Abstract
Cembrane-type diterpenoids are among the most frequently encountered natural products from the soft corals of the genus Lobophytum. In the course of our investigation to identify anti-inflammatory constituents from a wild-type soft coral Lobophytum crassum, two new cembranoids, lobophyolide A (1) and B (2), along with five known compounds (3–7), were isolated. The structures of these natural products were identified using NMR and MS spectroscopic analyses. Compound 1 was found to possess the first identified α-epoxylactone group among all cembrane-type diterpenoids. The in vitro anti-inflammatory effect of compounds 1–5 was evaluated. The results showed that compounds 1–5 not only reduced IL-12 release, but also attenuated NO production in LPS-activated dendritic cells. Our data indicated that the isolated series of cembrane-type diterpenoids demonstrated interesting structural features and anti-inflammatory activity which could be further developed into therapeutic entities.
Keywords: lobophyolides, α-epoxylactone group, cembranoids, IL-12 production, NO release
1. Introduction
Soft corals, which belong to the order Alcyonacea, are a group of corals missing a calcium carbonate skeleton. They usually grow on the hard surfaces of benthonic animals exposing their soft tissue. Therefore, these corals develop special chemical defense mechanisms and produce a large variety of secondary metabolites against predators, bacteria, and parasites, promoting their own protection and survival [1]. These secondary metabolites were found to possess a plethora of interesting biological activities at low concentrations, suggesting their potential application as therapeutic agents.
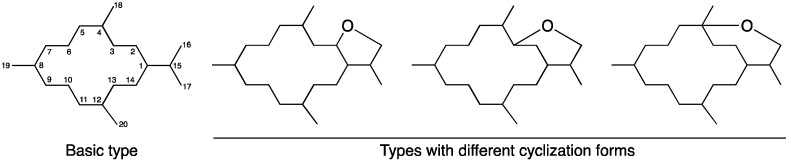
The soft corals of genus Lobophytum have been studied for decades since the first compound, lobophytolide, a cembrane-type diterpenoid, was identified from Lobophytum cristagalli [2]. Further investigation of this genus revealed more than 250 compounds categorized into cembrane-type diterpenoids [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58], other types of diterpenoids [59,60,61,62,63,64,65], lipids [66,67,68,69], steroids [44,69,70,71,72,73,74,75,76,77,78], tocopherols [79], triterpenoids [80], and zoanthamine-type alkaloids [81]. Our group has extensively studied cembrane-type diterpenoids over the past few years and has found that they demonstrate a wide structural diversity [82]. This class of compounds possesses a three-methyl substituted 14-memberd ring system with an isopropyl unit, whereas this isopropyl moiety can be replaced with several types of side rings including a γ-lactone or an unsaturated five-membered ring, a δ-lactone or an unsaturated six-membered ring, and an ɛ-lactone or an unsaturated seven-membered ring (Figure 1). Moreover, the structural changes such as epoxidation, allylic, and isopropyl oxidation or cyclization can be found in almost all unsaturated parts of the ring system [5,8,11].
Figure 1.
Structural characteristics of cembrane-type diterpenoids.
The pharmacological properties of cembranoids were extensively studied with special emphasis on their anti-inflammatory activity. Their effect was evaluated on iNOS (inducible nitric oxide synthase) [10,13], COX-2 (cyclooxygenase-2) [10,19], NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) [19,20], and neutrophil elastase release [15,18], as well as superoxide anion [15] and NO reduction [12,23]. Additionally, these compounds showed potent antiviral [31,49] and anti-bacterial activities [40,43], as well as brine shrimp [45] and fish toxicity [45]. The structural diversity and interesting biological activity of cembrane-type diterpenoids encouraged us to search for other members of this interesting compound from the soft corals of the genus Lobophytum. We isolated seven cembranoids from a wild-type soft coral Lobophytum crassum, including two new and five known compounds. Compound 1 demonstrated a unique α-epoxylactone group which has never been found as a side ring of cembrane-type diterpenoids. Compound 6 showed a 16-acetyl substitution, rendering it the first member of this group with such a structural feature to be isolated from natural sources. The anti-inflammatory activity of compounds 1–5 was evaluated using in vitro models for detecting LPS-induced interleukin 12 (IL-12) release and nitric oxide (NO) production in dendritic cells.
2. Results
2.1. Chemical Identification of Cembranoids
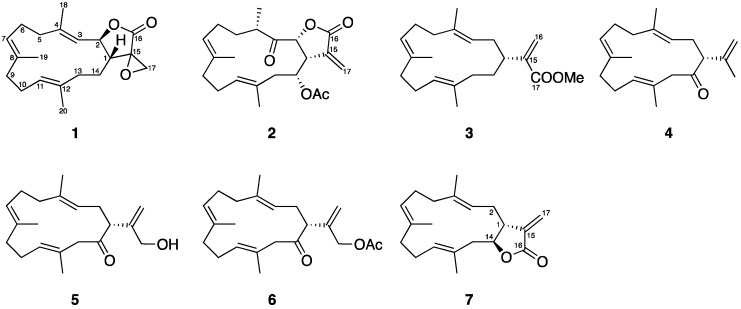
The EtOAc extract of the freeze-dried specimen of the soft coral Lobophytum crassum was chromatographed using normal phase silica gel and the eluting fractions were further separated and purified utilizing reversed phase HPLC to yield 1–7 (Figure 2). The new compounds were named as lobophyolide A (1) and B (2) and the known compounds were identified as 16-methoxycarbonyl-cembrene A (3) [50], sinarone (4) [83], sinulariol D (5) [83], 16-acetyl-sinulariol D (6) [83], and (E,E,E)-6,10,14-trimethy-3-methylene-trans-3α,4,7,8,11,12,15,15α-octahydrocy clotetradeca[β]furan-2(3H)-one (7) [3].
Figure 2.
Cembranoids isolated from the soft coral Lobophytum crassum.
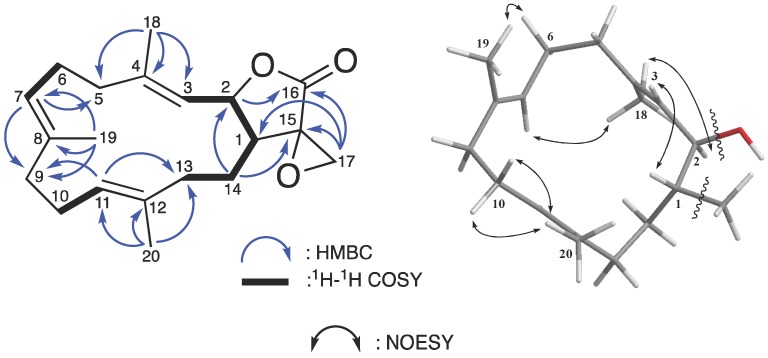
Compound 1 was isolated as a colorless oil. The analysis of its 13C NMR and HRESIMS data deduced its molecular formula, C20H28O3, which suggested seven degrees of unsaturation. The 1H and 13C NMR data (Table 1) demonstrated the signals of twenty-eight protons and twenty carbons sorted by DEPT and HSQC spectra. An ester carbonyl group (δC 173.8) was detected along with three pairs of C=C double bonds [δC/δH 142.5 (C-4), 133.8 (C-8), 131.4 (C-12), 125.2 (C-11)/4.93 (H-11, t, J = 5.0 Hz), 125.1 (C-7)/4.89 (H-7, t, J = 5.0 Hz), and 122.7 (C-3)/5.17 (H-3, d, J = 10.0 Hz)], an oxymethine [δC/δH 79.4 (C-2)/5.06 (H-2, dd, J = 10.0, 4.5 Hz)], an oxymethylene [δC 52.2 (C-17)/2.96 (H-17, d, J = 6.0 Hz), 3.30 (H-17, d, J = 6.0 Hz)], a sp3 quaternary carbon [δC 57.9 (C-15)], and three tertiary methyls [δH 1.52 (H-20, s), 1.59 (H-19, s), and 1.74 (H-18, s)]. Detailed analysis of these NMR data revealed three individual methyl-bearing trisubstituted double bond moieties which suggested the presence of a typical cembranoid skeleton (a three-methyl substituted 14-membered ring system) [84]. This partial structure was further confirmed by the HMBC cross-peaks from H-18 to C-3, C-4, and C-5; from H-19 to C-7, C-8, and C-9; from H-20 to C-11, C-12, and C-13; from H-3 and H-7 to C-5; from H-7 and H-11 to C-9; and from H-14 to C-2 (Table 1) (Figure 3).
Table 1.
1H, 13C, 1H–1H COSY, and HMBC NMR data of 1.
| Position | δH (J in Hz) a | δC (Mult.) b | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 2.26 m | 39.0 (CH) | H-2, H-14 | |
| 2 | 5.06 dd (10.0, 4.5) | 79.4 (CH) | H-1, H-3 | C-16 |
| 3 | 5.17 d (10.0) | 122.7 (CH) | C-5 | |
| 4 | 142.5 (C) | |||
| 5 | 2.22 m | 38.4 (CH2) | ||
| 6 | 2.21 m; 2.30 m | 24.1 (CH2) | H-7 | |
| 7 | 4.89 t (5.0) | 125.1 (CH) | H-6 | C-5, C-9 |
| 8 | 133.8 (C) | |||
| 9 | 1.99 m; 2.12 m | 39.0 (CH2) | ||
| 10 | 2.07 m; 2.20 m | 23.9 (CH2) | H-11 | |
| 11 | 4.93 t (5.0) | 125.2 (CH) | H-10 | C-9, C-13 |
| 12 | 131.4 (C) | |||
| 13 | 2.03 m | 35.1 (CH2) | H-14 | |
| 14 | 1.55 m; 1.77 m | 24.2 (CH2) | H-13, H-1 | C-2, C-15 |
| 15 | 57.9 (C) | |||
| 16 | 173.8 (C) | |||
| 17 | 2.96 d (6.0); 3.30 d (6.0) | 52.2 (CH2) | C-1, C-15, C-16 | |
| 18 | 1.74 s | 16.4 (CH3) | C-3, C-4, C-5 | |
| 19 | 1.59 s | 15.2 (CH3) | C-7, C-8, C-9 | |
| 20 | 1.52 s | 15.9 (CH3) | C-11, C-12, C-13 |
Spectra recorded at a 500 and b 125 MHz in CDCl3.
Figure 3.
Selective 1H–1H COSY, HMBC, and NOESY correlations of 1.
Excluding the four unsaturated groups from the main cembranoid structure, the additional three unsaturated degrees were attributed to an unsaturated ring system supported by the observation of a unique α-epoxylactone chemical shift pattern from the 13C NMR spectra (Table 1) [85]. Moreover, the IR spectrum showed characteristic absorptions at 927 cm−1 and 1785 cm−1, suggesting the presence of an epoxide [86] and a γ-lactone moiety [87]. The HMBC cross-correlations (from H-17 to C-1; and from H-14 to C-2 and C-15) connected these two units and the 1H–1H COSY and HMBC (from H-17 to C-15 and C-16) further revealed that 1 possessed one 1,1-disubstituted epoxide at C-17 (Figure 3), which implied a five-membered side ring cembranoidal diterpene derivative.
Assuming a β-oriented H-1 (δH 2.26 m) in compound 1, the relative configuration was determined by studying NOESY spectra (Figure 3). The presence of the COSY but not NOESY correlations between H-1 and H-2 indicated a trans-fused lactone with α-oriented H-2, which was also supported by the proton vicinal coupling constant of H-1/H-2 (J = 4.5 Hz) [88]. In this type of cembranoid, the E-form C=C double bond systems are usually found at C-3/C-4 and C-7/C-8 and sometimes at C-11/C-12 [23]. The missing NOESY correlations between H-20/H-11, H-19/H-7, and H-18/H-3 implied an E-configuration for these three C=C double bonds [84]. The up-fielded 13C NMR chemical shift of C-20 (δC 15.9) supported such a proposal because it differed from the previously reported Z-form olefinic methyl group (δC 20.1) [26]. According to the above data, the relative configuration of 1 was suggested as 1R*, 2R*, 3E, 7E, and 11E and the compound was named as lobophyolide A.
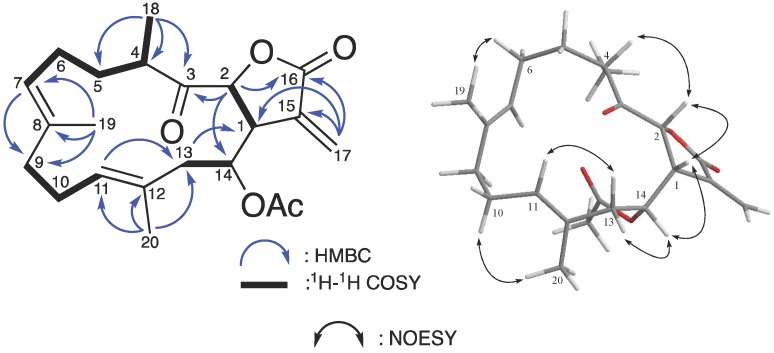
Compound 2, was obtained as colorless oil through the same procedure. The molecular formula (C22H30O5) was inferred from HRESIMS and 13C NMR data (Table 2), indicating eight degrees of unsaturation. The IR spectrum demonstrated the presence of several C=O functional groups based on the absorbed peaks at 1778, 1742, and 1719 cm−1. The 1H, 13C, and HSQC NMR revealed the presence of a ketone carbonyl carbon (δC 209.9), two ester carbonyl carbons (δC 170.0 and δC 169.5), three pairs of C=C double bonds [δC/δH 135.2 (C-8), 135.1 (C-15), 130.3 (C-11)/5.20 (H-11, t, J = 6.3 Hz), 129.3 (C-12), 125.7 (C-7)/4.98 (H-7, m), and 124.8 (C-3)/5.73 (Hb-3, d, J = 2.5 Hz), 6.42 (Ha-3, d, J = 2.5 Hz)], two oxymethines [δC/δH 80.2 (C-2)/4.97 (H-2, d, J = 3.5 Hz), 75.6 (C-14)/5.09 (H-14, dt, J = 11.0, 2.5 Hz)], three tertiary methyls [δH 2.02 (H-14-OAc, s), 1.72 (H-20, s), and 1.49 (H-19, s)], and a secondary methyl at δH 1.14 (H-18, d, J = 7.0 Hz) (Table 2). Detailed analysis of these NMR data suggested that 2 also displayed a type IIa cembranoid skeleton and was closely related to a previously reported compound, (1S,2S,3E,7E,11E)-3,7,11,15-cembratetraen-17,2-olide [80], with a missing C=C double bond moiety, as well as additional groups of a ketone carbonyl (δC 209.9) and an acetyl [δC/δH 170.0, 21.0/2.02 (s)]. According to the HMBC cross-peaks (from H-18 and H-2 to C-3; from H-2 to C-14), two additional oxygen bearing groups were added into the main 14-membered ring system, a ketone group at C-3 and an acetyl group at C-14 (Figure 4).
Table 2.
1H, 13C, 1H–1H COSY, and HMBC NMR data of 2.
| Position | δH (J in Hz) a | δC (Mult.) b | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 3.26 dd (3.0, 2.0) | 41.9 (CH) | H-2 | |
| 2 | 4.97 d (3.5) | 80.2 (CH) | H-1 | C-3, C-14 |
| 3 | 209.9 (C) | |||
| 4 | 2.66 m | 41.8 (CH) | H-5, H-18 | |
| 5 | 1.46 m; 1.95 m | 31.3 (CH2) | H-4, H-6 | |
| 6 | 1.83 m; 2.20 m | 26.0 (CH2) | H-5, H-7 | |
| 7 | 4.98 m | 125.7 (CH) | H-6 | C-9 |
| 8 | 135.2 (C) | |||
| 9 | 2.04 m; 2.18 m | 39.3 (CH2) | H-9, H-11 | |
| 10 | 2.13 m; 2.24 m | 24.4 (CH2) | H-11 | |
| 11 | 5.20 t (6.3) | 130.3 (CH) | H-10 | C-13 |
| 12 | 129.3 (C) | |||
| 13 | 2.31 d (13.0); 2.45 dd (15.0, 11.0) | 41.1 (CH2) | H-14 | C-1 |
| 14 | 5.09 dt (11.0, 2.5) | 75.6 (CH) | H-13 | |
| 15 | 135.1 (C) | |||
| 16 | 169.5 (C) | |||
| 17 | 5.73 d (2.5); 6.42 d (2.5) | 124.8 (CH2) | C-1, C-15, C-16 | |
| 18 | 1.14 d (7.0) | 17.7 (CH3) | C-3, C-4, C-5 | |
| 19 | 1.49 s | 15.0 (CH3) | C-7, C-8, C-9 | |
| 20 | 1.72 s | 16.1 (CH3) | C-11, C-12, C-13 | |
| 14-OAC | 2.02 s | 21.0 (CH3) | C-14-OAc | |
| 170.0 (C) |
Spectra recorded at a 500 and b 125 MHz in CDCl3.
Figure 4.
Selective 1H–1H COSY, HMBC, and NOESY correlations of 2.
The relative configuration of the four chiral centers in compound 2 was suggested based on the analysis of the NOESY data assuming a β-orientation of H-1 [δH 3.26 dd (3.0, 2.0)] (Figure 4). Since H-2 was correlated to H-1 and H-4 in the NOESY spectrum, the β- and α-orientations of H-2 and H-18 were suggested, respectively. The α-orientation of the 14-acetyl group was proposed based on the H-1/H-14 NOESY correlation. Moreover, the observation of the NOESY cross-peaks between H-6/H-19 and H-10/H-20 suggested an E configuration of these two C=C double bond systems (C-7/C-8 and C-11/C-12). Hence, the relative configuration was elucidated as 1R*, 2R*, 4S*, 14R*, 7E, and 11E, and the structure of compound 2 was established and named as lobophyolide B.
2.2. Anti-Inflammatory Activity of the Isolated Cembranoids
The anti-inflammatory activity of compounds 1–5 was evaluated using the in vitro models for the detection of LPS-induced interleukin 12 (IL-12) release and nitric oxide (NO) production in dendritic cells (Table 3). The survival rate of the treated dendritic cells was also tested to determine the cytotoxicity of the isolates. The results showed that compounds 1–3 and 5 (below 50 μg/mL) exhibited a potent inhibitory effect against cytokine IL-12 and NO production with inhibition rates ranging from 86.1% to 96.2%, but also showed considerable cytotoxicity. Compound 1 was found to be the best lead showing potent activity with minimum toxicity compared with the other tested cembranoids. These results suggested that further semisynthetic modifications are necessary for the introduction of a safe and potent anti-inflammatory cembranoid.
Table 3.
Inhibition of LPS-induced IL-12 release and NO production in dendritic cells.
| Compounds | LPS-Induced IL-12 Release | LPS-Induced NO Production | Survivals of DCs |
|---|---|---|---|
| (Inh%) a | (Inh%) a | (Survival%) b | |
| 1 | 93.4 ± 0.5 | 93.5 ± 6.5 | 76.0 ± 0.01 |
| 2 | 93.6 ± 0.0 | 95.9 ± 3.2 | 52.0 ± 0.04 |
| 3 | 86.3 ± 1.1 | 86.1 ± 2.2 | 75.0 ± 0.01 |
| 4 | 77.0 ± 1.5 | 54.9 ± 0.0 | 85.0 ± 0.08 |
| 5 | 92.6 ± 0.6 | 96.2 ± 2.2 | 51.0 ± 0.01 |
| Quercetin c | 86.4 ± 0.0 | 86.1 ± 3.0 | 85.0 ± 5.00 |
a Percentage of inhibition (Inh%) under the concentration of 50 μg/mL; b Survival percentage (Survival%) of DCs under the concentration of 50 μg/mL; c Positive control under the concentration of 50 μM.
3. Material and Methods
3.1. General Experimental Procedure
Optical rotation spectra were recorded on a JASCO P-1010 polarimeter (JASCO, Tokyo, Japan). IR spectra were obtained on a Fourier-transform IR spectrophotometer Varian Digilab FTS 1000 (Varian Inc., Palo Alto, CA, USA). NMR spectra were detected on a Varian Unity INOVA 500 FT-NMR instrument (Varian Inc., Palo Alto, CA, USA). High resolution electrospray ionization mass spectrometry (HRESIMS) analyses were performed on a Bruker APEX II instrument (Bruker Daltonik, Bremen, Germany). Gravity column chromatography was performed with 230–400 mesh silica gel (Merck, Darmstadt, Germany). TLC was performed on 0.25 mm thick precoated Kieselgel 60 F254 (Merck, Darmstadt, Germany) and/or 0.25 mm RP-18 F254S (Merck, Darmstadt, Germany) coated plates and was then visualized by spraying with 10% H2SO4 and heating on a hot plate. A Hitachi L-7100 pump, Rheodyne 7725 injection port, and a Hitachi L-2455 Photodiode Array Detector (Hitachi, Tokyo, Japan), along with a preparative normal phase column Supelco Ascentis® Si Cat #: 581514-U (25 cm × 10 mm, C18, 5 μm) and a reverse phase column Supelco Ascentis® C-18 Cat #: 581343-U, were used for RP-HPLC. All methods were carried out in accordance with the relevant guidelines and regulations.
3.2. Animal Material
Specimens of wild soft coral of Lobophytum crassum were collected by scuba diving at a depth of around 8 m off the coast of Pingtung, Taiwan (specimen No. 2016-11-14-SP). A voucher specimen was deposited in the National Museum of Marine Biology and Aquarium, Pingtung, Taiwan.
3.3. Extraction and Isolation
The wild soft coral of Lobophytum crassum (830 g, wet weight) was freeze-dried, and the resulting dry material (290 g) was then extracted exhaustively with EtOAc. The EtOAc extract was evaporated under reduced pressure to afford a residue (13.6 g). The residue was subjected to column chromatography on silica gel, using the mixtures of n-hexane, EtOAc, and acetone with increasing polarity (n-hexane:EtOAc:acetone, 50:1:0, 30:1:0, 20:1:0, 10:1:0, 8:1:0, 5:1:0, 3:1:0, 1:1:0, 1:2:0, 0:1:0, and 0:0:1) to yield 11 fractions. Fr-6 (3.7 g) was fractioned with LH-20 eluting with 100% acetone to afford eight subfractions (6-L1–6-L8). Fr-6-L5 and Fr-6-L6 were combined (540 mg) and then separated by normal-phase HPLC (n-hexane:acetone 3:1) to gain 19 more subfractions (6-L5-1–6-L5-19). Subfraction 6-L5-3 (2.7 mg) was purified by reversed-phase HPLC (90% MeOH) to afford 3 (0.5 mg) and 4 (0.7 mg). Subfractions 6-L5-4–6-L5-6 were combined (40.9 mg) and purified by reversed-phase HPLC (90% MeOH) to afford 1 (0.8 mg) and 7 (8.5 mg). Subfraction 6-L5-9 (34.3 mg) was also purified by reversed-phase HPLC (70% acetonitrile) to afford 2 (3.4 mg). Subfraction 6-L5-10 (44.5 mg) was purified by normal-phase HPLC (hexane:EtOAc:dichloromethane 8:1:1) to afford 5 (4.2 mg). Compound 6 (1.5 mg) was obtained from subfractions 6-L5-11–6-L5-13 (35.0 mg) using normal-phase HPLC (n-hexane:EtOAc 7:1).
Lobophyolide A (1): colorless oil; = −55.2 (c 0.1000, CHCl3); IR (neat, CHCl3) νmax 2921, 2852, 1785, 1441, 1385, 1314, 1295 cm−1; 13C (CDCl3, 125 MHz) and 1H (CDCl3, 500 MHz) NMR data, see Table 1; ESIMS m/z 339 [M + Na]+; HRESIMS m/z 339.19301 [M + Na]+ (calcd for C20H28O3Na, 339.19307).
Lobophyolide B (2): colorless oil; = +1.2 (c 0.0750, CHCl3); IR (neat, CHCl3) νmax 2923, 2854, 1778, 1742, 1719, 1437, 1374, 1271, 1228 cm−1; 13C (CDCl3, 125 MHz) and 1H (CDCl3, 500 MHz) NMR data, see Table 2; ESIMS m/z 397 [M + Na]+; HRESIMS m/z 397.19837 [M + Na]+ (calcd for C22H30O5Na, 327.22945).
16-Acetyl-sinulariol D (6): colorless oil; = +7.5 (c 0.0375, CHCl3); IR (neat, CHCl3) νmax 2923, 1778, 1651, 1436, 1373, 1227 cm−1; 13C (CDCl3, 125 MHz) and 1H (CDCl3, 500 MHz) NMR data, see supporting information; ESIMS m/z 353 [M + Na]+; HRESIMS m/z 353.24514 [M + Na]+ (calcd for C22H34O2Na, 353.24510).
3.4. Preparation of Mouse Bone Marrow-Derived Dendritic Cells (DCs)
C57BL/6 mice, which were purchased from Taiwan, were used in this study. All animals were housed in a specific pathogen-free facility in the Division of Laboratory Animals, China Medical University. All mice were maintained and handled according to standard protocols and the protocols were approved (103-156-N, 27 December 2012) by the Institutional Animal Care and Use Committee, China Medical University. The bones of mice were collected and bone marrow-derived dendritic cells (DCs) were prepared as previously described [89]. In brief, bone marrow cells were isolated from femurs and tibias and seeded on 24-well culture plates (Corning, New York, NY, USA) in 1 mL/well complete RPMI 1640 medium (Gibco, Waltham, MA, USA), and 10 ng/mL recombinant mouse GMCSF (Peprotech, Rocky Hill, NJ, USA). At day three, fresh medium (1 mL/well) containing 10 ng/mL GM-CSF was added. At day five, half of the cell-free supernatant was exchanged and fresh medium containing 10 ng/mL GMCSF was added. The seven-day-cultured DCs (>80% CD11c+ cells) were used for all experiments. In the T cell activation experiment, DCs were purified by an EasySep Positive Selection Kit (StemCell Technology, Vancouver, BC, Canada) according to the manufacturer’s instructions. The purity of CD11c+ cells was over 95%. The OT-II TCR-transgenic mice were provided by Dr. Clifford Lowell (UCSF, San Francisco, CA, USA). All animals were kept in a specific pathogen-free facility (NHRI, Miaoli, Taiwan) and handled according to protocols approved by the Institutional Animal Care and Use Committee of NHRI.
3.5. Measurement of Cytokines Production by DCs
Cytokine production was measured by an enzyme-linked immunosorbent assay (ELISA) as described previously [89]. The DCs were treated with lipopolysaccharide (LPS, 100 ng/mL) from Escherichia coli 055:B5 (Sigma, St. Louis, MO, USA) or LPS+testing cembranoidal compounds for 24 h for IL-12. The production of IL-12 was measured at 570 nm using the ELISA kit (eBioscience, San Diego, CA, USA).
3.6. Measurement of Nitric Oxide (NO) Production by DCs
The supernatants were collected from DCs (1 × 106 /mL) propagated in the presence or absence of testing cembranoidal compounds for 1 h. The cells were then stimulated with 100 ng/mL LPS. A total of 100 µL of cell culture supernatant was reacted with 100 µL of Griess reagent (1:1 mixture of 0.2% N-(1-naphthyl) ethylenediamine dihydrochloride in water and 2% sulfanilamide) in a 96-well plate and incubated at room temperature for 10 min. The absorbance at 540 nm was recorded using sandwich ELISA assays, according to the manufacturer’s specifications (PeproTech) [89]. Fresh medium was used as the blank. The results are expressed as the percentage of inhibition calculated relative to the cells treated with vehicle and LPS.
3.7. Cell Viability Assay
The cytotoxicity of the tested cembranoids (Sigma, St. Louis, MO, USA) was measured by Cell Counting Kit 8 (CCK-8 Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. In brief, 100 μL of 5 × 103 DCs was plated into each well of a 96-well plate and incubated overnight, and cells were then treated with various concentrations of naringenin (dissolved in DMSO and made a stock solution at 400 mM; Sigma-Aldrich, St. Louis, MO, USA) for an additional 24 h. Subsequently, 10 μL of the CCK-8 solution was added to each well and incubated at 37 °C for 3 h. The absorbance was recorded on a microplate reader (Tecan, Durham, NC, USA) at the wavelength of 450 nm.
3.8. Statistics
The results were expressed as the mean ± standard deviation (SD). Comparison in each experiment was performed using an unpaired Student’s t-test and a p value of less than 0.05 was considered to be statistically significant.
4. Conclusions
A series of cembrane-type diterpenoids were isolated from the ethyl extract of a wild-type soft coral Lobophytum crassum. Two new cembranoids, lobophyolide A (1) and B (2), as well as five known compounds (3–7), were identified. Compound 1 demonstrated a unique structural feature among all cembrane-type diterpenoids with an α-epoxylactone group, which might be derived from the terminal double bond moiety of (1S,14S)-(E,E,E)-15-methylene-3,7,11-trimethyl-17-oxabicyclo<12.3.01,14>heptadeca-2,6,10-trien-16-one [90,91]. The anti-inflammatory effect of compounds 1–5 was examined. Among all tested compounds, 1 showed the highest therapeutic index with less toxicity and potent activity in inhibiting IL-12 release and attenuating NO production from LPS-activated dendritic cells. These results suggest the potential application of this class of compounds as anti-inflammatory agents, especially with the recent advances in culture protocols for this soft coral developed by our group, as well as the success in the total synthesis of cembrane-type diterpenoids (7-acetylsinumaximol B) [92], which could supply necessary quantities of active cembrane-type diterpenoids for industrial development.
Acknowledgments
This research was supported by grants from the Ministry of Science and Technology (MOST 105-2320-B-291-001-MY3), Taiwan, awarded to J.-H. Su.
Author Contributions
K.-H.L. and J.-H.S. conceived and designed the experiments; W.-J.Y., Z.-J.L. and J.-H.S. performed the sample collections, extraction, isolation, and structures determination; the pharmacological experiments were carried out by C.-C.L.; J.-H.S. contributed reagents and analysis tools; K.-H.L. and M.E. participated in data interpretation, wrote the manuscript and revised the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Coll J.C., La Barre S., Sammarco P.W., Williams W.T., Bakus G.J. Chemical defences in soft corals (coelenterata: Octocorallia) of the great barrier reef: A study of comparative toxicities. Mar. Ecol. Prog. Ser. 1982;8:271–278. doi: 10.3354/meps008271. [DOI] [Google Scholar]
- 2.Tursch B., Braekman J.C., Daloze D., Hérin M., Karlsson R. Chemical studies of marine invertebrates. X. Lobophytolide, a new cembranolide diterpene from the soft coral lobophytum cristagalli (coelenterata, octocorallia, alcyonacea) Tetrahedron Lett. 1974;15:3769–3772. doi: 10.1016/S0040-4039(01)92004-0. [DOI] [Google Scholar]
- 3.Ahond A., Bowden B., Coll J., Fourneron J., Mitchell S. Further cembranolide diterpenes from lobophytum crassospiculatum and a correction of a previous stereochemical assignment. Aust. J. Chem. 1979;32:1273–1280. doi: 10.1071/CH9791273. [DOI] [Google Scholar]
- 4.Bowden B., Brittle J., Coll J., Liyanage N., Mitchell S., Stokie G. A new cembranolide diterpene from the soft coral lobophytum crassum. Tetrahedron Lett. 1977;18:3661–3662. doi: 10.1016/S0040-4039(01)83320-7. [DOI] [Google Scholar]
- 5.Kashman Y., Carmely S., Groweiss A. Further cembranoid derivatives from the red sea soft corals alcyonium flaccidum and lobophytum crassum. J. Org. Chem. 1981;46:3592–3596. doi: 10.1021/jo00331a003. [DOI] [Google Scholar]
- 6.Duh C.-Y., Wang S.-K., Huang B.-T., Dai C.-F. Cytotoxic cembrenolide diterpenes from the formosan soft coral lobophytum crassum. J. Nat. Prod. 2000;63:884–885. doi: 10.1021/np990620h. [DOI] [PubMed] [Google Scholar]
- 7.Yin S.-W., Shi Y.-P., Li X.-M., Wang B.-G. A novel hydroperoxyl substituted cembranolide diterpene from marine soft coral lobophytum crassum. Chin. Chem. Lett. 2005;16:1489–1491. [Google Scholar]
- 8.Yin S.W., Shi Y.P., Li X.M., Wang B.G. A new cembranoid diterpene and other related metabolites from the south china sea soft coral lobophytum crassum. Helv. Chim. Acta. 2006;89:567–572. doi: 10.1002/hlca.200690060. [DOI] [Google Scholar]
- 9.Zhang W., Krohn K., Ding J., Miao Z.-H., Zhou X.-H., Chen S.-H., Pescitelli G., Salvadori P., Kurtan T., Guo Y.-W. Structural and stereochemical studies of α-methylene-γ-lactone-bearing cembrane diterpenoids from a south china sea soft coral lobophytum crassm. J. Nat. Prod. 2008;71:961–966. doi: 10.1021/np800081p. [DOI] [PubMed] [Google Scholar]
- 10.Chao C.-H., Wen Z.-H., Wu Y.-C., Yeh H.-C., Sheu J.-H. Cytotoxic and anti-inflammatory cembranoids from the soft coral lobophytum crassum. J. Nat. Prod. 2008;71:1819–1824. doi: 10.1021/np8004584. [DOI] [PubMed] [Google Scholar]
- 11.Lin S.-T., Wang S.-K., Cheng S.-Y., Duh C.-Y. Lobocrasol, a new diterpenoid from the soft coral lobophytum crassum. Org. Lett. 2009;11:3012. doi: 10.1021/ol901070e. [DOI] [PubMed] [Google Scholar]
- 12.Wanzola M., Furuta T., Kohno Y., Fukumitsu S., Yasukochi S., Watari K., Tanaka C., Higuchi R., Miyamoto T. Four new cembrane diterpenes isolated from an okinawan soft coral lobophytum crassum with inhibitory effects on nitric oxide production. Chem. Pharm. Bull. 2010;58:1203–1209. doi: 10.1248/cpb.58.1203. [DOI] [PubMed] [Google Scholar]
- 13.Liao Z.-J., Su H.-J., Shyue Y.-C., Wen Z.-H., Sheu J.-H., Su J.-H. Two new cembranoids from the soft coral lobophytum crassum. Bull. Chem. Soc. Jpn. 2011;84:653–655. doi: 10.1246/bcsj.20100324. [DOI] [Google Scholar]
- 14.Tseng Y.-J., Wen Z.-H., Hsu C.-H., Dai C.-F., Sheu J.-H. Bioactive cembranoids from the dongsha atoll soft coral lobophytum crassum. Bull. Chem. Soc. Jpn. 2011;84:1102–1106. doi: 10.1246/bcsj.20110096. [DOI] [Google Scholar]
- 15.Kao C.-Y., Su J.-H., Lu M.-C., Hwang T.-L., Wang W.-H., Chen J.-J., Sheu J.-H., Kuo Y.-H., Weng C.-F., Fang L.-S. Lobocrassins a–e: New cembrane-type diterpenoids from the soft coral lobophytum crassum. Mar. Drugs. 2011;9:1319–1331. doi: 10.3390/md9081319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee N.-L., Su J.-H. Tetrahydrofuran cembranoids from the cultured soft coral lobophytum crassum. Mar. Drugs. 2011;9:2526–2536. doi: 10.3390/md9122526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin S.-T., Wang S.-K., Duh C.-Y. Cembranoids from the dongsha atoll soft coral lobophytum crassum. Mar. Drugs. 2011;9:2705–2716. doi: 10.3390/md9122705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C.-H., Kao C.-Y., Kao S.-Y., Chang C.-H., Su J.-H., Hwang T.-L., Kuo Y.-H., Wen Z.-H., Sung P.-J. Terpenoids from the octocorals menella sp.(plexauridae) and lobophytum crassum (alcyonacea) Mar. Drugs. 2012;10:427–438. doi: 10.3390/md10020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thao N.P., Luyen B.T.T., Ngan N.T.T., Song S.B., Cuong N.X., Nam N.H., Van Kiem P., Kim Y.H., Van Minh C. New anti-inflammatory cembranoid diterpenoids from the vietnamese soft coral lobophytum crassum. Bioorg. Med. Chem. Lett. 2014;24:228–232. doi: 10.1016/j.bmcl.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Cuong N.X., Thao N.P., Luyen B.T.T., Ngan N.T.T., Thuy D.T.T., Song S.B., Nam N.H., Van Kiem P., Kim Y.H., Van Minh C. Cembranoid diterpenes from the soft coral lobophytum crassum and their anti-inflammatory activities. Chem. Pharm. Bull. 2014;62:203–208. doi: 10.1248/cpb.c13-00805. [DOI] [PubMed] [Google Scholar]
- 21.Cheng S.-Y., Wang S.-K., Duh C.-Y. Secocrassumol, a seco-cembranoid from the dongsha atoll soft coral lobophytum crassum. Mar. Drugs. 2014;12:6028–6037. doi: 10.3390/md12126028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Minh C., Xuan Cuong N., Phuong Thao N., Thu Thuy D.T., Ngoc N.T., Van Thanh N., Hoai Nam N., Young Ho K., Van Kiem P. Cembranoid constituents from lobophytum crassum. Vietnam J. Chem. 2015;53:5–8. [Google Scholar]
- 23.Zhao M., Cheng S., Yuan W., Xi Y., Li X., Dong J., Huang K., Gustafson K.R., Yan P. Cembranoids from a chinese collection of the soft coral lobophytum crassum. Mar. Drugs. 2016;14:111. doi: 10.3390/md14060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed T.A., Elshamy A.I., Hussien T.A., Su J.-H., Sheu J.-H., Hegazy M.E.F. Lobophylins f-h: Three new cembrene diterpenoids from soft coral lobophytum crassum. J. Asian Nat. Prod. Res. 2017;19:201–207. doi: 10.1080/10286020.2016.1196673. [DOI] [PubMed] [Google Scholar]
- 25.Bowden B.F., Coll J., De Costa M., Mackay M., Mahendran M., De Silva E., Willis R. The structure determination of a new cembranolide diterpene from the soft coral lobophytum cristigalli (coelenterata, octocorallia, alcyonacea) Aust. J. Chem. 1984;37:545–552. doi: 10.1071/CH9840545. [DOI] [Google Scholar]
- 26.Uchio Y., Eguchi S., Kuramoto J., Nakayama M., Hase T. Denticulatolide, an ichthyotoxic peroxide-containing cembranolide from the soft coral lobophytum denticulatum. Tetrahedron Lett. 1985;26:4487–4490. doi: 10.1016/S0040-4039(00)88937-6. [DOI] [Google Scholar]
- 27.Uchio Y., Eguchi S., Fukazawa Y., Kodama M. 7-epidenticulatolide, a new cembranolide with a cyclic peroxide function from the soft coral lobophytum denticulatum. Bull. Chem. Soc. Jpn. 1992;65:1182–1184. doi: 10.1246/bcsj.65.1182. [DOI] [Google Scholar]
- 28.Cheng S.-Y., Wen Z.-H., Chiou S.-F., Hsu C.-H., Wang S.-K., Dai C.-F., Chiang M.Y., Duh C.-Y. Durumolides a-e, anti-inflammatory and antibacterial cembranolides from the soft coral lobophytum durum. Tetrahedron. 2008;64:9698–9704. doi: 10.1016/j.tet.2008.07.104. [DOI] [Google Scholar]
- 29.Cheng S.-Y., Wen Z.-H., Wang S.-K., Chiou S.-F., Hsu C.-H., Dai C.-F., Duh C.-Y. Anti-inflammatory cembranolides from the soft coral lobophytum durum. Bioorg. Med. Chem. 2009;17:3763–3769. doi: 10.1016/j.bmc.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 30.Cheng S.-Y., Wen Z.-H., Wang S.-K., Chiou S.-F., Hsu C.-H., Dai C.-F., Chiang M.Y., Duh C.-Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral lobophytum durum. J. Nat. Prod. 2008;72:152–155. doi: 10.1021/np800686k. [DOI] [PubMed] [Google Scholar]
- 31.Cheng S.-Y., Chen P.-W., Chen H.-P., Wang S.-K., Duh C.-Y. New cembranolides from the dongsha atoll soft coral lobophytum durum. Mar. Drugs. 2011;9:1307–1318. doi: 10.3390/md9081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchio Y., Toyota J., Nozaki H., Nakayama M., Nishizono Y., Hase T. Lobohedleolide and (7z)-lobohedleolide, new cembranolides from the soft coral lobophytum hedleyi whitelegge. Tetrahedron Lett. 1981;22:4089–4092. doi: 10.1016/S0040-4039(01)82073-6. [DOI] [Google Scholar]
- 33.Quang T.H., Ha T.T., Van Minh C., Van Kiem P., Huong H.T., Ngan N.T.T., Nhiem N.X., Tung N.H., Tai B.H., Thuy D.T.T. Cytotoxic and anti-inflammatory cembranoids from the vietnamese soft coral lobophytum laevigatum. Bioorg. Med. Chem. 2011;19:2625–2632. doi: 10.1016/j.bmc.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Coll J., Mitchell S.J., Stokie G.J. A novel cembrenoid diterpene from lobophytum michaelae. Aust. J. Chem. 1977;30:1859–1863. doi: 10.1071/CH9771859. [DOI] [Google Scholar]
- 35.Wang S.-K., Duh C.-Y., Wu Y.-C., Wang Y., Cheng M.-C., Soong K., Fang L.-S. Studies on formosan soft corals. Ii: Cytotoxic cembranolides from the soft coral lobphytum michaelae. J. Nat. Prod. 1992;55:1430–1435. doi: 10.1021/np50088a007. [DOI] [PubMed] [Google Scholar]
- 36.Wang L.-T., Wang S.-K., Soong K., Duh C.-Y. New cytotoxic cembranolides from the soft coral lobophytum michaelae. Chem. Pharm. Bull. 2007;55:766–770. doi: 10.1248/cpb.55.766. [DOI] [PubMed] [Google Scholar]
- 37.Wang S.-K., Duh C.-Y. New cytotoxic cembranolides from the soft coral lobophytum michaelae. Mar. Drugs. 2012;10:306–318. doi: 10.3390/md10020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada Y., Suzuki S., Iguchi K., Kikuchi H., Tsukitani Y., Horiai H. Two new cembranolides from the soft coral lobophytum pauciflorum (ehrenberg) Chem. Pharm. Bull. 1980;28:2035–2038. doi: 10.1248/cpb.28.2035. [DOI] [Google Scholar]
- 39.Yamada Y., Suzuki S., Iguchi K., Kikuchi H., Horiai H., Shibayama F. The stereochemistry of 13-membered carbocyclic cembranolide diterpenes from the soft coral lobophytum pauciflorum (ehrenberg) Tetrahedron Lett. 1980;21:3911–3914. doi: 10.1016/0040-4039(80)80214-0. [DOI] [Google Scholar]
- 40.Yan P., Deng Z., Ofwegen L.V., Proksch P., Lin W. Lobophytones o-t, new biscembranoids and cembranoid from soft coral lobophytum pauciflorum. Mar. Drugs. 2010;8:2837–2848. doi: 10.3390/md8112848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan P., Deng Z., Van Ofwegen L., Proksch P., Lin W. Lobophytones h-n, biscembranoids from the chinese soft coral lobophytum pauciflorum. Chem. Pharm. Bull. 2010;58:1591–1595. doi: 10.1248/cpb.58.1591. [DOI] [PubMed] [Google Scholar]
- 42.Yan P., Lv Y., van Ofwegen L., Proksch P., Lin W. Lobophytones a-g, new isobiscembranoids from the soft coral lobophytum pauciflorum. Org. Lett. 2010;12:2484–2487. doi: 10.1021/ol100567d. [DOI] [PubMed] [Google Scholar]
- 43.Yan P., Deng Z., Van Ofwegen L., Proksch P., Lin W. Lobophytones u–z1, biscembranoids from the chinese soft coral lobophytum pauciflorum. Chem. Biodivers. 2011;8:1724–1734. doi: 10.1002/cbdv.201000244. [DOI] [PubMed] [Google Scholar]
- 44.Lu Y., Lin Y.-C., Wen Z.-H., Su J.-H., Sung P.-J., Hsu C.-H., Kuo Y.-H., Chiang M.Y., Dai C.-F., Sheu J.-H. Steroid and cembranoids from the dongsha atoll soft coral lobophytum sarcophytoides. Tetrahedron. 2010;66:7129–7135. doi: 10.1016/j.tet.2010.06.094. [DOI] [Google Scholar]
- 45.Yamada K., Ryu K., Miyamoto T., Higuchi R. Three new cembrane-type diterpenoids from the soft coral lobophytum schoedei. J. Nat. Prod. 1997;60:798–801. doi: 10.1021/np960728m. [DOI] [Google Scholar]
- 46.Bowden B., Coll J., Mitchell S., Stokie G. Two new diterpenes from an unknown species of soft coral (genus lobophytum) Aust. J. Chem. 1978;31:1303–1312. doi: 10.1071/CH9781303. [DOI] [Google Scholar]
- 47.Bowden B., Coll J., Tapiolas D. New cembranoid diterpenes from a lobophytum species. Aust. J. Chem. 1983;36:2289–2295. doi: 10.1071/CH9832289. [DOI] [Google Scholar]
- 48.Subrahmanyam C., Rao C.V., Anjaneyulu V., Satyanarayana P., Rao P.S., Ward R.S., Pelter A. New diterpenes from a new species of lobophytum soft coral of the south andaman coast. Tetrahedron. 1992;48:3111–3120. doi: 10.1016/S0040-4020(01)92252-5. [DOI] [Google Scholar]
- 49.Rashid M.A., Gustafson K.R., Boyd M.R. Hiv-inhibitory cembrane derivatives from a philippines collection of the soft coral lobophytum species. J. Nat. Prod. 2000;63:531–533. doi: 10.1021/np990372p. [DOI] [PubMed] [Google Scholar]
- 50.Chen S.H., Huang H., Guo Y.W. Four new cembrane diterpenes from the hainan soft coral lobophytum sp. Chin. J. Chem. 2008;26:2223–2227. doi: 10.1002/cjoc.200890395. [DOI] [Google Scholar]
- 51.Chen S.H., Guo Y.W., Huang H., Cimino G. Six new cembranolides from the hainan soft coral lobophytum sp. Helv. Chim. Acta. 2008;91:873–880. doi: 10.1002/hlca.200890091. [DOI] [Google Scholar]
- 52.Chen S.-H., Huang H., Guo Y.-W. A new diterpenoid from the south china sea soft coral lobophytum sp. J. Asian Nat. Prod. Res. 2008;10:965–969. doi: 10.1080/10286020802217655. [DOI] [PubMed] [Google Scholar]
- 53.Fattorusso E., Romano A., Taglialatela-Scafati O., Irace C., Maffettone C., Bavestrello G., Cerrano C. Oxygenated cembranoids of the decaryiol type from the indonesian soft coral lobophytum sp. Tetrahedron. 2009;65:2898–2904. doi: 10.1016/j.tet.2009.02.008. [DOI] [Google Scholar]
- 54.Bonnard I., Jhaumeer-Laulloo S.B., Bontemps N., Banaigs B., Aknin M. New lobane and cembrane diterpenes from two comorian soft corals. Mar. Drugs. 2010;8:359–372. doi: 10.3390/md8020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hegazy M.E.F., Su J.-H., Sung P.-J., Sheu J.-H. Cembranoids with 3, 14-ether linkage and a secocembrane with bistetrahydrofuran from the dongsha atoll soft coral lobophytum sp. Mar. Drugs. 2011;9:1243–1253. doi: 10.3390/md9071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao M., Li X., Zhao F., Cheng S., Xiang Z., Dong J., Huang K., Yan P. Four new 7, 8-epoxycembranoids from a chinese soft coral lobophytum sp. Chem. Pharm. Bull. 2013;61:1323–1328. doi: 10.1248/cpb.c13-00612. [DOI] [PubMed] [Google Scholar]
- 57.Zhao M., Yin J., Jiang W., Ma M., Lei X., Xiang Z., Dong J., Huang K., Yan P. Cytotoxic and antibacterial cembranoids from a south china sea soft coral, lobophytum sp. Mar. Drugs. 2013;11:1162–1172. doi: 10.3390/md11041162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roy P.K., Ashimine R., Miyazato H., Taira J., Ueda K. New casbane and cembrane diterpenoids from an okinawan soft coral, lobophytum sp. Molecules. 2016;21:679. doi: 10.3390/molecules21050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chau V.M., Phan V.K., Nguyen X., Nguyen X.C., Nguyen P.T., Nguyen H.N., Hoang le T.A., Do C.T., Thuy D.T., Kang H.K., et al. Cytotoxic and antioxidant activities of diterpenes and sterols from the vietnamese soft coral lobophytum compactum. Bioorg. Med. Chem. Lett. 2011;21:2155–2159. doi: 10.1016/j.bmcl.2011.01.072. [DOI] [PubMed] [Google Scholar]
- 60.Matthée G.F., König G.M., Wright A.D. Three new diterpenes from the marine soft coral lobophytum crassum. J. Nat. Prod. 1998;61:237–240. doi: 10.1021/np970458n. [DOI] [PubMed] [Google Scholar]
- 61.Li L., Sheng L., Wang C.-Y., Zhou Y.-B., Huang H., Li X.-B., Li J., Mollo E., Gavagnin M., Guo Y.-W. Diterpenes from the hainan soft coral lobophytum cristatum tixier-durivault. J. Nat. Prod. 2011;74:2089–2094. doi: 10.1021/np2003325. [DOI] [PubMed] [Google Scholar]
- 62.Bowden B., Coll J., Liyanage N., Mitchell S., Stokie G., Altena I. A novel bicyclic diterpene from lobophytum hedleyi. Aust. J. Chem. 1978;31:163–170. doi: 10.1071/CH9780163. [DOI] [Google Scholar]
- 63.Edrada R.A., Proksch P., Wray V., Witte L., Van Ofwegen L. Four new bioactive lobane diterpenes of the soft coral lobophytum pauciflorum from mindoro, philippines. J. Nat. Prod. 1998;61:358–361. doi: 10.1021/np970276t. [DOI] [PubMed] [Google Scholar]
- 64.Govindam S.V., Yoshioka Y., Kanamoto A., Fujiwara T., Okamoto T., Ojika M. Cyclolobatriene, a novel prenylated germacrene diterpene, from the soft coral lobophytum pauciflorum. Bioorg. Med. Chem. 2012;20:687–692. doi: 10.1016/j.bmc.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 65.Lakshmana Raju B., Subbaraju G.V., Bheemasankara Rao C., Trimurtulu G. Two new oxygenated lobanes from a soft coral of lobophytum species of the andaman and nicobar coasts. J. Nat. Prod. 1993;56:961–966. doi: 10.1021/np50096a026. [DOI] [Google Scholar]
- 66.Carmely S., Kashman Y., Loya Y., Benayahu Y. New prostaglandin (pgf) derivatives from the soft coral lobophyton depressum. Tetrahedron Lett. 1980;21:875–878. doi: 10.1016/S0040-4039(00)71531-0. [DOI] [Google Scholar]
- 67.Chao C.-H., Huang H.-C., Wu Y.-C., Lu C.-K., Dai C.-F., Sheu J.-H. Glycolipids from the formosan soft coral lobophytum crassum. Chem. Pharm. Bull. 2007;55:1720–1723. doi: 10.1248/cpb.55.1720. [DOI] [PubMed] [Google Scholar]
- 68.Radhika P., Rao V.L., Laatsch H. Chemical constituents of a marine soft coral of the genus lobophytum. Chem. Pharm. Bull. 2004;52:1345–1348. doi: 10.1248/cpb.52.1345. [DOI] [PubMed] [Google Scholar]
- 69.Muralidhar P., Kumar M.M., Krishna N., Rao C.B., Rao D.V. New sphingolipids and a sterol from a lobophytum species of the indian ocean. Chem. Pharm. Bull. 2005;53:168–171. doi: 10.1248/cpb.53.168. [DOI] [PubMed] [Google Scholar]
- 70.Venkateswarlu Y., Rao M.R., Ramesh P. A new polyhydroxy sterol from the soft coral lobophytum crassum. J. Nat. Prod. 1997;60:1301–1302. doi: 10.1021/np970176n. [DOI] [Google Scholar]
- 71.Rama Rao M., Venkatesham U., Rami Reddy M.V., Venkateswarlu Y. An unusual novel c29 steroid from the soft coral lobophytum crassum. J. Nat. Prod. 1999;62:785–786. doi: 10.1021/np980500u. [DOI] [PubMed] [Google Scholar]
- 72.Quang T.H., Ngan N.T.T., Van Kiem P., Van Minh C., Kim Y.H. A new sterol from the soft coral lobophytum crassum. Notes. 2013;34:249. [Google Scholar]
- 73.Carmely S., Kashman Y. Isolation and structure elucidation of lobophytosterol, depresosterol and three other closely related sterols: Five new c28 polyoxygenated sterols from the red sea soft coral lobophytum depressum. Tetrahedron. 1981;37:2397–2403. doi: 10.1016/S0040-4020(01)88896-7. [DOI] [Google Scholar]
- 74.Hegazy M.-E.F., Mohamed T.A., Elshamy A.I., Hassanien A.A., Abdel-Azim N.S., Shreadah M.A., Abdelgawad I.I., Elkady E.M., Paré P.W. A new steroid from the red sea soft coral lobophytum lobophytum. Nat. Prod. Res. 2016;30:340–344. doi: 10.1080/14786419.2015.1046871. [DOI] [PubMed] [Google Scholar]
- 75.Tursch B., Hootele C., Kaisin M., Losman D., Karlsson R. Structure and absolute configuration of lobosterol, a novel polyoxygenated sterol from the alcyonacean lobophytum pauciflorum. Steroids. 1976;27:137–142. doi: 10.1016/0039-128X(76)90075-1. [DOI] [PubMed] [Google Scholar]
- 76.Yamada Y., Suzuki S., Iguchi K., Kikuchi H., Tsukitani Y., Horiai H., Nakanishi H. New polyhydroxylated sterols from the soft coral lobophytum pauciflorum (ehrenberg) Chem. Pharm. Bull. 1980;28:473–478. doi: 10.1248/cpb.28.473. [DOI] [Google Scholar]
- 77.Lu Q., Faulkner D.J. Two 11α-acetoxysterols from the palauan soft coral lobophytum cf. Pauciflorum. Nat. Prod. Lett. 1997;10:231–237. doi: 10.1080/10575639708041200. [DOI] [Google Scholar]
- 78.Morris L.A., Christie E.M., Jaspars M., van Ofwegen L.P. A bioactive secosterol with an unusual a and b ring oxygenation pattern isolated from an indonesian soft coral lobophytum sp. J. Nat. Prod. 1998;61:538–541. doi: 10.1021/np9705118. [DOI] [PubMed] [Google Scholar]
- 79.Cheng S.-Y., Lin S.-T., Wang S.-K., Duh C.-Y. Α-tocopherols from the formosan soft coral lobophytum crassum. Bull. Chem. Soc. Jpn. 2011;84:783–787. doi: 10.1246/bcsj.20110051. [DOI] [Google Scholar]
- 80.Tung N.H., Minh C., Kiem P., Huong H.T., Nam N.H., Cuong N.X., Quang T.H., Nhiem N.X., Hyun J.-H., Kang H.-K. Chemical components from the vietnamese soft coral lobophytum sp. Arch. Pharm. Res. 2010;33:503–508. doi: 10.1007/s12272-010-0402-3. [DOI] [PubMed] [Google Scholar]
- 81.Fattorusso E., Romano A., Taglialatela-Scafati O., Achmad M.J., Bavestrello G., Cerrano C. Lobozoanthamine, a new zoanthamine-type alkaloid from the indonesian soft coral lobophytum sp. Tetrahedron Lett. 2008;49:2189–2192. doi: 10.1016/j.tetlet.2008.02.028. [DOI] [Google Scholar]
- 82.Tsai T.C., Chen H.Y., Sheu J.H., Chiang M.Y., Wen Z.H., Dai C.F., Su J.H. Structural elucidation and structure-anti-inflammatory activity relationships of cembranoids from cultured soft corals sinularia sandensis and sinularia flexibilis. J. Agric Food Chem. 2015;63:7211–7218. doi: 10.1021/acs.jafc.5b01931. [DOI] [PubMed] [Google Scholar]
- 83.Kobayashi M., Hamaguchi T. Marine terpenes and terpenoids. Vi. Isolation of several plausible precursors of marine cembranolides, from the soft coral, sinularia mayi. Chem. Pharm. Bull. 1988;36:3780–3786. doi: 10.1248/cpb.36.3780. [DOI] [Google Scholar]
- 84.Hsiao T.H., Sung C.S., Lan Y.H., Wang Y.C., Lu M.C., Wen Z.H., Wu Y.C., Sung P.J. New anti-inflammatory cembranes from the cultured soft coral nephthea columnaris. Mar. Drugs. 2015;13:3443–3453. doi: 10.3390/md13063443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murray A.W., Reid R.G. Convenient synthesis of α-epoxylactones (4-oxo-1,5-dioxaspiro[2.4]heptanes and -[2.5]octanes) Synthesis. 1985;1:35–38. doi: 10.1055/s-1985-31096. [DOI] [Google Scholar]
- 86.Evtushenk Y.M., Lvanov V.M., Zaitsev B.E. Determination of epoxide and hydroxyl groups in epoxide resins by ir spectrometry. J. Anal. Chem. 2003;58:347–350. doi: 10.1023/A:1023297731492. [DOI] [Google Scholar]
- 87.Shriner R.L. The Systematic Identification of Organic Compounds. 8th ed. Wiley; Hoboken, NJ, USA: 2004. p. 723. [Google Scholar]
- 88.Marshall J.A., Crooks S.L., DeHoff B.S. Cembranolide total synthesis. Macrocyclization of (alpha-alkoxyallyl)stannane-acetylenic aldehydes as a route to cembrane lactones. J. Org. Chem. 1988;53:1616–1623. doi: 10.1021/jo00243a005. [DOI] [Google Scholar]
- 89.Lin M.K., Yu Y.L., Chen K.C., Chang W.T., Lee M.S., Yang M.J., Cheng H.C., Liu C.H., Chen Dz C., Chu C.L. Kaempferol from semen cuscutae attenuates the immune function of dendritic cells. Immunobiology. 2011;216:1103–1109. doi: 10.1016/j.imbio.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 90.Zhu D., Wang Y., Zhang M., Ikeda H., Deng Z., Cane D.E. Product-mediated regulation of pentalenolactone biosynthesis in Streptomyces species by the MarR/SlyA family activators PenR and PntR. J. Bacteriol. 2013;195:1255–1266. doi: 10.1128/JB.02079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uchio Y., Eguchi S., Nakayama M., Hase T. The isolation of two simple .GAMMA.-lactonic cembranolides from the soft coral Sinularia mayi. Chem. Lett. 1982;11:277–278. doi: 10.1246/cl.1982.277. [DOI] [Google Scholar]
- 92.McAulay K., Clark J.S. Total Synthesis of 7-epi-Pukalide and 7-Acetylsinumaximol B. Chemistry. 2017;23:9761–9765. doi: 10.1002/chem.201702591. [DOI] [PubMed] [Google Scholar]