Abstract
Object
Deep brain stimulation (DBS) of the lateral hypothalamic area (LHA) has been suggested as a potential treatment for intractable obesity. The authors present the 2-year safety results as well as early efficacy and metabolic effects in 3 patients undergoing bilateral LHA DBS in the first study of this approach in humans.
Methods
Three patients meeting strict criteria for intractable obesity, including failed bariatric surgery, under-went bilateral implantation of LHA DBS electrodes as part of an institutional review board– and FDA-approved pilot study. The primary focus of the study was safety; however, the authors also received approval to collect data on early efficacy including weight change and energy metabolism.
Results
No serious adverse effects, including detrimental psychological consequences, were observed with continuous LHA DBS after a mean follow-up of 35 months (range 30–39 months). Three-dimensional nonlinear transformation of postoperative imaging superimposed onto brain atlas anatomy was used to confirm and study DBS contact proximity to the LHA. No significant weight loss trends were seen when DBS was programmed using standard settings derived from movement disorder DBS surgery. However, promising weight loss trends have been observed when monopolar DBS stimulation has been applied via specific contacts found to increase the resting metabolic rate measured in a respiratory chamber.
Conclusions
Deep brain stimulation of the LHA may be applied safely to humans with intractable obesity. Early evidence for some weight loss under metabolically optimized settings provides the first “proof of principle” for this novel antiobesity strategy. A larger follow-up study focused on efficacy along with a more rigorous metabolic analysis is planned to further explore the benefits and therapeutic mechanism behind this investigational therapy.
Keywords: Obesity, Weight Loss, Deep Brain Stimulation, Energy Metabolism, Functional Neurosurgery, Respiratory Chamber
Obesity, which results from a long-term sustained positive energy balance, has become a global pandemic and a cause of poor health. 1,7,11 Decades of animal experimentation have implicated various hypothalamic subnuclei in regulating feeding behavior and body weight. The LHA has been dubbed the “feeding center” because both LHA lesioning and high-frequency electrical stimulation decrease food intake and may engender weight loss. 5,6,17 The smaller VMH of the hypothalamus, which lies inferomedial to the LHA, has been called the “satiety center” since both lesioning and high-frequency stimulation of this nucleus augment body weight and food consumption in animals. 8,12,15
Furthermore, there is evidence that the human brain acts to maintain energy homeostasis and body weight around specific “settling points” in individuals and may strongly buffer against weight loss by lowering metabolism in the setting of decreased food consumption. At the population level, such hard-wired evolutionary adaptation may have protected humans during millennia of intermittent caloric consumption, but it becomes a liability when weight loss strategies, including bariatric surgery and/or lifestyle changes, are implemented, contributing to frequent failures. Since high-frequency electrical stimulation to the LHA produced weight loss in animals despite stable or temporarily decreased food intake, 5,15 it has been hypothesized that the hypothalamic nucleus might provide a target for modulating human energy balance in cases of refractory obesity.18
Deep brain stimulation of several subcortical structures has become a well-established therapy for movement disorders, and hypothalamic DBS has recently been proven safe and effective for various chronic headache disorders including cluster headache. Deep brain stimulation is favored over lesioning for brain neuromodulation due to its nonablative, programmable, and reversible nature. However, only 1 case report has explored the impact of DBS for human obesity. 4 Here, we present safety and preliminary energy expenditure efficacy data from 3 patients undergoing bilateral LHA DBS as part of the first FDA-approved pilot study of LHA DBS for intractable human obesity.
Methods
Study Design
Through a physician-sponsored FDA-approved investigational device exemption (No. G070067) and with institutional review board approval, a pilot study of bilateral LHA DBS was initiated at West Virginia University. A total of 3 patients were studied with the primary outcome being safety. Patient selection involved a multidisciplinary team of neurosurgeons, neurologists, eating disorder specialists, physical therapists, psychologists, and psychiatrists. After obtaining informed consent, 3 patients with intractable obesity underwent bilateral LHA DBS implantation (Table 1). Inclusion criteria (Table 2) required that failed bariatric surgery had occurred in the patients, which was defined by the modified Reinhold classification as weighing more than 50% over ideal body weight despite a technically successful bariatric surgery. 14 Exclusion criteria are also listed in Table 2. Safety was verified by postoperatively monitoring adverse events for more than 2 years.
TABLE 1.
Patient demographics and treatment history*
| Case No. | Age (yrs), Sex | Pre-DBS Body Weight (lbs), BMI |
Prior Surgical Weight Loss Treatment, Yr |
Comorbidities |
|---|---|---|---|---|
| 1 | 60, F | 278.7, 49.4 | gastric bypass, 2001 | HTN |
| 2 | 50, F | 326, 48.1 | gastric bypass, 2001 | sleep apnea, DM2, HTN, migraine |
| 3 | 45, M | 314, 45.0 | gastric bypass, 2003 | lower-extremity edema |
BMI = body mass index; DM2 = diabetes mellitus type 2; HTN = hypertension.
TABLE 2.
Study inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| male & female patients ≥18 yrs | prior brain surgery |
| BMI ≥40 or ≥35 kg/m2 w/ a comorbid condition (HTN, cardiovascular disease, sleep apnea, DM2, dyslipidemia) | dementia or mini–mental state examination score <25 |
| failure of bariatric surgery (gastric banding or bypass). “Failed bariatric surgery” is determined using the modified Reinhold classification as patients who are still >50% over an ideal body weight after a technically successful surgery. | unable to fit into MRI or CT scanner (400-lb upper weight limit for CT scanner) |
| chronic obesity diagnosed by an eating disorder specialist w/expertise in the treatment of obesity | psychiatric disorder, including poorly controlled anxiety disorders, psychosis, bipolar disorder, active substance abuse, somatoform disorders, factitious disorders, dissociative disorders, & severe personality disorders, but excluding depression & binge eating |
| stable at present body weight for a 6-mo period | obesity as part of another medical condition, neurological injury or lesions, related to medication side effect, or as part of a genetic syndrome |
| psychiatric evaluation | unable to schedule follow-up clinic visits |
| Karnofsky Performance Scale score >60 |
Before enrollment, all patients were required to undergo a battery of psychological measures as well as visual field testing, blood monitoring tests of the sympathetic nervous system, neuropeptide profiles, and neuroendocrine levels.
Approval was obtained from the FDA and institutional review board to collect efficacy outcome data with body weight monitoring and energy metabolism testing in a respiratory chamber. This chamber is a 27,000-L open-circuit indirect calorimetric system that allows one to measure energy expenditure over a prolonged period of time.10 Energy expenditure is calculated from measures of oxygen consumption and carbon dioxide production. All 3 patients traveled to the Pennington Biomedical Research Center in Baton Rouge, Louisiana, for these metabolism experiments. The accuracy and precision of the calorimeters were determined by monthly propane combustion tests. Over the 24 months of the study, the accuracy of these chambers was 98.3% and 96.6% for oxygen and carbon dioxide, respectively.
Surgical Technique
Bilateral DBS electrodes were placed in the LHA using standard frame-based stereotaxy and microelectrode recording. A brain MRI study (1.5 T, Signa, GE) was obtained several weeks prior to surgery using a 1-mm slice thickness protocol for DBS. On the morning of surgery, the patient underwent placement of a Cosman-Roberts-Wells stereotactic headframe after administration of a local anesthetic. A noncontrast head CT (Somatom, Siemens) of the patient wearing the headframe was then obtained and fused with brain MRI using the StimPilot NeuroNavigation System (Medtronic, Inc.).
Prior to the day of surgery, preoperative planning was carried out using brain MRI. The LHA was targeted using a standard stereotactic atlas and previous reports regarding this hypothalamic target.13 A target approximately 6.5 mm lateral to the intercommissural line, 3 mm below the intercommissural line, and 4.5 mm posterior to the AC was chosen and then, in the Cases 2 and 3, slightly adjusted to be 4 mm lateral to the fornix and 1 mm posterior to the fornix. Akin to the refinement of ventralis intermedius nucleus targeting in movement disorder DBS by reference to the lateral wall of the third ventricle, we found that LHA targeting could be improved by regional indirect targeting based on the fornix. We therefore used the local fornix–LHA relationship to minimize the indirect targeting error introduced by variations in third ventricular anatomy. However, since mapping of postoperative electrode location with respect to an anatomical atlas in 1 patient (Case 1, shown below) still demonstrated that at least 1 of the 4 DBS lead contacts was well within the LHA, we did not think that fornix-based replacement of the DBS electrodes was required in this patient.
The patient underwent bilateral placement of DBS electrodes into the LHA using linear parasagittal incisions. Two bur holes were made anterior to the coronal suture using a 14-mm perforator drill bit. Prior to placement of the permanent electrode (model 3389, Medtronic, Inc.), microelectrode recording was performed to map the electrophysiological fingerprint of the surrounding brain areas. Microstimulation was then carried out at 1-mm contiguous intervals over a 12-mm course traversing the target. Pulse width and frequency were kept constant, and voltage was increased in 1-V increments. Clinical response to stimulation was then recorded.
Macrostimulation was then carried out after the DBS lead (model 3389) was placed at target depth. Each DBS lead had 4 platinum-iridium contacts that were numbered (0, 1, 2, and 3); Contact 0 was the most distal (deep) contact on the lead and Contact 3 was the most proximal (superficial) contact. Clinical responses were then recorded in a similar fashion to that for microstimulation.
The electrode was secured in place using an IGN bur hole cap (Medtronic, Inc.). The distal end of the electrode was attached to connecting wiring, which was externalized and connected to an external pulse generator. The wounds were closed, and a postoperative MR image was obtained using parameters safe for DBS electrodes. The patient remained in the hospital for 2 days for intensive electrode mapping. On the 3rd postoperative day, bilateral extensions (model 7482, Medtronic, Inc.) were tunneled from the scalp to the infraclavicular region where bilateral pulse generators (Soletra, Medtronic, Inc.) were implanted under the skin in the usual fashion.
Microstimulation
Microelectrode recording was performed using an 800- to 1200-ΚΩ tungsten microelectrode (FHC, Inc.), which was advanced with the aid of a microdrive. The majority of individual units isolated during microelectrode recording did not exhibit a discernable firing pattern. Two cells showed a fast rhythmic bursting pattern with 200- to 300-Hz bursts of 2–6 action potentials and an interburst frequency of 20–33 Hz. Microstimulation via the small-diameter microelectrode was performed at 15 1-mm contiguous nonoverlapping sites through the LHA target. Evoked responses proved more useful to confirm the target. Microstimulation within the LHA produced sensations of nausea and thermal responses while more ventromedial microstimulation, presumably within the VMH, engendered an anxiety or panic response.21
Postoperative Management and DBS Parameter Programming
The patient was observed weekly for the first 2 months with incremental electrical changes to the stimulator parameters. The patient’s weight was measured at each visit, and subjective feelings of mood, energy, and side effects were collected at each visit. The patient also kept a food intake diary. After the first 2 months, the patient was observed every 3 weeks. Respiratory chamber experiments were completed approximately 1 year after implantation.
Prior to having their measurements of energy metabolism obtained in the respiratory chamber, all patients had their LHA DBS programmed with standard settings derived from movement disorder DBS programming: monopolar or bipolar stimulation with a 90-µsec pulse width and 185-Hz frequency. One patient (Case 3) was noted to have a unilateral electrode fracture prior to respiratory chamber testing, which was replaced in the same fashion as described above for electrode placement.
Electrode Localization Technique
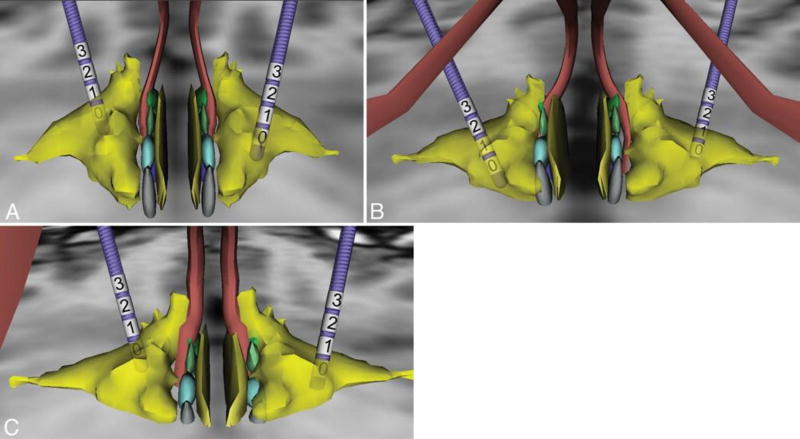
Postoperative head CT images were registered to the patients’ respective preoperative MR images using a standard linear affine registration. Next, a course 7-df registration was performed to optimize the alignment of the AC and PC of each preoperative image to the AC and PC in the high-resolution MRI data set that contains the 3D anatomical atlas. The 3D atlas was nonlinearly registered to the anatomy of the patient by a trained neuroanato-mist (K.W.F.) guided by visual landmarks and a thin-plate spline deformation algorithm modified to transform 3D polygonal objects.3 The anatomy of the hypothalamic nuclei and surrounding structures was derived from the Mai atlas of the human brain.9 Once satisfactory registration was achieved, 3D models of the model 3389 DBS leads were positioned at the location of the implant as visualized in the coregistered postoperative CT image and compared with the atlas data (Fig. 1). The middle of the target of DBS in these patients, the LHA, appeared to be most closely related to Contact 1 in all 3 patients.
Fig. 1.
Three-dimensional modeling of electrode contact location in 3 patients (Case 1 [A], Case 2 [B], and Case 3 [C]) undergoing bilateral LHA DBS. Yellow indicates the lateral hypothalamic area; rust, the fornices; light blue and light green, the medial hypothalamic nuclei. The numbers denote the contacts.
Respiratory Chamber Experiments
To assess the effect of selected stimulation parameters on energy expenditure, whole-body indirect calorimetry was performed at the Pennington Biomedical Research Center over 4 consecutive days. An accurate assessment of changes in energy expenditure over short periods was used to calculate the metabolic consequences of individual electrode contacts.10 On every test day the chamber was calibrated using standard gas mixtures.
Every morning, the patient reported to the metabolic chamber at 6:30 a.m. after an overnight fast with both DBS generators turned off for 8–10 hours. After voiding, the patient was weighed and then entered the metabolic chamber, where he/she received a light breakfast. After 60 minutes in the chamber, measurement of energy expenditure was initiated, first with both DBS generators off to measure the baseline metabolic rate. Next, using monopolar stimulation (contact cathode, pulse generator anode) with each of 4 contacts on the DBS lead with pulse width (90 µsec) and frequency (185 Hz) kept constant, the voltage was increased by 1 V every hour up to the maximum tolerable voltage or 7 V. A different contact was studied each day.
During testing, the patient was instructed to remain as inactive as possible (he/she was allowed to read, surf the Internet, or watch TV) and to refrain from sleeping. The patient was constantly monitored by a camera while in the chamber. Physical activity was assessed using a microwave motion sensor. Any subjective complaints or activity alterations were recorded as well. On the final day, the patient stayed overnight for a total of 13 consecutive hours in the chamber, while having DBS parameters activated at the optimal contact and voltage setting. During the overnight stay, the patient was instructed to fill out an activity log. Lights were turned off at 10:30 p.m., and the participant was woken up at 6:30 a.m. Before exiting the chamber, gas exchanges were measured during 60 minutes while the patient was performing sedentary activities.
At the end of each day, data were visually examined to determine respiratory steady-state periods with minimal spontaneous physical activity. The RMR in kilocalories per minute was calculated using the Weir equation over the selected periods and compared with the baseline RMR.19 The baseline RMR was defined as the average of all the DBS-off RMR measurements over 4 days. During the overnight stay, periods with known sedentary activities, as described in the activity log, were selected and analyzed similarly. Sleeping periods were excluded.
Results
Safety
After a mean follow-up of 35 months (range 30–39 months), no serious adverse events were observed with bilateral LHA DBS. Mild adverse events attributed to LHA DBS included nausea, anxiety, and sensations of “feeling too hot or flushed.” These mild adverse events were transient (lasting < 5 minutes) and usually were noted during programming changes. Besides the electrode fracture mentioned above, there were no other observed hardware-related complications including infection or wound erosion. Moreover, there were no adverse changes in psychological or biochemical testing as detailed below.
Psychological Analysis
All psychological testing was performed by an expert in eating disorders (J.S.M.). Using the Millon Behavioral Medicine Diagnostic assessment, which measures anxiety-tension, depression, cognitive dysfunction, emotional lability, and guardedness, all participants were in the normal range preoperatively, and postoperative testing revealed no consistent evidence that the DBS surgery had any negative effects on psychological function of the participants or on the self-reported cognitive function.
On the Gormally Binge Eating Scale, preoperatively 1 woman (Case 1) scored in the severe binge-eating range and the other 2 patients were in the moderate binge-eating range. At postoperative follow-up, 1 patient (Case 1) had improved such that her Binge Eating Scale score was within the normal range while the other 2 participants continued to score in the moderate binge-eating range. Thus, in terms of binge eating, DBS surgery did not make any participant worse and may have reduced binge-eating episodes in 1 patient.
The Cognitive Restraint subscale was used to assess dieting skills in these patients. Prior to surgery, all patients scored in the low range. After surgery, 1 patient (Case 1) improved to the high range while the other 2 patients remained in the low range. Thus, there was no evidence that DBS worsened participants’ dieting skills, and there was some evidence for improvement in dieting skills in 1 patient. Moreover, on the disinhibition scale (which measures emotional eating), DBS consistently had no negative effects on emotional eating.
The effects of DBS on hunger were studied with the hunger subscale and were variable. One patient (Case 3) remained in the clinical range on the hunger subscale, indicating that he continued to struggle with feelings of intense hunger. Another patient (Case 2) remained in the low hunger range after DBS, and the final patient (Case had a hunger score of 0 at postoperative follow-up and commented that this was the first time in her life that she did not have to fight constant hunger.
On the Body Shape Questionnaire, 2 participants (Cases 2 and 3) scored moderately higher than the general population, suggesting moderate distress about body shape and weight. After DBS, both of these participants’ Body Shape Questionnaire scores improved such that their scores were the same as the average score for the general population. There was no evidence that DBS led to any worsening of body image. Finally, on the Impact of Weight on Quality of Life–Lite Questionnaire, preoperative and postoperative testing revealed that DBS did not worsen participants’ quality of life. In conclusion, across a wide spectrum of psychological and eating/weight-related measures, LHA DBS did not appear to create negative effects in this small patient population.
Efficacy Data
Biochemical Analysis
Serial blood testing of the following nutritional studies, pituitary hormones, and neuroendocrine/neuropeptide studies did not reveal significant changes with LHA DBS stimulation: fasting glucose, hemoglobin A1C, serum calcium, serum magnesium, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, serum iron, thyroid-stimulating hormone, free T4, total T4, T3, follicle-stimulating hormone, LHA, serum cortisol, folate, vitamin B12, adrenocorticotropic hormone, fasting insulin, insulin-like growth factor, growth hormone, leptin, ghrelin, agouti-related peptide, neuropeptide Y, peptide tyrosine-tyrosine, and adiponectin.
Energy Metabolism Data
The energy metabolism measures revealed that stimulation via certain contacts and with certain voltages could significantly increase the RMR in 2 of 3 patients. The results of the monopolar stimulation experiments for each patient are shown in Table 3. The RMR was calculated over periods of time during which the patient was relatively stationary; however, patients were encouraged but not required to lie completely motionless during the metabolic chamber studies. Calculations were deemed indeterminate when patient motion made it difficult to ascertain whether RMR changes were due to stimulation or motion.
TABLE 3.
Average RMR changes with bilateral monopolar stimulation of individual DBS contacts during metabolic chamber experiments in 3 patients undergoing LHA DBS*
| Case No. | Contact 0 | Contact 1 | Contact 2 | Contact 3 |
|---|---|---|---|---|
| 1 | no change | 28% increase at 5.5 V | indeterminate | 0–5 V no change, >5 V indeterminate |
| 2 | indeterminate | indeterminate | indeterminate | indeterminate |
| 3 | indeterminate | 9% increase at >4 V | indeterminate | indeterminate |
Stimulation was performed in monopolar mode with the case or pulse generator always acting as the anode (positive contact). Pulse width (90 msec) and frequency (185 Hz) were kept constant during all stimulation settings. Calculations were deemed indeterminate when patient motion made it difficult to ascertain whether RMR changes were due to stimulation or motion.
Overall, monopolar stimulation at certain threshold voltages via Contact 1, which was anatomically most closely related to the mid-LHA, increased the RMR in one patient (Case 1) by 28% and in another (Case 3) by 9%. Contact 1 stimulation in the remaining patient (Case 1) was indeterminate.
Body Weight Data
Although this pilot study was not designed to assess the efficacy of LHA DBS for treating obesity, careful patient weight measurements were made at each follow-up visit and provided data for preliminary efficacy analysis (Table 4). We did not observe any consistent weight loss in any of the 3 patients when LHA DBS was delivered with standard movement disorder settings (data not shown). However, after LHA DBS was programmed to settings that appeared to augment RMR during metabolic chamber experiments, significant weight loss has been observed in 2 patients (Cases 2 and 3) while a stable weight has been observed in 1 patient (Case 1).
TABLE 4.
Body weight before and after metabolically optimized LHA DBS settings
| Case No. | Body Weight (kg) Prior to Optimized Settings |
Body Weight (kg) at Last Follow-Up |
Mos at Optimized Settings |
Change in Body Weight |
|---|---|---|---|---|
| 1 | 138.3 | 137 | 16 | 0.9% decrease |
| 2 | 147.4 | 129.3 | 11 | 12.3% decrease |
| 3 | 162.8 | 136.1 | 9 | 16.4% decrease |
Subjective Effects of LHA DBS
There were several subjective effects of DBS stimulation of the LHA reported in all 3 patients that appeared to be consistent, reproducible, and voltage dependent. During the metabolic chamber testing, Contact 3 consistently produced increased activity and increased arousal. Contact 0 produced a feeling of warmth that became uncomfortable at higher voltage settings. These effects were immediate and adjustable.
Long-term programming at the RMR-optimized settings resulted in the report of a decreased urge to eat that remained relatively constant over time and resolved when the stimulator was turned off, even in a blinded fashion. Increased energy levels were also reported as the voltage was increased during programming sessions. This effect tended to wear off between several days and several weeks after the adjustment was made. The feelings of transient nausea and warmth were often noted during programming sessions and affected the rate of voltage increase but resolved quickly as the subject adjusted to the voltage change.
Discussion
The primary goal of this pilot study was achieved since we have shown that bilateral LHA DBS may be performed safely in humans. After more than 2.5 years of follow-up in all 3 patients, there are no serious adverse events that can be attributed to bilateral LHA DBS including no detrimental changes seen with various psychological metrics and biochemical studies. Moreover, our novel finding that electrical brain stimulation may be able to augment the resting metabolic rate in humans provides a preliminary proof-of-principle that DBS should be further investigated as a potential therapeutic strategy for intractable obesity.
Due to the small size of LHA nuclei, we chose to use the commercially available DBS electrode with the most closely spaced contacts (model 3389, 0.5-mm spacing between each contact) since these electrodes have been successfully used in posterior hypothalamic DBS for cluster headache.2 However, since the LHA is significantly smaller than most current DBS targets, the development of new DBS technology with smaller electrodes may be necessary to improve results from LHA DBS. The fact that monopolar stimulation at specific settings via a specific contact increased the RMR in these patients while the other contacts had no appreciable effect on RMR has several implications. First, the stimulation efficacy zone within the hypothalamus that appears necessary and sufficient to raise RMR is quite small and appears centered within the LHA. Second, the compactness of the hypothalamic nuclear anatomy involved with metabolism and the titratable response seen with changing electric field strength suggests that, at least for the time being, it is prudent that further exploration of hypothalamic surgery for obesity proceed with stimulation technology rather than with irreversible and nonadjustable lesioning techniques such as radiosurgery and radiofrequency ablation.
From a metabolic rate standpoint, the most active contact (Contact 1) was located in the middle of the LHA nucleus based on postoperative MR images superimposed on a stereotactic brain atlas. However, other nearby nuclear structures and white matter tracts that could be influenced by the electric field of Contact 1 include the nucleus stria terminalis, perifornical nucleus, prereticular zone, medial preoptic area, medial forebrain bundle, and pallidohypothalamic tracts. We suspect that the inability to tolerate stimulation at levels higher than 5 V on the deepest contact (Contact 0), which induced anxiety and nausea, may have been due to current spread to the VMH; VMH stimulation has been shown to engender nausea, anxiety, and even panic attacks in humans. 20
The subjective reports of hunger urge modulation and increased energy with LHA DBS suggest both that the location is appropriate and that DBS can perhaps add a new dimension to the treatment of intractable obesity when compared with the present modes of treatment. However, we reaffirm that this was a safety-based study and our efficacy data are preliminary. The weight loss in 2 of 3 patients was modest and cannot be solely attributed to modulation of RMR by LHA DBS. In fact, since we did not observe a direct correlation between RMR reduction and weight loss, we cannot state that RMR reduction by LHA DBS will predict weight loss. Although RMR reduction cannot be touted as a metric of success, it has proven useful to guide DBS programming in this study. Respiratory chamber studies have provided a quantitative way to approach LHA DBS parameter programming, yet we suspect that optimal settings in each patient will not only augment RMR to some extent but will also reduce appetite and food cravings and possibly increase the overall level of energy. Future studies on these implanted patients are planned to continue monitoring weight loss trends and to assess the durability of RMR modulation with LHA DBS. Despite our small number of patients, our safety data are favorable and should facilitate FDA approval of larger patient series.
Although the increasing incidence of global obesity cannot be blamed on a low baseline RMR across the population, there is ample evidence to suggest that the physiological reduction in RMR, which occurs with all weight loss, contributes in a major way to the common failure of weight loss maintenance.16 We suspect that further metabolic studies will reveal that there is a wide variability in the RMR depression response among humans. This RMR adaptability, which may someday be quantified, represents how vigorously an individual’s metabolic settling point resists and buffers against changes in weight. By directly influencing RMR, DBS may be able to safely check the homeostatic mechanisms that have evolved to protect humans from sustained weight loss. The scope of the growing obesity problem and the myriad associated health comorbidities are of sufficient magnitude to merit further clinical investigation of brain-targeted obesity surgery for the most severe and intractable cases. The incorporation of detailed metabolic studies into the evaluation of patients with severe and refractory obesity may not only help to reduce the stigma surrounding this disease but may also reveal which patients—potentially those with subnormal baseline RMR or most rapidly depressing RMR in response to caloric deprivation—may be the best candidates for adjunctive DBS. Since obesity remains a problem of both energy input and output, electrical brain stimulation with DBS may be well poised to influence both ends of this energy imbalance disease.
Conclusions
The effects of LHA DBS in 3 patients with refractory obesity were investigated in a single-center pilot study. After 2.5 years of stimulation, no significant adverse effects were noted from LHA DBS. Nonblinded respiratory chamber studies demonstrated that certain stimulation parameters were able to augment the RMR. Although the study was not designed to evaluate the efficacy of LHA DBS for refractory obesity, follow-up data suggest that LHA DBS delivered at metabolically optimized settings may promote lower caloric intake and weight loss.
Acknowledgments
Disclosure
Medtronic Co. provided DBS hardware and financial support for this study. Medtronic Co. was not involved in preparation or submission of the manuscript. West Virginia University supplied grant support for this study. Dr. Tomycz participated on the Surgeon Advisory Board for St. Jude. Dr. Finnis is an employee of Medtronic. Dr. Oh is a consultant for St. Jude.
Abbreviations used in this paper
- AC
anterior commissure
- DBS
deep brain stimulation
- LHA
lateral hypothalamic area
- PC
posterior commissure
- RMR
resting metabolic rate
- VMH
ventromedial nucleus of the hypothalamus
Footnotes
A portion of these data was presented at the 2012 American Association of Neurological Surgeons Scientific Meeting in Miami, Florida, April 14–18, 2012.
Author contributions to the study and manuscript preparation include the following. Conception and design: D Whiting, Bailes, de Jonge, Oh. Acquisition of data: Tomycz, D Whiting, de Jonge, Lecoultre, Alcindor, Angle, Cantella, Mizes, Oh. Analysis and interpretation of data: Tomycz, D Whiting, Bailes, de Jonge, Lecoultre, Wilent, Alcindor, Prostko, Cheng, BB Whiting, Mizes, Ravussin, Oh. Drafting the article: Tomycz, de Jonge, Prostko, Cheng, BB Whiting, Ravussin. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Tomycz. Administrative/technical/material support: Angle, Cantella, Ravussin. Study supervision: Cheng, Ravussin, Oh. Design of figures: Finnis.
References
- 1.Centers for Disease Control and Prevention. [Accessed March 1, 2013];National Health and Nutrition Examination Survey: NHANES 2003–2004. ( http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/nhanes03_04.htm)
- 2.Franzini A, Ferroli P, Leone M, Broggi G. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery. 2003;52:1095–1101. [PubMed] [Google Scholar]
- 3.Gobbi DG, Peters TM. Generalized 3D nonlinear transformations for medical imaging: an object-oriented implementation in VTK. Comput Med Imaging Graph. 2003;27:255–265. doi: 10.1016/s0895-6111(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 4.Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63:119–123. doi: 10.1002/ana.21295. [DOI] [PubMed] [Google Scholar]
- 5.Harrell LE, Decastro JM, Balagura S. A critical evaluation of body weight loss following lateral hypothalamic lesions. Physiol Behav. 1975;15:133–136. doi: 10.1016/0031-9384(75)90292-9. [DOI] [PubMed] [Google Scholar]
- 6.Keesey RE, Powley TL. Self-stimulation and body weight in rats with lateral hypothalamic lesions. Am J Physiol. 1973;224:970–978. doi: 10.1152/ajplegacy.1973.224.4.970. [DOI] [PubMed] [Google Scholar]
- 7.Kuczmarski RJ, Carroll MD, Flegal KM, Troiano RP. Varying body mass index cutoff points to describe overweight prevalence among U.S. adults: NHANES III (1988 to 1994) Obes Res. 1997;5:542–548. doi: 10.1002/j.1550-8528.1997.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 8.Laćan G, De Salles AAF, Gorgulho AA, Krahl SE, Frighetto L, Behnke EJ, et al. Modulation of food intake following deep brain stimulation of the ventromedial hypothalamus in the vervet monkey. Laboratory investigation. J Neurosurg. 2008;108:336–342. doi: 10.3171/JNS/2008/108/2/0336. [DOI] [PubMed] [Google Scholar]
- 9.Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. 3. San Diego: Academic Press; 2008. pp. 135–159. [Google Scholar]
- 10.Nguyen T, de Jonge L, Smith SR, Bray GA. Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min. Med Biol Eng Comput. 2003;41:572–578. doi: 10.1007/BF02345320. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 12.Penicaud L, Larue-Achagiotis C, Le Magnen J. Endocrine basis for weight gain after fasting or VMH lesion in rats. Am J Physiol. 1983;245:E246–E252. doi: 10.1152/ajpendo.1983.245.3.E246. [DOI] [PubMed] [Google Scholar]
- 13.Quaade F, Vaernet K, Larsson S. Stereotaxic stimulation and electrocoagulation of the lateral hypothalamus in obese humans. Acta Neurochir (Wien) 1974;30:111–117. doi: 10.1007/BF01405759. [DOI] [PubMed] [Google Scholar]
- 14.Reinhold RB. Critical analysis of long term weight loss following gastric bypass. Surg Gynecol Obstet. 1982;155:385–394. [PubMed] [Google Scholar]
- 15.Sani S, Jobe K, Smith A, Kordower JH, Bakay RA. Deep brain stimulation for treatment of obesity in rats. J Neurosurg. 2007;107:809–813. doi: 10.3171/JNS-07/10/0809. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz A, Doucet E. Relative changes in resting energy expenditure during weight loss: a systematic review. Obes Rev. 2010;11:531–547. doi: 10.1111/j.1467-789X.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- 17.Thornton SN, Nicolaïdis S, Larue-Achagiotis C, Campfield A. Body weight gain after VMH lesions in adult female rats guanethidine-sympathectomized at birth. Appetite. 1991;17:47–53. doi: 10.1016/0195-6663(91)90083-5. [DOI] [PubMed] [Google Scholar]
- 18.Tomycz ND, Whiting DM, Oh MY. Deep brain stimulation for obesity—from theoretical foundations to designing the first human pilot study. Neurosurg Rev. 2012;35:37–43. doi: 10.1007/s10143-011-0359-9. [DOI] [PubMed] [Google Scholar]
- 19.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilent WB, Oh MY, Buetefisch CM, Bailes JE, Cantella D, Angle C, et al. Induction of panic attack by stimulation of the ventromedial hypothalamus. Case report. J Neurosurg. 2010;112:1295–1298. doi: 10.3171/2009.9.JNS09577. [DOI] [PubMed] [Google Scholar]
- 21.Wilent WB, Oh MY, Buetefisch C, Bailes JE, Cantella D, Angle C, et al. Mapping of microstimulation evoked responses and unit activity patterns in the lateral hypothalamic area recorded in awake humans. Technical note. J Neurosurg. 2011;115:295–300. doi: 10.3171/2011.3.JNS101574. [DOI] [PubMed] [Google Scholar]