Abstract
Overexpression of P-glycoprotein (Pgp) has been considered a primary cause for multidrug resistance in a variety of cancers for three decades. However, clinical translation of Pgp targeted therapeutics has been hindered by lack of patient preselection based on the Pgp presence in tumors. We aim to develop a molecularly targeted probe for imaging tumoral Pgp in vivo with positron emission tomography (PET) and fluorescence, and to provide a tool for preselecting the patients with tumoral Pgp expression. Thus, a Pgp monoclonal antibody 15D3 was chemically modified with IRDye800 (IR800) and DOTA chelator. The specificity of the antibody conjugates DOTA-Pab-IR800 was evaluated in Pgp-expressing 3T3-MDR1 and control 3T3 cells. After radiolabeling with 64Cu, the probe was applied in small animal PET imaging of Pgp in a mouse xenograft model of NCI/ADR-Res cells, which are chemoresistant through overexpression of Pgp. Quantification analysis of the PET images demonstrated that the tumor uptake of the radioactive probe was 9.9 ± 1.4, 12.1 ± 1.2, and 10.5 ± 1.0%ID/g at 4, 24, and 48h post injection. The tumor-to-muscle ratio was 20.9 at 48 h post injection based on biodistribution studies. Fluorescence imaging was performed following PET experiments, and it demonstrated excellent tumor accumulation of this dual-modality probe in the NCI/ADR-Res tumors. Further, an image-guided surgery was performed successfully using the fluorescence modality of the probe, demonstrating potential utility of this probe in image-guided surgical removal of Pgp-positive drug resistant tumors in the patients. In conclusion, this study clearly demonstrated that the Pgp-targeted antibody probe, 64Cu-DOTA-Pab-IR800, could provide a promising diagnosis tool for detection of Pgp-expressing tumors in vivo.
Graphical abstract

Introduction
Drug resistance remains a formidable challenge to cancer therapy. ATP-binding cassette (ABC) transporters, notably P-glycoprotein (Pgp), reduce cytotoxic effects of anticancer drugs by mediating their efflux from cancer cells, and thus become a major cause of multidrug resistance (MDR) of human cancers.1–5 Pgp is the first ABC transporter to be discovered,6 and is also the primary transporter causing MDR in cancers.5, 7 For example, Pgp was first identified to be responsible for MDR in ovarian cancer (OvCa).8 It is widely expressed in OvCa tumors with the positivity ranging from 40% to 92.8% across various studies, 8–15 and its expression is correlated with chemotherapy response and survival outcome.11,12 Strategies to overcome this resistance have been vigorously sought for more than 30 years, and three generations of small-molecule Pgp inhibitors have been developed to sensitize MDR tumor cells to chemotherapy agents.2, 16 However, they have yet to reach the oncology clinic.17
Lack of patient preselection is considered one of the main causes for the clinical failure of the small-molecule Pgp inhibitors. There are multiple causes for cancer MDR, and some mechanisms, including increased DNA repair capability and enhanced anti-apoptotic activity, are not caused by Pgp.1 The patients were not selected into the trials testing Pgp inhibitors based on assessment of the tumoral Pgp levels. However, most of the patients enrolled in these clinical trials had already undergone multiple lines of cancer therapeutics, including radiation therapy, chemotherapy, and targeted therapy, and therefore drug resistance was most likely caused by multiple mechanisms.17 Without patient preselection, the potential effects in patients with Pgp overexpression could be masked by the patients that do not express this transporter.17, 18 Thus, the success of any Pgp targeted therapeutics depends on a companion diagnostic test that can detect tumoral Pgp expression in a patient and to determine if favorable outcome will come from the specific treatments.
In this study, we aimed to construct a dual PET/Fluorescence probe with an anti-Pgp monoclonal antibody in order to image tumoral Pgp. We tested Pgp specificity of the probe using an in vitro immunostaining assay, and then examined its application in detection of Pgp expression with PET/fluorescence imaging in a mouse xenograft model of chemoresistant OvCa tumors. Further, we tested the feasibility of using the fluorescent moiety of this probe for an image-guided surgery.
Methods
Materials
The chelating agent 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) was purchased from Macrocyclics Inc. (Dallas, TX, USA). The fluorescence dye IRDye800-NHS (IR800-NHS) was purchased from LI-COR Inc. (Lincoln, NE, USA). 64Cu was produced at University of Wisconsin in the form of 64CuCl2 in 0.1 N HCl. Mouse IgG1 isotype control (IgG) was purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Anti-Pgp Antibody Production
Anti-Pgp monoclonal antibody (Pab) was produced in house using the hybridoma cell line 15D319 from ATCC (Rockville, MD, USA). Briefly, 15D3 hybridoma cells were initially cultured in DMEM media (Corning Inc., Corning, NY, USA) containing 10% fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, USA). The FBS content was reduced by serial dilution until culturing in serum-free hybridoma medium (Thermo Fisher Scientific, Rockford, IL, USA). The antibody-containing media was harvested every 24 h and the antibody was purified with HiTrap Protein G HP columns (GE Healthcare Life Sciences, Piscataway, NJ, USA). The identity and purity of the antibody was assessed by SDS-PAGE.
Chemistry and radiochemistry
Pab and control IgG were modified with DOTA using a method published previously.20 Briefly, DOTA was first reacted with EDC and Sulfo-NHS. Then 50-folded excess of activated DOTA was added to Pab or IgG and the pH was adjusted to 8.5 using borate buffer. Additional 3-folded excess of IR800-NHS was added to the Pab as well. Reaction mixture was incubated at 4 °C overnight followed by purification with a PD-10 desalting columns (GE). DOTA-Pab-IR800 and DOTA-IgG were then labeled with 64Cu as described previously.20 Briefly, 37 MBq of 64Cu was added to 60 μg of the DOTA-Pab-IR800 or IgG-DOTA and the pH was adjusted to 5.5 with 0.25 M NH4OAc. The mixture was incubated at 40 °C for 1 h with constant shaking and purified using a PD-10 column. The radioactivity of the eluted fractions was measured by a γ-counter (PerkinElmer, Waltham, MA, USA), and the radioactive fraction containing 64Cu-DOTA-Pab-IR800 or 64Cu-DOTA-IgG was collected for further experiments. The purity of the radioactive probes was examined by SDS-PAGE followed by autoradiography. SDS-PAGE was performed with a polyacrylamide gel (Bio-Rad, Hercules, CA, USA). After electrophoresis, the gel was stained with Coomassie Blue Staining buffer (Bio-Rad), and digitally scanned. The 64Cu radioactivity in the gel was detected using autoradiography.
Cell lines
Cell line 3T3-MDR1 is a mouse fibroblast cell line stably transfected with a cDNA coding for human Pgp (also called Multi-Drug Resistance Gene 1, MDR1) and was obtained from Dr. Gottesman’s lab at National Cancer Institute (NCI, Bethesda, MD, USA). This cell line was maintained in DMEM medium supplemented with 10% FBS, 400 IU/mL penicillin plus 100 μg/mL streptomycin (Corning Inc.), and 60 ng/ml colchicine. Adriamycin-resistant OvCa cell line NCI/ADR-RES was also obtained from Dr. Gottesman’s lab at NCI and was maintained in the same condition as 3T3-MDR1 cell line.
Cell immunostaining and flow cytometry
Immunostaining followed by flow cytometry was performed to determine Pgp specificity of DOTA-Pab-IR800. Briefly, 3T3-MDR1 and 3T3 cells were cultured overnight. After washed with PBS, the cells were detached using 0.25% Trypsin, 0.1% EDTA (Corning Inc.) and suspended in PBS buffer. Then, 1 × 106 of live cells were first blocked using 10% goat serum at room temperature for 10 min, and secondarily stained with 10 μg/ml of DOTA-Pab-IR800 or PBS at 4 °C for 30 min. Cell-associated fluorescence was detected on an LSRFortessa flow cytometer (BD Bioscience, CA, USA) and ten thousand cell events were analyzed with FlowJo software (FLOWJO LLC, OR, USA). Measurement of surface Pgp expression in NCI/ADR-RES and OVCAR8 cells was carried out by immunostaining with a PE-labeled anti-Pgp antibody (BD Biosciences) followed by flow cytometry.
Cytotoxicity
The cytotoxicity of taxol towards NCI/ADR-RES and OVCAR8 cells was assessed with the Alamar Blue assay as described previously.21 Briefly, NCI/ADR-RES and OVCAR8 cells were seeded in 96-well plates at 3,000 cells/well. After culture overnight, the cells were treated with increasing concentrations of taxol for 24 h. After washing thrice, cells were incubated in fresh medium for another 48 h. Alamar Blue reagent (Thermo Fisher Scientific) was then added and incubated for 2 h. The fluorescence of the samples was measured in a FLUOstar Omega microplate reader (BMG LABTECH, Cary, NC, USA) at 540 nm excitation and 590 nm emission wavelengths.
Animals
Nude mice from NCI were bred at the Division of Comparative Medicine at University of North Carolina at Chapel Hill (UNC). Female nude mice (4–6 weeks old) were selected to establish mouse xenograft model of OvCa. All animal studies were carried out according to the protocols approved by the UNC’s Institutional Animal Care and Use Committee. To establish tumor models, 5 × 106 NCI/ADR-RES cells were suspended in 0.1 ml PBS/Matrigel (BD Biosciences) (1/1, v/v) and inoculated subcutaneously into the bilateral flanks of nude mice. After four weeks, mice each carrying two NCI/ADR-RES tumors in similar size were selected for imaging experiments.
Immunostaining of Pgp in tumor tissues
Tumor tissues were harvested from NCI/ADR-RES-bearing nude mice and were fixed in 4% paraformaldehyde overnight at 4°C. After being immersed in 30% sucrose solution overnight, tumor tissues were sectioned in 7-μm thick. To detect the Pgp expression, slides were rinsed thoroughly with PBS, then blocked in 5% Normal Goat Serum in PBS for 30 min at room temperature, blotted off serum block, and incubated with anti-Pgp C219 monoclonal antibody (Thermo Fisher Scientific) at 10 μg/mL or PBS as negative control at room temperature for 60 min. After rinsed with PBS for three times, slides were incubated with AlexaFluor-594 goat anti-mouse IgG (H+L) secondary antibody (Thermo Fisher Scientific) at 5 μg/mL at room temperature for 30 min. The slides were then rinsed with PBS twice and stained with DAPI (Thermo Fisher Scientific). The fluorescence images were taken using Olympus FV1200 Spectral Confocal Microscope (Olympus, Tokyo, Japan). A section of tumor tissue that was only stained with the secondary antibody served as control.
In vivo PET Imaging
PET imaging was performed four weeks after tumor inoculation with a method described previously.20, 22 Briefly, each mouse was given 3.7 MBq of 64Cu-DOTA-Pab-IR800 or 64Cu-DOTA-IgG via the tail vein injection. The PET images were acquired at 4, 24, and 48 h post injection using a small animal PET/CT system (GE eXplore Vista) while the mice were under isoflurane-induced anesthesia. The regions of interest (ROIs) were calculated to percentage injected dose per gram tissue (%ID/g) based on the assumption of 1g/mL tissue density. Biodistribution study was performed in nude mice bearing NCI/ADR-RES tumors. Mice were given intravenously approximate 5.5 MBq of 64Cu-DOTA-Pab-IR800 and 64Cu-DOTA-IgG, and were sacrificed 48h post injection. Tissues and organs of interest were excised and weighed. Radioactivity was measured by a γ-counter (PerkinElmer) and the tracer uptake was calculated as %ID/g.
In vivo fluorescence imaging and image-guided surgery
Fluorescence imaging and image-guided surgery were performed with a method described previously.20 Mice were subjected to fluorescence imaging using an IVIS Kinetic optical imaging system (Caliper Life Sciences, Alameda, CA, USA) right after the microPET scan at each time point. The parameters used during the imaging was 10s exposure time (f/stop=4) with an ICG filter set. Image-guided surgery was performed following fluorescence imaging at 48 h after I.V. injection.
Statistical Analysis
Data are expressed as mean ± SD. Means were compared using one-way ANOVA followed by Tukey’s post-hoc analysis for multiple comparisons or Student’s t test for two-sample comparison. P values < 0.05 were considered statistically significant.
RESUTS
Chemistry and Radiochemistry
Pab and control IgG were conjugated with DOTA or IR800 through amino groups. We estimate that 2.4 DOTA molecules were conjugated to an antibody molecule using a titration method described previsously,23 and 1.8 molecules of IR800 were linked to the antibody by measurement of UV-Vis absorbance of the conjugates. The resultant DOTA-Pab-IR800 and IgG-DOTA were labeled with 64Cu with radiochemical yields of 30.4% and 32.0%, respectively. The calculated specific activity of 64Cu-DOTA-Pab-IR800 was 18.8 GBq/μmol while that of 64Cu-DOTA-IgG was 28.9 GBq/μmol. The radiochemical purity of the probes was examined with SDS-PAGE followed by autoradiography. As shown in Figure S1, only one radioactive band was observed for each probe in the autoradiographic image of the gel, and matched the Coomassie Blue stained SDS-PAGE gel, indicating high purity of the radioactive probes.
Pgp-specific binding of the antibody conjugates
Pgp-expressing 3T3-MDR1 and control 3T3 cells were used to examine Pgp specific binding of DOTA-Pab-IR800. After staining, 3T3-MDR1 cells showed a dramatic right shift when compared to ether PBS control or 3T3 cells stained with DOTA-Pab-IR800 (Figure 1), indicating that DOTA-Pab-IR800 could specifically bind to Pgp on the cell surface of 3T3-MDR1 cells, and conjugation with DOTA and IR800 did not compromise the Pgp specificity of the antibody.
Figure 1.
Flow cytometric analysis for Pgp specificity of DOTA-Pab-IR800. Both 3T3 and 3T3-MDR1 cells were stained with 10 μg/ml of DOTA-Pab-IR800 or PBS as negative control at 4 °C for 30 min. Flow cytometry was performed to measure cell associated IR800 fluorescence.
Chemoresistant OvCa model
We then validated Pgp expression in NCI/ADR-Res cells, a chemoresistant OvCa cell line.24, 25 As shown in Figure 2A, this cell line was 223-fold more resistant to taxol than the parent OVCAR8 cells. Accordingly, Pgp immunostaining followed by flow cytometry revealed that NCI/ADR-Res cells expressed significantly higher levels of Pgp than their parent OVCAR8 cells (Figure 2B). Further, NCI/ADR-Res tumor tissues were harvested from xenograft nude mice to detect the Pgp expression using Pgp specific C219 antibody. Immunostaining images in Fig 2C showed that Pgp was expressed in the NCI/ADR-Res tumor tissue, indicating that NCI/ADR-Res tumors were an appropriate model to examine Pgp-targeted molecular imaging.
Figure 2.
Validation of chemoresistant NCI/ADR-Res cell models. A. Cytotoxicity study showed over 223-fold higher IC50 value of NCI/ADR-Res cells to taxol than their parent OVCAR8 cells. B. Immunostaining with Pgp antibody followed by flow cytometry showed that NCI/ADR-Res cells have substantially higher surface expression of Pgp than their parent OVCAR8 cells. 3T3-MDR1 cells were used as a positive control. C. NCI/ADR-Res tumor tissues were harvest from xenograft nude mice to detect the Pgp expression using the Pgp specific C219 antibody. Pgp was localized in the NCI/ADR-Res tumor. A section of tumor tissue that was only stained with the secondary antibody served as control.
In vivo PET imaging
The targeting efficacy of 64Cu-DOTA-Pab-IR800 was evaluated in NCI/ADR-RES tumor-bearing mice with 64Cu-DOTA-IgG used as control. After intravenous injection of 64Cu-DOTA-Pab-IR800, the tumor xenografts on the bilateral flanks can be visualized with good tumor to background contrast through 48 h (Figure 3A). The tumor uptake of 64Cu-DOTA-Pab-IR800 was 9.9 ± 1.4 %ID/g, 12.1 ± 1.2 %ID/g and 10.5 ± 1.0 %ID/g at 4, 24 and 48 h post injection, respectively (Fig 3B). In the 64Cu-DOTA-IgG control group, the tumor uptake was only 6.2 ± 0.8 %ID/g, 7.2 ± 1.1 %ID/g and 6.0 ± 2.0 %ID/g at the same time points respectively (Fig 3B), which was significantly lower than those of 64Cu-DOTA-Pab-IR800 (p < 0.05). At 4 h post injection, both 64Cu-DOTA-Pab-IR800 and 64Cu-DOTA-IgG showed high blood pool accumulation (Figure 3A), likely due to long systemic circulation of antibodies. The liver showed high tracer uptake as well, indicating nonspecific liver uptake of the probes (Figure 3A). In the 64Cu-DOTA-Pab-IR800 group at 24 and 48 h post injection, the activity in the blood decreased together with the liver and kidney uptake while the tumor activity retained at a steady level, which further enhanced the tumor contrast (Figure 3B). On the contrary, 64Cu-DOTA-IgG maintained a relative high blood activity concentration at late time points likely due to the lack of targeted uptake in tumor tissues (Figure 3B). The muscle uptake of both tracers was below 1.5%ID/g and was not significantly different at all the time points tested (Figure 3B). Biodistribution study was also performed to further validate the ROI quantification. The tumor uptake of 64Cu-DOTA-Pab-IR800 and 64Cu-DOTA-IgG at 48 h post injection was 9.8 ± 0.3 %ID/g and 5.6 ± 0.5 %ID/g, respectively (Figure 4). The biodistribution results were consistent with the PET data.
Figure 3.
(A) PET images of NCI/ADR-RES tumor-bearing mice at 4, 24, and 48 h post injection of 64Cu-DOTA-Pab-IR800 and 64Cu-DOTA-IgG. The tumor sites are marked in red circles. (B) The quantitative analysis of tumor, liver, kidney, and muscle uptakes of 64Cu-DOTA-Pab-IR800 (upper) and 64Cu-DOTA-IgG (lower) derived from PET images.
Figure 4.
Biodistribution of 64Cu-DOTA-Pab-IR800 and 64Cu-DOTA-IgG in mice bearing NCI/ADR-RES xenograft. Mice were injected with 64Cu-DOTA-Pab-IR800 and 64Cu-DOTA-IgG, and were sacrificed 48h post injection. Tissues and organs of interest were excised and weighed. Radioactivity was measured and the tracer uptake was calculated as %ID/g. The tumor uptake of 64Cu-DOTA-Pab-IR800 was significantly higher than that of 64Cu-DOTA-IgG at 48 h post injection
Fluorescence imaging and image-guided surgery
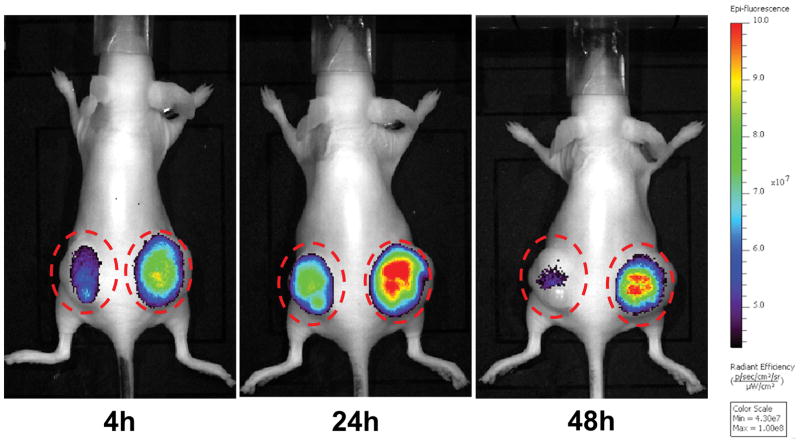
Fluorescence imaging was performed using an IVIS system following each PET scan. Similar to the PET imaging results, excellent tumor uptake was observed through 48 h (Figure 5). Different from PET images, the fluorescent signals in internal organs such as the liver were not observed. Then, an image-guided surgery was performed to remove the NCI/ADR-RES tumors following the last imaging at 48 h post injection. The two NCI/ADR-RES tumors were first located using fluorescence imaging (Figure 6). Then the subcutaneous tumor tissues were resected under image-guidance, and the second fluorescence imaging indicated complete removal of the tumors (Figure 6).
Figure 5.
Fluorescence imaging of mice bearing bilateral NCI/ADR-RES xenografts. The fluorescence imaging was performed right after each PET scan at 4, 24, and 48 h post injection of 64Cu-DOTA-Pab-IR800. Similar to the PET imaging results, excellent tumor uptake was shown at all time points. The tumor sites are marked in red circles.
Figure 6.
Fluorescent image-guided surgery of NCI/ADR-RES tumors. Female nude mouse bearing bilateral NCI/ADR-RES tumors was imaged at 48 h post injection of 64Cu-DOTA-Pab-IR800. The tumor lesion was localized by fluorescence imaging and was subsequently removed using fluorescent image-guided surgery. Upper: Fluorescent images that visualize the tumors and guide their surgical removal. Lower: Digital pictures of the surgical procedures.
Discussion
Pgp-mediated MDR remains as a main obstacle to effective cancer chemotherapy, and this important target is still undruggable after 3-decades of intensive efforts.1 Development of small-molecule inhibitors has been the main approach in those efforts; however, negative results came out of cancer clinical trials of Pgp inhibitors.16, 26 Lack of patient preselection based on tumoral Pgp expression has been considered one cause for these clinical failures.17 To avoid the same failure in future clinical trials, molecular imaging of tumoral Pgp can help select the patients, whose cancers present MDR through a Pgp-mediated mechanism, into the trials. The results of this study first demonstrated that the dual PET/Fluorescence probe made from anti-Pgp 15D3 antibody specifically targets Pgp. This dual-modality probe was successfully used to detect Pgp expression with PET/fluorescence imaging in a mouse xenograft model of chemoresistant OvCa tumors. Thus, this probe may provide a tool for preselecting the patients with tumoral Pgp expression.
In this study, after labelling the antibody with DOTA and fluorescence probe IR800, we examined the Pgp specificity of the antibody conjugates using a pair of 3T3-MDR1 and the control 3T3 cells. The immunostaining result shown in Figure 1 indicated that the DOTA-Pab-IR800 probe remained their Pgp-specific binding. We then validated our MDR model of NCI/ADR-Res cells. This cell line was 223-fold more resistant to taxol than their parent OVCAR8 cells, and accordingly, overexpression of Pgp was found in this cell line (Figure 2 A and B), indicating Pgp expression contributes to the chemoresistance of the cell line though it may not be the sole cause. Further, Pgp was expressed in the NCI/ADR-RES tumor in the xenograft nude mice (Figure 2C), indicating that NCI/ADR-RES tumor was an appropriate model to examine Pgp-targeted molecular imaging.
PET imaging of Pgp expression was studied with 64Cu-labelled Pab and control IgG antibody in tumor-bearing nude mice. The PET images after injection of Pgp targeted probe clearly visualized Pgp-expressing NCI/ADR-Res tumors in the xenograft mice through 2 days after I.V. administration (Figure 3). The radioactivity in the tumors after injection of 64Cu-DOTA-Pab-IR800 were quantified in the biodistribution experiment, which showed that the tumor-associated radioactivity in the 64Cu-DOTA-Pab-IR800 group was significantly higher than that in the 64Cu -DOTA-IgG group (p < 0.05, Figure 4), indicating that Pgp-medicated tumor uptake causes higher uptake of the targeted probe. The moderate tumor uptake of the control probe might be due to the enhanced permeability and retention (EPR) effect that is enjoyed by nanoparticles as well as macromolecules including antibody.27 Although the control antibody does not bind to Pgp, the EPR effect may cause nonspecific accumulation of this antibody in the tumor. Biodistribution of the two probes was also different in other tissues. For example, the 64Cu-DOTA-Pab-IR800 group showed lower radioactivity in the blood but higher activity in the liver than the 64Cu -DOTA-IgG group (Figure 4). This might be caused by different biodistribution profiles between Pab and the control IgG antibody as well as additional conjugation of IR800 to Pab.
Scintigraphic imaging has been used to assess Pgp activity in vivo using radiolabelled Pgp substrates such as 99mTc-sestamibi and 99mTc-tetrofosmin.28–33 Because Pgp transports these radioactive substrates out of the cell, the tumoral signals from the imaging are inversely proportional to the expression levels of Pgp in cancer cells.34 To assess Pgp activity in vivo, transporter inhibitors have to be given to detect the change of radioactive signal. Thus, a false positive result can be made when the inhibitors modulate Pgp activity in the liver and kidney and change the pharmacokinetics of the radiolabeled substrates.17 Further, many of these radiopharmaceuticals are substrates for multiple transporters,35 and this approach cannot identify the specific ABC transporter causing MDR.
Radiolabelled Pgp antibody is a superior probe for imaging Pgp to the small molecule substrates because monoclonal antibody is more specific to recognize Pgp than the radiopharmaceuticals that may be substrates for other ABC transporters. In addition, accumulation of the antibody is directly proportional to Pgp expression, which provides extra advantage for Pgp antibody. Two previous studies used radiolabelled antibodies to image Pgp in tumors. The first study used 125I-labelled MRK-16 antibody for in vivo imaging of tumoral Pgp.36 This probe showed significantly greater accumulation in Pgp-overexpressing neuroblastoma xenografts as compared to low Pgp expressing neuroblastoma xenografts.36 In another study, Pgp expression was examined in a tumor xenograft mouse model using an 111In- and 131I-labelled antibody.34 The biodistribution data demonstrated significantly higher probe uptake in Pgp-positive MES-SA/D×5 tumors than Pgp-negative MES-SA tumors.34 These two radiolabelled monoclonal antibodies reach peak accumulation 2–3 days after injection. However, our 64Cu-DOTA-Pab-IR800 showed highest tumor accumulation at 24 h post-injection (Figures. 3 and 5). Moreover, compared with the above mentioned radioisotopes, 64Cu used in our study has a shorter half-life (12.7 h) and is more suitable for antibody imaging by reducing the radiation exposure towards patients. The corresponding PET imaging modality also provide better imaging quality than previously used gamma camera imaging36 or scintigraphic imaging.34 Finally, our tracer showed lower blood uptake and higher tumor to muscle ratio which significantly reduced the background signals of the images (Figure 3).
The fluorescence imaging performed after each PET scan also showed excellent tumor uptake of 64Cu-DOTA-Pab-IR800 (Figure 5). However, the fluorescent signals in the internal organs such as the liver were substantially lower than the PET signals. PET has an advantage in imaging deep tissues due to the good tissue penetration of high energy photons (511 Kev). In contrast, fluorescent signals from these organs in dark color could not be absorbed or scattered, resulting in weak signal reaching the detector. Therefore, weak signal was observed from fluorescence imaging of these organs. In spite of low sensitivity in imaging deep tissues, fluorescence imaging has the advantage of high resolution, and thus is ideal for image-guided sugery.20 The purpose of using dual-modality PET/fluorescence probe is to combine the advantages of high sensitivity of PET imaging and excellent resolution of fluorescence imaging. When this probe is translated to clinic, PET imaging will first be used to preselect patients and localize MDR tumors, likely in the form of micrometastases buried in the normal tissues of human body; fluorescence imaging will then be used to mark the Pgp-positive tumors intraoperatively, which will enhance the possibility of resecting MDR tumors that would otherwise be resistant to the chemotherapy.
In conclusion, our results indicated that the Pgp-targeted probe, 64Cu-DOTA-Pab-IR800, could detect Pgp positive tumors in vivo with PET/fluorescence imaging. Thus, it may provide a promising tool for patient preselection based on tumoral Pgp expression as well as image-guided surgery of MDR tumors.
Supplementary Material
Acknowledgments
This work was supported by NIH grants 5R01CA194064 and 5R01EB014354. The authors would like to thank Dr. Michael Gottesman (NCI) for providing 3T3-MDR1 and NCI/ADR-Res cells, and Dr. Michael Miley (UNC Antibody Core Facility) for the assistance in production of anti-Pgp antibody.
References
- 1.Gottesman MM, Lavi O, Hall MD, Gillet JP. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annual review of pharmacology and toxicology. 2016;56:85–102. doi: 10.1146/annurev-pharmtox-010715-103111. [DOI] [PubMed] [Google Scholar]
- 2.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nature reviews Cancer. 2013;13(10):714–26. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nature reviews Cancer. 2010;10(2):147–56. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 4.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nature reviews Drug discovery. 2006;5(3):219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature reviews Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 6.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochimica et biophysica acta. 1976;455(1):152–62. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 7.Fu D. Where is it and How Does it Get There - Intracellular Localization and Traffic of P-glycoprotein. Frontiers in oncology. 2013;3:321. doi: 10.3389/fonc.2013.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell DR, Gerlach JH, Kartner N, Buick RN, Ling V. Detection of P-glycoprotein in ovarian cancer: a molecular marker associated with multidrug resistance. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1985;3(3):311–5. doi: 10.1200/JCO.1985.3.3.311. [DOI] [PubMed] [Google Scholar]
- 9.van der Zee AG, Hollema H, de Jong S, Boonstra H, Gouw A, Willemse PH, Zijlstra JG, de Vries EG. P-glycoprotein expression and DNA topoisomerase I and II activity in benign tumors of the ovary and in malignant tumors of the ovary, before and after platinum/cyclophosphamide chemotherapy. Cancer research. 1991;51(21):5915–20. [PubMed] [Google Scholar]
- 10.Itamochi H, Kigawa J, Sugiyama T, Kikuchi Y, Suzuki M, Terakawa N. Low proliferation activity may be associated with chemoresistance in clear cell carcinoma of the ovary. Obstetrics and gynecology. 2002;100(2):281–7. doi: 10.1016/s0029-7844(02)02040-9. [DOI] [PubMed] [Google Scholar]
- 11.Penson RT, Oliva E, Skates SJ, Glyptis T, Fuller AF, Jr, Goodman A, Seiden MV. Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecologic oncology. 2004;93(1):98–106. doi: 10.1016/j.ygyno.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 12.Raspollini MR, Amunni G, Villanucci A, Boddi V, Taddei GL. Increased cyclooxygenase-2 (COX-2) and P-glycoprotein-170 (MDR1) expression is associated with chemotherapy resistance and poor prognosis. Analysis in ovarian carcinoma patients with low and high survival International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2005;15(2):255–60. doi: 10.1111/j.1525-1438.2005.15212.x. [DOI] [PubMed] [Google Scholar]
- 13.Surowiak P, Materna V, Denkert C, Kaplenko I, Spaczynski M, Dietel M, Zabel M, Lage H. Significance of cyclooxygenase 2 and MDR1/P-glycoprotein coexpression in ovarian cancers. Cancer letters. 2006;235(2):272–80. doi: 10.1016/j.canlet.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Hao J, Wang L, Li Y. Coexpression of invasive markers (uPA, CD44) and multiple drug-resistance proteins (MDR1, MRP2) is correlated with epithelial ovarian cancer progression. British journal of cancer. 2009;101(3):432–40. doi: 10.1038/sj.bjc.6605185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu D, Shi HC, Wang ZX, Gu XW, Zeng YJ. Multidrug resistance-associated biomarkers PGP, GST-pi, Topo-II and LRP as prognostic factors in primary ovarian carcinoma. Br J Biomed Sci. 2011;68(2):69–74. doi: 10.1080/09674845.2011.11730326. [DOI] [PubMed] [Google Scholar]
- 16.Palmeira A, Sousa E, Vasconcelos MH, Pinto MM. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. Current medicinal chemistry. 2012;19(13):1946–2025. doi: 10.2174/092986712800167392. [DOI] [PubMed] [Google Scholar]
- 17.Yu M, Ocana A, Tannock IF. Reversal of ATP-binding cassette drug transporter activity to modulate chemoresistance: why has it failed to provide clinical benefit? Cancer metastasis reviews. 2013;32(1–2):211–27. doi: 10.1007/s10555-012-9402-8. [DOI] [PubMed] [Google Scholar]
- 18.Ween MP, Armstrong MA, Oehler MK, Ricciardelli C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Critical reviews in oncology/hematology. 2015;96(2):220–56. doi: 10.1016/j.critrevonc.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Shi T, Wrin J, Reeder J, Liu D, Ring DB. High-affinity monoclonal antibodies against P-glycoprotein. Clinical immunology and immunopathology. 1995;76(1 Pt 1):44–51. doi: 10.1006/clin.1995.1086. [DOI] [PubMed] [Google Scholar]
- 20.Deng HF, Wang H, Wang MZ, Li ZB, Wu ZH. Synthesis and Evaluation of Cu-64-DOTA-NT-Cy5. 5 as a Dual-Modality PET/Fluorescence Probe to Image Neurotensin Receptor-Positive Tumor. Molecular pharmaceutics. 2015;12(8):3054–3061. doi: 10.1021/acs.molpharmaceut.5b00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ming X, Feng L. Targeted delivery of a splice-switching oligonucleotide by cationic polyplexes of RGD-oligonucleotide conjugate. Mol Pharm. 2012;9(5):1502–10. doi: 10.1021/mp300113c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou B, Wang H, Liu R, Wang M, Deng H, Giglio BC, Gill PS, Shan H, Li Z. PET Imaging of Dll4 Expression in Glioblastoma and Colorectal Cancer Xenografts Using (64)Cu-Labeled Monoclonal Antibody 61B. Molecular pharmaceutics. 2015;12(10):3527–34. doi: 10.1021/acs.molpharmaceut.5b00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai W, Wu Y, Chen K, Cao Q, Tice DA, Chen X. In vitro and in vivo characterization of 64Cu-labeled Abegrin, a humanized monoclonal antibody against integrin alpha v beta 3. Cancer research. 2006;66(19):9673–81. doi: 10.1158/0008-5472.CAN-06-1480. [DOI] [PubMed] [Google Scholar]
- 24.Roschke AV, Tonon G, Gehlhaus KS, McTyre N, Bussey KJ, Lababidi S, Scudiero DA, Weinstein JN, Kirsch IR. Karyotypic complexity of the NCI-60 drug-screening panel. Cancer research. 2003;63(24):8634–47. [PubMed] [Google Scholar]
- 25.Batist G, Tulpule A, Sinha BK, Katki AG, Myers CE, Cowan KH. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. The Journal of biological chemistry. 1986;261(33):15544–9. [PubMed] [Google Scholar]
- 26.Binkhathlan Z, Lavasanifar A. P-glycoprotein Inhibition as a Therapeutic Approach for Overcoming Multidrug Resistance in Cancer: Current Status and Future Perspectives. Current cancer drug targets. 2013;13(3):326–346. doi: 10.2174/15680096113139990076. [DOI] [PubMed] [Google Scholar]
- 27.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of controlled release: official journal of the Controlled Release Society. 2000;65(1–2):271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 28.Bigott HM, Prior JL, Piwnica-Worms DR, Welch MJ. Imaging multidrug resistance P-glycoprotein transport function using microPET with technetium-94m-sestamibi. Mol Imaging. 2005;4(1):30–9. doi: 10.1162/15353500200504166. [DOI] [PubMed] [Google Scholar]
- 29.Mubashar M, Harrington KJ, Chaudhary KS, Lalani el N, Stamp GW, Sinnett D, Glass DM, Peters AM. 99mTc-sestamibi imaging in the assessment of toremifene as a modulator of multidrug resistance in patients with breast cancer. J Nucl Med. 2002;43(4):519–25. [PubMed] [Google Scholar]
- 30.Ballinger JR. 99mTc-tetrofosmin for functional imaging of P-glycoprotein modulation in vivo. Journal of clinical pharmacology. 2001;(Suppl):39S–47S. [PubMed] [Google Scholar]
- 31.Muzzammil T, Ballinger JR, Moore MJ. 99Tcm-sestamibi imaging of inhibition of the multidrug resistance transporter in a mouse xenograft model of human breast cancer. Nucl Med Commun. 1999;20(2):115–22. doi: 10.1097/00006231-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Derebek E, Capa G, Berk H, Sekeroglu B, Havitcioglu H, Degirmenci B, Alakavuklar M, Durak H. Tc-99m sestamibi imaging as an indicator of P-glycoprotein expression in metastatic pheochromocytoma. Clinical nuclear medicine. 1998;23(9):637–8. doi: 10.1097/00003072-199809000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Ballinger JR, Hua HA, Berry BW, Firby P, Boxen I. 99Tcm-sestamibi as an agent for imaging P-glycoprotein-mediated multi-drug resistance: in vitro and in vivo studies in a rat breast tumour cell line and its doxorubicin-resistant variant. Nucl Med Commun. 1995;16(4):253–7. doi: 10.1097/00006231-199504000-00156. [DOI] [PubMed] [Google Scholar]
- 34.van Eerd JE, de Geus-Oei LF, Oyen WJ, Corstens FH, Boerman OC. Scintigraphic imaging of P-glycoprotein expression with a radiolabelled antibody. European journal of nuclear medicine and molecular imaging. 2006;33(11):1266–72. doi: 10.1007/s00259-006-0152-0. [DOI] [PubMed] [Google Scholar]
- 35.Dizdarevic S, Peters AM. Imaging of multidrug resistance in cancer. Cancer imaging: the official publication of the International Cancer Imaging Society. 2011;11:1–8. doi: 10.1102/1470-7330.2011.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott AM, Rosa E, Mehta BM, Divgi CR, Finn RD, Biedler JL, Tsuruo T, Kalaigian H, Larson SM. In vivo imaging and specific targeting of P-glycoprotein expression in multidrug resistant nude mice xenografts with [125I]MRK-16 monoclonal antibody. Nuclear medicine and biology. 1995;22(4):497–504. doi: 10.1016/0969-8051(94)00127-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.