Abstract
Sea cucumbers belonging to echinoderm are traditionally used as tonic food in China and other Asian countries. They produce abundant biologically active triterpene glycosides. More than 300 triterpene glycosides have been isolated and characterized from various species of sea cucumbers, which are classified as holostane and nonholostane depending on the presence or absence of a specific structural unit γ(18,20)-lactone in the aglycone. Triterpene glycosides contain a carbohydrate chain up to six monosaccharide units mainly consisting of d-xylose, 3-O-methy-d-xylose, d-glucose, 3-O-methyl-d-glucose, and d-quinovose. Cytotoxicity is the common biological property of triterpene glycosides isolated from sea cucumbers. Besides cytotoxicity, triterpene glycosides also exhibit antifungal, antiviral and hemolytic activities. This review updates and summarizes our understanding on diverse chemical structures of triterpene glycosides from various species of sea cucumbers and their important biological activities. Mechanisms of action and structural–activity relationships (SARs) of sea cucumber glycosides are also discussed briefly.
Keywords: holostane, nonholostane, cucumarioside, cytotoxic, antifungal, glycosides
1. Introduction
Nature is the largest source of pharmaceutical lead drugs for the remedies of many diseases. Earlier scientists mainly focused on terrestrial samples (plants and microorganisms) for the discovery of lead bioactive compounds. With the passage of time, the search for new drugs or agrochemicals has been switching from land to ocean due to re-isolation of known natural products from terrestrial samples. Marine organisms produce diversified bioactive compounds because of large species biodiversities and living in extremely harsh environment.
Among so many sources, numerous bioactive metabolites have been isolated from marine invertebrates such as echinoderms with a broad spectrum of biological activities [1]. The echinoderms are divided into five classes, i.e., Holothuroidea (sea cucumbers), Asteroidea (starfishes), Echinoidea (sea urchins), Crinoidea (sea lilies), and Ophiuroidea (brittle stars and basket stars), which live exclusively in the marine habitat, distributed in almost all depths and latitudes, as well as reef environments or shallow shores [2,3]. The importance of these echinoderms as a potential source of bioactive compounds for the development of new therapeutic drugs/agrochemicals has been growing rapidly [1]. Compounds isolated from echinoderms showed numerous biological activities including antibacterial, anticoagulant, antifungal, antimalarial, antiprotozoal, anti-tuberculosis, anti-inflammatory, antitumor, and antiviral activities [1].
Sea cucumber traditionally has been used as tonic food in China and other Asian countries for thousands of years. Besides being used as food, sea cucumbers are also promising source of bioactive natural products which predominantly belong to triterpene glycosides exhibiting antifungal, cytotoxic, hemolytic, cytostatic, and immunomodulatory and antiviral activities [4]. Several monographs concerning the structures and biological properties of triterpene glycosides obtained from sea cucumbers have been published but not presented in a systematic way [5,6]. This report comprehensively reviews in depth structural features of sea cucumber glycosides with corresponding producing species. Important biological activities, mechanism of action, and structure–activity relationships (SARs) of the diverse glycosides produced by the different species of sea cucumber are also discussed briefly.
2. Taxonomy, Distribution and Nutritive Value of Sea Cucumbers
One of the predominant invertebrate lives in marine environment is sea cucumber, which belong to the class Holothuroidea under the phylum Echinodermata. Holothuroidea has been divided into three subclasses, Aspidochirotacea, Apodacea and Dendrochirotacea, and further into six orders, Apodida, Elasipodida, Aspidochirotida, Molpadida, Dendrochirotida and Dactylochirotida [7]. Majority of the harvestable species of sea cucumbers belong to three families, viz., Holothuriidae (genera Holothuria and Bohadschia), Stichopodidae (genera Stichopus, Actinopyga, Thelenota, Parastichopus and Isostichopus), and Cucumariidae (genus Cucumaria) [8].
Sea cucumbers are elongated tubular or flattened soft-bodied marine benthic invertebrates, typically with leathery skin, ranging in length from a few millimeters to a meter [9]. Holothuroids encompass 14,000 known species occur in most benthic marine habitats worldwide, in both temperate and tropical oceans, and from the intertidal zone to the deep sea, and are considered as the very important parts of oceanic ecosystem [10].
Economically, sea cucumbers are important in two reasons: first, some species produce triterpene glycosides that are interested to pharmaceutical companies finding their medical use and second, use as food item. About 70 species of sea cucumbers have been exploited worldwide; out of which 11 species have been found to be commercially important [11]. Sea cucumbers have been well recognized as a tonic and traditional remedy in Chinese and Malaysian literature for their effectiveness against hypertension, asthma, rheumatism, cuts and burns, impotency and constipation [12,13]. Nutritionally, sea cucumbers have an impressive profile of valuable nutrients such as vitamin A, vitamin B1 (thiamine),vitamin B2 (riboflavin), vitamin B3 (niacin), and minerals, especially calcium, magnesium, iron and zinc [14,15].
3. Extraction, Purification and Characterization
To extract glycosides, first sea cucumbers will be freeze dried, then cut into pieces and extracted twice with refluxing EtOH. The combined extracts will be concentrated under reduced temperature and the residue will be dissolved in H2O. Desalting will be carried out by passing this fraction through a Polychrom column (Teflon), eluting first the inorganic salts and crude polar impurities with H2O and then the glycosides fraction with 50% EtOH. The fraction will be sub-fractionated by silica gel column chromatography using suitable gradient solvent system. The glycosides from each sub-fraction can be purified by reverse phase HPLC developing suitable solvent system (MeOH-H2O).
Triterpene glycosides have two parts: carbohydrate and triterpene. The number of monosaccharide units present in the carbohydrate chain can be deduced by observing the number of anomeric carbons (~103 ppm) and protons (~5 ppm, d) resonances in 13C and 1H NMR spectra, respectively. The sequence of monosaccharide units in the carbohydrate chain can be established by the analysis of anomeric H/C correlations in the HMBC spectrum which can also be confirmed by NOE corrections between anomeric protons and MALDI-TOF mass spectroscopic data analysis. The position of attachment of glycone with aglycone can be confirmed by the HMBC experiment.
The presence of diverse types of monosaccharide units and their repetitions in the carbohydrate chain can be established by acid hydrolysis followed by GC-MS analysis of the corresponding aldononitrile peracetates [16]. The site of attachment of sulfate group at monosaccharide units can be determined by observing chemical shift of esterification carbon atoms. The chemical shifts of α (esterification) and β-carbons are shifted ~5 ppm downfield and ~2 ppm up field, respectively, compare to their corresponding nonsulfated derivatives.
The structure of the aglycone can be established based on its spectroscopic data (1H NMR, 13C NMR, COSY, HMBC, HSQC, and TOCSY) and by comparing with the literature data. Configuration can be determined by the analysis of NOE data, stable conformers, coupling constants and comparing chemical shifts of chiral centers with literature.
4. Structural Features of Triterpene Glycosides Isolated from Sea Cucumbers
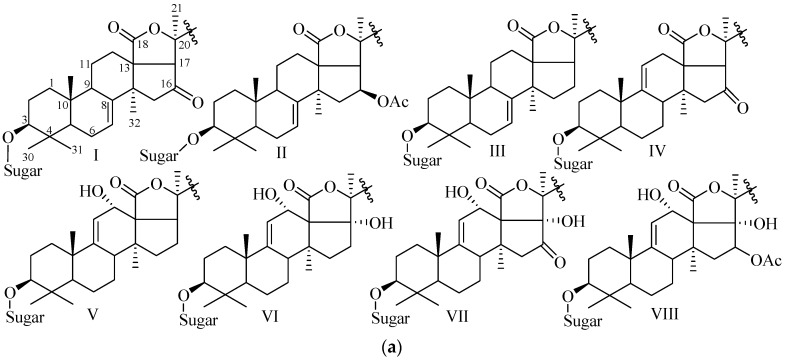
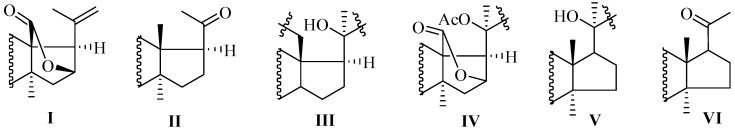
Triterpene glycosides, also known as holothurins or saponins, are secondary metabolites typically produced by sea cucumbers (class Holothuroidea). These glycosides are amphiphilic in nature having two parts: aglycone (lipophilic, lipid-soluble) and glycone (hydrophilic, water-soluble). The majority of the glycosides contain so called holostane type aglycone comprise of lanostane-3β-ol with a γ(18,20)-lactone in the E-ring of the pentacyclic triterpene [(3β,20S-dihydroxy-5α-lanostano- γ(18,20)-lactone] (Figure 1). A few of the glycosides contain nonholostane type aglycone which do not have γ(18,20)-lactone in the tetracyclic triterpene.
Figure 1.
Structures of lanostane, holostane and holostanol.
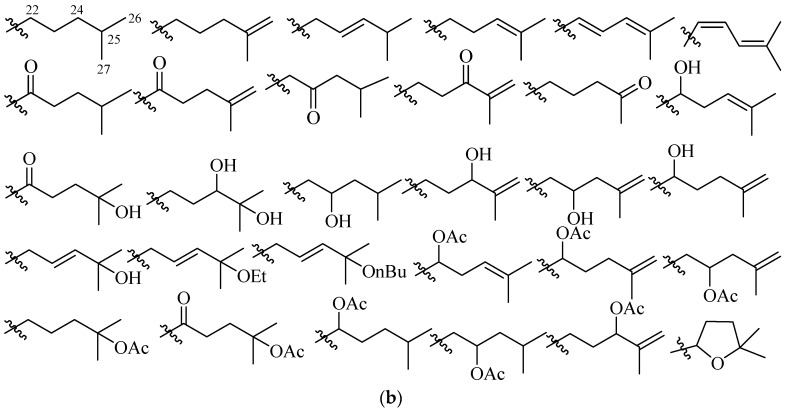
The glycone parts may contain up to six monosaccharide units covalently connected to C-3 of the aglycone. The sugar moieties mainly consist of d-xylose (Xyl), d-quinovose (Qui), d-glucose (Glc), 3-O-methyl-d-glucose (MeGlc), 3-O-methyl-d-xylose (MeXyl) (Figure 2) and sometimes 3-O-methyl-d-quinovose (MeQui), 3-O-methyl-d-glucuronic acid (MeGlcA) and 6-O-acetyl-d-glucose (AcGlc). In the carbohydrate chain, the first sugar unit is always a xylose and a majority case second is quinovose, whereas 3-O-methyl-d-glucose and/or 3-O-methyl-d-xylose are always the terminal monosaccharide units. The presence of two quinovose residues in a carbohydrate chain is unique for sea cucumber and starfish glycosides.
Figure 2.
Common sugar units present in sea cucumber glycosides.
In glycone part, the sugar units are generally arranged in a straight or branched chain (Figure 3). The majority of tetrasaccharides show a linear chain with the most common 3-O-Me-Glc-(1→3)-Glc-(1→4)-Qui-(1→2)-Xyl. Hexaglycosides are generally nonsulfated with a linear 3-O-Me-Glc (1→3)-Glc (1→4)-Xyl (2→1)-Qui (4→1)-Glc (3→1)-3-O-MeGlc unit. Pentasaccharides have a linear chain like tetrasaccharides but a branching at C-2 of quinovose (Figure 3).
Figure 3.
Some common carbohydrate architectures found in sea cucumber glycosides.
Sixty percent of the triterpene glycosides isolated so far from sea cucumbers have sulfate groups linked to the monosaccharide units of the carbohydrate chain. Most of them are monosulfsated, but many di- and trisulfated glycosides have also been isolated. Most tetrasaccharides and pentasaccharides are sulfated at C-4 of xylose unit. In both the cases, additional sulfate groups at C-6 of the 3-O-methylglucose and glucose units have also been found. The term “Ds” stands for desulfated. Sea cucumber triterpene glycosides are chemotaxonomic markers specific for groups of genera within each family.
Triterpene glycosides can be classified as holostane type having 3β-hydroxy-5α-lanostano- γ(18,20)-lactone structural feature and nonholostane type do not have a γ(18,20)-lactone but have other structural features like holostane type glycosides.
4.1. Holostane Type Triterpene Glycosides
Depending on the position of double bond in the B and C ring of the aglycone (Figure 1), holostane type glycosides can be further subdivided into three groups: glycosides with 3β-hydroxyholost-7(8)-ene, 3β-hydroxyholost-9(11)-ene, and 3β-hydroxyholost-8(9)-ene aglycone skeletons. There are eight pentacyclic triterpene and 30 alkane side chain aglycone architectures commonly found in holostane type glycosides (Figure 4). In these architectures, certain functional groups are generally attached to the specific carbons: keto and β-acetoxy groups at C-16, and α-hydroxy group at C-12 and C-17.
Figure 4.
Pentacyclic triterpene and alkane side chain skeletons are commonly found in holostane type glycosides. (a) Pentacylic triterpene skeletons. Substitution by selective functional groups and unsaturation generally take place in the alkane side chain (2-methylpentane) attached to C-20 of the E-ring of aglycone; (b) Alkane side chain architectures.
4.1.1. 3β-Hydroxyholost-7(8)-ene Skeleton Containing Holostane Glycosides
Substantial number of triterpene glycosides in this category is produced by sea cucumbers. The species Eupentacta fraudatrix, Holothuria lessoni, Bohadschia marmorata, Stichopus chloronotus and Staurocucumis liouvillei produce most of the compounds in this group. For convenience, the large number of compounds in this category can be further subdivided into four groups depending on the number of sugar units.
Holostane Glycosides with 3β-Hydroxyholost-7(8)-ene Skeleton and Six Sugar Units
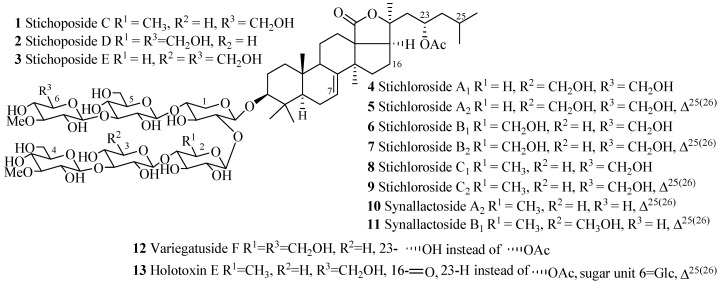
The name of the compounds in this group, their producing species, chemical structures and references are summarized in Table 1 and Figure 5. The most common features of glycosides in this category are the presence of α-acetoxy group at C-23, double bond at C-25(C-26) and terminal 3-O-methyl-d-glucose in carbohydrate chain. An interesting point to be noted in here is that the sulfate group is totally absent in this group of compounds.
Table 1.
Name and producing species of glycosides with 3β-hydroxyholost-7(8)-ene and sixs ugar units.
| Compound Name | Producing Species | Reference | Compound Name | Producing Species | Reference |
|---|---|---|---|---|---|
| Stichoposide C (1) | Thelenota anax | [17] | Stichoposide D (2) | Thelenota anax | [18] |
| Stichoposide E (3) | Stichopus chloronotus | [19] | Stichloroside A1 (4) | S. chloronotus | [20] |
| Stichloroside A2 (5) | S. chloronotus | [20] | Stichloroside B1 (6) | S. chloronotus | [20] |
| Stichloroside B2 (7) | S. chloronotus | [20] | Stichloroside C1 (8) | S. chloronotus | [20] |
| Stichloroside C2 (9) | S. chloronotus | [20] | Synallactoside A2 (10) | Synallactes nozawai | [16] |
| Synallactoside B1 (11) | S. nozawai | [16] | Variegatuside F (12) | S. variegates | [21] |
| Holotoxin E (13) | S. japonicus | [22] |
Figure 5.
Chemical structures of holostane glycosides with 3β-hydroxyholost-7(8)-ene and six sugar units.
Holostane Glycosides with 3β-Hydroxyholost-7(8)-ene Skeleton and Five Sugar Units
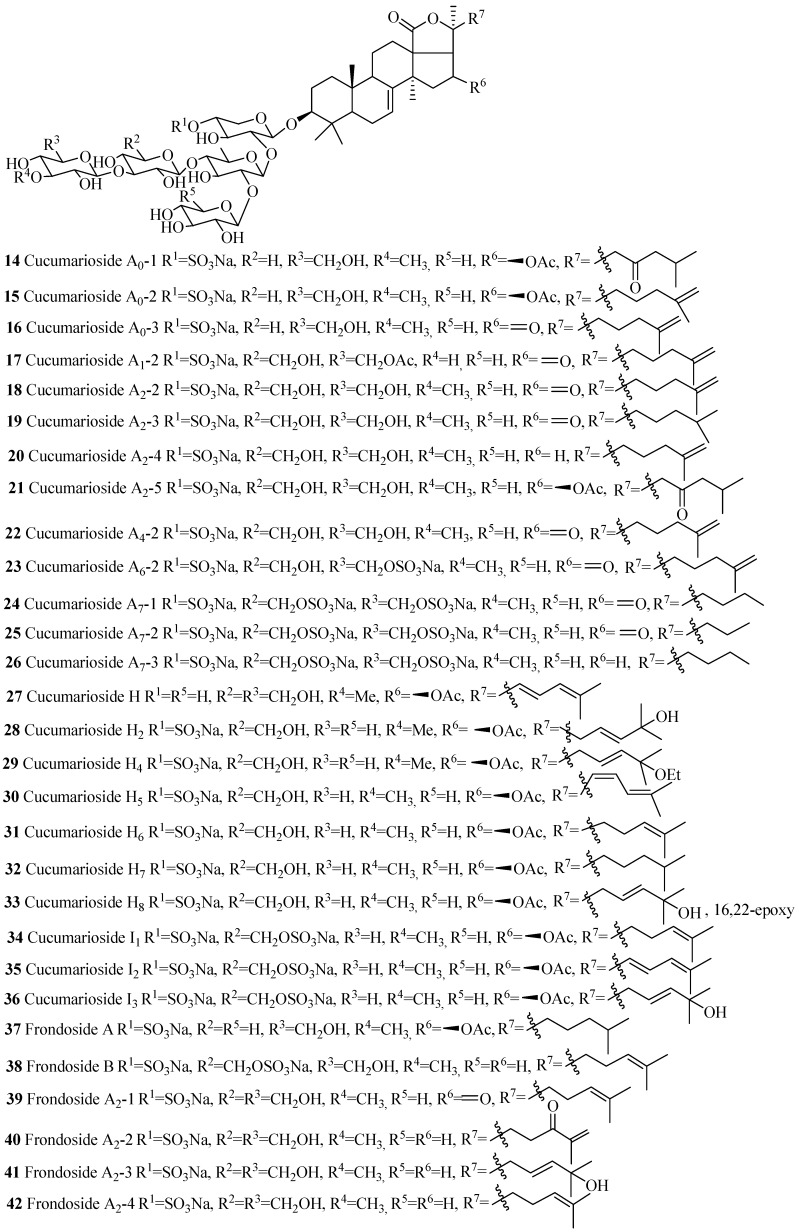
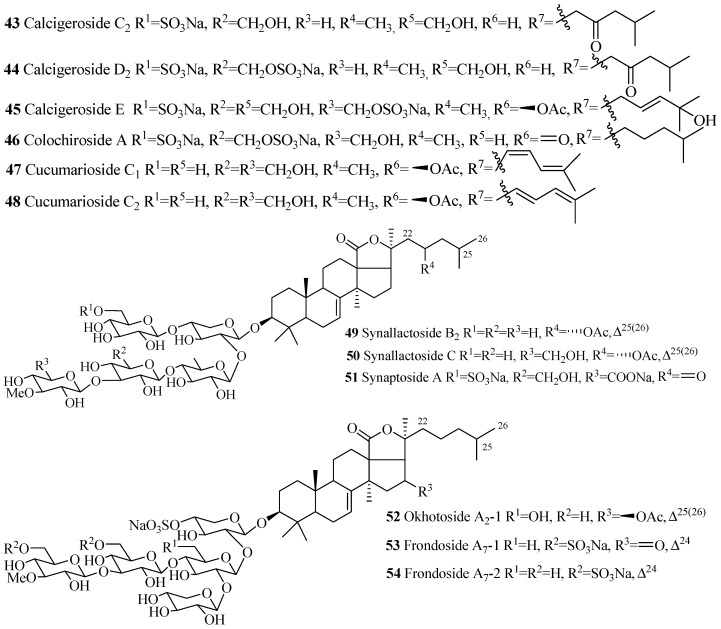
The name of the compounds in this group, their producing species, chemical structures and references are summarized in Table 2 and Figure 6. The most common structural features in this group are the sulfate groups at C-4 of xylose and C-6 of glucose and methylglucose with either β-acetoxy or keto group at C-16 and C-25(26) double bond. A quite number of compounds contain a keto group at C-23.The rare structural features of triterpene glycoside are the presence of 16,22-epoxy group (33), ethoxy group (29) and methylglucuronic acid (51). Cucumarioside A1-2 (17) is the only example of triterpene glycosides containing an acetate group at C-6 of the terminal sugar unit. Carbohydrate chain can be one branched (14–48 and 52–54) or straight (49–51). 3-O-methyl-d-xylose as a terminal monosaccharide unit that is a characteristic feature of all the glycosides isolated from Eupentacta fraudatrix.
Table 2.
Name and producing species of glycosides with 3β-hydroxyholost-7(8)-ene and five sugar units.
| Compound Name | Producing Species | Reference | Compound Name | Producing Species | Reference |
|---|---|---|---|---|---|
| Cucumarioside A0-1 (14) | Cucumaria japonica | [23] | Cucumarioside A0-2 (15) | C. japonica | [23] |
| Cucumarioside A0-3 (16) | C. japonica | [23] | Cucumarioside A1-2 (17) | C. japonica | [24] |
| Cucumarioside A2-2 (18) | C. japonica | [25] | Cucumarioside A2-3 (19) | C. japonica | [24] |
| Cucumarioside A2-4 (20) | C. japonica | [24] | Cucumarioside A2-5 (21) | C. conicospermium | [26] |
| Cucumarioside A4-2 (22) | C. japonica | [24] | Cucumarioside A6-2 (23) | C. japonica | [27] |
| Cucumarioside A7-1 (24) | C. japonica | [28] | Cucumarioside A7-2 (25) | C. japonica | [28] |
| Cucumarioside A7-3 (26) | C. japonica | [28] | Cucumarioside H (27) | E. fraudatrix | [29] |
| Cucumarioside H2 (28) | E. fraudatrix | [30] | Cucumarioside H4 (29) | E. fraudatrix | [30] |
| Cucumarioside H5 (30) | E. fraudatrix | [29] | Cucumarioside H6 (31) | E. fraudatrix | [29] |
| Cucumarioside H7 (32) | E. fraudatrix | [29] | Cucumarioside H8 (33) | E. fraudatrix | [29] |
| Cucumarioside I1 (34) | E. fraudatrix | [31] | Cucumarioside I2 (35) | E. fraudatrix | [32] |
| Cucumarioside I3 (36) | E. fraudatrix | [31] | Frondoside A (37) | C. frondosa | [33] |
| Frondoside B (38) | C. frondosa | [34] | Frondoside A2-1 (39) | C. frondosa | [35] |
| Frondoside A2-2 (40) | C. frondosa | [35] | Frondoside A2-3 (41) | C. frondosa | [35] |
| Frondoside A2-4 (42) | C. frondosa | [36] | Calcigeroside C2 (43) | P. calcigera | [37] |
| Calcigeroside D2 (44) | P. calcigera | [38] | Calcigeroside E (45) | P. calcigera | [38] |
| Colochiroside A (46) | C. anceps | [39] | Cucumarioside C1(47) | E. fraudatrix | [40] |
| Cucumarioside C2 (48) | E. fraudatrix | [40] | Synallactoside B2 (49) | S. nozawai | [16] |
| Synallactoside C (50) | S. nozawai | [16] | Synaptoside A (51) | Synapta maculata | [41] |
| Okhotoside A2-1 (52) | C. okhotensis | [42] | Frondoside A7-1 (53) | C. frondosa | [43] |
| Frondoside A7-2 (54) | C. frondosa | [43] |
Figure 6.
Chemical structures of holostane glycosides with 3β-hydroxyholost-7(8)-ene and five sugar units.
Holostane Glycosides with 3β-Hydroxyholost-7(8)-ene Skeleton and Four Sugar Units
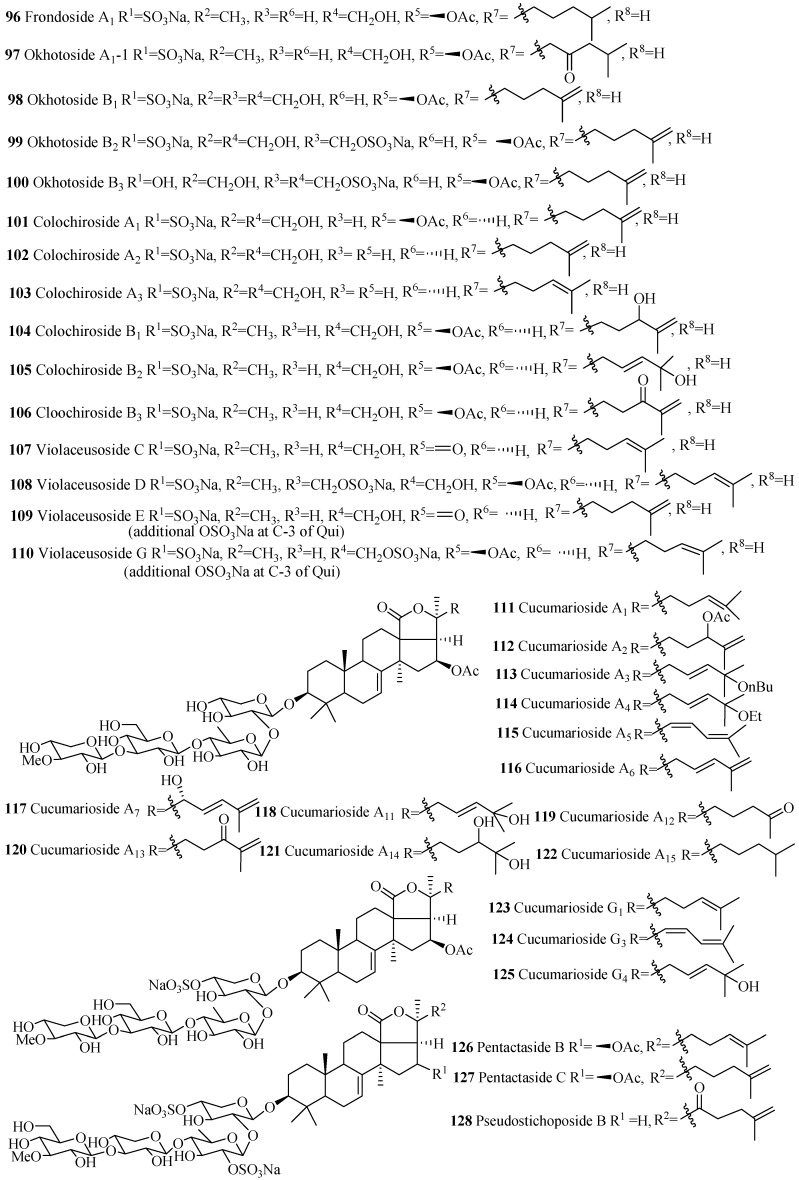
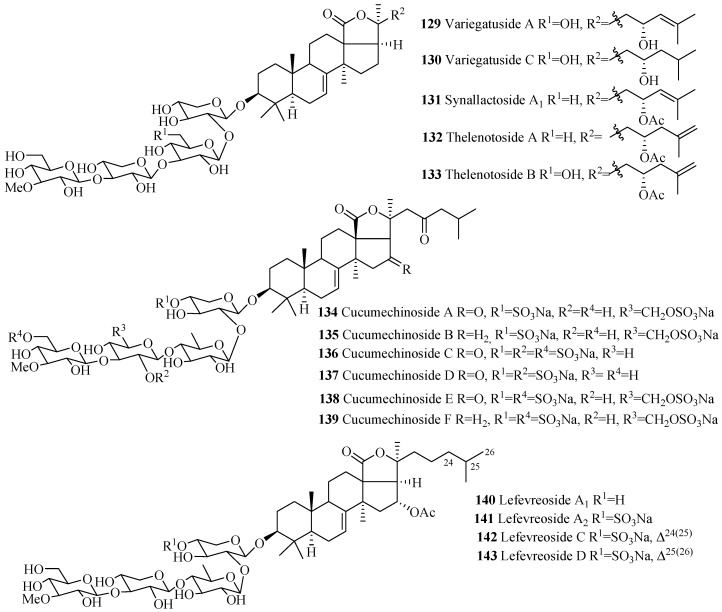
Several compounds in this group were isolated from the species of Staurocucumis liouvillei and Eupentacta fraudatrix (Table 3). The most common characteristic of glycosides in the group is the presence of sulfate at C-4 of xylose and either keto or β-acetoxy group at C-16 (Figure 7). Some of the compounds in this series, especially liouvillosides, violaceusosides and cucumechinosides, may contain up to three sulfates in their carbohydrate chain. The presence of α-hydroxy at C-12 and C-17 (78 and 79), artifact n-butoxy (113) and ethoxy (114) groups at C-25, and three consecutive xylose sugar units in carbohydrate chain (72) are rare structural features in this category. Cucumariosides A1 (111), A5 (115) and A11 (118) are the desulfated derivatives of cucumariosides G1 (123), G3 (124) and G4 (125), respectively.
Table 3.
Name and producing species of glycosides with 3β-hydroxyholost-7(8)-ene and four sugar units.
| Compound Name | Producing Species | Reference | Compound Name | Producing Species | Reference |
|---|---|---|---|---|---|
| Liouvilloside A (55) | Staurocucumis liouvillei | [44] | Liouvilloside A1 (56) | S. liouviellei | [45] |
| Liouvilloside A2 (57) | S. liouvillei | [45] | Liouvilloside A3 (58) | S. liouvillei | [45] |
| Liouvilloside A5 (59) | S. liouvillei | [46] | Liouvilloside B (60) | S. liouvillei | [44] |
| Liouvilloside B1 (61) | S. liouvillei | [45] | Liouvilloside B2 (62) | S. liouvillei | [45] |
| Violaceuside A (63) | P. violeceus | [47] | Violaceuside B (64) | P. violeceus | [47] |
| Violaceuside I (65) | P. violeceus | [48] | Violaceuside II (66) | P. violeceus | [48] |
| Violaceuside III (67) | P. violeceus | [48] | Intercedenside A (68) | M. intercedens | [49] |
| Intercedenside B (67) | Mensamria intercedens | [49] | Intercedenside C (70) | M. intercedens | [49] |
| Intercedenside D (71) | M. intercedens | [50] | Intercedenside E (72) | M. intercedens | [50] |
| Intercedenside F (73) | M. intercedens | [50] | Intercedenside G (74) | M. intercedens | [50] |
| Intercedenside H (75) | M. intercedens | [50] | Intercedenside I (76) | M. intercedens | [50] |
| Patagonicoside A (77) | Psolus patagonicus | [51] | Patagonicoside B (78) | P. patagonicus | [52] |
| Patagonicoside C (79) | P. patagonicus | [52] | Philinopside A (80) | P. quadrangularis | [53] |
| Philinopside B (81) | Pentacta quadrangularis | [53] | Philinopside E (82) | P. quadrangularis | [54] |
| Philinopside F (83) | P. quadrangularis | [54] | Mollisoside A (84) | A. mollis | [55] |
| Mollisoside B2 (85) | Australostichopus mollis | [55] | Eximisoside A (86) | P. eximius | [56] |
| Pseudostichoposide A (87) | Pseudostichopus trachus | [57] | Cucumarioside F1 (88) | E. fraudatrix | [58] |
| Cucumarioside F2 (89) | E. fraudatrix | [58] | Pseudocnoside A (90) | P. leoninus | [59] |
| Typicoside A1 (91) | Actinocucumis typica | [60] | Typicoside A2 (92) | A. typica | [60] |
| Typicoside B1 (93) | A. typica | [60] | Typicoside C1 (94) | A. typica | [60] |
| Typicoside C2 (95) | A. typica | [60] | Frondoside A1 (96) | C. okhotensis | [61] |
| Okhotoside A1-1 (97) | Cucumaria okhotensis | [61] | Okhotoside B1 (98) | C. okhotensis | [62] |
| Okhotoside B2 (99) | C. okhotensis | [62] | Okhotoside B3 (100) | C. okhotensis | [62] |
| Colochiroside A1 (101) | Colochirus robustus | [63] | Colochiroside A2 (102) | C. robustus | [63] |
| Colochiroside A3 (103) | C. robustus | [63] | Colochiroside B1 (104) | C. robustus | [64] |
| Colochiroside B2 (105) | C. robustus | [64] | Colochiroside B3 (106) | C. robustus | [64] |
| Violaceusosides C (107) | P. violaceus | [65] | Violaceusosides D (108) | P. violaceus | [65] |
| Violaceusosides E (109) | P. violaceus | [65] | Violaceusosides G (110) | P. violaceus | [65] |
| Cucumarioside A1 (111) | E. fraudatrix | [66] | Cucumarioside A2 (112) | E. fraudatrix | [67] |
| Cucumarioside A3 (113) | E. fraudatrix | [66] | Cucumarioside A4 (114) | E. fraudatrix | [66] |
| Cucumarioside A5 (115) | E. fraudatrix | [66] | Cucumarioside A6 (116) | E. fraudatrix | [66] |
| Cucumarioside A7 (117) | E. fraudatrix | [67] | Cucumarioside A11 (118) | E. fraudatrix | [67] |
| Cucumarioside A12 (119) | E. fraudatrix | [66] | Cucumarioside A13 (120) | E. fraudatrix | [67] |
| Cucumarioside A14 (121) | E. fraudatrix | [67] | Cucumarioside A15 (122) | E. fraudatrix | [66] |
| Cucumarioside G1 (123) | C. fraudatrix | [68] | Cucumarioside G3 (124) | E. fraudatrix | [69] |
| Cucumarioside G4 (125) | E. fraudatrix | [70] | Pentactaside B (126) | P. quadrangularis | [71] |
| Pentactaside C (127) | P. quadrangularis | [71] | Pseudostichoposide B (128) | P. trachus | [72] |
| Variegatuside A (129) | S. variegates | [73] | Variegatuside C (130) | S. variegates | [21] |
| Synallactoside A1(131) | S. nozawai | [16] | Thelenotoside A (132) | Thelenota ananas | [74] |
| Thelenotoside B (133) | T. ananas | [74] | Cucumechinoside A (134) | C. echinata | [75] |
| Cucumechinoside B (135) | C. echinata | [75] | Cucumechinoside C (136) | C. echinata | [75] |
| Cucumechinoside D (137) | C. echinata | [75] | Cucumechinoside E (138) | C. echinata | [75] |
| Cucumechinoside F (139) | C. echinata | [75] | Lefevreoside A1 (140) | C. lefevrei | [76] |
| Lefevreoside A2 (141) | C. lefevrei | [76] | Lefevreoside C (142) | C. lefevrei | [76] |
| Lefevreoside D (143) | C. lefevrei | [76] |
Figure 7.
Chemical structures of holostane glycosides with 3β-hydroxyholost-7(8)-ene and four sugar units.
Holostane Glycosides with 3β-Hydroxyholost-7(8)-ene Skeleton and 1–3 Sugar Units
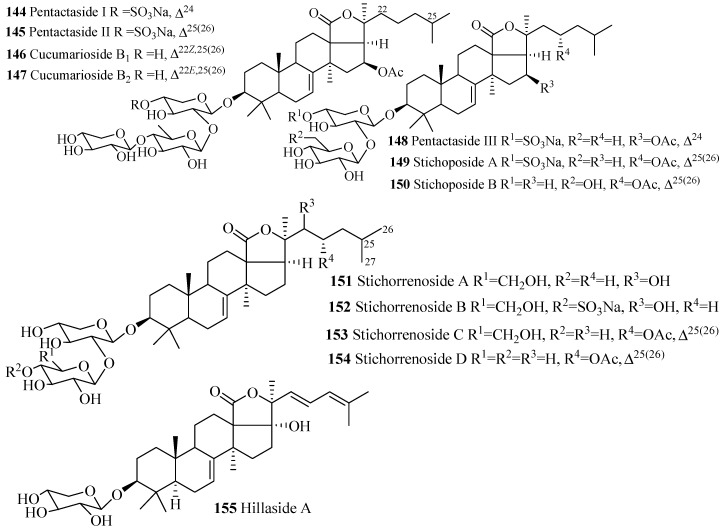
The name of the compounds in this group, their producing species, chemical structures and references are summarized in Table 4 and Figure 8. The most common feature of triterpene glycosides is the presence of double bond at C-25(26). Cucumarioside B1 (146) is the geometric isomer of cucumarioside B2 (142) and pentactaside III (148) is the positional isomer of stichoposide A (153).
Table 4.
Name and producing species of glycosides with 3β-hydroxyholost-7(8)-ene and 1–3 sugar units.
| Compound Name | Producing Species | Reference | Compound Name | Producing Species | Reference |
|---|---|---|---|---|---|
| Pentactaside I (144) | Pentacta quadrangularis | [77] | Pentactaside II (145) | P. quadrangularis | [77] |
| Cucumarioside B1 (146) | E. fraudatrix | [78] | Cucumarioside B2 (147) | E. fraudatrix | [78] |
| Pentactaside III (148) | P. quadrangularis | [77] | Stichoposide A (149) | S. cloronotus | [79] |
| Stichoposide B (150) | Stichopus cloronotus | [79] | Stichorrenoside A (151) | Stichopus horrens | [80] |
| Stichorrenoside B (152) | S. horrens | [80] | Stichorrenoside C (153) | S. horrens | [80] |
| Stichorrenoside D (154) | S. horrens | [80] | Hillaside A (155) | H. hilla | [81] |
Figure 8.
Chemical structures of holostane glycosides with 3β-hydroxyholost-7(8)-ene and 1–3 sugar units.
4.1.2. 3β-Hydroxyholost-9(11)-ene Skeleton Containing Holostane Glycosides
The species Holothuria lessoni, Bohadschia marmorata and Bohadschia bivittata produce most of the compounds in this group. For convenience, the large number of compounds in this category can also be further subdivided into four groups depending on the number of sugar units
Holostane Glycosides with 3β-Hydroxyholost-9(11)-ene Skeleton and Six Sugar Units
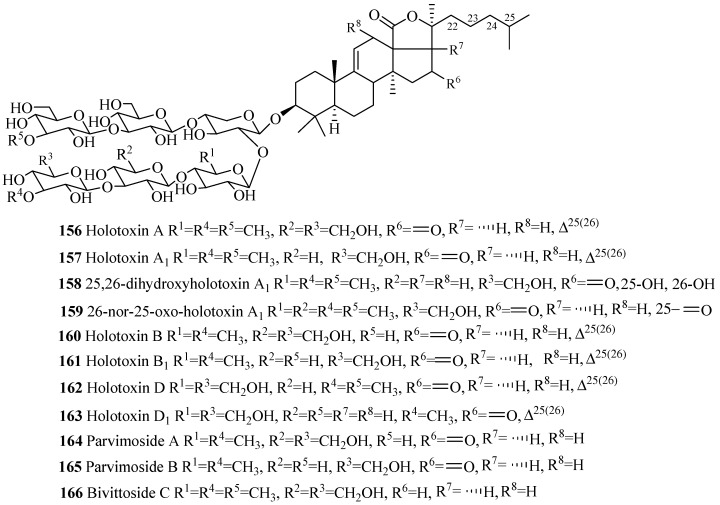
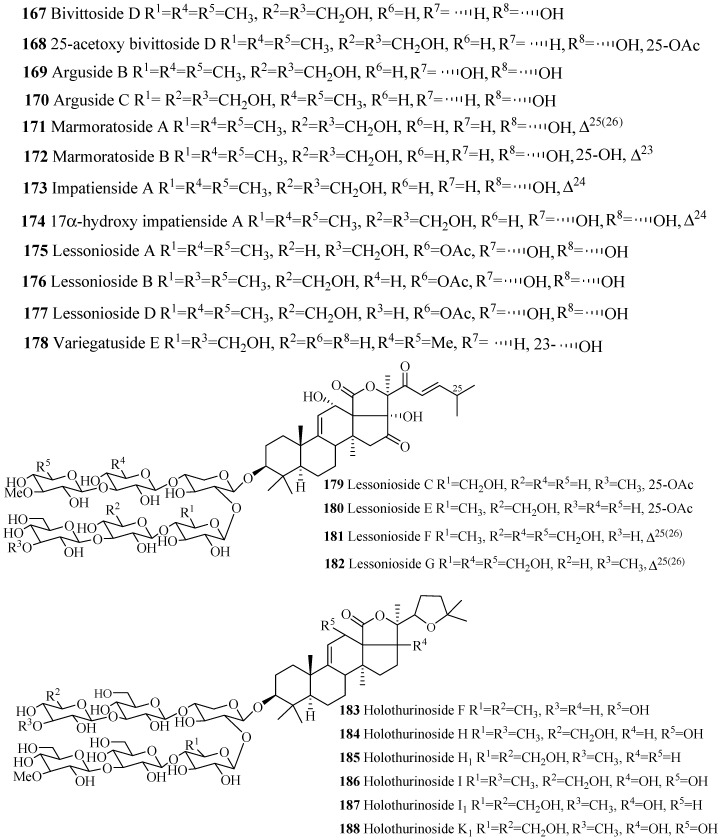
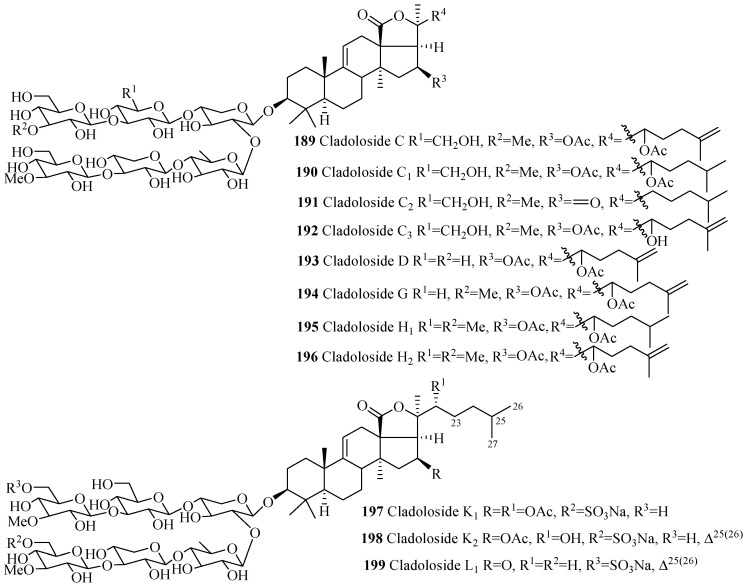
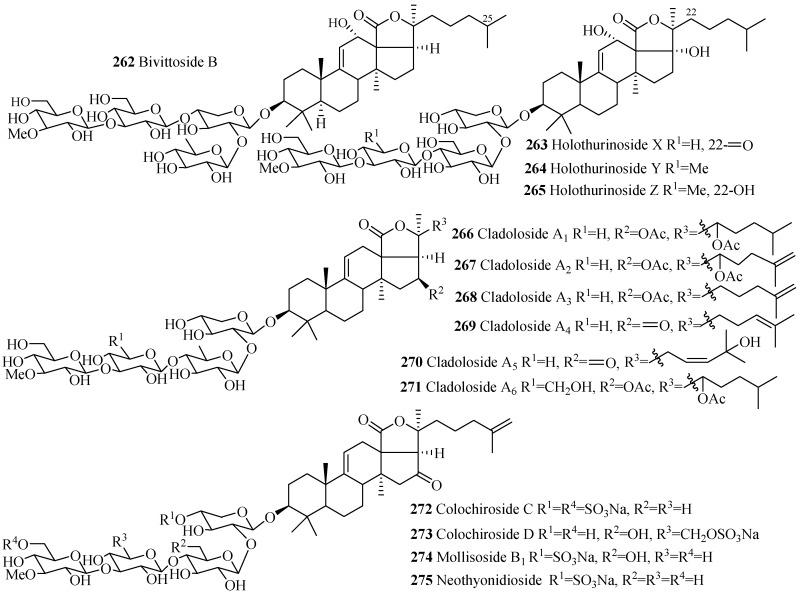
Similar to 3β-hydroxyholost-7(8)-ene skeleton with six sugar units (Figure 5), 3β-hydroxyholost-9(11)-ene skeleton with six sugar units glycosides also do not have any sulfate group in their carbohydrate chain (Figure 9 and Table 5) except cladolosides K1, K2 and L1 (197–199). The most common structural feature of triterpene glycosides in this category is the presence of 3-O-methyl-d-glucose at the both end of the straight carbohydrate chain. Holotoxin and parvimoside series (156–166) of compounds have a keto group at position C-16. Double bond at C-25(26) among holotoxins (156–163) is common, except 26-nor-25-oxo-holotoxin A1 (159), where the double bond is replaced by a keto group. The α-hydroxy groups at C-12 and C-17 are commonly found in the aglycone part of lessonioside series of glycosides (175–177). The α-hydroxy group at C-12 and C-17, and 22,25-epoxy are common structural characteristics of holothurinosides (183–188). Acetoxy group at C-16 and C-22 are frequently observed in cladoloside glycosides (189–199).
Figure 9.
Chemical structures of holostane glycosides with 3β-hydroxyholost-9(11)-ene and six sugar units.
Table 5.
Name and producing species of glycosides with 3β-hydroxyholost-9(11)-ene and six sugar units.
| Compound Name | Producing Species | Reference | Compound Name | Pro. Species | Reference |
|---|---|---|---|---|---|
| Holotoxin A (156) | Stichopus japonicus | [82] | Holotoxin A1 (157) | S. japonicus | [22] |
| 25,26-dihydroxyholotoxin A1 (158) | Apostichopus japonicus | [83] | Oxo-holotoxin A1 (159) | A. japonicus | [22] |
| Holotoxin B (160) | S. japonicus | [22] | Holotoxin B1 (161) | S. japonicus | [22] |
| Holotoxin D (162) | S. japonicus | [22] | Holotoxin D1 (163) | A. japonicus | [83] |
| Parvimoside A (164) | Stichopus parvimensis | [84] | Parvimoside B (165) | S. parvimensis | [84] |
| Bivittoside C (166) | Bohadschia bivittata | [85] | Bivittoside D (167) | B. bivittata | [85] |
| 25-acetoxybivittoside D (168) | Bohadschia marmorata | [86] | Arguside B (169) | B. argus | [87] |
| Arguside C (170) | B. argus | [87] | Marmoratoside A (171) | B. marmorata | [86] |
| Marmoratoside B (172) | B. marmorata | [86] | Impatienside A (173) | H. impatiens | [86] |
| 17α-hydroxyimpatienside A (174) | B. marmorata | [86] | Lessonioside A (175) | H. lessoni | [88] |
| Lessonioside B (176) | Holothuria lessoni | [88] | Lessonioside D (177) | H. lessoni | [88] |
| Variegatuside E (178) | S. variegates | [21] | Lessonioside C (179) | H. lessoni | [88] |
| Lessonioside E (180) | Holothuria lessoni | [88] | Lessonioside F (181) | H. lessoni | [88] |
| Lessonioside G (182) | H. lessoni | [88] | Holothurinoside F (183) | B. subrubra | [89] |
| Holothurinoside H (184) | B. subrubra | [89] | Holothurinoside H1 (185) | B. subrubra | [89] |
| Holothurinoside I (186) | B. subrubra | [89] | Holothurinoside I1 (187) | B. subrubra | [89] |
| Holothurinoside K1 (188) | B. subrubra | [89] | Cladoloside C (189) | C. schmeltzii | [90] |
| Cladoloside C1 (190) | Cladolabes schmeltzii | [90] | Cladoloside C2 (191) | C. schmeltzii | [90] |
| Cladoloside C3 (192) | C. schmeltzii | [91] | Cladoloside D (193) | C. schmeltzii | [90] |
| Cladoloside G (194) | C. schmeltzii | [91] | Cladoloside H1 (195) | C. schmeltzii | [91] |
| Cladoloside H2 (196) | C. schmeltzii | [91] | Cladoloside K1 (197) | C. schmeltzii | [92] |
| Cladoloside K2 (198) | C. schmeltzii | [92] | Cladoloside L1 (199) | C. schmeltzii | [92] |
Holostane Glycosides with 3β-Hydroxyholost-9(11)-ene Skeleton and Five Sugar Units
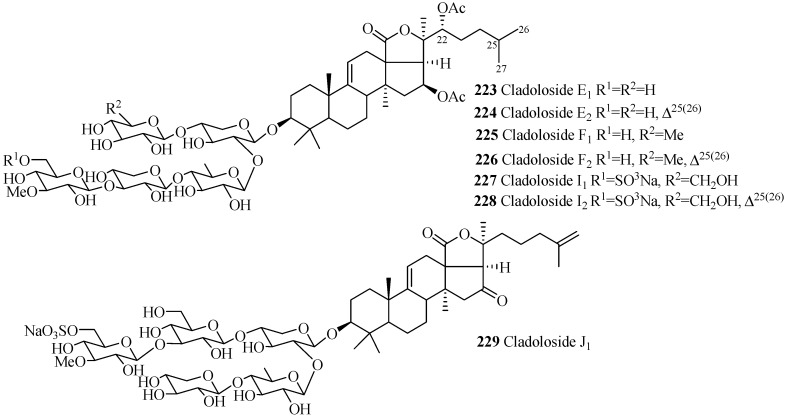
The carbohydrate chains of glycosides in this group are either straight (200–218 and 223–229) or branched (219–222) (Figure 10 and Table 6). The 22,25-epoxy (200–202, 213–215) and two acetoxy groups, one at C-16 and another at C-22 (211, 212, 223–228), are common in holothurinosides and cladolosides, respectively. Kolgaosides (204 and 205) and achlioniceosides (216–218) within their own groups have the same carbohydrate chains and the only difference is in their respective aglycone side chains.
Figure 10.
Chemical structures of holostane glycosides with 3β-hydroxyholost-9(11)-ene and five sugar units.
Table 6.
Name and producing species of glycosides with 3β-hydroxyholost-9(11)-ene and five sugar units.
| Compound Name | Producing Species | Reference | Compound Name | Producing Species | Reference |
|---|---|---|---|---|---|
| Holothurinoside A (200) | Holothuria forskalii | [93] | 17-dehydroxyholothurinoside A (201) | Holothuria grisea | [94] |
| Holothurinoside A1 (202) | H.lessoni | [95] | Holothurinoside B (203) | H. forskalii | [93] |
| Kolgaoside A (204) | Kolga hyalina | [96] | Kolgaoside B (205) | K. hyalina | [96] |
| Griseaside A (206) | H. grisea | [94] | Impatienside B (207) | H. axiloga | [97] |
| Arguside F (208) | Holothuria axiloga | [97] | Pervicoside D (209) | H. axiloga | [97] |
| Cladoloside B (210) | A. japonicus | [22] | Cladoloside B1 (211) | C. schmeltzii | [90] |
| Cladoloside B2 (212) | C. schmeltzii | [90] | Holothurinoside E (213) | H. lessoni | [95] |
| Holothurinoside E1 (214) | H. lessoni | [95] | Holothurinoside M (215) | H. lessoni | [95] |
| Achlioniceoside A1 (216) | A. violaecuspidata | [98] | Achlioniceoside A2 (217) | A. violaecuspidata | [98] |
| Achlioniceoside A3 (218) | A. violaecuspidata | [98] | Ds-penaustroside C (219) | P. australis | [99] |
| Ds-penaustroside D (220) | Pentacta australis | [99] | Frondoside A2-6 (221) | C. frondosa | [35] |
| Cladoloside E1 (222) | C. schmeltzii | [91] | Cladoloside E2 (223) | C. schmeltzii | [91] |
| Cladoloside F1 (224) | C. schmeltzii | [91] | Cladoloside F2 (225) | C. schmeltzii | [91] |
| Cercodemasoide A (226) | C. anceps | [100] | Cladoloside I1 (227) | C. schmeltzii | [92] |
| Cladoloside I2 (228) | C. schmeltzii | [92] | Cladoloside J1 (229) | C. schmeltzii | [92] |
Holostane Glycosides with 3β-Hydroxyholost-9(11)-ene Skeleton and Four Sugar Units
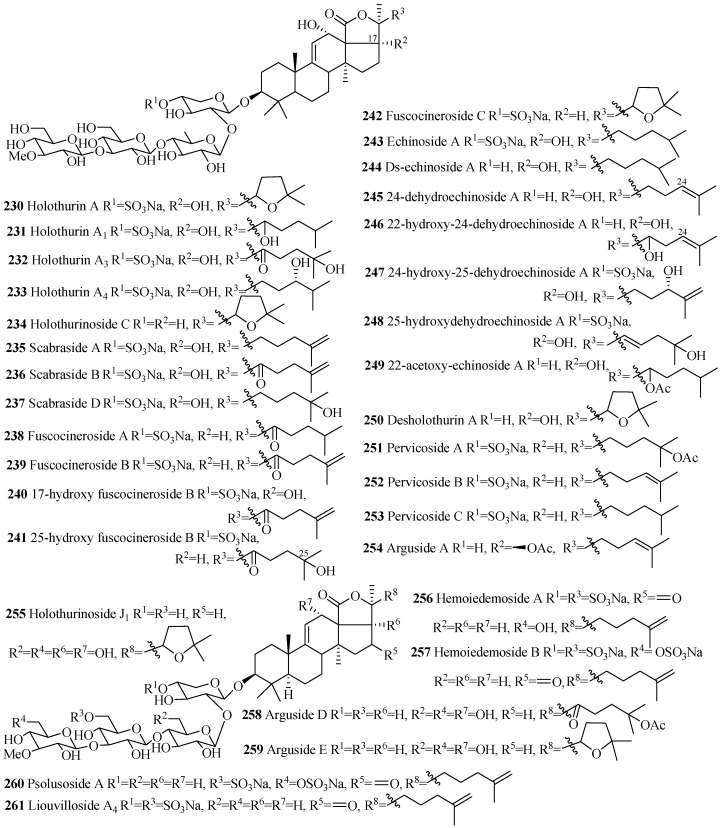
The names and structures of the glycosides belonging to this group are summarized in the Table 7 and Figure 11. Almost all the saponins in this group contain sulfate group at C-4 of xylose sugar. The most common features of holothurins (230–233), scabrasides (235–237) and echinosides (243–249) are the presence of hydroxy groups at C-12 and C-17 (Figure 11). Among the cladoloside series of compounds (266–271), either keto or acetoxy group is commonly found at position C-16 and 22. The uncommon linear sugar chain [3-O-MeGlc (1→3)-Glc (1→4)-Xyl (2→1)-Qui] is observed in bivittoside B (262). Another exceptional feature has been found in this category of compounds is the presence of three consecutive glucose unit in the linear carbohydrate chain (258 and 259).
Table 7.
Name and producing species of glycosides with 3β-hydroxyholost-9(11)-ene and four sugar units.
| Compound Name | Producing Species | Reference | Compound Name | Producing Species | Reference |
|---|---|---|---|---|---|
| Holothurin A (230) | Actinopyga agassizi | [101] | Holothurin A1 (231) | H. grisea | [102] |
| Holothurin A3 (232) | Holothuria scabra | [103] | Holothurin A4 (233) | H. scabra | [103] |
| Holothurinoside C (234) | H. forskalii | [93] | Scabraside A (235) | H. scabra | [104] |
| Scabraside B (236) | [104] | 104] | Scabraside D (237) | H. scabra | [105] |
| Fuscocineroside A (238) | H. fuscocinerea | [106] | Fuscocineroside B (239) | H. fuscocinerea | [106] |
| 17-hydroxy fuscocineroside B (240) | B. marmorata | [107] | 25-hydroxy-fuscocineroside B (241) | B. marmorata | [107] |
| Fuscocineroside C (242) | H. fuscocinerea | [106] | Echinoside A (243) | A. echinites | [108] |
| Ds-echinoside A (244) | P. graeffei | [109] | 24-dehydroechinoside A (245) | H. scabra | [105] |
| 22-hydroxy-24-dehydroechinoside A (246) | Actinopyga flammea | [110] | 24-hydroxy-25-dehydroechinoside A (247) | A. flammea | [110] |
| 25-hydroxydehydroechinoside A (248) | A. flammea | [110] | 22-acetoxyechinoside A (249) | A. flammea | [110] |
| Desholothurin A (250) | P. graeffei | [93] | Pervicoside A (251) | H. pervicax | [111] |
| Pervicoside B (252) | H. pervicax | [111] | Pervicoside C (253) | H. pervicax | [111] |
| Arguside A (254) | Bohadschia argus | [112] | Holothurinoside J1 (255) | P. graeffei | [95] |
| Hemoiedemoside A (256) | H. spectabilis | [113] | Hemoiedemoside B (257) | H. spectabilis | [113] |
| Arguside D (258) | B. argus | [114] | Arguside E (259) | B. argus | [114] |
| Psolusoside A (260) | Psolus fabricii | [115] | Liouvilloside A4 (261) | S. liouvillei | [46] |
| Bivittoside B (262) | Bohadschia bivitta | [85] | Holothurinoside X (263) | H. lessoni | [116] |
| Holothurinoside Y (264) | H.lessoni | [116] | Holothurinoside Z (265) | H. lessoni | [116] |
| Cladoloside A1 (266) | Cladolabes chmeltzii | [117] | Cladoloside A2 (267) | C. chmeltzii | [117] |
| Cladoloside A3 (268) | C. chmeltzii | [117] | Cladoloside A4 (269) | C. chmeltzii | [117] |
| Cladoloside A5 (270) | C. chmeltzii | [117] | Cladoloside A6 (271) | C. chmeltzii | [117] |
| Colochiroside C (272) | C. chmeltzii | [64] | Colochiroside D (273) | C. robustus | [63] |
| Mollisoside B1 (274) | A. mollis | [55] | Neothyonidioside (275) | A. mollis | [118] |
Figure 11.
Chemical structures of holostane glycosides with 3β-hydroxyholost-9(11)-ene and fours ugar units.
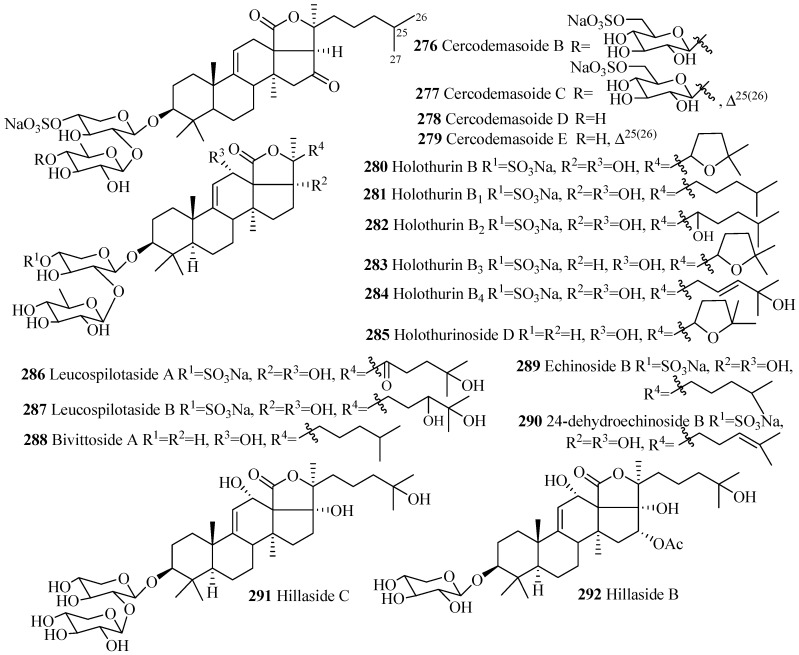
Holostane Glycosides with 3β-Hydroxyholost-9(11)-ene Skeleton and 1–3 Sugar Units
Only one type of carbohydrate chain, d-xylose-d-quinovose, is found in all glycosides in this group having two monosaccharide units (278–290), except 291 where carbohydrate chain is d-xylose-d-xylose (Figure 12 and Table 8); sulfate groups at C-4 of xylose units are also commonly found as well, except 285, 288 and 291. Hydroxy groups at either C-12 or C-17, or both positions, are observed in all the compounds in this category (Figure 12), except cercodemasoides (276–279).
Figure 12.
Chemical structure of holostane glycosides with 3β-hydroxyholost-9(11)-ene and 1–3 sugar units.
Table 8.
Name and producing species of glycosides with 3β-hydroxyholost-9(11)-ene and 1–3sugar units.
| Compound Name | Producing Species | Reference | Compound Name | Producing Species | Reference |
|---|---|---|---|---|---|
| Cercodemasoide B (276) | Cercodemas anceps | [100] | Cercodemasoide C (277) | C. anceps | [100] |
| Cercodemasoide D (278) | C. anceps | [100] | Cercodemasoide E (279) | C. anceps | [100] |
| Holothurin B (280) | Holothuria lessoni | [119] | Holothurin B1 (281) | H. lessoni | [120] |
| Holothurin B2 (282) | H. polii | [121] | Holothurin B3 (283) | H. polii | [121] |
| Holothurin B4 (284) | H. polii | [121] | Holothurinoside D (285) | H. forskalii | [93] |
| Leucospilotaside A (286) | H. leucospilota | [122] | Leucospilotaside B (287) | H. leucospilota | [122] |
| Bivittoside A (288) | Bohadschia bivittata | [85] | Echinoside B (289) | A. echinites | [108] |
| 24-dehydroechinoside B (290) | Actinopyga mauritiana | [123] | Hillaside C (291) | Holothuria hilla | [124] |
| Hillaside B (292) | H. hilla | [81] |
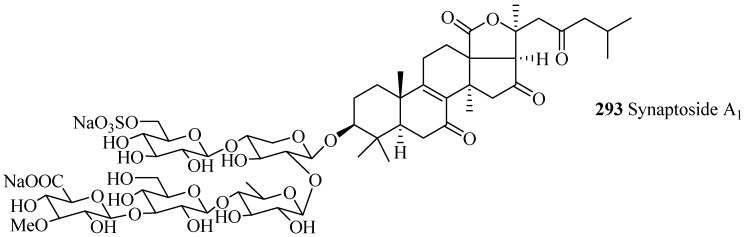
4.1.3. Holostane Glycosides with 3β-Hydroxyholost-8(9)-ene Skeleton
Only three glycosides belong to this group with carbohydrate chain consisting of 4–5 monosaccharide units (Table 9 and Figure 13). Among holostane sea cucumber glycosides, only one glycoside, synaptoside A1 (293), contains keto group at C-7.
Table 9.
Name and producing species of holostane glycosides with 3β-hydroxyholost-8(9)-ene skeleton.
Figure 13.
Chemical structures of holostane glycosides with 3β-hydroxyholost-8(9)-ene skeleton.
4.2. Nonholostane Glycosides
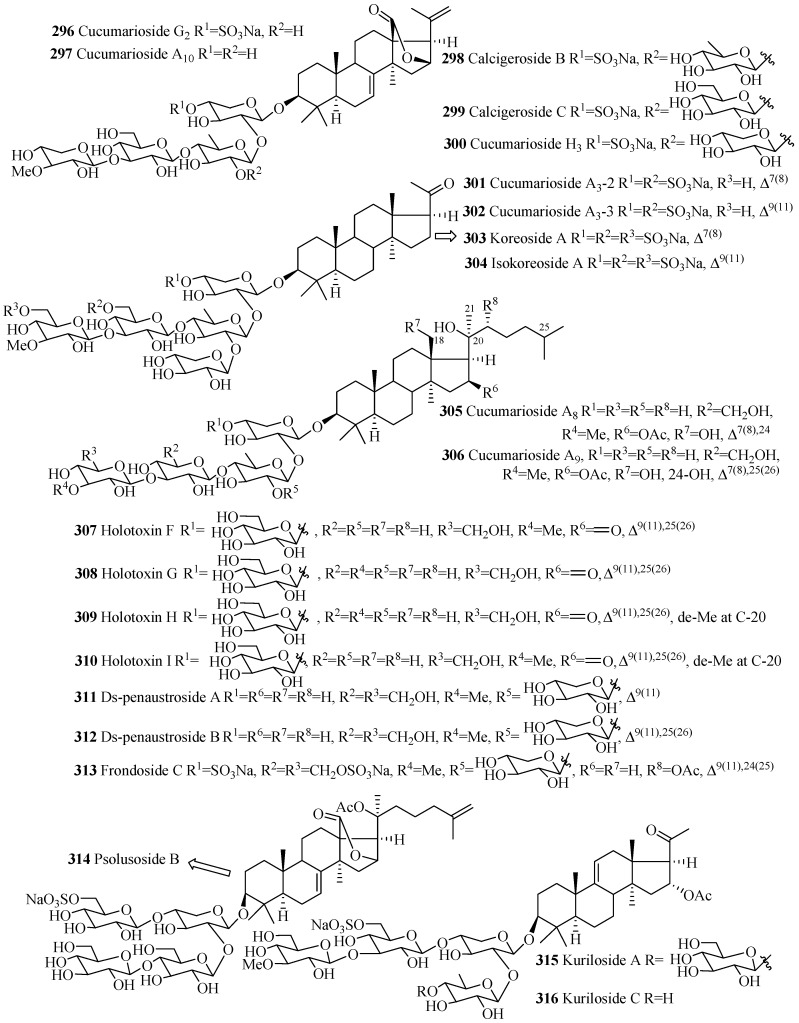
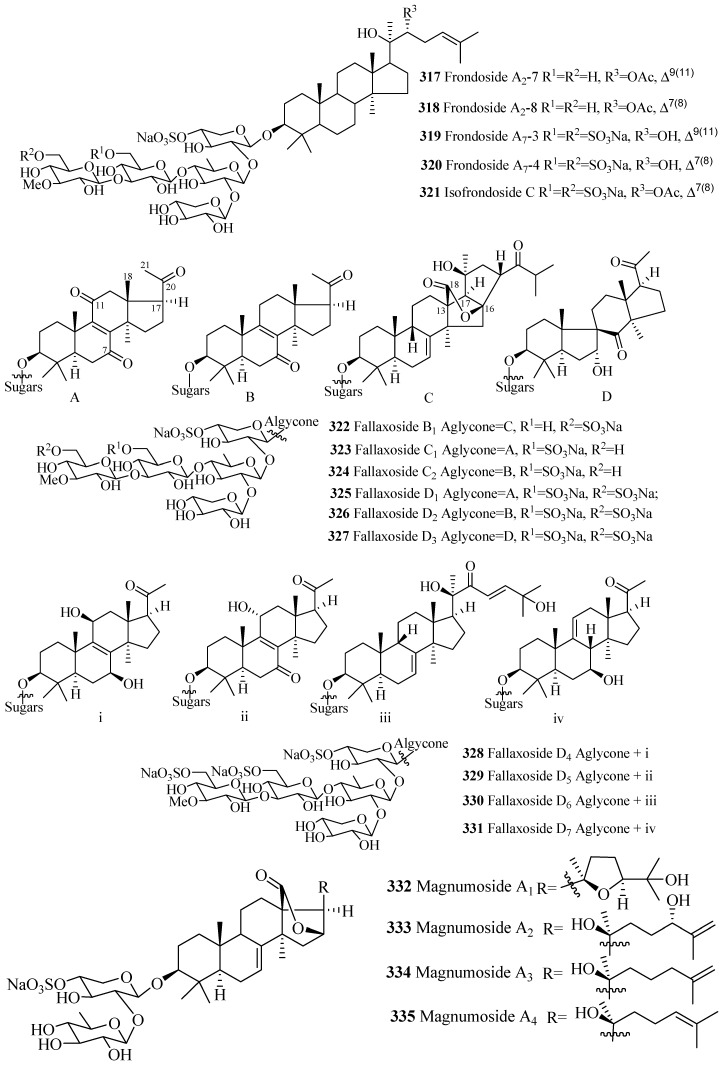
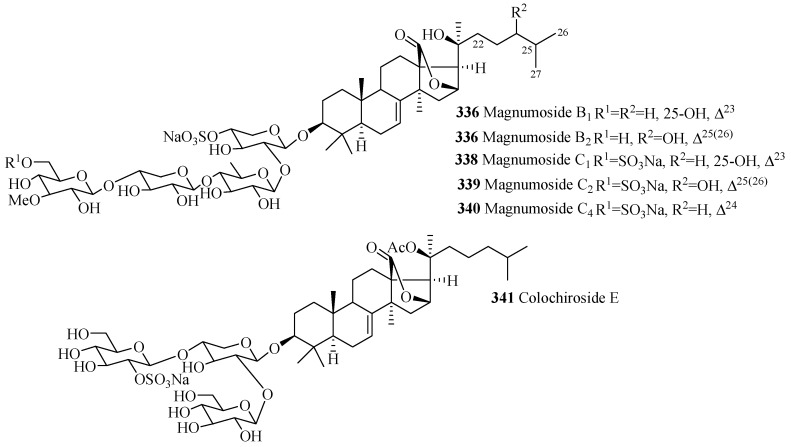
As mention earlier, like holostane glycosides, nonholostane glycosides do not have γ(18,20)-lactone structural unit (Figure 14 and Figure 15, Table 10). There are six different structural units (Figure 14) present in D- and E rings of aglycone in nonholostane glycosides. The aglycone side chain can be long or short, and may contain keto, methylene, hydroxy and acetoxy functional groups (Figure 15). Instead of γ(18,20)-lactone, some glycosides in this group contain γ(16,18)-lactone (296–300, 314, 322, 332–340 and 341). Cucumariosides A8 (305) and A9 (306) contain uncommon hydroxy group at C-18. Fallaxosides B1 (322) and D3 (327) are novel glycosides with unprecedented skeletons of aglycones. Psolusoside B (314) and Kuriloside C (316) have four members sugar architecture which are uncommon in both holostane and nonholostane glycosides. Another uncommon feature of this group of compounds is the presence of keto group at C-11 (323 and 325). Sulfate group is commonly found at C-4 of first xylose unit (Figure 15). Most of the nonholostane glycosides have branched five members carbohydrate chain (Figure 15).
Figure 14.
D- and E-ring structural architectures present in nonholotane glycosides.
Figure 15.
Chemical structures of nonholostane glycosides.
Table 10.
Name and producing species of nonholostane glycosides.
| Compound Name | Producing Species | Reference | Compound Name | Producing Species | Reference |
|---|---|---|---|---|---|
| Cucumarioside G2 (296) | E. fraudatrix | [125] | Cucumarioside A10 (297) | E. fraudatrix | [67] |
| Calcigeroside B (298) | P. calcigera | [37] | Calcigeroside C1 (299) | P. calcigera | [37] |
| Cucumarioside H3 (300) | E. fraudatrix | [30] | Cucumarioside A3-2 (301) | C. conicospermium | [26] |
| Cucumarioside A3-3 (302) | C. conicospermium | [26] | Koreoside A (303) | C. koraiensis | [126] |
| Isokoreoside A (304) | C. conicospermium | [26] | Cucumarioside A8 (305) | E. fraudatrix | [67] |
| Cucumarioside A9 (306) | E. fraudatrix | [67] | Holotoxin F (307) | A. japonicus | [22] |
| Holotoxin G (308) | A. japonicus | [22] | Holotoxin H (309) | S. japonicus | [127] |
| Holotoxin I (310) | S. japonicus | [127] | Ds-penaustroside A (311) | P. australis | [99] |
| Ds-penaustroside B (312) | P. australis | [99] | Frondoside C (313) | C. frondosa | [128] |
| Psolusoside B (314) | Psolus fabricii | [129] | Kuriloside A (315) | D. kurilensi | [130] |
| Kuriloside C (316) | D. kurilensi | [130] | Frondoside A2-7 (317) | C. frondosa | [36] |
| Frondoside A2-8 (318) | C. frondosa | [36] | Frondoside A7-3 (319) | C. frondosa | [43] |
| Frondoside A7-4 (320) | C. frondosa | [43] | Isofrondoside C (321) | C. frondosa | [43] |
| Fallaxoside B1 (322) | C. fallax | [131] | Fallaxoside C1 (323) | C. fallax | [132] |
| Fallaxoside C2 (324) | C. fallax | [132] | Fallaxoside D1 (325) | C. fallax | [132] |
| Fallaxoside D2 (326) | C. fallax | [132] | Fallaxoside D3 (327) | C. fallax | [131] |
| Fallaxoside D4 (328) | C. fallax | [133] | Fallaxoside D5 (329) | C. fallax | [133] |
| Fallaxoside D6 (330) | C. fallax | [133] | Fallaxoside D7 (331) | C. fallax | [133] |
| Magnumoside A1 (332) | Massinium magnum | [134] | Magnumoside A2 (333) | M. magnum | [134] |
| Magnumoside A3 (334) | M. magnum | [134] | Magnumoside A4 (335) | M. magnum | [134] |
| Magnumoside B1 (336) | M. magnum | [134] | Magnumoside B2 (337) | M. magnum | [134] |
| Magnumoside C1 (338) | M. magnum | [134] | Magnumoside C2 (339) | M. magnum | [134] |
| Magnumoside C4 (340) | M. magnum | [134] | Colochiroside E (341) | C. robustus | [135] |
5. The Important Biological Properties of Sea Cucumber Glycosides
Triterpene glycosides are the prime bioactive metabolites of sea cucumbers, and are commonly known as toxins of sea cucumbers to eukaryotic cells. These glycosides showed a wide range of biological activities including cytotoxic, antifungal, antiviral, hemolytic, antiprotozoal and immunomodulatory activities. Sea cucumbers produce some major glycosides in sufficient amount to carry out a wide range of biological activity tests [37,94]. Besides major glycosides, they also produce minor glycosides insufficient to test a range of biological activities [66,67]. The point to be noted here is that sea cucumber glycosides are able to exhibit biological activities in both in vitro and in vivo models [5]. The remarkable biological properties showed by some triterpene glycosides are summarized in Table 11. Triterpene glycosides do not exhibit antibacterial activity, indicating that these glycosides are probably produced by sea cucumbers for defence against eukaryotic predators.
Table 11.
Remarkable biological activities exhibited by some sea cucumber glycosides.
| Compound | Activity | Against/For | Activity Result | Reference |
|---|---|---|---|---|
| Hillaside C (285) | Cytotoxic | Human tumor cell lines | IC50: 0.15–3.20 µg/mL | [124] |
| Hemoiedemoside A (251) | Antifungal | C. cucumerinum | 20 µg/disc: 23 mm zone | [113] |
| Fuscocineroside C (237) | Cytotoxic | Human tumor cell lines | IC50: 0.88 and 0.58 µg/mL | [106] |
| Intercedenside A (66) | Cytotoxic | Human tumor cell lines | ED50: 0.96–4.0 µg/mL | [49] |
| Intercedenside B (67) | Cytotoxic | Human tumor cell lines | ED50: 0.61–2.0 µg/mL | [49] |
| Intercedenside C (68) | Cytotoxic | Human tumor cell lines | ED50: 0.96–4.0 µg/mL | [49] |
| Holothurinoside A (195) | Cytotoxic | Human tumor cell lines | IC50: 0.33–0.71 µg/mL | [93] |
| Holothurinoside C (229) | Cytotoxic | Human tumor cell lines | IC50: 0.16–0.93 µg/mL | [93] |
| Liouvilloside A (53) | Virucidal | Herpes simplex virus | <10 µg/mL | [44] |
| Leucospilotaside B (281) | Cytotoxic | Human tumor cell lines | IC50: 0.44–2.62 µg/mL | [122] |
| Arguside B (164) | Cytotoxic | Human tumor cell lines | IC50: 0.38–2.60 µg/mL | [87] |
| Arguside C (165) | Cytotoxic | Human tumor cell lines | IC50: 0.38–2.60 µg/mL | [87] |
| Philinopside A (78) | Cytotoxic | Human tumor cell lines | IC50: 1.70–3.50 µg/mL | [52] |
| Philinopside B (79) | Cytotoxic | Human tumor cell lines | IC50: 0.75–3.0 µg/mL | [53] |
| Cucumarioside A2-2 (18) | Hemolytic | Erythrocyte of mouse | ED50: 0.87 at 10−6 M | [136] |
| Holothurin B (274) | Antifungal | T. mentagrophytes | MIC-1.5 µg/mL | [137] |
| Holothurin A3 (227) | Cytotoxic | Human tumor cell lines | IC50 = 0.32–0.87 μg/mL | [103] |
| Holothurin A4 (228) | Cytotoxic | Human tumor cell lines | IC50 = 0.57–1.12 μg/mL | [103] |
| Scabraside A (230) | Antifungal | Eight pathogenic fungal strains | MIC80: 2–8 μg/mL | [138] |
| Echinoside A (238) | Antifungal | Eight pathogenic fungal strains | MIC80: 1–8 μg/mL | [108] |
| Cucumarioside A2-2 (18) | immunomodulatory | Increased lysosomal activity | 0.2–20 ng/mouse | [139] |
| Frondoside A (37) | immunomodulatory | Enhanced phagocytosis | 0.001 μg/mL | [140] |
| Philinopside E (80) | Cytotoxicity | Ten tumor cell lines | ED50: 0.75–3.50 μg/mL | [54] |
| Holotoxin A1 (152) | Antifungal | Five pathogenic fungi | MIC: 0.5–1.0 µg/mL | [22] |
| Cucumarioside A1 (106) | Hemolytic | Mouse erythrocytes | MEC100: 0.7 ± 0.1 µg/mL | [66] |
6. Mechanisms of Action
Natural products derived from marine organisms have incredible structural and functional diversity. The mechanism by which triterpene glycosides exhibit anticancer activity primarily involve induction of tumor cell apoptosis through the activation of intracellular caspase cell death pathways, arrest of the cell cycle at S or G2/M phases and increase of the sub-G0/G1 cell population; regulation of nuclear factor NF-κB expression; reduction in cancer cell adhesion; suppression of cell migration and tube formation; suppression of angiogenesis; inhibition of cell proliferation, colony formation, and tumor invasion [141]. However, the detailed mechanism(s) of the anticancer activities of these glycosides remains largely unclear.
Marked membranolytic effects such as increased membrane permeability, loss of barrier function, and the rupture of cell membrane are considered the basic mechanisms underlying a variety of biological activities exerted by triterpene glycosides of sea cucumbers. The glycosides form complex with ∆5(6)-sterols of cellular membrane especially cholesterol. This interaction induces significant changes in the physicochemical properties of cell membranes, such as variations in their stability, microviscosity, and permeability. Saponins form complexes with membrane sterols, leading to cell disruption by the formation of pores. Due to this irreversible interaction, the selective permeability of cell membranes is impaired and cell compounds are transferred into the extracellular matrix, ultimately resulting in cell death [142,143].
7. Structure–Activity Relationships (SARs)
Both glycone and aglycone parts are important for biological activities of sea cucumber glycosides. The structure–activity relationships among sea cucumber glycosides are presumably more complicated. The most important structural characteristics of glycosides that probably contribute in biological activities are mentioned below.
The cytotoxicity not only depends on the chemical structures of the glycosides but also cell types [144]. The presence of 12α-hydroxy and 9(11)-ene structural units in holostane aglycone play key role in cytotoxicity [144]. Number of monosaccharide units in sugar chains and the substitution in side chain of aglycone could affect cytotoxicity. The presence of hydroxy groups in the side chains of glycosides significantly reduces cytotoxicity of the glycosides with increasing distance of hydroxy group from the 18(20)-lactone [30,31]. Linear tetraoside unit plays important role in different biological activities of sea cucumber glycosides [144]. Hexaoside chain containing glycosides show stronger cytotoxic activity than pentaoside chain containing glycosides. Glycosides with hexaosides residue with xylose or quinovose in the fifth position are the most active cytotoxins [144]. Different activities test result indicates that the number of sulfate groups and their position in the carbohydrate chains affect cytotoxicity [144]. It has been shown that the sulfate group attached to C-6 of terminal 3-O-methylglucose unit greatly decrease and attached to C-6 of glucose (the third monosaccharide unit) generally increase membranotropic activity [145].
8. Conclusions
Sea cucumbers (or holothurians), a class of marine invertebrates, are used as human food and traditional medicine, especially in some parts of Asia. The majority of the sea cucumbers synthesize glycosides with a polycyclic aglycone that contain either 7(8)- or 9(11)-double bond with up to six monosaccharide residues containing carbohydrate chain. A few of them are known to synthesize aglycone with 8(9)-ene. Sea cucumber glycosides are cytotoxic to eukaryotes; probably produce for escaping from predation by marine eukaryotic organisms. These cucumber metabolites have shown profound cytotoxic and hemolytic activities against eukaryotic organisms but not prokaryotic organisms. Due to significant cytotoxic and antifungal activities, extensive differential SAR studies of these glycosides can be helpful to develop new drugs and agrochemicals.
Acknowledgments
We are also thankful to the World Bank for partial funding of this work through a subproject of Higher Education Quality Enhancement Project (HEQEP), Supplementary Complete Proposal #2071. This research was also supported in part by the Ministry of Oceans and Fisheries (Grant PM60300), Korea. Sincere thanks are due to Prodip Kumar Roy of OIST, Okinawa, Japan for help in literature collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gomes A.R., Freitas A.C., Rocha-Santos T.A.P., Duarte A.C. Bioactive compounds derived from echinoderms. RSC Adv. 2014;4:29365–29382. doi: 10.1039/C4RA03352C. [DOI] [Google Scholar]
- 2.Brusca R.C., Brusca G.J. Invertebrates. 2nd ed. Sinauer; Sunderland, MA, USA: 2003. pp. 808–826. [Google Scholar]
- 3.Leal M.C., Madeira C., Brandão C.A., Puga J., Calado R. Bioprospecting of marine invertebrates for new natural products-A chemical and zoogeographical perspective. Molecules. 2012;17:9842–9854. doi: 10.3390/molecules17089842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shixiu L., Nina A., Yongjun M., Shujuan S., Wenjing F., Song H. Bioactive compounds of sea cucumbers and their therapeutic effects. Chin. J. Oceanol. Limnol. 2016;34:549–558. doi: 10.1007/s00343-016-4334-8. [DOI] [Google Scholar]
- 5.Janakiram N.B., Mohammed A., Rao C.V. Sea cucumbers metabolites as potent anti-cancer agents. Mar. Drugs. 2015;13:2909–2923. doi: 10.3390/md13052909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caulier G., Dyck S., Gerbaux P., Eeckhaut I., Flammang P. Review of saponin diversity in sea cucumbers belonging to the family Holothuriidae. SPC Beche-de-mer Inf. Bull. 2011;31:48–54. [Google Scholar]
- 7.Hyman L.H. Echinodermata. Volume 4 McGraw Hill; New York, NY, USA: 1955. The Invertebrates. [Google Scholar]
- 8.Bruckner A.W., Johnson K., Field J. Conservation strategies for sea cucumbers: Can a CITES Appendix II listing promote sustainable international trade? SPC Beche-de-mer Inf. Bull. 2003;18:24–33. [Google Scholar]
- 9.Lawrence J. In: A Functional Biology of Echinoderms = Functional Biology Series 6. Calow P., editor. Croom Helm Ltd.; London, UK: 1987. p. 340. [Google Scholar]
- 10.Higgins M. Sea cucumbers in a deep pickle. Environmental News Network. Aug 30, 2000.
- 11.Purcell S.W., Samyn Y., Conand C. FAO Species Catalogue for Fishery Purposes. Volume 6. FAO; Rome, Italy: 2012. Commercially important sea cucumbers of the world; p. 150. [Google Scholar]
- 12.Weici T. Chinese medicinal materials from the sea. Abstr. Chin. Med. 1987;4:571–600. [Google Scholar]
- 13.Yaacob H.B., Kim K.H., Shahimi M., Aziz N.S., Sahil S.M. Malaysian sea cucumber (Gamat): A prospect in health food and therapeutic; Proceedings of the Asian Food Technology Seminar; Kuala Lumpur, Malaysia. 6–7 October 1997; p. 6. [Google Scholar]
- 14.Bordbar S., Anwar F., Saari N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs. 2011;9:1761–1805. doi: 10.3390/md9101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen J., Hu C., Fan S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agric. 2010;90:2469–2474. doi: 10.1002/jsfa.4108. [DOI] [PubMed] [Google Scholar]
- 16.Silchenko A.S., Avilov S.A., Antonov A.A., Kalinin V.I., Kalinovsky A.I., Smirnov A.V., Riguera R., Jimenez C. Triterpene Glycosides from the deep-water North-Pacific sea cucumber Synallactes nozawai Mitsukuri. J. Nat. Prod. 2002;65:1802–1808. doi: 10.1021/np0202881. [DOI] [PubMed] [Google Scholar]
- 17.Yun S.H., Park E.S., Shin S.W., Na Y.W., Han J.Y., Jeong J.S., Shastina V.V., Stonik V.A., Park J.I., Kwak J.Y. Stichoposide C induces apoptosis through the generation of ceramide in leukemia and colorectal cancer cells and shows in vivo antitumor activity. Clin. Cancer Res. 2012;18:5934–5948. doi: 10.1158/1078-0432.CCR-12-0655. [DOI] [PubMed] [Google Scholar]
- 18.Stonik V.A., Maltsev I.I., Kalinovsky A.I., Conde K., Elyakov G.B. Glycosides of marine-invertebrates. XI. Two novel triterpene glycosides from holothurians of Stichopodidae family. Chem. Nat. Prod. 1982;2:194–199. [Google Scholar]
- 19.Mal’tsev I.I., Stonik V.A., Kalinovskii A.I. Stichoposide E-A new triterpene glycoside from holothurians of the family Stichopodidae. Chem. Nat. Prod. 1983;19:292–295. doi: 10.1007/BF00579762. [DOI] [Google Scholar]
- 20.Kitagawa I., Kobuyashi M., Inamoto T., Yusuzawa T., Kyogoku Y., Kido M. The structures of six antifungal oligoglycosides, stichlorosides A1, A2, B1, B2, C1 and C2 from the sea cucumber Stichopus chloronotus (Brandt) Chem. Pharm. Bull. 1981;29:2387–2391. doi: 10.1248/cpb.29.2387. [DOI] [Google Scholar]
- 21.Wang X.-H., Zou Z.-R., Yi Y.-H., Han H., Li L., Pan M.-X. Variegatusides: New non-sulphated triterpene glycosides from the sea cucumber Stichopus variegates. Mar. Drugs. 2014;12:2004–2018. doi: 10.3390/md12042004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z., Zhang H., Yuan W., Gong W., Tang H., Liu B., Krohn K., Li L., Yi Y., Zhang W. Antifungal nortriterpene and triterpene glycosides from the sea cucumber Apostichopus japonicas Selenka. Food Chem. 2012;1:295–300. doi: 10.1016/j.foodchem.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 23.Drozdova O.A., Avilov S.A., Kalinovskii A.I., Stonik V.A., Mil’grom Y.M., Rashkes Y.V. New glycosides from the holothurian Cucumaria japonica. Chem. Nat. Compd. 1993;29:200–205. doi: 10.1007/BF00630115. [DOI] [Google Scholar]
- 24.Avilov S.A., Stonik V.A., Kalinovskii A.I. Structures of four new triterpene glycosides from the holothurian Cucumaria japonica. Chem. Nat. Compd. 1990;26:670–675. doi: 10.1007/BF00630079. [DOI] [Google Scholar]
- 25.Avilov S., Tishchenko L.Y., Stonik V.A. Structure of cucumarioside A2-2-A triterpene glycoside from the holothurian Cucumaria japonica. Chem. Nat. Compd. 1984;20:759–760. doi: 10.1007/BF00580052. [DOI] [Google Scholar]
- 26.Avilov S.A., Antonov A.S., Silchenko A.S., Kalinin V.I., Kalinovsky A.I., Dmitrenok P.S., Stonik V.A., Riguera R., Jimenez C. Triterpene Glycosides from the Far Eastern sea cucumber Cucumaria conicospermium. J. Nat. Prod. 2003;66:910–916. doi: 10.1021/np030005k. [DOI] [PubMed] [Google Scholar]
- 27.Drozdova O.A., Avilov S.A., Kalinin V.I., Kalinovsky A.I., Stonik V.A., Riguera R., Jiméne C. Cytotoxic triterpene glycosides from Far-Eastern sea cucumbers belonging to the genus Cucumaria. Liebigs Ann. Chem. 1997;11:2351–2356. doi: 10.1002/jlac.199719971125. [DOI] [Google Scholar]
- 28.Drozdova O.A., Avilov S.A., Kalinovskii A.I., Stonik V.A., Mil’grom Y.M., Rashkes Y.V. Trisulfated glycosides from the holothurians Cucumaria japonica. Chem. Nat. Compd. 1993;29:309–313. doi: 10.1007/BF00630529. [DOI] [Google Scholar]
- 29.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Yurchenko E.A., Kalinin V.I. Structure of cucumariosides H5, H6, H7 and H8, triterpene glycosides from the sea cucumber Eupentacta fraudatrix and unprecedented aglycone with 16,22-epoxy-group. Nat. Prod. Commun. 2011;6:1075–1082. [PubMed] [Google Scholar]
- 30.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Yurchenko E.A., Kalinin V.I. Structures and cytotoxic properties of cucumariosides H2, H3 and H4 from the sea cucumber Eupentacta fraudatrix. Nat. Prod. Res. 2012;26:1765–1774. doi: 10.1080/14786419.2011.602637. [DOI] [PubMed] [Google Scholar]
- 31.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Martyyas E.A., Kalinin V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of cucumariosides I1, I3, I4, three new minor disulfated pentaosides. Nat. Prod. Commun. 2013;8:1053–1058. [PubMed] [Google Scholar]
- 32.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Menchinskaya E.S., Aminin D.L., Kalinin V.I. Structure of cucumarioside I2 from the sea cucumber Eupentacta fraudatrix (Djakonov et Baranova) and cytotoxic and immunostimulatory activities of this saponin and relative compounds. Nat. Prod. Res. 2013;27:1776–1783. doi: 10.1080/14786419.2013.778851. [DOI] [PubMed] [Google Scholar]
- 33.Girard M., Bélanger J., ApSimon J.W., Garneau F.X., Harvey C., Brisson J.R. Frondoside A, a novel triterpene glycoside from the holothurians Cucumaria frondosa. Can. J. Chem. 1990;68:11–18. doi: 10.1139/v90-003. [DOI] [Google Scholar]
- 34.Findlay J.A., Yayli N., Radics L. Novel sulfated oligosaccharides from the sea cucumber Cucumaria frondosa. J. Nat. Prod. 1992;55:93–101. doi: 10.1021/np50079a014. [DOI] [PubMed] [Google Scholar]
- 35.Silchenko A.S., Avilov S.A., Antonov A.S., Kalinovsky A.I., Dmitrenok P.S., Kalinin V.I., Stonik V.A., Woodward C., Collin P.D. Glycosides from the sea cucumber Cucumaria frondosa. III. Structure of frondosides A2-1, A2-2, A2-3, and A2-6, four new minor monosulfated triterpene glycosides. Can. J. Chem. 2005;83:21–27. doi: 10.1139/v04-163. [DOI] [Google Scholar]
- 36.Silchenko A.S., Avilov S.A., Antonov A.S., Kalinovsky A.I., Dmitrenok P.S., Kalinin V.I., Stonik V.A., Woodward C., Collin P.D. Glycosides from the sea cucumber Cucumaria frondosa. IV. Structure of frondosides A2-4, A2-7, and A2-8, three new minor monosulfated triterpene glycosides. Can. J. Chem. 2005;83:2120–2126. doi: 10.1139/v05-243. [DOI] [Google Scholar]
- 37.Avilov S.A., Antonov A.S., Drozdova O.A., Kalinin V.I., Kalinovsky A.I., Stonik V.A., Riguera R., Lenis L.A., Jiménez C. Triterpene glycosides from the Far-Eastern sea cucumber Pentamera calcigera. 1. Monosulfated glycosides and cytotoxicity of their unsulfated derivatives. J. Nat. Prod. 2000;63:65–71. doi: 10.1021/np9903447. [DOI] [PubMed] [Google Scholar]
- 38.Avilov S.A., Antonov A.S., Drozdova O.A., Kalinin V.I., Kalinovsky A.I., Riguera R., Lenis L.A., Jiménez C. Triterpene Glycosides from the far eastern sea cucumber Pentamera calcigera II: Disulfated glycosides. J. Nat. Prod. 2000;63:1349–1355. doi: 10.1021/np000002x. [DOI] [PubMed] [Google Scholar]
- 39.Yong-Juan Z., Yang-Hua Y. Antitumor activities in vitro of the triterpene glycoside colochiroside A from sea cucumber Colochirus anceps. J. Mod. Oncol. 2011;2:205–207. [Google Scholar]
- 40.Afiyatullov S.S., Kalinovskii A.I., Stonik V.A. Structures of cucumariosides C1 and C2-Two new triterpene glycosides from the holothurians Eupentacta fraudatrix. Chem. Nat. Compd. 1987;23:691–696. doi: 10.1007/BF00596644. [DOI] [Google Scholar]
- 41.Avilov S.A., Silchenko A.S., Antonov A.S., Kalinin V.I., Kalinovsky A.I., Smirnov A.V., Dmitrenok P.S., Evtushenko E.V., Fedorov S.N., Savina A.S., et al. Synaptosides A and A1, triterpene glycosides from the sea cucumber Synapta maculata containing 3-O-methylglucuronic acid and their cytotoxic activity against tumor cells. J. Nat. Prod. 2008;71:525–531. doi: 10.1021/np070283+. [DOI] [PubMed] [Google Scholar]
- 42.Silchenko A.S., Avilov S.A., Kalinin V.I., Stonik V.A., Kalinovskiĭ A.I., Dmitrenok P.S., Stepanov V.G. Monosulfated triterpene glycosides from Cucumaria okhotensis Levin et Stepanov, a new species of sea cucumbers from sea of Okhotsk. Russ. J. Bioorg. Chem. 2007;33:73–82. doi: 10.1134/S1068162007010098. [DOI] [PubMed] [Google Scholar]
- 43.Silchenko A.S., Avilov S.A., Kalinovsky A.I., Dmitrenok P.S., Kalinin V.I., Morre J., Deinzer M.L., Woodward C., Collin P.D. Glycosides from the North Atlantic sea cucumber Cucumaria frondosa V-Structures of five new minor trisulfated triterpene oligoglycosides, frondosides A7-1, A7-2, A7-3, A7-4, and isofrondoside C. Can. J. Chem. 2007;85:626–636. doi: 10.1139/v07-087. [DOI] [Google Scholar]
- 44.Maier M.S., Roccatagliata A.J., Kuriss A., Chludil H., Seldes A.M., Pujol C.A., Damonte E.B. Two new cytotoxic and virucidal trisulfated triterpene glycosides from the Antarctic sea cucumber Staurocucumis liouvillei. J. Nat. Prod. 2001;64:732–736. doi: 10.1021/np000584i. [DOI] [PubMed] [Google Scholar]
- 45.Antonov A.S., Avilov S.A., Kalinovsky A.I., Anastyuk S.D., Dmitrenok P.S., Evtushenko E.V., Kalinin V.I., Smirnov A.V., Taboada S., Ballesteros M., et al. Triterpene glycosides from Antarctic sea cucumbers. 1. Structure of liouvillosides A1, A2, A3, B1, and B2 from the sea cucumber Staurocucumis liouvillei: New procedure for separation of highly polar glycoside fractions and taxonomic revision. J. Nat. Prod. 2008;71:1677–1685. doi: 10.1021/np800173c. [DOI] [PubMed] [Google Scholar]
- 46.Antonov A.S., Avilov S.A., Kalinovsky A.I., Dmitrenok P.S., Kalinin V.I., Taboada S., Ballesteros M., Avila C. Triterpene glycosides from Antarctic sea cucumbers III. Structures of liouvillosides A4 and A5, two minor disulphated tetraosides containing 3-O-methylquinovose as terminal monosaccharide units from the sea cucumber Staurocucumis liouvillei (Vaney) Nat. Prod. Res. 2011;25:1324–1333. doi: 10.1080/14786419.2010.531017. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S.Y., Yi Y.H., Tang H.F., Li L., Sun P., Wu J. Two new bioactive triterpene glycosides from the sea cucumber Pseudocolochirus violaceus. J. Asian Nat. Prod. Res. 2006;8:1–8. doi: 10.1080/10286020500034972. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S.Y., Yi Y.H., Tang H.F. Cytotoxic sulfated triterpene glycosides from the sea cucumber Pseudocolochirus violaceus. Chem. Biodivers. 2006;3:807–817. doi: 10.1002/cbdv.200690083. [DOI] [PubMed] [Google Scholar]
- 49.Zou Z.-R., Yi Y.-H., Wu H.-M., Wu J.-H., Liaw C.C., Lee K.H. Intercedensides A–C, three new cytotoxic triterpene glycosides from the sea cucumber Mensamaria intercedens Lampert. J. Nat. Prod. 2003;66:1055–1060. doi: 10.1021/np030064y. [DOI] [PubMed] [Google Scholar]
- 50.Zou Z., Yi Y., Wu H., Yao X., Du L., Jiuhong W., Liaw C.C., Lee K.H. Intercedensides D–I, cytotoxic triterpene glycosides from the sea cucumber Mensamaria intercedens Lampert. J. Nat. Prod. 2005;68:540–546. doi: 10.1021/np040205b. [DOI] [PubMed] [Google Scholar]
- 51.Murray A.P., Muniaín C., Seldes A.M., Maier M.S. Patagonicoside A: A novel antifungal disulfated triterpene glycoside from the sea cucumber Psolus patagonicus. Tetrahedron. 2001;57:9563–9568. doi: 10.1016/S0040-4020(01)00970-X. [DOI] [Google Scholar]
- 52.Careaga V.P., Muniain C., Maier M.S. Patagonicosides B and C, two antifungal sulfated triterpene glycosides from the sea cucumber Psolus patagonicus. Chem. Biodivers. 2011;8:467–475. doi: 10.1002/cbdv.201000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi Y.H., Xu Q.Z., Li L., Zhang S.L., Wu H.M., Ding J., Tong Y.G., Tan W.F., Li M.H., Tian F., et al. Philinopsides A and B, two new sulfated triterpene glycosides from the sea cucumber Pentacta quadrangularis. Helv. Chim. Acta. 2006;9:54–63. doi: 10.1002/hlca.200690012. [DOI] [Google Scholar]
- 54.Zhang S.L., Li L., Yi Y.H., Sun P. Philinopsides E and F, two new sulfated triterpene glycosides from the sea cucumber Pentacta quadrangularis. Nat. Prod. Res. 2006;20:399–407. doi: 10.1080/14786410500185584. [DOI] [PubMed] [Google Scholar]
- 55.Moraes G., Northcote P.T., Silchenko A.S., Antonov A.S., Kalinovsky A.I., Dmitrenok P.S., Avilov S.A., Kalinin V.I., Stonik V.A. Mollisosides A, B1, and B2: Minor triterpene glycosides from the New Zealand and South Australian sea cucumber Australostichopus mollis. J. Nat. Prod. 2005;68:842–847. doi: 10.1021/np050049o. [DOI] [PubMed] [Google Scholar]
- 56.Kalinin V.I., Avilov S.A., Kalinina E.Y., Korolkova O.G., Kalinovsky A.I., Stonik V.A., Riguera R., Jiménez C. Structure of eximisoside A, a novel triterpene glycoside from the far-eastern sea cucumber Psolus eximius. J. Nat. Prod. 1997;60:817–819. doi: 10.1021/np9701541. [DOI] [PubMed] [Google Scholar]
- 57.Kalinin V.I., Stonik V.A., Kalinovskii A.I., Isakov V.V. Structure of pseudostichoposide A-The main triterpene glycoside from the holothurian Pseudostichopus trachus. Chem. Nat. Compd. 1989;25:577–582. doi: 10.1007/BF00598079. [DOI] [Google Scholar]
- 58.Popov R.S., Avilov S.A., Silchenko A.S., Kalinovsky A.I., Dmitrenok P.S., Grebnev B.B., Ivanchina N.V., Kalinin V.I. Cucumariosides F1 and F2, two new triterpene glycosides from the sea cucumber Eupentacta fraudatrix and their LC-ESI MS/MS identification in the starfish Patiria pectinifera, a predator of the sea cucumber. Biochem. Syst. Ecol. 2014;57:191–197. doi: 10.1016/j.bse.2014.08.009. [DOI] [Google Scholar]
- 59.Careaga V.P., Bueno C., Muniain C., Alché L., Maier M.S. Pseudocnoside A, a new cytotoxic and antiproliferative triterpene glycoside from the sea cucumber Pseudocnus dubiosus leoninus. Nat. Prod. Res. 2014;28:213–220. doi: 10.1080/14786419.2012.751596. [DOI] [PubMed] [Google Scholar]
- 60.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Martyyas E.A., Kalinin V.I., Jayasandhya P., Rajan G.C., Padmakumar K.P. Structures and biological activities of typicosides A1, A2, B1, C1 and C2, triterpene glycosides from the sea cucumber Actinocucumis typica. Nat. Prod. Commun. 2013;8:301–310. [PubMed] [Google Scholar]
- 61.Aminin D.L., Silchenko A.S., Avilov S.A., Stepanov V.G., Kalinin V.I. Immunomodulatory action of monosulfated triterpene glycosides from the sea cucumber Cucumaria okhotensis: Stimulation of activity of mouse peritoneal macrophages. Nat. Prod. Commun. 2010;5:1877–1880. [PubMed] [Google Scholar]
- 62.Silchenko A.S., Avilov S.A., Kalinin V.I., Kalinovsky A.I., Dmitrenok P.S., Fedorov S.N., Stepanov V.G., Dong Z., Stonik V.A. Constituents of the sea cucumber Cucumaria okhotensis. Structures of okhotosides B1–B3 and cytotoxic activities of some glycosides from this species. J. Nat. Prod. 2008;71:351–356. doi: 10.1021/np0705413. [DOI] [PubMed] [Google Scholar]
- 63.Silchenkoa A.S., Kalinovskya A.I., Avilova S.A., Andryjaschenko P.V., Dmitrenok P.S., Yurchenko E.A., Kalinin V.I., Dolmatov I.Y. Colochirosides A1, A2, A3, and D, four novel sulfated triterpene glycosides from the sea cucumber Colochirus robustus (Cucumariidae, Dendrochirotida) Nat. Prod. Commun. 2016;11:381–386. [PubMed] [Google Scholar]
- 64.Silchenkoa A.S., Kalinovskya A.I., Avilova S.A., Andryjaschenko P.V., Dmitrenok P.S., Yurchenko E.A., Kalinin V.I., Dolmatov I.Y. Colochirosides B1, B2, B3 and C, novel sulfated triterpene glycosides from the sea cucumber Colochirus robustus (Cucumariidae, Dendrochirotida) Nat. Prod. Commun. 2015;10:687–694. [PubMed] [Google Scholar]
- 65.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Kalinin V.I., Yurchenko E.A., Dautov S.S. Structures of violaceusosides C, D, E and G, sulfated triterpene glycosides from the sea cucumber Pseudocolochirus violaceus (Cucumariidae, Dendrochirotida) Nat. Prod. Commun. 2014;9:391–399. [PubMed] [Google Scholar]
- 66.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Martyyas E.A., Kalinin V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of cucumariosides A1, A3, A4, A5, A6, A12 and A15, seven new minor non-sulfated tetraosides and unprecedented 25-keto, 27-norholostane aglycone. Nat. Prod. Commun. 2012;7:517–525. [PubMed] [Google Scholar]
- 67.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Martyyas E.A., Kalinin V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and cytotoxic action of cucumariosides A2, A7, A9, A10, A11, A13 and A14, seven new minor non-sulfated tetraosides and an aglycone with an uncommon 18-hydroxy group. Nat. Prod. Commun. 2012;7:845–852. [PubMed] [Google Scholar]
- 68.Afiyatullov S.S., Tishchenko L.Y., Stonik V.A., Kalinovskii A.I., Elyakov G.B. Structure of cucumarioside G1-A new triterpene glycoside from the holothurians Cucumaria fraudatrix. Chem. Nat. Compd. 1985;21:228–232. doi: 10.1007/BF00714918. [DOI] [Google Scholar]
- 69.Kalinin V.I., Avilov S.A., Kalinovskii A.I., Stonik V.A. Cucumarioside G3-A minor triterpene glycoside from the holothurian Eupentacta fraudatrix. Chem. Nat. Compd. 1992;28:635–636. doi: 10.1007/BF00630455. [DOI] [Google Scholar]
- 70.Kalinin V.I., Avilov S.A., Kalinovskii A.I., Stonik V.A., Mil’grom Y.M., Rashkes Y.V. Cucumarioside G4-A new triterpenglycoside from the holothurian Eupentacta fraudatrix. Chem. Nat. Compd. 1992;28:600–603. doi: 10.1007/BF00630438. [DOI] [Google Scholar]
- 71.Han H., Xu Q.Z., Yi Y.H., Gong W., Jiao B.H. Two new cytotoxic disulfated holostane glycosides from the sea cucumber Pentacta quadrangularis. Chem. Biodivers. 2010;7:158–167. doi: 10.1002/cbdv.200800324. [DOI] [PubMed] [Google Scholar]
- 72.Silchenko A.S., Avilov S.A., Kalinin V.I., Kalinovsky A.I., Stonik V.A., Smirnov A.V. Pseudostichoposide B-New triterpene glycoside with unprecedent type of sulfatation from the deep-water North-Pacific Sea cucumber Pseudostichopus trachus. Nat. Prod. Res. 2004;18:565–570. doi: 10.1080/14786410310001630591. [DOI] [PubMed] [Google Scholar]
- 73.Wang X.-H., Li L., Yi Y.-H., Sun P., Yan B., Pan M.-X., Han H., Wang X.-D. Two new triterpene glycosides from sea cucumber Stichopus variegatus. Chin. J. Nat. Med. 2006;4:177–180. [Google Scholar]
- 74.Stonik V.A., Mal’tsev I.I., Elyakov G.B. The structure of thelenotosides A and B from the holothurians Thelenota ananas. Chem. Nat. Compd. 1982;18:590–593. doi: 10.1007/BF00575045. [DOI] [Google Scholar]
- 75.Miyamoto T., Togawa K., Higuchi R., Komori T., Sasaki T. Constituents of holothuroidea, II. Six newly identified biologically active triterpenoid glycoside sulfates from the sea cucumber Cucumaria echinata. Liebigs Ann. Chem. 1990;5:453–460. doi: 10.1002/jlac.199019900186. [DOI] [Google Scholar]
- 76.Rodriguez J., Riguera R. Lefevreiosides: Four new triterpene glycosides from the sea cucumber Cucumaria lefevrei. J. Chem. Res. 1989;11:342–343. [Google Scholar]
- 77.Han H., Xu Q.Z., Tang H.F., Yi Y.H., Gong W. Cytotoxic holostane-type triterpene glycosides from the sea cucumber Pentacta quadrangularis. Planta Med. 2010;76:1900–1904. doi: 10.1055/s-0030-1249854. [DOI] [PubMed] [Google Scholar]
- 78.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Martyyas E.A., Kalinin V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological activity of cucumariosides B1 and B2, two new minor non-sulfated unprecedented triosides. Nat. Prod. Commun. 2012;7:1157–1162. [PubMed] [Google Scholar]
- 79.Sharypov V.F., Chumak A.D., Stonik V.A., Elyakov G.B. Glycosides of marine invertebrates. X. The structure of stichoposides A and B from the holothurians Stichopus cloronotus. Chem. Nat. Compd. 1981;17:139–142. doi: 10.1007/BF00634731. [DOI] [Google Scholar]
- 80.Cuong N.X., Vien L.T., Hoang L., Hanh T.T.H., Thao D.T., Thanh N.V., Nam N.H., Thung D.C., Kiem P.V., Minh C.V. Cytotoxic triterpene diglycosides from the sea cucumber Stichopus horrens. Bioorg. Med. Chem. Lett. 2017;27:2939–2942. doi: 10.1016/j.bmcl.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Wu J., Yi Y.H., Tang H.F., Wu H.M., Zhou Z.R. Hillasides A and B, two new cytotoxic triterpene glycosides from the sea cucumber Holothuria hilla Lesson. J. Asian Nat. Prod. Res. 2007;9:609–615. doi: 10.1080/10286020600882676. [DOI] [PubMed] [Google Scholar]
- 82.Dong P., Xue C.-H., Yu L.-F., Xu J., Chen S.-G. Determination of triterpene glycosides in sea cucumber (Stichopus japonicus) and its related products by high-performance liquid chromatography. J. Agric. Food Chem. 2008;56:4937–4942. doi: 10.1021/jf800893r. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z., Gong W., Sun G., Tang H., Liu B., Li L., Yi Y., Zhang W. New holostan-type triterpene glycosides from the sea cucumber Apostichopus japonicus. Nat. Prod. Commun. 2012;7:1431–1434. [PubMed] [Google Scholar]
- 84.Iñiguez-Martinez A.M., Guerra-Rivas G., Rios T., Quijano L. Triterpenoid oligoglycosides from the sea cucumber Stichopus parvimensis. J. Nat. Prod. 2005;68:1669–1673. doi: 10.1021/np050196m. [DOI] [PubMed] [Google Scholar]
- 85.Ohta T., Hikino H. Structures of four new triterpenoidal oligosides, bivittosides A, B, C and D from the sea cucumber Bohadschia bivittata Mitsukuri. Chem. Pharm. Bull. 1981;29:282–285. [Google Scholar]
- 86.Yuan W.H., Yi Y.H., Tang H.F., Liu B.S., Wang Z.L., Sun G.Q., Zhang W., Li L., Sun P. Antifungal triterpene glycosides from the sea cucumber Bohadschia marmorata. Planta Med. 2009;75:168–173. doi: 10.1055/s-0028-1088348. [DOI] [PubMed] [Google Scholar]
- 87.Liu B.S., Yi Y.H., Li L., Sun P., Yuan W.H., Sun G.Q., Han H., Xue M. Argusides B and C, two new cytotoxic triterpene glycosides from the sea cucumber Bohadschia argus. Chem. Biodivers. 2008;5:1288–1297. doi: 10.1002/cbdv.200890115. [DOI] [PubMed] [Google Scholar]
- 88.Bahrami Y., Franco C.M.M. Structure elucidation of new acetylated saponins, lessoniosides A, B, C, D, and E, and non-acetylated saponins, lessoniosides F and G, from the viscera of the sea cucumber Holothuria lessoni. Mar. Drugs. 2015;13:597–617. doi: 10.3390/md13010597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dyck S.V., Gerbaux P., Flammang P. Qualitative and quantitative saponin contents in five sea cucumbers from the Indian Ocean. Mar. Drugs. 2010;8:173–189. doi: 10.3390/md8010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Yurchenko E.A., Dolmatov I.Y., Kalinin V.I., Stonik V.A. Structure and biological action of cladolosides B1, B2, C, C1, C2 and D, six new triterpene glycosides from the sea cucumber Cladolabes schmeltzii. Nat. Prod. Commun. 2013;8:1527–1534. [PubMed] [Google Scholar]
- 91.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Yurchenko E.A., Dolmatov I.Y., Kalinin V.I. Structures and biological activities of cladolosides C3, E1, E2, F1, F2, G, H1 and H2, eight triterpene glycosides from the sea cucumber Cladolabes schmeltzii with one known and four new carbohydrate chains. Carbohydr. Res. 2015;23:22–31. doi: 10.1016/j.carres.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 92.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Chingizova E.A., Dolmatov I.Y., Kalinin V.I. Cladolosides I1, I2, J1, K1, K2 and L1, monosulfated triterpene glycosides with new carbohydrate chains from the sea cucumber Cladolabes schmeltzii. Carbohydr. Res. 2017;5:80–87. doi: 10.1016/j.carres.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 93.Rodriguez J., Castro R., Riguera R. Holothurinosides: New antitumour non sulphated triterpenoid glycosides from the sea cucumber Holothuria forskalii. Tetrahedron. 1991;47:4753–4762. doi: 10.1016/S0040-4020(01)86479-6. [DOI] [Google Scholar]
- 94.Sun G.-Q., Li L., Yi Y.-H., Yuan W.-H., Liu B.-S., Weng Y.-Y., Zhang S.-L., Sun P., Wang Z.-L. Two new cytotoxic nonsulfated pentasaccharide holostane (=20-hydroxylanostan-18-oic acid gamma-lactone) glycosides from the sea cucumber Holothuria grisea. Helv. Chim. Acta. 2008;9:1453–1460. doi: 10.1002/hlca.200890158. [DOI] [Google Scholar]
- 95.Bahrami Y., Zhang W., Franco C. Discovery of novel saponins from the viscera of the sea cucumber Holothuria lessoni. Mar. Drugs. 2014;12:2633–2667. doi: 10.3390/md12052633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjashchenko P.V., Fedorov S.N., Dmitrenok P.S., Yurchenko E.A., Kalinin V.I., Rogacheva A.V., Gebruk A.V. Kolgaosides A and B, two new triterpene glycosides from the Arctic deep water sea cucumber Kolga hyalina (Elasipodida: Elpidiidae) Nat. Prod. Commun. 2014;9:1259–1264. [PubMed] [Google Scholar]
- 97.Yuan W.H., Yi Y.H., Tan R.X., Wang Z.L., Sun G.Q., Xue M., Zhang H.W., Tang H.F. Antifungal triterpene glycosides from the sea cucumber Holothuria (Microthele) axiloga. Planta Med. 2009;75:647–653. doi: 10.1055/s-0029-1185381. [DOI] [PubMed] [Google Scholar]
- 98.Antonov A.S., Avilov S.A., Kalinovsky A.I., Anastyuk S.D., Dmitrenok P.S., Kalinin V.I., Taboada S., Bosh A., Avila C., Stonik V.A. Triterpene glycosides from Antarctic sea cucumbers. 2. Structure of achlioniceosides A1, A2, and A3 from the sea cucumber Achlionice violaecuspidata (=Rhipidothuria racowitzai) J. Nat. Prod. 2009;72:33–38. doi: 10.1021/np800469v. [DOI] [PubMed] [Google Scholar]
- 99.Miyamoto T., Togawa K., Higuchi R., Komori T., Sasaki T. Structures of four new triterpenoid oligoglycosides: DS-Penaustrosides A, B, C, and D from the sea cucumber Pentacta australis. J. Nat. Prod. 1992;55:940–946. doi: 10.1021/np50085a014. [DOI] [PubMed] [Google Scholar]
- 100.Cuong N.X., Vien L.T., Hanh T.T., Thao N.P., Thaodo T., Thanh N.V., Nam N.H., Thungdo C., Kiem P.V., Minh C.V. Cytotoxic triterpene saponins from Cercodemas anceps. Bioorg. Med. Chem. Lett. 2015;15:3151–3156. doi: 10.1016/j.bmcl.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 101.Chanley J.D., Ledeen R., Wax J., Nigrelli R.F., Sobotka H. Holothurin I. The isolation, properties and sugar components of holothurin A. J. Am. Chem. Soc. 1959;81:5180–5183. doi: 10.1021/ja01528a040. [DOI] [Google Scholar]
- 102.Oleinikova G.K., Kuznetsova T.A., Ivanova N.S., Kalinovskii A.I., Rovnykh N.V., Elyakov G.B. Glycosides of marine invertebrates. XV. A new triterpene glycoside-Holothurin A1-from Caribbean holothurians of the family Holothuriidae. Chem. Nat. Compd. 1982;18:430–434. doi: 10.1007/BF00579637. [DOI] [Google Scholar]
- 103.Dang N.H., Thanh N.V., Kiem P.V., Huong M., Minh C.V., Kim Y.H. Two new triterpene glycosides from the Vietnamese sea cucumber Holothuria scabra. Arch. Pharm. Res. 2007;30:1387–1391. doi: 10.1007/BF02977361. [DOI] [PubMed] [Google Scholar]
- 104.Han H., Yi Y., Xu Q., La M., Zhang H. Two new cytotoxic triterpene glycosides from the sea cucumber Holothuria scabra. Planta Med. 2009;75:1608–1612. doi: 10.1055/s-0029-1185865. [DOI] [PubMed] [Google Scholar]
- 105.Han H., Li L., Yi Y.-H., Wang X.-H., Pan M.-X. Triterpene glycosides from sea cucumber Holothuria scabra with cytotoxic activity. Chin. Herb. Med. 2012;4:183–188. doi: 10.3969/j.issn.1674-6384.2012.03.002. [DOI] [Google Scholar]
- 106.Zhang S.-Y., Yi Y.-H., Tang H.-F. Bioactive Triterpene glycosides from the sea cucumber Holothuria fuscocinerea. J. Nat. Prod. 2006;69:1492–1495. doi: 10.1021/np060106t. [DOI] [PubMed] [Google Scholar]
- 107.Yuan W., Yi Y., Tang H., Xue M., Wang Z., Sun G., Zhang W., Liu B., Li L., Sun P. Two new holostan-type triterpene glycosides from the sea cucumber Bohadschia marmorata. Chem. Pharm. Bull. 2008;56:1207–1211. doi: 10.1248/cpb.56.1207. [DOI] [PubMed] [Google Scholar]
- 108.Kitagawa I., Inamoto T., Fuchida M., Okada S., Kobayashi M., Nishino T., Kyogoku Y. Structures of echinosides A and B, two antifungal oligoglycosides from the sea cucumber Actinopyga echinites. Chem. Pharm. Bull. 1980;28:1651–1653. doi: 10.1248/cpb.28.1651. [DOI] [PubMed] [Google Scholar]
- 109.Zhao Q., Liu Z.-D., Xue Y., Wang J.F., Li H., Tang Q.J., Wang Y.M., Dong P., Xue C.H. Ds-echinoside A, a new triterpene glycoside derived from sea cucumber, exhibits antimetastatic activity via the inhibition of NF-κB-dependent MMP-9 and VEGF expressions. J. Zhejiang Univ. Sci. B. 2011;12:534–544. doi: 10.1631/jzus.B1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhatnagar S., Dudouet B., Ahond A., Poupat C., Thoison O., Clastres A., Laurent D., Potier P. Invertébrés marins du lagon néocalédonien. IV: Saponines et sapogénines d’une holothurie, Actinopyga flammea. Bull. Soc. Chim. Fr. 1985;1:124–129. (In French) [Google Scholar]
- 111.Kitagawa I., Kobayashi M., Son B.W., Siuzukia S., Kyogokub Y. Marine Natural Products. XIX. Pervicosides A, B, and C, lanostane-type triterpene-oligoglycoside sulfates from the sea cucumber Holothuria pervicax. Chem. Pharm. Bull. 1989;37:1230–1234. doi: 10.1248/cpb.37.1230. [DOI] [Google Scholar]
- 112.Liu B.-S., Yi Y.-H., Li L., Zhang S.L., Han H., Weng Y.Y., Pan M. Arguside A: A new cytotoxic triterpene glycoside from the sea cucumber Bohadschia argus. Chem. Biodivers. 2007;4:2845–2851. doi: 10.1002/cbdv.200790234. [DOI] [PubMed] [Google Scholar]
- 113.Chludil H.D., Muniain C.C., Seldes A.M., Maier M.S. Cytotoxic and antifungal triterpene glycosides from the patagonian sea cucumber Hemoiedema spectabilis. J. Nat. Prod. 2002;65:860–865. doi: 10.1021/np0106236. [DOI] [PubMed] [Google Scholar]
- 114.Liu B.-S., Yi Y.-H., Li L., Sun P., Han H., Sun G.Q., Wang X.H., Wang Z.L. Argusides D and E, two new cytotoxic triterpene glycosides from the sea cucumber Bohadschia argus. Chem. Biodivers. 2008;5:1425–1433. doi: 10.1002/cbdv.200890131. [DOI] [PubMed] [Google Scholar]
- 115.Kalinin V.I., Kalinovsky A.I., Stonik V.A. Structure of psolusoside A-the major triterpene glycoside from holothurian Psolus fabricii. Chem. Nat. Compd. 1985;21:197–202. doi: 10.1007/BF00714912. [DOI] [Google Scholar]
- 116.Bahrami Y., Zhang W., Chataway T., Franco C. Structural elucidation of novel saponins in the sea cucumber Holothuria lessoni. Mar. Drugs. 2014;12:4439–4473. doi: 10.3390/md12084439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Yurchenko E.A., Dolmatov I.Y., Savchenko A.M., Kalinin V.I. Triterpene glycosides from the sea cucumber Cladolabes schmeltzii. II. Structure and biological action of cladolosides A1–A6. Nat. Prod. Commun. 2014;9:1421–1428. [PubMed] [Google Scholar]
- 118.Yibmantasiri P., Leahy D.C., Busby B.P., Angermayr S.A., Sorgo A.G., Boeger K., Heathcott R., Barber J.M., Moraes G., Matthews J.H., et al. Molecular basis for fungicidal action of neothyonidioside, a triterpene glycoside from the sea cucumber, Australostichopus mollis. Mol. Biosyst. 2012;8:902–912. doi: 10.1039/c2mb05426d. [DOI] [PubMed] [Google Scholar]
- 119.Kitagawa I., Nishino T., Matsuno T., Akutsu H., Kyogoku Y. Structure of holothurin B-a pharmacologically active triterpene-oligoglycoside from the sea cucumber holothuria leucospilota (Brandt) Tetrahedron Lett. 1978;19:985–988. doi: 10.1016/S0040-4039(01)85431-9. [DOI] [Google Scholar]
- 120.Kuznetsova T.A., Kalinovskaya N.I., Kalinovskii A.I., Oleinikova G.K., Rovnykh N.V., Elyakov G.V. Glycosides of marine invertebrates. XIV. Structure of holothurin B1 from the holothurians Holothuria floridana. Chem. Nat. Compd. 1982;18:449–451. doi: 10.1007/BF00579642. [DOI] [Google Scholar]
- 121.Silchenko A.S., Stonik V.A., Avilov S.A., Kalinin V.I., Kalinovsky A.I., Zaharenko A.M., Smirnov A.V., Mollo E., Cimino G. Holothurins B2, B3, and B4, new triterpene glycosides from mediterranean sea cucumbers of the genus Holothuria. J. Nat. Prod. 2005;68:564–567. doi: 10.1021/np049631n. [DOI] [PubMed] [Google Scholar]
- 122.Hua H., Yi Y.-H., Li L., Liu B.-S., Pan M.-X., Yan B., Wang X.-H. Triterpene glycosides from sea cucumber Holothuria leucospilota. Chin. J. Nat. Med. 2009;7:346–350. doi: 10.3724/SP.J.1009.2009.00346. [DOI] [Google Scholar]
- 123.Kobayashi M., Hori M., Kan K., Yasuzawa T., Matsui M., Shigeki S., Kitagawa I. Marine Natural Products. XXVII. Distribution of lanostane-type triterpene oligoglycosides in ten kinds of okinawan sea cucumbers. Chem. Pharm. Bull. 1991;39:2282–2287. doi: 10.1248/cpb.39.2282. [DOI] [Google Scholar]
- 124.Wu J., Yi Y.H., Tang H.F., Zou Z.R., Wu H.M. Structure and cytotoxicity of a new lanostane-type triterpene glycoside from the sea cucumber Holothuria hilla. Chem. Biodivers. 2006;3:1249–1254. doi: 10.1002/cbdv.200690126. [DOI] [PubMed] [Google Scholar]
- 125.Avilov S.A., Kalinin V.I., Makarieva T.N., Stonik V.A., Kalinovsky A.I., Rashkes Y.W., Milgrom Y.M. Structure of cucumarioside G2, a novel nonholostane glycoside from the sea cucumber Eupentacta fraudatrix. J. Nat. Prod. 1994;57:1166–1171. doi: 10.1021/np50110a007. [DOI] [PubMed] [Google Scholar]
- 126.Avilov S.A., Kalinovsky A.I., Kalinin V.I., Stonik V.A., Riguera R., Jiménez C. Koreoside A, a new nonholostane triterpene glycoside from the sea cucumber Cucumaria koraiensis. J. Nat. Prod. 1997;60:808–810. doi: 10.1021/np970152g. [DOI] [PubMed] [Google Scholar]
- 127.Liu B.-S., Zhang H.-W., Zhang W., Yi Y.H., Li L., Tang H., Wang Z.-L., Yuan W.-H. Triterpene Glycosides-Antifungal Compounds of Sea Cucumber Holotoxins D-I and Preparation Method Thereof. CN101671385 B. Chinese Patent. 2012 May 30;
- 128.Avilov S.A., Drozdova O.A., Kalinin V.I., Kalinovsky A.I., Stonik V.A., Gudimova E.N., Riguera R., Jimenez C. Frondoside C, a new nonholostane triterpene glycoside from the sea cucumber Cucumaria frondosa: Structure and cytotoxicity of its desulfated derivative. Can. J. Chem. 1998;76:137–141. doi: 10.1002/chin.199840209. [DOI] [Google Scholar]
- 129.Kalinin V.I., Kalinovskii A.I., Stonik V.A., Dmitrenok P.S., El’kin Y.N. Structure of psolusoside B-a nonholostane triterpene glycoside of the holothurian genus Psolus. Chem. Nat. Compd. 1989;25:311–317. doi: 10.1007/BF00597709. [DOI] [Google Scholar]
- 130.Avilov S.A., Kalinovskii A.I., Stonik V.A. Two new triterpene glycosides from the holothurian Duasmodactyla kurilensis. Chem. Nat. Compd. 1991;27:188–192. doi: 10.1007/BF00629757. [DOI] [Google Scholar]
- 131.Silchenkoa A.S., Kalinovsky A.I., Avilov S.A., Dmitrenok P.S., Kalinin V.I., Berdyshev D.V., Chingizova E.A., Andryjaschenko P.V., Minin K.V., Stonika V. Fallaxosides B1 and D3, triterpene glycosides with novel skeleton types of aglycones from the sea cucumber Cucumaria fallax. Tetrahedron. 2017;73:2335–2341. doi: 10.1016/j.tet.2017.02.041. [DOI] [Google Scholar]
- 132.Silchenko A.S., Kalinovskya A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Kalinin V.I., Martyyas E.A., Minin K.V. Fallaxosides C1, C2, D1 and D2, unusual oligosulfated triterpene glycosides from the sea cucumber Cucumaria fallax (Cucumariidae, Dendrochirotida, Holothurioidea) and taxonomic status of this animal. Nat. Prod. Commun. 2016;11:939–945. [PubMed] [Google Scholar]
- 133.Silchenko A.S., Kalinovskya A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Kalinin V.I., Chingizova E.A., Minin K.V., Stonik V.A. Structures and biogenesis of fallaxosides D4, D5, D6 and D7, trisulfated non-holostane triterpene glycosides from the sea cucumber Cucumaria fallax. Molecules. 2016;21:939. doi: 10.3390/molecules21070939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Kalinin V.I., Andrijaschenko P.V., Dmitrenok P.S., Chingizova E.A., Ermakova S.P., Malyarenko O.S., Dautova T.N. Nine new triterpene glycosides, magnumosides A1–A4, B1, B2, C1, C2 and C4, from the Vietnamese sea cucumber Neothyonidium (=Massinium) magnum: Structures and activities against tumor cells independently and in synergy with radioactive irradiation. Mar. Drugs. 2017;16:256. doi: 10.3390/md15080256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Yurchenko E.A., Dolmatov I.Y., Dautov S.S., Stonik V.A., Kalinin V.I. Colochiroside E, an unusual non-holostane triterpene sulfated trioside from the sea cucumber Colochirus robustus and evidence of the impossibility of a 7(8)-double bond migration in lanostane derivatives having an 18(16)-lactone. Nat. Prod. Commun. 2016;11:741–746. [PubMed] [Google Scholar]
- 136.Kalinin V.I., Prkofieva N.G., Likhatskaya G.N. Hemolytic activities of triterpene glycosides from the holothurian order Dendrochirotida: Some trends in the evolution of this group of toxins. Toxicon. 1996;34:475–483. doi: 10.1016/0041-0101(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 137.Kumar R., Chaturvedi A.K., Shukla P.K., Lakshmi V. Antifungal activity in triterpene glycosides from the sea cucumber Actinopyga lecanora. Bioorg. Med. Chem. Lett. 2007;17:4387–4391. doi: 10.1016/j.bmcl.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 138.Hua H., Yang-hua Y., Li L., Liu B.S., La M.P., Zhang H.W. Antifungal active triterpene glycosides from sea cucumber Holothuria scabra. Acta Pharm. Sin. 2009;44:620–624. [PubMed] [Google Scholar]
- 139.Aminin D.L., Pislyagin E.A., Menchinskaya E.S., Silchenko A.S., Avilov S.A., Kalinin V.I. Immunomodulatory and anticancer activity of sea cucumber triterpene glycosides. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry. Volume 41. Elsevier Science; Amsterdam, The Netherlands: 2013. pp. 75–94. [Google Scholar]
- 140.Aminin D.L., Agafonova I.G., Kalinin V.I., Silchenko A.S., Avilov S.A., Stonik V.A., Collin P.D., Woodward C. Immunomodulatory properties of frondoside A, a major triterpene glycoside from the North Atlantic commercially harvested sea cucumber Cucumaria frondosa. J. Med. Food. 2008;11:443–453. doi: 10.1089/jmf.2007.0530. [DOI] [PubMed] [Google Scholar]
- 141.Wang J., Han H., Chen X., Yi Y., Sun H. Cytotoxic and apoptosis-inducing activity of triterpene glycosides from Holothuria scabra and Cucumaria frondosa against HepG2 cells. Mar. Drugs. 2014;24:4274–4290. doi: 10.3390/md12084274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Popov A., Kalinovskaia N., Kuznetsova T., Agafonova I., Anisimov M. Role of sterols in the membranotropic activity of triterpene glycosides. Antibiotiki. 1983;28:656–659. [PubMed] [Google Scholar]
- 143.Segal R., Schlosser E. Role of glycosides in the membranlytic, antifungal action of saponins. Arch. Microbiol. 1975;104:147–150. doi: 10.1007/BF00447315. [DOI] [PubMed] [Google Scholar]
- 144.Aminin D.L., Menchinskaya E.S., Pislyagin E.A., Silchenko A.S., Avilov S.A., Kalinin V.I. Sea cucumber triterpene glycosides as anticancer agents. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry. 1st ed. Volume 49. Elsevier Science; Amsterdam, The Netherlands: 2016. pp. 55–105. [Google Scholar]
- 145.Kalinin V.I., Aminin D.L., Avilov S.A., Silchenko A.S., Stonik V.A. Triterpene glycosides from sea cucumbers (Holothurioidae, Echinodermata), biological activities and functions. In: Atta-ur-Rahman, editor. Studies in Natural Product Chemistry (Bioactive Natural Products) Volume 35. Elsevier Science; Amsterdam, The Netherlands: 2008. pp. 135–196. [Google Scholar]