Abstract
In this paper, we fabricated a TiO2 homogeneous hybrid structure for application in perovskite solar cells (PSCs) under ambient conditions. Under the standard air mass 1.5 global (AM 1.5G) illumination, PSCs based on homogeneous hybrid structure present a maximum power conversion efficiency of 5.39% which is higher than that of pure TiO2 nanosheets. The enhanced properties can be explained by the better contact of TiO2 nanosheets/nanoparticles with CH3NH3PbI3 and fewer pinholes in electron transport materials. The advent of such unique structure opens up new avenues for the future development of high-efficiency photovoltaic cells.
Keywords: TiO2 homogeneous hybrid structure, CH3NH3PbI3, perovskite solar cells
1. Introduction
TiO2 is an optimized candidate for the development of nanoscale architectures due to its appropriate electronic band structure, photostability, non-toxicity and high chemical inertness [1–9]. Among diverse TiO2 nanostructure morphologies, TiO2 nanosheets (TiO2NSs) have been widely applied in the field of photovoltaic cells due to their sufficient surface area [10]. Many endeavours have been made to fabricate perovskite solar cells (PSCs) based on TiO2NSs films [11,12]. Although such PSCs based on TiO2NSs films show promising photovoltaic performance, poor properties are shown after the contact of CH3NH3PbI3 (MAPbI3) and fluorine-doped tin oxide (FTO) along the pinholes in the TiO2NSs films. Given all that, microscopic and high specific surface area TiO2 nanoparticles (TiO2NPs) can be introduced to wipe out the pinholes and retain high specific surface area of TiO2NSs [13–16]. Once TiO2NPs were deposited onto TiO2NSs, a peculiar TiO2NSs/NPs homogeneous hybrid structure can be formed. This structure is anticipated to simultaneously possess higher specific surface area and fewer pinholes. Hence, it will definitely increase the power conversion efficiency of PSCs. However, up to now, scarce relevant works have been reported on those issues.
Here, we report a TiO2NSs/NPs homogeneous hybrid structure as electron transport material (ETM) in PSCs, which was fabricated by the simple hydrothermal and chemical bath deposition (CBD) method. The photovoltaic properties of PSCs based on TiO2NSs/NPs homogeneous hybrid structure are superior to those based on bare TiO2NSs due to the introduction of TiO2NPs. This homogeneous hybrid structure may provide a new strategy for improving the performance of PSCs.
2. Experimental procedure
2.1. Experimental
The compact TiO2 (c-TiO2) and TiO2NSs films were synthesized by the simple CBD and hydrothermal method [17,18]. TiO2NSs films were fabricated by a hydrothermal method at 170°C for 3 h and annealed at 550°C for 2 h in air. To deposit TiO2NPs, the FTO/c-TiO2/TiO2NSs substrates were placed into an aqueous solution with 0.07 M TiCl4 at 70°C for 30 min. In addition, the TiO2NPs films were annealed at 450°C for 15 min in air atmosphere. The entire procedure of fabricating TiO2NPs films was termed as one cycle (C). Here, the procedure was repeated for 1C, 3C, 5C, 7C and 9C. Finally, the samples were annealed at 450°C for 30 min under ambient conditions and taken out after cooling down to room temperature.
The TiO2 substrates were treated by UV–ozone for 15 min before the MAPbI3 was deposited. Then, 50 µl PbI2 (462 mg ml−1 in dimethylformamide) was spin-coated onto the substrates (1.5 cm ×1.5 cm) with a low speed of 500 r.p.m. for 5 s and with a high speed of 4000 r.p.m. for 30 s. After that, the substrates were annealed at 100°C for 20 min. CH3NH3I (MAI) was synthesized by the technique as described in [19]. In the next step, 200 µl MAI (10 mg ml−1 in isopropanol) was spin-coated onto the substrates. After rinsing with isopropanol, the perovskite films were annealed at 100°C for 40 min. Thirty millilitre hole transport material (HTM) as described in [19] was spin-coated onto the perovskite films at 4000 r.p.m. for 10 s (0.073 g 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamine)-9,9′-spirobifluorene, 29 µl of 4-tert-butylpyridine, 18 µl of a lithium-bis(trifluoromethanesulfonyl)imide (Li-TFSI) solution (520 mg Li-TFSI/1 ml acetonitrile) were dissolved in 1 ml chlorobenzene) [20]. Finally, the Ag back electrode was deposited by thermal evaporation.
2.2. Characterization
The microstructure and morphology of the films were observed by a MAGELLAN 400 scanning electron microscope. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) measurements were conducted by a JEOL JEM-2100F microscope. The analysis of composition and crystal structure was conducted using X-ray diffraction (XRD; Rigaku D/max-2500) using Cu Kα radiation (λ = 1.5418 Å). Ultraviolet–visible (UV-vis) absorption spectra were measured in the range from 200 to 1000 nm by a UV-3150 double-beam spectrophotometer at room temperature. The current–voltage curves of PSCs were recorded by a Keithley 2400 source measure unit.
3. Results and discussion
3.1. Scanning electron microscope images
In figure 1, we present top-view scanning electron microscopy (SEM) images of the TiO2NSs array films after being combined with TiO2NPs for different cycles. TiO2NSs are coated with TiO2NPs uniformly, and the surface of the TiO2NSs becomes rough gradually. There was a notable increase in the adsorption of TiO2NPs deposited onto the TiO2NSs films, and the TiO2NPs architecture could result in a full TiO2NSs/NPs homogeneous hybrid film. The inset of figure 1b represents an enlarged image of TiO2NSs deposited with TiO2NPs of 1C. Because of the high surface energy and reactivity of {001} facets, the TiO2NSs can adsorb more particles on this facet. The inset of figure 1e represents an enlarged view of TiO2NPs deposited on TiO2NSs for 7C.
Figure 1.
Top-view SEM images of TiO2NPs deposited on TiO2NSs for (a) 0C, (b) 1C, (c) 3C, (d) 5C, (e) 7C and (f) 9C. The insets in (b) and (e) represent the enlarged images of TiO2NSs/NPs of 1C and 7C, respectively.
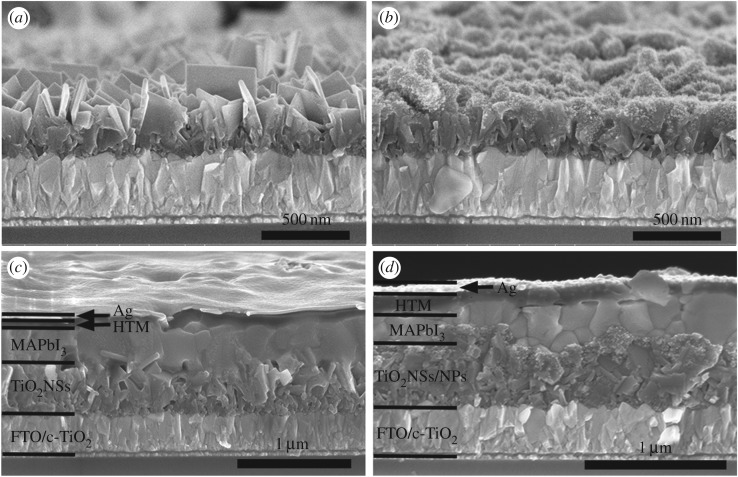
As portrayed in figure 2a, the aligned TiO2NSs films are relatively smooth and vertically grew on the FTO/c-TiO2 substrates, providing the transfer path for electrons. The thickness of the film is approximately 370 nm. Figure 2b is the SEM image of TiO2NSs film after the deposition of 7C TiO2NPs; obviously, the surface of the TiO2NSs became rougher, which benefits for the combination of MAPbI3 [21]. The cross-sectional SEM images of PSC devices with and without TiO2NPs are presented in figure 2c,d, respectively. MAPbI3 crystals are compactly arranged on the surface of TiO2 substrates. When the ETM films are not coated with TiO2NPs, some pinholes are seen, as shown in figure 2c. However, no pinholes are seen in the TiO2NSs/NPs/MAPbI3 films which could be ascribed to the full TiO2NSs/NPs film, and higher power conversion efficiency (PCE) was obtained.
Figure 2.
Cross-sectional SEM images of (a) TiO2NSs films grown on FTO at 170°C for 3 h, (b) TiO2NSs after coated with 7CNPs, (c) device based on TiO2NSs and (d) device based on TiO2NSs/7CNPs.
3.2. Transmission electron microscopy and high-resolution transmission electron microscopy observation
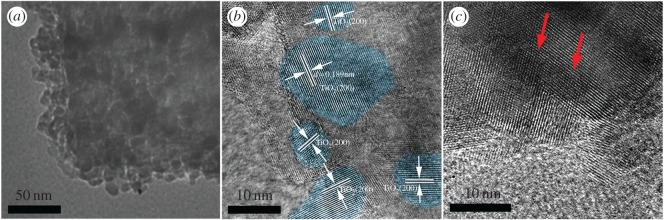
The detailed microscopic characterization of TiO2NSs/NPs nanostructures was performed by TEM, as shown in figure 3. Figure 3a clearly displays that TiO2NPs uniformly grew on TiO2NSs. This homogeneous structure was not damaged through ultrasonic processing, signifying that the TiO2NSs and TiO2NPs bonded firmly together. To clarify the growth mechanism, HRTEM was carried out. The inter-planar distance between the neighbouring lattice fringes of HRTEM is 0.189 nm which is corresponding to the inter-planar distance from {200} planes of TiO2NPs (JCPDS no. 21–1272), as shown in figure 3b. The TEM and HRTEM images indicate that TiO2NPs exhibit homogeneous fine grain microstructure, with an average grain size of approximately 10–20 nm. The staggered lattice of TiO2NSs and TiO2NPs was also observed by HRTEM and the joint parts are pointed out by the arrows in figure 3c. The plentiful growth interfaces inevitably will result in more charge transfer channels, which can enhance the electronic injection efficiency.
Figure 3.
(a) TEM bright-field image of TiO2NSs/NPs and (b,c) HRTEM images of TiO2NSs/NPs.
3.3. Phase composition and phase structure
The prepared TiO2 and TiO2/MAPbI3 films were examined by XRD to characterize the crystal structure (figure 4a). The diffraction pattern of TiO2NSs array film matches fairly well with the existing reference data available for this crystal from the Joint Committee on Powder Diffraction Standards (JCPDS no. 21–1272). In addition, after depositing with TiO2NPs for 7C, no diffraction lines of impurities were detected. The black curve shows the XRD pattern of FTO/c-TiO2/TiO2NSs/NPs/MAPbI3 film. Except the diffraction peaks of FTO, TiO2 and a weak peak at 12.7°of PbI2, other diffraction peaks all correspond to MAPbI3. This indicates that the MAPbI3 has a high purity and has been successfully coated onto the surface of TiO2 films. The peaks of MAPbI3 were sharp, indicating that MAPbI3 possesses good crystallinity.
Figure 4.

(a) X-ray diffraction patterns of (from bottom to top) FTO/c-TiO2/TiO2NSs, FTO/c-TiO2/TiO2NSs/7CNPs and FTO/c-TiO2/TiO2NSs/7CNPs/MAPbI3. The plot shows the X-ray intensity as a function of 2θ. (b) UV-vis absorption spectra of films.
3.4. Ultraviolet–visible absorption spectroscopy
The UV-vis absorption spectra of bare TiO2NSs array films, TiO2NSs/MAPbI3 films and TiO2NSs/NPs/MAPbI3 films are plotted in figure 4b as a function of wavelength. The peak maximum of TiO2NSs films occurs at approximately 370 nm and the films have no significant absorption for visible light due to the large band gap of TiO2 [22,23]. However, after coating with MAPbI3, the TiO2NSs/MAPbI3 films present excellent absorption capacity from 350 to 800 nm, and the absorption intensity increased substantially. The variation indicates that the deposited MAPbI3 has significantly extended the photoresponse of TiO2NSs films to the visible light region. The most striking thing is that after TiO2NSs films combined with TiO2NPs for 7 C, the TiO2NSs/NPs/MAPbI3 films exhibit stronger absorption than TiO2NSs/MAPbI3 films. This result indicates that the MAPbI3 films combine with TiO2NSs/NPs films better than TiO2NSs films.
3.5. Photovoltaic characterization of perovskite solar cells based on TiO2NSs/NPs films
To probe the effect of different amount of TiO2NPs on photovoltaic performance, successive deposition cycle (C) experiments of TiO2NPs were conducted. The working area of devices was 0.15 mm2. All current–voltage curves were recorded in air and derived from reverse scan (the bias scan rate was 100 mV s−1). Figure 5 shows the J–V characteristics of the PSCs under one sun AM 1.5G irradiance, and the corresponding parameters are summarized in table 1. The J–V characteristics are enhanced with the increase of TiO2NPs in the early cycles, suggesting that a higher incorporated amount of TiO2NPs induced more superior photovoltaic characteristics. Surprisingly, as the amount of TiO2NPs increased upon the cycles to seven, the PSCs exhibited a best PCE of 5.39%, with Voc = 0.82 V, Jsc = 17.06 mA cm−2 and FF = 0.40.
Figure 5.

J–V characteristic of the lead iodide perovskite solar cells based on TiO2NSs/NPs films of different deposition cycles.
Table 1.
Photovoltaic device parameters of the TiO2NSs/NPs/CH3NH3PbI3 solar cells.
| cycles | Jsc (mA cm−2) | Voc (V) | FF | PCE |
|---|---|---|---|---|
| 0 | 4.27 | 0.68 | 0.30 | 0.86 |
| 1 | 6.59 | 0.8 | 0.38 | 2.07 |
| 3 | 12.91 | 0.78 | 0.37 | 3.72 |
| 5 | 14.65 | 0.79 | 0.42 | 4.8 |
| 7 | 17.06 | 0.82 | 0.40 | 5.39 |
| 9 | 14.87 | 0.79 | 0.35 | 3.89 |
In the early 7 cycles, the Voc significantly increased along with the decreased pinholes which will generate charge loss. After TiO2NPs are deposited onto TiO2NSs, the MAPbI3 grain size tends to be larger than that of pure TiO2NSs. Larger grain size leads to fewer grain boundaries and, consequently, the corresponding Jsc improved [24]. With the increase of TiO2NPs, the surface of TiO2NSs array films appears rougher. This rougher surface is favourable for the combination of MAPbI3 and it will improve the fill factor of PSCs. However, when the deposited cycles are more than seven, the device performance of PSCs dropped. This phenomenon is presumably attributed to the excessive TiO2NPs grain boundaries. When TiO2NSs/NPs films transported photo-induced electrons from MAPbI3 to FTO, more grain boundaries caused more electron annihilation. This inevitably will decrease the J–V characteristics of PSCs. Etgar et al. [11] fabricated MAPbI3/TiO2NSs solar cell with Jsc = 16.1 mA cm−2, Voc = 0.631 V, corresponding to a PCE of 5.5% under glovebox conditions. Compared with their PSCs based on pure TiO2NSs, the TiO2 homogeneous hybrid structure PSCs synthesized under air conditions exhibit higher Jsc, Voc and compatible PCE.
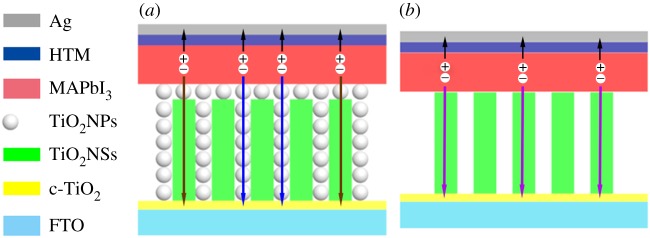
Holes are transported to the counter electrode by HTM in scheme 1a (the short black arrows). Electrons are transported to FTO by TiO2NPs (the long blue arrows) and TiO2NSs/NPs (the long brown arrows). Compared with pure TiO2NSs films, fewer pinholes exist in TiO2NSs/NPs homogeneous hybrid films, and these hybrid films have higher specific surface area. Therefore, the photovoltaic performance of PSCs based on TiO2NPs/NSs homogeneous hybrid structure is significantly enhanced.
Scheme 1.
Schematic of PSCs based on (a) TiO2NSs/NPs homogeneous hybrid structure films and (b) pure TiO2NSs films.
4. Conclusion
We fabricated a TiO2NSs/NPs homogeneous hybrid structure, the ETM in PSCs, by the hydrothermal and CBD method. When TiO2NPs are deposited onto TiO2NSs for 7 cycles, the PSCs based on TiO2NPs/NSs homogeneous hybrid structure exhibit photovoltaic performance with a best efficiency of 5.39% under one sun AM 1.5G solar spectrum, which is two and a half times that of bare TiO2NSs. Compared with pure TiO2NSs films, the MAPbI3 combined better with rough and free of pinholes TiO2NSs/NPs homogeneous hybrid films. Hence, it demonstrates that this extraordinary structure will have potential application for enhancing photovoltaic performance of PSCs in the future.
Supplementary Material
Acknowledgement
We acknowledge the constructive comments of the reviewers, which have improved the paper. We thank Shuailing Ma for the insightful discussions and helpful comments on an earlier version of the manuscript.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
P.S., W.F. and H.Y. conceived and designed the experiments, and analysed the data results. P.S., L.L., F.F. and S.F. measured the SEM images. P.S. and D.D. carried out the TEM experiments. P.S. carried out the XRD experiments. P.S. and Y.X. performed the device fabrication and photovoltaic performance measurements. W.F. and H.Y. helped draft the manuscript. All authors discussed the mechanism and gave their final approval for publication.
Competing interests
We declare that we have no competing interests.
Funding
This work was financially supported by National Natural Science Foundation of China (grant no. 51272086).
References
- 1.Liu X, Dang R, Dong W, Huang X, Tang J, Gao H, Wang G. 2017. A sandwich-like heterostructure of TiO2 nanosheets with MIL-100(Fe): a platform for efficient visible-light-driven photocatalysis. Appl. Catal. B: Environ. 209, 506–513. (doi:10.1016/j.apcatb.2017.02.073) [Google Scholar]
- 2.Jiang L, Zhu W, Wang C, Dong W, Zhang L, Wang G, Chen B, Li C, Zhang X. 2015. Preparation of hollow Ag/Pt heterostructures on TiO2 nanowires and their catalytic properties. Appl. Catal. B: Environ. 180, 344–350. (doi:10.1016/j.apcatb.2015.06.033) [Google Scholar]
- 3.Bavykin DV, Friedrich JM, Walsh FC. 2006. Protonated titanates and TiO2 nanostructured materials: synthesis, properties, and applications. Adv. Mater. 18, 2807–2824. (doi:10.1002/adma.200502696) [Google Scholar]
- 4.Lee JS, You KH, Park CB. 2012. Highly photoactive, low bandgap TiO2 nanoparticles wrapped by graphene. Adv. Mater. 24, 1084–1088. (doi:10.1002/adma.201104110) [DOI] [PubMed] [Google Scholar]
- 5.Sun WT, Yu Y, Pan HY, Gao XF, Chen Q, Peng LM. 2008. CdS quantum dots sensitized TiO2 nanotube-array photoelectrodes. J. Am. Chem. Soc. 130, 1124–1125. (doi:10.1021/ja0777741) [DOI] [PubMed] [Google Scholar]
- 6.Zheng Q, et al. 2008. Self-organized TiO2 nanotube array sensor for the determination of chemical oxygen demand. Adv. Mater. 20, 1044–1049. (doi:10.1002/adma.200701619) [Google Scholar]
- 7.Liu B, Aydil ES. 2009. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 131, 3985–3990. (doi:10.1021/ja8078972) [DOI] [PubMed] [Google Scholar]
- 8.Etgar L, Zhang W, Gabriel S, Hickey SG, Nazeeruddin MK, Eychmüller A, Liu B, Grätzel M. 2012. High efficiency quantum dot heterojunction solar cell using anatase (001) TiO2 nanosheets. Adv. Mater. 24, 2202–2206. (doi:10.1002/adma.201104497) [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Zhang Y, Xia T, Murowchick J, Liu G, Chen X. 2014. Lithium-ion battery performance of (001)-faceted TiO2 nanosheets vs. spherical TiO2 nanoparticles. Energy Technol. 2, 376–382. (doi:10.1002/ente.201300140) [Google Scholar]
- 10.You T, Jiang L, Han KL, Deng W. 2013. Improving the performance of quantum dot-sensitized solar cells by using TiO2 nanosheets with exposed highly reactive facets. Nanotechnology 24, 245401 (doi:10.1088/0957-4484/24/24/245401) [DOI] [PubMed] [Google Scholar]
- 11.Etgar L, Gao P, Xue Z, Peng Q, Chandiran AK, Liu B, Nazeeruddin MK, Grätzel M. 2012. Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. J. Am. Chem. Soc. 134, 17 396–17 399. (doi:10.1021/ja307789s) [DOI] [PubMed] [Google Scholar]
- 12.Guo D, Yu J, Fan K, Zou H, He B. 2017. Nanosheet-based printable perovskite solar cells. Sol. Energy Mater. Sol. Cells 159, 518–525. (doi:10.1016/j.solmat.2016.09.043) [Google Scholar]
- 13.Peng JD, Lin HH, Lee CT, Tseng CM, Suryanarayanan V, Vittal R, Ho KC. 2016. Hierarchically assembled microspheres consisting of nanosheets of highly exposed (001)-facets TiO2 for dye-sensitized solar cells. RSC Adv. 6, 14 178–14 191. (doi:10.1039/c5ra26307g) [Google Scholar]
- 14.Bai Y, Yu H, Li Z, Amal R, Lu GQMax, Wang L. 2012. In situ growth of a ZnO nanowire network within a TiO2 nanoparticle film for enhanced dye-sensitized solar cell performance. Adv. Mater. 24, 5850–5856. (doi:10.1002/adma.201201992) [DOI] [PubMed] [Google Scholar]
- 15.Suwanchawalit C, Patil AJ, Kumar RK, Wongnawa S, Mann S. 2009. Fabrication of ice-templated macroporous TiO2–chitosan scaffolds for photocatalytic applications. J. Mater. Chem. 19, 8478–8483. (doi:10.1039/b912698h) [Google Scholar]
- 16.Castro Y, Mosa J, Aparicio M, Pérez-Carrillo LA, Vílchez S, Esquena J, Durán A. 2015. Sol–gel hybrid membranes loaded with meso/macroporous SiO2, TiO2–P2O5 and SiO2–TiO2–P2O5 materials with high proton conductivity. Mater. Chem. Phys. 149–150, 686–694. (doi:10.1016/j.matchemphys.2014.11.028) [Google Scholar]
- 17.Sommeling P, O'Regan B, Haswell R, Smit H, Bakker N, Smits J, Kroon J, Roosmalen J. 2006. Influence of a TiCl4 post-treatment on nanocrystalline TiO2 films in dye-sensitized solar cells. J. Phys. Chem. B 110, 19 191–19 197. (doi:10.1021/jp061346k) [DOI] [PubMed] [Google Scholar]
- 18.Shang G, Wu J, Tang S, Liu L, Zhang X. 2013. Enhancement of photovoltaic performance of dye-sensitized solar cells by modifying tin oxide nanorods with titanium oxide layer. J. Phys. Chem. C 117, 4345–4350. (doi:10.1021/jp309193n) [Google Scholar]
- 19.Lee MM, Teuscher J, Miyasaka T, Murakami TN, Snaith HJ. 2012. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 338, 643–647. (doi:10.1126/science.1228604) [DOI] [PubMed] [Google Scholar]
- 20.Burschka J, Pellet N, Moon SJ, Humphry-Baker R, Gao P, Nazeeruddin MK, Grätzel M. 2013. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499, 316 (doi:10.1038/nature12340) [DOI] [PubMed] [Google Scholar]
- 21.Ito S, et al. 2005. Control of dark current in photoelectrochemical (TiO2/I−–I3−) and dye-sensitized solar cells. Chem. Commun. 34, 4351–4353. (doi:10.1039/b505718c) [DOI] [PubMed] [Google Scholar]
- 22.Prabakar K, Takahashi T, Nezuka T, Takahashi K, Nakashima T, Kubota Y, Fujishima A. 2008. Visible light-active nitrogen-doped TiO2 thin films prepared by DC magnetron sputtering used as a photocatalyst. Renewable Energy 33, 277–281. (doi:10.1016/j.renene.2007.05.018) [Google Scholar]
- 23.Zakrzewska K, Radecka M. 2017. TiO2-based nanomaterials for gas sensing—influence of anatase and rutile contributions. Nanoscale Res. Lett. 12, 89 (doi:10.1186/s11671-017-1875-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Im JH, Jang IH, Pellet N, Grätzel M, Park NG. 2014. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotechnol. 9, 927–932. (doi:10.1038/NNANO.2014.181) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.