Abstract
We recently developed a polyethylenimine (PEI) and polyethylene glycol (PEG) dual-functionalized reduced graphene oxide (GO) (PEG−nrGO−PEI, RGPP) for high-efficient gene delivery in HepG2 and Hela cell lines. To evaluate the feasibility and applicability of RGPP as a gene delivery carrier, we here assessed the transfection efficiency of RGPP on gene plasmids and siRNA in 11 different cell lines. Commercial polyalkyleneimine cation transfection reagent (TR) was used as comparison. In HepG2 cells, RGPP exhibited much stronger delivery ability for siRNA and large size plasmids than TR. For green fluorescent protein (GFP) plasmid, RGPP showed about 47.1% of transfection efficiency in primary rabbit articular chondrocytes, and about 27% of transfection efficiency in both SH-SY5Y and A549 cell lines. RGPP exhibited about 37.2% of GFP plasmid transfection efficiency in EMT6 cells and about 26.0% of GFP plasmid transfection efficiency in LO2 cells, but induced about 33% of cytotoxicity in both cell lines. In 4T1 and H9C2 cell lines, RGPP had less than 10% of GFP plasmid transfection efficiency. Collectively, RGPP is a potential nano-carrier for high-efficiency gene delivery, and needs to be further optimized for different cell lines.
Keywords: polyethylenimine, polyethylene glycol, graphene oxide, gene delivery, transfection efficiency
1. Introduction
Gene delivery provides a powerful tool for exploring gene function, generating transgenic organisms and treating gene-related diseases [1–3]. A variety of gene functional sensors have been designed to monitor intracellular dynamic processes such as activation of protein kinases, oligomerization of proteins and protein–protein interactions in single living cells [4–10]. Although RNA interference can only partially silence a target gene and has extensive off-target effects, it has been widely used for genome-wide loss-of-function screens and evaluating the function of genes and proteins [11–15]. CRISPR/Cas9, a versatile genome editing technology, can target nearly any DNA sequence, and the high efficiency of genome editing with Cas9 makes it possible to alter many targets in parallel, thereby enabling unbiased genome-wide functional screens to identify genes that play an important role in a phenotype of interest [15–17]. Moreover, CRISPR/Cas9 can generate cellular transgenic models for studying human polygenic diseases, generating transgenic animal models and repressing or activating gene transcription [15,18–23]. With high potential to treat diseases at genetic roots, gene therapy has long fascinated scientists and clinicians. In the past two decades, a series of phase I/II gene-therapy clinical trials have been reported to have significant efficacy and safety for the treatment of various severe inherited diseases of the immune, blood and nervous systems [24–28].
Safe and efficient carrier is critical for gene delivery [29]. Polyethylenimine (PEI), a cationic polymer with the highest cationic-charge-density potential, is a potential excellent carrier of gene delivery [30,31]. PEI can efficiently bind anionic DNA/RNA within the physiological pH range, protect DNA/RNA from degradation and trigger DNA/RNA release from endosome [32–37]. In contrast to the high cytotoxicity and poor biocompatibility of PEI, PEI-functionalized nanographene oxide (nGO−PEI) composites have an improved transfection efficiency with lower cytotoxicity [30,38–40]. Polyethylene glycol (PEG) can further improve the biocompatibility of nGO−PEI in the presence of saline or serum [41–45]. Moreover, PEG and PEI dual-functionalized GO (GO-PEI-PEG) also exhibited excellent transfection capacity and low cytotoxicity [42–48]. Recently, we developed a PEI and PEG dual-functionalized reduced GO (PEG−nrGO−PEI, RGPP) composite for high-efficiency gene delivery, exhibiting about 83.9% of transfection efficiency for green fluorescent protein (GFP) plasmid in Hela cells [45].
In this report, we evaluate the delivery ability of RGPP for siRNA and plasmids in 11 kinds of cell lines. Commercial polyalkyleneimine cation transfection reagent (TR) was used as comparison. In HepG2 cells, RGPP exhibited better gene delivery ability than TR for siRNA, large size for plasmids such as GFP-Bcl-xL (9.8 kb) and other functional gene plasmids including GFP-Bak, GFP-Puma, GFP-Bax 1–2/L-6 and GFP-NPM NLS1/2D. RGPP could efficiently deliver GFP plasmid into primary rabbit articular chondrocyte, SH-SY5Y, A549, EMT6 and LO2 cell lines. However, RGPP inefficiently delivered GFP plasmid into H9C2 and 4T1 cell lines.
2. Material and methods
2.1. Materials
GO was purchased from Nanjing XFNano Materials Tech Co. Ltd (Nanjing, China). Branched PEI (25 kDa) and N-(3-dimethylaminopropyl-N′-ethylcarbodiimide) hydrochloride (EDC) were purchased from Sigma-Aldrich (St Louis, USA). 8-arm-polyethylene glycol amine (10 kDa, PEG-NH2) was purchased from Shanghai Seebio Biotech, Inc. (Shanghai, China). Turbofect™ transfection regent (commercial polyalkyleneimine cation TR) was purchased from Thermo Fisher Scientific (Massachusetts, USA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Laboratories (Kumamoto, Japan). Dulbecco's modified Eagle's medium (DMEM) and RPMI 1640 medium were purchased from Gibco (Grand Island, USA). Fetal bovine serum (FBS) was purchased from Hangzhou Sijiqing Biological Engineering Materials Co. Ltd (Hangzhou, China).
Human hepatoma cell line HepG2 was purchased from the Experimental Animal Center of Sun Yat-Sen University (Guangzhou, China). Human neuroblastoma cell line SH-SY5Y, human breast cancer cell line MCF-7, mouse melanoma cell line B16 and high metastatic mouse melanoma cell line B16F10 were obtained from American Type Culture Collection. Human normal hepatocyte cell line LO2, human lung cancer cell line A549, mouse breast cancer cell line EMT6 and high metastatic mouse breast cancer cell line 4T1 were obtained from Jinan University (Guangzhou, China). Rat myocardial cell line H9C2 was obtained from Guangdong Provincial People's Hospital (Guangzhou, China). Primary rabbit articular chondrocytes were prepared according to our previously reported method and identified by using toluidine blue staining [49,50].
Venus (V, #27794), C32 V (Cerulean-32-Venus, #26396), VCV (Venus-Cerulean-Venus, #27788), VCVV (Venus-Cerulean-Venus-Venus, #27789), EGFP-Bak (#32564), EGFP-Puma (#16590), EGFP-Bax 1-2/L-6 (#30533), EGFP-Bcl-xL (GBX, #64123), GFP-Bcl2 (#17999), GFP-Bcl2-Cb5 (#18000), GFP-Bcl2-Maob (#18001), GFP-NPM WT (#17578), GFP-NPM NESD (#13283), GFP-NPM NLS1/2D (#13287) and EGFP (#74165) plasmids were obtained from Addgene (Cambridge, MA, USA). FAM-labelled siRNA was purchased from GenePharma (Shanghai, China).
2.2. Synthesis of PEG−nGO
GO was prepared by a modified Hummers method using expandable graphite flake [51,52]. GO (10 mg) was sonicated with Ultrasonic Cell Crusher (JY92-2D; Xinzhi Ultrasonic Equipment Co, Ningbo, China) for 1 h in ice bath to obtain nano-GO (nGO) suspension. NaOH (1.2 g) and ClCH2COOH (1.0 g) were added to the nGO suspension and sonicated for 30 min in ice bath to obtain carboxylation of nGO (nGO−COOH). The resulting nGO−COOH suspension was washed three to five times with deionized water by using 30 kDa molecular weight cut off (MWCO) ultrafiltration device (Millipore, Bedford, MA, USA) to remove ions.
PEG−nGO was obtained just as described previously [53]. Briefly, 2 ml of EDC aqueous solution (2.0 mg ml−1) was added to 10 ml of nGO suspension (1.0 mg ml−1), and pH of the mixture was adjusted to 8.0 by 5 mM NaOH and then sonicated for another 10 min. Next, 30 mg of 8-arm PEG-NH2 was added to the above suspension, and the mixture was sonicated for 10 min and then stirred for 12 h at room temperature to obtain nGO–PEG. The resulting nGO–PEG suspension was washed three to five times with deionized water by using ultrafiltration device (30 kDa MWCO) to remove the unreacted PEG and ions.
2.3. Synthesis of PEG−nrGO−PEI
RGPP was prepared as described previously [45]. Briefly, 2 ml PEG–nGO solution (1.0 mg ml−1) was mixed with 8 ml PEI (25 kDa) solution (0.75 mg ml−1) and then bathed at 80°C for 2 h to obtain RGPP solution. The resulting RGPP solution was dialysed against deionized water in a 30 kDa MWCO dialysis membrane for 2 days. The 630 nm ultraviolet–visible (UV–vis) absorbance of cuprammonium complex formed between PEI and copper(II) ion was measured by UV–vis spectrometer (Lambda 35; PerkinElmer, MA, USA) for determining the amount of modified PEI in RGPP [54].
2.4. Cell culture and cytotoxicity assay
HepG2, SH-SY5Y, MCF-7, B16, B16F10, A549 cells and primary rabbit articular chondrocytes were cultured in high-glucose DMEM supplemented with 10% FBS. H9C2 cells were cultured in low-glucose DMEM containing 10% FBS. LO2, EMT6 and 4T1 cells were cultured in RPMI 1640 containing 10% FBS. All cells were maintained in a humidified incubator with 5% CO2 at 37°C.
Cytotoxicity of RGPP and TR as well as plasmid was assessed by CCK-8 assay according to the manufacturer's protocol just as described previously [55]. Cells were collected and seeded in 96-well plates with a density of 1 × 104 cells well−1 and incubated for 24 h. Plasmid (0.2 µg) and different amount of RGPP or TR were mixed in 20 µl serum-free media and incubated at room temperature for 20 min. Plasmid/RGPP or plasmid/TR complexes were incubated with cells for 4 h in 100 µl serum-free media. Then, serum-free media were replaced by fresh media containing 10% FBS and the cells were incubated for another 44 h before CCK-8 assay.
2.5. Transfection assay of plasmids or siRNA
Cells were seeded in 24-well plates with a density of 1 × 105 cells/well and incubated for 24 h before transfection. For siRNA transfection, 0.369 µg of FAM-labelled siRNA and different amount of RGPP or TR were mixed in 100 µl serum-free media and incubated at room temperature for 20 min. Complexes of RGPP/siRNA or TR/siRNA were incubated with cells for 4 h in 500 µl serum-free media, and then siRNA transfection efficiency was determined by fluorescence microscope imaging (Olympus IX73 equipped with a CCD camera, Japan) and flow cytometry analysis (FCM, FACSCCanto II, BD Biosciences, NJ, USA). For plasmid transfection, 1 µg of plasmid and different amount of RGPP or TR were mixed in 100 µl serum-free media and incubated at room temperature for 20 min. After the complexes of RGPP/plasmid or TR/plasmid were incubated with cells for 4 h in 500 µl serum-free media, the serum-free media were replaced by fresh media containing 10% FBS and the cells were cultured for another 44 h before fluorescence microscope imaging or FCM analysis for transfection efficiency.
2.6. Statistics
Data were presented as mean ± s.d. from at least three independent experiments. The Student's t-test was used to evaluate the significance of difference between two groups. Statistical and graphic analyses were done using the software SPSS 19.0 (SPSS, Chicago) and Origin 8.5 (OriginLab Corporation). p < 0.05 was defined as statistically significant difference.
3. Results and discussion
3.1. Transfection efficiency of RGPP on plasmids and siRNA in HepG2 cells
After exposure of cells to RGPP or TR for 48 h, CCK-8 assay showed that both RGPP and TR exhibited dose-dependent cytotoxicity, and RGPP at N/P ratio of 60 and 0.4% (TR volume/medium volume) of TR had similar cytotoxicity (figure 1a,b). RGPP at N/P ratio of 60 and 0.4% of TR were used for plasmid transfection without indication.
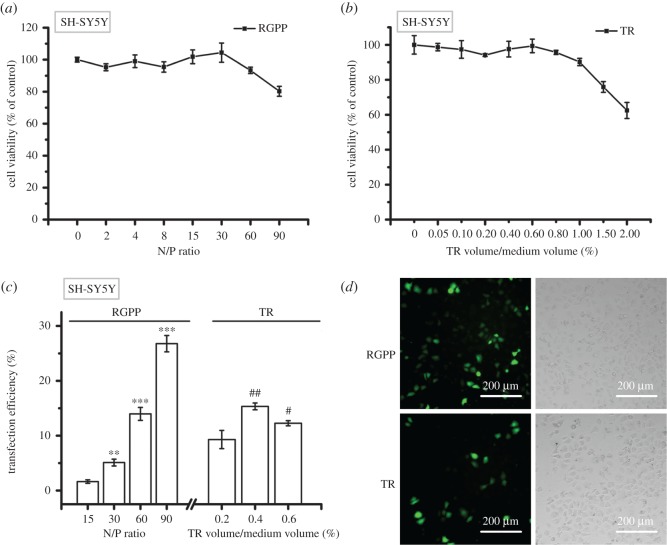
Figure 1.
Transfection efficiency of RGPP on plasmids and siRNA in HepG2 cells. (a,b) Relative cell viability of cells treated with different concentrations of RGPP or TR for 48 h determined by CCK-8 assay. (c) Transfection efficiency of RGPP or TR for various plasmids after transfection for 48 h determined by FCM analysis. *p < 0.05 and **p < 0.01, compared with RGPP group. (d) Fluorescence microscopic images of cells exclusively transfected with GFP-Bak, GFP-Puma, GFP-Bax 1-2/L-6, GFP-Bcl-xL and GFP-NPM NLS1/2D plasmids by using RGPP at N/P ratio of 60 or 0.4% of TR for 48 h. Scale bar, 200 µm. (e) Transfection efficiency of RGPP or TR on siRNA after transfection for 4 h determined by FCM analysis. ***p < 0.001, compared with RGPP at N/P ratio of 5; ##p < 0.01, compared with 0.1% of TR. (f) Fluorescence microscopic images of cells transfected with siRNA by using RGPP at N/P ratio of 15 or 0.2% of TR for 4 h. Scale bar, 200 µm.
We evaluated the transfection ability of RGPP/TR for 10 functional gene plasmids by using FCM analysis. FCM analysis showed that RGPP had higher transfection efficiency than TR for GFP-Bak (23.8 ± 2.6% for RGPP, 14.2 ± 4.2% for TR), GFP-Puma (20.9 ± 2.9% for RGPP, 6.3 ± 0.9% for TR), GFP-Bax 1-2/L-6 (25.5 ± 3.7% for RGPP, 15.1 ± 0.3% for TR), GFP-Bcl-xL (18.9 ± 0.6% for RGPP, 4.7 ± 0.4% for TR) and GFP-NPM NLS1/2D (30.3 ± 2.9% for RGPP, 20.8 ± 2.6% for TR) plasmids (figure 1c). Fluorescence microscopic images of cells exclusively transfected GFP-Bak, GFP-Puma, GFP-Bax 1-2/L-6, GFP-Bcl-xL and GFP-NPM NLS1/2D plasmids by using RGPP and TR for 48 h also demonstrated that RGPP showed higher delivery ability for these five plasmids than TR (figure 1d). We also determined the transfection ability of both RGPP and TR for V, C32 V, VCV and VCVV plasmids, and found that both RGPP and TR efficiently delivered these plasmids into cells, and the transfection ability of TR was slightly better than that of RGPP (figure 1c). After the complexes of RGPP/siRNA or TR/siRNA were incubated with cells for 4 h, FCM analysis showed that RGPP at N/P ratios of 10 and 15 exhibited 43.4 ± 2.1% and 62.8 ± 2.4% of siRNA transfection efficiency, much higher than that of 0.1% and 0.2% of TR (figure 1e). Fluorescence microscopic images also showed that RGPP at N/P ratio of 15 delivered more siRNA into cells than 0.2% of TR (figure 1f).
Size of plasmid is one of the elements influencing transfection efficiency during transient transfection assay [56,57]. Transfection efficiency of RGPP for GFP-Bcl-xL plasmid (9.8 kb) was about 18.9%, about threefold higher than TR (about 4.7%; figure 1c), suggesting that RGPP exhibited better delivery ability for large-size plasmid than TR. It was reported that huge aggregates (greater than 1000 nm) of DNA/transferrin-PEI exhibited much higher transfection efficiency than small aggregates in in vitro transfection assay [58]. Diameter of the GFP-Bcl-xL plasmid/RGPP complexes was about 987 nm, and the particle size of DNA/TR was 50–150 nm [59], which might be the reason why RGPP more efficiently delivered large-size plasmid into cells than TR. In addition, RGPP also exhibited better delivery ability for siRNA than TR (figure 1e,f), which might benefit from the inhibitory effect of RGPP on gene degradation [30].
3.2. Transfection efficiency of RGPP on GFP plasmid in human cancer cell lines
We evaluated the delivery ability of RGPP on GFP plasmid by using FCM analysis in three human cancer cell lines: human neuroblastoma cell line (SH-SY5Y), human lung cancer cell line (A549) and human breast cancer cell line (MCF-7). Figure 2a,b shows the cytotoxicity of different doses of RGPP or TR in SH-SY5Y cells, and RGPP at N/P ratios of 15, 30, 60 and 90, respectively, exhibited 2.1 ± 0.3%, 5.1 ± 0.6%, 14.0 ± 1.2% and 27.2 ± 1.5% of transfection efficiency, while 0.2%, 0.4% and 0.6% of TR, respectively, showed 9.3 ± 1.7%, 15.0 ± 0.6% and 12.3 ± 0.5% of transfection efficiency (figure 2c). RGPP at N/P ratio of 60 and 0.4% of TR exhibited similar cytotoxicity (about 4.5%) and transfection efficiency (about 14.0%) in SH-SY5Y cells (figure 2a–c). RGPP at N/P ratio of 90 showed the highest transfection efficiency (about 27.2%), about 1.8-fold that of 0.4% of TR (about 15.0%; figure 2c), suggesting that RGPP at N/P ratio of 90 was superior to 0.4% of TR as a gene delivery carrier for SH-SY5Y cells. Fluorescence microscopic images also showed that RGPP at N/P ratio of 90 delivered more GFP plasmid into SH-SY5Y cells than 0.4% of TR (figure 2d).
Figure 2.
Transfection efficiency of RGPP on GFP plasmid in SH-SY5Y cells. (a,b) Relative cell viability of cells treated with different concentrations of RGPP or TR for 48 h determined by CCK-8 assay. (c) Transfection efficiency of RGPP or TR on GFP plasmid after transfection for 48 h determined by FCM analysis. **p < 0.01 and ***p < 0.001, compared with RGPP at N/P ratio of 15; #p < 0.05 and ##p < 0.01, compared with 0.2% of TR. (d) Fluorescence microscopic images of cells transfected with GFP plasmid by using RGPP at N/P ratio of 90 or 0.4% of TR for 48 h. Scale bar, 200 µm.
The cytotoxicity and transfection efficiency of RGPP and TR in A549 and MCF-7 cells are listed in table 1. In A549 cells, RGPP at N/P ratios of 15, 30, 60 and 90, respectively, exhibited 9.7 ± 1.2%, 15.0 ± 1.0%, 27.3 ± 1.0% and 45.4 ± 3.6% of transfection efficiency with 13.2 ± 0.1%, 13.1 ± 2.4%, 17.8 ± 4.5% and 33.3 ± 8.5% of cytotoxicity, and 0.2% and 0.4% of TR showed 5.2 ± 0.4% and 30.1 ± 2.0% of transfection efficiency without cytotoxicity, and 0.6% of TR exhibited 18.0 ± 2.1% of transfection efficiency with 10.0 ± 2.0% of cytotoxicity (table 1). In MCF-7 cells, RGPP at N/P ratios of 15, 30 and 60, respectively, exhibited 5.5 ± 0.2%, 7.0 ± 1.0% and 11.4 ± 1.8% of transfection efficiency with 5.2 ± 4.6%, 7.7 ± 1.0% and 10.0 ± 5.2% of cytotoxicity, while 0.2%, 0.4% and 0.6% of TR, respectively, had 21.4 ± 0.3%, 18.9 ± 0.6% and 16.0 ± 1.0% of transfection efficiency with 14.1 ± 8.4%, 17.4 ± 6.6% and 14.3 ± 5.2% of cytotoxicity (table 1).
Table 1.
Transfection efficiency (TE) of RGPP on GFP plasmid in A549 and MCF-7 cell lines (CV, cell viability).
| RGPP (N/P ratio) |
TR (TR volume/medium volume) (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | 90 | 0.2 | 0.4 | 0.6 | ||
| A549 | CV (%) | 86.8 ± 0.1 | 86.9 ± 2.4 | 82.2 ± 4.5 | 66.7 ± 8.5 | 99.9 ± 3.7 | 95.4 ± 4.3 | 90.0 ± 2.0 |
| TE (%) | 9.7 ± 1.2 | 15.0 ± 1.0 | 27.3 ± 1.0 | 45.4 ± 3.6 | 5.2 ± 0.4 | 30.1 ± 2.0 | 18.0 ± 2.1 | |
| MCF-7 | CV (%) | 94.8 ± 4.6 | 92.3 ± 1.0 | 90.0 ± 5.2 | 79.1 ± 4.0 | 85.9 ± 8.4 | 82.6 ± 6.6 | 85.7 ± 5.2 |
| TE (%) | 5.5 ± 0.2 | 7.0 ± 1.0 | 11.4 ± 1.8 | 16.6 ± 0.1 | 21.4 ± 0.3 | 18.9 ± 0.6 | 16.0 ± 1.0 | |
3.3. Transfection efficiency of RGPP on GFP plasmid in mouse cancer cell lines
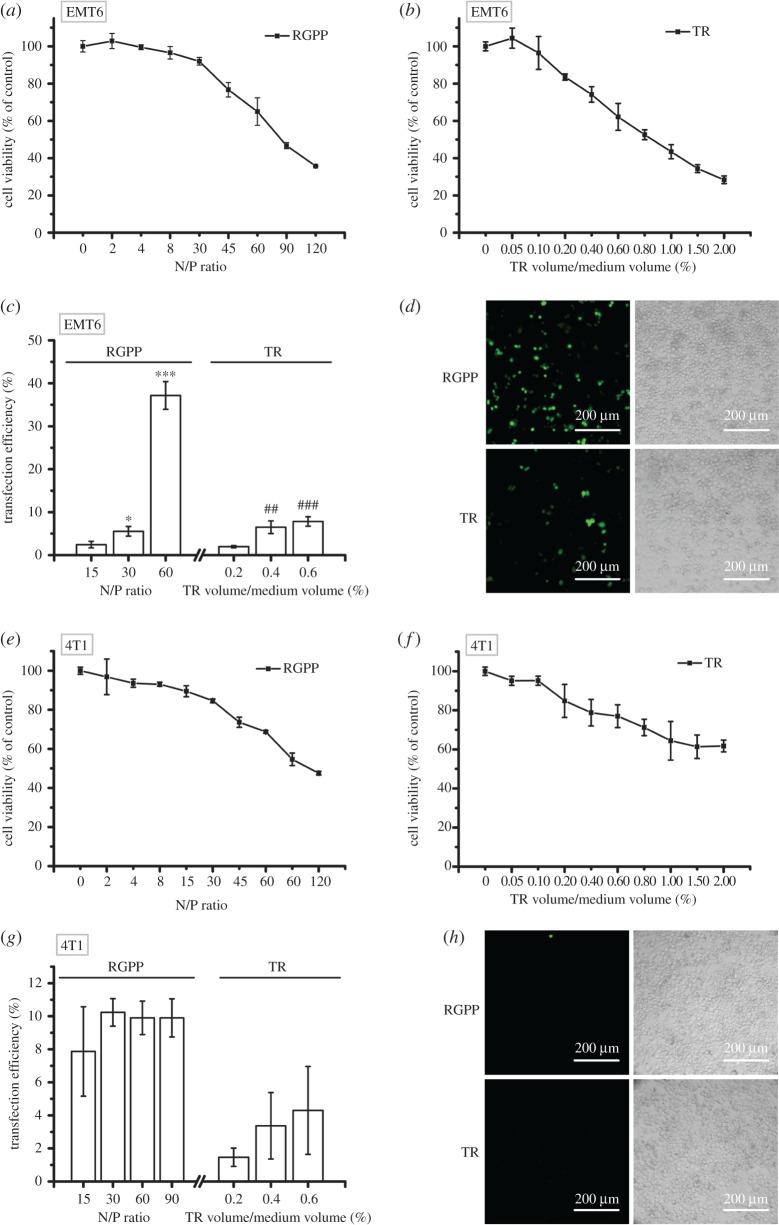
We next evaluated the delivery ability of RGPP on GFP plasmid in four mouse cancer cell lines: mouse breast cancer cell line (EMT6), high metastatic mouse breast cancer cell line (4T1), mouse melanoma cell line (B16) and high metastatic mouse melanoma cell line (B16F10). As shown in figure 3a,b, both RGPP and TR exhibited dose-dependent cytotoxicity in EMT6 cells, and RGPP at N/P ratios of 15, 30 and 60, respectively, had 2.4 ± 0.8%, 5.5 ± 1.1% and 37.2 ± 3.2% of transfection efficiency, and 0.2%, 0.4% and 0.6% of TR, respectively, had 2.0 ± 0.3%, 6.5 ± 1.5% and 7.8 ± 1.1% of transfection efficiency (figure 3c). In EMT6 cells, RGPP at N/P ratio of 60 and 0.6% of TR exhibited about 35% of cytotoxicity, but transfection efficiency of RGPP at N/P ratio of 60 (about 37.2%) was about fourfold higher than that of 0.6% of TR (about 7.8%; figure 3a–d). Similarly in 4T1 cells, both RGPP and TR also exhibited dose-dependent cytotoxicity (figure 3e,f), and RGPP at N/P ratios of 15, 30, 60 and 90, respectively, exhibited 7.9 ± 2.7%, 10.2 ± 0.8%, 9.9 ± 1.0% and 9.9 ± 1.2% of transfection efficiency, and 0.2%, 0.4% and 0.6% of TR, respectively, had 1.5 ± 0.6%, 3.4 ± 2.0% and 4.3 ± 2.7% of transfection efficiency (figure 3g). Both RGPP and TR showed less than 10% of transfection efficiency indicating that both RGPP and TR were inefficient in delivering plasmid into 4T1 cells. Fluorescence microscopic images also showed that both RGPP and TR were inefficient in delivering plasmid into 4T1 cells (figure 3h).
Figure 3.
Transfection efficiency of RGPP on GFP plasmid in mouse cancer cell lines. (a,b) Relative cell viability of EMT6 cells treated with different concentrations of RGPP or TR for 48 h determined by CCK-8 assay. (c) Transfection efficiency of RGPP or TR on GFP plasmid after transfection for 48 h in EMT6 cells determined by FCM analysis. *p < 0.05 and ***p < 0.001, compared with RGPP at N/P ratio of 15; ##p < 0.01 and ###p < 0.001, compared with 0.2% of TR. (d) Fluorescence microscopic images of EMT6 cells transfected with GFP plasmid by using RGPP at N/P ratio of 60 or 0.6% of TR for 48 h. Scale bar, 200 µm. (e,f) Relative cell viability of 4T1 cells treated with different concentrations of RGPP or TR for 48 h determined by CCK-8 assay. (g) Transfection efficiency of RGPP or TR on GFP plasmid after transfection for 48 h in 4T1 cells determined by FCM analysis. (h) Fluorescence microscopic images of 4T1 cells transfected with GFP plasmid by using RGPP at N/P ratio of 30 or 0.6% of TR for 48 h. Scale bar, 200 µm.
The cytotoxicity and transfection efficiency of RGPP and TR in B16F10 and B16 cells are listed in table 2. In B16F10 cells, RGPP at N/P ratios of 15, 30, 60 and 90, respectively, exhibited 8.7 ± 1.4%, 9.3 ± 0.7%, 18.1 ± 1.8% and 32.4 ± 1.3% of transfection efficiency with 17.3 ± 6.0%, 29.2 ± 4.6%, 36.9 ± 1.9% and 44.1 ± 1.2% of cytotoxicity, and 0.2%, 0.4% and 0.6% of TR, respectively, had 31.8 ± 1.9%, 33.7 ± 0.9% and 25.9 ± 0.1% of transfection efficiency with 20.3 ± 1.9%, 40.4 ± 3.2% and 40.9 ± 2.8% of cytotoxicity (table 2). In B16 cells, RGPP at N/P ratios of 30, 60 and 90, respectively, exhibited 3.4 ± 0.2%, 17.0 ± 0.1% and 32.8 ± 4.1% of transfection efficiency with 3.3 ± 9.1%, 9.5 ± 3.9% and 39.9 ± 5.4% of cytotoxicity, while 0.2%, 0.4% and 0.6% of TR, respectively, exhibited 4.3 ± 3.2%, 19.7 ± 0.4% and 16.2 ± 0.9% of transfection efficiency without cytotoxicity (table 2). Therefore, compared with RGPP, TR was more suitable as a gene delivery carrier in B16F10 (0.2% of TR) and B16 (0.4% of TR) cell lines.
Table 2.
Transfection efficiency (TE) of RGPP on GFP plasmid in B16F10 and B16 cell lines (CV, cell viability).
| RGPP (N/P ratio) |
TR (TR volume/medium volume) (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | 90 | 0.2 | 0.4 | 0.6 | ||
| B16F10 | CV (%) | 82.7 ± 6.0 | 70.8 ± 4.6 | 63.1 ± 1.9 | 55.9 ± 1.2 | 79.7 ± 1.9 | 59.6 ± 3.2 | 59.1 ± 2.8 |
| TE (%) | 8.7 ± 1.4 | 9.3 ± 0.7 | 18.1 ± 1.8 | 32.4 ± 1.3 | 31.8 ± 1.9 | 33.7 ± 0.9 | 25.9 ± 0.1 | |
| B16 | CV (%) | 108.0 ± 13.8 | 96.7 ± 9.1 | 90.5 ± 3.9 | 60.1 ± 5.4 | 106.5 ± 21.4 | 104.2 ± 12.6 | 116.5 ± 33.6 |
| TE (%) | 2.1 ± 0.4 | 3.4 ± 0.2 | 17.0 ± 0.1 | 32.8 ± 4.1 | 4.3 ± 3.2 | 19.7 ± 0.4 | 16.2 ± 0.9 | |
3.4. Transfection efficiency of RGPP on GFP plasmid in normal cells
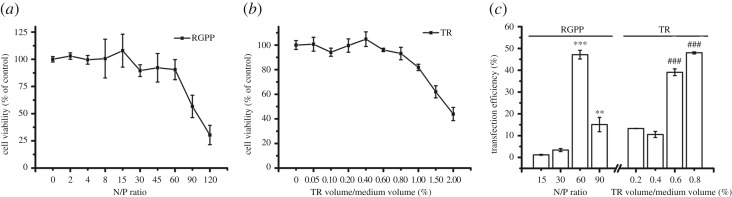
Finally, we evaluated the delivery ability of RGPP on GFP plasmid in normal cells including human normal hepatocyte cell line (LO2), rat myocardial cell line (H9C2) and primary rabbit articular chondrocytes. In LO2 cells, RGPP at N/P ratios of 15, 30 and 45, respectively, exhibited 1.6 ± 0.4%, 3.6 ± 0.3% and 26.1 ± 2.8% of transfection efficiency with 14.2 ± 7.1%, 27.0 ± 6.7% and 32.6 ± 0.3% of cytotoxicity, and 0.2%, 0.4% and 0.6% of TR, respectively, showed 6.2 ± 0.9%, 12.6 ± 2.9% and 16.3 ± 3.0% of transfection efficiency with 7.3 ± 6.3%, 32.6 ± 12.5% and 54.2 ± 4.6% of cytotoxicity (figure 4a–c). Both RGPP at N/P ratio of 45 and 0.4% of TR had about 32.6% of cytotoxicity, but the transfection efficiency of RGPP at N/P ratio of 45 was about 26.1%, about twofold that of 0.4% of TR (about 12.6%) in LO2 cells (figure 4a–c). In H9C2 cells, RGPP at N/P ratios of 15 and 30, respectively, exhibited only 1.4 ± 0.4% and 2.0 ± 1.0% of transfection efficiency with 7.5 ± 2.4% and 18.7 ± 0.6% of cytotoxicity, and 0.2% and 0.4% of TR exhibited 0.8 ± 0.3% and 3.2 ± 0.4% of transfection efficiency without cytotoxicity, and 0.6% of TR had 2.8 ± 0.8% of transfection efficiency with 7.9 ± 8.4% of cytotoxicity (figure 4d–f). Transfection efficiencies of RGPP and TR were less than 4% (figure 4f), indicating that both RGPP and TR were inefficient in delivering plasmid into H9C2 cells. In primary rabbit articular chondrocytes, RGPP at N/P ratios of 15, 30, 60 and 90, respectively, had 1.2 ± 0.2%, 3.4 ± 0.7%, 47.1 ± 2.0% and 15.1 ± 3.3% of transfection efficiency, and 0.2%, 0.4%, 0.6% and 0.8% of TR, respectively, exhibited 13.3 ± 0.1%, 10.5 ± 1.4%, 39.0 ± 1.5% and 48.0 ± 0.5% of transfection efficiency (figure 5c). RGPP at N/P ratio of 60 and 0.8% of TR exhibited similar cytotoxicity (about 8.5%) and transfection efficiency (47.1 ± 2.0% for RGPP, 48.0 ± 0.5% for TR) in primary rabbit articular chondrocytes (figure 5a–c). Therefore, both RGPP at N/P ratio of 60 and 0.8% of TR were excellent gene delivery carriers for primary rabbit articular chondrocytes.
Figure 4.
Transfection efficiency of RGPP on GFP plasmid in LO2 cells and H9C2 cells. (a,b) Relative cell viability of LO2 cells treated with different concentrations of RGPP or TR for 48 h determined by CCK-8 assay. (c) Transfection efficiency of RGPP or TR on GFP plasmid after transfection for 48 h in LO2 cells determined by FCM analysis. *p < 0.05 and ***p < 0.001, compared with RGPP at N/P ratio of 15; #p < 0.05 and ##p < 0.01, compared with 0.2% of TR. (d,e) Relative cell viability of H9C2 cells treated with different concentrations of RGPP or TR for 48 h determined by CCK-8 assay. (f) Transfection efficiency of RGPP or TR on GFP plasmid after transfection for 48 h in H9C2 cells determined by FCM analysis. #p < 0.05 and ##p < 0.01, compared with 0.2% of TR.
Figure 5.
Transfection efficiency of RGPP on GFP plasmid in primary rabbit articular chondrocyte. (a,b) Relative cell viability of rabbit articular chondrocyte treated with different concentrations of RGPP or TR for 48 h determined by CCK-8 assay. (c) Transfection efficiency of RGPP or TR on GFP plasmid after transfection for 48 h in rabbit articular chondrocyte determined by FCM analysis. **p < 0.01 and ***p < 0.001, compared with RGPP at N/P ratio of 15; ###p < 0.001, compared with 0.2% of TR.
4. Conclusion
The transfection efficiency of RGPP for GFP plasmid in 10 different cell lines summarized in table 3 demonstrates that RGPP is a potential gene delivery carrier. Especially, RGPP can efficiently deliver siRNA and large-size plasmids. RGPP exhibits efficient plasmid delivery ability in primary rabbit articular chondrocyte, SH-SY5Y, A549, EMT6 and LO2 cell lines. Moreover, based on the excellent photothermal efficiency of reduced GO (rGO), NIR enhances gene delivery ability of RGPP. Collectively, RGPP is a potential nano-carrier for high-efficiency gene delivery and further studies are needed to optimize its gene delivery ability for different cell lines.
Table 3.
Cytotoxicity and transfection efficiency (TE) of RGPP and TR at optimum dose in ten different cell lines. Cytotoxicity = 1 − (cell viability); RAC, rabbit articular chondrocytes.
| RGPP |
TR |
|||||
|---|---|---|---|---|---|---|
| cell line | N/P ratio | cytotoxicity (%) | TE (%) | TR volume/medium volume (%) | cytotoxicity (%) | TE (%) |
| SH-SY5Y | 90 | 20.0 ± 3.1 | 27.2 ± 1.5 | 0.4 | 3.0 ± 4.5 | 15.0 ± 0.6 |
| A549 | 60 | 18.0 ± 4.5 | 27.3 ± 1.0 | 0.4 | 4.6 ± 4.3 | 30.1 ± 2.0 |
| MCF-7 | 90 | 20.0 ± 4.0 | 16.6 ± 0.1 | 0.2 | 14.1 ± 8.4 | 21.4 ± 0.3 |
| EMT6 | 60 | 34.0 ± 7.4 | 37.2 ± 3.2 | 0.6 | 38.0 ± 7.2 | 7.8 ± 1.1 |
| 4T1 | 30 | 19.0 ± 1.0 | 10.2 ± 0.8 | 0.6 | 25.0 ± 5.8 | 4.3 ± 2.7 |
| B16F10 | 60 | 37.0 ± 1.9 | 18.1 ± 1.8 | 0.2 | 20.3 ± 1.9 | 31.8 ± 1.9 |
| B16 | 60 | 9.5 ± 3.9 | 17.0 ± 0.1 | 0.4 | −4.2 ± 12.6 | 19.7 ± 0.4 |
| LO2 | 45 | 32.6 ± 0.3 | 26.0 ± 2.8 | 0.6 | 54.2 ± 4.6 | 16.3 ± 3.0 |
| H9C2 | 30 | 20.0 ± 0.6 | 2.0 ± 1.0 | 0.4 | −2.0 ± 13.4 | 3.2 ± 0.4 |
| RAC | 60 | 9.5 ± 9.3 | 47.1 ± 2.0 | 0.8 | 8.0 ± 5.1 | 48.0 ± 0.5 |
Supplementary Material
Supplementary Material
Acknowledgements
We express our thanks to the Experimental Animal Center of Sun Yat-Sen University, American Type Culture Collection, Jinan University and Guangdong Provincial People's Hospital for providing the cell strains, and thanks to Addgene for providing the plasmids.
Data accessibility
Raw data for experiments are found in the electronic supplementary material.
Authors' contributions
L.P.W., X.P.W. and T.S.C. designed the study and wrote the manuscript. J.S.X. and T.L. synthesized and characterized the material. L.P.W., Z.H.M. and L.W. carried out the transfection experiments. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (61527825 and 81471699) and the Guangdong Province Science and Technology Plan Project (2014B090901060).
References
- 1.Wong JK, Mohseni R, Hamidieh AA, Maclaren RE, Habib N, Seifalian AM. 2017. Will nanotechnology bring new hope for gene delivery? Trends Biotechnol. 35, 434–451. (doi:10.1016/j.tibtech.2016.12.009) [DOI] [PubMed] [Google Scholar]
- 2.Mulligan RC. 1993. The basic science of gene therapy. Science 260, 926–932. (doi:10.1126/science.8493530) [DOI] [PubMed] [Google Scholar]
- 3.Dyer MR, Herrling PL. 2000. Progress and potential for gene-based medicines. Mol. Ther. 1, 213–224. (doi:10.1006/mthe.2000.0044) [DOI] [PubMed] [Google Scholar]
- 4.Onuki R, Nagasaki A, Kawasaki H, Baba T, Uyeda TQ, Taira K. 2002. Confirmation by FRET in individual living cells of the absence of significant amyloid β-mediated caspase 8 activation. Proc. Natl Acad. Sci. USA 99, 14 716–14 721. (doi:10.1073/pnas.232177599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takemoto K, Nagai T, Miyawaki A, Miura M. 2003. Spatio-temporal activation of caspase revealed by indicator that is insensitive to environmental effects. J. Cell Biol. 160, 235–243. (doi:10.1083/jcb.200207111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mochizuki N, Yamashita S, Kurokawa K, Ohba Y, Nagai T, Miyawaki A, Matsuda M. 2001. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 411, 1065–1068. (doi:10.1038/35082594) [DOI] [PubMed] [Google Scholar]
- 7.Gaidukov L, Nager AR, Xu S, Penman M, Krieger M. 2011. Glycine dimerization motif in the N-terminal transmembrane domain of the high density lipoprotein receptor SR-BI required for normal receptor oligomerization and lipid transport. J. Biol. Chem. 286, 18 452–18 464. (doi:10.1074/jbc.M111.229872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurel D, et al. 2008. Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat. Methods 5, 561–567. (doi:10.1038/nmeth.1213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins BC, Gillet LC, Rosenberger G, Röst HL, Vichalkovski A, Gstaiger M, Aebersold R. 2013. Quantifying protein interaction dynamics by SWATH mass spectrometry: application to the 14-3-3 system. Nat. Methods 10, 1246–1253. (doi:10.1038/nmeth.2703) [DOI] [PubMed] [Google Scholar]
- 10.Ivanusic D, Eschricht M, Denner J. 2014. Investigation of membrane protein-protein interactions using correlative FRET-PLA. Biotechniques 57, 188–198. (doi:10.2144/000114215) [DOI] [PubMed] [Google Scholar]
- 11.Rubinson DA, et al. 2003. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 33, 401–406. (doi:10.1038/ng1117) [DOI] [PubMed] [Google Scholar]
- 12.Nakayashiki H, Hanada S, Nguyen BQ, Kadotani N, Tosa Y, Mayama S. 2005. RNA silencing as a tool for exploring gene function in ascomycete fungi. Fungal Genet. Biol. 42, 275–283. (doi:10.1016/j.fgb.2005.01.002) [DOI] [PubMed] [Google Scholar]
- 13.Burch-Smith T, Anderson J, Martin GB, Dinesh-Kumar SP. 2004. Applications and advantages of virus-induced gene silencing for gene function studies in plants. Plant J. 39, 734–746. (doi:10.1111/j.1365-313X.2004.02158.x) [DOI] [PubMed] [Google Scholar]
- 14.Cerutti H, Ma X, Msanne J, Repas T. 2011. RNA-mediated silencing in algae: biological roles and tools for analysis of gene function. Eukaryot. Cell 10, 1164–1172. (doi:10.1128/EC.05106-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu PD, Lander ES, Zhang F. 2014. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278. (doi:10.1016/j.cell.2014.05.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalem O, et al. 2014. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87. (doi:10.1126/science.1247005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, Wei JJ, Sabatini DM, Lander ES. 2014. Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84. (doi:10.1126/science.1246981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sander JD, Joung JK. 2014. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355. (doi:10.1038/nbt.2842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. 2013. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918. (doi:10.1016/j.cell.2013.04.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niu Y, et al. 2014. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156, 836–843. (doi:10.1016/j.cell.2014.01.027) [DOI] [PubMed] [Google Scholar]
- 21.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183. (doi:10.1016/j.cell.2013.02.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH, Joung JK. 2013. CRISPR RNA-guided activation of endogenous human genes. Nat. Methods 10, 977–979. (doi:10.1038/nmeth.2598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM. 2013. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31, 833–838. (doi:10.1038/nbt.2675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufmann KB, Büning H, Galy A, Schambach A, Grez M. 2013. Gene therapy on the move. EMBO Mol. Med. 5, 1642–1661. (doi:10.1002/emmm.201202287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathwani AC, et al. 2014. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. New Engl. J. Med. 371, 1994–2004. (doi:10.1056/NEJMoa1407309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavazzana-Calvo M, et al. 2010. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 467, 318–322. (doi:10.1038/nature09328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burnett JC, Zaia JA, Rossi JJ. 2012. Creating genetic resistance to HIV. Curr. Opin. Immunol. 24, 625–632. (doi:10.1016/j.coi.2012.08.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplitt MG, et al. 2007. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet 369, 2097–2105. (doi:10.1016/S0140-6736(07)60982-9) [DOI] [PubMed] [Google Scholar]
- 29.Jin L, Zeng X, Liu M, Deng Y, He N. 2014. Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics 4, 240–255. (doi:10.7150/thno.6914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boussif O, Lezoualćh F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. 1995. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA 92, 7297–7301. (doi:10.1073/pnas.92.16.7297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li F, Wang Z, Huang Y, Xu H, He L, Deng Y, Zeng X, He N. 2015. Delivery of PUMA apoptosis gene usingpolyethyleneimine-SMCC-TAT/DNA nanoparticles: biophysical characterization and in vitro transfection into malignant, melanoma cells. J. Biomed. Nanotechnol. 11, 1776–1782. (doi:10.1166/jbn.2015.2151) [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Yadava P, Hughes J. 2004. Polyethylenimine strategies for plasmid delivery to brain-derived cells. Methods 33, 144–150. (doi:10.1016/j.ymeth.2003.11.004) [DOI] [PubMed] [Google Scholar]
- 33.Godbey WT, Wu KK, Mikos AG. 1999. Poly(ethylenimine) and its role in gene delivery. J. Control. Release 60, 149–160. (doi:10.1016/S0168-3659(99)00090-5) [DOI] [PubMed] [Google Scholar]
- 34.Merlin JL, ŃDoye A, Bouriez T, Dolivet G. 2002. Polyethylenimine derivatives as potent nonviral vectors for gene transfer. Drug News Perspect. 15, 445–451. (doi:10.1358/dnp.2002.15.7.840080) [DOI] [PubMed] [Google Scholar]
- 35.Godbey WT, Wu KK, Mikos AG. 1999. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc. Natl Acad. Sci. USA 96, 5177–5181. (doi:10.1073/pnas.96.9.5177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bieber T, Meissner W, Kostin S, Niemann A, Elsasser HP. 2002. Intracellular route and transcriptional competence of polyethylenimine-DNA complexes. J. Control. Release 82, 441–454. (doi:10.1016/S0168-3659(02)00129-3) [DOI] [PubMed] [Google Scholar]
- 37.Fischer D, Bieber T, Li Y, Elsässer HP, Kissel T. 1999. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 16, 1273–1279. (doi:10.1023/A:1014861900478) [DOI] [PubMed] [Google Scholar]
- 38.Feng L, Zhang S, Liu Z. 2011. Graphene based gene transfection. Nanoscale 3, 1252–1257. (doi:10.1039/c0nr00680g) [DOI] [PubMed] [Google Scholar]
- 39.Kim H, Namgung R, Singha K, Oh IK, Kim WJ. 2011. Graphene oxide-polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjug. Chem. 22, 2558–2567. (doi:10.1021/bc200397j) [DOI] [PubMed] [Google Scholar]
- 40.Yan L, et al. 2013. The use of polyethylenimine-modified graphene oxide as a nanocarrier for transferring hydrophobic nanocrystals into water to produce water-dispersible hybrids for use in drug delivery. Carbon 57, 120–129. (doi:10.1016/j.carbon.2013.01.042) [Google Scholar]
- 41.Zhang L, Wang Z, Lu Z, Shen H, Huang J, Liu M, He N, Zhang Z. 2013. PEGylated reduced graphene oxide as a superior ssRNA delivery system. J. Mater. Chem. B 1, 749–755. (doi:10.1039/c2tb00096b) [DOI] [PubMed] [Google Scholar]
- 42.Yin D, et al. 2013. Functional graphene oxide as a plasmid-based Stat3 siRNA carrier inhibits mouse malignant melanoma growth in vivo. Nanotechnology 24, 105102 (doi:10.1088/0957-4484/24/10/105102) [DOI] [PubMed] [Google Scholar]
- 43.Feng L, Yang X, Shi X, Tan X, Peng R, Wang J, Liu Z. 2013. Polyethylene glycol and polyethylenimine dual-functionalized nano-graphene oxide for photothermally enhanced gene delivery. Small 9, 1989–1997. (doi:10.1002/smll.201202538) [DOI] [PubMed] [Google Scholar]
- 44.Kim H, Kim WJ. 2014. Graphene oxide: photothermally controlled gene delivery by reduced graphene oxide-polyethylenimine nanocomposite. Small 10, 117–126. (doi:10.1002/smll.201202636) [DOI] [PubMed] [Google Scholar]
- 45.Li T, Wu L, Zhang J, Xi G, Pang Y, Wang X, Chen T. 2016. Hydrothermal reduction of polyethylenimine and polyethylene glycol dual-functionalized nanographene oxide for high-efficiency gene delivery. ACS Appl. Mater. Interfaces 8, 31 311–31 320. (doi:10.1021/acsami.6b09915) [DOI] [PubMed] [Google Scholar]
- 46.Wang C, et al. 2016. Multi-functionalized graphene oxide complex as a plasmid delivery system for targeting hepatocellular carcinoma therapy. RSC Adv. 6, 22 461–22 468. (doi:10.1039/c5ra21475k) [Google Scholar]
- 47.Yin D, et al. 2016. Plasmid-based Stat3 siRNA delivered by functional graphene oxide suppresses mouse malignant melanoma cell growth. Oncol. Res. 23, 229–236. (doi:10.3727/096504016X14550280421449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Feng L, Tan X, Shi X, Xu L, Liu Z, Peng R. 2013. Dual-polymer-functionalized nanoscale graphene oxide as a highly effective gene transfection agent for insect cells with cell-type-dependent cellular uptake mechanisms. Part. Part. Syst. Charact. 30, 794–803. (doi:10.1002/ppsc.201300107) [Google Scholar]
- 49.Quan YY, Qin GQ, Huang H, Liu YH, Wang XP, Chen TS. 2016. Dominant roles of Fenton reaction in sodium nitroprusside-induced chondrocyte apoptosis. Free Radic. Biol. Med. 94, 135–144. (doi:10.1016/j.freeradbiomed.2016.02.026) [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Liu X, Duan L, Li X, Zhang Y, Zhou Q. 2011. The effects of velvet antler polypeptides on the phenotype and related biological indicators of osteoarthritic rabbit chondrocytes. Acta Biochim. Pol. 58, 297–302. [PubMed] [Google Scholar]
- 51.Hummers WS Jr, Offeman RE. 1958. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339 (doi:10.1021/ja01539a017) [Google Scholar]
- 52.Dikin DA, Stankovich S, Zimney EJ, Piner RD, Dommett GH, Evmenenko G, Nguyen ST, Ruoff RS. 2007. Preparation and characterization of graphene oxide paper. Nature 448, 457–460. (doi:10.1038/nature06016) [DOI] [PubMed] [Google Scholar]
- 53.Li T, Liu H, Xi G, Pang Y, Wu L, Wang X, Chen T. 2016. One-step reduction and PEIylation of PEGylated nanographene oxide for highly efficient chemo-photothermal therapy. J. Mater. Chem. B 4, 2972–2983. (doi:10.1039/c6tb00486e) [DOI] [PubMed] [Google Scholar]
- 54.Ungaro F, De Rosa G, Miro A, Quaglia F. 2003. Spectrophotometric determination of polyethylenimine in the presence of an oligonucleotide for the characterization of controlled release formulations. J. Pharm. Biomed. Anal. 31, 143–149. (doi:10.1016/S0731-7085(02)00571-X) [DOI] [PubMed] [Google Scholar]
- 55.Zhao C, Qin G, Gao W, Chen J, Liu H, Xi G, Li T, Wu S, Chen T. 2014. Potent proapoptotic actions of dihydroartemisinin in gemcitabine-resistant A549 cells. Cell. Signal. 26, 2223–2233. (doi:10.1016/j.cellsig.2014.07.001) [DOI] [PubMed] [Google Scholar]
- 56.Yin W, Xiang P, Li Q. 2005. Investigations of the effect of DNA size in transient transfection assay using dual luciferase system. Anal. Biochem. 346, 289–294. (doi:10.1016/j.ab.2005.08.029) [DOI] [PubMed] [Google Scholar]
- 57.Xu X, Capito RM, Spector M. 2008. Plasmid size influences chitosan nanoparticle mediated gene transfer to chondrocytes. J. Biomed. Mater. Res. A 84, 1038–1048. (doi:10.1002/jbm.a.31479) [DOI] [PubMed] [Google Scholar]
- 58.Ogris M, Steinlein P, Kursa M, Mechtler K, Kircheis R, Wagner E. 1998. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 5, 1425–1433. (doi:10.1038/sj.gt.3300745) [DOI] [PubMed] [Google Scholar]
- 59.Lagunavicius ZA, Riauba S, Zaliauskiene L, Makuska L, Vareikis R, Bernadisiute AU. 2010. Transfection agent. US Patent no. 20100041739. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data for experiments are found in the electronic supplementary material.