A ligand-enabled Ir-catalyzed diastereoselective and enantioselective allylic alkylation of 3-substituted indoles is reported, providing indoline products containing three contiguous stereocenters in one step with high site-, regio-, diastereo- and enantioselectivities from a wide range of readily available starting materials.
A ligand-enabled Ir-catalyzed diastereoselective and enantioselective allylic alkylation of 3-substituted indoles is reported, providing indoline products containing three contiguous stereocenters in one step with high site-, regio-, diastereo- and enantioselectivities from a wide range of readily available starting materials.
Abstract
An Ir-catalyzed asymmetric allylic alkylation of 3-substituted indoles is reported. The reaction provides indoline products containing multiple contiguous stereocenters with high site-, regio-, diastereo- and enantioselectivities in one step from a wide range of readily available starting materials. The key to this method is the high level of diastereocontrol enabled by an iridium catalyst derived from a N-aryl phosphoramidite ligand (Me-THQphos, 1c).
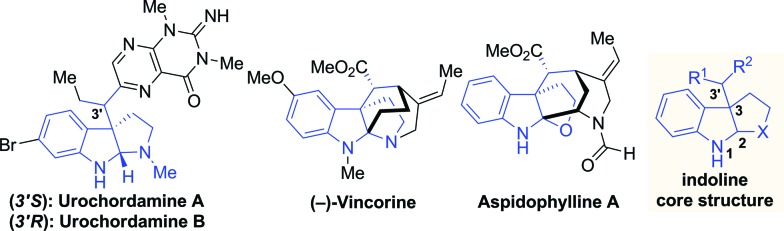
Polycyclic indoline skeletons are present in a variety of alkaloid natural products with diverse biological activities, and many of them bear multiple contiguous stereogenic centers.1 A large number of notable methods have been devised toward the synthesis of this privileged structure in the past several decades,2 including a chiral pool strategy from tryptophan derivatives,3 stereoselective functionalization of oxindoles followed by cyclizations,4 and recently, catalytic enantioselective tandem C3-functionalization/cyclization reactions of 3-substituted indoles,5 and others.6
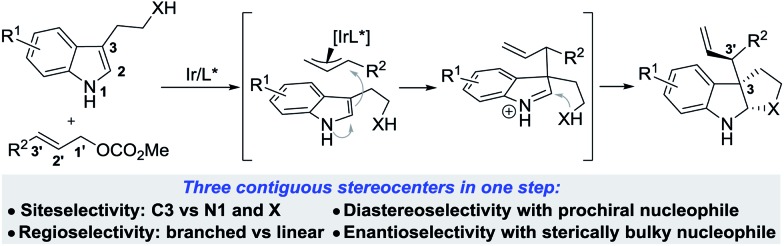
However, the existing methods are mainly limited to the construction of endocyclic vicinal stereocenters (C2 and C3) of indolines. Given the complexity and importance of indoline alkaloids with an additional tertiary stereocenter (C3′) adjacent to the C3 quaternary center (Fig. 1), the development of efficient methods to install these three contiguous stereocenters in one operation is highly appealing but remains a formidable challenge in organic synthesis.7 We envisioned that iridium-catalyzed reactions of 3-substituted indoles with allylic carbonates have the potential to create these stereogenic arrangements in a single step.
Fig. 1. Representative indoline natural products.
To achieve this, the reaction must show high levels of site selectivity for indoles with regard to N1, C3 and the side chain nucleophiles (X),8 regioselectivity toward branched or linear (C1′ vs. C3′), and enantioselectivity and diastereoselectivity with respect to the new C3–C3′ bond (Scheme 1). Despite the rapid growth of Ir-catalyzed allylic alkylations,9 control of the regio- and enantioselectivity of the reaction using sterically hindered nucleophiles has proven to be problematic. Two recently reported examples have provided direct access to 3-substituted pyrroloindolines in one step by iridium-catalysis,10 however, these reactions either afford only linear allylated products or lack enantioselectivity. In particular, the diastereocontrol of prochiral nucleophiles represents a significant challenge. To date, only a handful of examples of the intermolecular diastereoselective allylic alkylation of prochiral nucleophiles have been established,11–14 through the use of iridium catalysis combined with a judicious choice of additives,12,13 or synergistic iridium/amine dual catalysis.14 Moreover, the nucleophiles investigated in these accounts were limited to glycine derivatives, azlactones, and enolates. Our group has a strong focus on and interest in addressing the facial selectivity of prochiral nucleophiles by engineering new ligand structures. We have developed an iridacycle complex derived from 2-methyl-1,2,3,4-tetrahydroquinoline phosphoramidite (Me-THQPhos, 1c) and [Ir(COD)Cl]2,15 which was found to be an efficient catalyst enabling diastereocontrol in both intra- and intermolecular Ir-catalyzed allylic allylations, by our group16 and others.13 We recently found that the use of ligand 1c is crucial to the outcome with regard to regio-, diastereo-, and enantioselectivities in the synthesis of indolines (Scheme 1). Herein, we wish to report our preliminary results on this unprecedented Ir-catalyzed tandem allylic alkylation/cyclization reaction.
Scheme 1. Ir-catalyzed allylic alkylation of 3-substituted indoles.
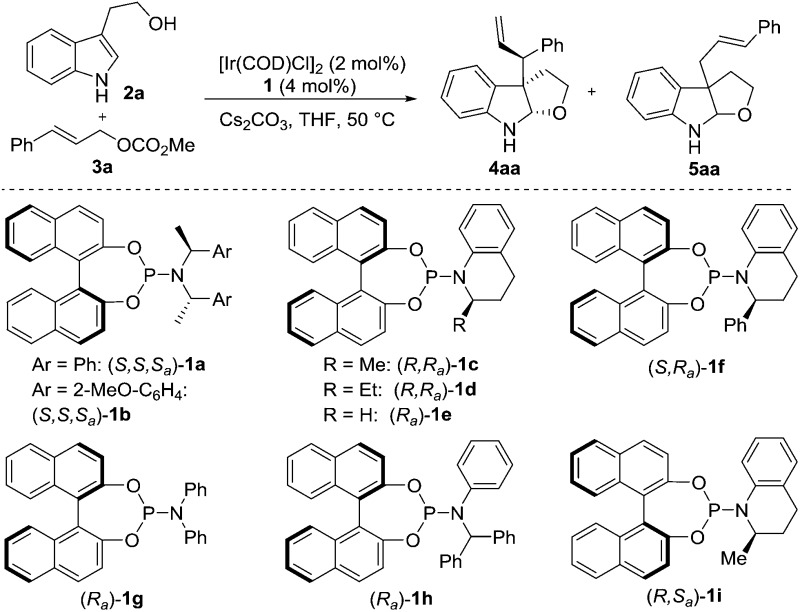
Since we hypothesized that the catalysts may play a key role in the stereoselectivity, our studies were initiated by investigating phosphoramidite ligands with tryptophol 2a and cinnamyl carbonate 3a as model substrates (Table 1). Exposure of the substrates to the catalyst derived from the Feringa ligand 1a (ref. 17a ) or the Alexakis ligand 1b (ref. 17b ) and 1 equiv. of Cs2CO3 in THF provided furoindoline 4aa in good yield as a mixture of diastereomers (2/1–3/1 dr, entries 1 and 2). We envisioned that the low diastereoselectivity may originate from the weak interaction between the sterically encumbered cyclometalated Ir–allyl complex18 and the bulky nucleophile. Efforts to improve the diastereoselectivity were made by conducting a survey of several N-aryl substituted ligands (entries 3–9). The superior diastereocontrol observed in the cases of the less bulky ligands (i.e., 1c–e, 1g and 1i) compared to that of the more bulky ligands (i.e., 1a, 1b, 1f and 1h) again suggested the importance of the ligand structure in the facial discrimination of indoles. However, if the ligand's steric bulk was too small (i.e., 1e and 1g), the reactivity tended to be lower (28–32% yields, entries 5 and 7). To our delight, ligand 1c finally emerged as the optimal one for both yield and diastereoselectivity (entry 3).
Table 1. Optimization of the reaction conditions a .
| |||||
| Entry | Ligand (1) | t (h) | dr (4aa) b | Yield (4aa) c (%) | ee d (%) |
| 1 | 1a | 12 | 2/1 | 50 | 86 |
| 2 | 1b | 3 | 3/1 | 76 | 90 |
| 3 | 1c | 5 | 5/1 | 84 | 87 |
| 4 | 1d | 9 | 5/1 | 84 | 86 |
| 5 | 1e | 24 | 10/1 | 28 | 90 |
| 6 | 1f | 3 | 1/1 | 72 | 90 |
| 7 | 1g | 24 | 9/1 | 32 | 92 |
| 8 e | 1h | 3 | 2/1 | 86 | 90 |
| 9 | 1i | 24 | 9/1 | 36 | –90 |
| 10 f | 1c | 6 | 8/1 | 82 | 90 |
| 11 f , g | 1c | 12 | 15/1 | 70 | 95 |
| 12 f , g , h | 1c | 12 | >20/1 | 82 | 95 |
aThe reaction was performed with 0.2 mmol of 2a, 1.5 equiv. of 3a and 1 equiv. of Cs2CO3. Ir-catalyst was prepared in situ with n-PrNH2.
bDetermined by 1H NMR of the crude reaction mixture. Unless otherwise noted, 4aa/5aa = >95/5.
cIsolated yield.
dee of 4aa was determined using HPLC analysis (Chiralpak IC).
e 4aa/5aa = 90/10.
f1 equiv. of Et3N was used with 2a/3a = 2/1.
gAt rt.
hIr-catalyst was prepared in situ with TBD.
Further optimization was conducted by screening various bases, temperatures, substrate ratios, and the activators used in the catalyst in situ preparation (entries 10–12).19–21 These results revealed that the use of excess nucleophile and the combination of Et3N and catalyst generated in situ by TBD could provide 4aa in 82% yield with >20/1 dr and 95% ee at room temperature (entry 12).
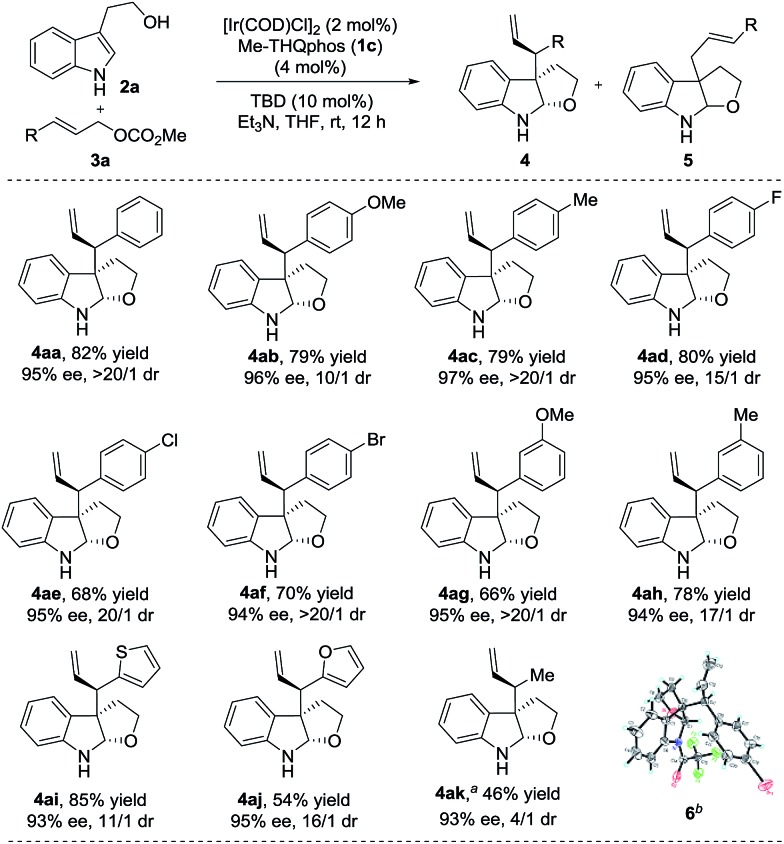
With the optimized reaction conditions in hand, we next studied the scope of different allylic carbonates amenable to the chemistry (Scheme 2). Carbonates containing either electron-donating (4-MeO, 4-Me, 3-MeO and 3-Me) or electron- withdrawing (4-F, 4-Cl and 4-Br) substituents on the phenyl ring were well tolerated and delivered the corresponding products efficiently (4aa–4ah, 66–82% yields, 10/1–>20/1 dr and 94–97% ee).
Scheme 2. Substrate scope of electrophiles. The reactions were performed under the conditions of Table 1, entry 12. The yields of isolated product 4 are shown. The dr was determined using 1H NMR of the crude reaction mixture and the ee was determined using chiral HPLC. Unless otherwise noted, 4/5 = >95/5. aReaction time was 18 h and 4/5 = 85/15. bThe absolute configuration of 4af was determined using X-ray analysis of its trifluoroacetyl-protected derivative 6.19 The absolute configuration of all other products was determined by analogy.
In addition, heteroaryl allylic carbonates, such as 2-thienyl and 2-furanyl, were also compatible with this transformation (4ai and 4aj, 11/1–16/1 dr and 93–95% ee). Gratifyingly, the reaction with methyl crotyl carbonate led to the desired product 4ak in excellent enantioselectivity (93% ee), albeit with a slightly lower dr than for aromatic substituted carbonates. To the best of our knowledge, this is the first example of the application of an aliphatic allylic carbonate in the Ir-catalyzed allylic alkylation of prochiral nucleophiles which can achieve good diastereo- and enantioselectivities.
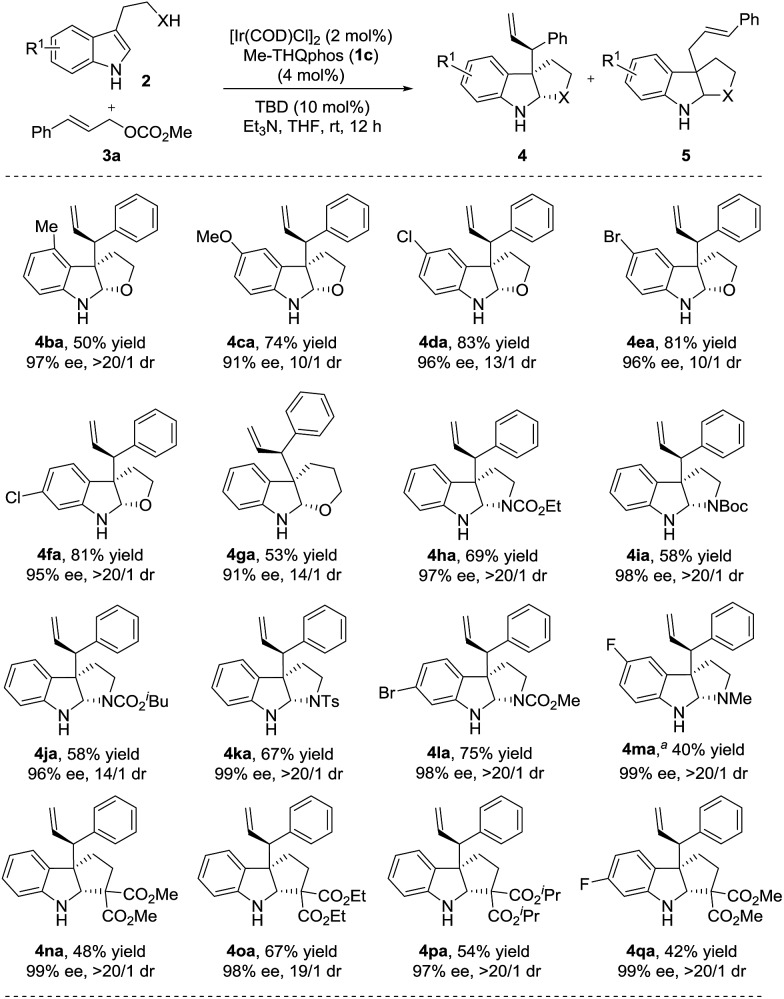
Subsequently, we set out to investigate the scope of nucleophiles. A variety of indoles with diverse substituents were examined and the results are summarized in Scheme 3.22 Different substituents (4-Me, 5-MeO, 5-Cl, 5-Br and 6-Cl) on the tryptophols were well tolerated in all cases delivering the desired furoindoline products smoothly (4ba–4fa, 50–83% yields, 10/1–>20/1 dr and 91–97% ee). Pyranoindoline 4ga was also obtained in good yield and selectivities. In addition, employing tryptamines with varied protecting groups (CO2Me, CO2Et, Boc, CO2 iBu and Ts) on the nitrogen of the linkage and halogen substituents on the aromatic ring all resulted in the corresponding products (58–75% yields, 4ha–4la). Upon treatment with LiAlH4, the N–Me substituted compound 4ma was obtained in 40% yield in two steps.19 The excellent diastereo- and enantioselectivities (14/1–>20/1 dr and 96–99% ee) of these particularly valuable pyrroloindolines demonstrate the potential of this method in the synthesis of complex alkaloid natural products. We also found that indoles with carbon nucleophile side chains were well compatible and proceeded to give cyclopentaneindoline products 4na–4qa under the same conditions in moderate yields (42–67%) with excellent stereoselectivities (19/1–>20/1 dr and 97–99% ee).23
Scheme 3. Substrate scope of nucleophiles. The reactions were performed under the conditions of Table 1, entry 12. The yields of isolated product 4 are shown. The dr was determined using 1H NMR of the crude reaction mixture and the ee was determined using chiral HPLC. Unless otherwise noted, 4/5 = >95/5. a 4ma was obtained through an allylic alkylation of methyl (2-(5-fluoro-1H-indol-3-yl)ethyl)carbamate and a subsequent reduction using LiAlH4.19 .
Remarkably, the reaction also tolerates chiral nucleophiles such as tryptophan derivatives. Subjecting the l-tryptophan derivative (–)-2r to the reaction conditions afforded the corresponding product cis-4ra with a good dr and in a moderate yield (Scheme 4a). Similarly, employment of the d-tryptophan derivative (+)-2r led to indoline trans-4ra in a slightly better yield, albeit as a 3.5/1 mixture of diastereoisomers (Scheme 4b). Both of the major isomers of the above reactions shared the identical stereochemical configuration at the three newly formed centers (C2, C3 and C3′), suggesting that ligand-enabled catalyst control is responsible for establishing the absolute stereochemistry at these three centers,15b while a pre-existing chiral center in the substrate could also bias the stereochemical outcomes albeit to a lesser extent.
Scheme 4. Allylic alkylation of N-Boc protected tryptophan methyl ester.
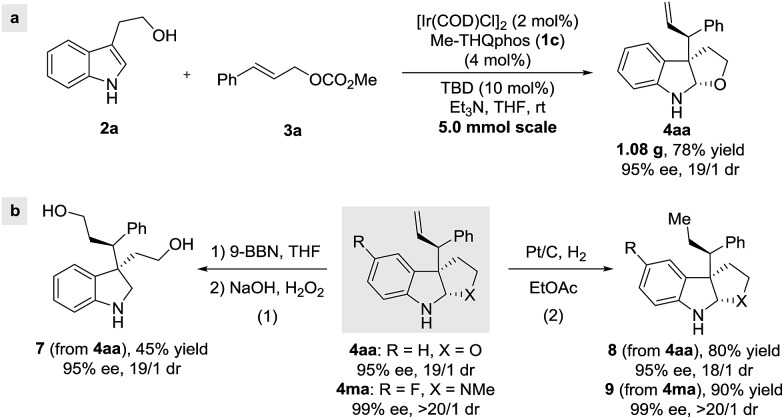
In order to demonstrate the utility of this method, a gram-scale reaction was carried out, achieving good yield and selectivities (Scheme 5a). Furthermore, hydroboration/oxidation of the terminal olefin in 4aa was achieved without a loss of enantiomeric purity (eqn (1), Scheme 5b). Finally, Pt/C-catalyzed hydrogenation afforded the corresponding furoindoline 8 and pyrroloindoline 9 (eqn (2), Scheme 5b), with excellent diastereoselectivities and enantioselectivities, showcasing the synthetic potential of this protocol.
Scheme 5. Gram-scale synthesis and product transformations.
Conclusions
In conclusion, we have developed an iridium-catalyzed intermolecular allylic alkylation reaction of 3-substituted indoles with high site-, regio-, diastereo-, and enantioselectivities. The transformation, in which three stereocenters are formed in a single step, provides efficient access to structurally complex polycyclic indolines from simple starting materials. We also demonstrate that stereoselectivity is mainly controlled by the catalyst when tryptophan derivatives are used. Critical to this strategy is the employment of the Me-THQPhos (1c) ligand. Further mechanistic studies and the application of this method in natural product synthesis is ongoing in our laboratory.
Acknowledgments
We thank the National Basic Research Program of China (973 Program 2015CB856600), the National Natural Science Foundation of China (21332009, 21421091), and the Chinese Academy of Sciences for generous financial support.
Footnotes
References
- (a) Anthoni U., Christophersen C. and Nielsen P. H., in Alkaloids: Chemical and Biological Perspectives, ed. S. W. Pelletier, Pergamon, Oxford, 1999, pp. 163–236. [Google Scholar]; (b) Tsukamoto S., Hirota H., Kato H., Fusetani N. Tetrahedron Lett. 1993;34:4819. [Google Scholar]; (c) Steven A., Overman L. E. Angew. Chem., Int. Ed. 2007;46:5488. doi: 10.1002/anie.200700612. [DOI] [PubMed] [Google Scholar]; (d) Schmidt M. A., Movassaghi M. Synlett. 2008:313. [Google Scholar]
- For reviews: ; (a) Zhuo C.-X., Zhang W., You S.-L. Angew. Chem., Int. Ed. 2012;51:12662. doi: 10.1002/anie.201204822. [DOI] [PubMed] [Google Scholar]; (b) Repka L. M., Reisman S. E. J. Org. Chem. 2013;78:12314. doi: 10.1021/jo4017953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Marsden S. P., Depew K. M., Danishefsky S. J. J. Am. Chem. Soc. 1994;116:11143. [Google Scholar]; (b) Crich D., Huang X. J. Org. Chem. 1999;64:7218. doi: 10.1021/jo982385y. [DOI] [PubMed] [Google Scholar]; (c) Kawahara M., Nishida A., Nakagawa M. Org. Lett. 2000;2:675. doi: 10.1021/ol005513p. [DOI] [PubMed] [Google Scholar]; (d) Shen L., Zhang M., Wu Y., Qin Y. Angew. Chem., Int. Ed. 2008;47:3618. doi: 10.1002/anie.200800566. [DOI] [PubMed] [Google Scholar]; (e) Kim J., Movassaghi M. J. Am. Chem. Soc. 2011;133:14940. doi: 10.1021/ja206743v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Boyer N., Movassaghi M. Chem. Sci. 2012;3:1798. doi: 10.1039/C2SC20270K. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Kieffer M. E., Chuang K. V., Reisman S. E. J. Am. Chem. Soc. 2013;135:5557. doi: 10.1021/ja4023557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Huang A., Kodanko J. J., Overman L. E. J. Am. Chem. Soc. 2004;126:14043. doi: 10.1021/ja046690e. [DOI] [PubMed] [Google Scholar]; (b) Trost B. M., Zhang Y. J. Am. Chem. Soc. 2006;128:4590. doi: 10.1021/ja060560j. [DOI] [PubMed] [Google Scholar]; (c) Ma S., Han X., Krishnan S., Virgil S. C., Stoltz B. M. Angew. Chem., Int. Ed. 2009;48:8037. doi: 10.1002/anie.200902943. [DOI] [PubMed] [Google Scholar]; (d) Zhang H., Hong L., Kang H., Wang R. J. Am. Chem. Soc. 2013;135:14098. doi: 10.1021/ja408336v. [DOI] [PubMed] [Google Scholar]; (e) Trost B. M., Osipov M., Krüger S., Zhang Y. Chem. Sci. 2015;6:349. doi: 10.1039/c4sc01826e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Austin J. F., Kim S.-G., Sinz C. J., Xiao W.-J., MacMillan D. W. C. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5482. doi: 10.1073/pnas.0308177101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Trost B. M., Quancard J. J. Am. Chem. Soc. 2006;128:6314. doi: 10.1021/ja0608139. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Repka L. M., Ni J., Reisman S. E. J. Am. Chem. Soc. 2010;132:14418. doi: 10.1021/ja107328g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhu S., MacMillan D. W. C. J. Am. Chem. Soc. 2012;134:10815. doi: 10.1021/ja305100g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Cera G., Chiarucci M., Mazzanti A., Mancinelli M., Bandini M. Org. Lett. 2012;14:1350. doi: 10.1021/ol300297t. [DOI] [PubMed] [Google Scholar]; (f) Zhang X., Han L., You S.-L. Chem. Sci. 2014;5:1059. [Google Scholar]; (g) Wang H., Reisman S. E. Angew. Chem., Int. Ed. 2014;53:6206. doi: 10.1002/anie.201402571. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Shao W., Li H., Liu C., Liu C.-J., You S.-L. Angew. Chem., Int. Ed. 2015;54:7684. doi: 10.1002/anie.201503042. [DOI] [PubMed] [Google Scholar]
- Selected examples: ; (a) Overman L. E., Paone D. V., Stearns B. A. J. Am. Chem. Soc. 1999;121:7702. [Google Scholar]; (b) Tanaka K., Taniguchi T., Ogasawara K. Tetrahedron Lett. 2001;42:1049. [Google Scholar]; (c) Pinto A., Jia Y., Neuville L., Zhu J. Chem.–Eur. J. 2007;13:961. doi: 10.1002/chem.200601016. [DOI] [PubMed] [Google Scholar]; (d) Dounay A. B., Humphreys P. G., Overman L. E., Wrobleski A. D. J. Am. Chem. Soc. 2008;130:5368. doi: 10.1021/ja800163v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Spangler J. E., Davies H. M. L. J. Am. Chem. Soc. 2013;135:6802. doi: 10.1021/ja4025337. [DOI] [PubMed] [Google Scholar]
- For an organocatalytic construction of pyrroloindolines bearing three contiguous stereocenters, see ref. 5a
- For Ir-catalyzed C3 and N1 allylic alkylation of indoles, see: ; (a) Liu W.-B., He H., Dai L.-X., You S.-L. Org. Lett. 2008;10:1815. doi: 10.1021/ol800409d. [DOI] [PubMed] [Google Scholar]; (b) Stanley L. M., Hartwig J. F., Angew. Chem., Int. Ed., 2009, 48 , 7841 , and ref. 5f. . [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bandini M., Melloni A., Piccinelli F., Sinisi R., Tommasi S., Umani-Ronchi A. J. Am. Chem. Soc. 2006;128:1424. doi: 10.1021/ja054109o. [DOI] [PubMed] [Google Scholar]; (d) Zhu Y., Rawal V. H. J. Am. Chem. Soc. 2012;134:111. doi: 10.1021/ja2095393. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Liu Y., Du H., Org. Lett., 2013, 15 , 740 , , and ref. 5b . [DOI] [PubMed] [Google Scholar]
- Selected reviews: ; (a) Helmchen G., Dahnz A., Dübon P., Schelwies M., Weihofen R. Chem. Commun. 2007:675. doi: 10.1039/b614169b. [DOI] [PubMed] [Google Scholar]; (b) Helmchen G., in Iridium Complexes in Organic Synthesis, ed. L. A. Oro and C. Claver, Wiley-VCH, Weinheim, 2009, pp. 211–250. [Google Scholar]; (c) Hartwig J. F., Pouy M. J. Top. Organomet. Chem. 2011;34:169. [Google Scholar]; (d) Liu W.-B., Xia J.-B., You S.-L. Top. Organomet. Chem. 2012;38:155. [Google Scholar]; (e) Tosatti P., Nelson A., Marsden S. P. Org. Biomol. Chem. 2012;10:3147. doi: 10.1039/c2ob07086c. [DOI] [PubMed] [Google Scholar]
- Carreira and coworkers recently reported an iridium-catalyzed reverse prenylation of 3-substituted indoles, affording the branched-selective indoline derivatives in excellent yields. The enantioselectivity was found to be <20% ee, see: Ruchti J., Carreira E. M., J. Am. Chem. Soc., 2014, 136 , 16756 , . For a recent Ir-catalyzed allylic alkylation of 3-substituted indoles affording linear-selective products, see ref. 5f . [DOI] [PubMed] [Google Scholar]
- Kanayama T., Yoshida K., Miyabe H., Takemoto Y. Angew. Chem., Int. Ed. 2003;42:2054. doi: 10.1002/anie.200250654. [DOI] [PubMed] [Google Scholar]
- (a) Chen W., Hartwig J. F. J. Am. Chem. Soc. 2013;135:2068. doi: 10.1021/ja311363a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen W., Hartwig J. F. J. Am. Chem. Soc. 2014;136:377. doi: 10.1021/ja410650e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen W., Chen M., Hartwig J. F. J. Am. Chem. Soc. 2014;136:15825. doi: 10.1021/ja506500u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Liu W.-B., Reeves C. M., Virgil S. C., Stoltz B. M. J. Am. Chem. Soc. 2013;135:10626. doi: 10.1021/ja4052075. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu W.-B., Reeves C. M., Stoltz B. M. J. Am. Chem. Soc. 2013;135:17298. doi: 10.1021/ja4097829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Krautwald S., Sarlah D., Schafroth M. A., Carreira E. M. Science. 2013;340:1065. doi: 10.1126/science.1237068. [DOI] [PubMed] [Google Scholar]; (b) Krautwald S., Schafroth M. A., Sarlah D., Carreira E. M. J. Am. Chem. Soc. 2014;136:3020. doi: 10.1021/ja5003247. [DOI] [PubMed] [Google Scholar]
- (a) Liu W.-B., He H., Dai L.-X., You S.-L. Synthesis. 2009:2076. [Google Scholar]; (b) Liu W.-B., Zheng C., Zhuo C.-X., Dai L.-X., You S.-L. J. Am. Chem. Soc. 2012;134:4812. doi: 10.1021/ja210923k. [DOI] [PubMed] [Google Scholar]
- (a) Wu Q.-F., He H., Liu W.-B., You S.-L. J. Am. Chem. Soc. 2010;132:11418. doi: 10.1021/ja105111n. [DOI] [PubMed] [Google Scholar]; (b) Zhuo C.-X., Liu W.-B., Wu Q.-F., You S.-L. Chem. Sci. 2012;3:205. [Google Scholar]
- (a) Teichert J. F., Feringa B. L. Angew. Chem., Int. Ed. 2010;49:2486. doi: 10.1002/anie.200904948. [DOI] [PubMed] [Google Scholar]; (b) Alexakis A., Polet D. Org. Lett. 2004;6:3529. doi: 10.1021/ol048607y. [DOI] [PubMed] [Google Scholar]
- (a) Madrahimov S. T., Markovic D., Hartwig J. F. J. Am. Chem. Soc. 2009;131:7228. doi: 10.1021/ja902609g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hartwig J. F., Stanley L. M. Acc. Chem. Res. 2010;43:1461. doi: 10.1021/ar100047x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Spiess S., Raskatov J. A., Gnamm C., Brödner K., Helmchen G. Chem.–Eur. J. 2009;15:11087. doi: 10.1002/chem.200902065. [DOI] [PubMed] [Google Scholar]; (d) Raskatov J. A., Spiess S., Gnamm C., Brödner K., Rominger F., Helmchen G. Chem.–Eur. J. 2010;16:6601. doi: 10.1002/chem.200903465. [DOI] [PubMed] [Google Scholar]
- See the ESI for details
- For the procedure of in situ generation of Ir-complex using n-PrNH2: ; (a) Ohmura T., Hartwig J. F. J. Am. Chem. Soc. 2002;124:15164. doi: 10.1021/ja028614m. [DOI] [PubMed] [Google Scholar]; (b) Kiener C. A., Shu C., Incarvito C., Hartwig J. F. J. Am. Chem. Soc. 2003;125:14272. doi: 10.1021/ja038319h. [DOI] [PubMed] [Google Scholar]
- For the procedure of in situ generation of Ir-complex using dry TBD: ; (a) Welter C., Koch O., Lipowsky G., Helmchen G. Chem. Commun. 2004:896. doi: 10.1039/b400023d. [DOI] [PubMed] [Google Scholar]; (b) Welter C., Dahnz A., Brunner B., Streiff S., Dübon P., Helmchen G. Org. Lett. 2005;7:1239. doi: 10.1021/ol047351t. [DOI] [PubMed] [Google Scholar]; (c) Gnamm C., Krauter C. M., Brödner K., Helmchen G. Chem.–Eur. J. 2009;15:2050. doi: 10.1002/chem.200802525. [DOI] [PubMed] [Google Scholar]; (d) Gnamm C., Brödner K., Krauter C. M., Helmchen G. Chem.–Eur. J. 2009;15:10514. doi: 10.1002/chem.200901316. [DOI] [PubMed] [Google Scholar]
- In some cases, the allylic etherification of 2 or the subsequent allylic amination was observed, which was responsible for the low yield of 4
- Synthesis of indoline with multiple stereocenters by Rh-, amine- or Cu-catalysis: ; (a) Lian Y., Davies H. M. L. J. Am. Chem. Soc. 2010;132:440. doi: 10.1021/ja9078094. [DOI] [PubMed] [Google Scholar]; (b) Cai Q., Zheng C., Zhang J.-W., You S.-L. Angew. Chem., Int. Ed. 2011;50:8665. doi: 10.1002/anie.201103937. [DOI] [PubMed] [Google Scholar]; (c) Xiong H., Xu H., Liao S., Xie Z., Tang Y. J. Am. Chem. Soc. 2013;135:7851. doi: 10.1021/ja4042127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.