Abstract
Embryonic germ cells are formed from embryonic progenitors through a highly complex differentiation process, recapitulation of which in vitro has proved challenging. Two new studies in The EMBO Journal report culture conditions for embryonic stem cell‐derived primordial germ cell‐like cells (PGCLCs) that enable global DNA demethylation (Ohta et al, 2017), and subsequent initiation of meiosis (Miyauchi et al, 2017), allowing future manipulations to elucidate mechanisms driving germ line differentiation.
Subject Categories: Development & Differentiation, Signal Transduction, Stem Cells
For many years, multiple groups have attempted to induce differentiation of mature gametes from cultured pluripotent cells in vitro. Initial attempts reported directed differentiation of germ cell‐like cells from embryonic stem cells of mice and humans; however, these approaches suffered from low efficiencies and high variability (Hübner et al, 2003). More recent studies have utilized knowledge of how germ cells are specified in vivo to recapitulate this process in vitro (Kurimoto & Saitou, 2015). Two studies from the laboratory of Mitinori Saitou published in The EMBO Journal now report new advances, producing cells that undergo two major germ cell‐specific processes: global DNA demethylation and meiotic initiation (Miyauchi et al, 2017; Ohta et al, 2017) (Fig 1).
Figure 1. Comparison of in vivo and in vitro differentiation of germ cells.
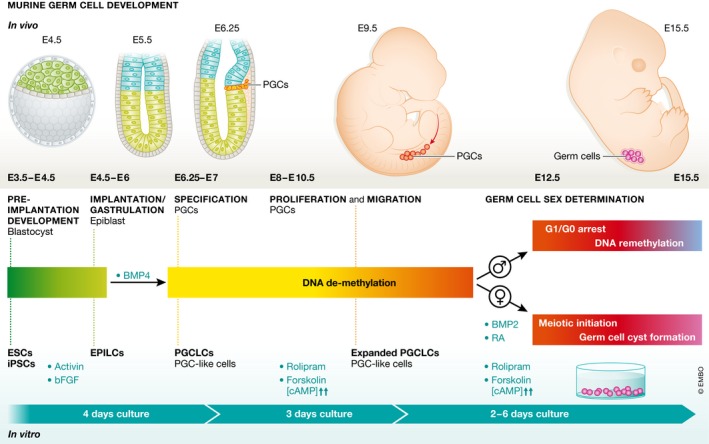
Developmental progression from pluripotent cells to sex committed early oocytes is depicted with crucial steps labeled. Time points and stages on top of timeline represent in vivo differentiation of germ cells in the mouse. Equivalent in vitro differentiation steps are listed below the timeline including added growth factors.
In mice, germ cells arise through an inductive mechanism from a small population of cells found in the posterior proximal epiblast. These cells then proliferate and migrate through the embryo toward their final home in the somatic gonad. Previous work established culture conditions in which pluripotent stem cells can be driven stepwise first into an epiblast‐like state and subsequently into a primordial germ cell‐like (PGCLC) state. These PGCLCs, when placed in an appropriate somatic gonadal milieu, are capable of producing mature gametes (Kurimoto & Saitou, 2015).
Ohta et al (2017) now show that treatment of PGCLCs with chemicals that stimulate cAMP signaling induces proliferation, allowing for an up to 50‐fold expansion, seemingly without alteration of potency, as these cells efficiently produce mature male germ cells when transplanted into a testis. Strikingly, PGCLCs induced to proliferate undergo global DNA demethylation, a process thought to be an essential landmark in gamete development prior to the onset of sex‐specific differentiation. This process leads to a resetting of DNA methylation at control elements of imprinted loci, enabling their subsequent sex‐specific re‐establishment. Two mechanisms for DNA demethylation have been proposed: (i) dilution via passive failure in maintenance DNA methylation over a series of cell divisions or (ii) an active enzymatically driven process. Data from several studies have suggested that the base excision repair pathway (BER), a pathway proposed to be involved in active demethylation, and oxidation of methylated DNA by enzymes of the TET family are active in PGCs in vivo (Hill et al, 2014). Other work argued for passive demethylation as the main mode of methylation erasure in PGCs (Kagiwada et al, 2012). PGCLCs induced to proliferate using Ohta et al (2017)'s protocol show decreased expression of proteins involved in maintenance (UHRF1) and de novo (DNMT3A, DNMT3B) DNA methylation, suggesting that passive demethylation occurs during in vitro differentiation. The relative contributions of passive and active mechanisms of methylation erasure and what roles BER and TET enzymes play in this process remain to be fully characterized.
Following the work of Ohta et al (2017), Miyauchi et al (2017) set out to identify conditions in which expanding PGCLCs could be induced to initiate female‐specific germ cell differentiation. Specification, migration and DNA demethylation of germ cells occur equivalently in both male and female embryos. After their arrival in the somatic gonad (which itself is at this stage initiating the primary sex determination pathway), germ cells respond to environmental cues to initiate male or female differentiation. A hallmark of early female differentiation is the initiation of meiosis. Male germ cells only initiate this process in postnatal life. Previous studies identified retinoic acid (RA) signaling as a driver of expression of a key meiotic regulator, Stra8, in the embryonic ovary (Spiller et al, 2017).
Using fluorescent reporters for expression of two late germ cell genes (Dazl and Ddx4), Miyauchi et al (2017) show that simultaneous treatment of proliferating PGCLCs with RA and BMP2 led to strong induction of these genes. Induced cells showed key features of meiosis, such as 4C DNA content, meiotic DNA break formation and repair, and synapsis of homologous chromosomes. Importantly, treatment of PGCLCs with RA alone is not sufficient to induce these features, while it is necessary and sufficient to drive Stra8 expression. The authors also show that chemical inhibition of BMP signaling during this crucial window of development in vivo severely affects the development of female, but not male, germ cells. These results show for the first time a role for BMP signaling in female germ line sex determination.
Though the role of BMP signaling in oogenesis was unknown, its role in the specification of primordial germ cells from the epiblast is well established (Lawson et al, 1999). Why do early PGCs not induce expression of meiotic genes? Miyauchi et al (2017) find that exposure to RA and BMP2 shortly after induction of the PGCLC state does not induce meiotic gene expression. Instead, it appears that the proliferative culture phase is required to enable a meiotic response to these signaling molecules. The authors suggest, though do not directly show, that DNA demethylation is a prerequisite for induction of the meiotic program.
In vivo, DNA demethylation occurs at least 1 day prior to the onset of meiotic initiation in female germ cells. What prevents these demethylated germ cells from responding to developmental cues and precociously initiating meiosis? One possibility could be the existence of redundant mechanisms of gene repression, for instance, via PRC2‐mediated histone H3 lysine 27 trimethylation (Ohta et al, 2017). In 2013, Yokobayashi and colleagues showed that reduced expression of core components of the polycomb repressive complex 1 (PRC1) in post‐migratory PGCs resulted in premature expression of RA‐sensitive germ line genes and accelerated entry into meiotic prophase in female but not male embryos. Interestingly, in ESCs, a variant PRC1 complex containing the factor PCGF6, targeted to chromatin by the transcription factors MAX and MGA, is required to maintain repression of germ line‐expressed genes controlled by DNA methylation in PGCs and soma (but not in ESCs) (Endoh et al, 2017). It remains to be seen whether a similar complex functions during PGC development and PGCLC differentiation.
Another question raised by Miyauchi et al's protocol for induction of meiotic prophase is the fact that while the number of cells entering meiosis is high, the percentage reaching the pachytene stage of prophase is low (1.8%). It is unknown which additional factors could drive prophase progression in the embryonic ovary. An interesting parallel for induction of meiosis occurs in testes of mice deficient for the RNA binding protein Nanos2. Nanos2 expression in male germ cells prevents activation of meiotic initiation; however, Nanos2‐deficient germ cells do not progress completely through prophase (Suzuki & Saga, 2008). Thus, it seems that in vivo as well as in vitro, initiation of meiosis is not sufficient to drive progression through prophase.
An ultimate goal of the approach is to produce fully in vitro‐derived gametes. The methods presented by Ohta et al and Miyauchi et al provide clear steps toward this goal; however, substantial barriers remain to be overcome. Mature oocytes are surrounded by ovarian somatic cells in structures known as follicles. These follicles arise from breakdown of cysts, groups of germ cells connected via intercellular bridges (Pepling & Spradling, 2001). The female germ cells produced by Miyauchi et al (2017) form cyst‐like structures, but whether an in vitro‐derived oocyte could progress further in its development without connections to follicular somatic cells or whether in vitro‐derived somatic granulosa cells could be incorporated into culture methods to produce more mature gametes remains unknown. Intriguingly, progression of oocytes into meiosis is not required for formation of follicles or other aspects of oogenesis (Dokshin et al, 2013), suggesting that additional pathways may need to be activated in vitro to fully realize the possibility of in vitro gametogenesis. Finally, while in vitro germ cell development offers an exciting opportunity to study these processes in human cells, a greatly understudied area, their possible use as a treatment for infertility lies far ahead. Addressing whether such cells could produce fully functional gametes poses both technical and ethical challenges.
See also: H Ohta et al (July 2017) and H Miyauchi et al (November 2017)
Contributor Information
Mark E Gill, Email: mark.gill@fmi.ch.
Antoine HFM Peters, Email: antoine.peters@fmi.ch.
References
- Dokshin GA, Baltus AE, Eppig JJ, Page DC (2013) Oocyte differentiation is genetically dissociable from meiosis in mice. Nat Genet 45: 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M, Endo TA, Shinga J, Hayashi K, Farcas A, Ma KW, Ito S, Sharif J, Endoh T, Onaga N, Nakayama M, Ishikura T, Masui O, Kessler BM, Suda T, Ohara O, Okuda A, Klose RJ, Koseki H (2017) PCGF6‐PRC1 suppresses premature differentiation of mouse embryonic stem cells by regulating germ cell‐related genes. eLife 6: e21064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PW, Amouroux R, Hajkova P (2014) DNA demethylation, Tet proteins and 5‐hydroxymethylcytosine in epigenetic reprogramming: an emerging complex story. Genomics 104: 324–333 [DOI] [PubMed] [Google Scholar]
- Hübner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF III, Boiani M, Schöler HR (2003) Derivation of oocytes from mouse embryonic stem cells. Science 300: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Kagiwada S, Kurimoto K, Hirota T, Yamaji M, Saitou M (2012) Replication‐coupled passive DNA demethylation for the erasure of genome imprints in mice. EMBO J 32: 340–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto K, Saitou M (2015) Mechanism and reconstitution in vitro of germ cell development in mammals. Cold Spring Harb Symp Quant Biol 80: 147–154 [DOI] [PubMed] [Google Scholar]
- Lawson K, Dunn NR, Roelen BAJ, Zeinstra LM, Davis AM, Wright CVE, Korving JPWFM, Hogan BLM (1999) Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13: 424–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi H, Ohta H, Nagaoka S, Nakaki F, Sasaki K, Hayashi K, Yabuta Y, Nakamura T, Yamamoto T, Saitou M (2017) Bone morphogenetic protein and retinoic acid synergistically specify female germ‐cell fate in mice. EMBO J 36: 3100–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Kurimoto K, Okamoto I, Nakamura T, Yabuta Y, Miyauchi H, Yamamoto T, Okuno Y, Hagiwara M, Shirane K, Sasaki H, Saitou M (2017) In vitro expansion of mouse primordial germ cell‐like cells recapitulates an epigenetic blank slate. EMBO J 36: 1888–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling M, Spradling A (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 234: 339–351 [DOI] [PubMed] [Google Scholar]
- Spiller C, Koopman P, Bowles J (2017) Sex determination in the mammalian germline. Annu Rev Genet 51: 265–285 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Saga Y (2008) Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev 22: 430–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokobayashi S, Liang CY, Kohler H, Nestorov P, Liu Z, Vidal M, van Lohuizen M, Roloff T, Peters AH (2013) PRC1 coordinates timing of sexual differentiation of female primordial germ cells. Nature 495: 236–240 [DOI] [PubMed] [Google Scholar]


