Abstract
N 6‐methyladenosine (m6A) is a highly dynamic RNA modification that has recently emerged as a key regulator of gene expression. While many m6A modifications are installed by the METTL3–METTL14 complex, others appear to be introduced independently, implying that additional human m6A methyltransferases remain to be identified. Using crosslinking and analysis of cDNA (CRAC), we reveal that the putative human m6A “writer” protein METTL16 binds to the U6 snRNA and other ncRNAs as well as numerous lncRNAs and pre‐mRNAs. We demonstrate that METTL16 is responsible for N 6‐methylation of A43 of the U6 snRNA and identify the early U6 biogenesis factors La, LARP7 and the methylphosphate capping enzyme MEPCE as METTL16 interaction partners. Interestingly, A43 lies within an essential ACAGAGA box of U6 that base pairs with 5′ splice sites of pre‐mRNAs during splicing, suggesting that METTL16‐mediated modification of this site plays an important role in splicing regulation. The identification of METTL16 as an active m6A methyltransferase in human cells expands our understanding of the mechanisms by which the m6A landscape is installed on cellular RNAs.
Keywords: methyltransferase, N6‐methyladenosine (m(6)A), pre‐mRNA splicing, RNA modification, snRNA
Subject Categories: RNA Biology
Introduction
Modifications in cellular RNAs (collectively termed the “epitranscriptome”) have emerged as important regulators of many aspects of gene expression. They expand the chemical and topological properties of the four basic nucleotides, thereby regulating the functions and fates of RNAs. So far, ~150 different types of RNA modifications have been identified in nature, ranging from methylations of different positions of the bases to complex modifications that are installed in multistep reactions by the coordinated action of several modification enzymes 1. While transfer RNAs (tRNAs) and ribosomal RNA (rRNAs) are the most highly modified RNA species 2, 3, the development of transcriptome‐wide mapping approaches for several RNA modifications 4, such as N 6‐methyladenosine (m6A; 5, 6, 7, 8), pseudouridine (Ψ; 9, 10, 11), N 1‐methyladenosine (m1A; 12, 13) and 5‐methylcytidine (m5C; 14, 15), has uncovered a complex landscape of modified sites in messenger RNAs (mRNAs) and many classes of non‐coding (nc) RNAs, including small nucleolar RNAs (snoRNAs), small nuclear RNAs (snRNAs), long non‐coding RNAs (lncRNAs) and microRNAs (miRNAs; reviewed in 16).
m6A is the most abundant internal mRNA modification and the majority of modifications lie within a RRACH sequence motif (R = A or G; H = A, C or U) in close proximity to stop codons 5, 6. Functionally, m6A modifications can have diverse effects on RNA secondary structure by either destabilising RNA duplexes or, when present in single‐stranded RNAs, promoting base stacking, thereby enhancing RNA stability 17. Alternatively, m6A modifications can be specifically recognised by proteins (“readers”), which then regulate the splicing, localisation, translation and/or stability of mRNAs 18, 19, 20, 21, 22. Furthermore, m6A modifications are installed dynamically and specific modifications have been suggested to be reversed by the action of the dioxygenases FTO and ALKBH5 23, 24. Such dynamic changes in the m6A profiles of mRNAs have been shown to influence mammalian circadian rhythms, stem cell differentiation and development, and promote tumorigenesis (e.g. 25, 26, 27, 28).
A complex composed of METTL3, METTL14, WTAP, RBM15/15B and KIAA1429 has been shown to possess m6A methyltransferase activity and be responsible for installing a multitude of m6A modifications in diverse cellular RNAs 29, 30, 31. Structural and biochemical analyses revealed that METTL3 is an active S‐adenosylmethionine (SAM)‐dependent RNA methyltransferase whereas METTL14 contributes to RNA substrate binding 32, 33. Transcriptome‐wide identification of the binding sites of the METTL3–METTL14 complex revealed an overlap with previously mapped m6A sites and a consensus binding motif that corresponds to the RRACH sequence 29. While the METTL3–METTL14 complex is a prominent mRNA m6A methyltransferase (“writer”), numerous known m6A modifications are present in different sequence contexts and were not identified in the METTL3–METTL14 crosslinking immunoprecipitation (CLIP) data sets, implying that other m6A methyltransferases exist in human cells.
The human genome encodes many putative methyltransferases, often designated as methyltransferase‐like (METTL) proteins, and here, we characterised METTL16 as an m6A “writer” protein. Using in vivo crosslinking and analysis of cDNA (CRAC), we provide insight into the RNA interactome and target spectrum of this protein, and uncover interactions with pre‐mRNAs, lncRNAs and several ncRNAs including the U6 snRNA. We show that METTL16 is responsible for N 6‐methylation of an adenine within an evolutionarily conserved U6 sequence that base pairs with 5′ splice sites of pre‐mRNAs. Furthermore, our data reveal that this modification is introduced during early stages in U6 snRNP biogenesis.
Results and Discussion
METTL16 binds directly to the U6 snRNA in vivo
The discovery that many m6A modifications in cellular RNAs do not map within the binding sites of the METTL3–METTL14 m6A “writer” complex indicates that other m6A methyltransferases exist in human cells. METTL16 represents a strong candidate for such activity due to its homology to the Escherichia coli YbiN protein that is responsible for N 6‐methylation of A1618 in the 23S ribosomal RNA 34. To identify RNA substrates of METTL16 in vivo, we performed crosslinking and analysis of cDNA experiments (CRAC; 35, 36, 37). HEK293 cells expressing C‐terminally His6‐PreScission protease site‐2x Flag (Flag)‐tagged METTL16 or the Flag tag were crosslinked using UV light at 254 nm (UV‐CRAC) or alternatively, were grown in the presence of the photoactivatable nucleoside analogue 4‐thiouridine prior to crosslinking at 365 nm (PAR‐CRAC). Crosslinked protein–RNA complexes were isolated, and RNAs were trimmed and radiolabelled before separation of complexes by polyacrylamide gel electrophoresis. After transfer to a nitrocellulose membrane, visualisation of radiolabelled RNAs by autoradiography revealed strong signals in both the UV‐CRAC and PAR‐CRAC METTL16‐Flag samples but not in the controls (Fig 1A), demonstrating that METTL16 associates with cellular RNAs. These RNAs were isolated, ligated to adaptors, and a cDNA library was generated and subjected to Illumina deep sequencing. The obtained sequence reads were mapped on the human genome and the distribution of reads mapping to different classes of RNA was analysed (Fig 1B). Interestingly, this revealed a significant increase in the proportion of sequences mapping to snRNA genes in the METTL16‐Flag samples compared to the controls, indicating association of METTL16 with snRNA(s) (Fig 1B). We also observed a modest increase in the proportion of reads mapping to rRNA sequences; however, analysis of the distribution of reads mapping to the rDNA unit encoding the 47S pre‐rRNA transcript did not reveal any specific crosslinking site of METTL16 and this enrichment therefore likely reflects non‐specific crosslinking arising from the presence of METTL16 in the nucleolus (Fig EV1; 38).
Figure 1. METTL16 crosslinks to the U6 snRNA in vivo .
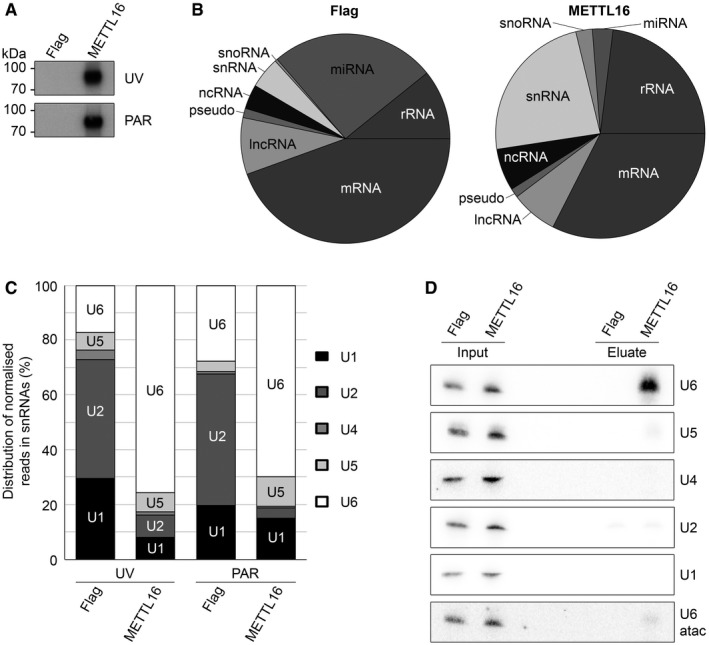
- HEK293 cells expressing METTL16‐Flag (METTL16) or the Flag tag (Flag) were crosslinked using UV light at 254 nm (UV) or grown in the presence of 4‐thiouridine prior to crosslinking at 365 nm (PAR). Protein–RNA complexes were affinity‐purified and bound RNAs trimmed, labelled at the 5′ end with 32P and ligated to sequencing adapters. Protein–RNA complexes were separated by polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane and visualised by autoradiography.
- RNAs isolated from the membranes described in (A) were subjected to reverse transcription and PCR amplification to generate cDNA libraries that were analysed by Illumina deep sequencing. The obtained sequence reads were mapped to the human genome and the relative proportion of UV‐CRAC reads mapping to different classes of RNAs is shown.
- The relative distribution of reads mapping to different snRNAs in the UV‐ and PAR‐CRAC data sets derived from cells expressing METTL16‐Flag or the Flag tag is shown. Only snRNAs to which > 5% of the total snRNA hits mapped are labelled within the columns.
- HEK293 cells expressing METTL16‐Flag or the Flag tag were UV crosslinked in vivo and protein–RNA complexes were isolated under denaturing conditions. Crosslinked RNAs were isolated, and inputs (0.2%) and eluates were analysed by Northern blotting using probes hybridising to the snRNAs indicated.
Figure EV1. METTL16 localises to the nucleoplasm and nucleolus.
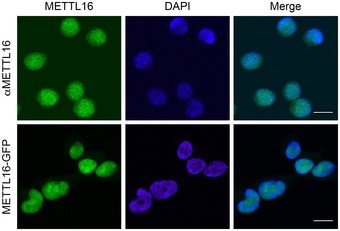
Immunofluorescence was performed using an antibody against METTL16 in HEK293 cells (αMETTL16; green; upper panel), or alternatively, HEK293 cells expressing METTL16‐GFP were fixed and directly analysed by fluorescence microscopy (METTL16‐GFP; green; lower panel). Nuclear material was visualised by DAPI staining (blue) and overlays of the different channels are presented on the right (Merge). The scale bar represents 10 μm.
To determine which snRNA was crosslinked to METTL16, we analysed the normalised number of sequence reads mapping to each of the snRNAs (Fig EV2) and the relative distribution of the sequence reads between the five snRNAs of the major spliceosome (Fig 1C). Notably, 75 and 70% of the reads mapped to the U6 snRNA in the METTL16 UV‐CRAC and PAR‐CRAC experiments, respectively, compared to only 17 and 27% in the corresponding control samples (Fig 1C), strongly suggesting that METTL16 interacts with the U6 snRNA. To confirm the specificity of this interaction, we performed additional crosslinking experiments in which cells expressing METTL16‐Flag or the Flag tag were UV crosslinked in vivo and after isolation of protein–RNA complexes, co‐precipitated RNAs were detected by Northern blotting (Fig 1D). While the U1, U2, U4 and U5 snRNAs were barely detected in any of the eluates, the U6 snRNA was strongly enriched in the eluate from the METTL16‐Flag, but not in the control. Notably, the U6atac snRNA of the minor spliceosome was also not enriched, implying a specific interaction of METTL16 with the U6 snRNA.
Figure EV2. METTL16 crosslinks to the U6 snRNA .
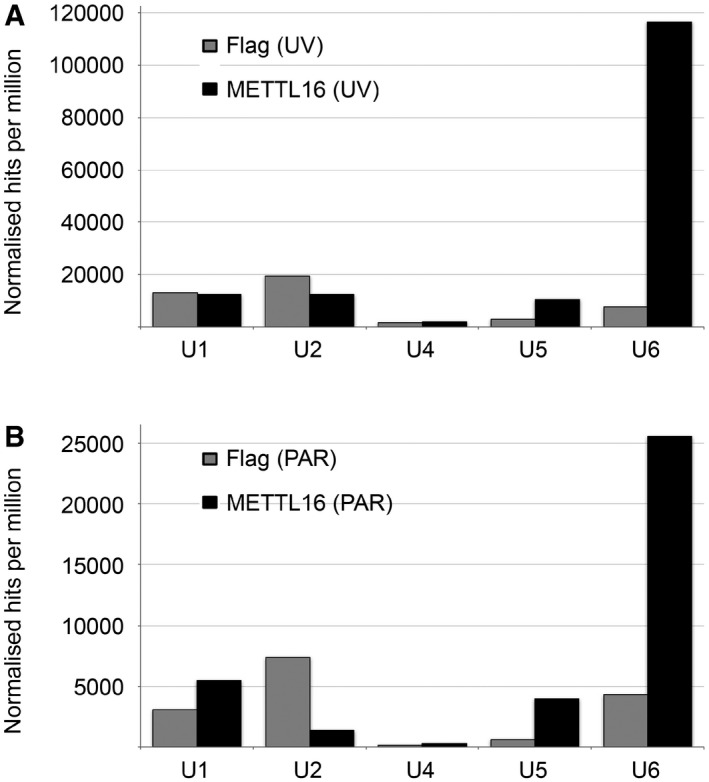
-
A, BThe normalised number of reads mapping to different snRNAs in the UV‐CRAC (A) and PAR‐CRAC (B) data sets derived from cells expressing METTL16‐Flag or the Flag tag is shown.
METTL16 is an m6A methyltransferase that modifies the U6 snRNA
The identification of the U6 snRNA as a direct interaction partner of the putative m6A methyltransferase METTL16 in vivo suggests that it may be a modification substrate. The U6 snRNA (but not U6atac) has been shown to carry an m6A at position 43 39, but the enzyme responsible for installing this modification long remained elusive. To determine whether METTL16 is responsible for N 6‐methylation of A43 in U6, we first examined the distribution of CRAC sequence reads mapping to the U6 snRNA (Fig 2A). This revealed that the majority of reads mapped to the central region of the U6 snRNA close to A43, supporting the hypothesis that METTL16 methylates this residue.
Figure 2. METTL16 is a human m6A methyltransferase.
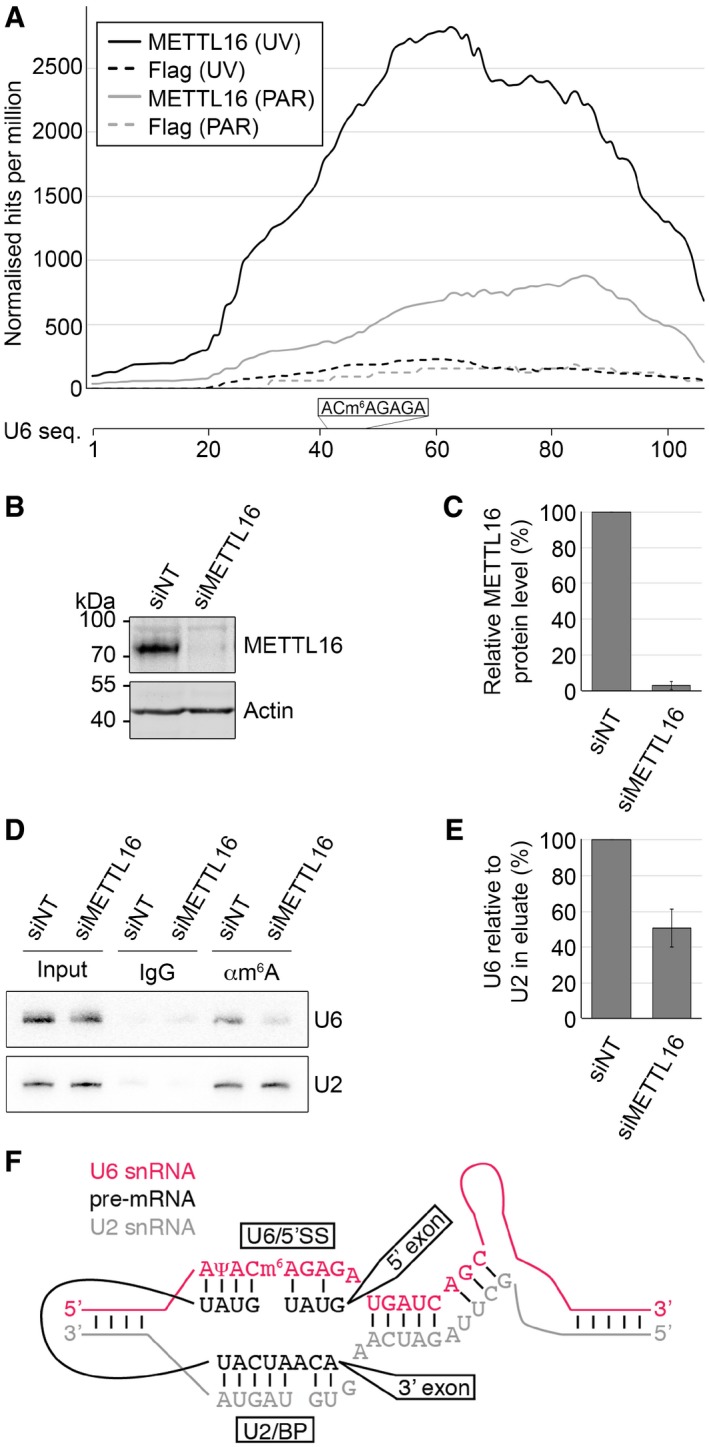
- The distribution of sequence reads, obtained from the METTL16‐Flag and Flag CRAC experiments, mapping to the U6 snRNA sequence is given as hits per million mapped reads. The sequence context of the m6A43 in the U6 snRNA is shown below.
- HeLa cells treated with non‐target siRNAs (siNT) or siRNAs targeting METTL16 (siMETTL16) were used to prepare whole‐cell extracts. Protein levels were analysed by Western blotting using antibodies against METTL16 or actin.
- The levels of METTL16 in siRNA‐treated cells analysed in (B) were quantified and normalised to actin. Data from three independent experiments are presented as mean ± SD.
- Total RNA extracted from siRNA‐treated HeLa cells (as in B) was incubated with an anti‐m6A antibody (αm6A) or with non‐specific IgGs (IgG). Complexes were retrieved on Protein G Sepharose and after thorough washing, co‐precipitated RNAs were isolated and analysed by Northern blotting using probes hybridising to the U6 or U2 snRNAs. Input = 0.5%.
- The amounts of U6 in the αm6A eluates were quantified, normalised to U2 levels and are shown as a bar graph. Data from three independent experiments are presented as mean ± SD.
- Schematic view of the base pairing interactions between the U6 snRNA (red), the U2 snRNA (grey) and a pre‐mRNA (black). The position of m6A43 within the evolutionarily conserved ACAGAG motif of the U6 snRNA is indicated. 5′SS—5′ splice site, BP—branch point.
In order to demonstrate the methyltransferase activity of METTL16 on U6, we first established RNAi against METTL16. After transfection of siRNAs specifically targeting METTL16, Western blotting using an antibody against endogenous METTL16 confirmed reduction in the protein level by up to 97% (Fig 2B and C). We then performed a methylated RNA immunoprecipitation (Me‐RIP) using an antibody that specifically recognises m6A nucleotides and RNA derived from cells that had been treated with control siRNAs or siRNAs targeting METTL16. Analysis of the Me‐RIP eluates by Northern blotting using probes hybridising to the U6 and U2 snRNAs revealed specific precipitation of both snRNAs in samples containing the anti‐m6A antibody but not non‐specific IgGs, confirming the presence of m6A modifications in these RNAs (Fig 2D; 39, 40). Interestingly, upon depletion of METTL16, the amount of U6 co‐precipitated with the m6A antibody was reduced by ~50%, while the amount of U2 present in the eluates was unaffected (Fig 2D and E), demonstrating that METTL16 is an m6A methyltransferase that specifically modifies U6. This finding is supported by a parallel study 41 where the action of METTL16 in installing the m6A43 modification of U6 was shown by an in vitro methylation assay. The twofold reduction in the amount of U6 precipitated by the m6A antibody may be due to the detection of modifications installed in the long‐lived U6 snRNA 42 prior to efficient METTL16 depletion or may reflect the presence of an additional METTL3‐mediated m6A modification at A76 of the U6 snRNA 29, 43. In contrast to the putative m6A76 modification, the m6A43 modification installed by METTL16 does not lie within a RRACH motif, suggesting that different sequence or structural elements influence modification target recognition by the two m6A methyltransferases.
Together with the U2 snRNA, U6 forms the catalytic core of the spliceosome 44, 45 and the m6A43 modification lies within a highly conserved ACAGAGA sequence of U6 that base pairs with the 5′ splice site of pre‐mRNAs (Fig 2F; 46, 47, 48). Mutations in this sequence impede such interactions and are lethal in yeast 49, 50, implying that the presence of the m6A within this part of the U6 snRNA plays an important role in the regulation of pre‐mRNA splicing. The precise function of this modification remains unknown; however, based on the recent structures of the human U4/U6.U5 tri‐snRNP and various (pre‐)catalytic spliceosomes (e.g. 45, 51, 52), it seems unlikely that this modification is reversible or that it exerts its effect via a reader protein, but rather it is anticipated to influence local secondary structure or base pairing interactions of this region of the U6 snRNA. Assembly of the spliceosome onto its substrate pre‐mRNAs is achieved by a combination of relatively weak interactions of both spliceosomal proteins and snRNAs with the pre‐mRNA. This suggests that U6‐m6A43 may fine‐tune snRNA–pre‐mRNA interactions, thereby regulating either 5′ splice site recognition or spliceosome assembly.
METTL16 associates with oligouridylated pre‐U6 snRNA during U6 snRNP assembly
Prior to its integration into the spliceosome, the U6 snRNA assembles as a U6 mono‐snRNP, then a U4/U6 di‐snRNP and a U4/U6.U5 tri‐snRNP (reviewed in 53). To determine in which context the m6A43 modification is introduced, we performed native RNA immunoprecipitation experiments using extracts from HEK293 cells expressing either METTL16‐Flag or the Flag tag. Co‐precipitated snRNAs were detected by Northern blotting, and while the U6 snRNA was efficiently recovered from the METTL16‐Flag extract but not from the control, the U4 and U5 snRNAs were not significantly enriched in either of the eluates (Fig 3A). This implies that METTL16 associates with the nascent U6 RNA or a U6 mono‐snRNP during the early stages of U6 maturation.
Figure 3. METTL16 interacts with oligouridylated pre‐U6 snRNA and co‐precipitates La, LARP7 and MEPCE .
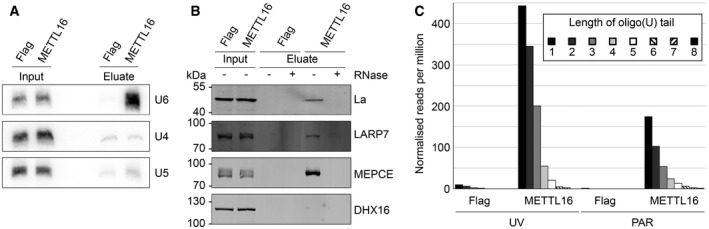
- Extracts from HEK293 cell lines expressing METTL16‐Flag or the Flag tag (Flag) were used in native immunoprecipitation experiments. Co‐precipitated RNAs were analysed alongside total RNA inputs (20%) by Northern blotting using probes hybridising to the indicated snRNAs.
- Extracts from HEK293 cell lines expressing METTL16‐Flag or the Flag tag (Flag) were used for immunoprecipitation experiments in the presence (+) or absence (−) of RNase A. Inputs (1%) and eluates containing co‐precipitated proteins were analysed by Western blotting using antibodies against La, LARP7, MEPCE and DHX16.
- The number of sequence reads containing non‐genomically encoded T's (U‐tails) mapping to the 3′ end of the U6 snRNA was determined in each of the CRAC data sets and is shown as the relative number of additional T's detected.
After transcription by RNA polymerase III in the nucleus, the pre‐U6 snRNA acquires a 5′ guanosine triphosphate cap 54 and undergoes 3′ oligouridylation 55, 56, leading to recruitment of the La protein to stabilise the transcript 57, 58. Formation of the mature 3′ end of U6 causes release of the La protein and recruitment of the LSm2‐8 complex. The U6 snRNP transits through the nucleolus where eight 2′‐O‐methyl groups and three pseudouridines are added by box C/D and box H/ACA snoRNPs, respectively 59, 60, before returning to the nucleoplasm. To further define the timing of m6A43 installation on the U6 snRNA, we next identified protein interaction partners of METTL16 by immunoprecipitation experiments followed by mass spectrometry. This revealed significant enrichment of the chaperone‐like La protein 61, the La‐associated protein LARP7 62 and the guanosine triphosphate capping enzyme MEPCE 63 in METTL16‐Flag eluates compared to the control samples. To confirm these interactions and ascertain whether they are formed via RNA, immunoprecipitation experiments were performed in the presence or absence of RNase A, using extracts from cells expressing METTL16‐Flag or the Flag tag, and the eluates were analysed by Western blotting using antibodies against endogenous La, LARP7, MEPCE or, as a control, the spliceosomal RNA helicase DHX16 (Fig 3B). This confirmed specific binding of METTL16 with La, LARP7 and MEPCE, but revealed that these interactions are RNA‐dependent (Fig 3B), implying that rather than reflecting direct protein–protein contacts, these interactions may be mediated by the U6 snRNA or other substrate RNAs of METTL16 that are also bound by these factors. The presence of MEPCE in METTL16 complexes could suggest that 5′ end capping and N 6‐methylation of A43 occur simultaneously. However, in the context of the 7SK RNP, binding of LARP7 to MEPCE has been shown to inhibit its catalytic activity and MEPCE then has a non‐catalytic function in promoting the interaction between LARP7 and the 7SK RNA 64. Although it is not yet known whether LARP7 and MEPCE form similar interactions on the U6 snRNA, by analogy, this could suggest that METTL16‐mediated modification of A43 takes place downstream of U6 5′ capping.
Since both La and LARP7 preferentially bind oligo(U) stretches at the 3′ ends of RNA polymerase III transcripts 57, 58, 65, we quantified the number of reads mapping to the 3′ end of U6 that contained additional, non‐genomically encoded T's (corresponding to U's in the METTL16‐associated RNAs; Fig 3C). This revealed that while only a very small number of sequences in the control CRAC data sets contained additional T's, sequence reads containing non‐genomically encoded T's were significantly more common in the CRAC data derived from METTL16‐Flag cells, with numerous reads containing up to eight additional T's (Fig 3C). These results strongly suggest that METTL16 associates with a nucleoplasmic, 5′‐capped, oligouridylated form of pre‐U6 that is stabilised by the presence of La, LARP7 and MEPCE.
METTL16 associates with various ncRNAs, lncRNAs and pre‐mRNAs
Interestingly, METTL16 has recently been reported to interact with the cancer‐associated MALAT1 lncRNA 38, 66, and the mRNA encoding the SAM synthetase MAT2A 41, indicating that METTL16 associates with additional cellular RNAs. To determine whether these RNAs are directly bound by METTL16, we analysed the number and distribution of sequence reads corresponding to these transcripts in the CRAC data sets derived from cells expressing METTL16‐Flag. We identified specific crosslinking sites of METTL16 in the MALAT1 lncRNA, with the highest density of mapped sequence reads occurring on the 3′ region that forms the RNA triple helix element required for nuclear expression (Fig 4A) 38. Direct binding of METTL16 to this sequence is supported by the identification of mutations and microdeletions that are introduced during cDNA library preparation at nucleotides crosslinked to amino acids (Fig 4B; 67). Consistent with the findings of a parallel study 41, we observed crosslinking of METTL16 to the 3′ exon of the MAT2A pre‐mRNA with the highest peaks observed over hairpin secondary structures required for METTL16‐dependent splicing of an otherwise retained intron (Fig 4C; 41).
Figure 4. Various pre‐mRNAs, lncRNAs and ncRNAs are bound by METTL16.
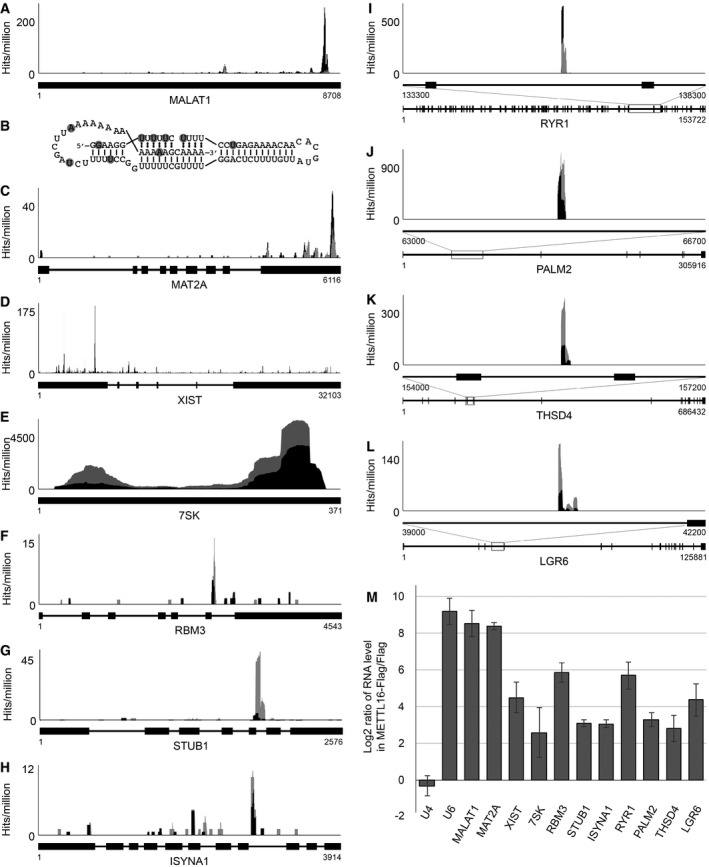
-
AThe relative distribution of CRAC sequence reads (UV‐CRAC—black; PAR‐CRAC—grey) mapping to the MALAT1 lncRNA is shown as hits per million mapped reads. Numbers below the schematic representation of the transcript indicate nucleotide positions.
-
BSecondary structure of the MALAT1 triple helix with nucleotides UV crosslinked to METTL16 highlighted with grey circles.
-
C–LThe relative distribution of CRAC sequence reads (UV‐CRAC—black; PAR‐CRAC—grey) mapping to the MAT2A mRNA (C), the XIST lncRNA (D), the 7SK ncRNA (E), and the RBM3 (F), STUB1 (G), ISYNA1 (H), RYR1 (I), PALM2 (J), THSD4 (K) and LGR6 (L) mRNAs is shown above schematic views of the introns (black lines) and exons (black boxes) present in the transcripts. For large mRNAs (I–L), a magnified view of the region of the pre‐mRNA that is crosslinked to METTL16 is shown (top, middle) above the schematic view of the whole mRNA.
-
MExtracts prepared from HEK293 cells expressing METTL16‐Flag or the Flag tag were used in immunoprecipitation experiments and co‐precipitated RNAs were isolated. RT–qPCR was performed to detect the indicated transcripts. Log2 fold changes between the levels of each transcript detected in the eluates of the METTL16‐Flag and the Flag control immunoprecipitations are shown. Data from three independent experiments are presented as mean ± SD.
To identify other METTL16‐bound RNAs, we performed peak calling on our CRAC data sets, and identified 355 mRNAs, 68 lncRNAs and nine ncRNAs that were specifically crosslinked to METTL16 in the UV‐ and PAR‐CRAC experiments (Tables EV1, EV2 and EV3). Interestingly, METTL16 crosslinking peaks were identified in the well‐characterised XIST lncRNA that participates in X chromosome inactivation during development (Fig 4D; 68), as well as the three vault RNAs (vtRNAs) and the four Y‐RNAs (Table EV3). Additional METTL16 substrates identified include the 7SL RNA of the signal recognition particle and the 7SK ncRNA, which is bound by MEPCE, LARP7 and La during its biogenesis (Fig 4E, Table EV3). Furthermore, METTL16 crosslinking peaks were identified in numerous mRNAs and notably, 93% of these peaks lie within introns, implying that METTL16 binds to a subset of pre‐mRNAs (see, for example, Fig 4F–L).
The RNAs identified by CRAC represent potential METTL16 modification substrates; however, it was shown that the presence of METTL16 on a pre‐mRNA can affect its splicing 41, raising the possibility that some METTL16‐associated RNAs may be bound, but not methylated. To highlight RNAs modified by METTL16, we compared the METTL16 binding sites identified by CRAC to previously identified m6A sites in the transcriptome 7, 8, 41, 43, 69, 70. This revealed that approximately 10, 25 and 83% of the CRAC peaks in mRNAs, lncRNAs and ncRNA, respectively, overlapped with m6A sites, implying that these transcripts are modified by METTL16. It is likely that particularly in the case of mRNAs, this represents an underestimate as the majority of the METTL16 interaction sites lie in introns, which are, compared to mature mRNA sequences, under‐represented in total RNA and therefore also in m6A‐seq data sets. In addition, the relatively low overlap between the available m6A data sets suggests that only a portion of the m6A sites in the transcriptome have been mapped so far. This may be especially relevant for the identification of METTL16‐mediated modifications, as in some m6A mapping approaches the positions reported as modified were determined based on the presence of a GAC or AAC motif that is recognised by METTL3–METTL14, but the mechanism of target recognition by METTL16 may be different.
To confirm the association of METTL16 with the proposed modification substrates as well as additional RNAs identified in the CRAC analyses, we performed RNA immunoprecipitation experiments using extracts from HEK293 cells expressing METTL16‐Flag or the Flag tag and monitored RNA enrichment by quantitative PCR. This confirmed highly significant increases in the amounts of U6 co‐precipitated with METTL16 compared to the control, while the amounts of U4 (which served as a control for non‐specifically precipitated RNAs) in the two eluates were not significantly different (Fig 4M). Furthermore, this revealed significant increases in the amounts of the XIST and MALAT1 lncRNAs, the 7SK ncRNA and eight tested pre‐mRNAs (MAT2A, RBM3, STUB1, ISYNA1, RYR1, PALM2, THSD4 and LGR6) co‐precipitated with METTL16 (Fig 4M), confirming that the targets identified by the CRAC experiments represent bona fide METTL16‐bound RNAs. Notably, for three of the selected pre‐mRNA targets, the crosslinking site of METTL16 was found to contain m6A modifications that were recently shown to be reduced in the absence of METTL16 41. While a reduction in m6A modification at many other sites will likely be due to the role of METTL16 in regulation of splicing of the SAM synthetase MAT2A that affects cellular SAM levels and thereby RNA methylation 41, the identification of METTL16 binding sites corresponding to the methylated positions on the RBM3, STUB1 and ISYNA1 pre‐mRNAs strongly supports these as METTL16‐mediated modifications.
Taken together, we demonstrate METTL16 as an active RNA methyltransferase that is responsible for N 6‐methylation of A43 of the U6 snRNA (Fig 5). While the precise influence of N 6‐methylation of A43 of the U6 snRNA remains unknown, the conservation of the ACm6AGAGA sequence throughout eukaryotes, its interactions with pre‐mRNA 5′ splice sites during the first catalytic step of splicing (Fig 5) and the finding that mutation of the modified position in yeast U6 is lethal 71 strongly suggest that METTL16‐mediated modification of this site is an essential step in human spliceosome assembly or function. The finding that METTL16 targets U6 and other highly structured lncRNAs and ncRNAs suggests that METTL16 may recognise specific secondary structure features in its substrates.
Figure 5. Model of the cellular functions of METTL16.
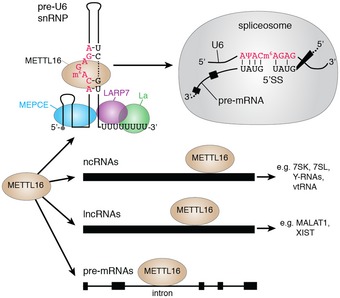
The m6A methyltransferase METTL16 binds diverse cellular RNAs, such as pre‐mRNAs, lncRNAs and several ncRNAs including the U6 snRNA. METTL16 associates with La, LARP7 and the methylphosphate capping enzyme MEPCE and installs U6‐m6A43 during the early stages of U6 snRNP biogenesis on an oligouridylated transcript. During splicing, A43 is involved in base pairing interactions with the 5′ splice site (5′SS) of pre‐mRNAs.
Transcriptome‐wide mapping of the binding sites of METTL16 on cellular RNAs also identified numerous pre‐mRNAs as putative targets (Fig 5). Interestingly, and in contrast to the enrichment of METTL3–METTL14‐mediated m6A modifications in close proximity to stop codons, the majority of METTL16 crosslinking sites were found in introns, implying that the two enzymes are responsible for distinct subsets of m6A modifications that likely have different cellular functions. While the functions of m6A in introns remain relatively unexplored, in Drosophila, it has been shown that an intronic m6A in the Slx (sex‐lethal) pre‐mRNA leads to alternative splicing and a sex bias towards maleness 72. Interestingly, the vast majority (87%) of the pre‐mRNA introns crosslinked by METTL16 are constitutively rather than alternatively spliced, suggesting that METTL16 might have diverse functions in regulating the fate of target RNAs. The identification of an additional m6A methyltransferase alongside the METTL3–METTL14 complex provides important insight into how the landscape of m6A modifications is installed in human cells and a basis for the dissection of the cellular functions of atypical m6A modifications.
Materials and Methods
Human cell culture, generation of stable cell lines and RNAi
For the generation of stable cell lines, the coding sequence of METTL16 (NM_024086.3) was cloned into a pcDNA5 vector for expression of the protein with a C‐terminal His6‐PreScission protease site‐2x Flag (Flag) tag or for expression of the protein with a C‐terminal GFP tag. HEK293 stable cell lines expressing METTL16‐Flag or the Flag tag alone were generated using the Flp‐In T‐Rex system (Thermo Fisher Scientific) according to the manufacturer's instructions. HEK293 Flp‐In T‐Rex and HeLa CCL2 cells were cultured in 1× Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal calf serum (FCS) and 2 mM glutamine at 37°C with 5% CO2. For RNAi, cells were transfected with non‐target siRNAs or siRNAs targeting METTL16 (40 nM; siNT 5′‐CGUACGCGGAAUACUUCGA‐3′, siMETTL16 5′‐ATGGCTGGTATTTCCTCGCAA‐3′) using Lipofectamine RNAiMAX (Thermo Fisher Scientific) reagent according to the manufacturer's instructions and cells were harvested after 5 days.
In vivo crosslinking experiments
UV‐ and PAR‐CRAC experiments were essentially performed as previously described 35, 36, 37. In brief, HEK293 cells expressing the Flag tag or METTL16‐Flag were grown in DMEM and crosslinked at 254 nm (UV‐CRAC), or alternatively were grown in DMEM supplemented with 100 μM 4‐thiouridine for 6 h prior to crosslinking at 365 nm (PAR‐CRAC). Samples were then processed and the obtained sequence reads were mapped to the human genome (GRCh37.p13) as previously described. Peak calling was performed using Piranha 73 with a bin size of 20 bp and a significance threshold of P < 0.05.
Detection of co‐precipitated, crosslinked snRNAs by Northern blotting was performed as previously described 74, 75 using specific probes (U1 5′‐GGTCAGCACATCCGGAGTGC‐3′, U2 5′‐CATTTAATATATTGTCCTCGG‐3′, U4 5′‐CCAGTGCCGACTATATTGC‐3′, U5 5′‐GACTCAGAGTTGTTCCTCTCC‐3′, U6 5′‐GAACGCTTCACGAATTTGCGTGTC‐3′, U6atac 5′‐GGTTAGATGCCACGAAGTAGGTG‐3′).
Immunoprecipitation of complexes
HEK293 stable cell lines were treated with 1 μg/ml doxycycline for 24 h to induce expression of METTL16‐Flag or the Flag tag. Cells were harvested and lysed by sonication in a buffer containing 50 mM Tris–HCl pH 7.4, 200 mM NaCl, 0.5 mM EDTA, cOmplete mini‐EDTA‐free protease inhibitor cocktail (Roche). Glycerol and Triton X‐100 were added to final concentrations of 10 and 0.35%, respectively, and the extract was supplemented with 1.5 mM MgCl2. The resulting lysate was cleared by centrifugation and then incubated with anti‐Flag M2 magnetic beads (Sigma‐Aldrich) for 2 h at 4°C in the presence or absence of 2 μM RNase A. Bound proteins or protein–RNA complexes were eluted from the beads by addition of 250 μg/ml Flag peptide (Sigma‐Aldrich) for 30 min at 4°C. Proteins were precipitated by addition of 20% trichloroacetic acid (TCA), separated by SDS–PAGE and analysed either by mass spectrometry 76 or by Western blotting using antibodies against the La protein (Proteintech, 11720‐1‐AP), LARP7 (Proteintech, 17067‐1‐AP), MEPCE (Proteintech, 14917‐1‐AP) and DHX16 (Bethyl, A301‐537A). Alternatively, RNA was extracted from the eluates using phenol:chloroform:isoamylalcohol (25:24:1) and ethanol‐precipitated. RNA was then either separated on a denaturing 10% polyacrylamide gel and analysed by Northern blotting using the specific probes listed above or alternatively, converted into cDNA using random hexamer primers and Superscript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Co‐precipitated mRNAs/lncRNAs were detected by quantitative RT–PCR in a LightCycler 480 using SYBR Green I Master (Roche) and the following primers: U6_F 5′‐GCTCGCTTCGGCAGCACATAT‐3′, U6_R 5′‐ATATGGAACGCTTCACGAATTTGC‐3′, U4_F 5′‐GCAGTGGCAGTATCGTAGCC‐3′, U4_R 5′‐AAAAATTGCCAGTGCCGACT‐3′, MALAT1_F 5′‐CAGGTGAACATAACAGACTTGGC‐3′, MALAT1_R 5′‐TGACCCTACTGAAGAGCATTGG‐3′, MAT2A_F 5′‐GGAGAAAGCTGACTTGGCTG‐3′, MAT2A_R 5′‐TAGGGCAAGCAGTCATGGAAT‐3′, XIST_F 5′‐CCCTACTGCGCTTTTTGCTG‐3′, XIST_R 5′‐AAATGCAAGGGCGACTGGTA‐3′, 7SK_F 5′‐CCAGGGTTGATTCGGCTGAT‐3′, 7SK_R 5′‐ATACCCTTGACCGAAGACCG‐3′, RMB3_F 5′‐TCGGCATTGAGTGAACCTGT‐3′, RBM3_R 5′‐TAGGTTGGCTGGGTGAGGTA‐3′, STUB1_F 5′‐GTGGATGAGAAGAGGAAGGTGAG‐3′, STUB1_R 5′‐CAGAGGCTGAAAAGGGGCACT‐3′, ISYNA1_F 5′‐CCAGCATCAGCTCCGAGGTA‐3′, ISYNA1_R 5′‐GCTGCACTCCAGGTGGTCAT‐3′, RYR1_F 5′‐GACCTCACTCAGGAAGCAGCATGG‐3′, RYR1_R 5′‐ACCAACGAGTGGCAGAGATGGG‐3′, PALM2_F 5′‐GTCCTTTTCCAAAGATAGCATAGTGGTTAAG‐3′, PALM2_R 5′‐AAAGCATACAGTTAGTAAGTGGCAGAGG‐3′, THSD4_F 5′‐ACAGTCACGAAGGGCACCA‐3′, THSD4_R 5′‐GCTGCATAAGGTCATAGGGCTCA‐3′, LGR6_F 5′‐TGCTGTGGTGTAATGGTTAAGTGTCTAG‐3′, LGR6_R 5′‐CCCTTGTTACAACTCCAACAGATAGGG‐3′.
Microscopy
Expression of METTL16‐GFP was induced in a HEK293 stable cell line by addition of 1 ng/ml doxycycline for 24 h before cells were fixed using 4% paraformaldehyde in PBS for 10 min at room temperature. Immunofluorescence was performed using a METTL16 antibody (Origene, TA504710) as previously described 77. Cells were mounted on coverslips using Vectashield® (Vector labs) and analysed by confocal microscopy using a ConfoCor2 (Carl Zeiss) microscope.
Anti‐m6A Me‐RIP
Isolation of m6A‐containing RNAs was essentially performed as described in Ref. 78; 20 μg of total RNA prepared from HeLa cells that had been treated with non‐target siRNAs or siRNAs against METTL16 as described above was resuspended in 50 mM Tris–HCl pH 7.4 and 750 mM NaCl and denatured by treatment at 70°C for 5 min before incubation on ice. The RNA samples were then incubated with 5 μg of anti‐m6A antibody (Synaptic systems, 202‐211) overnight at 4°C in IP Buffer [50 mM Tris–HCl pH 7.4, 750 mM NaCl, 0.5% Igepal CA‐630, 400 U/ml RiboLock (Thermo Fisher Scientific), cOmplete mini‐EDTA protease inhibitor cocktail (Roche)]. The samples were then incubated for 2.5 h at 4°C with rotation with 15 μl of Protein G Sepharose (GE Healthcare) that had been pre‐equilibrated in IP buffer. After thorough washing steps, co‐precipitated RNAs were extracted using TRI Reagent (Sigma‐Aldrich) and analysed by Northern blotting using probes hybridising to the U6 and U2 snRNAs.
Data availability
The primary high‐throughput sequencing data of the UV‐CRAC and PAR‐CRAC experiments have been submitted to the GEO SRA database and assigned the identifier GSE103948.
Author contributions
ASW and PH performed crosslinking experiments, and JK and MTB carried out bioinformatics analysis. ASW, JK and PH carried out immunoprecipitation experiments followed by Northern blotting, Western blotting and qPCRs. ASW generated stable cell lines and analysed protein localisation. CL and HU performed and analysed the mass spectrometry. KES, CH and MTB designed the study and analysed the data. MTB and CH acquired funding, and KES and MTB wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Review Process File
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (SPP1784: BO3442/2‐1 to M.T.B.; HO4436/2‐1 to C.H.) and the University Medical Center Göttingen (to M.T.B.).
EMBO Reports (2017) 18: 2004–2014
References
- 1. Motorin Y, Helm M (2011) RNA nucleotide methylation. Wiley Interdiscip Rev RNA 2: 611–631 [DOI] [PubMed] [Google Scholar]
- 2. Jackman JE, Alfonzo JD (2013) Transfer RNA modifications: nature's combinatorial chemistry playground. Wiley Interdiscip Rev RNA 4: 35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sloan KE, Warda AS, Sharma S, Entian KD, Lafontaine DLJ, Bohnsack MT (2017) Tuning the ribosome: The influence of rRNA modification on eukaryotic ribosome biogenesis and function. RNA Biol https://doi.org/10.1080/15476286.2016.1259781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helm M, Motorin Y (2017) Detecting RNA modifications in the epitranscriptome: predict and validate. Nat Rev Genet 18: 275–291 [DOI] [PubMed] [Google Scholar]
- 5. Dominissini D, Moshitch‐Moshkovitz S, Schwartz S, Salmon‐Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob‐Hirsch J, Amariglio N, Kupiec M et al (2012) Topology of the human and mouse m6A RNA methylomes revealed by m6A‐seq. Nature 485: 201–206 [DOI] [PubMed] [Google Scholar]
- 6. Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR (2012) Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149: 1635–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen K, Lu Z, Wang X, Fu Y, Luo GZ, Liu N, Han D, Dominissini D, Dai Q, Pan T et al (2015) High‐resolution N(6) ‐methyladenosine (m(6) A) map using photo‐crosslinking‐assisted m(6) A sequencing. Angew Chem Int Ed Engl 54: 1587–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Linder B, Grozhik AV, Olarerin‐George AO, Meydan C, Mason CE, Jaffrey SR (2015) Single‐nucleotide‐resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods 12: 767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carlile TM, Rojas‐Duran MF, Zinshteyn B, Shin H, Bartoli KM, Gilbert WV (2014) Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 515: 143–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, León‐Ricardo BX, Engreitz JM, Guttman M, Satija R, Lander ES et al (2014) Transcriptome‐wide mapping reveals widespread dynamic‐regulated pseudouridylation of ncRNA and mRNA. Cell 159: 148–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li X, Zhu P, Ma S, Song J, Bai J, Sun F, Yi C (2015) Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat Chem Biol 11: 592–597 [DOI] [PubMed] [Google Scholar]
- 12. Dominissini D, Nachtergaele S, Moshitch‐Moshkovitz S, Peer E, Kol N, Ben‐Haim MS, Dai Q, Di Segni A, Salmon‐Divon M, Clark WC et al (2016) The dynamic N(1)‐methyladenosine methylome in eukaryotic messenger RNA. Nature 530: 441–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li X, Xiong X, Wang K, Wang L, Shu X, Ma S, Yi C (2016) Transcriptome‐wide mapping reveals reversible and dynamic N(1)‐methyladenosine methylome. Nat Chem Biol 12: 311–316 [DOI] [PubMed] [Google Scholar]
- 14. Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T (2012) Widespread occurrence of 5‐methylcytosine in human coding and non‐coding RNA. Nucleic Acids Res 40: 5023–5033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang X, Yang Y, Sun BF, Chen YS, Xu JW, Lai WY, Li A, Wang X, Bhattarai DP, Xiao W et al (2017) 5‐methylcytosine promotes mRNA export ‐ NSUN2 as the methyltransferase and ALYREF as an m5C reader. Cell Res 27: 606–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roundtree IA, Evans ME, Pan T, He C (2017) Dynamic RNA modifications in gene expression regulation. Cell 169: 1187–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roost C, Lynch SR, Batista PJ, Qu K, Chang HY, Kool ET (2015) Structure and thermodynamics of N6‐methyladenosine in RNA: a spring‐loaded base modification. J Am Chem Soc 137: 2107–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G et al (2014) N6‐methyladenosine‐dependent regulation of messenger RNA stability. Nature 505: 117–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C (2015) N(6)‐methyladenosine modulates messenger RNA translation efficiency. Cell 161: 1388–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY et al (2016) Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell 61: 507–519 [DOI] [PubMed] [Google Scholar]
- 21. Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C (2017) YTHDF3 facilitates translation and decay of N6‐methyladenosine‐modified RNA. Cell Res 27: 315–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, Qi M, Lu Z, Shi H, Wang J et al (2017) Ythdc2 is an N6‐methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res 27: 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG et al (2013) N6‐methyladenosine in nuclear RNA is a major substrate of the obesity‐associated FTO. Nat Chem Biol 7: 885–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH et al (2013) ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49: 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fustin JM, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, Isagawa T, Morioka MS, Kakeya H, Manabe I et al (2013) RNA‐methylation‐dependent RNA processing controls the speed of the circadian clock. Cell 155: 793–806 [DOI] [PubMed] [Google Scholar]
- 26. Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, Bouley DM, Lujan E, Haddad B, Daneshvar K et al (2014) m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geula S, Moshitch‐Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon‐Divon M, Hershkovitz V, Peer E, Mor N, Manor YS et al (2015) m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 347: 1002–1006 [DOI] [PubMed] [Google Scholar]
- 28. Batista PJ (2017) The RNA modification N6‐methyladenosine and its implications in human disease. Genomics Proteomics Bioinformatics 15: 154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X et al (2014) A METTL3‐METTL14 complex mediates mammalian nuclear RNA N6‐adenosine methylation. Nat Chem Biol 10: 93–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS et al (2014) Mammalian WTAP is a regulatory subunit of the RNA N6‐methyladenosine methyltransferase. Cell Res 24: 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, Jaffrey SR (2016) m(6)A RNA methylation promotes XIST‐mediated transcriptional repression. Nature 537: 369–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang P, Doxtader KA, Nam Y (2016) Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell 63: 306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C et al (2016) Structural basis of N(6)‐adenosine methylation by the METTL3‐METTL14 complex. Nature 534: 575–578 [DOI] [PubMed] [Google Scholar]
- 34. Sergiev PV, Serebryakova MV, Bogdanov AA, Dontsova OA (2008) The ybiN gene of Escherichia coli encodes adenine‐N6 methyltransferase specific for modification of A1618 of 23 S ribosomal RNA, a methylated residue located close to the ribosomal exit tunnel. J Mol Biol 375: 291–300 [DOI] [PubMed] [Google Scholar]
- 35. Sloan KE, Leisegang MS, Doebele C, Ramírez AS, Simm S, Safferthal C, Kretschmer J, Schorge T, Markoutsa S, Haag S, et al (2015) The association of late‐acting snoRNPs with human pre‐ribosomal complexes requires the RNA helicase DDX21. Nucleic Acids Res 43: 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Memet I, Doebele C, Sloan KE, Bohnsack MT (2017) The G‐patch protein NF‐κB‐repressing factor mediates the recruitment of the exonuclease XRN2 and activation of the RNA helicase DHX15 in human ribosome biogenesis. Nucleic Acids Res 45: 5359–5374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haag S, Kretschmer J, Sloan KE, Bohnsack MT (2017) Crosslinking methods to identify RNA methyltransferase targets in vivo . Methods Mol Biol 1562: 269–281 [DOI] [PubMed] [Google Scholar]
- 38. Brown JA, Kinzig CG, DeGregorio SJ, Steitz JA (2016) Methyltransferase‐like protein 16 binds the 3′‐terminal triple helix of MALAT1 long noncoding RNA. Proc Natl Acad Sci 113: 14013–14018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shimba S, Bokar JA, Rottman F, Reddy R (1995) Accurate and efficient N‐6‐adenosine methylation in spliceosomal U6 small nuclear RNA by HeLa cell extract in vitro . Nucleic Acids Res 23: 2421–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bringmann P, Lührmann R (1987) Antibodies specific for N6‐methyladenosine react with intact snRNPs U2 and U4/U6. FEBS Lett 213: 309–315 [DOI] [PubMed] [Google Scholar]
- 41. Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, Conrad NK (2017) The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 169: 824–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sauterer RA, Feeney RJ, Zieve GW (1988) Cytoplasmic assembly of snRNP particles from stored proteins and newly transcribed snRNA's in L929 mouse fibroblasts. Exp Cell Res 176: 344–359 [DOI] [PubMed] [Google Scholar]
- 43. Sun WJ, Li JH, Liu S, Wu J, Zhou H, Qu LH, Yang JH (2016) RMBase: a resource for decoding the landscape of RNA modifications from high‐throughput sequencing data. Nucleic Acids Res 44: D259–D265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galej WP, Wilkinson ME, Fica SM, Oubridge C, Newman AJ, Nagai K (2016) Cryo‐EM structure of the spliceosome immediately after branching. Nature 537: 197–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bertram K, Agafonov DE, Liu WT, Dybkov O, Will CL, Hartmuth K, Urlaub H , Kastner B, Stark H, Lührmann R (2017) Cryo‐EM structure of a human spliceosome activated for step 2 of splicing. Nature 542: 318–323 [DOI] [PubMed] [Google Scholar]
- 46. Sawa H, Abelson J (1992) Evidence for a base‐pairing interaction between U6 small nuclear RNA and 5′ splice site during the splicing reaction in yeast. Proc Natl Acad Sci 89: 11269–11273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sawa H, Shimura Y (1992) Association of U6 snRNA with the 5′‐splice site region of pre‐mRNA in the spliceosome. Genes Dev 6: 244–254 [DOI] [PubMed] [Google Scholar]
- 48. Wassarman DA, Steitz JA (1992) Interactions of small nuclear RNA's with precursor messenger RNA during in vitro splicing. Science 257: 1918–1925 [DOI] [PubMed] [Google Scholar]
- 49. Peebles CL, Zhang M, Perlman PS, Franzen JS (1995) Catalytically critical nucleotide in domain 5 of a group II intron. Proc Natl Acad Sci 92: 4422–4426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keating KS, Toor N, Perlman PS, Pyle AM (2010) A structural analysis of the group II intron active site and implications for the spliceosome. RNA 16: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Agafonov DE, Kastner B, Dybkov O, Hofele RV, Liu WT, Urlaub H, Lührmann R, Stark H (2016) Molecular architecture of the human U4/U6.U5 tri‐snRNP. Science 351: 1416–1420 [DOI] [PubMed] [Google Scholar]
- 52. Zhang X, Yan C, Hang J, Finci LI, Lei J, Shi Y (2017) An atomic structure of the human spliceosome. Cell 169: 918–929 [DOI] [PubMed] [Google Scholar]
- 53. Mroczek S, Dziembowski A (2013) U6 RNA biogenesis and disease association. Wiley Interdiscip Rev RNA 4: 581–592 [DOI] [PubMed] [Google Scholar]
- 54. Singh and Reddy (1989) Gamma‐monomethyl phosphate: a cap structure in spliceosomal U6 small nuclear RNA. Proc Natl Acad Sci 86: 8280–8283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lund E, Dahlberg JE (1992) Cyclic 2′,3′‐phosphates and nontemplated nucleotides at the 3′ end of spliceosomal U6 small nuclear RNA's. Science 255: 327–330 [DOI] [PubMed] [Google Scholar]
- 56. Trippe R, Sandrock B, Benecke BJ (1998) A highly specific terminal uridylyl transferase modifies the 3′‐end of U6 small nuclear RNA. Nucleic Acids Res 26: 3119–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stefano JE (1984) Purified lupus antigen La recognizes an oligouridylate stretch common to the 3′ termini of RNA polymerase III transcripts. Cell 36: 145–154 [DOI] [PubMed] [Google Scholar]
- 58. Pannone BK, Xue D, Wolin SL (1998) A role for the yeast La protein in U6 snRNP assembly: evidence that the La protein is a molecular chaperone for RNA polymerase III transcripts. EMBO J 17: 7442–7453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Karijolich J, Yu YT (2010) Spliceosomal snRNA modifications and their function. RNA Biol 7: 192–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Watkins NJ, Bohnsack MT (2012) The box C/D and H/ACA snoRNPs: key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip Rev RNA 3: 397–414 [DOI] [PubMed] [Google Scholar]
- 61. Wolin SL, Cedervall T (2002) The La protein. Annu Rev Biochem 71: 375–403 [DOI] [PubMed] [Google Scholar]
- 62. Krueger BJ, Jeronimo C, Roy BB, Bouchard A, Barrandon C, Byers SA, Searcey CE, Cooper JJ, Bensaude O, Cohen EA et al (2008) LARP7 is a stable component of the 7SK snRNP while P‐TEFb, HEXIM1 and hnRNP A1 are reversibly associated. Nucleic Acids Res 36: 2219–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jeronimo C, Forget D, Bouchard A, Li Q, Chua G, Poitras C, Thérien C, Bergeron D, Bourassa S, Greenblatt J et al (2007) Systematic analysis of the protein interaction network for the human transcription machinery reveals the identity of the 7SK capping enzyme. Mol Cell 27: 262–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xue Y, Yang Z, Chen R, Zhou Q (2010) A capping‐independent function of MePCE in stabilizing 7SK snRNA and facilitating the assembly of 7SK snRNP. Nucleic Acids Res 38: 360–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Markert A, Grimm M, Martinez J, Wiesner J, Meyerhans A, Meyuhas O, Sickmann A, Fischer U (2008) The La‐related protein LARP7 is a component of the 7SK ribonucleoprotein and affects transcription of cellular and viral polymerase II genes. EMBO Rep 9: 569–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E et al (2003) MALAT‐1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early‐stage non‐small cell lung cancer. Oncogene 22: 8031–8041 [DOI] [PubMed] [Google Scholar]
- 67. Bohnsack MT, Tollervey D, Granneman S (2012) Identification of RNA helicase target sites by UV cross‐linking and analysis of cDNA. Methods Enzymol 511: 275–288 [DOI] [PubMed] [Google Scholar]
- 68. Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N (1996) Requirement for Xist in X chromosome inactivation. Nature 379: 131–137 [DOI] [PubMed] [Google Scholar]
- 69. Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, Haripal B, Zucker‐Scharff I, Moore MJ, Park CY et al (2015) A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev 29: 2037–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ke S, Pandya‐Jones A, Saito Y, Fak JJ, Vågbø CB, Geula S, Hanna JH, Black DL, Darnell JE Jr, Darnell RB (2017) m6A mRNA modifications are deposited in nascent pre‐mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev 31: 990–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Madhani HD, Bordonné R, Guthrie C (1990) Multiple roles for U6 snRNA in the splicing pathway. Genes Dev 4: 2264–2277 [DOI] [PubMed] [Google Scholar]
- 72. Haussmann IU, Bodi Z, Sanchez‐Moran E, Mongan NP, Archer N, Fray RG, Soller M (2016) m6A potentiates Sxl alternative pre‐mRNA splicing for robust Drosophila sex determination. Nature 540: 301–304 [DOI] [PubMed] [Google Scholar]
- 73. Uren PJ, Bahrami‐Samani E, Burns SC, Qiao M, Karginov FV, Hodges E, Hannon GJ, Sanford JR, Penalva LO, Smith AD (2012) Site identification in high‐throughput RNA‐protein interaction data. Bioinformatics 28: 3013–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sloan KE, Bohnsack MT, Watkins NJ (2013) The 5S RNP couples p53 homeostasis to ribosome biogenesis and nucleolar stress. Cell Rep 5: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Haag S, Warda AS, Kretschmer J, Günnigmann MA, Höbartner C, Bohnsack MT (2015) NSUN6 is a human RNA methyltransferase that catalyzes formation of m5C72 in specific tRNAs. RNA 21: 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Atanassov I, Urlaub H (2013) Increased proteome coverage by combining PAGE and peptide isoelectric focusing: comparative study of gel‐based separation approaches. Proteomics 13: 2947–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Warda AS, Freytag B, Haag S, Sloan KE, Görlich D, Bohnsack MT (2016) Effects of the Bowen‐Conradi syndrome mutation in EMG1 on its nuclear import, stability and nucleolar recruitment. Hum Mol Genet 25: 5353–5364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Delatte B, Wang F, Ngoc LV, Collignon E, Bonvin E, Deplus R, Calonne E, Hassabi B, Putmans P, Awe S et al (2016) Transcriptome‐wide distribution and function of RNA hydroxymethylcytosine. Science 351: 282–285 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Review Process File
Data Availability Statement
The primary high‐throughput sequencing data of the UV‐CRAC and PAR‐CRAC experiments have been submitted to the GEO SRA database and assigned the identifier GSE103948.


