Abstract
Since the discovery of penicillin, Penicillium has become one of the most attractive fungal genera for the production of bioactive molecules. Marine-derived Penicillium has provided numerous excellent pharmaceutical leads over the past decades. In this review, we focused on the cytotoxic metabolites * (* Cytotoxic potency was referred to five different levels in this review, extraordinary (IC50/LD50: <1 μM or 0.5 μg/mL); significant (IC50/LD50: 1~10 μM or 0.5~5 μg/mL); moderate (IC50/LD50: 10~30 μM or 5~15 μg/mL); mild (IC50/LD50: 30~50 μM or 15~25 μg/mL); weak (IC50/LD50: 50~100 μM or 25~50 μg/mL). The comparative potencies of positive controls were referred when they were available). produced by marine-derived Penicillium species, and on their cytotoxicity mechanisms, biosyntheses, and chemical syntheses.
Keywords: marine-derived Penicillium, natural products, cytotoxic metabolites, biosynthesis
1. Introduction
The oceans, which occupy more than 70% of the earth’s surface, undoubtedly support vast habitats and serve as prolific resources of various living organisms. Compared to terrestrial organisms, marine organisms often produce highly potent metabolites with unique structures to enable them to adapt to extremely challenging environments [1]. Developments and improvements made in biotechnology have led to a new era of bioprospecting for new marine products. Revolutionary target screening methods have improved the efficiency of drug discovery. In addition, leading edge genomics of biological symbiosis offer more opportunities to discover drug candidates and precursors. Marine endozoic microorganisms represent a new frontier in the discovery of pharmaceutical agents [2]. In particular, marine-derived fungi are excellent producers of biologically active secondary metabolites. Since the isolation of the broad-spectrum antibiotic, cephalosporin C from the marine-derived fungus Acremonium chrysogenum, thousands of bioactive metabolites have been discovered and evaluated [3].
Cancer is the second leading cause of death. Lung, prostate, colorectal, and digestive tract cancer are commonly encountered in males, whereas breast, lung, and cervical cancer are the major causes of female death. Marine microorganisms produce limited amounts of highly efficient toxic substances to protect their hosts from enemies, and these substances have been investigated as potential anticancer drug precursors. In particular, marine-derived Penicillium species represent a major source of cytotoxic metabolites. In this review, we list all cytotoxic or antitumor secondary metabolites isolated from marine-derived Penicillium species and classify them into distinct chemical groups. In addition, we summarize the cytotoxicity mechanisms and proposed biosyntheses of these metabolites. Overall, more than 200 natural products and their synthetic analogues are included in this review.
2. Alkaloids
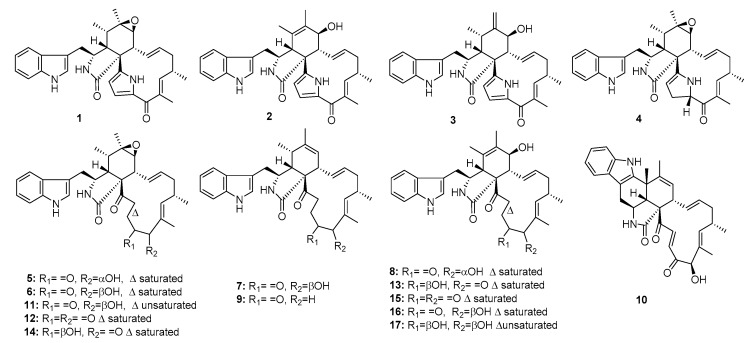
Cytochalasan alkaloids, characterized by a highly substituted perhydoisoindol-1-one fused to a macrocyclic ring, have been shown to possess potential cytotoxicity against diverse tumor cell lines [4,5]. Penochalasins, chaetoglobosins, and cytoglobosins are common classes of cytochalasan alkaloids. A series of cytochalasans, penochalasins A–J (1–10), chaetoglobosins A, C, E–G, O (11–16), and cytoglobosin C (17) (Figure 1) were isolated from the mangrove endophytic fungus P. chrysogenum [6] and from the marine alga Enteromorpha intestinalis [7,8]. Penochalasins A–H (1–8) and chaetoglobosins A, F, O (11, 14, 16) exhibited significant cytotoxic activity (ED50 = 0.4, 0.3, 0.5, 3.2, 2.1, 1.8, 1.9, 2.8, 0.6, 0.9, and 2.4 μg/mL, respectively) against P388 lymphocytic leukemia cells. Moreover, chaetoglobosin A (11) reportedly induced apoptosis of chronic lymphocytic leukemia (CLL) cells by targeting the cytoskeleton. The underlying mechanisms involve the induction of cell-cycle arrest and the inhibition of membrane ruffling and cell migration; therefore, it was proposed as a novel drug for CLL [9]. Penochalasin I (9) exhibited significant cytotoxic activities against MDA-MB-435 (human breast cancer cell line) and SGC-7901 (human gastric cancer cell line) with IC50 values of ~7 μM. Cytoglobosin C (17) showed potential cytotoxicity against both SGC-7901 and A549 (human lung adenocarcinoma) with IC50 values of 3–8 μM. Other cytochalasans, penochalasin J (10), chaetoglobosins C, E (12, 13), and chaetoglobosin G (15) showed moderate cytotoxicity against MDA-MB-435, SGC-7901, and A549 with IC50 values in the range of 10–40 μM (epirubicin was used as a positive control with IC50 values of 0.3~0.6 μM). A recent biosynthetic analysis showed that the fungal PKS-NRPS hybrid synthase, CheA, plays an essential role in cytochalasan formation [10].
Figure 1.
Chemical structures of compounds 1–17.
Gliotoxin induces cellular immunosuppression and apoptosis [11], and its analogues are disulfur or polysulfur-containing mycotoxins that belong to a class of naturally occurring epipolythio piperazines (ETP). In 2012, the marine fungus Penicillium sp. JMF034, which was isolated from a deep sea sediment in Japan, was found to produce seven gliotoxin-related compounds, (bis(dethio)-10a-methylthio-3a-deoxy-3,3a-didehydrogliotoxin (18), 6-deoxy-5a,6-dide hydrogliotoxin (19), bis(dethio) bis(methylthio)gliotoxin (20), bis(dethio)bis(methylthio)-5a,6-dide hydrogliotoxin (21), 5a,6-dide hydrogliotoxin (22), gliotoxin (23), and gliotoxin G (24) (Figure 2) [12], which potently killed P388 murine leukemia cells (IC50 = 3.4, 0.058, 0.11, 0.11, 0.056, 0.024, and 0.020 μM, respectively). Because of their extraordinary cytotoxicity, gliotoxin analogues are considered as antitumor leads [13]. Dimeric ETPs were reported to inhibit histone methyltransferase (HMT); in addition, compounds (22–24) with disulfide or tetrasulfide bonds showed significant inhibitory activities against HMT G9a (IC50 = 2.6, 6.4, and 2.1 μM, respectively) rather than HMT SET7/9 (IC50 > 100 μM). Gliotoxin G (24), isolated from the mangrove endophytic fungus P. brocae MA-231, was potently active against cisplatin-sensitive and resistant human ovarian cancer cell lines, A2780 and A2780 CisR, with IC50 values of 664 and 661 nM, respectively (cisplatin was used as a positive control with IC50 values of 1.67 and 12.63 μM, respectively) [14]. Compound 24 may be used as an anti-ovarian cancer agent, even in patients who are resistant to platinum compounds. Plausible hypotheses for the biosyntheses of ETPs have been previously reviewed [15].
Figure 2.
Chemical structures of compounds 18–24.
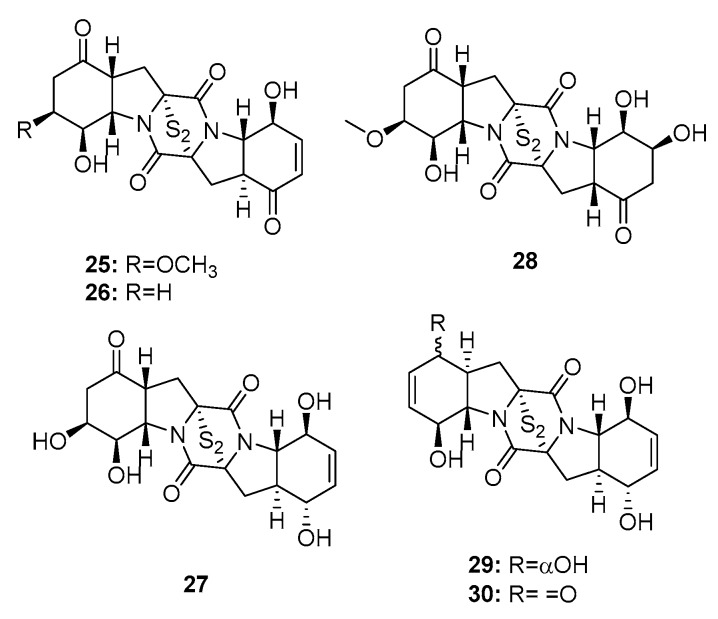
Four new cytotoxic bisthiodiketopiperazines (brocazines A–F) (25–30) (Figure 3), which share molecular similarities with gliotoxin, were isolated from a fungal strain of P. brocae MA-231, collected from the marine mangrove Avicennia marina [16]. Their cytotoxicity was investigated in human prostate cancer (DU145), human cervical carcinoma (Hela), human hepatoma (HepG2), human breast carcinoma (MCF-7), human large-cell lung carcinoma (NCI-H460), SGC-790, human pancreatic cancer (SW1990), human colon carcinoma (SW480), and human glioma (U251) cell lines. Brocazines A, B, E, and F (25, 26, 29, and 30) exhibited significant cytotoxic effects against most of the cell lines tested with IC50 values in the range of 0.89–9 μM (paclitaxel, cisplatin, cefitinib, doxorubicin, and gemcitabine were used as positive controls with IC50 values of 1~11 μM). In contrast, brocazines C and D (27 and 28), which lack the α, β unsaturated ketone group, had much lower cytotoxicity (IC50 > 20 μM), which suggests that the conjugated ketone system is crucial to the cytotoxic properties of bisthiodiketopiperazine analogues.
Figure 3.
Chemical structures of compounds 25–30.
Two bisthiodiketopiperazines, pretrichodermamide C (31) and N-methylpretrichodermamide B (32) (also called adametizine B and A, respectively) (Figure 4), were isolated from a marine sponge-derived fungus (P. adametzioides AS-53) [17], a hyper saline lake-derived Penicillium sp. [18], and a marine algicolous fungus (Penicillium sp. KMM4672) [19]. All three studies showed that compound 32, which contains chlorine, exhibited significant cytotoxicity, wherein it reduced the viability of L5178Y mouse lymphoma cells, human prostate cancer 22Rv1 cells, PC-3 cells, LNCaP cells, and brine shrimps (IC50 = 2, 0.51, 5.11, 1.76, and 4.8 μM, respectively; while kahalalide F, docetaxel, and colchicine were employed as positive controls with IC50 values of 4.3, 0.013, 0.015, 0.004, and 8.1 μM, respectively). Furthermore, it was found active in hormone-resistant 22Rv1 cells at nanomolar concentrations. In contrast, metabolite 31 was completely inactive in all bioassays with IC50 values > 100 μM. This remarkable difference in activity indicates that the halogen atom might improve the activity of the metabolite.
Figure 4.
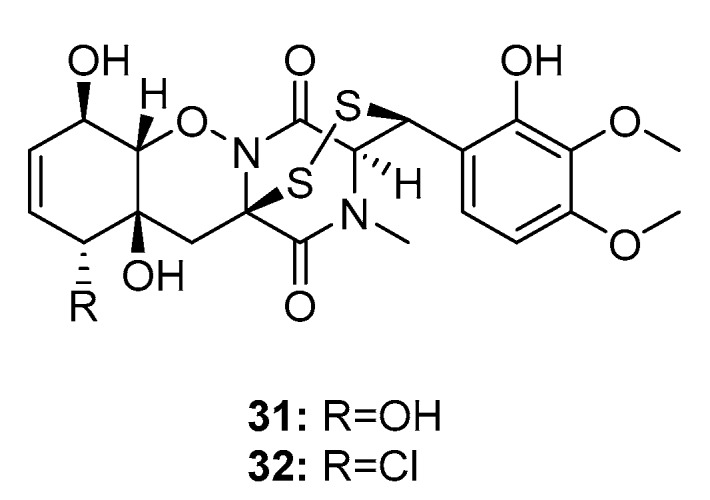
Chemical structures of compounds 31–32.
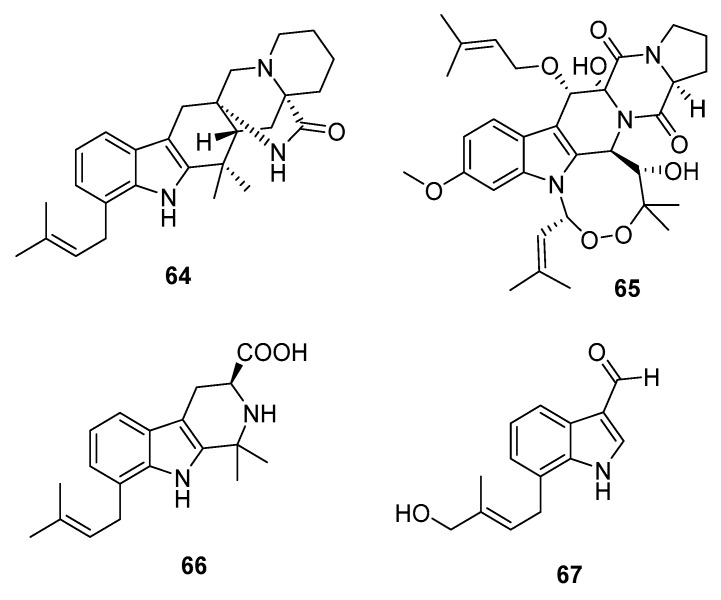
Roquefortine C (33) (Figure 5) is a potential neurotoxin that can activate P-glycoprotein and simultaneously inhibit P450-3A and other hemoproteins [20]. Roquefortine and meleagrin (38) analogues are considered biogenetically interrelated mycotoxins with promising cytotoxicity [21]. Recently, a series of roquefortine derivatives, roquefortines F–I (34–37), and meleagrin analogues, meleagrins B–E (39–42), were isolated from the deep ocean sediment-derived fungus Penicillium sp. [22], and most of these compounds (34, 35, and 39–42) were active against A549, HL-60 (human promyelocytic leukemia), BEL-7402 (human hepatoma), and MOLT-4 (human acute T lymphoblastic leukemia) cancer cell lines. Meleagrin B (39) was the most cytotoxic against these four cell lines with IC50 values in the range of 1.5–7 μM; the other compounds had IC50 values in the range of 4–50 μM. Meleagrin (38) was also isolated from a deep sea sediment-derived fungus, P. commune SD-118, and was found to be cytotoxic in HepG2, NCI-H460, Hela, MDA-MB-231 (human breast cancer cells), and DU145 human cancer cell lines (IC50 = 12, 22, 20, 11, and 5 μg/mL, respectively; while fluorouracil was employed as a positive control with IC50 values of 14, 1, 14, 8, and 0.4 μg/mL, respectively) [23].
Figure 5.
Chemical structures of compounds 33–42.
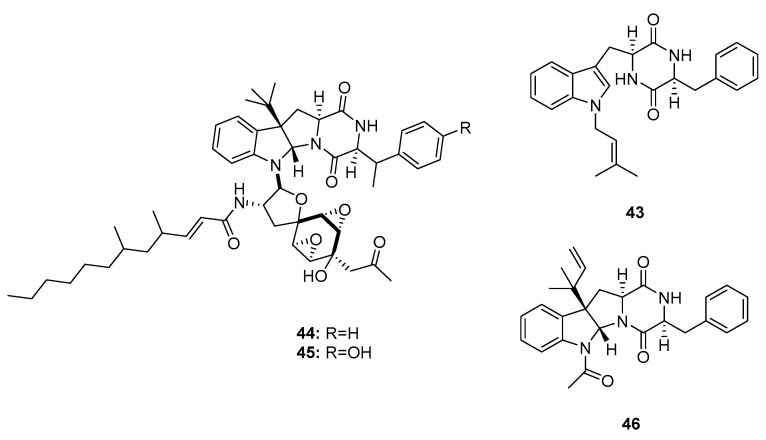
Penicimutanins A,B (43–45) and fructigenine A (46) (Figure 6) are structurally similar to roquefortines, and were first isolated from diethyl sulfate- or gentamicin-induced mutants of the marine-derived fungus P. purpurogenum G59 [24,25]. Mutation-based approaches can activate silent fungal gene clusters and afford more potent metabolites with unique structures. Compounds 44 and 45 are mutant cytotoxic products that showed potent activities against five human cancer cell lines: K562 (human chronic myelogenous leukemia), HL-60, Hela, BGC-823 (human gastric adenocarcinoma), and MCF-7 (IC50 values were 5–11 μM for 44 and 8–20 μM for 45). Compounds 43 and 46 also inhibited the proliferation of these cell lines (Inhibition Rate (IR)% = 22.6 and 20.8 (K562); 17.9 and 55.3 (HeLa); and 26.5 and 65.6% (MCF-7) at 100 μg/mL, respectively; while 5-fluorouracil was employed as a positive control with IR% of 48.5, 37.4, and 47.4 μg/mL at 100 μg/mL, respectively).
Figure 6.
Chemical structures of compounds 44–46.
Since the isolation of (+)-chaetocin A (47) and (+)-verticillin A (48) (Figure 7) in 1970, dimeric epidithiodiketopiperazine alkaloids have received much attention owing to their diverse biological activities and complex molecular structures [26,27]. In 1999, two additional dimeric epidithiodiketopiperazine alkaloids, (+)-11,11′-dideoxyverticillin A (49) and (+)-11′-deoxyverticillin A (50), were isolated from a marine alga-derived fungus Penicillium sp. and were found to exhibit extraordinary cytotoxicity against HCT-116 cells (human colon cancer) with IC50 of 30 ng/mL [28]. Chaetocin A (47) was the first compound reported to inhibit HMT, and to have specific effects on HMT SU(VAR)3-9 in vitro and in vivo [29]. (+)-11,11′-Dideoxyverticillin A (49), an alkaloid, exhibited diverse antitumor activities in vitro and in vivo [30]; in addition, it potently inhibited the phosphorylation of epidermal growth factor receptor in human breast cancer (MDA-MB-468) [31]. Movassaghi et al. used a concise enantioselective method for the total synthesis of (+)-11,11′-dideoxyverticillin A (49) in 2009 [32] based on mimicking the biosynthetic pathway; in addition, they used this approach to synthesize various dimeric epidithiodiketopiperazines [33].
Figure 7.
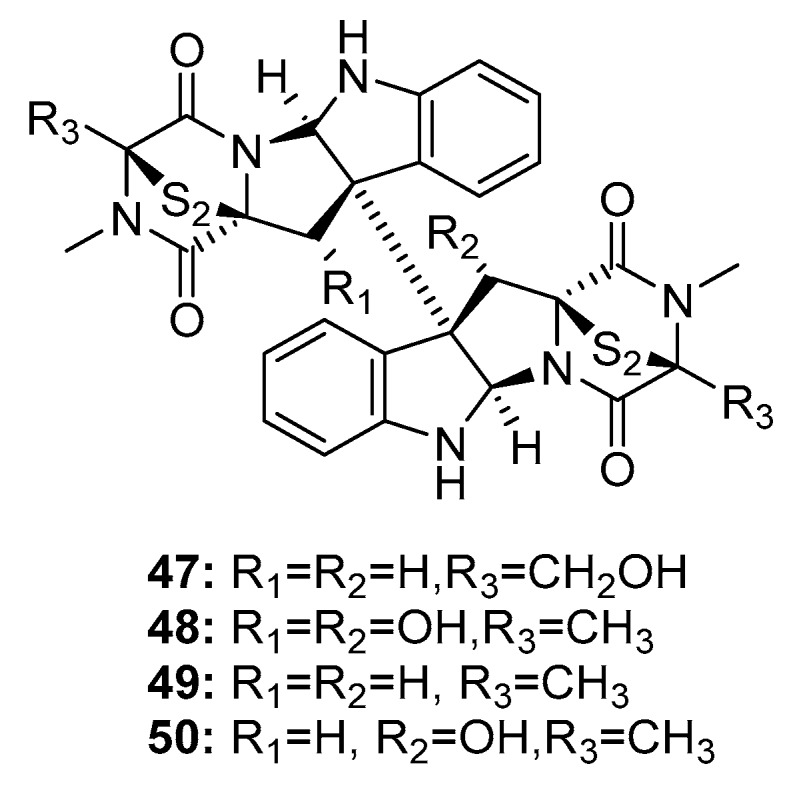
Chemical structures of compounds 47–50.
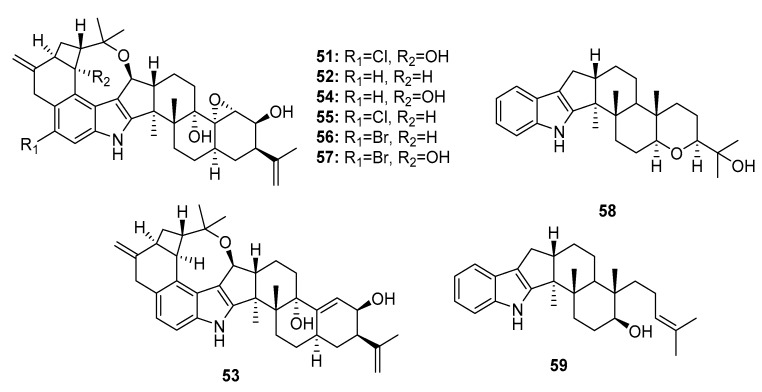
Seven cytotoxic indole diterpene alkaloids, penitrems A,B (51–52), D–F (53–55), paspaline (58), and emindole SB (59) (Figure 8) were isolated from a marine Penicillium sp. KBr-induced mutation of this fungus produced two bromo-substituted indole alkaloids, 6-bromopenitrems B and E (56–57) [34]. Compounds (51–59) showed potent antiproliferative (IC50 = 5–20 μM for MCF-7; 8–30 μM for MDA-MB-231), anti-migratory (IC50 = 7–35 μM for MDA-MB-231) and anti-invasive properties (IR% = 10–75% at 15 μM) against human breast cancer cells. In addition, penitrems A, B, and E (51–52, 54) were evaluated in 60 human tumor cell lines as a part of the Development Therapeutics Program of the National Cancer Institute (NCI60). Penitrem B (52) exhibited the strongest mean growth inhibitory effect in the 60 human cancer cells (IR% = 41.05% at 10 μM) and was considered a potential selective inhibitory agent for leukemia cells. The nematode Caenorhabditis elegans was used to assess the brain’s Maxi-K (BK) channel inhibitory activity and toxicity in vivo [35,36]. Penitrem A (51) and 6-bromopenitrem E (57) displayed BK channel inhibition, comparable to that of a knockout strain. A pharmacophore study on the effects of the penitrem skeleton on the antiproliferative activity against MCF-7 cells indicated that less structural complexity of the penitrems, paspaline (58), and emindole SB (59) better maintained the molecular alignment and pharmacophoric features. Penitrem A (51) was also considered a neurotoxin that antagonizes BK channels [37].
Figure 8.
Chemical structures of compounds 51–59.
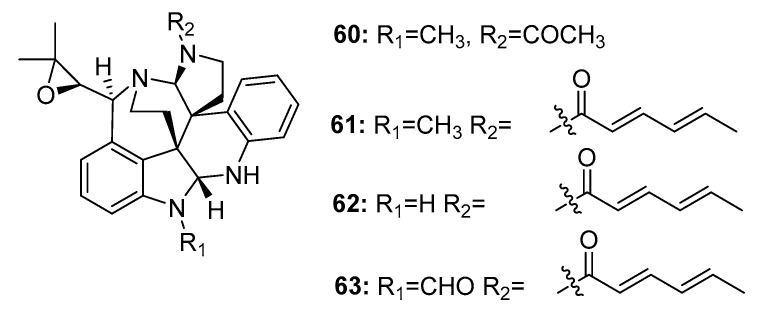
Another large family of indole alkaloid mycotoxins, comprising communesins A–D (60–63) (Figure 9), was isolated from marine-derived Penicillium sp. from a marine alga [38], marine sponge [39], and marine sediment [40]. Communesin B (61) (also called nomofungin) was more cytotoxic to P388 lymphocytic leukemia cells (ED50 = 0.45 μg/mL) than communesin A (60) (ED50 = 3.5 μg/mL). The antiproliferative activity of communesins B–D (61–63) was further evaluated in six lymphocytic leukemia cell lines (U-937, THP-1, NAMALWA, L-428, MOLT-3, and SUP-B15). They steadily and effectively inhibited the proliferation of five of these cell lines with ED50 values ranging from 7 to 16 μg/mL; however, they were inactive in L-428 cells. The total synthesis of communesin B (61) was previously reported [41].
Figure 9.
Chemical structures of compounds 60–63.
Four new cytotoxic prenylated indole alkaloid derivatives, penioxamide (64) [42], 13-O-prenyl-26-hydroxyverruculogen (65) [43], and penipalines B and C (66–67) (Figure 10) [44], were isolated from marine mangrove-derived P. oxalicum EN-201, marine sediment-derived P. brefeldianum SD-273, and marine sediment-derived P. paneum SD-44, respectively. Metabolites 64–65 showed significant lethality in brine shrimps with LD50 values of 5.6 and 9.4 μM, respectively (colchicine was employed as a positive control with an LD50 value of 7.8 μM). Metabolites 66–67 induced moderate cytotoxicity against A549 (IC50 = 20.44 and 21.54 μM, respectively) and HCT-116 cell lines (IC50 = 14.88 and 18.54 μM, respectively).
Figure 10.
Chemical structures of compounds 64–67.
In addition, three 1,4-diazepane derivatives, terretriones A, C, and D (68–70) (Figure 11), obtained from marine sponge-derived P. vinaceum [45] and marine tunicate-derived Penicillium sp. CYE-87 [46], moderately inhibited the migratory activity of MDA-MB-231 cells with IC50 values of 17.7, 17.6, and 16.5 μM, respectively (Z-4-ethylthio-phenylmethylene hydantoin was used as a positive control with an IC50 value of 43.4 μM). These findings indicate that terretriones might be potential anti-metastatic breast cancer candidates.
Figure 11.
Chemical structures of compounds 68–70.
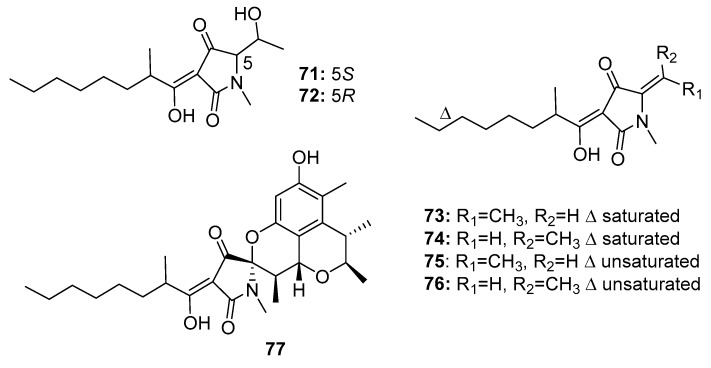
Six tetramic acid derivatives, penicillenols A1, A2, B1, B2, D1, and D2 (71–76) (Figure 12), were isolated from a marine sediment-derived fungus P. citrinum. Penicillenol B2 (74) exhibited the strongest cytotoxic activity against A-375 human malignant melanoma cell line (IC50 = 0.97 μg/mL), whereas the IC50 values of compounds 71–73 were 3.2, 13.8, and 2.8 μg/mL, respectively [47,48]. Penicillenols D1 and D2 (75–76) showed moderate cytotoxicity against A549 cells with IC50 values of 17.2 and 12.1 μg/mL, respectively. However, penicillenols A1 and B1 (71, 73) showed significant cytotoxicity in HL-60 cells (IC50 = 0.76 and 3.2 μM, respectively) [49]. A novel tetramic acid derivative, penicitrinine A (77), which contains a citrinin-like group, was isolated [50]. The combination of two cytotoxic fragments in this metabolite might contribute to its extensive antiproliferative activity in diverse tumor cell lines, particularly A-375 cells. Penicitrinine A (77) not only induced A-375 cell apoptosis by upregulating Bax and downregulating Bcl-2, but also inhibited A-375 cell metastatic activity by suppressing matrix metalloproteinase 9 (MMP-9) and promoting the expression of its specific inhibitor, tissue inhibitor of metalloproteinases-1 (TIMP-1). These findings suggest that penicitrinine A (77) is a potential lead compound.
Figure 12.
Chemical structures of compounds 71–77.
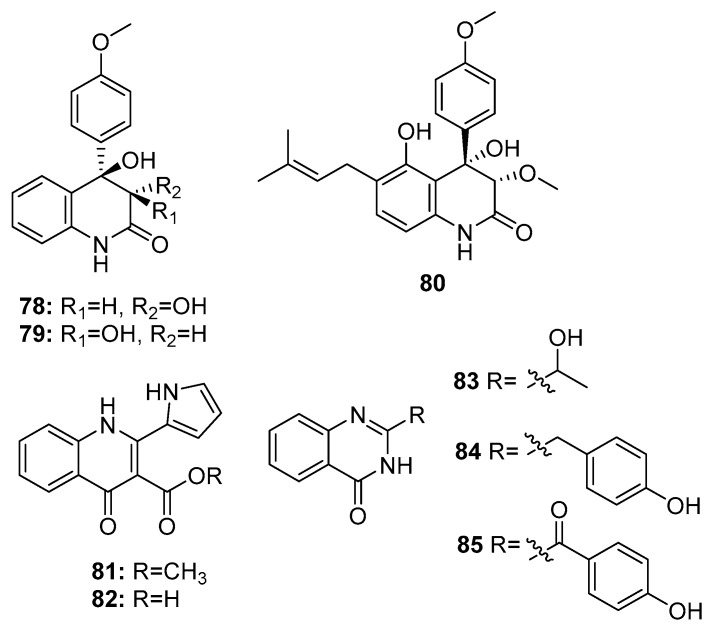
Quinolinone and quinazolinone alkaloids have unique pharmacophores that allow their binding to multiple sites with high affinity; moreover, they possess various biological properties [51]. Some cytotoxic quinolinone (78–82) and quinazolinone alkaloids (83–85) (Figure 13) were isolated from marine-derived members of the Penicillium genus, such as P. janczewskii, Penicillium sp. ghq208, P. oxalicum 0312F1, P. chrysogenum EN-118, and P. commune SD-118 [23]. 2-quinolinone metabolites (78–79) exhibited IR% values of 50–60% at 10 μg/mL. Interestingly, compound 80, which has an additional prenyl chain, showed significant cytotoxicity against MDA-MB-231 and HT-29 (human colon carcinoma) cell lines with IR% values of 92–96% at 10 μg/mL [52]. In addition, a 4-quinolinone derivative (82) exhibited significant cytotoxicity against the human lung cancer cell line 95-D (IC50 = 0.57 μg/mL). Both compounds 81 and 82 exhibited similar cytotoxicity (IC50 = 11.3 and 13.2 μM, respectively) against HepG2 cells [53,54]. However quinazolinone derivatives (83–85) showed only moderate cytotoxicity (compound 83, IC50 = 20 μg/mL in SW1990 cell line; compound 84, IC50 = 8 μg/mL in DU145, A549, and Hela cell lines; and compound 85, IR% = 35–40 at 200 μg/mL in SGC-7901 and BEL-7404 cell lines) [55,56].
Figure 13.
Chemical structures of compounds 78–85.
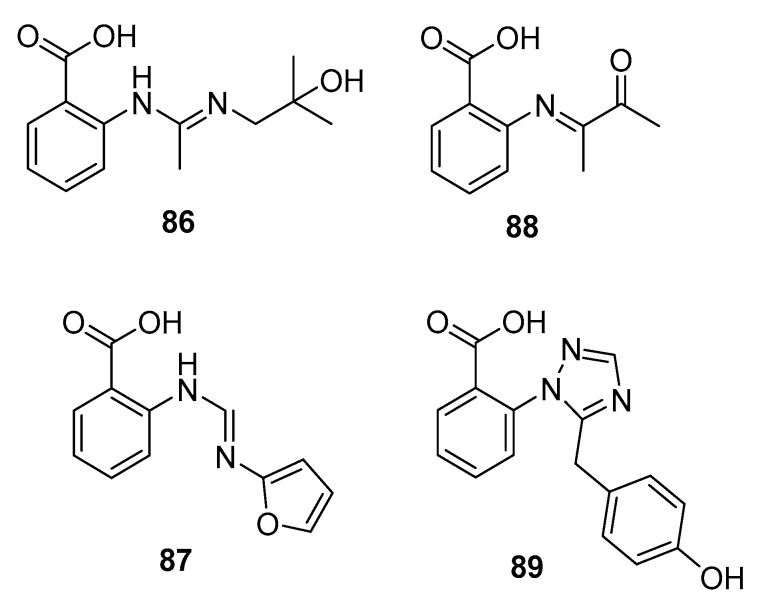
In an ongoing study that aims to produce new active metabolites from P. paneum SD-44 (a deep sea sediment-derived fungus) using culture variations, three amidine anthranilic acid analogues (86–88) and one triazole anthranilic acid derivative, penipanoid A (89) (Figure 14), were obtained after culture in malt and rice medium, respectively. Compounds 86 and 87 strongly inhibited RKO human colon cancer cell viability (IC50 = 8.4 and 9.7 μM, respectively). In addition, compound 88 was cytotoxic to Hela cells (IC50 = 6.6 μM) [57], whereas compound 89 with a triazole group only weakly inhibited SMMC-7721 cell viability (human hepatocarcinoma) (IC50 = 54.2 μM) while fluorouracil was used as a positive control for three cell lines with IC50 values of 25.0, 14.5, and 13.0 μM, respectively [58].
Figure 14.
Chemical structures of compounds 86–89.
An azaphilone analogue, bis-sclerotioramin (90) (Figure 15), obtained from a marine mangrove endophytic fungus, Penicillium 303#, was found to possess moderate cytotoxicity against MDA-MB-435 cell line (IC50 = 7.13 μg/mL), while epirubicin was used as a positive control with an IC50 value of 0.325 μg/mL [59]. Another novel alkaloid, the sorbicilin-derived sorbicillactone A (91), was first isolated from a Mediterranean sponge-derived fungus, P. chrysogenum. Sorbicillactone A (91) exhibited a selective antileukemic activity in L5178Y cells (murine leukemic lymphoblast) with an IC50 of 2.2 μg/mL, as well as in other tumor cell lines (IC50 > 10 μg/mL). The biosynthesis of sorbicillactone A (91) was investigated using 13C-labeled precursor feeding experiments, which showed that the its skeleton was derived from acetate, alanine, and methionine [60]. Furthermore, a new strategy for the large-scale biotechnological production of sorbicilin-derived alkaloids was developed for preclinical screening and a structure-activity relationship (SAR) study [61]. In addition, a 4-oxoquinoline derivative, brocaeloid B (92), isolated from the mangrove endophytic fungus P. brocae, showed mild lethality against brine shrimps with an LD50 of 36.7 μM, while colchicine was used as a positive control with an LD50 value of 87.6 μM [62]. Li et al. cultured the marine mangrove fungus P. varibile with the DNA methyltransferase inhibitor 5-azacytidine to identify novel responsive molecules by gene silencing. A highly modified fatty acid amide, varitatin A (93), exhibited significant cytotoxicity against HCT-116 cells (IC50 = 2.8 μM, while doxorubicin was used as a positive control with an IC50 value of 0.2 μM) and potently inhibited protein tyrosine kinases, platelet-derived growth factor receptor-beta (PDGFR-β), and ErbB4 with IR% values of 50 and 40%, respectively, at a concentration of 1 μM [63]. In addition, a new pyridinyl-α-pyrone alkaloid, 18-hydroxydecaturin B (94), was isolated from an endophytic fungus, P. oxalicum EN-201, derived from the marine mangrove Rhizophora stylosa. Compound 94 showed significant lethality in brine shrimps (LD50 = 2.3 μM, while colchicine was used as a positive control with an LD50 value of 7.8 μM) [42]. A previous study showed that the metabolites of decaturin, a potent insecticide, were cytotoxic [64]. The isocyanide alkaloid, xantocillin X (95), which is a known antiviral and antibiotic agent [65], was first isolated from P. notatum in 1950 [66]. Recently, compound 95 was isolated from the deep sea sediment-derived fungus P. commune SD-118, and showed moderate cytotoxicity in six cancer cell lines (MCF-7, HepG2, NCI-H460, Hela, DU145, and MDA-MB-231) with IC50 values of 12, 7, 10, 10, 8, and 8 μg/mL, respectively, while fluorouracil was used as a positive control with IC50 values of 4, 14, 1, 14, 0.4, and 8 μg/mL, respectively [23]. A later pharmacological study on human HepG2 cells showed that compound 95 induced apoptosis and autophagy by inhibiting the MEK/EPK signaling pathway and activating the class III PI3K/Beclin 1 signaling pathway [67].
Figure 15.
Chemical structures of compounds 90–95.
3. Terpenes, Meroterpenes, and Steroids
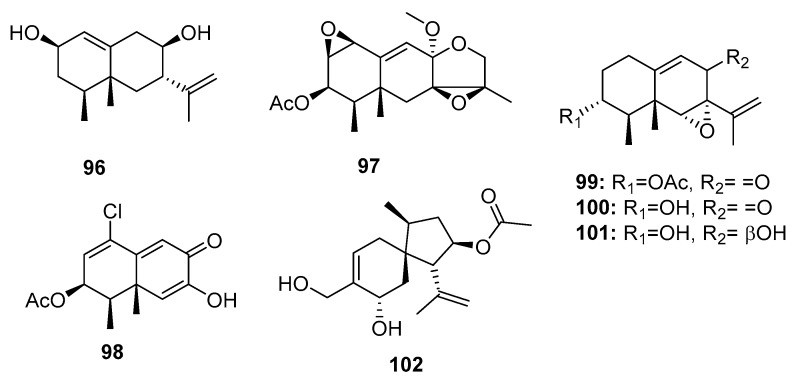
The genus Penicillium is a well-known producer of eremophilane-type sesquiterpenes with phytotoxic, mycotoxic, and phytohormonic activities [68,69]. Chemical investigation of an Antarctic deep sea-derived fungus, Penicillium sp. PR19 N-1, yielded three new cytotoxic eremophilane-type sesquiterpenes (96–98) (Figure 16), which were moderately cytotoxic to HL-60 (IC50 = 45.8, 28.3, and 11.8 μM, respectively) and A549 (IC50 = 82.8, 5.2, and 12.2 μM, respectively) cancer cell lines [70,71]. Three other eremophilane-type sesquiterpenes (99–101) were isolated from a sea mud-derived fungus, Penicillium sp. BL 27-2. Of these, compound 99 was the most cytotoxic to P388, A549, HL-60, and BEL-7402 cell lines (IC50 = 0.073, 0.096, 0.065, and 4.59 μM, respectively), whereas compounds 100 and 101 had IC50 values in the range of 3–12 μM [72]. These results suggest that the epoxide ring is essential for the cytotoxicity of eremophilane-type sesquiterpenes and that the presence of an acetyl group enhances the cytotoxicity. A new acorane sesquiterpene, adametacorenol B (102), isolated from a marine sponge-derived fungus, P. adametzioides AS-53, displayed selective cytotoxicity against NCI-H446 cell lines (IC50 = 5 μM), compared to its cytotoxicity against the other 13 tumor cell lines tested (A549, DU145, HeLa, HepG2, Huh-7 (human hepatocarcinoma), L02 (human hepatocarcinoma), LM3 (murine breast cancer), MA (mouse Leydig tumor), MCF-7, SGC-7901, SW1990, SW480, and U251) (IC50 > 10 μM) [17].
Figure 16.
Chemical structures of compounds 96–102.
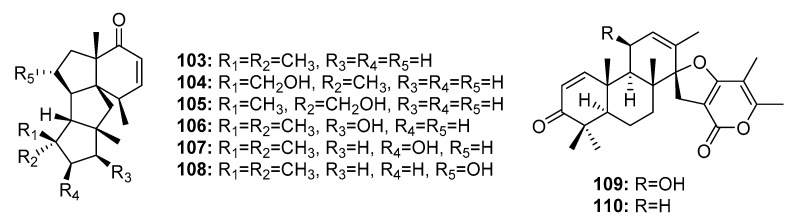
The deep sea sediment-derived fungus Penicillium sp. was reported to be a good source of cytotoxic diterpenes. Six tetracyclic diterpenes, conidiogenones B–G (103–108) (Figure 17), exhibited cytotoxicity against HL-60, A549, BEL-7402, and MOLT-4 cell lines. Conidiogenone C (104) was potently cytotoxic against HL-60 and BEL-7402 cells with IC50 values of 0.038 and 0.97 μM, respectively; however, it was not cytotoxic against A549 and MOLT-4 cell lines at 50 μM. Other conidiogenones (103, 105–108) had moderate cytotoxicity with IC50 values ranging from 1 to 50 μM [22]. The spiroditerpenes, breviones I and A (109–110) were also obtained from this fungus and showed cytotoxicity comparable to that of cisplatin (the positive control) against MCF-7 cells (IC50 = 7.44 and 28.4 μM, respectively, versus 8.04 μM for cisplatin) [73].
Figure 17.
Chemical structures of compounds 103–110.
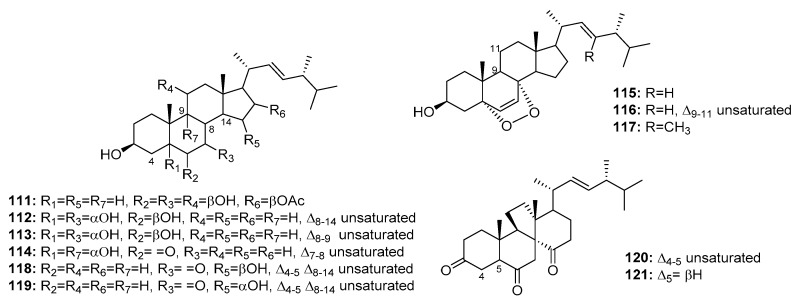
Although several marine-derived steroids have been isolated, few have been found to be bioactive. A cytotoxic polyoxygenated steroid, penicisteroide A (111) (Figure 18), was isolated from a marine alga-derived fungus, P. chrysogenum QEN-24S. Penicisteroide A (111) displayed moderate cytotoxicity against Hela, SW1990, and NCI-H460 cell lines with IC50 values of 15, 31, and 40 μg/mL, respectively [74]. Three other polyoxygenated steroids (112–114) and two epidioxygenated steroids (115–116) were isolated from the marine moss-derived fungus Penicillium sp. These steroids moderately inhibited HepG2 cell line growth (IC50 values = 10.4, 15.6, 20.7, 16.8, and 21.3 μg/mL, respectively) [75]. In addition, an epidioxygenated steroid (117), produced by a sea squirt-derived fungus, P. stoloniferum QY2-10, was cytotoxic to P388 cells with an IC50 of 4.07 μM [76]. Moreover, a marine Penicillium sp. fungus collected from the inner tissues of an unidentified sponge is reportedly the source of two epimeric steroids (118–119) and two cytotoxic steroids of a new class, dankasterone A (120) and B (121). Dankasterone A (120) was more effective than the positive control, adriamycin (IC50 = 0.98 μM) against HL-60, Hela, and K562 cancer cell lines with IC50 values of 0.78, 4.11, and 7.57 μM, respectively. Compounds 118–119 and 121 also significantly inhibited K562 cell growth (IC50 = 4.38, 5.54, and 7.89 μM, respectively) [77].
Figure 18.
Chemical structures of compounds 111–121.
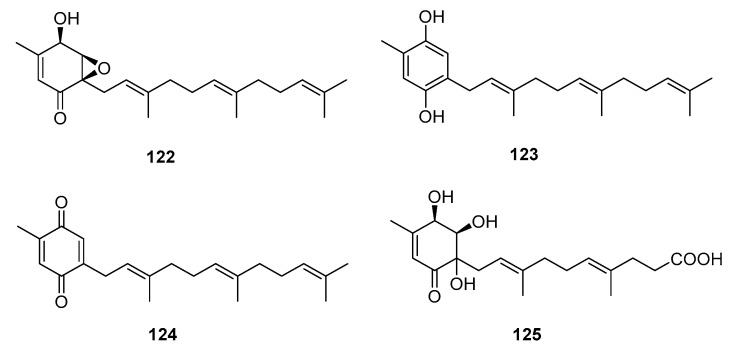
Meroterpenes are widely distributed in the marine environment, particularly in brown algae and microorganisms. Terpene-quinone and -hydroquinone are the major bioactive members because they produce reactive oxygen species (ROS) [78]. Three quinone- and hydroquinone-type meroterpenes (122–124) (Figure 19) were isolated from a marine-derived Penicillium sp. Compounds 122 and 123 exhibited extensive cytotoxicity against five cancer cell lines (A549, SKOV-3 (human ovary adenocarcinoma), SKMEL-2 (human skin cancer), XF498 (human CNS cancer), and HCT15 (human colon cancer)) with IC50 values in the range of 3–10 μg/mL, whereas compound 124 had IC50 values ranging from 20 to 40 μg/mL (doxorubicin was used as a positive control with IC50 values of 0.02~0.8 μg/mL). These results suggest that the quinone form tends to be less cytotoxic [79]. Penicillone A (125), isolated from marine-derived Penicillium sp. F11., contains a carboxylic acid group instead of the isoprenyl tail, which resulted in mild cytotoxicity against fibrosarcoma (HT1080) and human nasopharyngeal carcinoma (Cne2) cell lines (IC50 = 45.8 and 46.2 μM, respectively) [80].
Figure 19.
Chemical structures of compounds 122–125.
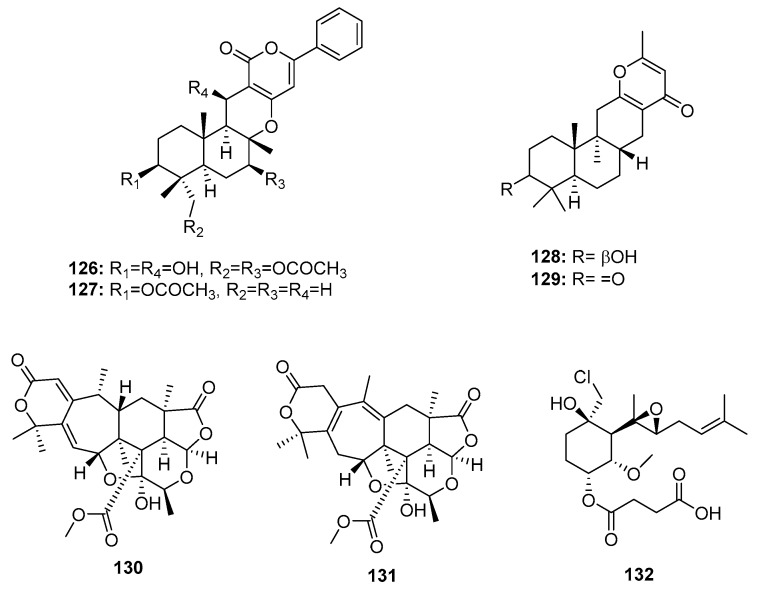
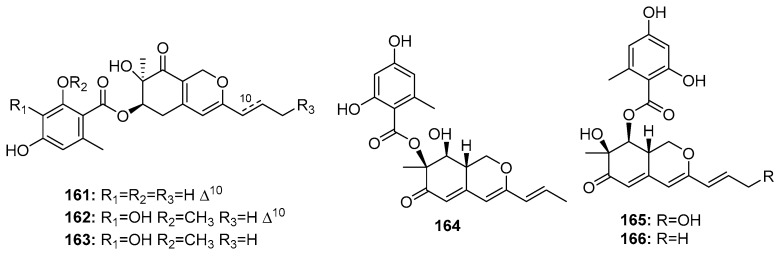
Two sesquiterpene α-pyrones, phenylpyropenes E and F (126–127) (Figure 20), were isolated from the marine-derived fungus P. concentricum ZLQ-69 and displayed moderate and selective cytotoxicity against MGC-803 cells (human gastric cancer) with IC50 values of 19.1 and 13.6 μM, respectively (doxorubicin was used as a positive control with an IC50 value of 0.37 μM) [81]. Furthermore, the marine sediment-derived fungus Penicillium sp. F446 yielded two new sesquiterpene γ-pyrone-type meroterpenes, penicillipyrone A and B (128–129), which were moderately cytotoxic against A549 cells (IC50 = 15 and 17 μM, respectively, while doxorubicin was used as a positive control with an IC50 value of 1.2 μM) [82]. Two polycyclic α-pyrone-type meroterpenes (130–131), isolated from the marine mangrove endophytic fungus Penicillium 303#, exhibited IC50 values of 20–30 μg/mL in four cancer cell lines (MDA-MB-435, HepG2, HCT-116, and A549), while epirubicin was used as a positive control with IC50 values of 0.2~0.6 μg/mL [59]. Fumagillin was first isolated from Aspergillus fumigatus in 1949, and has been used as an antimicrobial [83]. Recently, ligerin (132), a natural chlorinated merosesquiterpene related to fumagillin, was obtained from a marine-derived Penicillium sp., and showed selective in vitro antiproliferative activity against osteosarcoma cell lines (IC50 = 117 nM against POS1 cells, which is 20 times greater than the IC50 in other cancer cell lines), while doxorubicin was used as a positive control with IC50 values of 0.04~2 μM [84]. Ligerin analogues were semi-synthesized in an SAR study, which showed that chlorohydrin and C6 substituents were crucial for cytotoxic activities. Furthermore, ligerin (132) exhibited stronger cytotoxicity against human osteosarcoma SaOS2 and MG63 cancer cell lines. However, its cytotoxicity was less than that of TNP470 (a positive control and fumagillin analogue) [85].
Figure 20.
Chemical structures of compounds 126–132.
4. Polyketides
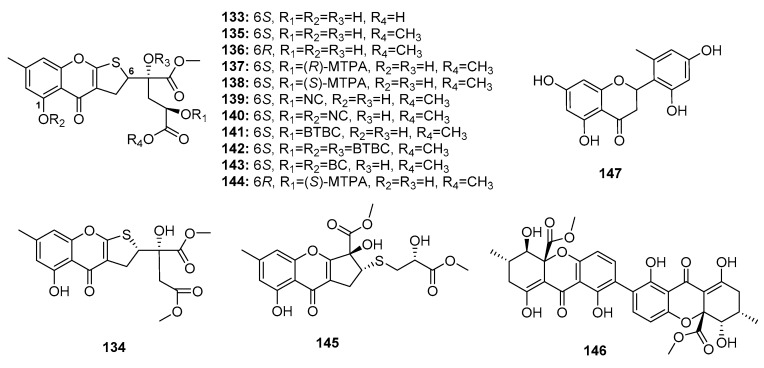
Chromone derivatives are abundantly present in nature and are considered potential immunomodulatory, anticancer, and anti-inflammatory agents. Chromone scaffolds were reported to possess outstanding pharmacological properties [86]. A Chinese research group recently isolated four dihydrothiophene-condensed chromones, oxalicumones D, E (133–134) and A, B (135–136) (Figure 21) from a marine gorgonian-derived fungus, P. oxalicum SCSGAF 0023. Similar to synthetic dihydrothiophene-condensed chromones (137–144), these four natural chromones (133–136) displayed significant cytotoxicity against eight carcinoma cell lines (human lung adenocarcinoma (H1975), human lymphoma (U937), K562, BGC823, MOLT-4. MCF-7, HL-60, and Huh-7) (IC50 < 10 μM). Of these, oxalicumone A (135) was the most cytotoxic against MOLT-4 cell line (IC50 = 0.30 μM). An SAR study showed that the 2,3-dihydrothiophene unit was crucial for activity and that the presence of 1-OH and absolute configuration at C-6 contributed to cytotoxicity [87,88]. Subsequent pharmacological studies showed that oxalicumone A (135) inhibited leukemia cell growth and induced apoptosis, in part, via the induction of the endoplasmic reticulum stress pathway by upregulating calnexin and Bax and activating unfolded protein response [89]. Another study found that oxalicumone A (135) could induce oxidative stress injury in the mitochondria, and thus promote human renal epithelial cell death [90]. Chromosulfine (145), a novel cyclopentachromone sulfide which is structurally similar to dihydrothiophene-condensed chromones, was isolated from a neomycin-resistant mutant of the marine-derived fungus P. purpurogenum G59, and showed selective cytotoxicity against HL-60 cancer cell line (IC50 = 16.7 μM) [91]. Secalonic acid F (146), a chiral dimeric tetrahydroxanthone, was first isolated from Aspergillus sp. before discovering that the deep sea sediment-derived fungus Penicillium sp. F11 is a good source of this compound. Compound 146 induced HL-60 cell apoptosis by modulating the Rho GDP dissociation inhibitor 2 pathway [92]. Recent studies showed that secalonic acid F (146) could induce apoptosis by activating caspase 3 and 9 through the mitochondrial pathway in hepatocellular carcinoma, wherein it was found to be more effective than 5-fluorouracil [93]. Furthermore, a flavone, namely penimethavone A (147), obtained from a gorgonian-derived fungus, P. chrysogenum, exhibited selective cytotoxicity against Hela and rhabdomyosarcoma cell lines (IC50 = 8.41 and 8.18 μM, respectively) while adriamycin was used as a positive control with IC50 values of 0.43 and 0.09 μM, respectively [94].
Figure 21.
Chemical structures of compounds 133–147.
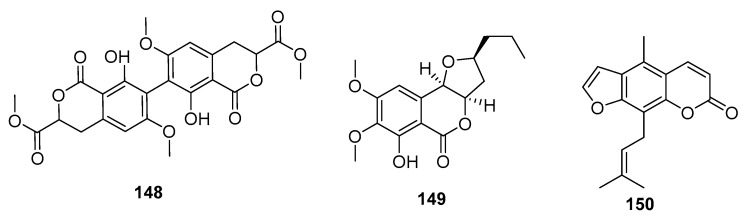
Coumarin derivatives of the chromone isomers (148–150) (Figure 22) were also isolated from the deep sea sediment-derived fungus (P. chrysogenum SCSIO 41001), a marine sponge-derived fungus Penicillium sp., and a mangrove endophytic fungus (Penicillium sp. ZH16), respectively. The dimeric isocoumarin, bipenicillisorin (148), displayed significant cytotoxicity against K562, A549, and Huh-7 cell lines (IC50 = 6.78, 6.94, and 2.59 μM, respectively), while taxol was used as a positive control with IC50 values of 3.44, 2.61, and 14.70 nM, respectively [95]. The dihydroisocoumarin monocerin (149) exhibited significant cytotoxicity against L5178Y cells (a murine lymphoma cell line) with an IC50 value of 8.4 μM (kahalalide F was used as a positive control with an IC50 value of 4.3 μM) [96]. Moreover, furanocoumarin (150) showed moderate cytotoxicity against human nasopharyngeal carcinoma (KB and KBv200) cell lines (IC50 = 5 and 10 μg/mL, respectively) [97].
Figure 22.
Chemical structures of compounds 148–150.
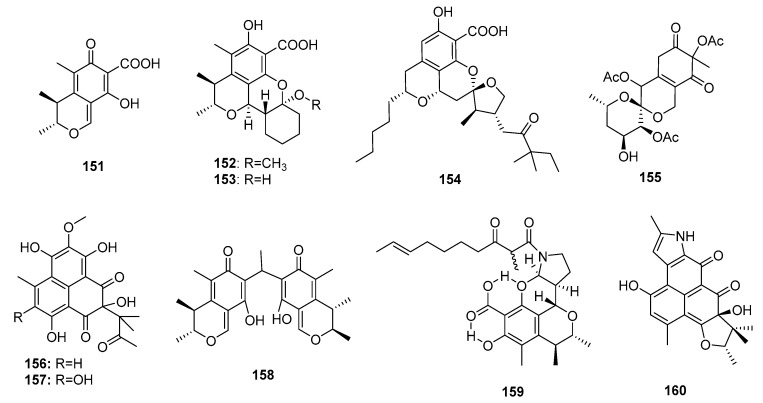
Citrinin (151) (Figure 23), a typical azaphilone polyketide mycotoxin, was first found in P. citrinum in 1931 [98]. Compound 151 is strongly nephrotoxic because of its inhibition of respiration complex III [99]. The biosynthesis pathway of compound 151 was further investigated [100]. Interestingly, the marine sponge-derived fungus Penicillium sp. FF001 was found to be a good source of unique and potent citrinin derivatives [101]. Two new citrinin derivatives, penicitrinols L and M (152–153), isolated from the marine sediment-derived fungus P. citrinum, showed moderate cytotoxicity against a human Caucasian colon adenocarcinoma cell line (SW-620) (IC50 = 25.6 and 20.9 μM, respectively) [48]. One penicitrinol analogue, berkelic acid (154), with a novel spiroketal structure, isolated from an acid mine lake fungal extremophile Penicillium sp., showed selective and extraordinary cytotoxicity against a human ovarian carcinoma cell line (OVCAR-3) at nanomolar concentrations (GI50 = 91 nm) [102]. The total synthesis of (–)-berkelic acid (154) was previously described [103]. An alga-derived fungus, P. thomii, yielded a new citrinin analogue, sargassopenilline C (155), which possessed a unique 6,6-spiroketal skeleton and inhibited the transcription of oncogenic nuclear factor, AP-1 (IC50 = 15 μM) [104]. Two phenalenone-skeleton citrinin analogues, sculezonones A and B (156–157), isolated from a marine sponge-derived fungus Penicillium sp., inhibited both DNA polymerases (α and β) [105]. Dicitrinone B (158), a marine sediment-derived fungal metabolite (P. citrinum) containing a rare carbon-bridge citrinin dimer, induced A-375 cell apoptosis by generating ROS via a caspase-related pathway [106]. In another study, two novel skeletal metabolites (159–160) possibly biogenetically derived from citrinin were found. Perinadine A (159), a scalusamide A-type pyrrolidine isolated from a fish gastrointestinal fungus, P. citrinum, exhibited mild cytotoxicity against a murine leukemia L1210 cell line (IC50 = 20 μg/mL) [107]. However, herqueiazole (160), obtained from a marine sediment-derived fungus, Penicillium sp. F011, possessed a novel pyrrole-containing phenalenone moiety and demonstrated weak cytotoxicity against A549 cells (IC50 = 67.3 μM), while doxorubicin was used as a positive control with an IC50 value of 3.3 μM [108].
Figure 23.
Chemical structures of compounds 151–160.
Other fungal azaphilone polyketides include comazaphilones D–F (161–163) (Figure 24), pinophilins A, B, and Sch 725680 (164–166), which were isolated from a marine sediment-derived fungus, P. commune QSD-17 (comazaphilones D–F), and a marine seaweed-derived P. pinophilum Hedgcok (pinophilins A-B and Sch 725680). Comazaphilones D–F (161–163) showed selective but weak cytotoxicity against SW1990 cell line (IC50 = 51, 26, and 53 μM, respectively), while fluoruoracil was used as a positive control with an IC50 value of 120 μM) [109]. Azaphilone derivatives (164–166) were suggested to suppress cancer cell proliferation by inhibiting DNA replication via the inhibition of mammalian DNA polymerases A, B, and Y [110].
Figure 24.
Chemical structures of compounds 161–166.
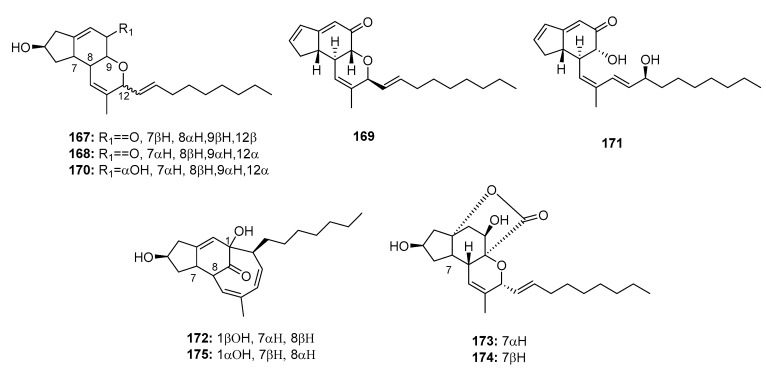
Penicillium sp. strain OUPS-79, which is derived from the marine alga Enteromorpha intestinalis, yielded various cytotoxic polyketides, including penostatins A–C, E–I (167–169, 171–175) (Figure 25) [111,112]. They were found to be significantly cytotoxic to P388 lymphocytic leukemia cells (ED50 = 0.8, 1.2, 1.0, 0.9, 1.4, 0.5, 0.8, and 1.2 μg/mL, respectively). However, penostatin D (170) exhibited moderate cytotoxicity (ED50 = 11.0 μg/mL), which may be attributed to the absence of the cyclic conjugated enone system. Moreover, penostatin C (169) exhibited significant cytotoxicity in seven of the 36 cell lines tested with ED50 values ranging from 1 to 2 μg/mL. Recent studies have shown that penostatins A–C (167–169) may be tyrosine phosphatase 1B (PTP1B) inhibitors, which can be used to treat type II diabetes and other associated metabolic diseases (IC50 = 15.87, 33.65, and 0.37 μM, respectively), while sodium orthovanadate was used as a positive control with an IC50 value of 0.65 μM [113]. The total synthesis of penostatins A, B, and F (167, 168, 172) was previously reported [114,115].
Figure 25.
Chemical structures of compounds 167–174.
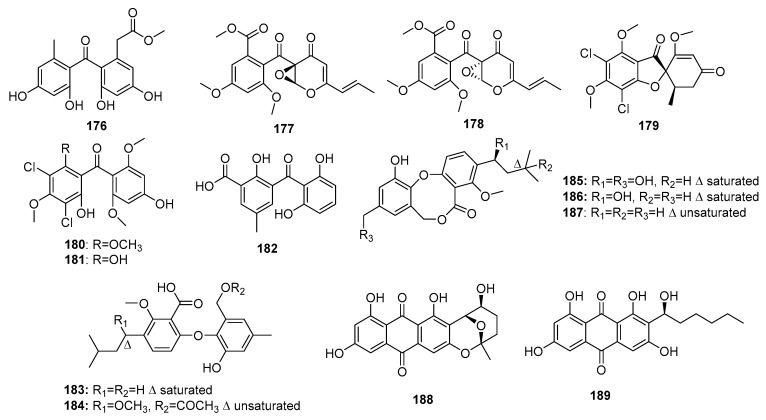
Fungal phenolic polyketides have diverse biological activities and unique structures [116]. A weak DNA topoisomerase Ι inhibitor, compound (176) (Figure 26), was obtained from the marine sediment-derived P. oxalicum HSY05 [117], whereas a racemic mixture (177–178) was obtained from the co-cultivation of marine mangrove-derived Penicillium sp. WC-29-5 and Streptomyces fradiae 007. Compounds 177–178 displayed significant cytotoxicity against H1975 cell lines (IC50 = 3.97 and 5.73 μM, respectively). Moreover, compound 178 exhibited cytotoxicity against HL-60 cells (IC50 = 3.73 μM) [118]. Using a bioinformatics tool, Marine Halogenated Compound Analysis (MeHaloCoA), three halogenated bioactive metabolites, (+)-5-chlorogriseofulvin (179) as well as griseophenones I and G (180–181), were isolated from a marine-derived P. canescens. They inhibited the growth of KB cells at a concentration of 0.6 μM (IR% = 49, 58, and 47%, respectively) [119]. Furthermore, one benzophenone, iso-monodictyphenone (182), and two diphenyl ether derivatives, penikellides A and B (183–184), were isolated from a mangrove endogenous fungus, Penicillium sp. MA-37. These three metabolites exhibited moderate brine shrimp lethality (LD50 = 25.3, 14.2, and 39.2 μM, respectively), while colchicine was used as a positive control with an LD50 value of 1.22 μM [120]. Penicillide (185), a multifunctional metabolite produced by a marine sediment-derived Penicillium sp. strain, was shown to be an acyl-CoA cholesterol acyltransferase (ACAT) [121], nonpeptide calpain inhibitor [122], and oxytocin antagonist [123]. Furthermore, compound 185 was found to exhibit cytotoxic, antibiotic, and plant growth inhibitory properties. Recently, two marine fungi, P. pinophilum (derived from a gorgonian) and Penicillium sp. ZLN29 (derived from a sediment), were found to produce penicillide (185) and penicillide derivatives (186–187) that exhibited potent cytotoxicity against HepG2 cell line (IC50 = 9.7 and 9.9 μM for 185–186, respectively); moreover, compound 187 showed additional cytotoxicity against Hela cell line (IC50 = 6.1 μM) [124,125]. Two anthraquinone derivatives, nidurufin (188) and averantin (189), isolated from a marine sediment-derived fungus, P. flavidorsum SHK1-27, were cytotoxic against K562 cell line (IC50 = 12.6 and 27.7 μM, respectively), while adriamycin was used as a positive control with an IC50 value of 1.5 μM. Nidurufin (188) was suggested to induce cell cycle arrest at the G2/M transition in a time-dependent manner [126]. The total synthesis of (±)-nidurufin (188), an aflatoxin precursor, was previously described [127].
Figure 26.
Chemical structures of compounds 176–189.
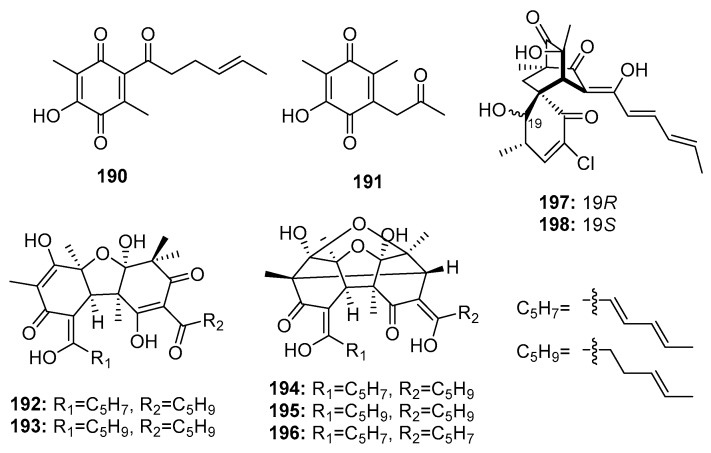
Members of the sorbicillinoid family are hexaketide metabolites isolated from various fungi. In 2005, Zhu et al. found two sorbicillin analogues (benzoquinone (190–191)), two bisvertinolones (192–193), and three bridged bicyclic bisorbicillinoids (194–196) (Figure 27) in a marine sediment-derived fungus, P. terrestre. Dihydrobisvertinolone (192) and trichodimerol (196) demonstrated the strongest cytotoxic effects (IC50 = 0.52 μM in A549, IC50 = 0.33 μM in P388, respectively), while etoposide was used as a positive control with IC50 values of 1.4 and 0.064 μM, respectively [128,129]. The preliminary SAR showed that an intact sorbyl side chain played a decisive role [130]. Further investigation of this strain yielded two additional chlorinated sorbicillinoids (197–198). Interestingly, the configuration at C-19 was found to largely determine the cytotoxicity, wherein chloctanspirone A (197) (R configuration) was 4-fold more active than chloctanspirone B (198) (S configuration) in HL-60 and A549 cancer cell lines [131].
Figure 27.
Chemical structures of compounds 190–198.
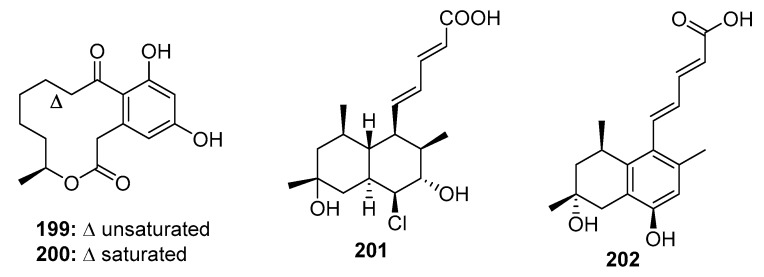
Macrolides represent a well-known class of antibiotics, and curvularin (200) (Figure 28) is a heat shock protein (HSP90) inhibitor [132]. (10E, 15S)-10,11-Dehydrocurvularin (199) was isolated from marine sponge-derived Penicillium sp. DRF2 and Curvularia sp. It exhibited significant cytotoxicity with mean IC50 values ranging from 0.28 to 6 μM in 14 different solid tumors (36 tumor cell lines) [133,134]. Penicillium fungi are also a good source of tanzawaic acid polyketides, which exhibit antibiotic resistance [135], as well as anti-inflammatory [136] and cytotoxic activities. Tanzawaic acid P (201), isolated from a marine-derived fungus, Penicillium sp. CF07370, was selectively toxic to U937 cancer cells via the activation of the mitochondrial apoptotic pathway [137]. Computational ligand-protein-DNA binding analysis revealed that tanzawaic acid D (202), isolated from P. steckii, effectively and selectively bound to the transcription factor, forkhead box O1 (FOXO1), which can regulate epidermal growth factor receptor (EFGR) signaling, suppress cell cycle progression, and stabilize the conformation of FOXO1-DNA [138].
Figure 28.
Chemical structures of compounds 199–202.
5. Lipopeptides
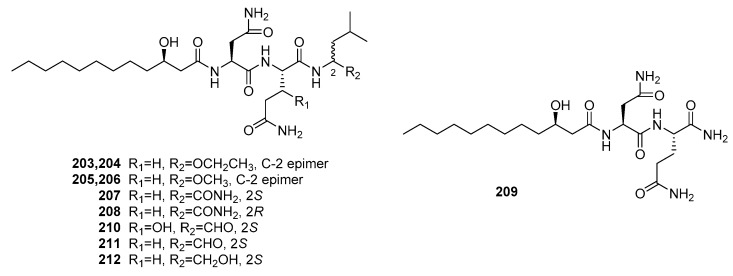
Fellutamides A and B (210–211) (Figure 29) were the first cytotoxic lipopeptides isolated from fish-derived P. fellutanum [139]. Compounds 210 and 211 exhibited significant cytotoxicity against murine leukemia P388 (IC50 = 0.2 and 0.1 μg/mL, respectively), L1210 (IC50 = 0.8 and 0.7 μg/mL, respectively), and KB cells (IC50 = 0.5 and 0.7 μg/mL, respectively). Recently, seven new similar lipopeptides, penicimutalides A–G (203–209) and fellutamides B and C (211–212) were isolated from a diethyl sulfate-induced mutant of the marine fungus, P. purpurogenum G59 [140]. They were cytotoxic against five human cancer cell lines (K562, HL-60, Hela, BGC-823, and MCF-7). Compounds 203–209 and 212 exhibited weak cytotoxicity (IR% = 10–50% at 100 μg/mL, while 5-fluoruoracil as a positive control with the IR% of 37~50% at 100 μg/mL). However, fellutamide B (211) with a C-terminal aldehyde group was more potent with IC50 values that ranged from 20 to 80 μg/mL, which indicated that the C-terminal aldehyde group improves the cytotoxicity.
Figure 29.
Chemical structures of compounds 203–212.
6. Miscellaneous Compounds
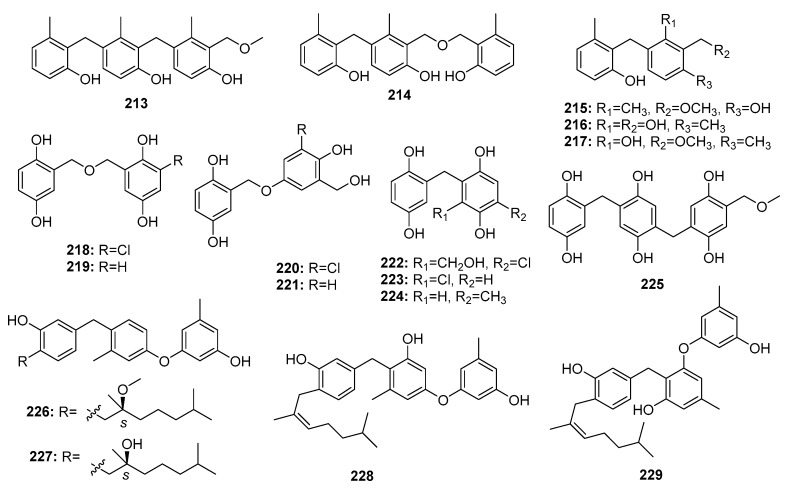
Polyphenol derivatives are the most abundant fungal secondary metabolites. Unsurprisingly, marine Penicillium sp. is a good source of polyphenol derivatives. Two trimeric peniphenylanes A, B (213–214) and three dimeric peniphenylanes D, F, G (215–217) (Figure 30) were isolated from the deep sea sediment-derived fungus, P. fellutanum HDN14-323. Peniphenylane D (215) displayed more potent and extensive cytotoxicity with IC50 values in the range of 9–30 μM in three cancer cell lines (Hela, HL-60, and HCT-116), while doxorubicin was used as a positive control with the IC50 values of 0.2, 0.6, and 0.2 μM, respectively [141]. The marine sediment-derived fungus, P. terrestre was found to produce several gentisyl alcohol derivatives, including trimeric terrestrol A (225) and dimeric terrestrols B–H (218–224), which were found to be cytotoxic against HL-60, MOLT-4, BEL-7402, and A549 cancer cell lines with IC50 values in the range of 5–65 μM [142]. Interestingly, the marine mangrove endogenous P. expansum 091006 yielded four novel cytotoxic phenolic bisabolane sesquiterpenoids (expansols A–C; E (226–229)) with IC50 values of 15.7, 5.4, 18.2, and 20.8 μM, respectively, in HL-60 cells. In addition, expansol B (227) showed significant cytotoxicity against A549 cells (IC50 = 1.9 μM), while etoposide was used as a positive control with IC50 values of 0.042 and 0.63 μM for two cell lines, respectively [143,144].
Figure 30.
Chemical structures of compounds 213–229.
Patulin (230) (Figure 31) is a mycotoxin commonly found in rotting fruits, and is used as a potassium-uptake inhibitor or inducer of ion flux across cell membranes. An alga-derived Penicillium sp. was found to produce patulin (230) along with (+)-epiepoxydon (231), both of which exhibited extraordinary cytotoxic effects in P388 cells (IC50 = 0.06 and 0.2 μg/mL, respectively). Furthermore, (+)-epiepoxydon (231) had significant cytotoxicity against seven other cancer cell lines with IC50 values in the range of 0.3–1.5 μg/mL [111]. The isobenzofurannone derivative (232) isolated from a mangrove endophytic Penicillium sp. displayed moderate cytotoxicity against KB and KBV200 cells (IC50 = 6 and 10 μg/mL, respectively) [145], whereas the penicillic acid (233), isolated from marine-derived Penicillium strain, exhibited moderate cytotoxicity against POS1, AT6-1(murine prostatic carcinoma), and L929 (murine fibroblasts) cell lines (IC50 = 7.8, 29.4, and 12.9 μM, respectively) while doxorubicin was used as a positive control with IC50 values of 0.04~2 μM [84].
Figure 31.
Chemical structures of compounds 230–233.
7. Conclusions
The rapid development of marine biotechnology and ever increasing needs of industrial applications resulted in the emergence of marine natural products as alternative drug sources in the early 1990s [146]. Marine-associated microorganisms are sensitive to culture conditions; therefore, strains living in extremely competitive environments tend to provide high potency leads (compound 154 in this review inhibited OVCAR-3 cell line at nanomolar concentrations). Furthermore, the activation of silent gene clusters may activate new biosynthetic pathways that produce compounds with novel structure, which provide equally valid leads (compounds 44 and 45, which have unique skeletons, had cytotoxic effects in the five cancer cell lines with IC50 values of ~10 μM). Interestingly, the halogenation of compound 31, which was completely inactive, produces compound 32, which exhibited a much greater potency (compound 32 had significant cytotoxicity in 22Rv1 cells at nanomolar levels) [147].
The genus Penicillium has been explored for antitumor leads in recent years [148]. However, the marine ecological diversity of this genus offers more opportunities for drug discovery. This review includes more than 200 cytotoxic or antitumor compounds isolated from marine Penicillium fungus and chemically synthesized analogues. Of these, the major metabolites are alkaloids, particularly diketopiperazine alkaloids and indole alkaloids (Appendix A, Table A1). Cytochalasan alkaloids, which are indole alkaloids, constitute a large class of mycotoxins that exhibit significant cytotoxicity against P388 cells (IC50 < 1 μg/mL). Furthermore, a series of diketopiperazine alkaloids, gliotoxin analogues, and roquefortine analogues with remarkable cytotoxicity at nanomolar levels are potential anticancer leads. Terpenoid metabolites appear to be more effective against cancer cell lines than steroids; in particular, compounds 99, 104, and 132 were effective at nanomolar levels. Furthermore, citrinins (chromone analogues) and their derivatives, which are polyketide mycotoxins, possess excellent cytotoxic activities. Penostatins (cytotoxic polyketides) are cytotoxic to P388 cells with IC50 values of ~1 μg/mL. With the exception of 210 and 211, lipopeptides exhibited moderate cytotoxicity. In addition, the Penicillium genus can produce polyphenolic compounds (terrestrols) with pronounced cytotoxicity.
Although our review includes most of the cytotoxic metabolites described in the literature, more compounds are yet to be identified in marine Penicillium sp. Different marine hosts and environments can also affect the biosynthesis of metabolites by endozoic fungi. Notably, over 99% of the symbiotic microorganisms cannot be cultured. Further investigations may utilize metagenome libraries of the host organisms to identify more metabolites produced by symbiotic microorganisms [149]. Additionally, further studies are needed to explore the functional mechanisms of the bioactive compounds and to optimize their production.
Acknowledgments
This research was a part of a project entitled ‘Omics based fishery disease control technology development and industrialization’ funded by the Korean Ministry of Oceans and Fisheries.
Appendix A
Table A1.
Secondary metabolites from Penicillium strain of marine origin. Items are listed according to the metabolite numbers used in this review.
| Metabolites | Producing Stain | Environment Source | Type | Cell Lines/Brine Shrimp | IC50, LD50, or IR (%) | Target | Reference |
|---|---|---|---|---|---|---|---|
| Penochalasin A (1) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 0.4 μg/mL | [8] | |
| Penochalasin B (2) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 0.3 μg/mL | [8] | |
| Penochalasin C (3) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 0.5 μg/mL | [8] | |
| Penochalasin D (4) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 3.2 μg/mL | [7] | |
| Penochalasin E (5) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 2.1 μg/mL | [7] | |
| Penochalasin F (6) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 1.8 μg/mL | [7] | |
| Penochalasin G (7) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 1.9 μg/mL | [7] | |
| Penochalasin H (8) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 2.8 μg/mL | [7] | |
| Penochalasin I (9) | P. chrysogenum V11 | Mangrove | Indole alkaloid | MDA-MB-435, SGC-7901, A549 | (7.55, 7.32, 16.13) μM | [6] | |
| Penochalasin J (10) | P. chrysogenum V11 | Mangrove | Indole alkaloid | MDA-MB-435, SGC-7901, A549 | (36.68, 37.70, 35.93) μM | [6] | |
| Chaetoglobosin A (11) | P. chrysogenum V11 | Mangrove | Indole alkaloid | P388, MDA-MB-435, SGC-7901, A549 | 0.6 μg/mL (37.56, 7.84, 6.56) μM | Cell-cycle arrest induction, membrane ruffling inhibition, and cell migration | [6,8,9] |
| Chaetoglobosin C (12) | P. chrysogenum V11 | Mangrove | Indole alkaloid | MDA-MB-435, SGC-7901, A549 | (19.97, 15.36, 17.82) μM | [6] | |
| Chaetoglobosin E (13) | P. chrysogenum V11 | Mangrove | Indole alkaloid | A549 | 36.63 μM | [6] | |
| Chaetoglobosin F (14) | P. chrysogenum V11 | Mangrove | Indole alkaloid | P388, MDA-MB-435, SGC-7901, A549 | 0.9 μg/mL, (37.77, 26.53, 27.72) μM | [6,8] | |
| Chaetoglobosin G (15) | P. chrysogenum V11 | Mangrove | Indole alkaloid | MDA-MB-435, SGC-7901, A549 | (38.77, 25.86, 27.63) μM | [6] | |
| Chaetoglobosin O (16) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 2.4 μg/mL | [7] | |
| Cytoglobosin C (17) | P. chrysogenum V11 | Mangrove | Indole alkaloid | MDA-MB-435, SGC-7901, A549 | (12.58, 8.15, 3.35) μM | [6] | |
| 18 | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 3.4 μM | [12] | |
| 19 | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 0.058 μM | HMT G9a (IC50 = 55 μM) | [12] |
| 20 | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 0.11 μM | [12] | |
| 21 | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 0.11 μM | HMT G9a (IC50 = 58 μM) | [12] |
| 22 | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 0.056 μM | HMT G9a (IC50 = 2.6 μM) | [12] |
| Gliotoxin (23) | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 0.024 μM | HMT G9a (IC50 = 6.4 μM) Dual inhibitor of farnesyltransferase and geranylgeranyltransferase I |
[12,13] |
| Gliotoxin G (24) |
Penicillium sp. JMF034 P. brocae MA-231 |
Deep sea sediment Mangrove | Diketopiperazine | P388 A2780, A2780 CisR | 0.02 μM (0.664, 0.661) μM | HMT G9a (IC50 = 2.1 μM) | [12] [14] |
| Brozazine A (25) | P. brocae MA-231 | Mangrove | Diketopiperazine | Du145, Hela, HepG2, MCF-7, NCI-H460, SGC-7901, SW1990, SW480, U251 | (4.2, 6.8, 6.4, 5.5, 4.9, 2.6, 6.0, 2.0, 5.2) μM | [16] | |
| Brozazine B (26) | P. brocae MA-231 | Mangrove | Diketopiperazine | Du145, Hela, HepG2, MCF-7, NCI-H460, SGC-7901, SW1990, SW480, U251 | (3.6, 5.3, 5.5, 6.1, 4.0, 2.4, 6.4, 1.2, 3.5) μM | [16] | |
| Brozazine E (29) | P. brocae MA-231 | Mangrove | Diketopiperazine | Du145, Hela, HepG2, MCF-7, NCI-H460, SGC-7901, SW1990, U251 | (11.2, 4.3, 5.6, 9.0, 12.4, 3.3, 2.1, 6.1) μM | [16] | |
| Brozazine F (30) | P. brocae MA-231 | Mangrove | Diketopiperazine | Du145, Hela, HepG2, MCF-7, NCI-H460, SGC-7901, SW1990, U251 | (1.7, 6.9, 2.9, 3.0, 0.89, 8.0, 5.9, 5.3) μM | [16] | |
| N-methylpretrichodermam ide B/adametizines A (32) |
P. adametzioides AS-53 Penicillium sp. |
Marine sponge/sediment/alga | Diketopiperazine | Artemia salina | 4.8 μM | [17,18,19] | |
| L5178Y, 22Rv1, PC-3, LNCaP | (2, 0.51, 5.11, 1.76) μM | ||||||
| Roquefortine C (33) | Penicillium sp. | Deep sea sediment | Diketopiperazine | Activate P-glycoprotein and inhibit P450-3A and other haemoproteins | [20,22] | ||
| Roquefortine F (34) | Penicillium sp. | Deep sea sediment | Diketopiperazine | A549, HL-60, BEL-7402, MOLT-4 | (14.0, 33.6, 13.0, 21.2) μM | [22] | |
| Roquefortine G (35) | Penicillium sp. | Deep sea sediment | Diketopiperazine | A549, HL-60 | (42.5, 36.6) μM | [22] | |
| Meleagrin (38) |
Penicillium sp. P. commune SD-118 |
Deep sea sediment | Indole alkaloid | A549, HL-60 HepG2, NCI-H460, Hela, DU145, MDA-MB-231, | (19.9, 7.4) μM (12.0, 22.0, 20.0, 11.0, 5.0) μg/mL | Arrest the cell cycle through G2/M phase Inhibitor of tubulin polymerization |
[21,22,23] |
| Meleagrin B (39) | Penicillium sp. | Deep sea sediment | Indole alkaloid | A549, HL-60, BEL-7402, MOLT-4 | (2.7, 6.7, 1.8, 2.9) μM | [21,22] | |
| Meleagrin C (40) | Penicillium sp. | Deep sea sediment | Indole alkaloid | A549, BEL-7402, MOLT-4 | (9.9, 10.0, 4.7) μM | [22] | |
| Meleagrin D (41) | Penicillium sp. | Deep sea sediment | Indole alkaloid | A549 | 32.2 μM | [21] | |
| Meleagrin E (42) | Penicillium sp. | Deep sea sediment | Indole alkaloid | A549 | 55.9 μM | [21] | |
| Penicimutanin (43) | Mutant P. purpurogenum G59 | Marine soil | Diketopiperazine | K562, Hela, MCF-7 | IR% (100 μg/mL): 22.6%, 17.9%, 26.5% | [24] | |
| Penicimutanin A (44) | Mutant P. purpurogenum G59 | Marine soil | Diketopiperazine | K562, HL-60, Hela, BGC-823, MCF-7 | (11.4, 5.4, 9.5, 8.0, 5.4) μM | [24] | |
| Penicimutanin B (45) | Mutant P. purpurogenum G59 | Marine soil | Diketopiperazine | K562, HL-60, Hela, BGC-823, MCF-7 | (19.9, 12.1, 17.7, 16.6, 8.0) μM | [24] | |
| Fructigenine A (46) | Mutant P. purpurogenum G59 | Marine soil | Diketopiperazine | K562, Hela, MCF-7, BGC-823 | IR% (100μg/mL): 20.8%, 55.3%, 65.6%, 34.8% | [24,25] | |
| 11,11′-dideoxyverticillin A (49) | Penicillium sp. | Marine alga | Diketopiperazine | HCT-116 | 30 ng/mL | Induce G2/M arrest through p38 MAPK pathway; Epidermal growth factor receptor tyrosine kinase inhibitor |
[28,30,31] |
| 11′-deoxyverticillin A (50) | Penicillium sp. | Marine alga | Diketopiperazine | HCT-116 | 30 ng/mL | [28] | |
| Penitrem A (51) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) MDA-MB-231 (anti-invasion) |
(11.9, 9.8) μM 8.7 μM IR% (15 μM)> 75% |
BK channel inhibitor | [34] |
| Penitrem B (52) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) |
(5.5, 13.7) μM 10.3 μM |
[34] | |
| Penitrem D (53) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) |
(8.3, 29.7) μM 9.2 μM |
[34] | |
| Penitrem E (54) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) |
(17.5, 25.4) μM 20.3 μM |
[34] | |
| Penitrem F (55) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) |
(15.0, 13.8) μM 35.0 μM |
[34] | |
| 6-bromopenitrem B (56) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) MDA-MB-231 (anti-invasion) |
(19.3, 18.8) μM 30.3 μM IR%(15 μM) > 40% |
[34] | |
| 6-bromopenitrem E (57) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) |
(16.7, 8.5) μM 9.6 μM |
BK channel inhibitor | [34] |
| Paspaline (58) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) |
(12.8, 12.4) μM 7.6 μM |
[34] | |
| Emnidole SB (59) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) |
(10.1, 21.3) μM 19.0 μM |
[34,37] | |
| Communesin A (60) | Penicillium sp. | Marine alga/Sediment | Indole alkaloid | P388 | 3.5 μg/mL | [38,40] | |
| Communesin B (61) | Penicillium sp. | Marine alga/Sponge/Sediment | Indole alkaloid | P388, U-937, THP-1, NAMALWA, MOLT-3, SUP-B15 | (0.45, 10.4, 11.4, 9.9, 8.1, 7.2) μg/mL | [38,39,40] | |
| Communesin C (62) | Penicillium sp. | Marine sponge | Indole alkaloid | U-937, THP-1, NAMALWA, MOLT-3, SUP-B15 | (11.3, 13.1, 8.2, 8.6, 10.8) μg/mL | [39] | |
| Communesin D (63) | Penicillium sp. | Marine sponge | Indole alkaloid | U-937, THP-1, NAMALWA, MOLT-3, SUP-B15 | (13.1, 16.2, 14.6, 9.9, 9.0) μg/mL | [39] | |
| Penioxamide (64) | P. oxalicum EN-201 | Mangrove | Indole alkaloid | A. salina | 5.6 μM | [42] | |
| 65 | P. brefeldianum SD-273 | Marine sediment | Indole alkaloid | A. salina | 9.4 μM | [43] | |
| Penipaline B (66) | P. paneum SD-44 | Marine sediment | Indole alkaloid | A549, HCT-116 | (20.44, 14.88) μM | [44] | |
| Penipaline C (67) | P. paneum SD-44 | Marine sediment | Indole alkaloid | A549, HCT-116 | (21.54, 18.54) μM | [44] | |
| Terretrione A (68) | P. vinaceum | Marine sponge | 1,4-diazepane alkaloid | MDA-MB-231 | 17.7 μM | [45] | |
| Terretrione C (69) | Penicillium sp.CYE-87 | Marine tunicate | 1,4-diazepane alkaloid | MDA-MB-231 | 17.6 μM | [46] | |
| Terretrione D (70) | Penicillium sp.CYE-87 | Marine tunicate | 1,4-diazepane alkaloid | MDA-MB-231 | 16.5 μM | [46] | |
| Penicillenol A1 (71) | Penicillium sp. GQ-7/P. citrinum | Mangrove/Marine sediment | Pyrrolidinone alkaloid | A-375, HL-60, A549, BEL-7402, P388 | 3.2 μg/mL (0.76, 23.8, 13.03, 8.85) μM | [47,49] | |
| Penicillenol A2 (72) | Penicillium sp. GQ-7/P. citrinum | Mangrove/Marine sediment | Pyrrolidinone alkaloid | A-375 HL-60 |
13.8 μg/mL 16.26 μM |
[47,49] | |
| Penicillenol B1 (73) | Penicillium sp. GQ-7/P. citrinum | Mangrove/Marine sediment | Pyrrolidinone alkaloid | A-375 HL-60 |
2.8 μg/mL 3.2 μM |
[47,49] | |
| Penicillenol B2 (74) | Penicillium sp. GQ-7/P. citrinum | Mangrove/Marine sediment | Pyrrolidinone alkaloid | A-375 HL-60 |
0.97 μg/mL 7.65 μM |
[47,49] | |
| Penicillenol D1 (75) | P. citrinum | Marine sediment | Pyrrolidinone alkaloid | A549, HL-60 | (17.2, 18.5) μg/mL | [48] | |
| Penicillenol D2 (76) | P. citrinum | Marine sediment | Pyrrolidinone alkaloid | A549, HL-60 | (12.1, 14.5) μg/mL | [48] | |
| Penitrinine A (77) | P. citrinum | Marine sediment | Pyrrolidinone alkaloid | A-375, SPC-A1, HGC-27 | (20.12, 28.67, 29.49) μM | Upregulate Bax, downregulate Bcl-2, suppress MMP-9 and TIMP-1 | [50] |
| 78 | P. janczewskii | Sea water | Quinolinone | MDA-MB-231, DU-145, SKOV-3, HT-29, A549, CAKI-1, SK-MEL-2, K562 | IR % (10 μg/mL) = 20~50% | [52] | |
| 79 | P. janczewskii | Sea water | Quinolinone | MDA-MB-231, DU-145, SKOV-3, HT-29, A549, CAKI-1, SK-MEL-2, K562 | IR % (10 μg/mL) = 30~90% | [52] | |
| 80 | P. janczewskii | Sea water | Quinolinone | MDA-MB-231, DU-145, SKOV-3, HT-29 | IR % (10 μg/mL) = 91.6%, 69.2%, 79.8%, 96.0% | [52] | |
| 81 | Penicillium sp. ghq208/Penicillium sp. | Marine sediment/Mangrove | Quinolinone | 95-D, HepG2 | (0.57, 6.5) μg/mL | [53,54] | |
| 82 | Penicillium sp. ghq208 | Marine sediment | Quinolinone | HepG2 | 13.2 μM | [53] | |
| 83 | P. commune SD-118 | Deep sea sediment | Quinazolinone | SW1990 | 20 μg/mL | [23] | |
| 84 | P. chrysogenum EN-118 | Marine alga | Quinazolinone | DU145, A549, Hela | 8 μg/mL | [56] | |
| 85 | P. oxalicum 0312F1 | Marine (not clear) | Quinazolinone | SGC-7901, BEL-7404 | IR % (200 μg/mL) = 30~40% | [55] | |
| Penipacid A (86) | P. paneum SD-44 | Deep sea sediment | Amidine alkaloid | RKO | 8.4 μM | [57] | |
| Penipacid E (87) | P. paneum SD-44 | Deep sea sediment | Amidine alkaloid | RKO | 9.7 μM | [57] | |
| 88 | P. paneum SD-44 | Deep sea sediment | Imine alkaloid | Hela | 6.6 μM | [57] | |
| Penipanoid A (89) | P. paneum SD-44 | Deep sea sediment | Triazole alkaloid | SMMC-7721 | 54.2 μM | [58] | |
| Bis-sclerotioramin (90) | Penicillium 303# | Mangrove | Azaphilone alkaloid | MDA-MB-231 | 7.13 μM | [59] | |
| Sorbicillactone (91) | P. chrysogenum | Marine sponge | Miscellaneous Alkaloid | L5178Y | 2.2 μg/mL | Selective anti-leukemic | [60] |
| Brocaeloid B (92) | P. brocae | Mangrove | Miscellaneous Alkaloid | A. salina | 36.7 μM | [62] | |
| Varitatin (93) | Mutant P. varibile | Mangrove | Amide alkaloid | HCT-116 | 2.8 μM | IR%(1μM) = 50% and 40% (PDGFR-βand ErbB4) | [63] |
| 18-hydroxydecaturin B (94) | P. oxalicum EN-201 | Mangrove | Pyridinyl-α-pyrone alkaloid | A. salina | 2.3 μM | [42] | |
| Xantocillin X (95) | P. commune SD-118 | Deep sea sediment | Isocyanide alkaloid | MCF-7, HepG2, NCI-H460, Hela, DU145, MDA-MB-231 | (12, 7, 10, 10, 8, 8) μg/mL | Inhibit MEK/EPK pathway and activate class III PI3K/Beclin 1 pathway | [23,67] |
| 96 | Penicillium sp. PR19 N-1 | Marine sludge | Sesquiterpene | HL-60, A549 | (45.8, 82.8) μM | [70] | |
| 97 | Penicillium sp. PR19 N-1 | Marine sludge | Sesquiterpene | HL-60, A549 | (28.3, 5.2) μM | [70] | |
| 98 | Penicillium sp. PR19 N-1 | Marine sludge | Sesquiterpene | HL-60, A549 | (11.8, 12.2) μM | [71] | |
| 99 | Penicillium sp. BL 27-2 | Sea mud | Sesquiterpene | P388, A549, HL-60, BEL-7402 | (0.073, 0.096, 0.065, 4.59) μM | [72] | |
| Sporogen-AO 1 (100) | Penicillium sp. BL 27-2 | Sea mud | Sesquiterpene | P388, A549, HL-60, BEL-7402 | (10.1, 8.81, 10.4, 5.7) μM | [72] | |
| 101 | Penicillium sp. BL 27-2 | Sea mud | Sesquiterpene | P388, A549, HL-60, BEL-7402 | (8.71, 3.51, 7.75, 11.8) μM | [72] | |
| Adametacorenol B (102) | P. adametzioides AS-53 | Marine sponge | Diterpene | NCI-H446 | 5.0 μM | [17] | |
| Conidiogenone B (103) | Penicillium sp. | Deep sea sediment | Diterpene | A549, HL-60 | (40.3, 28.2) μM | [22] | |
| Conidiogenone C (104) | Penicillium sp. | Deep sea sediment | Diterpene | HL-60, BEL-7402 | (0.038, 0.97) μM | [22] | |
| Conidiogenone D (105) | Penicillium sp. | Deep sea sediment | Diterpene | A549, HL-60, BEL-7402, MOLT-4 | (9.3, 5.3, 11.7, 21.1) μM | [22] | |
| Conidiogenone E (106) | Penicillium sp. | Deep sea sediment | Diterpene | A549, HL-60, MOLT-4 | (15.1, 8.5, 25.8) μM | [22] | |
| Conidiogenone F (107) | Penicillium sp. | Deep sea sediment | Diterpene | A549, HL-60, BEL-7402 | (42.2,17.8, 17.1) μM | [22] | |
| Conidiogenone G (108) | Penicillium sp. | Deep sea sediment | Diterpene | A549, HL-60, BEL-7402, MOLT-4 | (8.3, 1.1, 43.2, 4.7) μM | [22] | |
| Brevione I (109) | Penicillium sp. | Deep sea sediment | Diterpene | MCF-7 | 7.44 μM | [73] | |
| Brevione A (110) | Penicillium sp. | Deep sea sediment | Diterpene | MCF-7 | 28.4 μM | [73] | |
| Penicisteroid A (111) | P. chrysogenum QEN-24S | Marine alga | Steroid | Hela, SW1990, NCI-H460 | (15, 31, 40) μg/mL | [74] | |
| 112 | Penicillium sp. | Marine moss | Steroid | HepG2 | 10.4 μg/mL | [75] | |
| 113 | Penicillium sp. | Marine moss | Steroid | HepG2 | 15.6 μg/mL | [75] | |
| 114 | Penicillium sp. | Marine moss | Steroid | HepG2 | 20.7 μg/mL | [75] | |
| 115 | Penicillium sp. | Marine moss | Steroid | HepG2 | 16.8 μg/mL | [75] | |
| 116 | Penicillium sp. | Marine moss | Steroid | HepG2 | 21.3 μg/mL | [75] | |
| 117 | P. stoloniferum QY2-10 | Sea squirt | Steroid | P388 | 4.07 μM | [76] | |
| 118 | Penicillium sp. | Marine sponge | Steroid | K562 | 5.54 μM | [77] | |
| 119 | Penicillium sp. | Marine sponge | Steroid | K562 | 4.38 μM | [77] | |
| Dankasterone A (120) | Penicillium sp. | Marine sponge | Steroid | HL-60, Hela, K562 | (0.78, 4.11, 7.57) μM | [77] | |
| Dankasterone B (121) | Penicillium sp. | Marine sponge | Steroid | HL-60, Hela, K562 | (3.25, 4.74, 7.89) μM | [77] | |
| 7-deacetoxyyanuthone (122) | Penicillium sp. | Marine (not clear) | Meroterpene | A549, SKOV-3, SKMEL-2, XF498, HCT-15 | (7.74, 6.35, 3.86, 10.04, 10.07) μg/mL | [79] | |
| Farnesylbenzenediol (123) | Penicillium sp. | Marine (not clear) | Meroterpene | A549, SKOV-3, SKMEL-2, XF498, HCT-15 | (4.73, 5.31, 4.80, 5.94, 6.11) μg/mL | [79] | |
| Farnesylquinone (124) | Penicillium sp. | Marine (not clear) | Meroterpene | A549, SKOV-3, SKMEL-2, XF498, HCT-15 | (25.44, 37.29, 18.41, 38.07, 42.56) μg/mL | [79] | |
| Penicillone A (125) | Penicillium sp. F11 | Marine (not clear) | Meroterpene | HT1080, Cne2 | (45.8, 46.2) μM | [80] | |
| Phenylpyropene E (126) | P. concentricum ZLQ-69 | Sea water | Sesquiterpene | MGC-803 | 19.1 μM | [81] | |
| Phenylpyropene F (127) | P. concentricum ZLQ-69 | Sea water | Sesquiterpene | MGC-803 | 13.6 μM | [81] | |
| Penicillipyrone A (128) | Penicillium sp. F446 | Marine sediment | Sesquiterpene | K562, A549 | (28, 15) μM | [82] | |
| Penicillipyrone B (129) | Penicillium sp. F446 | Marine sediment | Sesquiterpene | K562, A549 | (50, 17) μM | [82] | |
| 130 | Penicillium 303# | Mangrove | Meroterpene | MDA-MB-435, HepG2, HCT-116, A549 | (34.25, 24.56, 33.72, 37.82) μg/mL | [59] | |
| 131 | Penicillium 303# | Mangrove | Meroterpene | MDA-MB-435, HepG2, HCT-116, A549 | (31.32, 23.87, 29.19, 34.06) μg/mL | [59] | |
| Ligerin (132) | Penicillium sp. | Sea water | Merosesquiterpene | POS1, SaOS2, MG63 | (117/78, 137, 1459) nM | [84,85] | |
| Oxalicumone D (133) | P. oxalicum SCSGAF 0023 | Marine gorgonian | Chromone | BGC823, MOLT-4 | (10.10, 5.74) μM | [87] | |
| Oxalicumone E (134) | P. oxalicum SCSGAF 0023 | Marine gorgonian | Chromone | H1975, U937, K5652, BGC823, MOLT-4, MCF-7, HL-60, Huh-7 | (5.45, 4.16, 8.80, 1.96, 1.36, 4.32, 2.96, 6.33) μM | [87] | |
| Oxalicumone A (135) | P. oxalicum SCSGAF 0023 | Marine gorgonian | Chromone | H1975, U937, K5652, BGC823, MOLT-4, MCF-7, HL-60, Huh-7, A375, A549, Hela, HepG2, SW-620, L-02 | (10.38, 2.35, 4.53, 4.89, 0.30, 11.30, 2.55, 9.49, 11.7, 41.9, 46.2, 77.8, 22.6, 99.0) μM | [87,88] | |
| Oxalicumone B (136) | P. oxalicum SCSGAF 0023 | Marine gorgonian | Chromone | U937, MOLT-4, HL-60, A375, Hela, SW-620 | (5.00, 2.30, 6.41, 27.8, 60.9, 40.6) μM | [87,88] | |
| Chromosulfine (145) | Mutant P. purpurogenum G59 | Marine (not clear) | Chromone | K562, HL-60, BGC-823, Hela, MCF-7 | (60.8, 16.7, 73.8, 75.4, 59.2) μM | [91] | |
| Secalonic acid F (146) | Penicillium sp. | Deep sea sediment | Xanthone | HL-60 | Modulate Rho GDP dissociation inhibitor 2 Activate caspase 3 and caspase 9 | [92,93] | |
| Penimethavone A (147) | P. chrysogenum | Marine gorgonian | Flavone | Hela, rhabdomyosarcoma | (8.41, 8.18) μM | [94] | |
| Bipenicillisorin (148) | P. chrysogenum SCSIO 41001 | Deep sea sediment | Coumarin | K562, A549, Huh-7 | (6.78, 6.94, 2.59) μM | [95] | |
| Monocerin (149) | Penicillium sp. | Marine sponge | Coumarin | L5178Y | 8.4 μM | [96] | |
| 150 | Penicillium sp. ZH16 | Mangrove | Coumarin | KB, KBv200 | (5, 10) μg/mL | [97] | |
| Citrinin (151) | Penicillium sp FF001 | Marine sponge | Azaphilone polyketide | Inhibit respiration complex III | [99,101] | ||
| Penicitrinol L (152) | P. citrinum | Marine sediment | Azaphilone polyketide | SW-620 | 25.6 μM | [48] | |
| Penicitrinol M (153) | P. citrinum | Marine sediment | Azaphilone polyketide | SW-620 | 20.9 μM | [48] | |
| Berkelic acid (154) | Penicillium sp. | Acid mine lake | Azaphilone polyketide | OVCAR-3 (in NCI60) | 91 nM | Inhibit MMP-3 (GI50 = 1.87 μM) Inhibit caspase 1 (GI50 = 98 μM) | [102] |
| Sargassopenilline C (155) | P. thomii | Marine alga | Azaphilone polyketide | Inhibit the oncogenic nuclear factor AP-1 (IC50 = 15 μM) | [104] | ||
| Sculezonone A (156) | Penicillium sp. | Marine sponge | Azaphilone polyketide | Inhibit both DNA polymerases (α and β) | [105] | ||
| Sculezonone B (157) | Penicillium sp. | Marine sponge | Azaphilone polyketide | Inhibit both DNA polymerases (α and β) | [105] | ||
| Dicitrinone B (158) | P. citrinum | Marine sediment | Azaphilone polyketide | Induce apoptosis through ROS-related caspase pathway | [106] | ||
| Perinadine A (159) | P. citrinum | Marine fish | Azaphilone polyketide | L1210 | 20 μg/mL | [107] | |
| Herqueiazole (160) | Penicillium sp F011 | Marine sediment | Azaphilone polyketide | A549 | 67.3 μM | [108] | |
| Comazaphilone D (161) | P. commune QSD-17 | Marine sediment | Azaphilone polyketide | SW1990 | 51 μM | [109] | |
| Comazaphilone E (162) | P. commune QSD-17 | Marine sediment | Azaphilone polyketide | SW1990 | 26 μM | [109] | |
| Comazaphilone F (163) | P. commune QSD-17 | Marine sediment | Azaphilone polyketide | SW1990 | 53 μM | [109] | |
| Pinophilin A (164) | P. pinophilum Hedgcok | Marine seaweed | Azaphilone polyketide | A549, BALL-1, HCT-116, Hela, NUGC-3 | (52.5, 50.2, 51.3, 55.6, 54.7) μM | Inhibit the mammalian DNA polymerases A, B, Y family | [110] |
| Pinophilin B (165) | P. pinophilum Hedgcok | Marine seaweed | Azaphilone polyketide | A549, BALL-1, HCT-116, Hela, NUGC-3 | (93.1, 90.4, 92.5, 99.0, 96.8) μM | Inhibit the mammalian DNA polymerases A, B, Y family | [110] |
| Sch 725680 (166) | P. pinophilum Hedgcok | Marine seaweed | Azaphilone polyketide | A549, BALL-1, HCT-116, Hela, NUGC-3 | (65.7, 62.0, 64.6, 68.8, 66.4) μM | Inhibit the mammalian DNA polymerases A, B, Y family | [110] |
| Penostatin A (167) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 0.8 μg/mL | PTP1B inhibitor (IC50 = 15.87 μM) | [111,113] |
| Penostatin B (168) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 1.2 μg/mL | PTP1B inhibitor (IC50 = 33.65 μM) | [111,113] |
| Penostatin C (169) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388, BSY-1, MCF-7, HCC2998, NCI-H522, DMS114, OVCAR-3, MKN1 | (1.0, 2.0, 1.6, 2.0, 2.5, 1.9, 2.4, 1.7) μg/mL | PTP1B inhibitor (IC50 = 0.37 μM) | [111,113] |
| Penostatin D (170) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 11.0 μg/mL | [111] | |
| Penostatin E (171) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 0.9 μg/mL | [111] | |
| Penostatin F (172) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 1.4 μg/mL | [112] | |
| Penostatin G (173) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 0.5 μg/mL | [112] | |
| Penostatin H (174) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 0.8 μg/mL | [112] | |
| Penostatin I (175) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 1.2 μg/mL | [112] | |
| 176 | P. oxalicum HSY05 | Marine sediment | Phenolic polyketide | DNA topoisomerase I inhibitor | [117] | ||
| 177 | Co-cultured Penicillium sp. WC-29-5 | Mangrove | Phenolic polyketide | H1975 | 3.97 μM | [118] | |
| 178 | Co-cultured Penicillium sp. WC-29-5 | Mangrove | Phenolic polyketide | H1975, HL-60 | (5.73, 3.73) μM | [118] | |
| (+)-5-chlorogriseofulvin (179) | P. canescens MMS460 | Sea water | Phenolic polyketide | KB | IR% (0.6 μM) = 49% | [119] | |
| Griseophenone I (180) | P. canescens MMS460 | Sea water | Phenolic polyketide | KB | IR% (0.6 μM) = 58% | [119] | |
| Griseophenone G (181) | P. canescens MMS460 | Sea water | Phenolic polyketide | KB | IR% (0.6 μM) = 47% | [119] | |
| Iso-monodictyphenone (182) | Penicillium sp. MA-37 | Mangrove | Phenolic polyketide | A. salina | 25.3 μM | [120] | |
| Penikellide A (183) | Penicillium sp. MA-37 | Mangrove | Phenolic polyketide | A. salina | 14.2 μM | [120] | |
| Penikellide B (184) | Penicillium sp. MA-37 | Mangrove | Phenolic polyketide | A. salina | 39.2 μM | [120] | |
| Penicillide (185) | Penicillium sp. ZLN29 | Marine sediment | Phenolic polyketide | HepG2 | (6.7/9.7, 7.8) μM | ACAT and nonpeptide calpain inhibitor | [121,122,124,125] |
| Prepenicillide (186) | Penicillium sp. ZLN29 | Marine sediment | Phenolic polyketide | HepG2, RD | 9.9 μM | [124] | |
| Hydroxypenicillide (187) | P. pinophilum | Marine gorgonian | Phenolic polyketide | Hela | 6.1 μM | [125] | |
| Nidurufin (188) | P. flavidorsum SHK1-27 | Marine sediment | Anthraquinone | K562 | 12.6 μM | Induce cell cycle arrest at G2/M transition | [126] |
| Averantin (189) | P. flavidorsum SHK1-27 | Marine sediment | Anthraquinone | K562 | 12.6 μM | [126] | |
| 190 | P. terrestre | Marine sediment | Polyketide | A549, P388 | (5.3, 15.7) μM | [128] | |
| 191 | P. terrestre | Marine sediment | Polyketide | A549, P388 | (7.6, 10.5) μM | [128] | |
| Dihydrobisvertinolone (192) | P. terrestre | Marine sediment | Polyketide | A549, P388 | (0.52, 1.7) μM | [128] | |
| 193 | P. terrestre | Marine sediment | Polyketide | A549 | 1.4 μM | [128] | |
| 194 | P. terrestre | Marine sediment | Polyketide | A549, P388 | (2.1, 2.8) μM | [129] | |
| 195 | P. terrestre | Marine sediment | Polyketide | A549, P388 | (4.3, 8.8) μM | [129] | |
| Trichodimerol (196) | P. terrestre | Marine sediment | Polyketide | A549, P388 | (4.7, 0.33) μM | [129] | |
| Chloctanspirone A (197) | P. terrestre | Marine sediment | Polyketide | HL-60, A549 | (9.2, 39.7) μM | [131] | |
| Chloctanspirone B (198) | P. terrestre | Marine sediment | Polyketide | HL-60 | 37.8 μM | [131] | |
| (10E,15S)-10,11-Dehydrocurvularin (199) | Penicillium sp. DRF2 | Marine sponge | Macrolide | 36 tumor cell lines | 0.28~6 μM | [133,134] | |
| Curvularin (200) | Penicillium sp. DRF2 | Marine sponge | Macrolide | HSP90 inhibitor | [132] | ||
| Tanzawaic acid P (201) | Penicillium sp. CF07370 | Marine sediment | Polyketide | Jurkat, K562, Raji | (28.6, 30.2, 20.3) μM | Active the mitochondrial apoptotic pathway | [137] |
| Tanzawaic acid D (202) | P. steckii | Marine (not clear) | Polyketide | Bind to the FOXO1 which regulates EFGR signaling and stabilizes the FOXO1-DNA conformation | [138] | ||
| Penicimutamide A (203) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 10~40% | [140] | |
| Penicimutamide B (204) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 25~40% | [140] | |
| Penicimutamide C (205) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 10~40% | [140] | |
| Penicimutamide D (206) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 20~40% | [140] | |
| Penicimutamide E (207) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 10~45% | [140] | |
| Penicimutamide F (208) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 10~50% | [140] | |
| Penicimutamide G (209) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 10~20% | [140] | |
| Fellutamide A (210) | P. fellutanum | Marine fish | Lipopepetide | P388, L1210 | (0.2, 0.8) μg/mL | [139] | |
| Fellutamide B (211) | P. fellutanum | Marine fish | Lipopepetide | P388, L1210 | (0.1, 0.7) μg/mL | [139] | |
| Fellutamide C (212) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 30~50% | [140] | |
| Peniphenylane A (213) | P. fellutanum HDN14-323 | Deep sea sediment | Polyphenol | Hela | 14.5 μM | [141] | |
| Peniphenylane B (214) | P. fellutanum HDN14-323 | Deep sea sediment | Polyphenol | Hela, HCT-116 | (11.4, 15.8) μM | [141] | |
| Peniphenylane D (215) | P. fellutanum HDN14-323 | Deep sea sediment | Polyphenol | Hela, HL-60, HCT-116 | (9.3, 18.2, 31.7) μM | [141] | |
| Peniphenylane F (216) | P. fellutanum HDN14-323 | Deep sea sediment | Polyphenol | Hela | 29.3 μM | [141] | |
| Peniphenylane G (217) | P. fellutanum HDN14-323 | Deep sea sediment | Polyphenol | Hela, HL-60, HCT-116 | (16.6, 23.2, 24.7) μM | [141] | |
| Terrestol B (218) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (6.1, 5.8, 18.3, 62.3) μM | [142] | |
| Terrestol C (219) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (5.5, 5.6, 18.2, 57.3) μM | [142] | |
| Terrestol D (220) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (5.3, 5.5, 14.3, 38.5) μM | [142] | |
| Terrestol E (221) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (54.7, 6.4, 9.6, 59.0) μM | [142] | |
| Terrestol F (222) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (55.0, 58.1, 13.8, 63.2) μM | [142] | |
| Terrestol G (223) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (5.1, 6.5, 5.7, 6.0) μM | [142] | |
| Terrestol H (224) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (6.3, 5.8, 33.8, 61.9) μM | [142] | |
| Terrestol A (225) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (33.3, 5.5, 23.5, 57.0) μM | [142] | |
| Expansol A (226) | P. expansum 091006 | Mangrove | Polyphenol | HL-60 | 15.7 μM | [143] | |
| Expansol B (227) | P. expansum 091006 | Mangrove | Polyphenol | HL-60, A549 | (5.4, 1.9) μM | [143,144] | |
| Expansol C (228) | P. expansum 091006 | Mangrove | Polyphenol | HL-60 | 18.2 μM | [143] | |
| Expansol E (229) | P. expansum 091006 | Mangrove | Polyphenol | HL-60 | 20.8 μM | [143] | |
| Patulin (230) | Penicillium sp. | Marine alga | Other | P388, BSY-1, MCF-7, HCC2998, NCI-H522, DMS114, OVCAR-3, MKN1 | (0.06, 0.34, 0.65, 1.54, 0.30, 0.57, 0.37, 0.39) μg/mL | Potassium-uptake inhibitor Ion flux across cell membranes inducer |
[111] |
| (+)-Epiepoxydon (231) | Penicillium sp. | Marine alga | Other | P388 | 0.2 μg/mL | [111] | |
| 232 | Penicillium sp. | Mangrove | Other | KB, KBv200 | (6, 10) μg/mL | [145] | |
| Penicillic acid (233) | Penicillium sp. | Sea water | Other | POS1, AT6-1, L299 | (7.8, 29.4, 12.9) μM | [84] |
Author Contributions
Sen Liu and Jee H. Jung conceived and designed the study. Sen Liu collected and assessed the references. Sen Liu, Mingzhi Su, Shao-Jiang Song, and Jee H. Jung reviewed the manuscript. Sen Liu, and Jee H. Jung wrote the manuscript.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- 1.Lindequist U. Marine-Derived Pharmaceuticals—Challenges and Opportunities. Biomol. Ther. 2016;24:561–571. doi: 10.4062/biomolther.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H., Hong J., Yin J., Moon H.R., Liu Y., Wei X., Oh D.-C., Jung J.H. Dimeric Octaketide Spiroketals from the Jellyfish-Derived Fungus Paecilomyces variotii J08NF-1. J. Nat. Prod. 2015;78:2832–2836. doi: 10.1021/acs.jnatprod.5b00594. [DOI] [PubMed] [Google Scholar]
- 3.Lee Y.M., Kim M.J., Li H., Zhang P., Bao B., Lee K.J., Jung J.H. Marine-derived Aspergillus species as a source of bioactive secondary metabolites. Mar. Biotechnol. 2013;15:499–519. doi: 10.1007/s10126-013-9506-3. [DOI] [PubMed] [Google Scholar]
- 4.Ding G., Song Y.C., Chen J.R., Xu C., Ge H.M., Wang X.T., Tan R.X. Chaetoglobosin U, a cytochalasan alkaloid from endophytic Chaetomium globosum IFB-E019. J. Nat. Prod. 2006;69:302–304. doi: 10.1021/np050515+. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J., Ge H.M., Jiao R.H., Li J., Peng H., Wang Y.R., Wu J.H., Song Y.C., Tan R.X. Cytotoxic chaetoglobosins from the endophyte Chaetomium globosum. Planta Med. 2010;76:1910–1914. doi: 10.1055/s-0030-1249936. [DOI] [PubMed] [Google Scholar]
- 6.Huang S., Chen H., Li W., Zhu X., Ding W., Li C. Bioactive Chaetoglobosins from the Mangrove Endophytic Fungus Penicillium chrysogenum. Mar. Drugs. 2016;14:172. doi: 10.3390/md14100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwamoto C., Yamada T., Ito Y., Minoura K., Numata A. Cytotoxic cytochalasans from a Penicillium species separated from a marine alga. Tetrahedron. 2001;57:2997–3004. doi: 10.1016/S0040-4020(01)00153-3. [DOI] [Google Scholar]
- 8.Numata A., Takahashi C., Ito Y., Minoura K., Yamada T., Matsuda C., Nomoto K. Penochalasins, a novel class of cytotoxic cytochalasans from a Penicillium species separated from a marine alga: Structure determination and solution conformation. J. Chem. Soc. Perkin 1. 1996;3:239–245. doi: 10.1039/p19960000239. [DOI] [Google Scholar]
- 9.Knudsen P.B., Hanna B., Ohl S., Sellner L., Zenz T., Dohner H., Stilgenbauer S., Larsen T.O., Lichter P., Seiffert M. Chaetoglobosin A preferentially induces apoptosis in chronic lymphocytic leukemia cells by targeting the cytoskeleton. Leukemia. 2014;28:1289–1298. doi: 10.1038/leu.2013.360. [DOI] [PubMed] [Google Scholar]
- 10.Schümann J., Hertweck C. Molecular basis of cytochalasan biosynthesis in fungi: Gene cluster analysis and evidence for the involvement of a PKS-NRPS hybrid synthase by RNA silencing. J. Am. Chem. Soc. 2007;129:9564–9565. doi: 10.1021/ja072884t. [DOI] [PubMed] [Google Scholar]
- 11.McDougall J. Antiviral action of gliotoxin. Arch. Virol. 1969;27:255–267. doi: 10.1007/BF01249648. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y., Takada K., Takemoto Y., Yoshida M., Nogi Y., Okada S., Matsunaga S. Gliotoxin analogues from a marine-derived fungus, Penicillium sp., and their cytotoxic and histone methyltransferase inhibitory activities. J. Nat. Prod. 2011;75:111–114. doi: 10.1021/np200740e. [DOI] [PubMed] [Google Scholar]
- 13.Vigushin D., Mirsaidi N., Brooke G., Sun C., Pace P., Inman L., Moody C., Coombes R. Gliotoxin is a dual inhibitor of farnesyltransferase and geranylgeranyltransferase I with antitumor activity against breast cancer in vivo. Med. Oncol. 2004;21:21–30. doi: 10.1385/MO:21:1:21. [DOI] [PubMed] [Google Scholar]
- 14.Meng L.-H., Wang C.-Y., Mándi A., Li X.-M., Hu X.-Y., Kassack M.U., Kurtán T., Wang B.-G. Three Diketopiperazine Alkaloids with Spirocyclic Skeletons and One Bisthiodiketopiperazine Derivative from the Mangrove-Derived Endophytic Fungus Penicillium brocae MA-231. Org. Lett. 2016;18:5304–5307. doi: 10.1021/acs.orglett.6b02620. [DOI] [PubMed] [Google Scholar]
- 15.Jiang C.-S., Guo Y.-W. Epipolythiodioxopiperazines from fungi: Chemistry and bioactivities. Mini Rev. Med. Chem. 2011;11:728–745. doi: 10.2174/138955711796355276. [DOI] [PubMed] [Google Scholar]
- 16.Meng L.-H., Li X.-M., Lv C.-T., Huang C.-G., Wang B.-G. Brocazines A–F, cytotoxic bisthiodiketopiperazine derivatives from Penicillium brocae MA-231, an endophytic fungus derived from the marine mangrove plant Avicennia marina. J. Nat. Prod. 2014;77:1921–1927. doi: 10.1021/np500382k. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Li X.-M., Meng L.-H., Jiang W.-L., Xu G.-M., Huang C.-G., Wang B.-G. Bisthiodiketopiperazines and acorane sesquiterpenes produced by the marine-derived fungus Penicillium adametzioides AS-53 on different culture media. J. Nat. Prod. 2015;78:1294–1299. doi: 10.1021/acs.jnatprod.5b00102. [DOI] [PubMed] [Google Scholar]
- 18.Orfali R.S., Aly A.H., Ebrahim W., Abdel-Aziz M.S., Müller W.E., Lin W., Daletos G., Proksch P. Pretrichodermamide C and N-methylpretrichodermamide B, two new cytotoxic epidithiodiketopiperazines from hyper saline lake derived Penicillium sp. Phytochem. Lett. 2015;11:168–172. doi: 10.1016/j.phytol.2014.12.010. [DOI] [Google Scholar]
- 19.Yurchenko A.N., Smetanina O.F., Ivanets E.V., Kalinovsky A.I., Khudyakova Y.V., Kirichuk N.N., Popov R.S., Bokemeyer C., Von Amsberg G., Chingizova E.A. Pretrichodermamides D–F from a Marine Algicolous Fungus Penicillium sp. KMM 4672. Mar. Drugs. 2016;14:122. doi: 10.3390/md14070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aninat C., Andre F., Delaforge M. Oxidative metabolism by P450 and function coupling to efflux systems: Modulation of mycotoxin toxicity. Food Addit. Contam. 2005;22:361–368. doi: 10.1080/02652030500073287. [DOI] [PubMed] [Google Scholar]
- 21.Du L., Feng T., Zhao B., Li D., Cai S., Zhu T., Wang F., Xiao X., Gu Q. Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities. J. Antibiot. 2010;63:165–170. doi: 10.1038/ja.2010.11. [DOI] [PubMed] [Google Scholar]
- 22.Du L., Li D., Zhu T., Cai S., Wang F., Xiao X., Gu Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron. 2009;65:1033–1039. doi: 10.1016/j.tet.2008.11.078. [DOI] [Google Scholar]
- 23.Shang Z., Li X., Meng L., Li C., Gao S., Huang C., Wang B. Chemical profile of the secondary metabolites produced by a deep sea sediment-derived fungus Penicillium commune SD-118. Chin. J. Oceanol. Limnol. 2012;30:305–314. doi: 10.1007/s00343-012-1075-1. [DOI] [Google Scholar]
- 24.Fang S.-M., Wu C.-J., Li C.-W., Cui C.-B. A practical strategy to discover new antitumor compounds by activating silent metabolite production in fungi by diethyl sulphate mutagenesis. Mar. Drugs. 2014;12:1788–1814. doi: 10.3390/md12041788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai Y.-J., Cui C.-B., Li C.-W., Wu C.-J., Tian C.-K., Hua W. Activation of the dormant secondary metabolite production by introducing gentamicin-resistance in a marine-derived Penicillium purpurogenum G59. Mar. Drugs. 2012;10:559–582. doi: 10.3390/md10030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauser D., Weber H., Sigg H. Isolation and configuration of Chaetocin. Helv. Chim. Acta. 1970;53:1061–1073. doi: 10.1002/hlca.19700530521. [DOI] [PubMed] [Google Scholar]
- 27.Katagiri K., Sato K., Hayakawa S., Matsushima T., Minato H. Verticillin A, a new anti-biotic from Verticillium sp. J. Antibiot. 1970;23:420–422. doi: 10.7164/antibiotics.23.420. [DOI] [PubMed] [Google Scholar]
- 28.Son B., Jensen P., Kauffman C., Fenical W. New Cytotoxic Epidithiodioxopiperazines Related to Verticillin A From A Marine Isolate of the Fungus Penicillium. Nat. Prod. Lett. 1999;13:213–222. doi: 10.1080/10575639908048788. [DOI] [Google Scholar]
- 29.Greiner D., Bonaldi T., Eskeland R., Roemer E., Imhof A. Identification of a specific inhibitor of the histone methyltransferase SU (VAR) 3-9. Nat. Chem. Biol. 2005;1:143–145. doi: 10.1038/nchembio721. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Miao Z.-H., Zhao W.-M., Ding J. The p53 pathway is synergized by p38 MAPK signaling to mediate 11,11′-dideoxyverticillin-induced G2/M arrest. FEBS Lett. 2005;579:3683–3690. doi: 10.1016/j.febslet.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y.-X., Chen Y., Guo X.-N., Zhang X.-W., Zhao W.-M., Zhong L., Zhou J., Xi Y., Lin L.-P., Ding J. 11,11′-dideoxy-verticillin: A natural compound possessing growth factor receptor tyrosine kinase-inhibitory effect with anti-tumor activity. Anti-Cancer Drugs. 2005;16:515–524. doi: 10.1097/00001813-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Kim J., Ashenhurst J.A., Movassaghi M. Total synthesis of (+)-11,11′-dideoxyverticillin A. Science. 2009;324:238–241. doi: 10.1126/science.1170777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J., Movassaghi M. Biogenetically-inspired total synthesis of epidithiodiketopiperazines and related alkaloids. Acc. Chem. Res. 2015;48:1159–1171. doi: 10.1021/ar500454v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallam A.A., Houssen W.E., Gissendanner C.R., Orabi K.Y., Foudah A.I., El Sayed K.A. Bioguided discovery and pharmacophore modeling of the mycotoxic indole diterpene alkaloids penitrems as breast cancer proliferation, migration, and invasion inhibitors. MedChemComm. 2013;4:1360–1369. doi: 10.1039/c3md00198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sings H., Singh S. Tremorgenic and nontremorgenic 2,3-fused indole diterpenoids. Alkaloids Chem. Biol. 2003;60:51–163. doi: 10.1016/s0099-9598(03)60002-7. [DOI] [PubMed] [Google Scholar]
- 36.Saikia S., Nicholson M.J., Young C., Parker E.J., Scott B. The genetic basis for indole-diterpene chemical diversity in filamentous fungi. Mycol. Res. 2008;112:184–199. doi: 10.1016/j.mycres.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Cavanagh J., Holton J., Nolan C., Ray D., Naik J., Mantle P. The effects of the tremorgenic mycotoxin penitrem A on the rat cerebellum. Vet. Pathol. 1998;35:53–63. doi: 10.1177/030098589803500105. [DOI] [PubMed] [Google Scholar]
- 38.Numata A., Takahashi C., Ito Y., Takada T., Kawai K., Usami Y., Matsumura E., Imachi M., Ito T., Hasegawa T. Communesins, cytotoxic metabolites of a fungus isolated from a marine alga. Tetrahedron Lett. 1993;34:2355–2358. doi: 10.1016/S0040-4039(00)77612-X. [DOI] [Google Scholar]
- 39.Jadulco R., Edrada R.A., Ebel R., Berg A., Schaumann K., Wray V., Steube K., Proksch P. New Communesin Derivatives from the Fungus Penicillium sp. Derived from the Mediterranean Sponge Axinella v errucosa. J. Nat. Prod. 2004;67:78–81. doi: 10.1021/np030271y. [DOI] [PubMed] [Google Scholar]
- 40.Vansteelandt M., Kerzaon I., Blanchet E., Tankoua O.F., Du Pont T.R., Joubert Y., Monteau F., Le Bizec B., Frisvad J.C., Pouchus Y.F. Patulin and secondary metabolite production by marine-derived Penicillium strains. Fungal Biol. 2012;116:954–961. doi: 10.1016/j.funbio.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Crawley S.L., Funk R.L. A synthetic approach to nomofungin/communesin B. Org. Lett. 2003;5:3169–3171. doi: 10.1021/ol034407v. [DOI] [PubMed] [Google Scholar]
- 42.Zhang P., Li X.-M., Liu H., Li X., Wang B.-G. Two new alkaloids from Penicillium oxalicum EN-201, an endophytic fungus derived from the marine mangrove plant Rhizophora stylosa. Phytochem. Lett. 2015;13:160–164. doi: 10.1016/j.phytol.2015.06.009. [DOI] [Google Scholar]
- 43.An C.-Y., Li X.-M., Li C.-S., Xu G.-M., Wang B.-G. Prenylated indolediketopiperazine peroxides and related homologues from the marine sediment-derived fungus Penicillium brefeldianum SD-273. Mar. Drugs. 2014;12:746–756. doi: 10.3390/md12020746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C.S., Li X.M., An C.Y., Wang B.G. Prenylated Indole Alkaloid Derivatives from Marine Sediment-Derived Fungus Penicillium paneum SD-44. Helv. Chim. Acta. 2014;97:1440–1444. doi: 10.1002/hlca.201400035. [DOI] [Google Scholar]
- 45.Asiri I.A., Badr J.M., Youssef D.T. Penicillivinacine, antimigratory diketopiperazine alkaloid from the marine-derived fungus Penicillium vinaceum. Phytochem. Lett. 2015;13:53–58. doi: 10.1016/j.phytol.2015.05.014. [DOI] [Google Scholar]
- 46.Shaala L.A., Youssef D.T. Identification and bioactivity of compounds from the fungus Penicillium sp. CYE-87 isolated from a marine tunicate. Mar. Drugs. 2015;13:1698–1709. doi: 10.3390/md13041698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L., Huang K., Zhong P. Tumonoic Acids K and L, Novel Metabolites from the Marine-Derived Fungus Penicillium citrinum. Heterocycles. 2012;85:413–419. doi: 10.3987/COM-11-12380. [DOI] [Google Scholar]
- 48.Chen L., Zhou T., Zhao Y.-Y. Four new penicitrinols and two new penicillenols from the marine-derived fungus Penicillium citrinum. Heterocycles. 2015;91:1007–1016. doi: 10.3987/COM-15-13178. [DOI] [Google Scholar]
- 49.Lin Z.-J., Lu Z.-Y., Zhu T.-J., Fang Y.-C., Gu Q.-Q., Zhu W.-M. Penicillenols from Penicillium sp. GQ-7, an endophytic fungus associated with Aegiceras corniculatum. Chem. Pharm. Bull. 2008;56:217–221. doi: 10.1248/cpb.56.217. [DOI] [PubMed] [Google Scholar]
- 50.Liu Q.-Y., Zhou T., Zhao Y.-Y., Chen L., Gong M.-W., Xia Q.-W., Ying M.-G., Zheng Q.-H., Zhang Q.-Q. Antitumor effects and related mechanisms of penicitrinine A, a novel alkaloid with a unique spiro skeleton from the marine fungus Penicillium citrinum. Mar. Drugs. 2015;13:4733–4753. doi: 10.3390/md13084733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jafari E., Khajouei M.R., Hassanzadeh F., Hakimelahi G.H., Khodarahmi G.A. Quinazolinone and quinazoline derivatives: Recent structures with potent antimicrobial and cytotoxic activities. Res. Pharm. Sci. 2016;11:1–14. [PMC free article] [PubMed] [Google Scholar]
- 52.He J., Lion U., Sattler I., Gollmick F.A., Grabley S., Cai J., Meiners M., Schünke H., Schaumann K., Dechert U. Diastereomeric Quinolinone Alkaloids from the Marine-Derived Fungus Penicillium j anczewskii. J. Nat. Prod. 2005;68:1397–1399. doi: 10.1021/np058018g. [DOI] [PubMed] [Google Scholar]
- 53.Gao H., Zhang L., Zhu T., Gu Q., Li D. Unusual pyrrolyl 4-quinolinone alkaloids from the marine-derived fungus Penicillium sp. ghq208. Chem. Pharm. Bull. 2012;60:1458–1460. doi: 10.1248/cpb.c12-00487. [DOI] [PubMed] [Google Scholar]
- 54.Shao C.-L., Wang C.-Y., Gu Y.-C., Wei M.-Y., Pan J.-H., Deng D.-S., She Z.-G., Lin Y.-C. Penicinoline, a new pyrrolyl 4-quinolinone alkaloid with an unprecedented ring system from an endophytic fungus Penicillium sp. Bioorg. Med. Chem. Lett. 2010;20:3284–3286. doi: 10.1016/j.bmcl.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 55.Shen S., Li W., Wang J. A novel and other bioactive secondary metabolites from a marine fungus Penicillium oxalicum 0312f1. Nat. Prod. Rep. 2013;27:2286–2291. doi: 10.1080/14786419.2013.827190. [DOI] [PubMed] [Google Scholar]
- 56.An C.Y., Li X.M., Li C.S., Gao S.S., Shang Z., Wang B.G. Triazoles and Other N-Containing Metabolites from the Marine-Derived Endophytic Fungus Penicillium chrysogenum EN-118. Helv. Chim. Acta. 2013;96:682–687. doi: 10.1002/hlca.201200433. [DOI] [Google Scholar]
- 57.Li C.-S., Li X.-M., Gao S.-S., Lu Y.-H., Wang B.-G. Cytotoxic anthranilic acid derivatives from deep sea sediment-derived fungus Penicillium paneum SD-44. Mar. Drugs. 2013;11:3068–3076. doi: 10.3390/md11083068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C.-S., An C.-Y., Li X.-M., Gao S.-S., Cui C.-M., Sun H.-F., Wang B.-G. Triazole and dihydroimidazole alkaloids from the marine sediment-derived fungus Penicillium paneum SD-44. J. Nat. Prod. 2011;74:1331–1334. doi: 10.1021/np200037z. [DOI] [PubMed] [Google Scholar]
- 59.Li J., Yang X., Lin Y., Yuan J., Lu Y., Zhu X., Li J., Li M., Lin Y., He J. Meroterpenes and azaphilones from marine mangrove endophytic fungus Penicillium 303. Fitoterapia. 2014;97:241–246. doi: 10.1016/j.fitote.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Bringmann G., Lang G., Gulder T.A., Tsuruta H., Mühlbacher J., Maksimenka K., Steffens S., Schaumann K., Stöhr R., Wiese J. The first sorbicillinoid alkaloids, the antileukemic sorbicillactones A and B, from a sponge-derived Penicillium chrysogenum strain. Tetrahedron. 2005;61:7252–7265. doi: 10.1016/j.tet.2005.05.026. [DOI] [Google Scholar]
- 61.Bringmann G., Gulder T.A., Lang G., Schmitt S., Stöhr R., Wiese J., Nagel K., Imhoff J.F. Large-scale biotechnological production of the antileukemic marine natural product sorbicillactone A. Mar. Drugs. 2007;5:23–30. doi: 10.3390/md502023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang P., Meng L.H., Mándi A., Kurtán T., Li X.M., Liu Y., Li X., Li C.S., Wang B.G. Brocaeloids A–C, 4-Oxoquinoline and Indole Alkaloids with C-2 Reversed Prenylation from the Mangrove-Derived Endophytic Fungus Penicillium brocae. Eur. J. Org. Chem. 2014;2014:4029–4036. doi: 10.1002/ejoc.201400067. [DOI] [Google Scholar]
- 63.He X., Zhang Z., Chen Y., Che Q., Zhu T., Gu Q., Li D. Varitatin A, a Highly Modified Fatty Acid Amide from Penicillium variabile Cultured with a DNA Methyltransferase Inhibitor. J. Nat. Prod. 2015;78:2841–2845. doi: 10.1021/acs.jnatprod.5b00742. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y., Li C., Swenson D.C., Gloer J.B., Wicklow D.T., Dowd P.F. Novel antiinsectan oxalicine alkaloids from two undescribed fungicolous Penicillium spp. Org. Lett. 2003;5:773–776. doi: 10.1021/ol0340686. [DOI] [PubMed] [Google Scholar]
- 65.Takatsuki A., Suzuki S., Ando K., Tamura G., Arima K. New antiviral antibiotics; xanthocillin X mono-and dimethylether, and methoxy-xanthocillin X dimethylether. I: Isolation and characterization. J. Antibiot. 1968;21:671–675. doi: 10.7164/antibiotics.21.671. [DOI] [PubMed] [Google Scholar]
- 66.Rothe W. Vorläufige Mitteilung über eine neues Antibiotikum. Pharmazie. 1950;5:190. [Google Scholar]
- 67.Zhao Y., Chen H., Shang Z., Jiao B., Yuan B., Sun W., Wang B., Miao M., Huang C. SD118-xanthocillin X (1), a novel marine agent extracted from Penicillium commune, induces autophagy through the inhibition of the MEK/ERK pathway. Mar. Drugs. 2012;10:1345–1359. doi: 10.3390/md10061345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sugawara F., Hallock Y., Bunkers G., Kenfield D., Strobel G., Yoshida S. Phytoactive eremophilanes produced by the weed pathogen Drechslera gigantea. Biosci. Biotechnol. Biochem. 1993;57:236–239. doi: 10.1271/bbb.57.236. [DOI] [PubMed] [Google Scholar]
- 69.Tirilly Y., Kloosterman J., Sipma G., Kettenes-Van den Bosch J.J. A fungitoxic sesquiterpene from Hansfordia pulvinata. Phytochemistry. 1983;22:2082–2083. doi: 10.1016/0031-9422(83)80051-X. [DOI] [Google Scholar]
- 70.Lin A., Wu G., Gu Q., Zhu T., Li D. New eremophilane-type sesquiterpenes from an Antarctic deep sea derived fungus, Penicillium sp. PR19 N-1. Arch. Pharm. Res. 2014;37:839–844. doi: 10.1007/s12272-013-0246-8. [DOI] [PubMed] [Google Scholar]
- 71.Wu G., Lin A., Gu Q., Zhu T., Li D. Four new chloro-eremophilane sesquiterpenes from an Antarctic deep sea derived fungus, Penicillium sp. PR19N-1. Mar. Drugs. 2013;11:1399–1408. doi: 10.3390/md11041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang Y.F., Qiao L., Lv A.L., Pei Y.H., Tian L. Eremophilane sesquiterenes from the marine fungus Penicillium sp. BL27–2. Chin. Chem. Lett. 2008;19:562–564. doi: 10.1016/j.cclet.2008.03.018. [DOI] [Google Scholar]
- 73.Li Y., Ye D., Shao Z., Cui C., Che Y. A sterol and spiroditerpenoids from a Penicillium sp. isolated from a deep sea sediment sample. Mar. Drugs. 2012;10:497–508. doi: 10.3390/md10020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao S.-S., Li X.-M., Li C.-S., Proksch P., Wang B.-G. Penicisteroids A and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus Penicillium chrysogenum QEN-24S. Bioorg. Med. Chem. Lett. 2011;21:2894–2897. doi: 10.1016/j.bmcl.2011.03.076. [DOI] [PubMed] [Google Scholar]
- 75.Sun Y., Tian L., Huang J., Li W., Pei Y.-H. Cytotoxic sterols from marine-derived fungus Pennicillium sp. Nat. Prod. Rep. 2006;20:381–384. doi: 10.1080/14786410600661229. [DOI] [PubMed] [Google Scholar]
- 76.Xin Z.-H., Zhu T.-J., Wang W.-L., Du L., Fang Y.-C., Gu Q.-Q., Zhu W.-M. Isocoumarin derivatives from the sea squirt-derived fungus Penicillium stoloniferum QY2-10 and the halotolerant fungus Penicillium notatum B-52. Arch. Pharm. Res. 2007;30:816–819. doi: 10.1007/BF02978830. [DOI] [PubMed] [Google Scholar]
- 77.Qi J., Shao C.-L., Liu M., Qi X., Wang C.-Y. Bioactive steroids from a marine-derived fungus Penicillium sp. from the South China Sea. Chem. Nat. Compd. 2014;3:568–570. doi: 10.1007/s10600-014-1020-y. [DOI] [Google Scholar]
- 78.Menna M., Imperatore C., D’Aniello F., Aiello A. Meroterpenes from marine invertebrates: Structures, occurrence, and ecological implications. Mar. Drugs. 2013;11:1602–1643. doi: 10.3390/md11051602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X., Choi H.D., Kang J.S., Lee C.-O., Son B.W. New polyoxygenated farnesylcyclohexenones, deacetoxyyanuthone A and its hydro derivative from the marine-derived fungus Penicillium sp. J. Nat. Prod. 2003;66:1499–1500. doi: 10.1021/np030231u. [DOI] [PubMed] [Google Scholar]
- 80.Zhuang P., Tang X.-X., Yi Z.-W., Qiu Y.-K., Wu Z. Two new compounds from marine-derived fungus Penicillium sp. F11. J. Asian Nat. Prod. Res. 2012;14:197–203. doi: 10.1080/10286020.2011.634279. [DOI] [PubMed] [Google Scholar]
- 81.Ding Z., Zhang L., Fu J., Che Q., Li D., Gu Q., Zhu T. Phenylpyropenes E and F: New meroterpenes from the marine-derived fungus Penicillium concentricum ZLQ-69. J. Antibiot. 2015;68:748–751. doi: 10.1038/ja.2015.64. [DOI] [PubMed] [Google Scholar]
- 82.Liao L., Lee J.-H., You M., Choi T.J., Park W., Lee S.K., Oh D.-C., Oh K.-B., Shin J. Penicillipyrones A and B, meroterpenoids from a marine-derived Penicillium sp. fungus. J. Nat. Prod. 2014;77:406–410. doi: 10.1021/np400826p. [DOI] [PubMed] [Google Scholar]
- 83.Hanson F., Eble T. An antiphage agent isolated from Aspergillus sp. J. Bacteriol. 1949;58:527–529. doi: 10.1128/jb.58.4.527-529.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vansteelandt M., Blanchet E., Egorov M., Petit F., Toupet L., Bondon A., Monteau F., Le Bizec B., Thomas O.P., Pouchus Y.F. Ligerin, an antiproliferative chlorinated sesquiterpenoid from a marine-derived Penicillium strain. J. Nat. Prod. 2013;76:297–301. doi: 10.1021/np3007364. [DOI] [PubMed] [Google Scholar]
- 85.Blanchet E., Vansteelandt M., Bot R.L., Egorov M., Guitton Y., Pouchus Y.F., Grovel O. Synthesis and antiproliferative activity of ligerin and new fumagillin analogs against osteosarcoma. Eur. J. Med. Chem. 2014;79:244–250. doi: 10.1016/j.ejmech.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 86.Keri R.S., Budagumpi S., Pai R.K., Balakrishna R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014;78:340–374. doi: 10.1016/j.ejmech.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 87.Bao J., Luo J.-F., Qin X.-C., Xu X.-Y., Zhang X.-Y., Tu Z.-C., Qi S.-H. Dihydrothiophene-condensed chromones from a marine-derived fungus Penicillium oxalicum and their structure-bioactivity relationship. Bioorg. Med. Chem. Lett. 2014;24:2433–2436. doi: 10.1016/j.bmcl.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 88.Sun Y.-L., Bao J., Liu K.-S., Zhang X.-Y., He F., Wang Y.-F., Nong X.-H., Qi S.-H. Cytotoxic dihydrothiophene-condensed chromones from the marine-derived fungus Penicillium oxalicum. Planta Med. 2013;79:1474–1479. doi: 10.1055/s-0033-1350805. [DOI] [PubMed] [Google Scholar]
- 89.Wang J., Wang Q.-L., Nong X.-H., Zhang X.-Y., Xu X.-Y., Qi S.-H., Wang Y.-F. Oxalicumone A, a new dihydrothiophene-condensed sulfur chromone induces apoptosis in leukemia cells through endoplasmic reticulum stress pathway. Eur. J. Pharmacol. 2016;783:47–55. doi: 10.1016/j.ejphar.2016.04.056. [DOI] [PubMed] [Google Scholar]
- 90.Shi S., Guo K., Wang X., Chen H., Min J., Qi S., Zhao W., Li W. Toxicity study of oxalicumone A, derived from a marine-derived fungus Penicillium oxalicum, in cultured renal epithelial cells. Mol. Med. Rep. 2017;15:2611–2619. doi: 10.3892/mmr.2017.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yi L., Cui C.-B., Li C.-W., Peng J.-X., Gu Q.-Q. Chromosulfine, a novel cyclopentachromone sulfide produced by a marine-derived fungus after introduction of neomycin resistance. RSC Adv. 2016;6:43975–43979. doi: 10.1039/C6RA06250D. [DOI] [Google Scholar]
- 92.Li N., Yi Z., Wang Y., Zhang Q., Zhong T., Qiu Y., Wu Z., Tang X. Differential proteomic analysis of HL60 cells treated with secalonic acid F reveals caspase 3-induced cleavage of Rho GDP dissociation inhibitor 2. Oncol. Rep. 2012;28:2016–2022. doi: 10.3892/or.2012.2062. [DOI] [PubMed] [Google Scholar]
- 93.Gao X., Sun H., Liu D., Zhang J., Zhang J., Yan M., Pan X. Secalonic acid-F inhibited cell growth more effectively than 5-fluorouracil on hepatocellular carcinoma in vitro and in vivo. Neoplasma. 2017;64:344–350. doi: 10.4149/neo_2017_304. [DOI] [PubMed] [Google Scholar]
- 94.Hou X.-M., Wang C.-Y., Gu Y.-C., Shao C.-L. Penimethavone A, a flavone from a gorgonian-derived fungus Penicillium chrysogenum. Nat. Prod. Rep. 2016;30:2274–2277. doi: 10.1080/14786419.2016.1163695. [DOI] [PubMed] [Google Scholar]
- 95.Chen S., Wang J., Wang Z., Lin X., Zhao B., Kaliaperumal K., Liao X., Tu Z., Li J., Xu S. Structurally diverse secondary metabolites from a deep sea-derived fungus Penicillium chrysogenum SCSIO 41001 and their biological evaluation. Fitoterapia. 2017;117:71–78. doi: 10.1016/j.fitote.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Chen H., Aktas N., Konuklugil B., Mandi A., Daletos G., Lin W., Dai H., Kurtan T., Proksch P. A new fusarielin analogue from Penicillium sp. isolated from the Mediterranean sponge Ircinia oros. Tetrahedron Lett. 2015;56:5317–5320. doi: 10.1016/j.tetlet.2015.07.072. [DOI] [Google Scholar]
- 97.Huang Z., Yang J., Cai X., She Z., Lin Y. A new furanocoumarin from the mangrove endophytic fungus Penicillium sp.(ZH16) Nat. Prod. Rep. 2012;26:1291–1295. doi: 10.1080/14786419.2011.569502. [DOI] [PubMed] [Google Scholar]
- 98.Hetherington A.C., Raistrick H. On the production and chemical constitution of a new yellow colouring matter, citrinin, produced from glucose by Penicillium citrinum Thom. Philos. Trans. R. Soc. Lond. 1931;220:269–295. doi: 10.1098/rstb.1931.0025. [DOI] [Google Scholar]
- 99.Chagas G.M., Campello A.P., Klüppel M., Lúcia W. Mechanism of citrinin-induced dysfunction of mitochondria. I. Effects on respiration, enzyme activities and membrane potential of renal cortical mitochondria. J. Appl. Toxicol. 1992;12:123–129. doi: 10.1002/jat.2550120209. [DOI] [PubMed] [Google Scholar]
- 100.He Y., Cox R.J. The molecular steps of citrinin biosynthesis in fungi. Chem. Sci. 2016;7:2119–2127. doi: 10.1039/C5SC04027B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Subramani R., Kumar R., Prasad P., Aalbersberg W. Cytotoxic and antibacterial substances against multi-drug resistant pathogens from marine sponge symbiont: Citrinin, a secondary metabolite of Penicillium sp. Asian Pac. J. Trop. Biomed. 2013;3:291–296. doi: 10.1016/S2221-1691(13)60065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stierle A.A., Stierle D.B., Kelly K. Berkelic acid, a novel spiroketal with selective anticancer activity from an acid mine waste fungal extremophile. J. Org. Chem. 2006;71:5357–5360. doi: 10.1021/jo060018d. [DOI] [PubMed] [Google Scholar]
- 103.Wu X., Zhou J., Snider B.B. Synthesis of (−)-Berkelic Acid. Angew. Chem. Int. Ed. 2009;48:1283–1286. doi: 10.1002/anie.200805488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhuravleva O.I., Sobolevskaya M.P., Afiyatullov S.S., Kirichuk N.N., Denisenko V.A., Dmitrenok P.S., Yurchenko E.A., Dyshlovoy S.A. Sargassopenillines A–G, 6,6-spiroketals from the alga-derived fungi Penicillium thomii and Penicillium lividum. Mar. Drugs. 2014;12:5930–5943. doi: 10.3390/md12125930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perpelescu M., Kobayashi J.I., Furuta M., Ito Y., Izuta S., Takemura M., Suzuki M., Yoshida S. Novel phenalenone derivatives from a marine-derived fungus exhibit distinct inhibition spectra against eukaryotic DNA polymerases. Biochemistry. 2002;41:7610–7616. doi: 10.1021/bi020115a. [DOI] [PubMed] [Google Scholar]
- 106.Chen L., Gong M.-W., Peng Z.-F., Zhou T., Ying M.-G., Zheng Q.-H., Liu Q.-Y., Zhang Q.-Q. The marine fungal metabolite, dicitrinone B, induces A375 cell apoptosis through the ROS-related caspase pathway. Mar. Drugs. 2014;12:1939–1958. doi: 10.3390/md12041939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sasaki M., Tsuda M., Sekiguchi M., Mikami Y., Kobayashi J.I. Perinadine A, a Novel Tetracyclic Alkaloid from Marine-Derived Fungus Penicillium citrinum. Org. Lett. 2005;7:4261–4264. doi: 10.1021/ol051695h. [DOI] [PubMed] [Google Scholar]
- 108.Julianti E., Lee J.-H., Liao L., Park W., Park S., Oh D.-C., Oh K.-B., Shin J. New polyaromatic metabolites from a marine-derived fungus Penicillium sp. Org. Lett. 2013;15:1286–1289. doi: 10.1021/ol4002174. [DOI] [PubMed] [Google Scholar]
- 109.Gao S.-S., Li X.-M., Zhang Y., Li C.-S., Cui C.-M., Wang B.-G. Comazaphilones A–F, azaphilone derivatives from the marine sediment-derived fungus Penicillium commune QSD-17. J. Nat. Prod. 2011;74:256–261. doi: 10.1021/np100788h. [DOI] [PubMed] [Google Scholar]
- 110.Myobatake Y., Takeuchi T., Kuramochi K., Kuriyama I., Ishido T., Hirano K., Sugawara F., Yoshida H., Mizushina Y. Pinophilins A and B, inhibitors of mammalian A-, B-, and Y-family DNA polymerases and human cancer cell proliferation. J. Nat. Prod. 2012;75:135–141. doi: 10.1021/np200523b. [DOI] [PubMed] [Google Scholar]
- 111.Iwamoto C., Minoura K., Oka T., Ohta T., Hagishita S., Numata A. Absolute stereostructures of novel cytotoxic metabolites, penostatins A–E, from a Penicillium species separated from an Enteromorpha alga. Tetrahedron. 1999;55:14353–14368. doi: 10.1016/S0040-4020(99)00884-4. [DOI] [Google Scholar]
- 112.Iwamoto C., Minoura K., Hagishita S., Nomoto K., Numata A. Penostatins F–I, novel cytotoxic metabolites from a Penicillium species separated from an Enteromorpha marine alga. J. Chem. Soc. Perkin 1. 1998;3:449–456. doi: 10.1039/a706853k. [DOI] [Google Scholar]
- 113.Chen Y.-P., Yang C.-G., Wei P.-Y., Li L., Luo D.-Q., Zheng Z.-H., Lu X.-H. Penostatin derivatives, a novel kind of protein phosphatase 1B inhibitors isolated from solid cultures of the entomogenous fungus Isaria tenuipes. Molecules. 2014;19:1663–1671. doi: 10.3390/molecules19021663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Snider B.B., Liu T. Total Synthesis of (±)-Deoxypenostatin A. Approaches to the Syntheses of Penostatins A and B. J. Org. Chem. 2000;65:8490–8498. doi: 10.1021/jo000850x. [DOI] [PubMed] [Google Scholar]
- 115.Barriault L., Ang P.J., Lavigne R.M. Rapid Assembly of the Bicyclo [5.3.1] undecenone Core of Penostatin F: A Successive Diels-Alder/Claisen Reaction Strategy with an Efficient Stereochemical Relay. Org. Lett. 2004;6:1317–1319. doi: 10.1021/ol049680r. [DOI] [PubMed] [Google Scholar]
- 116.Crawford J.M., Townsend C.A. New insights into the formation of fungal aromatic polyketides. Nat. Rev. Microbiol. 2010;8:879–889. doi: 10.1038/nrmicro2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu B., Wang H.-F., Zhang L.-H., Liu F., He F.-J., Bai J., Hua H.-M., Chen G., Pei Y.-H. New compound with DNA Topo I inhibitory activity purified from Penicillium oxalicum HSY05. Nat. Prod. Rep. 2015;29:2197–2202. doi: 10.1080/14786419.2015.1008472. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y., Wang L., Zhuang Y., Kong F., Zhang C., Zhu W. Phenolic polyketides from the co-cultivation of marine-derived Penicillium sp. WC-29–5 and Streptomyces fradiae 007. Mar. Drugs. 2014;12:2079–2088. doi: 10.3390/md12042079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roullier C., Guitton Y., Valery M., Amand S., Prado S., Robiou du Pont T., Grovel O., Pouchus Y.F. Automated detection of natural halogenated compounds from LC-MS profiles–Application to the isolation of bioactive chlorinated compounds from marine-derived fungi. Anal. Chem. 2016;88:9143–9150. doi: 10.1021/acs.analchem.6b02128. [DOI] [PubMed] [Google Scholar]
- 120.Luo H., Li X.-M., Li C.-S., Wang B.-G. Diphenyl ether and benzophenone derivatives from the marine mangrove-derived fungus Penicillium sp. MA-37. Phytochem. Lett. 2014;9:22–25. doi: 10.1016/j.phytol.2014.03.012. [DOI] [Google Scholar]
- 121.Nishida H., Tomoda H., Cao J., Araki S., Okuda S., Omura S. Purpactins, new inhibitors of Acyl-CoA: Cholesterol acyltransferase produced by Penicillium purpurogenum. J. Antibiot. 1991;44:152–159. doi: 10.7164/antibiotics.44.152. [DOI] [PubMed] [Google Scholar]
- 122.Chung M.-C., Lee H., Chun H., Kho Y. Penicillide, a nonpeptide calpain inhibitor, produced by Penicillium sp. J. Microbiol. Biotechnol. 1998;8:188–190. [Google Scholar]
- 123.Salituro G.M., Pettibone D.J., Clineschmidt B.V., Williamson J.M., Zink D.L. Potent, non-peptidic oxytocin receptor antagonists from a natural source. Bioorg. Med. Chem. Lett. 1993;3:337–340. doi: 10.1016/S0960-894X(01)80905-7. [DOI] [Google Scholar]
- 124.Gao H., Zhou L., Li D., Gu Q., Zhu T.J. New Cytotoxic Metabolites from the Marine-Derived Fungus Penicillium sp. ZLN29. Helv. Chim. Acta. 2013;96:514–519. doi: 10.1002/hlca.201200596. [DOI] [Google Scholar]
- 125.Zhao D.-L., Shao C.-L., Zhang Q., Wang K.-L., Guan F.-F., Shi T., Wang C.-Y. Azaphilone and diphenyl ether derivatives from a gorgonian-derived strain of the fungus Penicillium pinophilum. J. Nat. Prod. 2015;78:2310–2314. doi: 10.1021/acs.jnatprod.5b00575. [DOI] [PubMed] [Google Scholar]
- 126.Ren H., Liu W.-W. Nidurufin as a new cell cycle inhibitor from marine-derived fungus Penicillium flavidorsum SHK1-27. Arch. Pharm. Res. 2011;34:901–905. doi: 10.1007/s12272-011-0606-1. [DOI] [PubMed] [Google Scholar]
- 127.O’Malley G.J., Murphy R.A., Jr., Cava M.P. Aflatoxin precursors: Total synthesis of (.+−.)-averufin and (.+−.)-nidurufin. J. Org. Chem. 1985;50:5533–5537. doi: 10.1021/jo00350a020. [DOI] [Google Scholar]
- 128.Liu W., Gu Q., Zhu W., Cui C., Fan G. Two new benzoquinone derivatives and two new bisorbicillinoids were isolated from a marine-derived fungus Penicillium terrestre. J. Antibiot. 2005;58:441–446. doi: 10.1038/ja.2005.57. [DOI] [PubMed] [Google Scholar]
- 129.Liu W., Gu Q., Zhu W., Cui C., Fan G. Dihydrotrichodimerol and tetrahydrotrichodimerol, two new bisorbicillinoids, from a marine-derived Penicillium terrestre. J. Antibiot. 2005;58:621–624. doi: 10.1038/ja.2005.85. [DOI] [PubMed] [Google Scholar]
- 130.Harned A.M., Volp K.A. The sorbicillinoid family of natural products: Isolation, biosynthesis, and synthetic studies. Nat. Prod. Rep. 2011;28:1790–1810. doi: 10.1039/c1np00039j. [DOI] [PubMed] [Google Scholar]
- 131.Li D., Chen L., Zhu T., Kurtán T., Mándi A., Zhao Z., Li J., Gu Q. Chloctanspirones A and B, novel chlorinated polyketides with an unprecedented skeleton, from marine sediment derived fungus Penicillium terrestre. Tetrahedron. 2011;67:7913–7918. doi: 10.1016/j.tet.2011.08.037. [DOI] [Google Scholar]
- 132.Janin Y.L. Heat shock protein 90 inhibitors. A text book example of medicinal chemistry? J. Med. Chem. 2005;48:7503–7512. doi: 10.1021/jm050759r. [DOI] [PubMed] [Google Scholar]
- 133.De Castro M.V., Ióca L.P., Williams D.E., Costa B.Z., Mizuno C.M., Santos M.F., de Jesus K., Ferreira E.V.L., Seleghim M.H., Sette L.D. Condensation of Macrocyclic Polyketides Produced by Penicillium sp. DRF2 with Mercaptopyruvate Represents a New Fungal Detoxification Pathway. J. Nat. Prod. 2016;79:1668–1678. doi: 10.1021/acs.jnatprod.6b00295. [DOI] [PubMed] [Google Scholar]
- 134.Greve H., Schupp P.J., Eguereva E., Kehraus S., Kelter G., Maier A., Fiebig H.H., König G.M. Apralactone A and a New Stereochemical Class of Curvularins from the Marine Fungus Curvularia sp. Eur. J. Org. Chem. 2008;30:5085–5092. doi: 10.1002/ejoc.200800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Getino M., Fernández-López R., Palencia-Gándara C., Campos-Gómez J., Sánchez-López J.M., Martínez M., Fernández A., de la Cruz F. Tanzawaic acids, a chemically novel set of bacterial conjugation inhibitors. PLoS ONE. 2016;11:e0148098. doi: 10.1371/journal.pone.0148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shin H.J., Pil G.B., Heo S.-J., Lee H.-S., Lee J.S., Lee Y.-J., Lee J., Won H.S. Anti-inflammatory activity of tanzawaic acid derivatives from a marine-derived fungus Penicillium steckii 108YD142. Mar. Drugs. 2016;14:14. doi: 10.3390/md14010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cardoso-Martínez F., José M., Díaz-Marrero A.R., Darias J., Cerella C., Diederich M., Cueto M. Tanzawaic acids isolated from a marine-derived fungus of the genus Penicillium with cytotoxic activities. Org. Biomol. Chem. 2015;13:7248–7256. doi: 10.1039/C5OB00773A. [DOI] [PubMed] [Google Scholar]
- 138.Sun Y., Ai X., Hou J., Ye X., Liu R., Shen S., Li Z., Lu S. Integrated discovery of FOXO1–DNA stabilizers from marine natural products to restore chemosensitivity to anti-EGFR-based therapy for metastatic lung cancer. Mol. Biosyst. 2017;13:330–337. doi: 10.1039/C6MB00678G. [DOI] [PubMed] [Google Scholar]
- 139.Shigemori H., Wakuri S., Yazawa K., Nakamura T., Sasaki T., Kobayashi J.I. Fellutamides A and B, cytotoxic peptides from a marine fish-possessing fungus Penicillium fellutanum. Tetrahedron. 1991;47:8529–8534. doi: 10.1016/S0040-4020(01)82396-6. [DOI] [Google Scholar]
- 140.Wu C.-J., Li C.-W., Cui C.-B. Seven new and two known lipopeptides as well as five known polyketides: The activated production of silent metabolites in a marine-derived fungus by chemical mutagenesis strategy using diethyl sulphate. Mar. Drugs. 2014;12:1815–1838. doi: 10.3390/md12041815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Z., Guo W., He X., Che Q., Zhu T., Gu Q., Li D. Peniphenylanes A–G from the Deep sea-Derived Fungus Penicillium fellutanum HDN14-323. Planta Med. 2016;82:872–876. doi: 10.1055/s-0042-102885. [DOI] [PubMed] [Google Scholar]
- 142.Chen L., Fang Y., Zhu T., Gu Q., Zhu W. Gentisyl alcohol derivatives from the marine-derived fungus Penicillium terrestre. J. Nat. Prod. 2007;71:66–70. doi: 10.1021/np070421v. [DOI] [PubMed] [Google Scholar]
- 143.Wang J., Lu Z., Liu P., Wang Y., Li J., Hong K., Zhu W. Cytotoxic Polyphenols from the Fungus Penicillium expansum 091 006 Endogenous with the Mangrove Plant Excoecaria agallocha. Planta Med. 2012;78:1861–1866. doi: 10.1055/s-0032-1315395. [DOI] [PubMed] [Google Scholar]
- 144.Lu Z., Zhu H., Fu P., Wang Y., Zhang Z., Lin H., Liu P., Zhuang Y., Hong K., Zhu W. Cytotoxic polyphenols from the marine-derived fungus Penicillium expansum. J. Nat. Prod. 2010;73:911–914. doi: 10.1021/np100059m. [DOI] [PubMed] [Google Scholar]
- 145.Yang J., Huang R., Qiu S.X., She Z., Lin Y. A new isobenzofuranone from the mangrove endophytic fungus Penicillium sp.(ZH58) Nat. Prod. Rep. 2013;27:1902–1905. doi: 10.1080/14786419.2013.784870. [DOI] [PubMed] [Google Scholar]
- 146.Kornprobst J.-M., La Barre S. New Trends in Marine Natural Products. J. Oceanogr. Mar. Res. 2014;2:1000e109. [Google Scholar]
- 147.Zeng Y., Wang H., Kamdem R.S., Orfali R.S., Dai H., Makhloufi G., Janiak C., Liu Z., Proksch P. A new cyclohexapeptide, penitropeptide and a new polyketide, penitropone from the endophytic fungus Penicillium tropicum. Tetrahedron Lett. 2016;57:2998–3001. doi: 10.1016/j.tetlet.2016.05.095. [DOI] [Google Scholar]
- 148.Koul M., Singh S. Penicillium spp.: Prolific producer for harnessing cytotoxic secondary metabolites. Anti-Cancer Drugs. 2017;28:11–30. doi: 10.1097/CAD.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 149.Brady S.F., Simmons L., Kim J.H., Schmidt E.W. Metagenomic approaches to natural products from free-living and symbiotic organisms. Nat. Prod. Rep. 2009;26:1488–1503. doi: 10.1039/b817078a. [DOI] [PMC free article] [PubMed] [Google Scholar]