Abstract
Opiate withdrawal/negative reinforcement has been implicated as one of the mechanisms for the progression from impulsive to compulsive drug use. Increase in the intracellular cAMP level and protein kinase A (PKA) activities within the neurocircuitry of addiction has been a leading hypothesis for opiate addiction. This increase requires the phosphorylation of μ‐opioid receptor (MOR) at Tyr336 by Src after prolonged opiate treatment in vitro. Here, we report that the Src‐mediated MOR phosphorylation at Tyr336 is a prerequisite for opiate withdrawal in mice. We observed the recruitment of Src in the vicinity of MOR and an increase in phosphorylated Tyr336 (pY336) levels during naloxone‐precipitated withdrawal. The intracerebroventricular or stereotaxic injection of a Src inhibitor (AZD0530), or Src shRNA viruses attenuated pY336 levels, and several somatic withdrawal signs. This was also observed in Fyn−/− mice. The stereotaxic injection of wild‐type MOR, but not mutant (Y336F) MOR, lentiviruses into the locus coeruleus of MOR −/− mice restored somatic withdrawal jumping. Regulating pY336 levels during withdrawal might be a future target for drug development to prevent opiate addictive behaviors.
Keywords: lentivirus injection, locus coeruleus, naloxone‐precipitated opiate withdrawal, opiate addiction, Src‐mediated phosphorylation of MOR at Tyr336
Subject Categories: Neuroscience
Introduction
Cravings for opioids have been linked to the initial rewards associated with the drugs as well as to withdrawal and the adverse motivational state (Koob, 2009). The neurocircuitry involved in the three stages of the addiction cycle has been mapped, with the extended amygdala as the focal point in the withdrawal/negative affect stage (Stinus et al, 1990; Nestler, 2004; Koob & Volkow, 2010). The extended amygdala is composed of the central nucleus of the amygdala, the bed nucleus of the stria terminalis (BNST), and the medial (shell) subregion of the nucleus accumbens (NAc). It receives projections from limbic structures such as the basolateral amygdala and hippocampus, and sends fibers to brain areas that interface with limbic structures with output to the extrapyramidal motor system (Alheid et al, 1995). A challenge is to identify the temporal processes and neural substrates that are involved in the plasticity of this neural circuit during opioid addiction. Furthermore, to develop a successful treatment for opioid addiction, the cellular events involved in the drug withdrawal episodes that enhance an opioid's incentive value to the extent that compulsive drug‐taking and drug‐seeking take over one's behavior need to be elucidated.
Among other neural substrates, protein kinase‐mediated alterations have been reported in neural circuitries within limbic structures involved in motivational withdrawal including the NAc, amygdala (Hand et al, 1988; Stinus et al, 1990; Heinrichs et al, 1995), and BNST (Delfs et al, 2000). They have also been found within the mesencephalic and autonomic brain areas involved in somatic withdrawal including the locus coeruleus (LC) and periaqueductal gray area (Maldonado et al, 1992). Specifically, the D1‐dopaminergic receptor activity‐dependent increase in cAMP‐dependent protein kinase (PKA) activity that is observed following acute opioid treatment and that is enhanced in opiate withdrawal states at the NAc and dorsal striatum (Punch et al, 1997; Borgkvist et al, 2007) resulted in an increase in the phosphorylation of the transcription factor cAMP response element binding protein (pCREB) in brain regions implicated in drug addiction and in brain structures involved in behavioral plasticity associated with addiction (Shaw‐Lutchman et al, 2002; Olson et al, 2005; Walters et al, 2005). The activation of transcription factors such as CREB can result in an increase in dynorphin expression in the NAc, leading to a reduction in drug rewards (Carlezon et al, 1998). This in turn counteracts the effects of ∆FosB by decreasing the transcription of dynorphin and thus contributing to drug rewards (Nestler et al, 2001). Although the reciprocal effects of CREB and ∆FosB on the same gene could complicate the final addictive behavioral responses, the alteration in neuronal cAMP levels that leads to PKA‐mediated changes is a direct consequence of the opioid receptor signaling process.
An opiate‐induced increase in PKA‐mediated phosphorylation and the activation of the cAMP/PKA/DARPP‐32 signaling pathway have been shown in several brain regions. The involvement of adenylyl cyclase (AC) in chronic morphine action was demonstrated with AC5, AC1, and AC8 knockout mice, which showed the attenuation of some somatic withdrawal signs (Kim et al, 2006; Zachariou et al, 2008). An increase in cAMP levels is related to context‐specific withdrawal, and several investigators have suggested that opiate withdrawal symptoms reflect classic conditioned responses (O'Brien et al, 1977; Hinson & Siegel, 1982; Schulteis et al, 2005). The inhibition of PKA activity, which disrupts memory reconsolidation, results in the weakening of motivational withdrawal (Taubenfeld et al, 2010). In fact, an increase in GluR1S845 phosphorylation by PKA was observed in the NAc and amygdala during spontaneous withdrawal following heroin self‐administration (Edwards et al, 2009). Although somatic withdrawal from opiates can be attenuated with the use of PKA‐selective inhibitors (Punch et al, 1997), the use of such inhibitors might not be the ideal approach for the treatment of opioid addiction. Indeed, because of PKA interactions with multiple signaling pathways, PKA inhibitors attenuates a myriad of kinase actions that are unrelated to drug addiction (Parsons & Parsons, 2004; Babus et al, 2011; Sen & Johnson, 2011). In summary, in acute pain conditions, opioids induce analgesia by diminishing neuronal excitability by triggering intracellular signaling events that leads to the inhibition of adenylyl cyclase (AC) activity, activation of inwardly rectifying K+ current, and/or inhibition of Ca2+ conductance (Law et al, 2000). When pain persists, chronic opioid exposure not only leads to a blunting of these intracellular responses but also results in a compensatory increase in intracellular cAMP levels and AC activity in response to the excessive action of the agonist (Zhang et al, 2009, 2013). Upon the removal of the opioid from the cellular environment or the addition of an antagonist such as naloxone, the compensatory increase in AC activity becomes particularly significant and unopposed and contributes to the activation of neurons during withdrawal (Nestler, 1997). This AC superactivation phenomenon, generally referred to in the literature as the upregulation of the cAMP pathway (Nestler, 2004), has been well established as being one of the accepted molecular mechanisms of drug dependence and withdrawal (Koob & Bloom, 1988; Nestler, 2004).
Adenylyl cyclase superactivation has been demonstrated after in vivo opioid administration and subsequent naloxone injection in neurons in several regions of the rat brain, including the LC (Nestler & Aghajanian, 1997; He et al, 2009). The compensatory increase in AC activity is isozyme‐specific (Avidor‐Reiss et al, 1996, 1997; Yang et al, 2016). AC1, AC5, AC6, and AC8 are the isoforms that have been demonstrated to be superactivated after chronic morphine exposure in transfected cells (Avidor‐Reiss et al, 1996, 1997). In mice, AC1, AC5, and AC8 are more specifically implicated in the behavioral expression of withdrawal (Kim et al, 2006; Zachariou et al, 2008).
Recently, we delineated a non‐canonical signaling pathway that can account for the observed superactivation of AC in cell model systems (Zhang et al, 2013). During acute morphine treatment, the μ‐opioid receptor (MOR) interacts with Gαi2 (Zhang et al, 2006) and causes an inhibition of AC activity and a decrease in the intracellular cAMP level. Under prolonged treatment, the MOR non‐canonical signaling pathway is activated. It involves the recruitment of Src kinase by the MOR signal complex within which Src kinase is activated. The activated Src phosphorylates MOR at Tyr336, although it remains to be demonstrated whether the phosphorylation is direct or indirect. In turn, the phosphorylated Tyr336 serves as a docking site for growth factor receptor‐bound protein (Grb)/son of sevenless (SOS), leading to the recruitment and activation of Ras. Activated Ras binds to Raf‐1 and recruits Src kinase that will activate Raf‐1 in the membrane proximity resulting in Raf‐1 increase in activity (Baccarini, 2005). This leads to the subsequent phosphorylation and activation of AC5/6 by Raf‐1 or, in other words, the AC superactivation. Hence, the direct or indirect phosphorylation of MOR at Tyr336 by Src kinase serves as the switch for converting a classic Gi/Go‐coupled receptor into a receptor tyrosine kinase‐like entity, resulting in a non‐canonical signaling pathway even after the canonical Gi/Go signaling has been attenuated. This non‐canonical signaling pathway seems to be responsible for the switching from MOR‐mediated initial AC inhibition to subsequent AC activation (Zhang et al, 2013).
If the PKA‐mediated neural plasticity within the addiction circuit that is a direct result of AC superactivation is a probable cause for compulsive drug use due to withdrawal/negative reinforcement, then PKA‐mediated neural plasticity can be modulated by regulating the pre‐required Src‐mediated phosphorylation of MOR at Tyr336. Whether such changes in Src activity and MOR phosphorylation occur in vivo during opiate withdrawal is unknown. To establish the relationship between Tyr336 phosphorylation and opiate withdrawal, we developed an antigen‐purified rabbit polyclonal antibody against the phosphorylated Y336 residue of MOR. Here, we demonstrate that MOR is phosphorylated at Tyr336 during naloxone‐precipitated withdrawal. Using Fyn−/− mice and the Src kinase inhibitor AZD0530, we show that the phosphorylation of MOR is mediated by the Src family kinases (SFKs). Furthermore, we reveal that the naloxone‐precipitated somatic withdrawal signs observed after chronic morphine treatment are dependent on the Src‐mediated phosphorylation of MOR at Tyr336. Our findings suggest that small molecules that modulate the Src‐mediated phosphorylation of MOR might prevent the transition to the withdrawal/negative reinforcement stage of the opiate addiction cycle.
Results
MOR is phosphorylated at Tyr336 during naloxone‐precipitated withdrawal
To demonstrate that MOR is phosphorylated at Tyr336, we developed an affinity‐purified polyclonal antibody against phosphorylated Tyr336 (anti‐pMORY336). The specificity of the antibody was demonstrated in clonal cell lines expressing a wild‐type (WT) or Y336F mutant MOR. As shown in Appendix Figs S1A–D and S2A–D, this antibody detected the phosphorylated, but not the non‐phosphorylated, Y336 residue of MOR in both Western and immunofluorescence analyses. Although the peptide sequence used to immunize the rabbits (NPVLYAFLDEN) is conserved in all three of the opioid receptors, anti‐pMORY336 did not detect the non‐phosphorylated MOR or KOR, and exhibited only slight reactivity toward the phosphorylated DOR. The antibody is specific because it does not detect any pY336 in HEK293 cells expressing the Y336F mutant in both Western and immunofluorescence analyses (Appendix Figs S1A–D and S2A–D). Anti‐pMORY336 is specific also because of the disappearance of pY336 immunoreactivity when the pMORY336 antibody was pre‐incubated with the immunoprecipitated MOR complex that had been extracted from the LC of morphine‐dependent WT mice undergoing naloxone‐precipitated withdrawal (Fig 1B) and with the corresponding phospho‐peptide (Fig 2A). The pSrcY416 antibody is well characterized and used in many reported studies, especially for Western blot (Koreckij et al, 2009; Megison et al, 2014).
Figure 1. Increase in Src activation and MOR phosphorylation at Tyr336 during naloxone‐precipitated withdrawal.
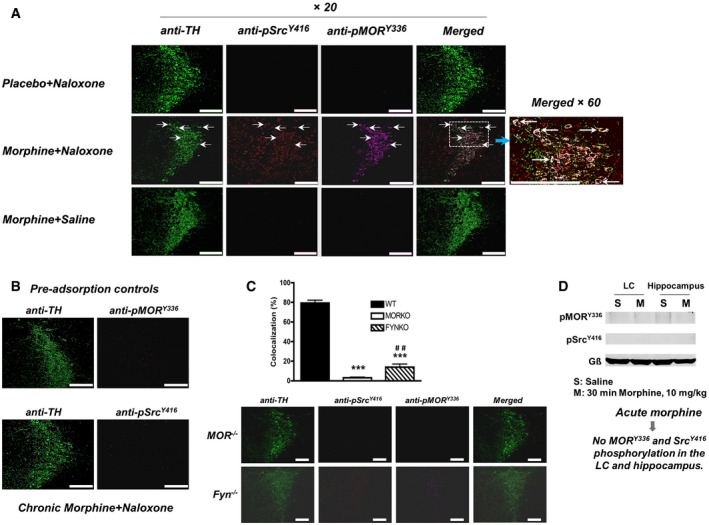
- Low (×20) and high‐magnification (×60) photomicrographs showing the colocalization of pMORY336 and pSrcY416 in the TH+ neurons of the LC (arrows) from a placebo‐and‐naloxone‐treated mouse (Placebo + Naloxone), a morphine‐and‐naloxone‐treated mouse (Morphine + Naloxone), and a morphine‐and‐saline‐treated (Morphine + Saline) mouse. The green, red, and magenta colors represent TH, pSrcY416, and pMORY336, respectively. Scale bar = 70 μm.
- The specificity of the pMORY336 and pSrcY416 antibodies was confirmed by the disappearance of pMORY336 and pSrcY416 immunoreactivity when pMORY336 and pSrcY416 antibodies were pre‐incubated with the immunoprecipitated MOR complex that had been extracted from the LC of morphine‐dependent WT mice with naloxone‐precipitated withdrawal (Morphine + Naloxone). Scale bar = 70 μm.
- The bar graph shows the colocalization analysis of the WT, MOR−/−, and Fyn−/− mice (Fig EV1). The percentage of colocalized pMORY336, pSrcY416, and TH results from the quantification in three mice/genotype, four slices/mouse. Significant differences among the groups (WT, MOR−/−, and Fyn−/−) were determined using one‐way ANOVA, followed by Duncan's post hoc comparison. ***P = 0.0001 MOR−/− versus WT; ***P = 0.0001 Fyn−/− versus WT; ## P = 0.002729 significant differences between MOR−/− and Fyn−/−. The photomicrographs show minimal colocalization between pMORY336 and pSrcY416 in the TH+ neurons of the LC from MOR−/− or Fyn−/− mice treated with chronic morphine (pellets for 3 days) and injected with naloxone after morphine pellets removal on the 4th day. The green, red, and magenta colors represent TH, pSrcY416 and pMORY336, respectively. Data are presented as means ± SEM. Scale bar = 70 μm.
- The phosphorylation of MORY336 (pMORY336) and SrcY416 (pSrcY416) was not detected within the MOR complex in the LC and the hippocampus of WT mice after acute injection of morphine (30 min, 10 mg/kg). An affinity‐purified polyclonal antibody against the MOR N‐terminus (conjugated to Dynabeads) was employed to IP pSrc and pMOR as described in the Materials and Methods and Fig 2A. The immunoblots were probed with anti‐pSrcY416 (1:1,000), anti‐pMORY336 (1:500), or anti‐Gβ (Santa Cruz sc‐378, 1:500).
Figure 2. Src is recruited to and activated within the MOR complex during naloxone‐precipitated withdrawal.
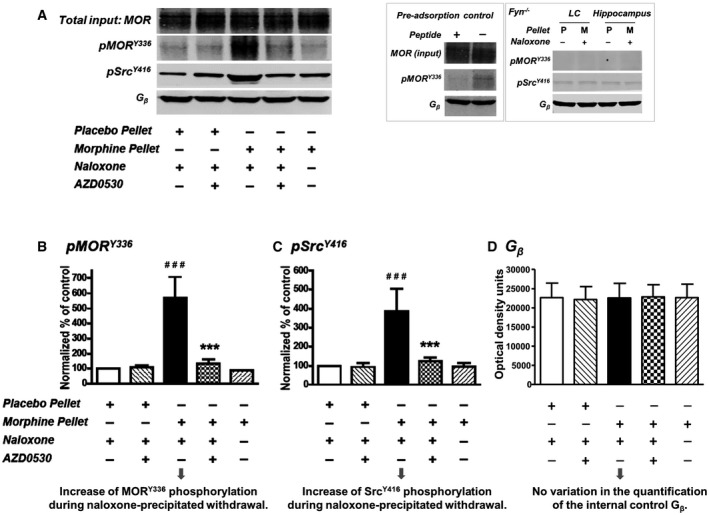
- An affinity‐purified polyclonal antibody against the MOR N‐terminus (conjugated to Dynabeads) was employed to IP pSrc and pMOR as described in the Materials and Methods. The immunoblots were probed with anti‐pSrcY416 (1:1,000), anti‐pMORY336 (1:500), or anti‐Gβ (Santa Cruz sc‐378, 1:500). In the middle panel, a Western blot analysis shows the disappearance of the pMORY336 immunoreactivity when the pMORY336 antibody was pre‐incubated with the corresponding phospho‐peptide (GeneTex PJ90076 NPVL(pY)AFLDENC). This pre‐adsorption control was performed with the immunoprecipitated MOR complex that had been extracted from the LC of morphine‐dependent WT mice with naloxone‐precipitated withdrawal. On the right, another Western blot shows residual Src activities in the Fyn−/− mice (Src needs to be phosphorylated at Tyr416 before activation). The reduced bands correlate with the sparsely detectable pSrcY416 immunoreactivity in the photomicrographs of Figs 1C and EV1B. This should be a clear indication of the specificity of the anti‐pSrcY416.
- The bar graphs show the densitometric quantification and statistical analysis of the changes in pMORY336 from the experiment presented in (A, left panel).
- The bar graphs show the densitometric quantification and statistical analysis of the changes in pSrcY416 from the experiment presented in (A, left panel).
- The bar graphs show no significant differences in the densitometric quantification of Gβ (from the experiment presented in A, left panel) among the conditions (the internal control, n = 3 mice).
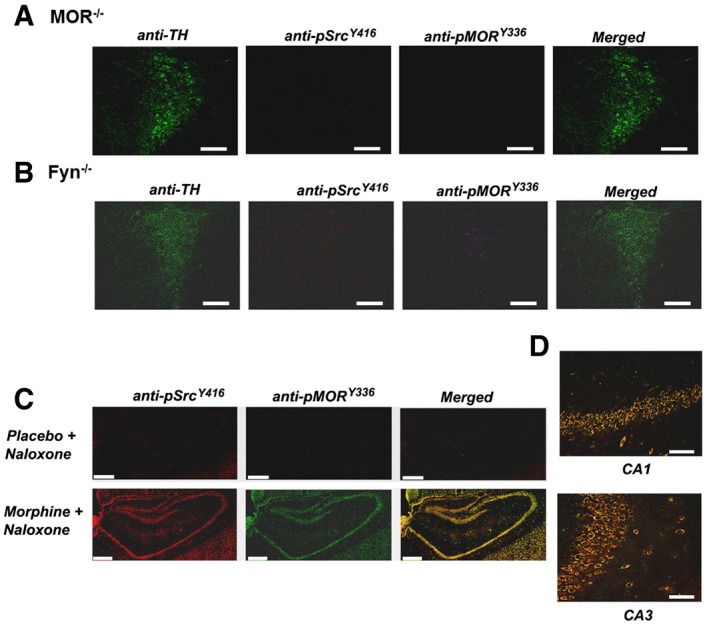
In order to establish the causal relationship between the increase in pMORY336 and naloxone‐precipitated somatic opiate withdrawal, we examined whether anti‐pMORY336 can detect an increase in MOR phosphorylation in the LC. The LC has been reported to be the primary anatomical site responsible for the expression of the motor components of opiate withdrawal, such as jumping, rearing, and locomotor activity, in studies using electrolytic lesions, PKA inhibitors, or PKA activators (Maldonado et al, 1992; Maldonado & Koob, 1993; Punch et al, 1997). As shown in Fig 1A, increases in both the pSrcY416 and the pMORY336 immunofluorescence signals in the LC were observed in mice implanted with a morphine pellet only after naloxone‐precipitated withdrawal. The increases were not observed in mice with morphine pellets in place or in mice implanted with placebo pellets and subjected to naloxone treatment. Similar increases in both pSrcY416 and pMORY336 levels were observed in mice chronically treated with morphine according to progressive dose injection methods, although a lower level of pMORY336 immunofluorescence was observed (data not shown). A colocalization analysis indicated that 79.4 ± 12.0% of the tyrosine hydroxylase‐positive (TH+) cells exhibited both pSrcY416 and pMORY336 immunofluorescence, suggesting that the increase in Src activity and MORY336 phosphorylation occurred in TH+ neurons within the LC during somatic withdrawal (F (2,19) = 303.9, P < 0.0001, one‐way ANOVA; Fig 1C). Other regions such as the hippocampus also exhibited similar increases in pSrcY416 and pMORY336 levels during naloxone‐precipitated somatic withdrawal (Fig EV1C). The colocalization of the pSrcY416 and pMORY336 immunofluorescent signals was observed at proximity of the cell surface at the CA1 and CA3 regions of the hippocampus, suggesting that Src may be recruited to the vicinity of the surface‐localized MOR (Fig EV1D).
Figure EV1. Effect of naloxone‐precipitated somatic opiate withdrawal on the increase in Src activation and MORY 336 phosphorylation in WT, MOR −/−, or Fyn−/− mice.
-
A, BMinimal colocalization between pMORY336 and pSrcY416 was observed in the TH+ neurons of the LC from MOR−/− (A) or Fyn−/− (B) mice treated with chronic morphine (pellets for 3 days) and injected with naloxone after morphine pellet removal on the 4th day. The green, red, and magenta colors represent TH, pSrcY416, and pMORY336, respectively.
-
CColocalization of pMORY336 (green) and pSrcY416 (red) in the hippocampus of WT mice implanted with either placebo or morphine pellets. The yellow denotes overlap.
-
DA magnified view of the CA1 (upper) and CA3 (lower) regions from merged images of morphine‐ and naloxone‐treated animals.
The increase in the pMORY336 immunofluorescent signal requires the presence of MOR and Src. MOR−/− mice implanted with a morphine pellet and subjected to naloxone‐precipitated opiate withdrawal showed a minimal increase in pSrcY416 and pMORY336 levels (Fig EV1A). The residual 3.17 ± 0.53% colocalization of pSrcY416 and pMORY336 in MOR−/− mice (Fig 1C) might represent the cross‐reactivity of the anti‐pMORY336 toward pDOR that was detected in the Western and immunofluorescence analyses (Appendix Figs S1A–D and S2A–D). As for the requirement of Src, five members of the SFK, N‐Src, Fyn, Lyn, Yes, and Lck, are present at substantial levels in the adult mammalian brain especially in the amygdala, NAc, VTA, and midbrain (Ohnishi et al, 2011). However, Fyn is the major subtype that has been shown to be implicated in multiple neural functions, including the regulation of NMDA receptor activity (Miyakawa et al, 1997) and behavior in response to ethanol exposure (Boehm et al, 2003). Thus, Fyn−/− mice were used to examine whether naloxone‐precipitated somatic withdrawal after chronic morphine pellet implantation would elicit a similar increase in pSrcY416 and pMORY336 levels. As summarized in Fig 1A, an increase in pSrcY416 immunofluorescence was observed in the LC of the mice implanted with the morphine pellets and subjected to naloxone treatment compared to that observed in the LC of mice implanted with the placebo pellets and similarly treated with naloxone. Such an increase was anticipated since 50% of the total Src kinase enzymatic activity remained in the Fyn−/− mice brain homogenates. Nevertheless, as with the increase in pSrcY416, a minimal increase in pMORY336 immunofluorescence was observed, and only 13.9 ± 3.2% of this signal colocalized with pSrcY416 in Fyn−/− mice subjected to naloxone‐precipitated opiate withdrawal (Fig 1C). It is likely that Fyn is the major Src subtype that contributes to the increase in pMORY336 levels during somatic withdrawal.
Src is recruited to the MOR signaling complex during naloxone‐precipitated withdrawal
For the Src‐mediated phosphorylation of MOR to occur, the tyrosine kinase has to be recruited in the vicinity of the receptor (Zhang et al, 2013). We conjugated a peptide affinity‐purified MOR rabbit polyclonal antibody directed against the MOR N‐terminal sequence SHVDGNQSDPCGLNRTGLGGNDSLCPQTGSPSMVTHRD to Dynabeads®M‐270 Epoxy beads (Life Technologies) to immunoprecipitate (IP) the receptor complex. The specificity of the MOR antibody was demonstrated by its inability to IP any receptor complex in MOR−/− mice. AC superactivation has been shown to be independent from agonist‐induced receptor internalization and to require the location of both MOR and the Gαi2 proteins at lipid rafts (Zhao et al, 2006). Since immunoprecipitated MOR was reported to be phosphorylated at Tyr336 by Src kinase within lipid rafts (Zhang et al, 2009), the pSrcY416 or pMORY336 detected with our Western blots are assumed to be from MOR‐Gαi2‐Src signaling complex located within lipid rafts and pulled down with the MOR N‐terminus antibody. As shown in Fig 2A, the IP from the midbrain extracts from mice implanted with a placebo pellet revealed the presence of basal pSrcY416 but not of pMORY336 (lane 1). The IP from the extracts from mice implanted with a 75‐mg morphine pellet for 4 days and subjected to naloxone‐precipitated withdrawal showed an increase in the amount of pSrcY416 associated with MOR and phosphorylated MORY336 (Fig 2A, lane 3). No detectable increase in either pSrcY416 or pMORY336 was observed in extracts from mice that were implanted with a morphine pellet but did not undergo naloxone‐precipitated withdrawal (Fig 2A, lane 5). The increases in pMORY336 and pSrcY416 levels within the receptor complex after naloxone‐precipitated withdrawal were determined to be 5.69 ± 1.38‐fold and 3.86 ± 1.10‐fold, respectively, over the placebo control (Fig 2B and C; n = 3 blots, P = 0.001 in both cases). Hence, the MOR non‐canonical signaling pathway exists in the mouse brain and parallels that observed in cell models.
Inhibition of Src kinase activity with AZD0530 blocked signs of naloxone‐precipitated somatic withdrawal
If Src‐mediated increase in pMORY336 levels is key to induction of naloxone‐precipitated withdrawal, then the in vivo inhibition of Src activity should blunt withdrawal. Narita et al (2006) reported that the intracerebroventricular (i.c.v.) injection of the water‐insoluble Src kinase inhibitor PP2 can block the reward effect and hyperlocomotion induced by morphine. Because the stereotaxic injection of the inhibitor PP2, which is dissolvable only in organic solvents such as DMSO, damaged the LC, we used the water‐soluble Src kinase inhibitor Saracatinib (AZD0530). Moreover, AZD0530 is one of the four SFK inhibitors [including Dasatinib, Bosutinib (SKI‐606), and KX2–391] that are currently undergoing clinical evaluation in oncology (Puls et al, 2011), and has >250‐fold selectivity for the SFK over other tyrosine kinase families (Green et al, 2005, 2009). AZD0530 inhibits Src with a potency of 2 nM and inhibits Abl at 30 nM (Hennequin et al, 2006). However, the selective inhibition of Src over Abl when mice are i.c.v. injected with 50 μg of AZD0530 has been demonstrated. The observed increase in Src enzymatic activity that occurs during naloxone‐precipitated withdrawal was blunted after the i.c.v. injection of AZD0530, while the observed increase in Abl activity was not altered (Fig 3). A parallel decrease in pSrcY416 associated with the MOR complex and the inhibition of pMORY336 was also observed in mice i.c.v. injected with 50 μg of AZD0530 (Fig 2A, lane 4). It is likely that in vivo i.c.v. doses of AZD0530 < 50 μg inhibit Src kinase activity without affecting Abl. The observed alteration in pMORY336 levels is the direct consequence of Src kinase activity modulated by AZD0530.
Figure 3. Selective inhibition of Src but not Abl activity in mice i.c.v. injected with AZD0530.
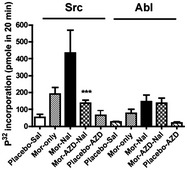
Mice were implanted either with placebo or morphine, and AZD0530 or saline was then injected i.c.v. Then, the mice were s.c. injected with or without naloxone (Nal) as detailed in the Materials and Methods. The midbrains of the mice in each treatment group were dissected and used in a Src or Abl kinase activity assay as described (Zhang et al, 2013). ***P = 0.00093 for Mor‐AZD‐Nal treatment compared with placebo‐Sal as determined by Student's t‐test. Each experiment was repeated three times. Data are presented as means ± SEM.Source data are available online for this figure.
The ability of AZD0530 to selectively inhibit Src kinase activity allowed us to investigate whether the Src‐mediated phosphorylation of MORY336 is required for naloxone‐precipitated withdrawal. Mice that had been implanted with a morphine pellet for 4 days were subjected to naloxone‐precipitated withdrawal. Thirty minutes prior to the injection of naloxone, 50 μg of AZD0530 was injected into the third ventricle via an implanted cannula. The injection of AZD0530 blocked the increase in the immunofluorescence of pSrcY416 and pMORY336 in the LC after naloxone‐precipitated opiate withdrawal (Fig 4A). Several somatic withdrawal signs such as diarrhea, jumping, mastication, paw tremors, and wet dog shakes (WDS) were significantly blunted after the AZD0530 injection; others such as grooming and body weight change were not altered by AZD0530 injection (Figs 5A and EV2). The number of jumps observed after naloxone injection decreased from 89.7 ± 27.0 in control mice to 23.7 ± 13.0 in mice injected with AZD0530 (F (2,21) = 9.29, P = 0.0013, one‐way ANOVA; P = 0.006141, Duncan's post hoc test). A significant decrease in global withdrawal scores from 101 ± 12.8 to 21 ± 6.7 (F (2,21) = 45.01, P < 0.001, one‐way ANOVA; P = 0.000147, Duncan's post hoc test) was observed in the presence of AZD0530 (Fig 5A). Similarly, the AZD0530‐mediated inhibition of naloxone‐precipitated withdrawal signs was observed in mice chronically treated with progressive morphine doses. The effect of AZD0530 was dose‐dependent and blunted the signs of withdrawal when stereotaxically injected into the LC (Appendix Fig S3). The stereotaxic injection of 5 μg of AZD0530 bilaterally into the LC eliminated the pSrcY416 and pMORY336 immunofluorescent signals in the LC after naloxone‐precipitated withdrawal (data not shown), but it did not affect the signals in other brain areas such as the hippocampus (Fig 4B). The stereotaxic injection of AZD0530 into the LC also blunted several, but not all, the naloxone‐precipitated withdrawal signs. The affected withdrawal signs included: jumping, mastication, paw tremors, and WDS (Figs 5B and EV3). A significant decrease in the global withdrawal score from 60 ± 4.3 (n = 11) to 27 ± 6.9 (n = 13) (unpaired Student's t‐test, P = 0.000656) was observed in the presence of AZD0530 (Fig 5B). The somatic withdrawal signs inhibited by the stereotaxic injection of AZD0530 are in accordance with the withdrawal signs shown to be associated with the LC (Maldonado et al, 1992). Thus, the AZD0530‐mediated selective inhibition of global Src kinase activity blocked increases in pSrcY416 and pMORY336 levels and subsequently diminished some of the naloxone‐precipitated somatic withdrawal signs.
Figure 4. Inhibition of Src activity and MORY 336 phosphorylation in the LC, but not in the hippocampus, of mice stereotaxically injected in the LC with AZD0530.
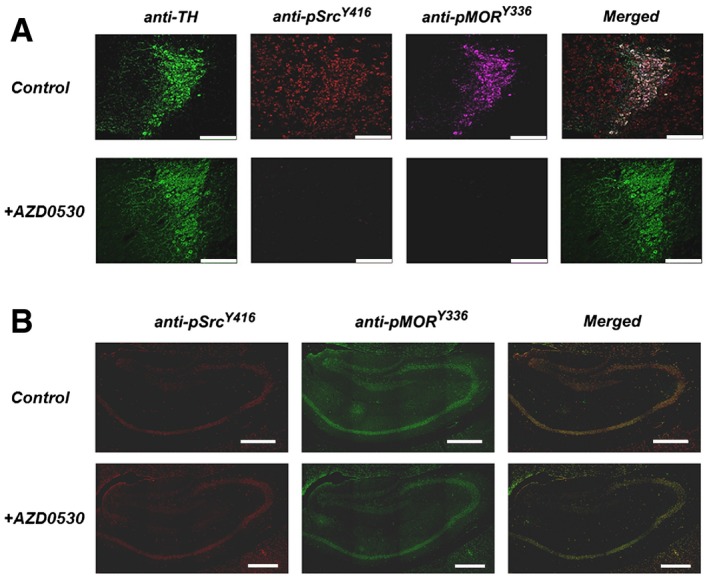
- Immunostaining of TH (green), pSrcY416 (red), or MORY336 (magenta) in the LC following the injection of saline (upper) or AZD0530 (lower) into the LC. Scale bar = 70 μm.
- Immunostaining of pSrcY416 (red) or MORY336 (green) in the hippocampus following the injection of saline (upper) or AZD0530 (lower) into the LC. The yellow denotes overlap. Scale bar = 100 μm.
Figure 5. The effect of Src activity or levels on naloxone‐precipitated somatic withdrawal signs.
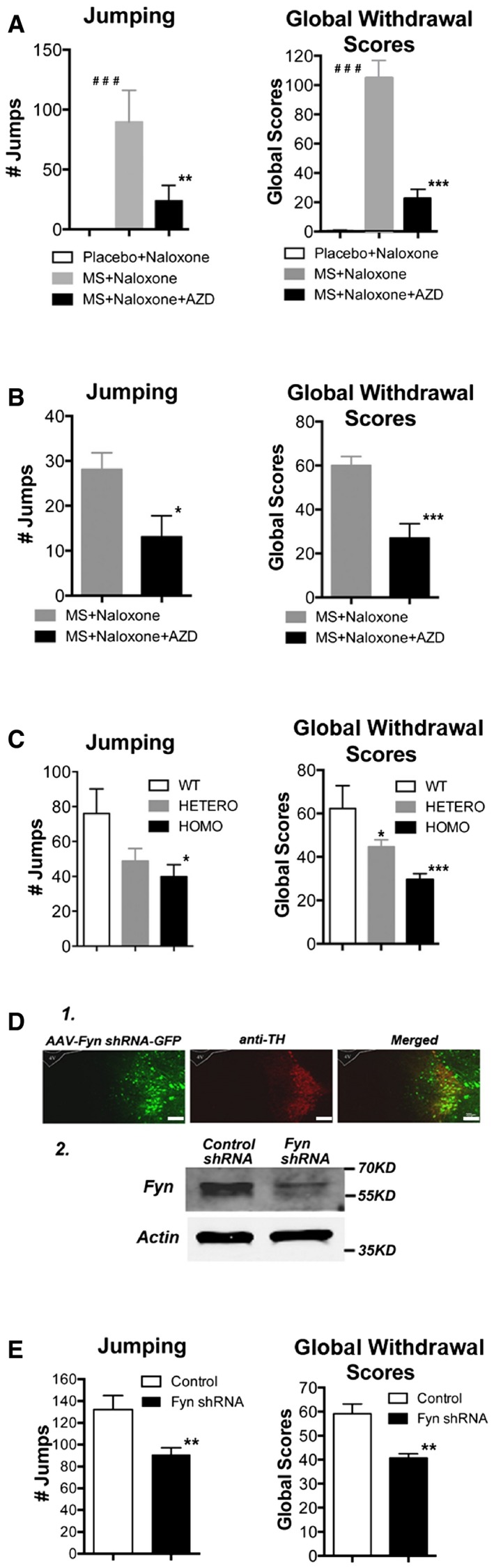
- The effect of AZD0530‐mediated inhibition of Src activity on naloxone‐precipitated somatic withdrawal behavior. Fifty micrograms of AZD0530 was i.c.v. injected into WT mice as described in the Materials and Methods. The inhibition of Src activity induces a significant decrease in naloxone‐precipitated jumping, other withdrawal signs (Fig EV2), and global withdrawal scores. One‐way ANOVA followed by Duncan's post hoc comparison revealed significant differences between the morphine‐and‐naloxone‐treated mice (MS + Naloxone, n = 8) and morphine‐naloxone‐and‐AZD0530‐treated mice (MS + Naloxone + AZD, n = 8) in the number of jumps **P = 0.006141 and global withdrawal scores ***P = 0.000147. Significant differences between the placebo‐and‐naloxone‐treated mice (Placebo + Naloxone, n = 8) and morphine‐and‐naloxone‐treated mice (MS + Naloxone) were shown in the number of jumps ### P = 0.000677 and global withdrawal scores ### P = 0.00007.
- Effect of Src inhibition on naloxone‐precipitated somatic withdrawal signs following the stereotaxic injection of AZD0530 (7.5 μg/μl) into the LC. The LC‐specific inhibition of Src activity causes a significant decrease in naloxone‐precipitated jumping, other withdrawal signs (Fig EV3), and global withdrawal scores. Significant differences between the morphine‐and‐naloxone‐treated mice (MS + Naloxone, n = 11) and morphine‐naloxone‐and‐AZD‐treated mice (MS + Naloxone + AZD, n = 13) were determined using Student's t‐test: *P = 0.0190 for jumping and ***P = 0.000656 for global withdrawal scores.
- The reduction in naloxone‐precipitated withdrawal signs in heterozygous (HETERO) and homozygous (HOMO) Fyn−/− mice (n = 6/group). The jumping and global withdrawal scores were significantly blunted in the HOMO Fyn−/− mice. One‐way ANOVA followed by Duncan's post hoc test revealed significant differences between the WT and HOMO mice in the number of jumps *P = 0.017939 and the global withdrawal scores ***P = 0.000409. There are also significant differences between the WT and HETERO mice in the global withdrawal scores *P = 0.022127.
- The reduction in Fyn levels following the stereotaxic injection of Fyn shRNA virus. (1.) Immunofluorescence analysis showing the colocalization of AAV‐Fyn‐shRNA‐GFP (green) with TH (red), a marker for adrenergic neurons in the LC. Scale bar = 200 μm. (2.) Representative Western blot image of the in vivo knockdown of Fyn expression.
- The knockdown of Fyn in the LC significantly attenuated naloxone‐precipitated morphine withdrawal signs. One microliter of Fyn shRNA AAV virus (titer: 2.09 × 1012 v.g./ml) was injected bilaterally into the LC (n = 12–13) as described in the Materials and Methods. Fyn knockdown induced a significant decrease in naloxone‐precipitated jumping, other withdrawal signs (Fig EV5), and global withdrawal scores. Student's t‐tests showed significant differences between the control (GFP‐transferred) and Fyn shRNA‐transferred WT mice in the number of jumps **P = 0.009429 and global withdrawal scores **P = 0.001723.
Figure EV2. Naloxone‐precipitated withdrawal signs in WT mice (n = 8/group) i.c.v. injected with AZD0530.
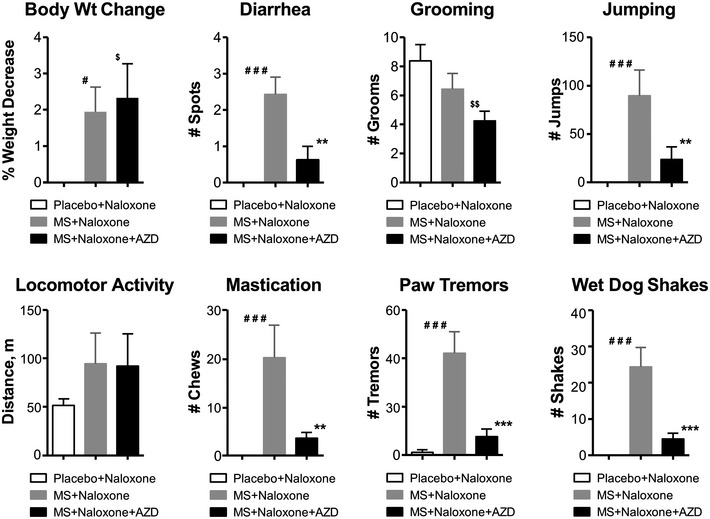
This presentation allows a comparative view of all the withdrawal signs including jumping presented in Fig 5A. The inhibition of Src activity after the i.c.v. injection of 50 μg of AZD0530, induces a significant decrease in naloxone‐precipitated diarrhea, jumping, mastication, paw tremors, and wet dog shakes (WDS). Body weight (Body Wt Change), grooming, and locomotor activity remain unchanged. Significant differences among the groups were determined using one‐way ANOVA, followed by Duncan's post hoc comparison. ***P < 0.001 and **P < 0.01 significant differences between the morphine‐and‐naloxone‐treated mice (MS + Naloxone, n = 8) and morphine‐naloxone‐and‐AZD‐treated mice (MS + Naloxone + AZD, n = 8). ### P < 0.001 and # P < 0.05 significant differences between the placebo‐and‐naloxone‐treated mice (Placebo + Naloxone, n = 8) and morphine‐and‐naloxone‐treated mice (MS + Naloxone). $$ P < 0.01 and $ P < 0.05 significant differences between the placebo‐and‐naloxone‐treated mice (Placebo + Naloxone) and morphine‐naloxone‐and‐AZD0530‐treated mice (MS + Naloxone + AZD0530). The bars and errors represent the means ± SEM. Exact P‐values are in Appendix Table S3.
Figure EV3. Naloxone‐precipitated withdrawal signs in WT mice in which AZD0530 was stereotaxically injected into the LC .
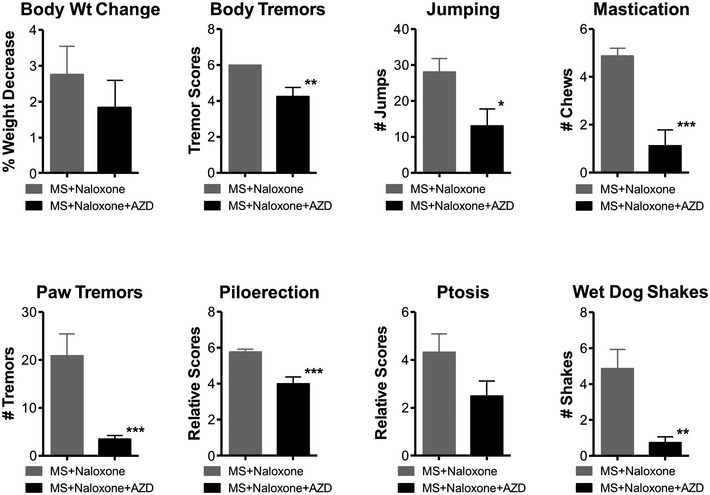
This presentation allows a comparative view of all the withdrawal signs including jumping presented in Fig 5B. The inhibition of Src activity following the injection of 7.5 μg/μl AZD0530 into the LC, induces a significant decrease in naloxone‐precipitated body tremors, jumping, mastication, paw tremors, piloerection, and wet dog shakes. Body weight (Body Wt Change) and ptosis remain unchanged. Significant differences between the morphine‐and‐naloxone‐treated mice (MS + Naloxone, n = 11) and morphine‐naloxone‐and‐AZD‐treated mice (MS + Naloxone + AZD, n = 13) were determined using the unpaired Student's t‐test. ***P < 0.001, **P < 0.01 and *P < 0.05 significant differences between MS + Naloxone and MS + Naloxone + AZD. The bars and errors represent the means ± SEM. Exact P‐values are in Appendix Table S4.
Naloxone‐precipitated somatic withdrawal signs are dependent on Fyn levels
The central role of Src activity and naloxone‐precipitated somatic withdrawal was further demonstrated in Fyn−/− mice. WT, heterozygous, and homozygous Fyn−/− mice were implanted with morphine pellets for 4 days and subjected to naloxone‐precipitated withdrawal. As summarized in Figs 1C and EV1B, increases in pSrcY416 and pMORY336 levels after naloxone‐precipitated withdrawal were not observed in Fyn−/− mice. A parallel decrease in naloxone‐precipitated withdrawal signs was observed. Some somatic withdrawal signs such as diarrhea, jumping, mastication, paw tremors, and WDS were blunted in the Fyn−/− mice (Figs 5C and EV4). These alterations in somatic withdrawal signs in the Fyn−/− mice are similar to those observed with AC1−/− but not AC8−/− mice (Zachariou et al, 2008). The incomplete blockade of somatic withdrawal signs could be due to the presence of other Src isoforms, as suggested by the presence of residual Src kinase activity and pSrcY416 immunofluorescence within the LC structure of the Fyn−/− mice (Fig EV1B). Nonetheless, the same signs that were blunted in the WT mice injected with AZD0530 were also reduced in the Fyn−/− mice. The blunting of the withdrawal signs appears to be dependent on gene dosage. Overall global withdrawal signs were significantly decreased from 62.3 ± 10.5 in the WT mice to 29.5 ± 2.8 in the homozygotes (F (2,15) = 11.40, P = 0.000978, one‐way ANOVA; P = 0.000409, post hoc; Fig 5C).
Figure EV4. Naloxone‐precipitated withdrawal signs in WT, HETERO, and HOMO of Fyn−/− mice.
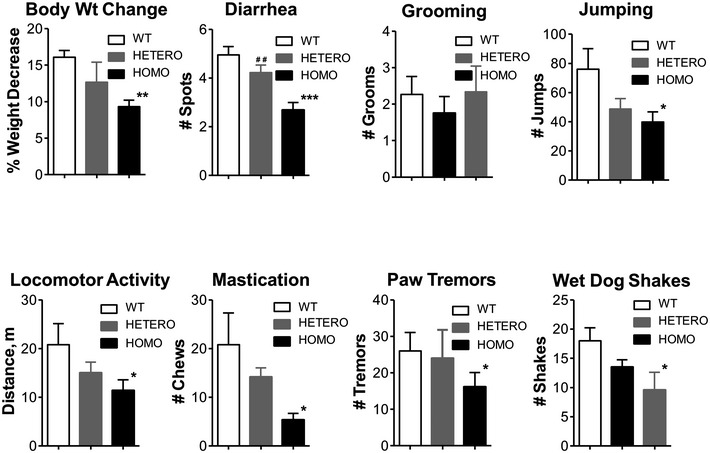
The significant reduction in body weight, diarrhea, jumping, mastication, paw tremors, and wet dog shakes observed in the HOMO Fyn−/− mice suggests the involvement of Fyn in the manifestation of these naloxone‐precipitated withdrawal signs. Also, HETERO Fyn+/− mice display a significant attenuation of the number of jumps. Locomotor activity and grooming are not affected by the reduction or absence of Fyn in Fyn+/− or −/− mice, respectively. Significant differences among the groups were determined using one‐way ANOVA, followed by Duncan's post hoc comparison (n = 6/group). ***P < 0.001, **P < 0.01, and *P < 0.05 relative to WT mice; ## P < 0.01 significant differences between HETERO Fyn+/− mice and HOMO Fyn−/− mice. The bars and errors represent the means ± SEM. Exact P‐values are in Appendix Table S5.
The probable involvement of Fyn in naloxone‐precipitated withdrawal can be further substantiated by the decrease in total Fyn levels following injection of Fyn shRNA. Regardless of the viral vehicle, either 5.0 × 108 TU/ml for lentivirus or 2.1 × 1012 TU/ml for AAV2/9, the injection of the Fyn shRNA virus into the LC (Fig 5D, panel 1.) resulted in a 70.8 ± 4.5% (n = 8, P = 0.01) decrease in total Fyn levels as demonstrated by Western blot analysis (Fig 5D, panel 2.). When naloxone‐precipitated withdrawal signs were monitored in mice injected with Fyn shRNA virus and compared to those injected with GFP virus, significant reductions in several withdrawal signs such as jumping, mastication, and paw tremors were observed (Figs 5E and EV5). The reduction in jumping was significant, but was much less than that observed following AZD0530 injection or in the Fyn−/− mice (GFPv = 132.2 ± 12.77, n = 13; Fyn shRNAv = 91.0 ± 6.12, n = 12; P = 0.009429 unpaired Student's t‐test). Furthermore, the WDS somatic withdrawal sign that was significantly reduced in AZD0530‐injected mice or in Fyn−/− homozygotes remained unchanged in the Fyn shRNA‐injected mice. This discrepancy could be due to incomplete knockdown resulting in a residual level of Fyn in the LC (70.8% decrease). Nevertheless, there was a significant decrease in the global withdrawal scores, from 59.1 ± 4.1 to 40.7 ± 1.8 (GFPv, n = 13; Fyn shRNA, n = 12; P = 0.001723 unpaired Student's t‐test).
Figure EV5. Naloxone‐precipitated withdrawal signs in WT mice injected in which control (GFP) or Fyn shRNA was injected into the LC .
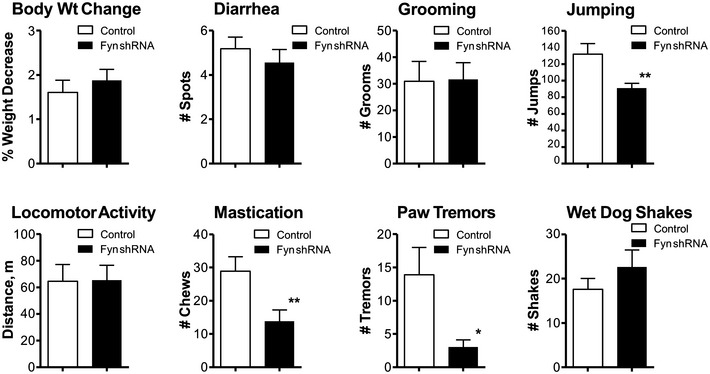
One microliter of Fyn shRNA AAV2/9 virus (titer: 2.09 × 1012 v.g./ml) was injected bilaterally into the LC (n = 12) as described in the Materials and Methods. The same number of control mice was injected bilaterally with the control GFP virus. This presentation allows a comparative view of all the withdrawal signs including jumping presented in Fig 5E. Fyn knockdown induced a significant decrease in naloxone‐precipitated jumping, mastication, and paw tremors. Body weight, diarrhea, grooming, locomotor activity, and wet dog shakes remain unchanged. Significant differences between the control (GFP‐transferred) and Fyn shRNA‐transferred WT mice were determined using Student's t‐test. **P < 0.01 and *P < 0.05 significant differences between the control (GFP‐transferred) and Fyn shRNA‐transferred WT mice. The bars and errors represent the means ± SEM. Exact P‐values are in Appendix Table S6.
Phosphorylation of MOR at Tyr336 is a prerequisite for naloxone‐precipitated withdrawal
The role of the Src‐mediated phosphorylation of MOR at Tyr336 in naloxone‐precipitated withdrawal was further substantiated in MOR−/− mice. The absence of any morphine dependence or somatic withdrawal signs in the MOR−/− mice is unequivocal. If the MOR non‐canonical signaling pathway is critical during naloxone‐precipitated withdrawal, we should be able to delineate differences in the somatic withdrawal in MOR−/− mice after the WT or the Y336F mutant MOR is reintroduced into the brain regions involved in opiate withdrawal. Lentiviruses in which a TH promoter fragment (kindly provided by Uwe Maskos, Pasteur Institute, Paris, FR) was used to control the expression of the transgene in the TH+ neurons of the LC were constructed (Toiu et al, 2010). Equal amounts of lentivirus, 3.82 × 105 TU/ml with the TH promoter driving the expression of either GFP (TH‐GFPv), WT MORGFP (TH‐MORGFPv), or mutant MORY336FGFP (TH‐Y336FGFPv), were injected bilaterally into the LC 1 week prior to the implantation of a morphine pellet. The mice were subsequently subjected to naloxone‐precipitated withdrawal 4 days later. Immunofluorescence analysis indicated that the expression of the transgenes was localized within the LC structure (Appendix Fig S4A–F). 46.34 ± 15.36% and 47.82 ± 13.68% of the TH+ neurons expressed the TH‐MORGFPv and mutant TH‐Y336FGFPv, respectively. All the somatic withdrawal signs we graded and monitored, we observed the restoration of withdrawal jumping and WDS in all the MOR−/− mice that had been stereotaxically injected with the TH‐MORGFPv (Fig 6). Although the naloxone‐precipitated jumping in the MOR−/− mice injected with the TH‐MORGFPv was significantly lower than that observed in the WT mice treated similarly (79.67 ± 17.61 jumps in WT mice (n = 6) vs. 13.25 ± 5.46 jumps in MOR−/− mice with TH‐MORGFPv (n = 8) (P = 0.001564, Duncan's post hoc test; F (3,22) = 17.35, P = 0.000005, one‐way ANOVA with “MOR gene expression” as the variable), withdrawal jumping was not observed in MOR−/− mice that had been stereotaxically injected with either TH‐GFPv or TH‐Y336FGFPv (Fig 6). The restoration of the WDS in MOR−/− mice appeared to be more robust than the restoration of the jumping. The MOR−/− mice that had been stereotaxically injected with the WT MORGFP virus exhibited a greater number of shakes (20.25 ± 4.59 shakes) than the WT mice treated with MS (15 ± 2.99 shakes) (Fig 6; P = 0.393488, Duncan's post hoc test). The MOR−/− mice injected with TH‐GFPv did not exhibit any restoration of WDS. However, WDS were observed in five out of six MOR−/− mice that had been injected with TH‐Y336FGFPv (and in no mice that had been injected with TH‐GFPv). The average was 5.00 ± 1.77 shakes in TH‐Y336FGFPv mice, which is significantly lower than that observed in WT (P = 0.016420, Duncan's post hoc test) or MOR−/− mice injected with TH‐MORGFPv (P = 0.005544, Duncan's post hoc test; Fig 6; F (3,22) = 8.19, P = 0.000761, one‐way ANOVA with “MOR gene expression” as the variable). MOR gene expression levels were similar between the TH‐MORGFPv‐ and TH‐Y336FGFPv‐transferred MOR−/− mice as verified by RT–qPCR (Fig 7A). Regression analyses of the MOR mRNA levels correlating with the withdrawal scores were performed with several MOR‐transferred MOR−/− mice that were taken randomly from the experiment presented in Fig 6. However, the sample is not sufficiently large to detect precisely the relationship between the WT or mutant MOR mRNA levels and the corresponding scores of each withdrawal signs (Fig 7B and C), although there seems to be a strong correlation between the level of MOR gene expression and the number of WDS in the TH‐MORGFPv‐transferred MOR−/− mice. Hence, the mutation of Tyr336 to Phe, which blocked Src‐mediated phosphorylation and the initiation of the MOR non‐canonical signaling pathway, attenuated naloxone‐precipitated withdrawal.
Figure 6. Restoration of naloxone‐precipitated somatic withdrawal signs in MOR −/− mice with WT MOR but not MORY336F lentivirus injected into the LC .
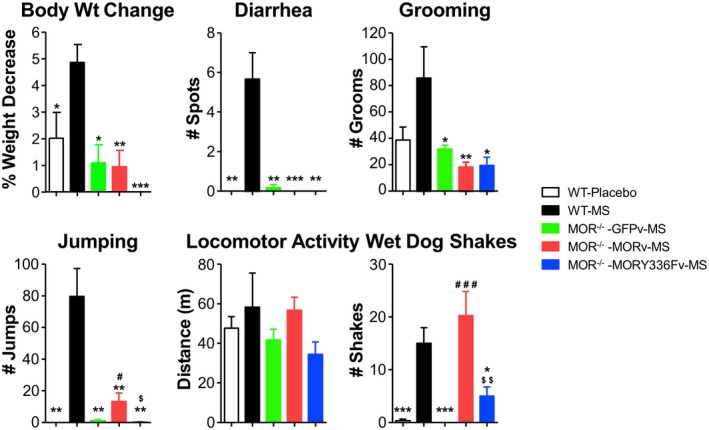
Construction of the lentiviruses containing the TH promoter that controls the expression of GFP (control), WT MORGFP, or MORY336F‐GFP was as described in the Materials and Methods. MOR−/− mice were stereotaxically injected with the GFPv (n = 6), MORv (n = 8), or MORY336Fv (n = 6) 1 week prior to chronic treatment with morphine and the subsequent monitoring of the naloxone‐precipitated somatic withdrawal signs as described. In parallel, chronic morphine treatment and naloxone‐precipitated withdrawal were carried out in WT mice (WT‐MS, n = 6). Placebo‐implanted WT mice injected with naloxone do not show withdrawal (WT‐Placebo, n = 6). The somatic withdrawal signs of body weight change, diarrhea, grooming, jumping, locomotor activity, and WDS were assessed for 30 min. Significant differences among the groups (MOR−/− ‐GFPv‐MS, MOR−/− ‐MORv‐MS, and MOR−/− ‐MORY336F‐MS) were determined using one‐way ANOVA, followed by Duncan's post hoc tests. ***P < 0.001, **P < 0.01 and *P < 0.05 relative to WT‐MS; ### P < 0.001 and # P < 0.05 significant differences between MOR−/− ‐GFPv‐MS and MOR−/− ‐MORv‐MS; $$ P < 0.01 and $ P < 0.05 significant differences between MOR−/− ‐MORv‐MS and MOR−/− ‐MORY336Fv‐MS. The bars and errors represent the means ± SEM. Exact P‐values are in Appendix Table S1.
Figure 7. Similar MOR gene expression between the WT MOR‐ and MORY336F‐transferred MOR −/− mice and regression analyses.
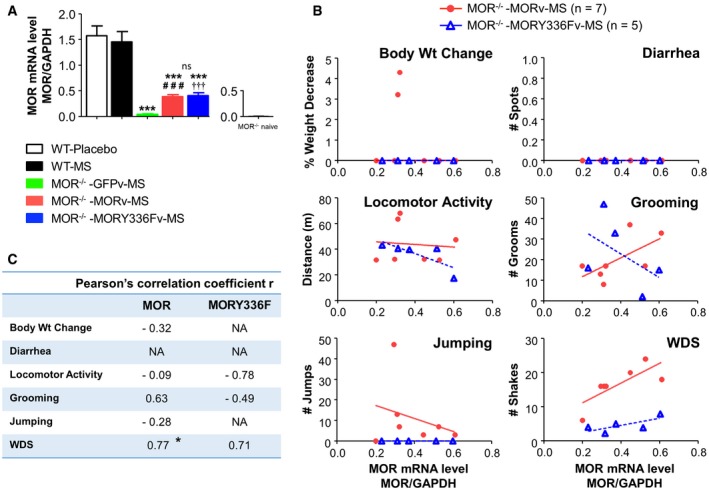
- Quantitative real‐time PCR (RT–qPCR) showing the level of MOR gene expression in the LC of placebo‐treated WT mice (WT‐Placebo, n = 3), and morphine‐dependent mice undergoing naloxone‐precipitated withdrawal such as WT (WT‐MS, n = 3), GFP‐transferred MOR−/− (MOR−/− ‐GFPv‐MS, n = 4), WT MORGFP‐transferred MOR−/− (MOR−/− ‐MORv‐MS, n = 7), and MORY336F‐GFP‐transferred MOR−/− (MOR−/− ‐MORY336Fv‐MS, n = 5) mice presented in Fig 6. MOR mRNA levels are expressed relative to GAPDH mRNA levels in the LC. Each value represents the mean ± SEM of at least four independent experiments. Significant differences among the groups (MOR−/− ‐GFPv‐MS, MOR−/− ‐MORv‐MS, and MOR−/− ‐MORY336Fv‐MS) were determined using one‐way ANOVA, followed by Duncan's post hoc comparison. ***P < 0.001 relative to WT‐MS; ### P < 0.001 significant differences between MOR−/− ‐GFPv‐MS and MOR−/− ‐MORv‐MS; ††† P < 0.001 significant differences between MOR−/− ‐GFPv–MS and MOR−/− ‐MORY336Fv‐MS; ns, no significant differences between MOR−/− ‐MORv‐MS and MOR−/− ‐MORY336Fv‐MS.
- Regression analyses of the MOR expression levels correlated with the withdrawal scores in several mice that were taken randomly from the MOR−/− ‐MORv‐MS and MOR−/− ‐MORY336Fv‐MS groups presented in Fig 6.
- The Pearson's correlation coefficient r showed a significant and strong correlation between the level of MOR gene expression and the number of wet dog shakes (WDS) in the TH‐MORGFPv‐transferred MOR−/− mice. *P < 0.01, significant r in TH‐MORGFPv‐transferred MOR−/− mice.
Discussion
The increase in AC activity above the basal level during naloxone‐precipitated withdrawal has long been a working hypothesis for the PKA activation that leads to alterations in the DARPP‐32 signaling pathway in various brain regions and to the subsequent behavioral responses observed during opiate withdrawal. The exact mechanism of AC superactivation was not established until our recent observations that identified the requirement for the Src‐mediated phosphorylation of MOR at Tyr336. The phosphorylation of MOR resulted in the formation of a signaling complex that consisted of Grb/SOS/Ras/Raf‐1 and resembled that of the tyrosine kinase receptor (Zhang et al, 2013). Such a non‐canonical MOR signaling pathway was demonstrated in a cell model system where receptor mutagenesis studies were performed in conjunction with the use of a p‐Tyr pan‐antibody. In the current studies, we could demonstrate the in vivo phosphorylation of MOR at Tyr336 with the affinity‐purified antibody anti‐pMORY336. The specificity of this antibody was clearly demonstrated by its inability to detect any MOR phosphorylation in HEK293 cells expressing a MORY336F mutant and by the pre‐adsorption controls (Figs 1B and 2A). Although this antibody could detect the phosphorylation of DOR at Tyr318 in HEK293 cells due to the peptide sequence NPVLpY that was used to develop the antibody, the minimal signal detected in MOR−/− mice after chronic morphine treatment (Fig EV1A) confirms our conclusion that anti‐pMORY336 is indeed detecting the phosphorylation of MOR at Tyr336. A definitive conclusion regarding the phosphorylation of MOR at Tyr336 could come from the mass spectrometric analysis of the phospho‐peptides isolated from the enzymatic digest of purified MOR. However, due to the location of Tyr336, obtaining an optimal digest of MOR is not trivial, and we have yet to successfully generate the peptide fragments required for mass spectrometry. Nevertheless, our results generated using the WT receptor, the Y336F mutant and Fyn−/− and MOR−/− mice collectively demonstrate that the pMORY336 antibody is indeed detecting the Src‐mediated phosphorylation of MOR at Tyr336. We also did not observe basal levels of pMORY336 in mice implanted with a placebo pellet (Fig 2A). This result is in line with previous studies that showed that there is a basal MOR phosphorylation of Ser363 and Thr370, but not Tyr336, in the absence of morphine or with naloxone alone (El Kouhen et al, 2001; Zhang et al, 2009). Western blots from the LC from mice implanted with either placebo or morphine pellets revealed basal levels of pSrcY416 (Fig 2A). This observation does not affect the key fact that morphine pellets implantation and subsequent naloxone treatment provoke a significant increase of pSrcY416 concomitant with MORY336 phosphorylation. More importantly, this increase in SrcY416 phosphorylation was totally blocked by the Src inhibitor AZD0530 (Fig 2A, lane 4).
It is of interest to note that the in vivo phosphorylation of MOR at Tyr336 and the formation of the non‐canonical signaling complex occur only after chronic morphine treatment and naloxone‐precipitated withdrawal (Figs 1A–D and 2A–D), since we did not detect pMORY336 and increases of pSrcY416 from the LC of mice in the presence of morphine pellet alone. However, according to previous in vitro results, there may be a pre‐existing complex of Src and MOR in the presence of chronic morphine treatment only. Indeed, the prolonged morphine treatment of cultured cells caused a transitory increase in pSrc that reached a maximum level after 2 h of treatment but decreased 4 h later (Zhang et al, 2009). When pSrc decreased, Src was still highly present in the complex in the presence of morphine. Then, the consecutive exposure to naloxone not only amplified the phosphorylation but also the quantity of Src in this pre‐existing complex suggesting recruitment of the kinase (Zhang et al, 2009). If such an in vivo increase in MOR phosphorylation and signal complex formation will lead to AC superactivation as observed with in vitro clonal cell lines (Zhang et al, 2013), then the subsequent aberrant increase in PKA and CREB activities that leads to the adaptation to chronic exposure to opiates will not occur as long as agonists such as morphine remain bound to the receptor. Also, if withdrawal and the adverse motivational state are the keys to drug addiction as suggested (Koob, 2009; Koob & Volkow, 2010), then the modulation of the neural substrates and circuitry that contribute to drug craving will not occur unless the agonist is dissociated from the receptor either by naloxone competition or the decrease in agonist concentration that occurs in opiate abstinence. To define the transcripts that are involved in withdrawal and the adverse motivational state and therefore drug addiction, the transcripts that have roles in such a state must be distinguished from those that participate in the other states of the addiction cycle. Although the alteration of PKA or CREB activity in the NAc clearly modulates the morphine reward (Self et al, 1998; Barrot et al, 2002), the observed alterations in the downstream targets of PKA and CREB might not reflect those participating in the withdrawal/adverse motivational state due to the prolonged activation of PKA or CREB that occurs when the agonist remains bound to the receptor.
To avoid unwanted modifications of the downstream targets of PKA, CREB, or Src kinase, studying the effects of the inhibition of Src kinase activity on naloxone‐precipitated withdrawal whether with the use of AZD0530, Fyn−/− mice, or Fyn shRNA becomes significant and coherent only when it is combined with the use of phosphorylation‐deficient Y336F mutant MOR in MOR−/− mice. The aim is essentially to block MORY336 phosphorylation. We assume that the reduction in the morphine withdrawal symptoms (i.e., jumping) when MORY336 phosphorylation is blocked or inhibited by Src inhibitors or receptor mutation, prevents the formation of the complex Grb/SOS/Ras/Raf‐1 with the receptor and, thus, phosphorylation and activation of AC5/6 by Raf‐1. Because Tyr336 of MOR is itself one of the substrates of Src kinase, the alteration in opiate withdrawal signs resulting from the direct phosphorylation of Src kinase of MORY336 is one possibility; whether the tyrosine phosphorylation in the NPXXY motif serves as a new docking site to directly recruit SH2/SH3 domain‐containing proteins (i.e., Src kinase) to activate AC remains to be determined. However, Src kinase activity can affect indirectly the MORY336 phosphorylation levels through other signaling molecules. For example, Src kinase phosphorylates phospholipase Cγ (PLCγ) and the GPCR‐kinase‐interacting protein‐1 (GIT1) after the activation of angiotensin II type 1 receptor and epidermal growth factor receptor (Haendeler et al, 2003). GIT1 has been shown to be important for GPCR internalization and acts as an integrator of Src‐dependent signal transduction activated by GPCRs and receptor tyrosine kinases (Haendeler et al, 2003).
Some existing treatments for drug relapse focus on modulating the glutamatergic activities that regulate the dopaminergic output within the NAc‐VTA reward pathway. However, if the incentive‐motivational theory in which drug withdrawal episodes enhance the drug's incentive value to the extent that compulsive drug‐taking and drug‐seeking take over the behavioral repertoire is correct (Hutcheson et al, 2001), then the modulation of withdrawal episodes will definitely have a pronounced effect on behavior associated with addiction. Although the use of protein kinase inhibitors such as those for PKA (Taubenfeld et al, 2010) and the Src kinase inhibitor PP2 (Narita et al, 2006) could modulate various aspects of chronic drug effects, these kinase inhibitors may also attenuate, in a maladaptive manner, a myriad of kinase actions that are unrelated to drug addiction such as synaptic plasticity, immune cells development, or cell division (Parsons & Parsons, 2004; Babus et al, 2011; Sen & Johnson, 2011). For example, intracerebral microinjections with PP2 significantly suppressed the morphine‐induced rewarding effect and hyperlocomotion in a dose‐dependent manner (Narita et al, 2006) and reduced the ethanol self‐administration in WT animals (Wang et al, 2007). In humans, a genetic study correlated a mutation of the fyn gene with increased alcohol consumption (Schumann et al, 2003). A once‐daily administration of SU‐6656, another selective SFK markedly and dose‐dependently attenuated the naloxone‐precipitated withdrawal syndrome in morphine‐dependent mice (Rehni & Singh, 2011). However, treatment with Src inhibitors such as Dasatinib increases the infection rate in patients with cancer, and this was suggested to occur because Dasatinib affects the immune system by reduction of neutrophil adhesion and recruitment into injured tissue (Parsons & Parsons, 2004; Zarbock, 2012). Thus, our results not only strongly suggest that the MOR non‐canonical signaling pathway, particularly with the focal event of MORY336 phosphorylation, may be critical for AC superactivation during the withdrawal/negative affective stage of addiction, but they also provide a new pharmacological approach for blunting opiate withdrawal episodes without altering kinase activity. If the phosphorylation of MOR at Tyr336 residue within the NPVLY motif can be blunted with small molecules that do not inhibit protein kinases such as PKA and Src that are critical in overall memory consolidation, only the behavioral repertoire associated with drug‐taking and drug‐seeking should be modified. These small molecules could lead to future novel treatment paradigms for opiate addiction.
Materials and Methods
Animals
Homozygous Fyn knockout (Fyn−/−) s129 mice were obtained from Jackson Laboratory (Bar Harbor, ME) and backcrossed to C57BL/6J mice until F‐13 before use. WT, heterozygous, and homozygous mice from the same litter were used in the studies. The μ‐opioid receptor knockout (MOR−/−) C57BL/6J mice were generous gifts from Dr. John Pintar (Robert Wood Johnson Medical School, Piscataway, New Jersey, USA) and were crossed with C57BL/6J mice to produce the WT and MOR−/− mice for the experiments. The mice were housed in a temperature‐controlled (21–23°C) environment with a 12‐h light/dark cycle and with food and water ad libitum. The experiments were conducted on 2‐month‐old male mice (30–35 g). All the animal procedures followed the National Institutes of Health (NIH) guidelines and were approved by the University of Minnesota Institutional Animal Care (Protocol# IACUC1303A30454) and Use Committee and the Bioethics Committee of the Shanghai Institute of Materia Medica (Shanghai, China). Animals were randomly included into experimental groups according to genotyping. The experimenters were blinded for mouse treatment or transferred gene during behavior testing.
Development and validation of rabbit polyclonal anti‐pMORY336
Antigen‐purified polyclonal antibodies against the phosphorylated Tyr336 of MOR (anti‐pMORY336) were developed by GeneTex, Inc (Irvine, CA) by immunizing rabbits with the conjugated synthetic peptide NPVLY*AFLDENC. The ability of anti‐pMORY336 to detect phosphorylated MOR, but not non‐phosphorylated MOR, was established by carrying out Western analyses and immunofluorescence studies on HEK293 cells stably expressing either WT‐HAMOR (MOR with the hemagglutinin epitope at N‐terminus), HAMORY336F, HADOR, or FlagKOR. HEK293 cells expressing these receptors were treated with 1 μM morphine (WT and mutant HAMOR), 1 μM DPDPE (HADOR), or 1 μM U50, 488 (FlagKOR) for 2 h followed by treatment with or without 10 μM naloxone (HAMOR and HADOR) or with 10 μM nor‐BNI (FlagKOR). Then, the cells were either transferred to ice‐cold detergent buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 10 mM disodium pyrophosphate, 1% Nonidet P‐40 [less hydrophilic than Triton X‐100 and most commonly used for immunoprecipitation], 0.5% sodium deoxycholate and 0.1% SDS containing protease and phosphatase inhibitors) for homogenization and Western analysis, or the cells were fixed with 4% paraformaldehyde in 0.1 M PBS, pH 7.4, with 4% sucrose at 4°C for immunofluorescence analysis. For Western analysis, the receptor in the supernatants (16,000 × g, 30 min) was immunoprecipitated using protein G‐agarose and mouse monoclonal anti‐HA (Covance MMS‐101P, 1:200) or anti‐Flag (Sigma‐Aldrich F‐3165, 1:200) antibodies. The proteins were eluted from the beads by heating them using the SDS–PAGE treatment buffer at 65°C for 30 min and then resolved on an 8% SDS–PAGE gel. The levels of pMORY336 and total epitope‐tagged receptors were examined by Western blot analysis using anti‐pMORY336 (1 μg/ml), rabbit anti‐HA (Covance PRB‐101P, 1:1,000), or anti‐Flag (Sigma‐Aldrich F‐3165, 1:1,000) antibodies with chemifluorescence performed using a Storm 860 (Molecular Dynamics). As for the immunofluorescence studies, after fixing the cells with paraformaldehyde at 4°C for 10 min and quenching the fixation by washing with 0.1 M glycine in PBS, the cells were permeabilized by the addition of 0.25% Triton X‐100 for 5 min. The levels of total and phosphorylated receptors were determined by incubating with anti‐HA (Covance MMS‐101P, 1:500), or anti‐Flag (Sigma‐Aldrich F‐3165, 1:200) and anti‐pMORY336 (2 μg/ml) antibodies at RT for 1 h, and then incubating with Alexa Fluor 488‐conjugated goat anti‐mouse (Invitrogen A‐10680, 1:5,000) and Alexa Fluor 594‐conjugated goat anti‐rabbit (Invitrogen R37117, 1:5,000) antibodies. Immunofluorescence was visualized using a CCD camera connected to a Leica DM5500 B upright microscope (Leica, Germany). Leica Application Suite (Leica, Germany) and Metamorph (Sunnyvale, CA) software were used to analyze colocalization.
Chronic morphine treatment and naloxone‐induced withdrawal signs
Mice were chronically exposed to morphine by implanting a 75‐mg morphine pellet (National Institute on Drug Abuse) or a placebo pellet subcutaneously (s.c.) for 4 days. The evening before the implantation of the morphine pellet, the mice were injected with a single dose of 2.4 mg/kg of morphine sulfate (the ED50 value for tail‐flick antinociception) to ensure the survival of all the mice. On the 4th day after pellet implantation, naloxone hydrochloride (10 mg/kg) was injected intraperitoneally (i.p.) 4 h after the pellet was removed as per the method of Yano & Takemori (1977) and Seth et al (2011). The morphine analgesic tolerance previously measured with the tail‐flick test (Patrick et al, 1975; Yoburn et al, 1985; Kibaly et al, 2017) is complete at 72 h after the implantation of a 75‐mg morphine pellet and persists for at least 24 h after pellet removal (Patrick et al, 1975). The mice were then placed in a locomotor chamber (18″ × 18″), and the withdrawal signs were tracked and recorded with the ANY‐maze software (Stoelting Co., Wood Dale, IL) for the next 30 min. In the Src kinase inhibitor and Fyn shRNA experiments, parallel studies were carried out by chronically exposing the mice to morphine by pellet implantation, or by exposing the mice to progressively increasing morphine doses via s.c. injections. In the latter case, the mice were injected with either progressive doses of 10, 20, 30, 40, and 50 mg/kg of morphine hydrochloride or saline twice a day for 5 days. On day 6, the mice were injected with saline or morphine hydrochloride (60 mg/kg), and withdrawal signs were induced by the i.p. injection of naloxone hydrochloride (10 mg/kg) 4 h later and recorded. Somatic withdrawal signs such as jumping, WDS, and weight loss were graded, and signs such as diarrhea, mastication, grooming, paw tremors, ptosis, and piloerection were monitored. The locomotor activity of the mice was also recorded during the 30‐min withdrawal period. The global withdrawal signs were then determined as described (Papaleo & Contarino, 2006).
Intracerebroventricular (i.c.v.) injection of AZD0530 difumarate
The Src kinase inhibitor was injected intracerebrally into the third ventricle. The mice were anesthetized by i.p. injecting a mixture of ketamine and xylazine (final dose: 90 mg/kg ketamine and 10 mg/kg xylazine). The mice were then mounted on a stereotaxic frame (Stoelting). After exposing the skull and removing the connective tissues using the bregma as the zero coordinate, a 0.5‐mm burr hole was drilled at the anteroposterior (AP) coordinate – 0.94 mm. A metal cannula with a plug was then secured at this position with dental cement. The mice were then allowed to recover from the procedure for 1 week prior to implanting either a placebo or a morphine (75 mg) pellet s.c. On the 4th day after pellet implantation, the pellet was removed. The mice were then anesthetized with isoflurane 3.5 h after pellet removal and mounted on the stereotaxic frame. A 10‐μl Hamilton syringe was filled with AZD0530 difumarate (Axon MedChem, Reston, VA). With the implanted cannula as the guide, the syringe was lowered to 2.25 mm below the skull in a 2‐min period. Two microliters of the AZD0530 solution was injected with a Quintessential Stereotaxic Injector (Stoelting) in a 10‐min period so as to deliver 50 μg of the inhibitor per mouse. The syringe was kept in place for an additional 5 min before it was gradually withdrawn to prevent backflow. Thirty minutes after the injection of AZD0530, the mice were i.p. injected with naloxone (10 mg/kg). The naloxone‐precipitated somatic withdrawal signs were checked and recorded.
Viral constructs
Fyn shRNA‐GFP lentivirus (titer: 5.0 × 106 TU/ml) and Fyn shRNA‐GFP AAV2/9 (titer: 2.09 × 1012 v.g./ml) were purchased from Santa Cruz Biotechnology (sc‐35425‐V) (Dallas, TX) and OBio (AAV‐830010H Y2692) (Shanghai, China), respectively. The WT MORGFP and MORY336FGFP mutant lentivirus with the tyrosine hydroxylase (TH) promoter were constructed by cloning the WT MORGFP fragments into the pCDH‐CMV promoter lentiviral vector system (System Biosciences, Mountain View, CA). Site‐directed mutagenesis was used to introduce a Y336F mutation into MORGFP using the primers: 5′‐TTTCATCCAGGAATGCGAAAAGAACTGGATTCAGGCAGCT‐3′ and 5′‐AGCTGCCTGAATCCAGTTCTTTTCGCATTCCTGGATGAAA‐3′ (QuikChange II, Agilent Technologies, Santa Clara, CA). The 2.5‐kb Nhe‐EcoRI TH promoter fragment, provided by Dr. Uwe Maskos (Pasteur Institute, Paris, FR), were cloned into pCDH‐CMV after the removal of the CMV promoter with the SnaBI/EcoRI endonucleases. Lentivirus particles were produced by the Lipofectamine‐mediated transfection of HEK293T cells with four‐plasmid mixtures: pLP1, pLP2, pLP/VSVG, and pCDH‐TH‐MORGFP or pCDH‐TH‐MORY336F‐GFP (ratio: 3:1:1:5.5), and were purified with iodixanol gradients and tittered by flow cytometry using HT‐1080 cells as described previously (Lin et al, 2012).
Stereotaxic injection into the locus coeruleus
For the microinjections at the LC, mice were anesthetized by the i.p. injection of a mixture of ketamine and xylazine (final dose: 90 mg/kg ketamine and 10 mg/kg xylazine). After mounting the mice on the stereotaxic frame, a midsagittal incision was made to expose the bregma and lambda. With the bregma as zero, the coordinates for the LC were anteroposterior (AP) – 5.15 mm, mediolateral (ML) ± 1.20 mm, and dorsoventral (DV) – 3.62 mm (Franklin & Paxinos, 2008). For the AZD0530 difumarate injection, the mice were implanted bilaterally with a metal guide cannulae at the same coordinates 1 week prior to the initiation of chronic morphine treatment. One microliter of either AZD0530 difumarate or virus solution was microinjected through a 31‐gauge Hamilton microsyringe on each side at a rate of 0.2 μl/min using the Quintessential Stereotaxic Injector (Stoelting). The syringe needle was kept in place for an additional 5 min before it was withdrawn to prevent backflow. For the virus injection, the mice could recover for 1 week in their home cage prior to morphine exposure. For the AZD0530 difumarate injection, the Src kinase inhibitor was injected using the implanted metal cannulae as a guide 3.5 h after pellet removal or the last dose of morphine, and 30 min prior to the i.p. injection of naloxone.
Quantifying Fyn levels in the locus coeruleus by Western analysis
Three weeks after virus infusion, the mice were euthanized by decapitation. Coronal brain sections (1 mm thick) were obtained using a mouse brain slicer (Braintree Scientific, Braintree, MA). Bilateral LC punches were pooled from two mice (four punches). The tissue was then homogenized in 100 μl of buffer containing 1% Triton, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, and protease and phosphatase inhibitors cocktail. The insoluble materials were removed by centrifugation at 10,000 × g for 10 min at 4°C. The protein concentration of the supernatant was determined using the BCA assay kit. Then, 20 μl of supernatant (~20 μg) was applied to the SDS–PAGE gel for Western analysis using the rabbit polyclonal antibodies against all the Src subtypes (Thermo Fisher Scientific, Waltham, MA).
Immunohistofluorescence analysis
The mice were anesthetized with 100 mg/kg sodium pentobarbital and then perfused with Tyrode's solution (116 mM NaCl, 5.36 mM KCl, 2 mM MgCl2.6H2O, 0.406 mM MgSO4.7H2O, 1.23 mM NaH2PO4, 3 mM glucose, 26.2 mM NaHCO3, pH 7.2), followed by 4% paraformaldehyde (Sigma‐Aldrich) in 0.1 M phosphate buffer (75 mM KH2PO4, 85 mM Na2HPO4.7H2O). The brains were post‐fixed in 4% paraformaldehyde overnight at 4°C, immersed in 10 and 30% sucrose until they sank to the bottom and frozen in OCT (Sakura Finetek, Tokyo, Japan). Thirty‐micrometer coronal tissue sections were prepared with a cryostat (Leica Microsystems, Wetzlar, Germany) and mounted on Superfrost Plus glass slides (Thermo Fisher Scientific). The sections were incubated with either rabbit anti‐TH (1:1,000, for the identification of Fyn shRNA injections and WT MOR or MORY336F injections), goat anti‐TH (GeneTex GTX113016, 1:1,000), mouse anti‐pSrcY416 (Millipore clone 9A6 #05‐677, 1:500), or peptide affinity‐purified rabbit anti‐pMORY336 (1:500) overnight at 4°C. All primary antibodies were diluted in PBS, pH 7.4, 0.5% Triton X‐100, 1% BSA, and 3% donkey serum (depending on the host species of the secondary antibody). After the overnight incubation with the primary antibodies, the slides were incubated with the respective anti‐rabbit, anti‐goat, or anti‐mouse secondary antibodies (1:2,500) conjugated to either Alexa Fluor 488, Alexa Fluor 594, or Alexa Fluor 647. Immunofluorescence was visualized using a CCD camera connected to a Leica DM5500 B upright microscope (Leica, Germany). Leica Application Suite (Leica, Germany) and Metamorph (Sunnyvale, CA) software were used to perform the colocalization analysis of the images.
Quantitative RT–PCR (RT–qPCR)
RT–qPCR was performed according to the same procedure described by Song et al (2007) (Appendix Supplementary Materials and Methods). The specific primer sequences for MOR1 were: forward, 5′‐CATGGCCCTCTATTCTATCGTGT‐3′, and reverse, 5′‐CAGCGTGCTAGTGGCTAAGG‐3′ (Invitrogen, Carlsbad, CA, USA). The primers for the housekeeping gene glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) were as follows: forward, 5′‐GGTGAAGGTCGGTGTGAACG‐3′, and reverse, 5′‐CTCGCTCCTGGAAGATGGTG‐3′ (Invitrogen, Carlsbad, CA, USA).
Statistical analysis
The results were analyzed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). The data are presented as means ± SEM. Sample size was chosen because of previous experience regarding data variability in similar models. No statistical method was used to predetermine sample size. Statistical differences between values from two groups were determined using unpaired Student's t‐tests, whereas statistical differences between three or more groups were analyzed using ANOVAs followed by Duncan's post hoc comparisons. A difference of P < 0.05 was considered statistically significant.
Author contributions
LZ, CK, Y‐JW, CX, KYS: design, acquisition of data, analysis and interpretation of data, drafting and revising the article; PWM: acquisition of data, analysis, and interpretation of data; HHL: contribution unpublished essential data or reagents; J‐GL: conception and design, analysis and interpretation of data, drafting and revising the article; P‐YL: conception and design, analysis and interpretation of data, writing and revising the article.
Conflict of interest
The authors declare that they have no conflict of interest.
The paper explained.
Problem
Upregulation of the cAMP pathway mediated via AC superactivation (i.e., the compensatory increase in AC activity) in response to chronic opiate administration has been shown to be one important mechanism involved in physical opiate dependence and withdrawal. We recently demonstrated that AC superactivation after prolonged opiate treatment in cell models requires the recruitment and activation of a tyrosine kinase protein, Src, and subsequent phosphorylation of the MOR at Tyr336 by Src. Thus, our hypothesis is that elimination of MOR‐Tyr336 phosphorylation will prevent the appearance of withdrawal symptoms in mice after interruption of long‐term opioid treatment or addition of an opioid antagonist (e.g., naloxone).
Results
We observed the recruitment of Src in the vicinity of MOR and an increase in phosphorylated Tyr336 (pY336) levels during naloxone‐precipitated withdrawal in mice. The intracerebroventricular or stereotaxic injection into the locus coeruleus (LC) of a Src inhibitor (AZD0530), or Src shRNA viruses, attenuated pY336 levels, several somatic withdrawal signs, and overall global withdrawal scores in WT mice. Similar results were observed in Fyn−/− mice that do not express Fyn, a Src kinase subtype. The stereotaxic injection of wild‐type (WT) MOR, but not the phosphorylation‐deficient (Y336F) MOR, lentiviruses into the LC of MOR−/− mice restored somatic withdrawal jumping. These observations suggest that the Src‐mediated phosphorylation of MOR at Tyr336 is a prerequisite for opiate withdrawal.
Impact
Treatment for opiate abuse involves substituting heroin or other opiates with another agonist (methadone) or a partial agonist (buprenorphine). However, both drugs also have potential for abuse. Our findings form the basis for the development of small allosteric ligands for the μ‐opioid receptor that can inhibit receptor phosphorylation without inhibiting Src kinase activity or interfering with the other cellular functions of the Src kinase. Thus, they provide an alternative approach for the future development of drugs for the treatment of opiate abuse.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Source Data for Figure 1
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 7
Acknowledgements
This work was supported by grants from the National Institutes of Health DA031442, USA (PYL, LZ, CK, CX, KYS, PWM, HHL); from the Ministry of Science and Technology of China 2013CB835100 and 2015CB553502 (JGL); from the National Natural Science Foundation of China 81130087, 81671322 (JGL), and 81401107 (YJW); from the President's International Fellowship Initiative Program of the Chinese Academy of Sciences 2011T2S29 (PYL); and from the Youth Innovation Promotion Association of the Chinese Academy of Sciences 2017334 (YJW).
EMBO Mol Med (2017) 9: 1521–1536
Contributor Information
Cherkaouia Kibaly, Email: kibal001@umn.edu.
Jing‐Gen Liu, Email: jgliu@simm.ac.cn.
Ping‐Yee Law, Email: lawxx001@umn.edu.
References
- Alheid GF, De Olmos JS, Beltramino CA (1995) Amygdala and extended amygdala In The rat nervous system, Paxinos G. (ed.), pp 495–578. San Diego, CA: Academic Press; [Google Scholar]
- Avidor‐Reiss T, Nevo I, Levy R, Pfeuffer T, Vogel Z (1996) Chronic opioid treatment induces adenylyl cyclase V superactivation. Involvement of Gbetagamma. J Biol Chem 271: 21309–21315 [DOI] [PubMed] [Google Scholar]
- Avidor‐Reiss T, Nevo I, Saya D, Bayewitch M, Vogel Z (1997) Opiate‐induced adenylyl cyclase superactivation is isozyme‐specific. J Biol Chem 272: 5040–5047 [DOI] [PubMed] [Google Scholar]
- Babus LW, Little EM, Keenoy KE, Minami SS, Chen E, Song JM, Caviness J, Koo SY, Pak DT, Rebeck GW et al (2011) Decreased dendritic spine density and abnormal spine morphology in Fyn knockout mice. Brain Res 1415: 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini M (2005) Second nature: biological functions of the Raf‐1 “kinase”. FEBS Lett 579: 3271–3277 [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC et al (2002) CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA 99: 11435–11440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SLI, Peden L, Chang R, Harris RA, Blednov YA (2003) Deletion of the Fyn‐kinase gene alters behavioral sensitivity to ethanol. Alcohol Clin Exp Res 27: 1033–1040 [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Usiello A, Greengard P, Fisone G (2007) Activation of the cAMP/PKA/DARPP‐32 signaling pathway is required for morphine psychomotor stimulation but not for morphine reward. Neuropsychopharmacology 32: 1995–2003 [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Thome J, Olson VG, Lane‐Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ (1998) Regulation of cocaine reward by CREB. Science 282: 2272–2275 [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston‐Jones G (2000) Noradrenaline in the ventral forebrain is critical for opiate withdrawal‐induced aversion. Nature 403: 430–434 [DOI] [PubMed] [Google Scholar]
- Edwards S, Graham DL, Whisler KN, Self DW (2009) Phosphorylation of GluR1, ERK and CREB during spontaneous withdrawal from chronic heroin self‐administration. Synapse 63: 224–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kouhen R, Burd AL, Erickson‐Herbrandson LJ, Chang CY, Law PY, Loh HH (2001) Phosphorylation of Ser363, Thr370, and Ser375 residues within the carboxyl tail differentially regulates mu‐opioid receptor internalization. J Biol Chem 276: 12774–12780 [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (2008) The mouse brain in stereotaxic coordinates, compact, 3rd edn, Elsevier (ed). Amsterdam, London: Academic Press; [Google Scholar]
- Green T, Hennequin LF, Ple PA, Jones RJ, Clack G, Gallagher N (2005) Pre‐clinical and early clinical activity of the highly selective, orally available, dual Src/Abl kinase inhibitor AZD0530. Proc Am Assoc Cancer Res 46: abst SY13‐3 [Google Scholar]
- Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, Logie A, Hargreaves J, Hickinson DM, Wilkinson RW et al (2009) Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol Oncol 3: 248–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendeler J, Yin G, Hojo Y, Saito Y, Melaragno M, Yan C, Sharma VK, Heller M, Aebersold R, Berk BC (2003) GIT1 mediates Src‐dependent activation of phospholipase Cgamma by angiotensin II and epidermal growth factor. J Biol Chem 278: 49936–49944 [DOI] [PubMed] [Google Scholar]
- Hand TH, Koob GF, Stinus L, Le Moal M (1988) Aversive properties of opiate receptor blockade: evidence for exclusively central mediation in naive and morphine‐dependent rats. Brain Res Rev 474: 364–368 [DOI] [PubMed] [Google Scholar]
- He L, Kim JA, Whistler JL (2009) Biomarkers of morphine tolerance and dependence are prevented by morphine‐induced endocytosis of a mutant μ‐opioid receptor. FASEB J 23: 4327–4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs S, Menzaghi F, Schulteis G, Koob GF, Stinus L (1995) Suppression of corticotropin‐releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol 6: 74–80 [PubMed] [Google Scholar]
- Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, Lambert‐van der Brempt C, Morgentin R, Norman RA, Olivier A et al (2006) N‐(5‐Chloro‐1,3‐benzodioxol‐4‐yl)‐7‐[2‐(4‐methylpiperazin‐1‐yl)ethoxyl]‐5‐(tetrahydro‐2H‐pyran‐4‐yloxoy0quinazolin‐4‐amine, a novel, highly selective, orally available, dual‐specific c‐Src/Abl kinase inhibitor. J Med Chem 49: 6465–6488 [DOI] [PubMed] [Google Scholar]
- Hinson RE, Siegel S (1982) Nonpharmacological bases of drug tolerance and dependence. J Psychosom Res 26: 495–503 [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A (2001) The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci 4: 943–947 [DOI] [PubMed] [Google Scholar]
- Kibaly C, Lin HY, Loh HH, Law PY (2017) Spinal or supraspinal phosphorylation deficiency at the MOR C‐terminus does not affect morphine tolerance in vivo . Pharmacol Res 119: 153–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Lee KW, Lee KW, Im JY, Yoo JY, Kim SW, Lee JK, Nestler EJ, Han PL (2006) Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc Natl Acad Sci USA 103: 3908–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE (1988) Cellular and molecular mechanisms of drug dependence. Science 242: 715–723 [DOI] [PubMed] [Google Scholar]
- Koob GF (2009) Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56(Suppl 1): 18–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreckij T, Nguyen H, Brown LG, Yu EY, Vessella RL, Corey E (2009) Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br J Cancer 101: 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH (2000) Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol 40: 389–430 [DOI] [PubMed] [Google Scholar]
- Lin HY, Law PY, Loh HH (2012) Activation of protein kinase C (PKC)α or PKCɛ as an approach to increase morphine tolerance in respiratory depression and lethal overdose. J Pharmacol Exp Ther 341: 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Stinus L, Gold LH, Koob GF (1992) Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther 261: 669–677 [PubMed] [Google Scholar]
- Maldonado R, Koob GF (1993) Destruction of the locus coeruleus decreases physical signs of opiate withdrawal. Brain Res 605: 128–138 [DOI] [PubMed] [Google Scholar]
- Megison ML, Gillory LA, Stewart JE, Nabers HC, Mrozcek‐Musulman E, Beierle EA (2014) FAK inhibition abrogates the malignant phenotype in aggressive pediatric renal tumors. Mol Cancer Res 12: 514–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H (1997) Fyn‐kinase as a determinant of ethanol sensitivity: relation to NMDA‐receptor function. Science 278: 698–701 [DOI] [PubMed] [Google Scholar]
- Narita M, Kato H, Kasukawa A, Narita M, Suzuki M, Takeuchi T, Suzuki T (2006) Role of Src family kinase in the rewarding effect and hyperlocomotion induced by morphine. NeuroReport 17: 115–119 [DOI] [PubMed] [Google Scholar]
- Nestler EJ (1997) Molecular mechanisms of opiate and cocaine addiction. Curr Opin Neurobiol 7: 713–719 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK (1997) Molecular and cellular basis of addiction. Science 278: 58–63 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW (2001) ∆FosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA 98: 11042–11046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2004) Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci 25: 210–218 [DOI] [PubMed] [Google Scholar]
- O'Brien C, Testa T, O'Brien TJ, Brady JP, Wells B (1977) Conditioned narcotic withdrawal in humans. Science 195: 1000–1002 [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Murata Y, Okazawa H, Matozaki T (2011) Src family kinases: modulators of neurotransmitter receptor function and behavior. Trends Neurosci 34: 629–637 [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ (2005) Regulation of drug reward by cAMP response element‐binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci 25: 5553–5562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Contarino A (2006) Gender‐ and morphine dose‐linked expression of spontaneous somatic opiate withdrawal in mice. Behav Brain Res 170: 110–118 [DOI] [PubMed] [Google Scholar]
- Parsons SJ, Parsons JT (2004) Src family kinases, key regulators of signal transduction. Oncogene 23: 7906–7909 [DOI] [PubMed] [Google Scholar]
- Patrick GA, Dewey WL, Spaulding TC, Harris LS (1975) Relationship of brain morphine levels to analgesic activity in acutely treated mice and rats and in pellet implanted mice. J Pharmacol Exp Ther 193: 876–883 [PubMed] [Google Scholar]
- Puls LN, Eadens M, Messersmith W (2011) Current status of SRC inhibitors in solid tumor malignancies. Oncologist 16: 566–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punch LJ, Self DW, Nestler EJ, Taylor JR (1997) Opposite modulation of opiate withdrawal behaviors on microinfusion of a protein kinase A inhibitor versus activator into the locus coeruleus or periaqueductal gray. J Neurosci 17: 8520–8527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehni AK, Singh N (2011) Modulation of src‐kinase attenuates naloxone‐precipitated opioid withdrawal syndrome in mice. Behav Pharmacol 22: 182–190 [DOI] [PubMed] [Google Scholar]
- Schulteis G, Liu J, Amitai N, Tzeng S (2005) Context‐ and cue‐conditioned potentiation of acute morphine dependence and withdrawal. Pharmacol Biochem Behav 82: 82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Rujescu D, Kissling C, Soyka M, Dahmen N, Preuss UW, Wieman S, Depner M, Wellek S, Lascorz J et al (2003) Analysis of genetic variations of protein tyrosine kinase fyn and their association with alcohol dependence in two independent cohorts. Biol Psychiatry 54: 1422–1426 [DOI] [PubMed] [Google Scholar]
- Self D, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ (1998) Involvement of cAMP‐dependent protein kinase in the nucleus accumbens in cocaine self‐administration and relapse of cocaine‐seeking behavior. J Neurosci 18: 1848–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B, Johnson FM (2011) Regulation of SRC family kinases in human cancers. J Signal Transduct 2011: 865819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth V, Upadhyaya P, Moghe V, Ahmad M (2011) Role of calcium in morphine dependence and naloxone‐precipitated withdrawal in mice. J Exp Pharmacol 3: 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw‐Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, Duman RS, Storm D, Nestler EJ (2002) Regional and cellular mapping of cAMP response element‐mediated transcription during naltrexone‐precipitated morphine withdrawal. J Neurosci 22: 3663–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KY, Hwang CK, Kim CS, Choi HS, Law PY, Wei LN, Loh HH (2007) Translational repression of mouse mu opioid receptor expression via leaky scanning. Nucleic Acid Res 35: 1501–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinus L, Le Moal M, Koob GF (1990) Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience 37: 767–773 [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Muravieva EV, Garcia‐Osta A, Alberini CM (2010) Disrupting the memory of places induced by drugs of abuse weakens motivational withdrawal in a context‐dependent manner. Proc Natl Acad Sci USA 107: 12345–12350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toiu S, Avale ME, Nakatani H, Pons S, Parnaudeau S, Tronche F, Vogt A, Monyer H, Vogel R, de Chaumont F et al (2010) A versatile system for the neuronal subtype specific expression of lentiviral vectors. FASEB J 24: 723–730 [DOI] [PubMed] [Google Scholar]
- Walters CL, Godfrey M, Li X, Blendy JA (2005) Alterations in morphine‐induced reward, locomotor activity, and thermoregulation in CREB‐deficient mice. Brain Res Rev 1032: 193–199 [DOI] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D (2007) Ethanol induces long‐term facilitation of NR2B‐NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci 27: 3593–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Nagpure BV, Bian JS (2016) Opioid Dependence and the Adenylyl Cyclase/cAMP Signaling In Neuropathology of drug addiction and substance misuse, Preedy VR. (ed), Vol. 3, pp 449–456. London: King's College London, Academic Press; [Google Scholar]
- Yano I, Takemori AE (1977) Inhibition by naloxone of tolerance and dependence in mice treated acutely and chronically with morphine. Res Commun Chem Pathol Pharmacol 16: 721–734 [PubMed] [Google Scholar]
- Yoburn BC, Chen J, Huang T, Inturrisi CE (1985) Pharmacokinetics and pharmacodynamics of subcutaneous morphine pellets in the rat. J Pharmacol Exp Ther 235: 282–286 [PubMed] [Google Scholar]
- Zachariou V, Liu R, LaPlant Q, Xiao G, Renthal W, Chan GC, Storm DR, Aghajanian G, Nestler EJ (2008) Distinct roles of adenylyl cyclase 1 and 8 in opiate dependence: behavioral, electrophysiological, and molecular studies. Biol Psychiatry 63: 1013–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A (2012) The shady side of dasatinib. Blood 119: 4817–4818 [DOI] [PubMed] [Google Scholar]
- Zhang L, Tetrault J, Wang W, Loh HH, Law PY (2006) Short‐ and long‐term regulation of adenylyl cyclase activity by delta‐opioid receptor are mediated by Galphai2 in neuroblastoma N2A cells. Mol Pharmacol 69: 1810–1819 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao H, Qiu Y, Loh HH, Law PY (2009) Src phosphorylation of mu‐receptor is responsible for the receptor switching from an inhibitory to a stimulatory signal. J Biol Chem 284: 1990–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Loh HH, Law PY (2013) A novel noncanonical signaling pathway for the mu‐opioid receptor. Mol Pharmacol 84: 844–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Loh HH, Law PY (2006) Adenylyl cyclase superactivation induced by long‐term treatment with opioid agonist is dependent on receptor localized within lipid rafts and is independent of receptor internalization. Mol Pharmacol 69: 1421–1432 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File
Source Data for Figure 1
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 7


