Abstract
Fish discards are of major concern in new EU policies. Alternatives for the management of the new biomass that has to be landed is compulsory. The production of bioactive compounds from fish protein hydrolysates (FPH) has been explored in recent years. However, the viability of Scyliorhinus canicula discards, which might account for up to 90–100% of captures in mixed trawler, gillnet, and longline industrial fisheries, to produce FPH from the muscle with bioactivities has still not been studied in terms of the optimization of the experimental conditions to enhance its production. The effect of pH and temperature on the hydrolysis of the S. canicula muscle was mediated by three commercial proteases using response surface methodology. Temperatures of 64.6 °C and 60.8 °C and pHs of 9.40 and 8.90 were established as the best hydrolysis conditions for Alcalase and Esperase, respectively. Optimization of the best conditions for the maximization of antihypertensive and antioxidant activities was performed. Higher Angiotensin-converting enzyme (ACE) activity was found with Esperase. The pH optimum and temperature optimum for antioxidants were 55 °C/pH8.0 for ABTS/DPPH-Esperase, 63.1 °C/pH9.0 for DPPH-Alcalase, and 55 °C/pH9.0 for ABTS-Alcalase. No hydrolysis was detected when using Protamex.
Keywords: Common Fishery Policy, fish discards, Scyliorhinus canicula muscle by-products, fish protein hydrolysates, enzyme hydrolysis, response surface methodology, antihypertensive activity, antioxidant activity
1. Introduction
The new European marine policy framework that aims to achieve a sustainable use of marine resources to ensure high long-term fishing yields has been included in the last reform of the Common Fishery Policy, along with new guidelines to reduce the wasteful practice of discarding by the introduction of a landing obligation (Regulation EU N° 1380/2013). The need of implementing alternatives for the management of the new biomass that should be landed is therefore compulsory. Such an approach is also consistent with the Blue Growth initiative of European Horizon 2020, which aims to ensure and support the sustainable growth of natural marine resources. The success of this blue strategy depends on the development of a blue technology focused on the exploration of bioactive compounds obtained from marine organisms including discards and by-products with potential interest in the food/feed/pharmaceutical/cosmetical industries.
Scyliorhinus canicula (small-spotted catshark) is generally captured as a non-target (by-catch) alongside the target species in mixed trawler, gillnet, and longline industrial fisheries, with very high discard rates reaching 90–100% [1]. Discards of this species in the Cantabrian Sea and North Portugal waters account for approximately 1200 tn/year [1,2]. The huge amount of potential discards of this species, together with the elevated percentage of muscle fraction in S. canicula (43.56 ± 3.78%), makes the hydrolysis of muscle proteins an excellent alternative to efficiently upgrading this biomass [2]. The preparation and characterization of fish protein hydrolysates (FPH) covering different species, enzymes, or hydrolysis conditions have been extensively studied [3]. The presence of essential nutrients and bioactive compounds in FPH transforms them into high value added products with potential to be used in a wide range of industrial sectors such as food [4,5] or pharmacy [6]. Lipid oxidation and cardiovascular diseases are of particular concern for the food industry and international public health organizations, respectively. FPH from other fish substrates and by-products have been cited to possess antihypertensive and antioxidant bioactivities [7,8,9,10]. Only one reference about the production of enzymatic hydrolysates with biological properties from S. canicula has been reported [11]. However, the optimization of the experimental conditions to enhance the production of FPH and bioactivities from small-spotted catshark muscle has not been still explored.
In the present work, the effect of pH and temperature on the hydrolysis of S. canicula muscle mediated by three commercial proteases, Alcalase, Esperase, and Protamex, was studied using response surface methodology (RSM). Optimization of the best conditions for the maximization of hydrolysis degree and the production of bioactive peptides with antihypertensive and antioxidant properties was performed. These objectives are consistent with the current policy framework above mentioned.
2. Results and Discussion
In previous reports, different parts of S. canicula by-products have been valorised obtaining various products and biocompounds of medium-high added value. Cartilages from fins, skeletons, and heads were processed to isolate chondroitin sulphate [12]; marine peptones used as microbial nutrients were recovered from viscera [13], proteolytic activities including trypsin analogues were extracted from pancreas [14], and collagen and derivatives were produced from skins [15]. The corresponding muscle from S. canicula discards that cannot be used for human consumption may be also a valuable substrate for the production of FPH and bioactive peptides.
2.1. Composition of S. canicula Muscle
The average (±SD) chemical composition of muscle from S. canicula, expressed as a percentage of wet weight, was 77.79 ± 0.70, 1.11 ± 0.06, 22.19 ± 0.46, and 0.35 ± 0.06 of moisture, ash, protein, and lipids respectively. The protein content was higher, the lipid content lower, and the ash content similar to values reported for the edible portion of most common finfish and crustacean species [16]. When compared to other elasmobranchs, protein and ash content of small-spotted catshark were higher (except when compared to smooth hound which presented a higher content of ashes), while lipid content was lower [17,18]. The low lipid content found in the flesh of small-spotted catshark muscle has a positive impact on the overall quality of this species (low rancidity, low oxidation, and enhanced texture of the flesh).
2.2. Hydrolysis Process and Production of FPH
Three commercial proteases, Alcalase, Esperase, and Protamex, were selected for the hydrolysis of the present by-products. They were chosen due to their excellent ability to digest several substrates from different fish by-products and their low cost when applied in industrial processes [3,19,20]. The experimental and predicted profiles of hydrolysis degrees, executed under the conditions specified in Table S1, are displayed in Figures S1 and S2. In various combinations of pH and temperature, no detectable degrees of hydrolysis were observed (for example, highest pH in Alcalase or lowest temperature in Esperase). Additionally, the results for Protamex catalysis were not presented since no hydrolysis was detected and therefore discarded for the evaluation of bioactivities. This last issue was unexpected because Protamex has reported good hydrolytic ability on muscle of, among others, catfish, small croaker, and Pacific hake [21,22]. It may be due to the possible presence of inhibitor compounds of Protamex activity in the muscle discards of S. canicula.
Experimental data of enzyme hydrolysis were accurately modelled by Weibull equation [5] with coefficients of determination ranging from 0.951 to 0.999 (Table 1).
Table 1.
Parametric estimations corresponding to hydrolysis data modelling by Weibull equation of the experimental conditions studied (Table S1). Independent variables are expressed in natural values in brackets. Numerical values of the parameters are shown with their confident intervals. Determination coefficients (R2) and p-values from Fisher F-test are also summarized. NHD: non hydrolysis detected.
| Experimental Conditions | Hm (%) | vm (% min−1) | τ (min) | β | R2 | p-Value | |
|---|---|---|---|---|---|---|---|
| Alcalase | T:−1 (37.3 °C)/pH:−1 (6.9) | 11.39 ± 0.32 | 0.068 ± 0.006 | 42.41 ± 2.95 | 0.73 ± 0.05 | 0.982 | <0.001 |
| T:1 (72.7 °C)/pH:−1 (6.9) | 13.52 ± 0.01 | 0.811 ± 0.012 | 4.08 ± 0.09 | 0.71 ± 0.01 | 0.999 | <0.001 | |
| T:−1 (37.3 °C)/pH:1 (11.1) | NHD | NHD | NHD | NHD | NHD | NHD | |
| T:1 (72.7 °C)/pH:1 (11.1) | 29.26 ± 1.34 | 0.063 ± 0.005 | 122.2 ± 11.6 | 0.76 ± 0.03 | 0.998 | <0.001 | |
| T:−1.41 (30.0 °C)/pH:0 (9.0) | 12.57 ± 0.29 | 0.053 ± 0.003 | 75.53 ± 3.22 | 0.92 ± 0.04 | 0.991 | <0.001 | |
| T:1.41 (80.0 °C)/pH:0 (9.0) | 12.79 ± 9.30 | 0.002 ± 0.001 | 826.5 (NS) | 0.34 ± 0.06 | 0.966 | <0.001 | |
| T:0 (55.0 °C)/pH:−1.41 (6.0) | 5.61 ± 0.01 | 1.67 ± 0.30 | 0.44 ± 0.11 | 0.38 ± 0.03 | 0.974 | <0.001 | |
| T:0 (55.0 °C)/pH:1.41 (12.0) | NHD | NHD | NHD | NHD | NHD | NHD | |
| T:0 (55.0 °C)/pH:0 (9.0) | 21.66 ± 0.39 | 0.125 ± 0.007 | 31.56 ± 1.59 | 0.53 ± 0.02 | 0.994 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (9.0) | 24.25 ± 0.27 | 0.207 ± 0.009 | 17.06 ± 0.62 | 0.42 ± 0.01 | 0.997 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (9.0) | 29.19 ± 5.17 | 0.042 ± 0.025 | 95.71 ± 64.35 | 0.39 ± 0.05 | 0.971 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (9.0) | 29.22 ± 4.42 | 0.056 ± 0.038 | 55.45 ± 40.32 | 0.31 ± 0.04 | 0.975 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (9.0) | 21.85 ± 2.50 | 0.089 ± 0.042 | 30.35 ± 14.03 | 0.36 ± 0.05 | 0.951 | <0.001 | |
| Esperase | T:−1 (37.3 °C)/pH:−1 (6.6) | NHD | NHD | NHD | NHD | NHD | NHD |
| T:1 (72.7 °C)/pH:−1 (6.6) | NHD | NHD | NHD | NHD | NHD | NHD | |
| T:−1 (37.3 °C)/pH:1 (9.4) | 12.95 ± 0.13 | 0.149 ± 0.007 | 24.13 ± 1.26 | 0.80 ± 0.05 | 0.972 | <0.001 | |
| T:1 (72.7 °C)/pH:1 (9.4) | 30.0 ± 18.04 | 0.004 (NS) | 604.8 (NS) | 0.25 ± 0.05 | 0.969 | <0.001 | |
| T:−1.41 (30.0 °C)/pH:0 (8.0) | 5.41 ± 0.08 | 0.041 ± 0.003 | 122.5 ± 2.62 | 2.66 ± 0.19 | 0.978 | <0.001 | |
| T:1.41 (80.0 °C)/pH:0 (8.0) | 11.73 ± 0.02 | 1.43 ± 0.06 | 2.10 ± 0.16 | 0.74 ± 0.05 | 0.981 | <0.001 | |
| T:0 (55.0 °C)/pH:−1.41 (6.0) | NHD | NHD | NHD | NHD | NHD | NHD | |
| T:0 (55.0 °C)/pH:1.41 (10.0) | 20.54 ± 0.05 | 0.532 ± 0.012 | 8.43 ± 0.29 | 0.63 ± 0.02 | 0.993 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (8.0) | 24.45 ± 0.18 | 0.249 ± 0.007 | 16.20 ± 0.40 | 0.48 ± 0.01 | 0.997 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (8.0) | 29.42 ± 0.54 | 0.209 ± 0.014 | 22.04 ± 0.93 | 0.45 ± 0.02 | 0.993 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (8.0) | 25.25 ± 0.23 | 0.281 ± 0.010 | 14.79 ± 0.47 | 0.48 ± 0.01 | 0.995 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (8.0) | 29.78 ± 0.29 | 0.318 ± 0.012 | 15.67 ± 0.52 | 0.48 ± 0.01 | 0.995 | <0.001 | |
| T:0 (55.0 °C)/pH:0 (8.0) | 27.83 ± 0.21 | 0.322 ± 0.010 | 14.52 ± 0.40 | 0.49 ± 0.01 | 0.996 | <0.001 |
The experimental and theoretical profiles were almost indistinguishable. In almost all cases the maximum hydrolysis was reached at 2–4 h (stationary phase); therefore, S. canicula kinetics could be shorted and the ratio S:L and enzyme concentration could be also reduced. In fact, using a S:L ratio of (1:2), Alcalase concentration of 0.5% (v/w) and run kinetics for 4 h at 60 °C/pH = 8.60, hydrolysis of 24%, was achieved (data not shown). The robustness of Weibull equation to predict the present patterns was also confirmed in all kinetics (p < 0.001 from Fisher F-test) and the numerical parameters were always statistically significant (Student t-test). The validity of the Weibull equation proposed has been again revealed. Habitually used for the modelling of dose-response curves, animal growths, and chemical and enzymatic reactions without mechanistical basis [7,23], that mathematical model has been recently applied to the description of enzyme proteolysis of fish cartilages [24] and squid pen [25] with excellent outcomes. Two parameters (Hm and vm) from equation [5] were selected as responses (dependent variables) to assess the effect of pH and T on proteolysis.
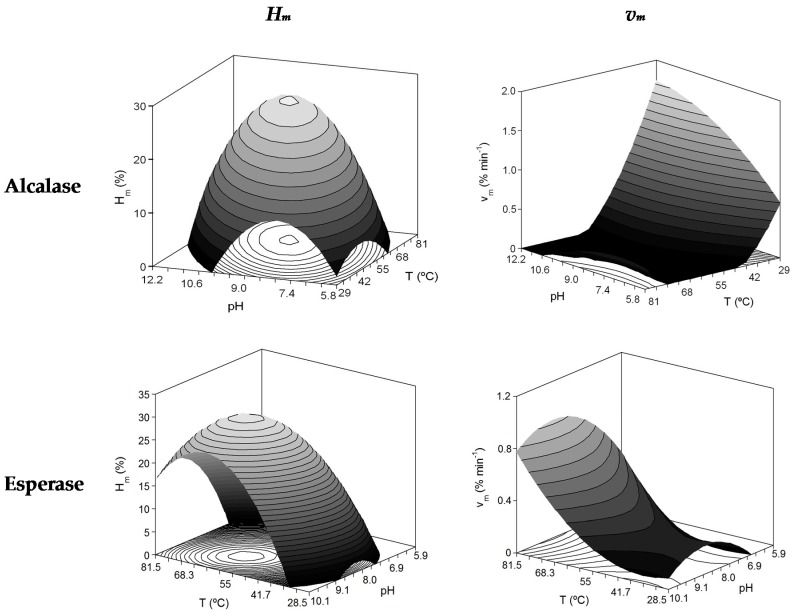
Figure 1 illustrates the plots corresponding with the 3D-surfaces predicted by the second-order equations as a function of T and pH (Equation (1)) using RSM and the multivariable analysis summarized in Table 2.
Figure 1.
Theoretical response surfaces describing the combined effects of the temperature and pH on the Weibull parameters obtained by Alcalase and Esperase proteolysis of S. canicula muscle and summarized in Table 1.
Table 2.
Empirical models describing the combined effect of temperature (T) and pH on the enzyme hydrolysis parameters and the antihypertensive activities (ACE-inhibitory activity (IACE) in % and IC50 in μg/mL) of S. canicula muscle hydrolysates. Optima values of the two variables (Topt and pHopt) to obtain the maximum responses (Ymax) from the empirical equations are summarized. The coefficients of determination R2 and the results of Fisher F-tests (F1, F2, F3, and F4) are also shown. S: significant; NS: non-significant.
| Enzyme/Activity | Polynomial Equations | R2 | Fisher F-Test | Topt (°C) | pHopt | Ymax |
|---|---|---|---|---|---|---|
| Alcalase/Hydrolysis | Hm (%) = 25.22 + 3.97 T + 6.78 T pH − 4.82 T2 − 9.79 pH2 | 0.698 | F1: S; F2: S; F3: NS; F4: NS | 64.6 | 9.40 | 26.3% |
| vm (% min−1) = 0.104 + 0.092 T − 0.398 pH − 0.170 T pH − 0.088 T2 + 0.318 pH2 | 0.693 | F1: S; F2: S; F3: NS; F4: NS | 53.9 | 10.29 | 1.6% min−1 | |
| Esperase/Hydrolysis | Hm (%) = 27.34 + 3.25 T + 9.02 pH + 4.26 T pH − 9.09 T2 − 8.23 pH2 | 0.943 | F1: S; F2: S; F3: S; F4: S | 60.8 | 8.90 | 30.7% |
| vm (% min−1) = 0.277 + 0.228 T + 0.113 pH + 0.115 T2 − 0.122 pH2 | 0.413 | F1: S; F2: S; F3: NS; F4: NS | 80 | 8.65 | 0.90% min−1 | |
| Alcalase/Antihypertensive | IACE (%) = 74.51 + 7.61 T + 3.48 pH − 6.03 T2 − 6.50 pH2 | 0.746 | F1: S; F2: S; F3: NS; F4: NS | 66.2 | 11.5 | 90.7% |
| * IC50 (μg/mL) = 117.42 − 23.61 T + 47.81 T2 | 0.739 | F1: S; F2: S; F3: NS; F4: NS | 59.4 | non effect | 114.5 μg/mL | |
| Esperase/Antihypertensive | IACE (%) = 74.42 − 3.87 T | 0.496 | F1: S; F2: S; F3: NS; F4: NS | 30.0 | non effect | 79.9% |
| * IC50 (μg/mL) = 111.41 + 94.34 T + 80.12 T2 | 0.583 | F1: S; F2: S; F3: NS; F4: NS | 44.6 | non effect | 83.64 μg/mL |
The values for the non-hydrolysis detected (NHD) were assumed as zero. The coefficients of determination of the theoretical surfaces ranged from 0.413 to 0.943. Very poor proportion of variability was explained for the case of vm with Esperase (41%), but remarkable fittings were obtained in the other responses (>69%). The consistence of the polynomial equations was also demonstrated in all situations (F1 and F2 were always confirmed).
The coefficients present in the equations defined in Table 2 indicated that the quadratic effects of both variables were statistically significant in all cases and always with negative sign for pH. The quadratic term of T was also negative for the maximum degree of hydrolysis (Hm) and the interaction pH × T was significant for this parameter. Thus, the theoretical surfaces show the typical form of convex dome with clear maximum at unique values of pH and T. In order to calculate those optimal levels, mathematical optimization using numerical derivation of empirical equations was performed. The optima values of both variables (pHopt and Topt) and the corresponding maximum value for each response (Ymax) are also represented in Table 2. Temperatures of 64.6 °C and 60.8 °C and pHs of 9.40 and 8.90 were established as the best hydrolysis conditions for Alcalase and Esperase, respectively. These levels are much higher than commonly applied (50 °C/pH = 7.0–8.0) in the production of FPH production using other fish substrates but without previous optimisation of both variables [8,25]. Higher capacity of hydrolysis of Esperase (30.7%) in comparison to Alcalase (26.3%) was predicted under the optimal conditions mentioned (Table 2). Our Hm data for Alcalase are in general similar or higher to other species as Yellow stripe trevally, Pacific whiting, Cape hake and horse mackerel [8,10,26]. However, greater degree of hydrolysis (44%) was described for salmon-FPH using an acid fungal protease [27] and anchovy sprat-FPH formulated with Papain or Bromelain [25]. The results of Esperase can not be compared with literature data because our study is the first report addressing the production of FPH catalysed by such endoprotease.
The case of vm was more heterogeneous; equations described different surfaces depending on the enzyme used, and the highest maximum rate of hydrolysis were achieved at high pH and low T for Alcalase and high T and pH = 8.65 for Esperase. Nevertheless, the reliability of the equations is much lower than in the case of Hm. This kinetic parameter (vm) is not applied in the studies concerning FPH since the modelling of FPH kinetics is habitually inexistent.
2.3. Determination of the In Vitro Antihypertensive Activities of the Produced FPH
The samples of hydrolysates at the end of the enzyme process (6 h) were analysed quantifying the ACE activity as percentage of inhibition and as the value of IC50 [28]. The experimental and expected data from the factorial design are compiled in Tables S2 and S3. The corresponding polynomial equations calculated from such data demonstrated the significant effect of T and the non effect of pH in three of the four cases evaluated (Table 2). The explanatory capacity of those models was higher for the case of Alcalase (>74%) than Esperase (>50%).
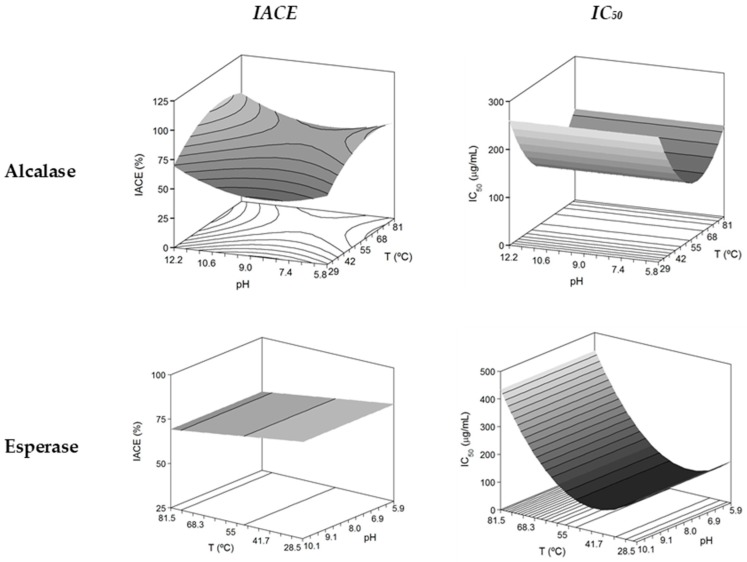
The multivariate analysis for the experimental data of Esperase predicted a surface in the form of a sheet; only linear coefficient of T was significant, producing greater IACE values at high temperatures (Figure 2). The case of Alcalase was somewhat more complex with better conditions of operation at high and low temperatures and pHs in a characteristic surface with structure of saddle. The optimal values were 66 °C and pH = 11.5.
Figure 2.
Theoretical response surfaces describing the combined effects of the temperature and pH of enzymatic hydrolysis on antihypertensive activities of S. canicula muscle hydrolysates and summarized in Table S2.
The theoretical surfaces show similar form in the IC50-response for both enzymes, that is, constant values for any level of pH and concave sheet with a minimum at 59.4 °C for Alcalase and 44.6 °C for Esperase (Figure 2 and Table 2). Thus, lower value of IC50 (higher activity) was found employing Esperase (83.6 μg/mL) than using Alcalase as catalyst (114.5 μg/mL). The values of Hm in the optimal conditions for maximizing IC50 can be calculated from the polynomial equations listed in Table 2 and were 25.9% and 23.1% for Alcalase and Esperase, respectively. As it was reported by [29], the presence of shorter peptides (obtained at high degrees of hydrolysis) in blue whiting hydrolysates lead to highest ACE-inhibitory activity (lower values of IC50); thus, our results are in concordance with that assumption: high Hm values, but not the highest, generated almost the best bioactivities (lower IC50 values and levels of IACE greater than 75%).
In addition, IC50 data obtained in the current study are much lower (higher antihypertensive capacity) than exhibited by other authors. Values ranging 0.25–7.4 mg/mL were observed for hydrolysates prepared from horse mackerel, sardinelle, blue whiting, trevally and goby by-products, and discards employing various types of proteases [11,17,29,30,31]. Our crude hydrolysates are also more active (83.6 and 114.5 μg/mL) than crude FPH produced from S. canicula muscle (302 μg/mL) by the simultaneous combination of subtilisin and trypsin [11]. These authors improved the bioactivite of the hydrolysates through the isolation of peptide fractions of lower molecular weights by size-exclusion chromatography (SEC). Two fractions of 470–1210 Da and <470 Da yielded values of 72 and 27 μg/mL, respectively, and were associated to the presence, among others, of the following peptides: ELVGV, LVAPAN, and VAMPF. These peptides may be responsible for the excellent antihypertensive activities reported here.
2.4. Determination of Antioxidant Activities of the Produced FPH
Because the mechanism of inhibition of oxidation process in biological samples is dependent on the chemical features of the antioxidant and the matrix, different types of antioxidant activities were measured in each sample obtained from the experimental design. In the present work, four protocols were applied (DPPH, ABTS, crocin, and β-carotene bleaching assays). Experiment data and numerical expected values obtained from second-order equations are summarized in Tables S2 and S3.
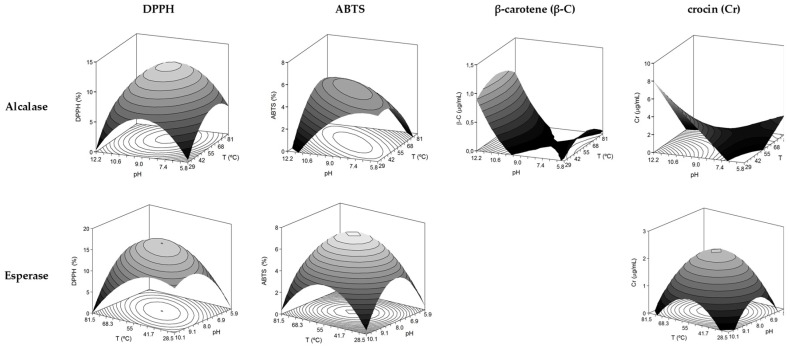
The outcomes of DPPH and ABTS for both enzymes were quite similar: convex domes were the 3D-surfaces expected (Figure 3) due to the negative terms obtained for the quadratic effect of T and pH (Table 3).
Figure 3.
Theoretical response surfaces describing the combined effects of the temperature and pH of enzymatic hydrolysis on antioxidant activities of S. canicula muscle hydrolysates and summarized in Table S3.
Table 3.
Empirical models describing the combined effect of temperature (T) and pH on the antioxidant activities (β-carotene: β-C, crocin: Cr, DPPH and ABTS methods) of S. canicula muscle hydrolysates. Optima values of the two variables (Topt and pHopt) to obtain the maximum responses (Ymax) from the empirical equations are summarized. The coefficients of determination R2 and the results of Fisher F-test (F1, F2, F3 and F4) are also shown. S: significant; NS: non-significant; Tr: Trolox.
| Enzyme | Polynomial Equations | R2 | Fisher F-Test | Topt (°C) | pHopt | Ymax |
|---|---|---|---|---|---|---|
| Alcalase | β-C (μg BHT/mL) = 0.08 + 0.28 pH − 0.09 T2 + 0.26 pH2 | 0.680 | F1: S; F2: S; F3: NS; F4: NS | 55.0 | 12.0 | 1.09 μg BHT/mL |
| Cr (μg Trolox/mL) = 1.63 − 0.95 T + 0.98 pH − 1.53 T pH | 0.543 | F1: S; F2: S; F3: NS; F4: NS | 30.0 | 12.0 | 7.96 μg Tr/mL | |
| DPPH (%) = 12.07 + 1.44 T − 1.57 T2 − 2.68 pH2 | 0.833 | F1: S; F2: S; F3: NS; F4: NS | 63.1 | 9.0 | 12.4% | |
| ABTS (%) = 5.10 + 1.24 T pH − 0.85 T2 − 0.82 pH2 | 0.578 | F1: S; F2: S; F3: NS; F4: NS | 55.0 | 9.0 | 5.1% | |
| Esperase | Cr (μg Trolox/mL) = 2.23 − 0.62 T2 − 0.61 pH2 | 0.625 | F1: S; F2: S; F3: NS; F4: NS | 55.0 | 8.0 | 2.23 μg Tr/mL |
| DPPH (%) = 16.02 − 2.31 T pH − 2.26 T2 − 2.49 pH2 | 0.840 | F1: S; F2: S; F3: NS; F4: NS | 55.0 | 8.0 | 16.0% | |
| ABTS (%) = 7.30 − 1.44 T2 − 1.64 pH2 | 0.752 | F1: S; F2: S; F3: NS; F4: NS | 55.0 | 8.0 | 7.3% |
The coefficients of determination were also greater for DPPH than ABTS response. The values of pHopt and Topt were found closer to the center of the experimental domain, 55 °C/pH8.0 for ABTS/DPPH-Esperase, 63.1 °C/pH9.0 for DPPH-Alcalase, and 55 °C/pH9.0 for ABTS-Alcalase. DPPH and ABTS maximum values varied among 12.4% and 5.1% for Alcalase hydrolysates and 16% and 7.3% for Esperase hydrolysates. These antioxidant activities are lower than those addressed for FPH formulated with different materials and using several enzymes: horse mackerel and subtilisin [10], red scorpionfish and Flavourzyme [32], or threadfin bream and Papain [33].
The crocin response with Esperase followed an identical pattern in terms of the significant response surface defined by the significant second-order model and the corresponding optima conditions calculated. On the contrary, crocin results for Alcalase displayed the best operatory values when protease was exposed to lower temperature and higher level of pH. The hydrolysates generated by Esperase did not show any type of capacity to slow down the β-carotene bleaching reaction. Finally, the maximum response for β-C and Alcalase was established at 55 °C and high value of pH.
3. Materials and Methods
3.1. S. canicula Discards
Discards from S. canicula were obtained in a local market (Vigo, Spain) and stored at −4 °C until use. Specimens were thawed and the muscle tissue removed, mixed thoroughly, ground, separated into different batches, and stored in sealed plastic bags at −20 °C.
Proximate Composition of S. canicula Muscle
Muscle was analysed for crude protein (N × 6.25) by Kjeldahl method in a DigiPREP 500 fully automatic steam distillation (SCP Science, Baie-D’Urfe, QC, Canada) and a TitroLine Easy Unit (Metrohm AG, Ionenstrasse, Switzerland). Lipid content was determined by the methodology of [34]. Moisture was determined after heating the sample at 105 °C for 24 h, and ash content was determined after heating the sample 24 h at 550 °C.
3.2. Experimental Design
The simultaneous effect of two factors, temperature (T) and pH, on the enzymatic hydrolysis of S. canicula muscle by-products was evaluated by a rotatable second order design [35]. The values of the variables tested for each protease and the procedure of codification-decodification of the variables is summarized in Table S1. The commercial proteases were Alcalase 2.4 L (2.4 Anson Unit/g, AU/g), Esperase 8 L (8 KNovo Protease Unit/g, KNPU/g) and Protamex (Novozymes, Nordisk, Bagsvaerd, Denmark), and the concentration employed was 1% (v/w of muscle) in all cases. The rest of the experimental conditions were maintained constant: ratio solid:liquid (1:5) and 200 rpm of agitation. The kinetics of hydrolysis were performed in a controlled pH-Stat system with a 100 mL glass-reactor and extended up to 6 h. At the end of hydrolysis, the samples were heated at 90 °C for 15 min to inactivate the proteases and were stored at −20 °C until analysis.
Orthogonal least-squares calculation on factorial design data were used to obtain the empirical equations describing the different dependent variables (hydrolysis kinetic parameters and bioactivities) assessed (Y) in function of the independent variables (T and pH):
| (1) |
where Y represents the parameters to be modelled; b0 is the constant coefficient, b1 and b2 are the coefficient of linear effects, b12 is the coefficient of interaction effect among pH and T, and b11 and b22 are the coefficients of quadratic effects. The Student t-test (α = 0.05) was employed to determine the statistical significance of the coefficients. The goodness-of-fit was established as the determination coefficient (R2) and the model consistency by the Fisher F-test (α = 0.05) using the following mean squares ratios:
| the model is acceptable when | |
| F1 = Model/Total error | |
| F2 = (Model + Lack of fitting)/Model | |
| F3 = Total error/Experimental error | |
| F4 = Lack of fitting/Experimental error |
are the theoretical values to α = 0.05 with the corresponding degrees of freedom for numerator (num) and denominator (den). The equation is acceptable when F1 and F2 are validated. F3 and F4 were additionally calculated to improve the degree of robustness and consistency of the empirical equations obtained. A Microsoft Excel spreadsheet was employed for the procedures of numerical fittings, coefficient estimates, and statistical evaluations.
3.3. Enzyme Proteolysis of Muscle Discards
The hydrolysis degree (H, in %) was determined by the pH-Stat method defined by Adler-Nissen [36] by applying the following equation:
| (2) |
where B is the volume (mL) of 2 M NaOH consumed during hydrolysis; Nb is the normality of NaOH; Mp is the mass (g) of initial protein (N × 6.25); htot is the total number of peptide bonds available for proteolytic hydrolysis (8.6 meq/g), and α is the average degree of dissociation of the amino groups in the protein substrate, and it was determined with:
| (3) |
The pK value is dependent on the temperature of hydrolysis (in K degrees) and can be estimated according to the expression:
| (4) |
Finally, the kinetics data of the S. canicula muscle hydrolysis (H) were modelled by means of the Weibull equation [24]:
| (5) |
where H is the degree of hydrolysis (%); t is the time of hydrolysis (min); Hm is the maximum degree of hydrolysis (%); β is a parameter related to the maximum slope of muscle hydrolysis (dimensionless); τ is the time required to achieve the semi-maximum degree of hydrolysis (min) and vm is the maximum hydrolysis rate at the τ-time (% min−1).
3.4. Antihypertensive Activities and Angiotensin I-Converting Enzyme (ACE) Inhibition Assay
The antihypertensive activity of S. canicula hydrolysates was determined using N-[3-(2-Furyl) acryloyl]-l-phenylalanyl-glycyl-glycine (FAPGG) as substrate according the modifications reported by Estévez et al. [28] ACE-inhibitory activity (IACE) of hydrolysates was calculated as a function of the average slope of decrease in Absorbance with time and expressed as percentage inhibition of the enzyme, according to the following expression:
| (6) |
where IACE is the ACE-inhibitory capacity (%), rAh is the slope of decrease in Absorbance at 340 nm in the presence of inhibitor (hydrolysate), and rAc is the slope decrease in Absorbance at 340 nm in the absence of inhibitor (control). The protein-hydrolysate concentration that generates a 50% of IACE (IC50) was calculated by fitting the dose-response relationship between IACE vs. hydrolysate to a Weibull equation [37]:
| (7) |
where K is the maximum IACE (%), C is the protein-hydrolysate concentration (μg/mL), IC50 is the concentration for semi-maximum IACE (μg/mL), and a is the form parameter related to the maximum slope of the function (dimensionless).
3.5. Antioxidant Activity Determinations
3.5.1. 1,1-Diphenyl-2-Picryhydrazyl (DPPH) Radical-Scavenging Capacity
The antioxidant activity as radical-scavenging capacity was determined with DPPH as a free radical by the microplate method described by Prieto et al. [38]. The DPPH activity was calculated as a percentage of DPPH discoloration using the equation:
| (8) |
where Asample is the Absorbance at 515 nm of the DPPH in the presence of the hydrolysate, and Acontrol is the Absorbance at 515 nm of the DPPH solution in its absence.
3.5.2. ABTS Bleaching Method
The ABTS (2,2′-azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid) radical scavenging activities were evaluated according the protocol previously reported [38]. The ABTS activity was calculated as a percentage of ABTS discoloration using the equation:
| (9) |
where Asample is the absorbance at 414 nm of the ABTS in the presence of the hydrolysate, and Acontrol is the absorbance at 414 nm of the ABTS solution in its absence.
3.5.3. β-Carotene Bleaching Method
The kinetics of the β-carotene (β-C) bleaching assay were performed according the protocol described for its use in microplate spectrophotometer developed by Prieto et al. [39].
3.5.4. Crocin Bleaching Method
The kinetics of the crocin (Cr) bleaching assay were based on the protocol optimized by Prieto et al. [40] using crocin and 2,2′-azobis-2-amidinopropane (AAPH) as reagents and carried out in microplate spectrophotometer.
In these last two methods the kinetics of reaction were performed in triplicate. For each series, reversed curves were obtained by subtracting the Absorbance at time t from the Absorbance value at time 0. The area under the curves (AUC) can be calculated by the following function [37]:
| (10) |
where y0 to yn are the n + 1 y-values defining the curve, and Δt is the sampling interval (min).
Calculated areas of controls concentrations (Trolox for Cr and BHT for β-C) were fitted by linear regression. Calculated areas of hydrolysate dilutions were plotted against controls (equivalents) and the antioxidant activities (as equivalents in μg of BHT or Trolox per mL of hydrolysate) were defined by means of the EC50 values obtained by fitting the data of equivalents versus sample concentrations to a similar Weibull equation [7] (but replacing IC50 by EC50).
3.6. Numerical and Statistical Analyses
Fitting procedures and parametric estimations calculated from the non-linear equations were carried out by minimising the sum of quadratic differences between the observed and model-predicted values and using the non-linear least-squares (quasi-Newton) method provided by the macro-‘Solver’ of the Microsoft Excel spreadsheet. Confidence intervals from the parametric estimates (Student t-test) and consistence of mathematical models (Fisher F-test) were evaluated by “SolverAid” macro (Levie’s Excellaneous website: http://www.bowdoin.edu/~rdelevie/excellaneous).
4. Conclusions
In the present report, we have optimised the production of FPH with potentially bioactive peptides, including antioxidant and antihypertensive properties, from the S. canicula muscle by enzymatic hydrolysis using the response surface methodology. The optimal conditions for the highest proteolysis were established in 60.8 °C/pH8.9 and 64.6 °C/pH9.4 for Esperase and Alcalase, respectively. No hydrolysis was, however, detected when using Protamex. The lower IC50 values (higher ACE activity) were achieved with hydrolysates obtained at 59.4 °C for Alcalase and 44.6 °C for Esperase, and any level of pH. In such conditions, the Esperase hydrolysate was more active than the Alcalase one. For antioxidants, ABTS and DPPH activities were maximized in the range of 55–63.1 °C and pH of 8.0–9.0. Nevertheless, further studies should be conducted in order to characterise and isolate the bioactive peptides that produce the best antioxidant and antihypertensive activities.
Acknowledgments
We appreciate the excellent technical support of Ana I. Durán and Araceli Menduíña (IIM-CSIC). We also thank to Ramiro Martínez (Novozymes A/S, Spain) for supplying us with proteases. This research was funded by the project iSEAS LIFE13 ENV/ES/000131 (LIFE+ Programme, EU). The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007-2013) under REA grant agreement n° 600391.
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/15/10/306/s1, Figure S1: Proteolysis kinetics of S. canicula muscle wastes mediated by Esperase, Figure S2: Proteolysis kinetics of S. canicula muscle wastes mediated by Alcalase, Table S1: Experimental domain values, Table S2: Experimental results for hydrolysates obtained by Alcalase, Table S3: Experimental results for hydrolysates obtained by Esperase.
Author Contributions
José A. Vázquez, Ricardo I. Pérez-Martín and Maria Blanco conceived and designed the experiments; Maria Blanco, Agueda E. Massa and Isabel Rodríguez-Amado performed the experiments; José A. Vázquez, Ricardo I. Pérez-Martín and Maria Blanco analyzed the data; José A. Vázquez and Maria Blanco wrote the paper. Ricardo I. Pérez-Martín critically revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rodríguez-Cabello C., Fernández A., Olaso I., Sánchez F., Gancedo R., Punzón A., Cendrero O. Overview of continental shelf elasmobranch fisheries in the Cantabrian Sea. J. Northwest Atl. Fish. Sci. 2005;35:375–385. [Google Scholar]
- 2.Blanco M. Ph.D. Thesis. Universidad de Vigo; Vigo, Spain: 2015. Valorización de Descartes y Subproductos de Pintarroja (Scyliorhinus canicula) [Google Scholar]
- 3.Chalamaiah M., Dinesh Kumar B., Hemalatha R., Jyothirmayi T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012;135:3020–3038. doi: 10.1016/j.foodchem.2012.06.100. [DOI] [PubMed] [Google Scholar]
- 4.Sila A., Bougatef A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. J. Funct. Foods. 2016;21:10–26. doi: 10.1016/j.jff.2015.11.007. [DOI] [Google Scholar]
- 5.Blanco M., Sotelo C.G., Pérez-Martín R.I. Hydrolysis as a valorization strategy for unused marine food biomass: Boarfish and small-spotted catshark discards and by-products. J. Food Biochem. 2015;39:368–376. doi: 10.1111/jfbc.12141. [DOI] [Google Scholar]
- 6.Roblet C., Akhartar M.J., Mikhaylin S., Pilon G., Gill T., Marette A., Bazinet L. Enhanceent of glucose uptake in muscular cell by peptide fractions separated by electrodialysis with filtration membrane from salmon frame protein hydrolysate. J. Funct. Foods. 2016;22:337–346. doi: 10.1016/j.jff.2016.01.003. [DOI] [Google Scholar]
- 7.Amado I.R., Vázquez J.A., González M.P., Murado M.A. Production of antihypertensive and antioxidante activities by enzymatic hydrolysis of protein concentrates recovered by ultrafiltration from cuttlefish processing wastewaters. Biochem. Eng. J. 2013;76:43–54. doi: 10.1016/j.bej.2013.04.009. [DOI] [Google Scholar]
- 8.Pires C., Clement T., Batista I. Functional properties of protein hydrolysates from Cape hake by-products prepared by three different methodologies. J. Sci. Food Agric. 2013;93:771–780. doi: 10.1002/jsfa.5796. [DOI] [PubMed] [Google Scholar]
- 9.Chi C.F., Wang B., Hu F.Y., Wang Y.M., Zhang B., Deng S.G., Wu C.W. Purification and identification of three novel antioxidante peptides from protein hydrolysates of bluefin leatherjacket (Novadon septentrionalis) skin. Food Res. Int. 2015;73:124–129. doi: 10.1016/j.foodres.2014.08.038. [DOI] [Google Scholar]
- 10.Morales-Medina R., Pérez-Gálvez R., Guadix A., Guadix E.M. Multiobjective optimization of the antioxidant activities of horsemackerel hydrolysates produced with protease mixtures. Process Biochem. 2017;52:149–158. doi: 10.1016/j.procbio.2016.11.001. [DOI] [Google Scholar]
- 11.García-Moreno P.J., Espejo-Carpio F.J., Guadix A., Guadix E.M. Production and identification of angiotensin I-converting enzyme (ACE) inhibitory peptides from Mediterranean fish discards. J. Funct. Foods. 2015;18:95–105. [Google Scholar]
- 12.Blanco M., Fraguas J., Sotelo C.G., Pérez-Martín R.I., Vázquez J.A. Production of chondroitin sulphate from head, skeleton and fins of Scyliorhinus canicula by-products by combination of enzymatic, chemical precipitation and ultrafiltration methodologies. Mar. Drugs. 2015;13:3287–3308. doi: 10.3390/md13063287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vázquez J.A., Pastrana L., Piñeiro C., Teixeira J.A., Pérez-Martín R.I., Amado I.R. Production of hyaluronic acid by Streptococcus zooepidemicus on protein substrates obtained from Scyliorhinus canicula discards. Mar. Drugs. 2015;13:6537–6549. doi: 10.3390/md13106537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco M., Simpson B.K., Pérez-Martín R.I., Sotelo C.G. Isolation and partial characterization of trypsin from pancreas of small-spotted catshark (Scyliorhinus canicula) J. Food Biochem. 2014;38:196–206. doi: 10.1111/jfbc.12038. [DOI] [Google Scholar]
- 15.Sotelo C.G., Blanco M., Ariza P.R., Pérez-Martín R.I. Characterization of collagen from different discarded fish species of the west coast of the Iberian Peninsula. J. Aquat. Food Prod. Technol. 2016;25:388–399. doi: 10.1080/10498850.2013.865283. [DOI] [Google Scholar]
- 16.Silva J.L., Chamul R. Composition of marine and freshwater finfish and shellfish species and their products. In: Martin R.E., Carter E.P., Flick G.J., Davis L.M., editors. Marine and Freshwater Products Handbook. CRC Press; Boca Raton, FL, USA: 2000. pp. 31–46. [Google Scholar]
- 17.Bougatef A., Nedjar-Arroume N., Ravallec-Plé R., Leroy Y., Guillochon D., Barkia A., Nasri M. Angiotensin I-converting enzyme (ACE) inhibitory activities of sardinelle (Sardinella aurita) by-products protein hydrolysates obtained by treatment with microbial and visceral fish serine proteases. Food Chem. 2008;111:350–356. doi: 10.1016/j.foodchem.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 18.Diniz F.M., Martin A.M. Optimization of nitrogen recovery in the enzymatic hydrolysis of dogfish (Squalus acanthias) protein. Composition of the hydrolysates. Int. J. Food Sci. Nutr. 2009;48:191–200. doi: 10.3109/09637489709012592. [DOI] [PubMed] [Google Scholar]
- 19.Batista I., Ramos C., Coutinho J., Bandarra N.M., Nunes M.L. Characterization of protein hydrolysates and lipids obtained from black scabbardfish (Aphanopus carbo) by-products and antioxidative activity of the hydrolysates produced. Process Biochem. 2010;45:18–24. doi: 10.1016/j.procbio.2009.07.019. [DOI] [Google Scholar]
- 20.Mosquera M., Giménez B., Da Silva I.M., Boelter J.F., Montero P., Gómez-Guillén M.C., Brandelli A. Nanoencapsulation of an active peptidic fraction from sea bream scales collagen. Food Chem. 2014;156:144–150. doi: 10.1016/j.foodchem.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Cinq-Mars C.D., Li-Chan E.C.Y. Optimizing angiotensin I-convertingenzyme inhibitory activity of pacific hake (Merluccius productus) fillet hydrolysate using response surface methodology and ultrafiltration. J. Agric. Food Chem. 2007;55:9380–9388. doi: 10.1021/jf0713354. [DOI] [PubMed] [Google Scholar]
- 22.Choi Y.J., Hur S., Choi B.D., Konno K., Park J.W. Enzymatic hydrolysis of recovered protein from frozen small croaker and functional properties of its hydrolysates. J. Food Sci. 2009;74:17–24. doi: 10.1111/j.1750-3841.2008.00988.x. [DOI] [PubMed] [Google Scholar]
- 23.Rial D., Vázquez J.A., Menduiña A., García A.M., González M.P., Mirón J., Murado M.A. Toxicity of binary mixtures of oil fractions to sea urchin embryos. J. Hazard. Mater. 2013;263:431–440. doi: 10.1016/j.jhazmat.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 24.Vázquez J.A., Blanco M., Fraguas J., Pastrana L., Pérez-Martín R.I. Optimisation of the extraction and purification of chondroitin sulphate from head by-products of Prionace glauca by environmental friendly processes. Food Chem. 2016;198:28–35. doi: 10.1016/j.foodchem.2015.10.087. [DOI] [PubMed] [Google Scholar]
- 25.Ovissipour M., Rasco B., Shiroodi S.G., Modanlow M., Gholami S., Nemati M. Antioxidant activity of protein hydrolysates from whole anchovy sprat (Clupeonella engrauliformis) prepared using endogenous enzymes and commercial proteases. J. Food Sci. Agric. 2013;93:1718–1726. doi: 10.1002/jsfa.5957. [DOI] [PubMed] [Google Scholar]
- 26.Pacheco-Aguilar R., Mazorra-Manzano M.A., Ramirez-Suarez J.C. Functional properties of fish protein hydrolysates from Pacific whiting (Merluccius productus) muscle produced by a commercial protease. Food Chem. 2008;109:782–789. doi: 10.1016/j.foodchem.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 27.Nurdiani R., Dissanayake M., Street W.E., Donkor O.N., Singh T.K., Vasiljevic T. In vitro study of selected physiological and physicochemical properties of fish protein hydrolysates from 4 Australian fish species. Int. Food Res. J. 2016;23:2042–2053. [Google Scholar]
- 28.Estévez N., Fuciños P., Sobrosa A.C., Pastrana L., Pérez N., Rúa M.L. Modeling the angiotensin-converting enzyme inhibitory activity of peptide mixtures obtained from cheese whey hydrolysates using concentration-response curves. Biotechnol. Prog. 2012;28:1197–1206. doi: 10.1002/btpr.1587. [DOI] [PubMed] [Google Scholar]
- 29.García-Moreno P.J., Pérez-Gálvez R., Espejo-Carpio F.J., Ruiz-Quesada C., Pérez-Morilla A.I., Martínez-Agustín O., Guadix A., Guadix E.M. Functional, bioactive and antigenicity properties of blue whiting protein hydrolysates: Effect of enzymatic treatment and degree of hydrolysis. J. Sci. Food Agric. 2017;97:299–308. doi: 10.1002/jsfa.7731. [DOI] [PubMed] [Google Scholar]
- 30.Salampessy J., Reddy N., Kailasapathy K., Phillips M. Functional and potential therapeutic ACE-inhibitory peptides derived from bromelain hydrolysis of trevally proteins. J. Funct. Foods. 2015;14:716–725. doi: 10.1016/j.jff.2015.02.037. [DOI] [Google Scholar]
- 31.Nasri R., Younes I., Jridi M., Trigui M., Bougatef A., Nedjar-Arroume N., Dhulster P., Nasri M., Karra-Châabouni M. ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophio-cephalus) fish protein hydrolysates: Effect on meat lipid oxidation. Food Res. Int. 2013;54:552–561. doi: 10.1016/j.foodres.2013.07.001. [DOI] [Google Scholar]
- 32.Aissaoui N., Abidi F., Marzouki M.N. ACE inhibitory and antioxidant activities of red scorpionfish (Scorpaena notata) protein hydrolysates. J. Food Sci. Technol. 2015;52:7092–7102. doi: 10.1007/s13197-015-1862-8. [DOI] [Google Scholar]
- 33.Gajanan P.G., Elavarasan K., Shamasundar B.A. Bioactive and functional properties of protein hydrolysates from fish frame processing waste using plant proteases. Environ. Sci. Pollut. Res. 2016;23:24901–24911. doi: 10.1007/s11356-016-7618-9. [DOI] [PubMed] [Google Scholar]
- 34.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 35.Box G.E., Hunter J.S., Hunter W.G. Statistics for Experimenters: Design, Innovation, and Discovery. 2nd ed. John Wiley & Sons, Inc.; New York, NY, USA: 2005. [Google Scholar]
- 36.Adler-Nissen J. Enzymatic Hydrolysis of Food Proteins. Elsevier; London, UK: 1986. pp. 132–142. [Google Scholar]
- 37.Amado I.R., González M.P., Murado M.A., Vázquez J.A. Shrimp wastewater as a source of astaxanthin and bioactive peptides. J. Chem. Technol. Biotechnol. 2016;91:793–805. doi: 10.1002/jctb.4647. [DOI] [Google Scholar]
- 38.Prieto M.A., Curran T., Gowen A., Vázquez J.A. An efficient methodology for quantification of synergy and antagonism in single electron transfer antioxidant assays. Food Res. Int. 2015;67:284–298. doi: 10.1016/j.foodres.2014.11.030. [DOI] [Google Scholar]
- 39.Prieto M.A., Rodríguez-Amado I., Vázquez J.A., Murado M.A. β-Carotene assay revisited. Application to characterize and quantify antioxidant and prooxidant activities in a microplate. J. Agric. Food Chem. 2012;60:8983–8993. doi: 10.1021/jf302218g. [DOI] [PubMed] [Google Scholar]
- 40.Prieto M.A., Vázquez J.A., Murado M.A. Crocin bleaching antioxidant assay revisited. Application to microplate to analyse antioxidant and prooxidant activities. Food Chem. 2015;167:299–310. doi: 10.1016/j.foodchem.2014.06.114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.