Abstract
Background and Objective
We saw 2 patients who lost their sense of taste, which was restored by pharmacologic doses of biotin. The key objective is to describe the 2 case reports and suggest a potential treatment for unexplained loss of taste.
Methods and Design
The first patient was a 67-year-old woman who lost her sense of taste taking Juvenon, a dietary herbal supplement containing acyl-L-carnitine, lipoic acid, calcium, phosphorus, and biotin 300 μg per day. The second patient was a 60-year-old man who lost his sense of taste after a sleeve gastrectomy for obesity.
Results
The first patient did not respond to 5 mg per day of biotin, but taste was restored with 10 mg of biotin per day. Biotin was prescribed based on information that lipoic acid bound to the biotin transporter. Baseline urine gave no evidence of a pre-existing biotin deficiency. The second patient did not have restoration of taste after taking biotin 5 mg per day for 7 weeks but did have taste restoration on biotin 20 mg per day. Neither subject had an abnormal biotinidase level.
Conclusions
Further research into the relationship of biotin to taste is clearly indicated. Loss of taste was very distressing and significantly altered the quality of life for both patients. Since biotin up to 40 mg per day has been shown to be safe, a therapeutic trial of pharmacologic doses of biotin should be considered as a potentially curative treatment in patients who present with a loss of taste that has no obvious cause.
Keywords: biotin, Juvenon, lipoic acid, taste, sleeve gastrectomy
INTRODUCTION
Egg white injury syndrome was created in animals by Bateman in 1916 [1], and reversal of the egg white damage by biotin was demonstrated by Boas in 1927 [2]. These findings set the stage for Sydenstricker et al. to demonstrate egg white injury in humans and its reversal by biotin in 1942 [3]. Egg whites contain avidin, which binds biotin, creating a deficiency state with symptoms of dermatitis, anemia, grayish pallor, changes in mental status, myalgia, hyperesthesia, localized paresthesia, anorexia, and nausea [4].
We now understand that biotin is not only an essential nutrient that humans cannot make but is also contained in our food and is made by intestinal bacteria. The richest sources of biotin in the diet are cow’s milk and liver, but biotin is distributed in many foods at a low concentration. The first understanding of the importance of biotin came from knowledge that it is a cofactor for 5 holocarboxylases: propionyl-CoA carboxylase, methylcrotonyl-CoA carboxylase, pyruvate carboxylase, and 2 forms of acetyl-CoA carboxylase. These enzymes catalyze key reactions in gluconeogenesis, fatty acid metabolism, and amino acid catabolism [5].
More recently, a carrier system has been isolated for biotin, human sodium-dependent multivitamin transporter (hSMVT). This transport system is saturable at nanomolar concentrations, sodium dependent, and ATP energy dependent, but it is chloride independent. Calcium and calmodulin are involved in the mediation of biotin uptake. This transport system is not specific to biotin. The uptake of biotin is inhibited by pantothenic acid, lipoic acid, desthiobiotin, and valeric acid at milimolar concentrations. The thiophane portion in the biotin molecule plays an important role in binding to the biotin transporter (Fig. 1). The keto group at the second position of the imidazole ring in biotin is essential for the substrate to interact with the transporter, but lipoic acid does not have the keto group and desthiobiotin does not have the tetrahydrothiophene ring, and both still bind to the biotin transporter. In addition, the terminal carboxyl groups of the valeric acid side chain as well as the length of the side chain determine its interaction with the biotin transporter [6].
Fig. 1.
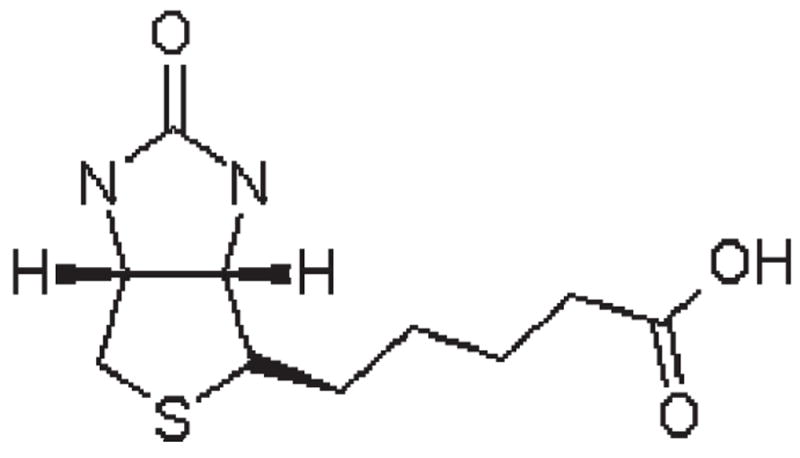
The chemical structure of biotin.
It is known that biotinidase removes biotin from the histones on DNA just as it removes biotin from the holocarboxylases [7]. This insight into the additional role that biotin plays in genetic regulation may change the outlook we have about the optimal nutritional intake of biotin and its importance to biological processes. Measuring 3-hydroxyisovaleric acid has been shown to be the most sensitive marker of biotin deficiency [8]. Using this marker, it was demonstrated that marginal biotin deficiency without any symptoms exists in normal pregnancy and that 300 μg per day of biotin orally only partially restored biotin levels toward normal. Minor biotin deficiency in animals that does not cause obvious evidence of deficiency is associated with birth defects. In a reproductive mouse experiment, 94% of all pups were malformed with a cleft palate, and half also had micrognathia. These studies have obvious implications for biotin supplementation in human pregnancy and may prompt reevaluation of optimal biotin intake [9].
Biotin deficiency has been described primarily in the context of raw egg white consumption. Biotin deficiency has also been reported in protein-energy malnutrition and long- term treatment with certain anticonvulsants [8]. Genetic defects in the holocarboxylase enzymes that use biotin as a cofactor and defects in biotinidase are also rare causes associated with defects in biotin metabolism. To this point, we are not aware that loss of taste has been reported to accompany biotin deficiency. Herein, we describe 2 patients who lost their taste acutely and had taste sensation restored by treatment with pharmacologic doses of biotin.
MATERIALS AND METHODS
Case 1
J.K. was a 67-year-old Caucasian woman who volunteered for a study to evaluate the effect of the dietary supplement Juvenon on cognition and cerebral glucose metabolism. Juvenon is taken as 2 pills per day orally with meals and delivers a daily total of acetyl L-carnitine 1 g, alpha lipoic acid 400 mg, biotin 300 μg, calcium 154 mg, and phosphorus 117 mg. This combination of acetyl L-carnitine and alpha lipoic acid has been shown to improve mitochondrial function in the brain, improve memory loss, and improve metabolic function in aging rats [10-12]. The volunteers in this crossover study took Juvenon or placebo for 8 weeks followed by a 4-week washout and then had another 8 weeks of treatment with the condition they did not have in the first 8-week period.
J.K. had hypertension controlled on valsartan/hydrochlorothiazide 80/12.5 mg/d and osteoarthritis with shoulder and hip pain treated with meloxicam 7.5 mg/d. She had a basal cell carcinoma removed from her face at the age of 48 years but had no serious injuries or transfusions. She had an allergy to penicillin that resulted in a rash and hallucinations. Although she experienced hives and itching with alcohol, she had 1 to 2 drinks per week. She had 3 pregnancies and 3 live births with normal pap smears and mammograms every 2 years. She was a nonsmoker who was not sexually active and had no toxic exposures or history of intravenous drug use. Her mother was an alcoholic, her son had hypertension, and her children were recovering from alcohol and drug abuse. Her father died of a myocardial infarction at the age of 60 years, and she had 2 sisters who were living and well. On physical examination, she was 74.3 kg, 160 cm, had a body mass index (BMI) of 28.4 kg/ m2, had a blood pressure of 125/85 mmHg, and had a pulse rate of 76 beats per minute (bpm). She wore glasses, had high- frequency hearing loss and sciatic pain, and complained of intermittent sinus stuffiness.
Case 2
W.J.B. was a 60-year-old Caucasian man who underwent a sleeve gastrectomy for obesity on March 22, 2010, and volunteered for a study comparing 3 types of obesity surgery. He was overweight since childhood, tried exercise and diets without success, and participated in sports until 1981. After stopping sports, he gained weight to be 159 kg with a BMI of kg/m2 at the time of surgery.
He had dyslipidemia since 2005 and was treated with fenofibrate 135 mg/d. He suffered from intermittent reflux esophagitis since 2000, which was treated with pantoprazole 40 mg as needed. He had deep venous thrombosis in 1995 and 2007, for which he took Coumadin 5 mg/d. He had arthritis and low-back pain, for which he took oxycodone/acetaminophen 10/325 mg as needed. He had insomnia, for which he took zolpidem 5 mg/d, and he also took some vitamins and minerals daily. He had multiple surgeries for his arthritis (back surgery, right knee × 4, left knee × 3, right foot × 2, thumb × 4, shoulder × 2, and elbow × 2). He also had a septoplasty × 4, ventral hernia × 2, and a cholecystectomy in 2005. He was allergic to nonsteroidal anti-inflammatory drugs, which gave him skin ulcers; morphine; and wasp stings, which caused swelling. He injured his right knee in a motor vehicle accident in 2000, at which time he required transfusions, which were not accompanied by hepatitis or reactions. He had a 5 pack-year history of smoking cigarettes, but he quit smoking in the 1980s. He did not drink alcohol, was not sexually active, did not use intravenous drugs, and had been a metal worker who was working as an athletic event organizer. His father had diabetes and hypertension, his mother had bipolar disorder, one brother had diabetes, and another brother had Down syndrome. He had a brother and 2 children who were living and well.
On physical examination, he was 195.6 cm tall and had a BMI of 40.5 kg/m2, a blood pressure of 124/76 mmHg, and a pulse rate of 70 bpm. He was centrally obese, had osteoarthritic changes in his joints, bilateral carpel tunnel syndrome, and bilateral pedal edema.
RESULTS
Case 1
J.K. began the blinded study medication on November 5, 2009, and completed the first 8-week treatment condition on January 8, 2010, without any complaints. Her first treatment condition was the placebo, and the second treatment was Juvenon. She began the second blinded treatment phase on February 1, 2010. On February 25, 2010, she complained that everything tasted badly or altered. She attributed the taste problem to her study medication and stopped taking it. Her blood pressure medication was changed on the chance that it might have been responsible, but there were no improvements in taste. She was seen again on March 18, 2010, and could taste hot, cold, and peppermint but felt that milk and sweets were being altered in taste due to something in her saliva. Since the study medication contained alpha lipoic acid, which can inhibit the biotin transporter, she was advised to take 5 mg/d of biotin on March 29, 2010. She took one 5-mg biotin tablet per day for 4 days and had no change in her taste. She increased her dose to 5 mg twice daily, and 3 days later, her taste returned to 90% of normal, with everything tasting normal except for candy and very sweet foods, which still tasted altered. She continued on the 5-mg twice-daily dose, and in another 1 to 2 weeks, her taste fully returned to normal. She continued the biotin until the end of May. She has now been off biotin for more than 3 months, and her taste remains normal. Her biotinidase level was 9.4 U/L (reference range, 3.5–13.8 U/L). Her baseline urine was tested for 3-hydroxyisovaleric acid to assess for pre-existing biotin deficiency, but no evidence of biotin deficiency was found.
Case 2
The patient awoke from anesthesia after his sleeve gastrectomy surgical procedure without a sense of taste. He was started on biotin 5 mg per day as soon as he was able to take solid material orally. This amount of biotin is routinely prescribed by the surgeon to minimize the potential for any hair loss that might be attributable to a biotin deficiency. Despite taking 5 mg of biotin per day for 7 weeks, he had only a metallic taste. None of his medications were reported to give a loss of taste. Since case 1 had a loss of taste that responded to biotin at higher doses than 5 mg/d, the patient was advised to increase the biotin to 5 mg 3 times a day orally. He actually took 5 mg 4 times a day and called 36 hours later to state that his taste had returned. He then reduced the dose to biotin 5 mg twice a day, and his taste became only metallic again. Upon increasing the biotin dose to 20 mg per day, his taste returned gradually over a few days and has remained so for more than a month. His biotinidase was 8.0 U/L (reference range, 3.5–13.8 U/L). No baseline urine was available for measurement of 3-hydroxyisovaleric acid, an indicator of biotin deficiency.
DISCUSSION
Loss of taste was very distressing to both of these patients. Juvenon contains lipoic acid, which has been described to compete with the biotin transporter hSMVT [6]. This suggested that a deficiency of biotin might be responsible for the loss of taste in case 1. Since biotin has been administered to humans in doses up to 40 mg/d without untoward effects, a trial of high- dose biotin seemed to have a reasonable rationale, with every indication of having a large safety margin. Although both patients responded to high-dose biotin with restoration of their sense of taste, both appeared to require doses greater than 5 mg per day to experience taste improvement. In fact, Juvenon contains the recommended daily allowance of 300 lg of biotin. Case 2 had been taking 5 mg per day of biotin for 7 weeks without an improvement in taste, so the effect of biotin in these 2 cases must represent a pharmacologic effect rather than a classical vitamin deficiency. Since we were not able to find an explanation for the loss of taste and had seen case 1 respond to a higher dose of biotin, we increased the biotin dose of case 2 with almost immediate success in restoring his taste sensation. We do not have an explanation as to why this patient developed the taste abnormality or why he might have responded to biotin.
It has been shown that gurmarin, a peptide that suppresses sweet taste, binds to the circumvallate papillae in rats [13]. Otsuka Long-Evans Tokushima fatty rats have a greater chorda tympani nerve response to sucrose compared with Long-Evans Tokushima lean rats, without a difference in other taste stimuli. A high-biotin diet reduced this elevated response to sucrose [14]. The intestinal bypass, an obesity operation that is no longer performed, gave a reduction in the preference for concentrated sucrose and glucose solutions, returning the heightened preference for sweets seen in obese individuals to the level seen in normal-weight individuals [15]. Similar findings have been documented with the gastric bypass, the most popular obesity surgery in the United States [16]. Case 2 had the sleeve gastrectomy operation, and it is not known why he lost his sense of taste. There are, however, recognized changes in sweet taste after other obesity surgeries, a relationship of taste with biotin in an animal model of obesity, and a known peptide that suppresses sweet taste in rodents, which could be involved in the altered sweet taste preferences after obesity surgery.
Since both of these patients responded only to pharmacologic doses of biotin, and since biotin deficiency has not been previously associated with loss of taste, these patients appear not to have had biotin deficiency in the classical sense. More research is certainly needed to explore the reasons why pharmacologic doses of biotin restored taste in these patients, to explore how taste and biotin may interact, and to investigate the extent to which other people suffering from a loss of taste may respond to pharmacologic doses of biotin.
CONCLUSIONS
Regardless of the reason for the restoration of taste with oral pharmacologic biotin therapy, the observation is important from a clinical perspective. Loss of taste is very distressing to patients and impairs their quality of life to a very significant degree. In fact, case 2 was becoming distressed that he had undergone the obesity surgery, blaming the surgery for his loss of taste and feeling that this loss was not a tradeoff he would have been willing to make for the weight loss he experienced. Although we encourage more research into the relationship between biotin and taste, we encourage those treating patients with a loss of taste that defies an obvious explanation to attempt a trial of pharmacologic doses of biotin as a safe and possibly curative therapy.
Acknowledgments
A research grant from Juvenon, Inc. was awarded to the Pennington Biomedical Research Center. Partial grant support was received from Juvenon and Ethicon.
Footnotes
Author contributions: Frank L. Greenway prepared the article. Donald K. Ingram conceived the idea for the study of Juvenon and edited the article. Eric Ravussin conceived of the surgical study and edited the article. Mark Hausmann performed the sleeve gastrectomy on one of the subjects and edited the article. Steven R. Smith provided medical oversight for the patient in the Juvenon study and edited the article. Lauren Cox coordinated the Juvenon study, interacted with the patient, and edited the article. Katie Tomayko coordinated the surgery study, interacted with the patient, and edited the article. Benjamin V. Treadwell provided the Juvenon mentioned in the study and edited the article.
Contributor Information
Frank L. Greenway, Pennington Biomedical Research Center, Louisiana State University System.
Donald K. Ingram, Pennington Biomedical Research Center, Louisiana State University System.
Eric Ravussin, Pennington Biomedical Research Center, Louisiana State University System.
Mark Hausmann, Surgical Specialty Group, Baton Rouge, Louisiana.
Steven R. Smith, Pennington Biomedical Research Center, Louisiana State University System.
Lauren Cox, Pennington Biomedical Research Center, Louisiana State University System.
Katie Tomayko, Pennington Biomedical Research Center, Louisiana State University System.
Benjamin V. Treadwell, Juvenon Cellular Health Supplement, Scientific Advisory Board, Marco Island, Florida.
References
- 1.Bateman WG. The digestibility and utilization of egg proteins. J Biol Chem. 1916;26:263–291. [Google Scholar]
- 2.Boas MA. The effect of desiccation upon the nutritive properties of egg-white. Biochem J. 1927;21:712–724. doi: 10.1042/bj0210712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sydenstricker VP, Singal SA, Briggs AP, Devaughn NM, Isbell H. Preliminary observations on “egg white injury” in man and its cure with a biotin concentrate. Science. 1942;95:176–177. doi: 10.1126/science.95.2459.176. [DOI] [PubMed] [Google Scholar]
- 4.Roth KS. Biotin in clinical medicine—a review. Am J Clin Nutr. 1981;34:1967–1974. doi: 10.1093/ajcn/34.9.1967. [DOI] [PubMed] [Google Scholar]
- 5.Pacheco-Alvarez D, Solorzano-Vargas RS, Del Rio AL. Biotin in metabolism and its relationship to human disease. Arch Med Res. 2002;33:439–447. doi: 10.1016/s0188-4409(02)00399-5. [DOI] [PubMed] [Google Scholar]
- 6.Kansara V, Luo S, Balasubrahmanyam B, Pal D, Mitra AK. Biotin uptake and cellular translocation in human derived retinoblastoma cell line (Y-79): a role of hSMVT system. Int J Pharm. 2006;312:43–52. doi: 10.1016/j.ijpharm.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 7.Gravel RA, Narang MA. Molecular genetics of biotin metabolism: old vitamin, new science. J Nutr Biochem. 2005;16:428–431. doi: 10.1016/j.jnutbio.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Mock DM, Henrich-Shell CL, Carnell N, Stumbo P, Mock NI. 3-Hydroxypropionic acid and methylcitric acid are not reliable indicators of marginal biotin deficiency in humans. J Nutr. 2004;134:317–320. doi: 10.1093/jn/134.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zempleni J, Mock DM. Marginal biotin deficiency is teratogenic. Proc Soc Exp Biol Med. 2000;223:14–21. doi: 10.1046/j.1525-1373.2000.22303.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci U S A. 2002;99:1876–1881. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagen TM, Liu J, Lykkesfeldt J, Wehr CM, Ingersoll RT, Vinarsky V, Bartholomew JC, Ames BN. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci U S A. 2002;99:1870–1875. doi: 10.1073/pnas.261708898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshie S, Miyasaka A, Imoto T. Histological localization of the sweet taste receptor in rat taste buds by the use of gurmarin, a sweet taste-suppressing peptide. Arch Histol Cytol. 1994;57:531–534. doi: 10.1679/aohc.57.531. [DOI] [PubMed] [Google Scholar]
- 14.Tsunoda K, Osada K, Komai M, Zhang H, Morimoto K, Suzuki H, Furukawa Y. Effects of dietary biotin on enhanced sucrose intake and enhanced gustatory nerve responses to sucrose seen in diabetic OLETF rat. J Nutr Sci Vitaminol (Tokyo) 1998;44:207–216. doi: 10.3177/jnsv.44.207. [DOI] [PubMed] [Google Scholar]
- 15.Rodin J, Moskowitz HR, Bray GA. Relationship between obesity, weight loss, and taste responsiveness. Physiol Behav. 1976;17:591–597. doi: 10.1016/0031-9384(76)90157-8. [DOI] [PubMed] [Google Scholar]
- 16.Miras AD, le Roux CW. Bariatric surgery and taste: novel mechanisms of weight loss. Curr Opin Gastroenterol. 2009;26:140–145. doi: 10.1097/MOG.0b013e328333e94a. [DOI] [PubMed] [Google Scholar]


