Abstract
Astaxanthin is a carotenoid of significant commercial value due to its superior antioxidant potential and wide applications in the aquaculture, food, cosmetic and pharmaceutical industries. A higher ratio of astaxanthin to the total carotenoids is required for efficient astaxanthin production. β-Carotene ketolase and hydroxylase play important roles in astaxanthin production. We first compared the conversion efficiency to astaxanthin in several β-carotene ketolases from Brevundimonas sp. SD212, Sphingomonas sp. DC18, Paracoccus sp. PC1, P. sp. N81106 and Chlamydomonas reinhardtii with the recombinant Escherichia coli cells that synthesize zeaxanthin due to the presence of the Pantoea ananatis crtEBIYZ. The B. sp. SD212 crtW and P. ananatis crtZ genes are the best combination for astaxanthin production. After balancing the activities of β-carotene ketolase and hydroxylase, an E. coli ASTA-1 that carries neither a plasmid nor an antibiotic marker was constructed to produce astaxanthin as the predominant carotenoid (96.6%) with a specific content of 7.4 ± 0.3 mg/g DCW without an addition of inducer.
Keywords: astaxanthin, Escherichia coli, metabolic engineering, β-carotene ketolase, β-carotene hydroxylase
1. Introduction
Astaxanthin is a carotenoid of significant commercial value due to its superior antioxidative, anti-inflammatory and anticancer features [1]. It has wide applications in the aquaculture, food, cosmetic and pharmaceutical industries. Currently, commercial astaxanthin is mainly synthesized chemically or extracted from natural producers such as the green algae Haematococcus pluvialis or the red yeast Xanthophyllomyces dendrorhous. Considering the limited productivity of astaxanthin via extraction and the biosafety issues of chemical synthesis, microbial production of astaxanthin via metabolic engineering has become an attractive alternative [2,3].
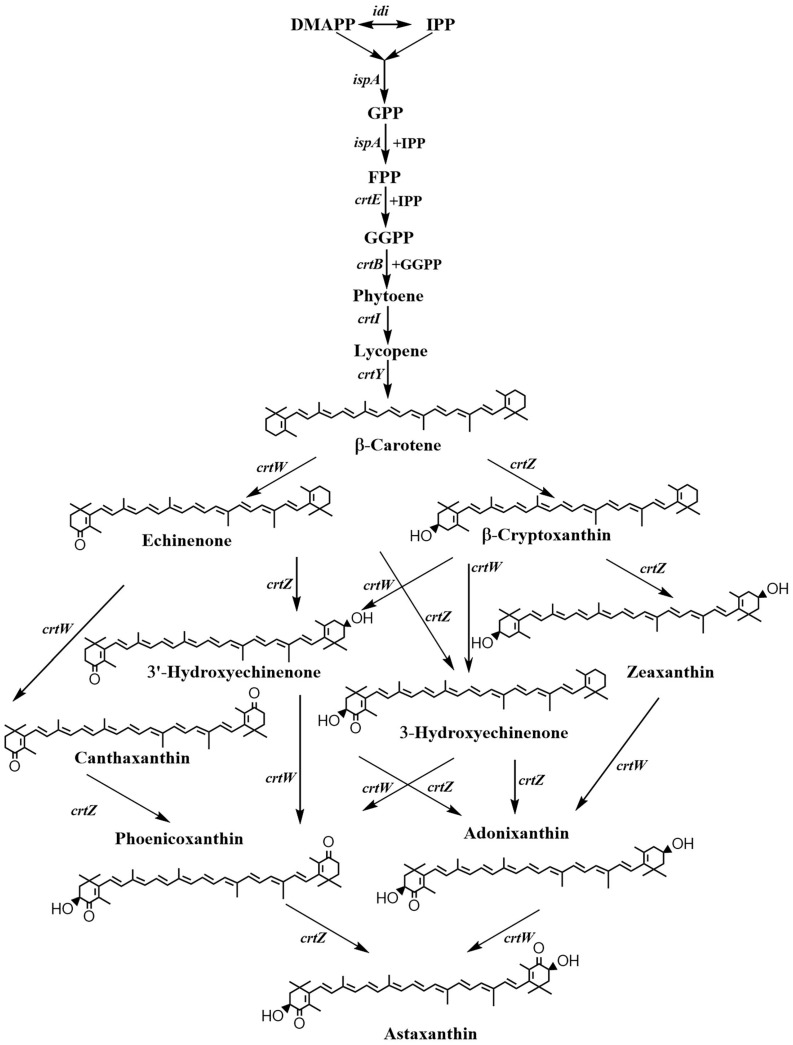
In recent years, Escherichia coli [4], Saccharomyces cerevisiae [5,6] and Corynebacterium glutamicum [7] have been used as a host strain for astaxanthin production by the introduction of the astaxanthin biosynthesis pathway (Figure 1) into these non-carotenogenic microorganisms. Metabolic engineering E. coli for astaxanthin production has been widely reported in recent years. It has been demonstrated that the pathway from β-carotene to astaxanthin is a crucial step in astaxanthin synthesis [8]. The pathway requires two enzymes, β-carotene ketolase CrtW and β-carotene hydroxylase CrtZ. It has been shown that many bacterial CrtWs and CrtZs are bifunctional, with respect to their substrate [9,10]. They can accept β-carotene as well as its hydroxylated or ketolated products as a substrate, resulting in the formation of eight carotenoid intermediates which affect astaxanthin conversion as measured by the percentage of astaxanthin produced relative to the total carotenoid content (Figure 1). The astaxanthin ratio affects the production costs. To increase the astaxanthin ratio, many bacterial CrtWs and CrtZs have been identified and characterized [4,8,11,12,13,14,15,16,17]. However, the ratio reported in the above papers was lower than 90%. Thus, to increase the astaxanthin ratio, we first compared the conversion efficiency to astaxanthin in several CrtWs, which had a higher efficiency for astaxanthin production reported in the literature, with recombinant E. coli cells that synthesize zeaxanthin. Then, balancing the expressions of the two enzymes was carried out to obtain a plasmid-free E. coli, which produced astaxanthin of 7.4 ± 0.3 mg/g dry cell weight (DCW) with the astaxanthin ratio of 96.6% without the addition of an inducer.
Figure 1.
Pathway for biosynthesizing astaxanthin. DMAPP: Dimethylallyl diphosphate; GPP: Geranyl pyrophosphate; FPP: Farnesyl pyrophosphate; GGPP: Geranylgeranyl pyrophosphate; ispA: FPP synthase gene; crtE: GGPP synthase gene; crtB: Phytoene synthase gene; crtI: Carotene desaturase gene; crtY: Lycopene β-cyclase gene; crtZ: β-carotene hydroxylase gene; crtW: β-carotene ketolase gene.
2. Results and Discussion
2.1. Screening of β-Carotene Ketolase
It has been demonstrated that astaxanthin biosynthesis proceeds from β-carotene through hydroxylation by CrtZ and then ketolation by CrtW [14]. Moreover, our previous study has proven that Pantoea ananatis crtZ is superior to that of P. agglomerans or H. pluvialis for zeaxanthin production [18]. Thus, we first compared the catalytic efficiency for ketolating zeaxanthin to astaxanthin by different CrtWs. We selected four β-carotene ketolases with higher efficiencies for astaxanthin production reported in the literature as candidates. The four ketolases were Brevundimonas sp. SD212 CrtW [11,12], Sphingomonas sp. DC18 CrtWF213L/R203W [8], Paracoccus sp. N81106 CrtWL175W [16] and Chlamydomonas reinhardtii β-carotene ketolase (Bkt) [17]. The plasmids containing the β-carotene ketolase gene were transferred into an engineered zeaxanthin-producing strain E. coli ZEAX [19]. One copy of P. ananatis crtZ under the control of the P37 promoter was integrated into the chromosome of the β-Carotene producing strain E. coli BETA-1 [18]. Table 1 presents the results of astaxanthin production by the different engineered E. coli. Among the four β-carotene ketolase genes, the strain harboring B. sp. SD212 crtW produced a higher level of astaxanthin (2.7 ± 0.1 mg/g DCW), indicating that B. sp. SD212 crtW and P. ananatis crtZ genes are the best combinations for astaxanthin production. Misawa’s group also demonstrated that B. sp. SD212 crtW and P. ananatis crtZ genes are a combination of the most promising gene candidates for astaxanthin production [10,11,12]. Then we assembled two genes into one plasmid to increase the dose of the gene using BglBrick assembly technology and investigated its effect on the combination of different genes on astaxanthin production. As shown in Table 1, increasing the dose of the gene indeed enhanced astaxanthin production. E. coli ZEAX (pZS-2crtWBsp) produced 4.6 ± 0.1 mg/g DCW of astaxanthin.
Table 1.
Effect of the overexpression of different β-carotene ketolase genes on astaxanthin production in Escherichia coli ZEAX.
| Plasmid | OD600 * | Astaxanthin Concentration, mg/L | Astaxanthin Content, mg/gDCW |
|---|---|---|---|
| Single gene | |||
| pZS-crtWBsp | 9.55 ± 0.16 | 8.1 ± 0.1 | 2.7 ± 0.1 |
| pZS-crtWPsp | 8.05 ± 0.47 | 1.3 ± 0.1 | 0.5 ± 0.1 |
| pZS-crtWSsp | 8.61 ± 0.21 | 0.8 ± 0.1 | 0.3 ± 0.1 |
| pZS-bkt | 11.07 ± 0.20 | 5.0 ± 0.2 | 1.4 ± 0.1 |
| Double genes | |||
| pZS-2crtWBsp | 19.67 ± 0.33 | 28.8 ± 0.2 | 4.6 ± 0.1 |
| pZS-2bkt | 22.15 ± 0.33 | 20.9 ± 1.6 | 3.5 ± 0.1 |
| pZS-2crtWPsp | 16.82 ± 0.56 | 7.0 ± 0.1 | 1.3 ± 0.1 |
| pZS-2crtWSsp | 14.91 ± 0.31 | 1.1 ± 0.6 | 0.2 ± 0.1 |
| Mixed genes | |||
| pZS-crtWBspcrtWPsp | 19.93 ± 0.38 | 12.6 ± 0.6 | 2.0 ± 0.1 |
| pZS-crtWBspcrtWSsp | 21.73 ± 0.19 | 24.6 ± 0.4 | 3.5 ± 0.1 |
| pZS-crtWBspbkt | 19.9 ± 1.27 | 20.9 ± 1.6 | 3.3 ± 0.1 |
| pZS-crtWPspcrtWSsp | 16.6 ± 0.17 | 8.5 ± 0.2 | 1.6 ± 0.2 |
| pZS-crtWPspbkt | 20.01 ± 0.12 | 10.5 ± 1.6 | 1.6 ± 0.1 |
| pZS-crtWSspbkt | 21.57 ± 0.38 | 11.2 ± 0.7 | 1.6 ± 0.1 |
* The OD600 value was expressed as cell growth.
It has been shown that ketolase activity on zeaxanthin is the limiting step of astaxanthin biosynthesis in a bacterial and plant system [4,17]. To increase the astaxanthin ratio and produce efficiency, many bacterial CrtWs have been characterized and compared. It has been reported that the CrtW enzyme from B. sp. SD212 had a higher efficiency for converting zeaxanthin to astaxanthin than that from P. sp. PC1 and P. sp. N81106 [11]. Of the three β-carotene ketolase enzymes from H. pluvialis, Chlorella zofingien and C. reinhardtii, C. reinhardtii β-carotene ketolase had the highest activity for the conversion of zeaxanthin to astaxanthin [17]. Among Rhodococcus erythropolis PR4 CrtO, Synechosistis sp. PCC6803 CrtO and B. sp. SD212 CrtW, only B. sp. SD212 CrtW could synthesize astaxanthin from zeaxanthin [12]. Comparative analysis of the CrtO and CrtW revealed that CrtW was more efficient for the conversion of carotene to canthaxanthin than CrtO [14]. The conversion efficiency of Gloeobacter violaceus PCC 7421, Anabaena (also known as Nostoc) sp. PCC 7120 and Nostoc punctiforme PCC 73102 CrtW was compared in engineered E. coli [13]. The results demonstrated that the CrtW from A. sp. PCC 7120 as well as N. punctiforme PCC 73102 (CrtW148) can convert not only β-carotene but also zeaxanthin into canthaxanthin and astaxanthin, respectively [13].
Protein engineering of CrtW has been successfully used to improve astaxanthin production in recombinant E. coli cells that synthesize zeaxanthin. To improve S. sp. DC18 CrtW activity in hydroxylated carotenoids for astaxanthin production, S. sp. DC18 CrtW was evolved to obtain the R203W/F213L double mutant that yielded the highest improvement for astaxanthin production [8]. The strain harboring the double mutant produced astaxanthin as the predominant carotenoid (88%) [8]. By using random mutagenesis, P. sp. N81106 crtW mutants were generated [16]. The zeaxanthin producer E. coli harboring the crtWL175 mutant produced 78% of astaxanthin in the total carotenoid [16].
2.2. Balancing the Activities of β-Carotene Ketolase and Hydroxylase
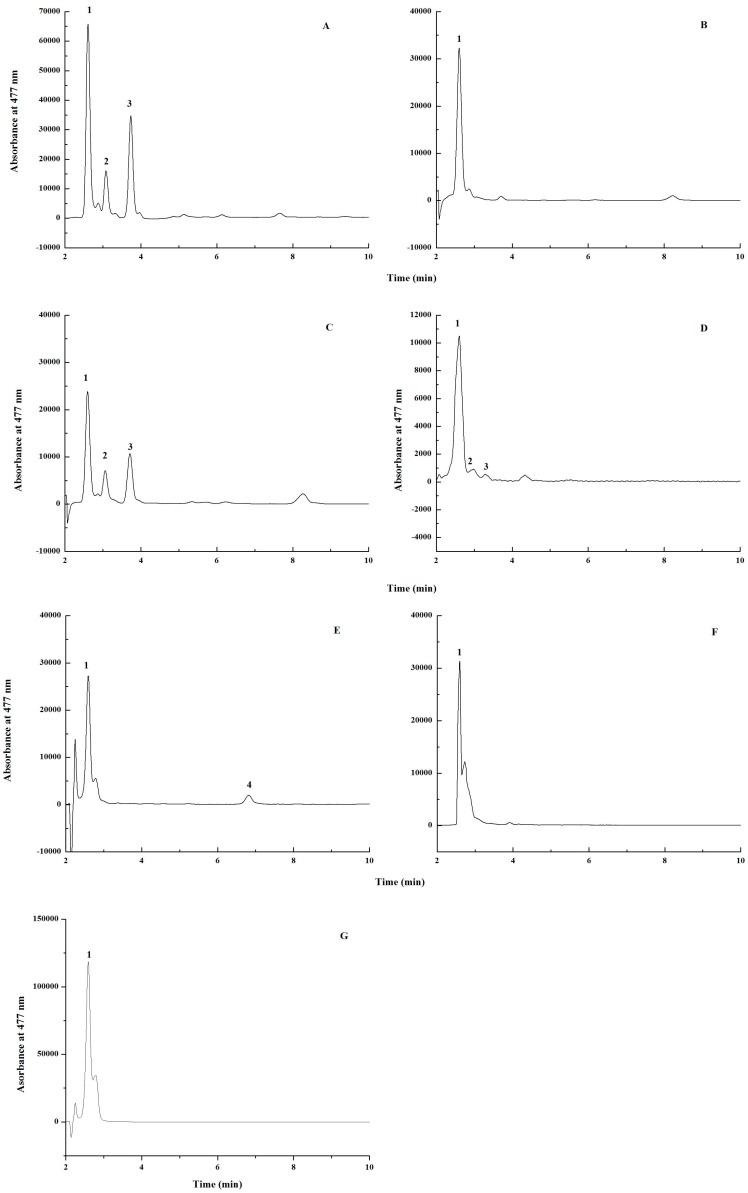
We analyzed the accumulated carotenoids in E. coli ZEAX (pZS-2crtWBsp) as shown in Figure 2A. The engineered strain produced 51.9% astaxanthin, 13.4% phoenicoxanthin and 30.4% canthaxanthin. From the biosynthetic pathway as shown in Figure 1, canthaxanthin and phoenicoxanthin are the intermediates of the pathway through first ketolation and then hydroxylation. Their accumulation indicates that the expression level of the hydroxylase gene crtZ may be low in this strain. Thus, we expressed pBAD-crtZ in E. coli ZEAX (pZS-2crtWBsp) to verify our hypothesis. As shown in Figure 2B, co-expressing pBAD-crtZ with pZS-2crtWBsp in E. coli ZEAX indeed increased the astaxanthin ratio to the total carotenoid content from 51.9% to 87.5%. We also co-overexpressed pBAD-crtWBsp with pZS-2crtWBsp in E. coli ZEAX. As shown in Figure 2C, the co-overexpression decreased the astaxanthin ratio from 51.9% to 46.4% and increased the canthaxanthin and phoenicoxanthin ratio. This stands in contrast to the study by Lemuth et al. [4], who found that increasing the ketolase activity or decreasing the hydroxylase activity would be necessary for astaxanthin production. Our results demonstrated that increasing CrtZ activity would be necessary for producing astaxanthin as the predominant carotenoid. Thus, to increase the expression level of crtZ, we integrated two copies of the crtZ into the chromosome of the β-carotene producer E. coli BETA to generate the zeaxanthin producer E. coli ZEAX-4. The recombinant E. coli ZEAX-4 harboring pZS-2crtWBsp produced 88.6% astaxanthin, 3.9% phoenicoxanthin and 3.0% canthaxanthin (Figure 2D).
Figure 2.
HPLC analysis of carotenoid products extracted from E. coli ZEAX (pZS-2crtWBsp) (A), E. coli ZEAX (pZS-2crtWBsp, pBAD-crtZ) (B), E. coli ZEAX (pZS-2crtWBsp, pBAD-crtWBsp) (C), E. coli ZEAX-4 (pZS-2crtWBsp) (D), E. coli ASTA (E), E. coli ASTA-1 (F) and standard astaxanthin (G). 1. astaxanthin; 2. Phoenicoxanthin; 3. Canthaxanthin; 4. Lycopene.
To reduce the metabolic burden and to avoid antibiotic markers resulting from the plasmid, we integrated B. sp. SD212 crtW into the chromosome of the zeaxanthin producer E. coli ZEAX-4 to generate an astaxanthin producer E. coli ASTA. The resulting strain E. coli ASTA produced 92.6% astaxanthin (Figure 2E). However, lycopene was also detected in E. coli ASTA. We guess the phenomenon may be due to the lower expression level of the crtY. Thus, we integrated another copy of the crtY into the chromosome of E. coli ASTA to obtain E. coli ASTA-1. This integration enhanced the astaxanthin ratio to 96.6% (Figure 2F). E. coli ASTA-1 produced astaxanthin as the predominant carotenoid (96.6%) with a specific content of 7.4 ± 0.3 mg/g DCW (Figure 2F).
It is supposed that β-carotene hydroxylase and ketolase compete for their substrate and that only a balanced expression of these two enzymes might result in a complete conversion of β-carotene to astaxanthin [4,14,20,21]. To allow a variable expression of crtZ compared to the tac-promoter controlled N. punctiforme PCC 73102 crtW148 and P. ananatis crtEBIY, P. ananatis crtZ was expressed under the control of the rhamnose-promoter [4]. The engineered strain E. coli BW-ASTA produced astaxanthin as the predominant carotenoid (95%) at a concentration of 1.4 mg/g DCW in minimal medium with glucose and Isopropyl β-d-thiogalactoside (IPTG) [4]. The E. coli strain with the pTrcCrtW-pBADCrtZ dual expression systems had an increased selectivity for astaxanthin production (1.99 mg/g DCW, about 90%) [14]. Our study also suggests that appropriate activities of β-carotene hydroxylase and ketolase are important for astaxanthin production.
Astaxanthin production by microorganisms is summarized in Table 2. Although Lemuth et al. first engineered a plasmid-free E. coli strain for astaxanthin production, the strain produced astaxanthin of 1.4 mg/g DCW with an astaxanthin ratio of 95% only with IPTG induction [4]. In this study, we engineered a plasmid-free E. coli for astaxanthin production, which reached 7.4 ± 0.3 mg/g DCW with the astaxanthin ratio of 96.6% without the addition of an inducer. From Table 2, we can see that the astaxanthin ratio obtained in this study is the highest value. However, the astaxanthin yield obtained in this study is slightly lower than that (8.64 mg/g DCW) in E. coli reported by Ma et al., which is the highest astaxanthin yield reported to date [22]. In their study, the upper mevalonate (MEV) pathway operon from S. cerevisiae, the lower MEV pathway operon from S. cerevisiae, plus E. coli idi and the optimized astaxanthin biosynthetic pathway genes were expressed on three different plasmids [22]. The optimized astaxanthin biosynthetic pathway genes contain P. ananatis crtEBI under the control of the PT7 promoter, P. agglomerans crtY and crtZ, B. sp. SD212 crtW and E. coli idi under the control of the PT7 promoter [22]. Thus, the introduction of an MEV pathway in our strain E. coli ASTA-1 may further increase astaxanthin production.
Table 2.
Astaxanthin production by different microorganisms.
| Strain | Astaxanthin Yield | Astaxanthin Ratio (%) | Reference |
|---|---|---|---|
| E. coli | 5.8 mg/g DCW | N.D. * | [23] |
| E. coli | 8.64 mg/g DCW | N.D. | [22] |
| E. coli | 1.4 mg/g DCW | 95 | [4] |
| E. coli | 1.99 mg/g DCW | 90 | [14] |
| E. coli | 7.4 ± 0.3 mg/g DCW | 96.6 | This study |
| S. cerevisiae | 4.7 mg/g DCW | N.D. | [5] |
| S. cerevisiae | 8.10 mg/g DCW | N.D. | [6] |
| C. glutamicum | 0.4 mg/L/h | N.D. | [7] |
* N.D. = not determined.
3. Materials and Methods
3.1. Strains, Plasmids and Primers
Strains, plasmids and primers used in this study were listed in Table 3.
Table 3.
Strains and plasmids used in this study.
| Name | Description | Reference/Sources |
|---|---|---|
| Strain | ||
| E. coli BETA-1 | β-Carotene producing strain | [18] |
| E. coli ZEAX | Zeaxanthin producing strain, one copy of Pantoea ananatis crtZ under the control of the P37 promoter was integrated into E. coli BETA-1 chromosome | [19] |
| E. coli ZEAX-4 | Zeaxanthin producing strain, two copies of P. ananatis crtZ under the control of the P37 promoter was integrated into E. coli BETA-1 chromosome | This study |
| E. coli ASTA | Astaxanthin producer, one of B. sp. SD212 crtW under the control of the P37 promoter was integrated into E. coli ZEAX-4 chromosome | This study |
| E. coli ASTA-1 | Astaxanthin producer, another copy of P. ananatis crtY under the control of the P37 promoter was integrated into E. coli ASTA-1 chromosome | This study |
| Plasmid | ||
| pZSABP | Constitute expression vector, pSC101 ori, P37 promoter, Ampr, BglBrick, ePathBrick containing four isocaudamer (AvrII, NheI, SpeI and XbaI) | [18] |
| pBAD33 | Expression vector, PBAD, p15A ori, Cmr | [24] |
| pZS-crtWBsp | pZSABP containing Brevundimonas sp. SD212 crtW under the control of the P37 promoter | This study |
| pZS-crtWPsp | pZSABP containing Paracoccus sp. N81106 crtWL175W under the control of the P37 promoter | This study |
| pZS-crtWSsp | pZSABP containing Sphingomonas sp. DC18 crtWF213L/R203W under the control of the P37 promoter | This study |
| pZS-bkt | pZSABP containing Chlamydomonas reinhardtii bkt under the control of the P37 promoter | This study |
| pZS-2crtWBsp | pZSABP containing two copies of B.s sp. SD212 crtW under the control of the P37 promoter | This study |
| pZS-2bkt | pZSABP containing two copies of C. reinhardtii bkt under the control of the P37 promoter | This study |
| pZS-2crtWPsp | pZSABP containing two copies of P. sp. N81106 crtWL175W under the control of the P37 promoter | This study |
| pZS-2crtWSsp | pZSABP containing two copies of S. sp. DC18 crtWF213L/R203W under the control of the P37 promoter | This study |
| pZS-crtWBspcrtWPsp | pZSABP containing B.s sp. SD212 crtW under the control of the P37 promoter and P. sp. N81106 crtWL175W under the control of the P37 promoter | This study |
| pZS-crtWBspcrtWSsp | pZSABP containing B.s sp. SD212 crtW under the control of the P37 promoter and S. sp. DC18 crtWF213L/R203W under the control of the P37 promoter | This study |
| pZS-crtWBspbkt | pZSABP containing B.s sp. SD212 crtW under the control of the P37 promoter and C. reinhardtii bkt under the control of the P37 promoter | This study |
| pZS-crtWPspcrtWSsp | pZSABP containing P. sp. N81106 crtWL175W under the control of the P37 promoter and S. sp. DC18 crtWF213L/R203W under the control of the P37 promoter | This study |
| pZS-crtWPspbkt | pZSABP containing P. sp. N81106 crtWL175W under the control of the P37 promoter and C. reinhardtii bkt under the control of the P37 promoter | This study |
| pZS-crtWSspbkt | pZSABP containing S. sp. DC18 crtWF213L/R203W under the control of the P37 promoter and C. reinhardtii bkt under the control of the P37 promoter | This study |
| pBAD-crtZ | pBAD33 containing P. ananatis crtZ | This study |
| pBAD-crtWBsp | pBAD33 containing B.s sp. SD212 crtW | This study |
3.2. Genetic Methods
After codon optimization for E. coli codon usage by using the 31C method reported by Boël et al. [25], B. sp. SD212 crtW, S. sp. DC18 crtWF213L/R203W, P. sp. N81106 crtWL175W and C. reinhardtii bkt genes were synthesized by Suzhou GENEWIZ, Inc. (Suzhou, China) and ligated into pUC57. The gene fragment was then digested and inserted into the NheI/KpnI sites of pZSBP [18] to obtain pZS-crtWBSP, pZS-crtWSSP, pZS-crtWPSP and pZS-bkt, respectively. The BglBrick standard assembling method was used to assemble the above any two genes into a plasmid.
3.3. Astaxanthin Production in Shake Flasks
A single colony was inoculated into 5 mL of Luria-Bertani (LB) medium supplemented with 5 g/L KAc in a falcon tube which was incubated overnight at 37 °C. The overnight seed culture was then inoculated into 50 mL of the Super Broth with ammonium and sucrose (SBMSN) medium with an initial OD600 of 0.1. The SBMSN medium (pH 7.0) contained 5 g/L sucrose, 12 g/L peptone, 24 g/L yeast extract, 1.7 g/L KH2PO4, 11.42 g/L K2HPO4, 1 g/L MgCl2·6H2O, 1.42 g/L ammonium oxalate, and 2 g/L Tween-80. The cultures were incubated at 37 °C for 48 h in a rotary shaking incubator set to 150 rpm. Cell growth was measured according to the OD600 and converted into DCW (g/L) using a standard curve.
3.4. Extraction and Quantification of Carotenoids
Cells were extracted with acetone to isolate carotenoids as described previously [9]. E. coli cultures (250 μL) were harvested by centrifugation at 12,000 rpm for 5 min. The cell pellet was washed with water and extracted with 1 mL of acetone at 55 °C for 15 min with intermittent vortexing. The acetone supernatant after centrifugation was transferred to a new tube. Carotenoids were analyzed by HPLC (Shimadzu HPLC system, Model LC-20A, Shimadzu, Japan) using an Inertsil ODS-SP column (5 μm, 4.6 × 150 mm, GL Sciences Inc., Tokyo, Japan). The mobile phase of acetonitrile-methanol (65:35 v/v) at a flow rate of 1 mL/min was used. The absorbance of carotenoids at 477 nm was detected using a photodiode array detector (SPD-M20A). Carotenoid compounds were identified on the basis of their retention times relative to standard compounds (Sigma-Aldrich, St. Louis, MO, USA). Astaxanthin was quantified by comparing the integrated peak areas with that of authentic standards. The contents of total carotenoids were approximated via application of the astaxanthin curve.
3.5. Statistical Analysis
All experiments were performed in triplicate, and the data are presented as the mean of the three experiments ± standard deviation. Tukey’s test was carried out for the statistical analysis using the OriginPro (version 7.5) package. Statistical significance was defined as p < 0.05.
Acknowledgments
We are grateful to the National Natural Science Foundation of China (Grant No. 21276289, J1310025), the Natural Science Foundation of Guangdong Province (No. 2015A030311036), the Project of the Scientific and Technical Program of Guangdong Province (No. 2015A010107004) and the Project of the Scientific and Technical Program of Guangzhou (No. 201607010028) for their financial support.
Abbreviations
| B. sp. | Brevundimonas sp. |
| S. sp. | Sphingomonas sp. |
| P. sp. | Paracoccus sp. |
| P. ananatis | Pantoea ananatis |
| H. pluvialis | Haematococcus pluvialis |
| P. agglomerans | Pantoea agglomerans |
| C. reinhardtii | Chlamydomonas reinhardtii |
| A. sp. | Anabaena sp. |
| N. punctiforme | Nostoc punctiforme |
| MEV | mevalonate |
Author Contributions
Q.L. and Y.-F.B. performed the experiments. They contributed equally to this work. J.-Z.L. directed the project and wrote the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Hussein G., Sankawa U., Goto H., Matsumoto K., Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006;69:443–449. doi: 10.1021/np050354+. [DOI] [PubMed] [Google Scholar]
- 2.Lee P.C., Schmidt-Dannert C. Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl. Microbiol. Biotechnol. 2002;60:1–11. doi: 10.1007/s00253-002-1101-x. [DOI] [PubMed] [Google Scholar]
- 3.Ye V.M., Bhatia S.K. Pathway engineering strategies for production of beneficial carotenoids in microbial hosts. Biotechnol. Lett. 2012;34:1405–1414. doi: 10.1007/s10529-012-0921-8. [DOI] [PubMed] [Google Scholar]
- 4.Lemuth K., Steuer K., Albermann C. Engineering of a plasmid-free Escherichia coli strain for improved in vivo biosynthesis of astaxanthin. Microb. Cell Fact. 2011;10:29. doi: 10.1186/1475-2859-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P.P., Ye L.D., Xie W.P., Lv X.M., Yu H.W. Highly efficient biosynthesis of astaxanthin in Saccharomyces cerevisiae by integration and tuning of algal crtZ and bkt. Appl. Microbiol. Biotechnol. 2015;99:8419–8428. doi: 10.1007/s00253-015-6791-y. [DOI] [PubMed] [Google Scholar]
- 6.Zhou P.P., Xie W.P., Li A.P., Wang F., Yao Z., Bian Q., Zhu Y.Q., Yu H.W., Ye L.D. Alleviation of metabolic bottleneck by combinatorial engineering enhanced astaxanthin synthesis in Saccharomyces cerevisiae. Enzyme Microb. Technol. 2017;100:28–36. doi: 10.1016/j.enzmictec.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Henke N.A., Heider S.A.E., Peters-Wendisch P., Wendisch V.F. Production of the marine carotenoid astaxanthin by metabolically engineered Corynebacterium glutamicum. Mar. Drugs. 2016;14:124. doi: 10.3390/md14070124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao L.A., Wilczek J., Odom J.M., Cheng Q.O. Engineering a β-carotene ketolase for astaxanthin production. Metab. Eng. 2006;8:523–531. doi: 10.1016/j.ymben.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Martin J.F., Gudina E., Barredo J.L. Conversion of β-carotene into astaxanthin: Two separate enzymes or a bifunctional hydroxylase-ketolase protein? Microb. Cell Fact. 2008;7:3. doi: 10.1186/1475-2859-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misawa N. Carotenoid beta-ring hydroxylase and ketolase from marine bacteria-promiscuous enzymes for synthesizing functional xanthophylls. Mar. Drugs. 2011;9:757–771. doi: 10.3390/md9050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi S.K., Nishida Y., Matsuda S., Adachi K., Kasai H., Peng X., Komemushi S., Miki W., Misawa N. Characterization of beta-carotene ketolases, CrtW, from marine bacteria by complementation analysis in Escherichia coli. Mar. Biotechnol. 2005;7:515–522. doi: 10.1007/s10126-004-5100-z. [DOI] [PubMed] [Google Scholar]
- 12.Choi S.K., Harada H., Matsuda S., Misawa N. Characterization of two beta-carotene ketolases, CrtO and CrtW, by complementation analysis in Escherichia coli. Appl. Microbiol. Biotechnol. 2007;75:1335–1341. doi: 10.1007/s00253-007-0967-z. [DOI] [PubMed] [Google Scholar]
- 13.Makino T., Harada H., Ikenaga H., Matsuda S., Takaichi S., Shindo K., Sandmann G., Ogata T., Misawa N. Characterization of cyanobacterial carotenoid ketolase CrtW and hydroxylase CrtR by complementation analysis in Escherichia coli. Plant Cell Physiol. 2008;49:1867–1878. doi: 10.1093/pcp/pcn169. [DOI] [PubMed] [Google Scholar]
- 14.Scaife M.A., Burja A.M., Wright P.C. Characterization of cyanobacterial β-carotene ketolase and hydroxylase Genes in Escherichia coli, and their application for astaxanthin biosynthesis. Biotechnol. Bioeng. 2009;103:944–955. doi: 10.1002/bit.22330. [DOI] [PubMed] [Google Scholar]
- 15.Scaife M.A., Ma C.A., Ninlayarn T., Wright P.C., Armenta R.E. Comparative analysis of beta-carotene hydroxylase genes for astaxanthin biosynthesis. J. Nat. Prod. 2012;75:1117–1124. doi: 10.1021/np300136t. [DOI] [PubMed] [Google Scholar]
- 16.Ye R.W., Stead K.J., Yao H., He H.X. Mutational and functional analysis of the beta-carotene ketolase involved in the production of canthaxanthin and astaxanthin. Appl. Environ. Microb. 2006;72:5829–5837. doi: 10.1128/AEM.00918-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong Y.J., Huang J.C., Liu J., Li Y., Jiang Y., Xu Z.F., Sandmann G., Chen F. Functional characterization of various algal carotenoid ketolases reveals that ketolating zeaxanthin efficiently is essential for high production of astaxanthin in transgenic Arabidopsis. J. Exp. Bot. 2011;62:3659–3669. doi: 10.1093/jxb/err070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X.R., Tian G.Q., Shen H.J., Liu J.Z. Metabolic engineering of Escherichia coli to produce zeaxanthin. J. Ind. Microbiol. Biotechnol. 2015;42:627–636. doi: 10.1007/s10295-014-1565-6. [DOI] [PubMed] [Google Scholar]
- 19.Shen H.J., Cheng B.Y., Zhang Y.M., Tang L., Li Z., Bu Y.F., Li X.R., Tian G.Q., Liu J.Z. Dynamic control of the mevalonate pathway expression for improved zeaxanthin production in Escherichia coli and comparative proteome analysis. Metab. Eng. 2016;38:180–190. doi: 10.1016/j.ymben.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Fraser P.D., Miura Y., Misawa N. In vitro characterization of astaxanthin biosynthetic enzymes. J. Biol. Chem. 1997;272:6128–6135. doi: 10.1074/jbc.272.10.6128. [DOI] [PubMed] [Google Scholar]
- 21.Fraser P.D., Shimada H., Misawa N. Enzymic confirmation of reactions involved in routes to astaxanthin formation, elucidated using a direct substrate in vitro assay. Eur. J. Biochem. 1998;252:229–236. doi: 10.1046/j.1432-1327.1998.2520229.x. [DOI] [PubMed] [Google Scholar]
- 22.Ma T., Zhou Y.J., Li X.W., Zhu F.Y., Cheng Y.B., Liu Y., Deng Z.X., Liu T.G. Genome mining of astaxanthin biosynthetic genes from Sphingomonas sp. ATCC 55669 for heterologous overproduction in Escherichia coli. Biotechnol. J. 2016;11:228–237. doi: 10.1002/biot.201400827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zelcbuch L., Antonovsky N., Bar-Even A., Levin-Karp A., Barenholz U., Dayagi M., Liebermeister W., Flamholz A., Noor E., Amram S., et al. Spanning high-dimensional expression space using ribosome-binding site combinatorics. Nucleic Acids Res. 2013;41 doi: 10.1093/nar/gkt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzman L.M., Belin D., Carson M.J., Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boel G., Letso R., Neely H., Price W.N., Wong K.H., Su M., Luff J.D., Valecha M., Everett J.K., Acton T.B., et al. Codon influence on protein expression in E. coli correlates with mRNA levels. Nature. 2016;529:358–363. doi: 10.1038/nature16509. [DOI] [PMC free article] [PubMed] [Google Scholar]