-colonized population (Cairns, Queensland: 17°56′ S, 145°56′ E; more than 76 years post-colonization [51]; mean annual rainfall: 1999.7 mm, mean annual maximum temperature: 29.0°) and half from a range-front population (Oombulgurri, Western Australia: 15°10′45′′ S,127°52′36′′ E; less than 3 years post-colonization [56]; mean annual rainfall: 809.4 mm, mean annual maximum temperature: 35.1° for Kununurra; Australian Government Bureau of Meteorology (www.bom.gov.au) 2016). Toads were collected from three sub-populations within each location, hence the years since colonization (above) are means for each area. Our collection and transportation procedures are described in Gruber et al. [55]. Toads were kept at the University of Sydney's Tropical Ecology Research Facility at Middle Point, Northern Territory (12°34′ S, 131°18′ E; 11 years post-colonization; mean annual rainfall: 1421.7 mm, mean annual maximum temperature: 33.1°; Australian Government Bureau of Meteorology (www.bom.gov.au) 2016). Upon collection, toads were weighed to the nearest 0.1 g on a digital scale and measured (snout–urostyle length (SUL)) to the nearest 0.01 mm using digital callipers.
2.2. Rearing of toads in captivity
Our common-garden toads were bred in captivity from wild-caught Western Australian and Queensland toads (for collection details and breeding protocols, see [57,58]). All dams and sires were bred only once, hence all F1 individuals from each clutch were full-siblings [58]. We used individuals from 13 clutches from Western Australian and 16 clutches from Queensland ancestry. At the time of testing, these captive-raised toads were approximately 18 months of age and had been raised for their entire lives under standard conditions at the same site (our Northern Territory research station, as described above). All toads were weighed and measured before being housed as described below.
2.3. Methods for general husbandry
At the research station, toads were transferred from their large outdoor pens [58] to smaller containers (100 l plastic tubs with mesh lids) where they were housed in groups of two or three. Each tub had a wood-shaving substrate and contained two shelters (each shelter was large enough to hold three toads) and a large shallow water dish. Toads were fed live crickets four times per week and were provided with fresh water ad libitum. Toads were weighed and measured regularly to monitor their health (average toad SUL 98 mm; average toad mass 120 g). None showed any signs of illness or weight loss. Handling of toads was kept to a minimum and, to reduce stress, toads were transferred to and from trial arenas in dark plastic tubs. To minimize stress, toads were left undisturbed during non-trial periods.
2.4. Trial methods
As adult toads are most active at night [59,60], we conducted behavioural trials between 18.30 and 01.00 h. Each toad was tested in three different behavioural trials: (i) exploration of a novel arena, (ii) risk-taking (emergence from a shelter into a test arena), and (iii) neophilia (response to a novel object). Trials ran for 30 min and four trials with one toad per trial arena were conducted simultaneously in each trial round. Trials were split over 3 days with six trial rounds on the first two nights (24 toads tested on each night) and five (20 toads tested) on the third night. All toads were exposed to each behavioural trial in the same sequence, that is, an exploratory trial, a risk-taking trial and a neophilia trial with 2 days of rest in between trial types (while the other sets of toads were assayed). An equal number of toads from range-front and range-core populations and each sex were randomly allocated a trial time and arena within each trial day. Toads from the common-garden and wild-caught populations were assayed consecutively rather than simultaneously due to logistical and space constraints.
Trial arenas were large (120 × 120 × 83 cm) hexagonal pens made from waterproof fabric with an open top to allow filming from above. The PVC substrate (and shelters, etc.) of each arena were wiped with diluted ethanol before each trial to eliminate scent. We measured the arena floor temperature before each trial (range: 29–31°C). We included an empty container in all trials to disambiguate hiding behaviour from interest in a novel object (for consistency, empty containers were also included in exploration and emergence trials). At the start of each exploratory and neophilia trial, toads were given 5 min before their resting shelter was removed and trials began. During risk-taking trials, toads began the trial in the safety of the shelter and the entrance was covered for five minutes before the cover was removed. All trials were recorded using CCTV cameras and we scored videos using Ethovision XT10 behavioural analysis software. Ethovision scored all videos in a standardized way without information on the population of origin (to ensure blind scoring). The investigator left the room during trials to avoid affecting toad behaviour.
2.4.1. Exploratory trial
To test exploration and space use in a novel environment [3,13], we measured the time spent moving and rate of movement [61,62]. We provided a shelter in the arena to give toads the option to hide during trials. This allowed us to distinguish bold exploratory behaviour from fear-driven escape behaviour [63]. An empty container was placed next to the shelter and both were positioned equidistant to one another at the opposite end of the arena from the start point.
2.4.2. Emergence trial
To score risk-taking behaviour, we recorded whether or not a toad emerged from its shelter, and latency to emerge (s). Emergence during the trial and a shorter latency to emerge indicates higher risk-taking propensity [3,19]. Two empty containers were placed at the opposite end of the arena from the start point.
2.4.3. Novel object trial
To test neophilia, we used a silicone fishing lure (20 mm × 10 mm, mimicking a squid) driven by a small motor to move up and down every 2 s as a novel object. The novel object was housed inside a clear container to prevent the toad from consuming the plastic lure. The novel object was placed at the opposite end of the arena to the start point.
2.5. Statistical analysis
We used linear models to analyse the effects of population source (Western Australia–Queensland), population type (common-garden–wild-caught) and population source within population type separately (common-garden toads from Western Australian versus Queensland ancestry and wild-caught toads from Western Australia and Queensland) on behavioural traits. We did not incorporate clutch as a factor in our analysis as we were not able to obtain information on relatedness in wild toads. The specific measurements collected from each trial were as follows:
(1) exploratory trials—total time spent moving and rate of movement (as quantified by the residual scores from a simple linear regression of total distance moved against total time spent moving);
(2) emergence trials—emergence (binomial, whether or not individuals emerged during trials) and latency to emerge; and
(3) novel object trials—whether or not toads approached to within 90 mm (the minimum body length required for toads to be classed as adults [64]; thus, toads were recorded as being in the ‘novel object zone’ when within one body length of the focal object) of the novel object (binomial), latency to approach and time spent within 90 mm of the novel object.
Repeatabilities of these behaviours (obtained from a larger sample of toads subjected to these trials on two occasions) ranged from R = 0.01 (±95% CI 0, 0.56) p = 0.50 for activity to R = 0.49 (±95% CI 0.31, 0.81) p = 0.001 for latency to emerge (Gruber et al. 2017, unpublished data). We included data from captive-raised and wild-caught toads in a single model to examine the effects of population (range-core and range-edge), source (captive-raised and wild-caught) and their interaction on toad behaviour. We used top-down stepwise model selection, starting with a full model including all factors, covariates and their interactions, and sequentially deleted non-significant terms. Sex, mass, arena temperature, arena number, time of trial and day of trial had non-significant effects on behavioural traits and thus were excluded from the final models. We did not detect significant interactions between any factors, hence all interaction terms were also removed from the final models.
3. Results
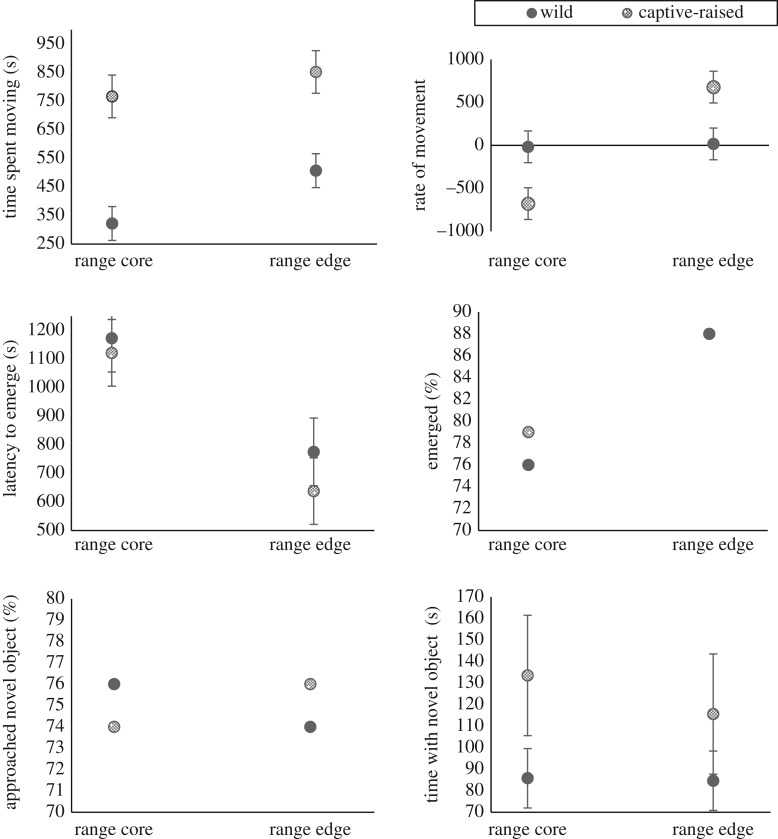
Overall, toads from the range edge were more exploratory and had a higher propensity to take risks than did toads from the range core (table 1). We found no significant interaction effect between population (range-edge versus range-core) and prior experience (captive-raised versus wild-caught) on any behavioural traits. Range-edge and range-core toads exhibited similar rates of movement during exploration trials, were similarly likely to emerge during risk-taking trials and responded in similar ways to the novel object during neophilia trials (table 1).
Table 1.
Effects of population (range-core versus range-edge), source (captive-raised versus wild-caught) and their interaction (population × source) on behavioural traits during exploration (time spent moving, rate of movement), risk-taking (proportion to emerge and latency to emerge) and neophilia (proportion to approach novel object and time spent with novel object) trials. Results for main effects are based on analyses after exclusion of non-significant interaction terms. Statistically significant values (p < 0.05) are highlighted in bold text.
| variable | population | captive-raised versus wild | population × source |
|---|---|---|---|
| time spent moving (s) | F1,135 = 4.40 | F1,135 = 35.19 | F1,135 = 0.45 |
| p < 0.04 | p d 0.0001 | p = 0.50 | |
| movement rate | F1,135 = 0.42 | F1,135 = 31.43 | F1,135 = 0.11 |
| p = 0.52 | p < 0.0001 | p = 0.74 | |
| proportion to emerge | χ2 = 1.57 | χ2 = 0.00 | χ2 = 0.03 |
| p = 0.21 | p = 0.1 | p = 0.86 | |
| emergence latency (s) | F1,135 = 12.77 | F1,135 = 0.68 | F1,135 = 0.026 |
| p = 0.0005 | p = 0.41 | p = 0.87 | |
| proportion to approach novel object | χ2 = 0.08 | χ2 = 0.16 | χ2 = 0.16 |
| p = 0.78 | p = 0.78 | p = 0.69 | |
| time spent with novel object (s) | F1,135 = 0.17 | F1,135 = 3.79 | F1,135 = 0.06 |
| p = 0.68 | p = 0.054 | p = 0.80 |
During exploration trials, captive-raised toads spent more time moving and moved faster than did wild-caught conspecifics (table 1; figure 1). The proportion of toads to emerge during risk-taking trials and to approach the novel object during neophilia trials did not differ between captive-raised versus wild-caught toads (table 1; figure 1). Captive-raised toads were quicker to emerge from the shelter during emergence trials and spent more time with the novel object during novel object trials than did wild-caught toads, but these results did not reach α < 0.05 (table 1).
Figure 1.
Behavioural traits of cane toads from wild-caught and captive-raised populations of Western Australian (range-edge) and Queensland (range-core) origin. Graphs show mean values and associated standard errors (where relevant) for traits measured during trials of exploratory behaviour (time spent moving, rate of movement), risk-taking (emerged from shelter, latency to emerge) and neophilia (approached novel object, time with novel object).
4. Discussion
Our findings are consistent with those of previous studies on behavioural divergence across the cane toad's Australian invasion range [55]: range-edge toads were more exploratory and willing to take risks than were conspecifics from range-core populations. Importantly, the divergence in risk-taking behaviour was seen in captive-raised toads as well as wild-caught animals (and was statistically significant in analyses restricted only to data from captive-raised animals). The divergence in risk-taking behaviour in toads from different ancestral populations, even after the animals were raised in identical conditions, indicates a heritable component to this behaviour. Hence, behavioural divergence across the invasion range in this species cannot be due entirely to plastic responses to different environments.
The distinctive behavioural traits seen at expanding range edges may result from both adaptive and non-adaptive processes. First, traits that enhance dispersal and adaptation to novel environments may confer fitness advantages at the range edge, and hence evolve via natural selection [16]. For example, ‘risk-taking’ individuals may be more likely to disperse into novel environments where competition for resources (such as food and shelter) and the numbers of pathogens and parasites are reduced by low densities of conspecifics [16,17,41,65]. High levels of exploration, risk-taking and neophilia also may enable individuals to exploit novel niches and resources [21,66,67]. Even in the absence of such adaptive advantages, however, distinctive behavioural phenotypes may accumulate at the invasion front because of spatial sorting [43]. That is, the increasingly fast-moving invasion front inevitably is dominated by fast-dispersing individuals, whose interbreeding produces even faster-dispersing offspring [5,43,68]. The evolution of behavioural traits across an invasion range may also be indirectly affected by the rapid evolution of morphological, physiological or life-history traits if there are genetic links between behaviour and these traits (e.g. the ‘Pace of Life Syndrome’ hypothesis [69]). Other processes such as genetic drift, surfing of deleterious mutations [70,71] and density-dependent selection [57] may also influence the frequency of inherited traits at the invasion front.
Previous research using captive-raised (common-garden) cane toads has documented significant heritability for traits such as dispersal rate [57], limb morphology [58] and locomotor performance [72]. Similarly, heritability of dispersal-related behavioural traits has been documented in the great tit (Parus major) [34,65,73,74], the red-cockaded woodpecker (Picoides borealis) [75], the collared flycatcher (Ficedula albicollis) [76] and the Glanville fritillary butterfly (Melitaea cinxia) [77]. Our results on cane toads suggest a heritable component to behavioural traits that enhance dispersal, but we cannot quantify heritability without breeding experiments to allow cross-generational comparisons of behavioural traits between parents and offspring.
Heritability and plasticity are not mutually exclusive mechanisms [78]. Indeed, behavioural plasticity is driven both by environmental conditions and by the constraints of evolved (genetically based) norms of reaction [66]. However, these two responses are closely related, with reaction norms evolving such as to generate the most appropriate response to any given set of environmental conditions [79]. Behavioural plasticity often may be beneficial in a novel environment, if it induces an individual to alter its behaviour appropriately and at a rate that fits the prevailing environmental conditions [27,29,80].
Adaptive activational plasticity occurs when an animal has the ability to express a trait (such as neophilia) but only does so in response to the appropriate stimulus [66]. Activational plasticity may explain why neophilia varied so little among toads in this study (in wild-caught as well as captive-bred populations, and from the range edge as well as the range core). The potential advantage of neophilia at the range edge is clear, as it promotes movement through novel areas and exploitation of novel resources [11,28,67]. Although cane toads from the range core are in a familiar environment, they nonetheless must often encounter novelty (especially, in urban habitats) and neophilic traits may also enhance fitness in this situation. If so, range-core individuals may possess neophilic traits but not express them unless they are exposed to novelty (e.g. during the neophilia trial) [1].
In this study, we investigated whether the behavioural divergence documented in cane toads across their Australian invasion range [55] is solely due to plastic responses to the profoundly different climatic, physical and demographic environments experienced by range-front versus range-core individuals, or if other mechanisms have a role to play. In keeping with the earlier report, we found that range-edge toads were more exploratory and willing to take risks than were toads from long-colonized areas—and importantly, that divergence was evident even if the toads had been raised in captivity. We thus infer that at least part of the behavioural divergence between range-core and range-edge cane toads in Australia is a result of rapid evolutionary change (i.e. heritable factors, that differ among populations), as has been documented also for a wide range of morphological and physiological traits [5,41,48,50–52]. Further work is required to measure heritability in behavioural traits and to determine whether the shifts in these traits during the course of the toads' Australian invasion have been driven by adaptive or non-adaptive processes.
Supplementary Material
Acknowledgements
We thank Michael Crossland for support during experiments, Georgia Ward-Fear and Dan Selechnik for assistance with toad collection, Andrea West for assistance with husbandry, and Adam McKiernan and Moe McGruber for support and extensive discussions during the preparation of this manuscript.
Ethics
This research was conducted under approval no. 2013/5805 from the University of Sydney Animal Care and Ethics Committee.
Data accessibility
The dataset supporting this article has been uploaded as part of the electronic supplementary material.
Authors' contributions
J.G. co-designed experiments, carried out animal collection, experimental procedures, video scoring and data analyses, and drafted the manuscript. R.S. co-designed the experiment, made contributions to all stages of the manuscript draft and assisted with data analyses. G.B. assisted with data analyses and helped draft the manuscript. M.J.W. contributed to the experimental design and helped draft the manuscript. All the authors reviewed and edited the manuscript, and gave their final approval for publication.
Competing interests
We have no competing interests.
Funding
R.S. was supported by the Australian Research Council (FL120100074).
References
- 1.Chuang A, Peterson CR. 2016. Expanding population edges: theories, traits, and trade-offs. Glob. Change Biol. 22, 494–512. (doi:10.1111/gcb.13107) [DOI] [PubMed] [Google Scholar]
- 2.Kelehear C, Brown GP, Shine R. 2012. Rapid evolution of parasite life history traits on an expanding range-edge. Ecol. Lett. 15, 329–337. (doi:10.1111/j.1461-0248.2012.01742.x) [DOI] [PubMed] [Google Scholar]
- 3.Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A. 2010. Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc. R. Soc. B 277, 1571–1579. (doi:10.1098/rspb.2009.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laparie M, Renault D, Lebouvier M, Delattre T. 2013. Is dispersal promoted at the invasion front? Morphological analysis of a ground beetle invading the Kerguelen Islands, Merizodus soledadinus (Coleoptera, Carabidae). Biol. Invasions 15, 1641–1648. (doi:10.1007/s10530-012-0403-x) [Google Scholar]
- 5.Phillips BL, Brown GP, Webb JK, Shine R.. 2006. Invasion and the evolution of speed in toads. Nature 439, 803 (doi:10.1038/439803a) [DOI] [PubMed] [Google Scholar]
- 6.Matlack GR. 1987. Diaspore size, shape, and fall behavior in wind-dispersed plant species. Am. J. Bot. 74, 1150–1160. (doi:10.2307/2444151) [Google Scholar]
- 7.Monty A, Mahy G. 2010. Evolution of dispersal traits along an invasion route in the wind-dispersed Senecio inaequidens (Asteraceae). Oikos 119, 1563–1570. (doi:10.1111/j.1600-0706.2010.17769.x) [Google Scholar]
- 8.Murray BR, Phillips ML. 2010. Investment in seed dispersal structures is linked to invasiveness in exotic plant species of south-eastern Australia. Biol. Invasions 12, 2265–2275. (doi:10.1007/s10530-009-9637-7) [Google Scholar]
- 9.Wolf M, Weissing FJ. 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 27, 452–461. (doi:10.1016/j.tree.2012.05.001) [DOI] [PubMed] [Google Scholar]
- 10.Carere C, Gherardi F. 2013. Animal personalities matter for biological invasions. Trends Ecol. Evol. 28, 5–6. (doi:10.1016/j.tree.2012.10.006) [DOI] [PubMed] [Google Scholar]
- 11.Chapple DG, Simmonds SM, Wong BBM. 2012. Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol. Evol. 27, 57–64. (doi:10.1016/j.tree.2011.09.010) [DOI] [PubMed] [Google Scholar]
- 12.Dingemanse NJ, Wright J, Kazem AJN, Thomas DK, Hickling R, Dawnay N. 2007. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138. (doi:10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- 13.Fraser DF, Gilliam JF, Daley MJ, Le AN, Skalski GT. 2001. Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am. Nat. 158, 124–135. (doi:10.1086/321307) [DOI] [PubMed] [Google Scholar]
- 14.Cote J, Clobert J, Brodin T, Fogarty S, Sih A. 2010. Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Phil. Trans. R. Soc. B 365, 4065–4076. (doi:10.1098/rstb.2010.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole EF, Quinn JL. 2012. Personality and problem-solving performance explain competitive ability in the wild. Proc. R. Soc. B 279, 1168–1175. (doi:10.1098/rspb.2011.1539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myles-Gonzalez E, Burness G, Yavno S, Rooke A, Fox MG. 2015. To boldly go where no goby has gone before: boldness, dispersal tendency, and metabolism at the invasion front. Behav. Ecol. 26, 1083–1090. (doi:10.1093/beheco/arv050) [Google Scholar]
- 17.Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED. 2012. Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav. Ecol. 23, 960–969. (doi:10.1093/beheco/ars059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atwell JW, Cardoso GC, Whittaker DJ, Price TD, Ketterson ED. 2014. Hormonal, behavioral, and life-history traits exhibit correlated shifts in relation to population establishment in a novel environment. Am. Nat. 184, E147–E160. (doi:10.1086/678398) [DOI] [PubMed] [Google Scholar]
- 19.Brodin T, Lind MI, Wiberg MK, Johansson F. 2013. Personality trait differences between mainland and island populations in the common frog (Rana temporaria). Behav. Ecol. Sociobiol. 67, 135–143. (doi:10.1007/s00265-012-1433-1) [Google Scholar]
- 20.Liebl AL, Martin LB. 2012. Exploratory behaviour and stressor hyper-responsiveness facilitate range expansion of an introduced songbird. Proc. R. Soc. B 279, 4375–4381. (doi:10.1098/rspb.2012.1606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebl AL, Martin LB. 2014. Living on the edge: range edge birds consume novel foods sooner than established ones. Behav. Ecol. 25, 1089–1096. (doi:10.1093/beheco/aru089) [Google Scholar]
- 22.Martin LB II, Fitzgerald L. 2005. A taste for novelty in invading house sparrows, Passer domesticus. Behav. Ecol. 16, 702–707. (doi:10.1093/beheco/ari044) [Google Scholar]
- 23.Duckworth RA, Badyaev AV. 2007. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022. (doi:10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duckworth RA. 2008. Adaptive dispersal strategies and the dynamics of a range expansion. Am. Nat. 172(Suppl.), S4–S17. (doi:10.1086/588289) [DOI] [PubMed] [Google Scholar]
- 25.Cote J, Fogarty S, Brodin T, Weinersmith K, Sih A. 2011. Personality-dependent dispersal in the invasive mosquitofish: group composition matters. Proc. R. Soc. B 278, 1670–1678. (doi:10.1098/rspb.2010.1892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster SA. 2013. Evolution of behavioural phenotypes: influences of ancestry and expression. Anim. Behav. 85, 1061–1075. (doi:10.1016/j.anbehav.2013.02.008) [Google Scholar]
- 27.Dall SRX, Houston AI, McNamara JM. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739. (doi:10.1111/j.1461-0248.2004.00618.x) [Google Scholar]
- 28.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 29.Wright TF, Eberhard JR, Hobson EA, Avery ML. 2010. Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethol. Ecol. Evol. 22, 393–404. (doi:10.1080/03949370.2010.505580) [Google Scholar]
- 30.Sol D, Timmermans S, Lefebvre L. 2002. Behavioural flexibility and invasion success in birds. Anim. Behav. 63, 495–502. (doi:10.1006/anbe.2001.1953) [Google Scholar]
- 31.Aisner R, Terkel J. 1992. Ontogeny of pine cone opening behaviour in the black rat, Rattus rattus. Anim. Behav. 44, 327–336. (doi:10.1016/0003-3472(92)90038-B) [Google Scholar]
- 32.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465. (doi:10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouchard TJ Jr, Loehlin JC. 2001. Genes, evolution, and personality. Behav. Genet. 31, 243–273. (doi:10.1023/A:1012294324713) [DOI] [PubMed] [Google Scholar]
- 34.Drent PJ, van Oers K, van Noordwijk AJ. 2003. Realized heritability of personalities in the great tit (Parus major). Proc. R. Soc. Lond. B 270, 45–51. (doi:10.1098/rspb.2002.2168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Oers K, Drent PJ, de Jong G, van Noordwijk AJ. 2004. Additive and nonadditive genetic variation in avian personality traits. Heredity 93, 496–503. (doi:10.1038/sj.hdy.6800530) [DOI] [PubMed] [Google Scholar]
- 36.Dingemanse NJ, Both C, Drent PJ, Tinbergen JM. 2004. Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. Lond. B 271, 847–852. (doi:10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell AM. 2005. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473. (doi:10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- 38.van Oers K, de Jong G, van Noordwijk AJ, Kempenaers B, Drent PJ. 2005. Contribution of genetics to the study of animal personalities: a review of case studies. Behaviour 142, 1185–1206. (doi:10.1163/156853905774539364) [Google Scholar]
- 39.Dochtermann NA, Schwab T, Sih A (eds). 2015. The contribution of additive genetic variation to personality variation: heritability of personality. Proc. R. Soc. B 282, 20142201 (doi:10.1098/rspb.2014.2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkins AT, Phillips BL, Baskett ML, Hastings A. 2013. Evolution of dispersal and life history interact to drive accelerating spread of an invasive species. Ecol. Lett. 16, 1079–1087. (doi:10.1111/ele.12136) [DOI] [PubMed] [Google Scholar]
- 41.Brown GP, Kelehear C, Shine R. 2013. The early toad gets the worm: cane toads at an invasion front benefit from higher prey availability. J. Anim. Ecol. 82, 854–862. (doi:10.1111/1365-2656.12048) [DOI] [PubMed] [Google Scholar]
- 42.Bonte D, et al. 2012. Costs of dispersal. Biol. Rev. 87, 290–312. (doi:10.1111/j.1469-185X.2011.00201.x) [DOI] [PubMed] [Google Scholar]
- 43.Shine R, Brown GP, Phillips BL. 2011. An evolutionary process that assembles phenotypes through space rather than through time. Proc. Natl Acad. Sci. USA 108, 5708–5711. (doi:10.1073/pnas.1018989108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trumbo DR, Epstein B, Hohenlohe PA, Alford RA, Schwarzkopf L, Storfer A. 2016. Mixed population genomics support for the central marginal hypothesis across the invasive range of the cane toad (Rhinella marina) in Australia. Mol. Ecol. 25, 4161–4176. (doi:10.1111/mec.13754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kearney M, Phillips BL, Tracy CR, Christian KA, Betts G, Porter WP. 2008. Modelling species distributions without using species distributions: the cane toad in Australia under current and future climates. Ecography 31, 423–434. (doi:10.1111/j.0906-7590.2008.05457.x) [Google Scholar]
- 46.Ducatez S, Crossland M, Shine R. 2016. Differences in developmental strategies between long-settled and invasion-front populations of the cane toad in Australia. J. Evol. Biol. 29, 335–343. (doi:10.1111/jeb.12785) [DOI] [PubMed] [Google Scholar]
- 47.Indermaur L, Schmidt BR, Tockner K, Schaub M. 2010. Spatial variation in abiotic and biotic factors in a floodplain determine anuran body size and growth rate at metamorphosis. Oecologia 163, 637–649. (doi:10.1007/s00442-010-1586-4) [DOI] [PubMed] [Google Scholar]
- 48.Hudson CM, McCurry MR, Lundgren P, McHenry CR, Shine R. 2016. Constructing an invasion machine: the rapid evolution of a dispersal-enhancing phenotype during the cane toad invasion of Australia. PLoS ONE 11, e0156960 (doi:10.1371/journal.pone.0156950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tingley R, Greenlees MJ, Shine R. 2012. Hydric balance and locomotor performance of an anuran (Rhinella marina) invading the Australian arid zone. Oikos 121, 1959–1965. (doi:10.1111/j.1600-0706.2012.20422.x) [Google Scholar]
- 50.Phillips BL. 2009. The evolution of growth rates on an expanding range edge. Biol. Lett. 5, 802–804. (doi:10.1098/rsbl.2009.0367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips BL, Brown GP, Greenlees M, Webb JK, Shine R. 2007. Rapid expansion of the cane toad (Bufo marinus) invasion front in tropical Australia. Austral Ecol. 32, 169–176. (doi:10.1111/j.1442-9993.2007.01664.x) [Google Scholar]
- 52.Lindström T, Brown GP, Sisson SA, Phillips BL, Shine R. 2013. Rapid shifts in dispersal behavior on an expanding range edge. Proc. Natl Acad. Sci. USA 110, 13 452–13 456. (doi:10.1073/pnas.1303157110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown GP, Phillips BL, Dubey S, Shine R. 2015. Invader immunology: invasion history alters immune system function in cane toads (Rhinella marina) in tropical Australia. Ecol. Lett. 18, 57–65. (doi:10.1111/ele.12390) [DOI] [PubMed] [Google Scholar]
- 54.Brown GP, Phillips BL, Shine R. 2014. Directional dispersal has not evolved during the cane toad invasion. Funct. Ecol. 29, 830–838. (doi:10.1111/1365-2435.12397) [Google Scholar]
- 55.Gruber J, Brown G, Whiting MJ, Shine R. 2017. Geographic divergence in dispersal-related behaviour in cane toads from range-front versus range-core populations in Australia. Behav. Ecol. Sociobiol. 71, 1–7. (doi:10.1007/s00265-017-2266-8) [Google Scholar]
- 56.Ward-Fear G, Pearson DJ, Brown GP, Rangers B, Shine R. 2016. Ecological immunization: in situ training of free-ranging predatory lizards reduces their vulnerability to invasive toxic prey. Biol. Lett. 12, 20150863 (doi:10.1098/rsbl.2015.0863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phillips BL, Brown GP, Shine R. 2010. Evolutionarily accelerated invasions: the rate of dispersal evolves upwards during the range advance of cane toads. J. Evol. Biol. 23, 2595–2601. (doi:10.1111/j.1420-9101.2010.02118.x) [DOI] [PubMed] [Google Scholar]
- 58.Hudson CM, Brown GP, Shine R. 2016. It is lonely at the front: contrasting evolutionary trajectories in male and female invaders. R. Soc. open sci. 3, 160687 (doi:10.1098/rsos.160687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zug GR, Zug PB.. 1979. The marine toad, Bufo marinus: a natural history resume of native populations. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 60.Lever C. 2001. The cane toad: the history and ecology of a successful colonist. Otley, UK: Westbury Academic & Scientific Pub. [Google Scholar]
- 61.Millot S, Bégout M-L, Chatain B. 2009. Exploration behaviour and flight response toward a stimulus in three sea bass strains (Dicentrarchus labrax L.). Appl. Anim. Behav. Sci. 119, 108–114. (doi:10.1016/j.applanim.2009.03.009) [Google Scholar]
- 62.Adriaenssens B, Johnsson JI. 2010. Shy trout grow faster: exploring links between personality and fitness-related traits in the wild. Behav. Ecol. 22, 135–143. (doi:10.1093/beheco/arq185) [Google Scholar]
- 63.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. Camb. Philos. Soc. 82, 291–318. (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 64.Alford R, Cohen M, Crossland M, Hearnden M, Schwarzkopf L. 1995. Population biology of Bufo marinus in northern Australia. In Wetland research in the wet-dry tropics of Australia (ed. CM Finlayson), pp. 173–181. Canberra, Australia: Office of the Supervising Scientist. [Google Scholar]
- 65.Dingemanse NJ, Both C, van Noordwijk AJ, Rutten AL, Drent PJ. 2003. Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747. (doi:10.1098/rspb.2002.2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Foster SA, Sih A. 2013. Behavioural plasticity and evolution. Anim. Behav. 85, 1003 (doi:10.1016/j.anbehav.2013.04.006) [Google Scholar]
- 67.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. (doi:10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 68.Phillips BL, Brown GP, Travis JMJ, Shine R. 2008. Reid's paradox revisited: the evolution of dispersal kernels during range expansion. Am. Nat. 172(Suppl.), S34–S48. (doi:10.1086/588255) [DOI] [PubMed] [Google Scholar]
- 69.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. (doi:10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Excoffier L, Foll M, Petit R. 2009. Genetic consequences of range expansions. Ann. Rev. Ecol., Evol. Syst. 40, 481–501. (doi:10.1146/annurev.ecolsys.39.110707.173414) [Google Scholar]
- 71.Travis JMJ, Münkemüller T, Burton OJ, Best A, Dytham C, Johst K. 2007. Deleterious mutations can surf to high densities on the wave front of an expanding population. Mol. Biol. Evol. 24, 2334–2343. (doi:10.1093/molbev/msm167) [DOI] [PubMed] [Google Scholar]
- 72.Llewelyn J, Phillips BL, Alford RA, Schwarzkopf L, Shine R. 2010. Locomotor performance in an invasive species: cane toads from the invasion front have greater endurance, but not speed, compared to conspecifics from a long-colonised area. Oecologia 162, 343–348. (doi:10.1007/s00442-009-1471-1) [DOI] [PubMed] [Google Scholar]
- 73.Verbeek MEM, Drent PJ, Wiepkema PR. 1994. Consistent individual differences in early exploratory behaviour of male great tits. Anim. Behav. 48, 1113–1121. (doi:10.1006/anbe.1994.1344) [Google Scholar]
- 74.Dingemanse NJ, Both C, Drent PJ, van Oers K, van Noordwijk AJ. 2002. Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 64, 929–938. (doi:10.1006/anbe.2002.2006) [Google Scholar]
- 75.Pasinelli G, Schiegg K, Walters Jeffrey R. 2004. Genetic and environmental influences on natal dispersal distance in a resident bird species. Am. Nat. 164, 660–669. (doi:10.1086/424765) [DOI] [PubMed] [Google Scholar]
- 76.Doligez B, Gustafsson L, Pärt T. 2009. ‘Heritability’ of dispersal propensity in a patchy population. Proc. R. Soc. B 276, 2829–2836. (doi:10.1098/rspb.2009.0454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saastamoinen M. 2008. Heritability of dispersal rate and other life history traits in the Glanville fritillary butterfly. Heredity 100, 39–46. (doi:10.1038/sj.hdy.6801056) [DOI] [PubMed] [Google Scholar]
- 78.Mery F, Burns JG. 2010. Behavioural plasticity: an interaction between evolution and experience. Evol. Ecol. 24, 571–583. (doi:10.1007/s10682-009-9336-y) [Google Scholar]
- 79.Tuomainen U, Candolin U. 2011. Behavioural responses to human-induced environmental change. Biol. Rev. 86, 640–657. (doi:10.1111/j.1469-185X.2010.00164.x) [DOI] [PubMed] [Google Scholar]
- 80.Sih A. 2013. Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav. 85, 1077 (doi:10.1016/j.anbehav.2013.02.017) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article has been uploaded as part of the electronic supplementary material.