Abstract
Nuclear actin regulates transcriptional programmes in a manner dependent on its levels and polymerisation state. This dynamics is determined by the balance of nucleocytoplasmic shuttling, formin‐ and redox‐dependent filament polymerisation. Here, using Xenopus egg extracts and human somatic cells, we show that actin dynamics and formins are essential for DNA replication. In proliferating cells, formin inhibition abolishes nuclear transport and initiation of DNA replication, as well as general transcription. In replicating nuclei from transcriptionally silent Xenopus egg extracts, we identified numerous actin regulators, and disruption of actin dynamics abrogates nuclear transport, preventing NLS (nuclear localisation signal)‐cargo release from RanGTP–importin complexes. Nuclear formin activity is further required to promote loading of cyclin‐dependent kinase (CDK) and proliferating cell nuclear antigen (PCNA) onto chromatin, as well as initiation and elongation of DNA replication. Therefore, actin dynamics and formins control DNA replication by multiple direct and indirect mechanisms.
Keywords: actin, cyclin‐dependent kinase, DNA replication, formin, nuclear transport
Subject Categories: Cell Adhesion, Polarity & Cytoskeleton; DNA Replication, Repair & Recombination
Introduction
Mounting evidence suggests that nuclear actin is an important regulator of transcription in mammalian cells (Huet et al, 2012). Monomeric actin binds RNA polymerase complexes (Rando et al, 2002) and promotes transcription by all three RNA polymerases (Hofmann et al, 2004; Hu et al, 2004; Philimonenko et al, 2004). In epithelial cells, loss of nuclear actin triggers quiescence by disrupting binding of RNA polymerases to their transcription sites (Spencer et al, 2011). Nuclear actin is also involved in co‐repressor eviction from promoters (Huang et al, 2011), and it can bind to gene regulatory regions (Miyamoto et al, 2011, 2013). The most studied transcriptional roles of nuclear actin are in the serum response, which is regulated by dynamics of actin nuclear transport and polymerisation. The involvement of nuclear actin dynamics in serum response factor (SRF)‐dependent transcription is complex and remains incompletely understood. Nuclear actin levels are generally low and are regulated by active transport of monomers between the nucleus and cytoplasm (Stüven et al, 2003; Dopie et al, 2012), and by polymerisation (Vartiainen et al, 2007). Monomeric actin promotes export of the SRF cofactor MAL/MRTF, extinguishing SRF (Vartiainen et al, 2007). Yet both nuclear actin polymerisation, triggered by nuclear formin mDia2 (Baarlink et al, 2013), and depolymerisation, brought about via Met44 oxidation by MICAL‐2 (Lundquist et al, 2014), can induce SRF‐dependent transcription by depleting nuclear monomeric actin.
The form of nuclear actin remains poorly characterised due to difficulties in staining nuclear actin with phalloidin (Grosse & Vartiainen, 2013) and the large amounts of actin in the cytoplasm. Polymeric nuclear actin is observed in several pathologies (de Lanerolle, 2012) and can be induced by various manipulations, including heat shock and DMSO treatment (Sanger et al, 1980; Iida et al, 1986); increasing nuclear actin concentrations (Stüven et al, 2003; Kalendová et al, 2014); activating nuclear mDia2 (Baarlink et al, 2013); overexpressing NLS‐tagged IQGAP1 (Johnson et al, 2013) or supervillin (Serebryannyy et al, 2016); or knockdown of MICAL‐2 (Lundquist et al, 2014). In specific settings, like the giant quiescent nuclei of amphibian oocytes, a filamentous actin network has scaffolding functions and links nuclear pore complexes to the nuclear interior (Clark & Rosenbaum, 1979; Gounon & Karsenti, 1981; Kiseleva et al, 2004; Feric & Brangwynne, 2013). In somatic cells, sub‐populations of nuclear actin have distinct mobilities, suggesting existence of polymeric forms (McDonald et al, 2006; Dopie et al, 2012), and several regulators of actin polymerisation have been found in nuclei (Wu et al, 2006; Yoo et al, 2007; Khoudoli et al, 2008; Obrdlik & Percipalle, 2011; Miyamoto et al, 2013; Dopie et al, 2015). However, dynamic nuclear actin polymerisation has only been described upon serum stimulation of mouse fibroblasts (Baarlink et al, 2013).
While roles of nuclear actin dynamics in transcription are well established, less is known about its involvement in other nuclear processes. Monomeric actin is also a functional component of the INO80 chromatin remodelling complex (Kapoor et al, 2013), which promotes both transcription and DNA replication (Conaway & Conaway, 2009; Kurat et al, 2017). Interestingly, replication stress strongly stimulates nuclear import of actin and actin regulators (Johnson et al, 2013).
Whether nuclear actin dynamics is involved in DNA replication is not yet known. In the first step of DNA replication, licensing, pre‐replication complexes (pre‐RC) are assembled via the origin recognition complex (ORC). ORC, Cdc6 and Cdt1 recruit minichromosome maintenance (MCM) proteins 2–7, the main component of the replicative helicase. Pre‐RC assembly is inhibited by CDK activity and does not require a nuclear envelope. In the second step, pre‐RC are converted to pre‐initiation complexes (pre‐IC), which contain Cdc45, the sliding clamp PCNA and the replicative DNA polymerases. This step requires an intact nuclear envelope and activity of two key kinases, Cdc7 and CDK (for review, see Labib, 2010).
Many of these mechanisms were identified by studying DNA replication in nuclei formed in Xenopus egg extracts (XEE; Arias & Walter, 2004), a system that has also been instrumental in identification of nuclear assembly pathways (Hetzer et al, 2005). Unlike Xenopus oocytes, which are quiescent but transcriptionally active, Xenopus eggs have undergone meiotic maturation, during which they acquire replication competence and transcription becomes repressed. Egg activation by fertilisation or calcium mobilisation triggers onset of rapid embryonic cell cycles that consist entirely of successions of S‐phase and mitosis without intervening G1 or G2 phases, and in the total absence of transcription. XEE are undiluted extracts from calcium‐activated eggs, and recapitulate early embryonic cell cycles in vitro upon the addition of demembranated sperm nuclei. Nuclei assemble autonomously before replicating, and resemble somatic cell nuclei in most respects, although they are transcriptionally silent and do not have a G1 phase. Highly concentrated nucleoplasmic extracts (NPE) of nuclei formed in XEE can promote DNA replication in the absence of a nuclear envelope (Walter et al, 1998), suggesting that nuclear transport is required to establish a threshold concentration of replication‐promoting factors, possibly including CDK.
Here, using XEE and human somatic cells, we show that actin dynamics is required for nuclear transport and initiation of DNA replication. Nuclear actin binds RanGTP–importin complexes, affecting importin–cargo release. Furthermore, nuclear formin activity promotes chromatin loading and activation of DNA replication factors, as well as replication elongation.
Results
Nuclear actin dynamics during the cell cycle
To analyse the behaviour of nuclear actin during the cell cycle in human cells, we generated a tool for live nuclear actin imaging. We modified a camelid antibody‐based probe, actin chromobody (Chromotek®), by appending a nuclear localisation signal (NLS—see Materials and Methods). An identical tool was independently developed recently (Plessner et al, 2015). We concurrently followed the DNA replication programme using a second chromobody to visualise endogenous PCNA, whose patterns change during S‐phase progression (Burgess et al, 2012). We found that in U2OS cells, a dynamic network of actin filaments formed in most early G1 nuclei (Movie EV1; Fig 1A). Using confocal imaging and long exposures, we found that these G1 actin filaments could be stained with phalloidin (Fig 1A), which could also detect a G1 nuclear actin network in cells without any ectopic chromobody expression (Fig 1B). Chromobody‐visualised filaments disassembled after an average of 200 min, in mid‐late G1 (Fig 1C).
Figure 1. Perturbing nuclear actin dynamics inhibits DNA replication in human somatic cells.
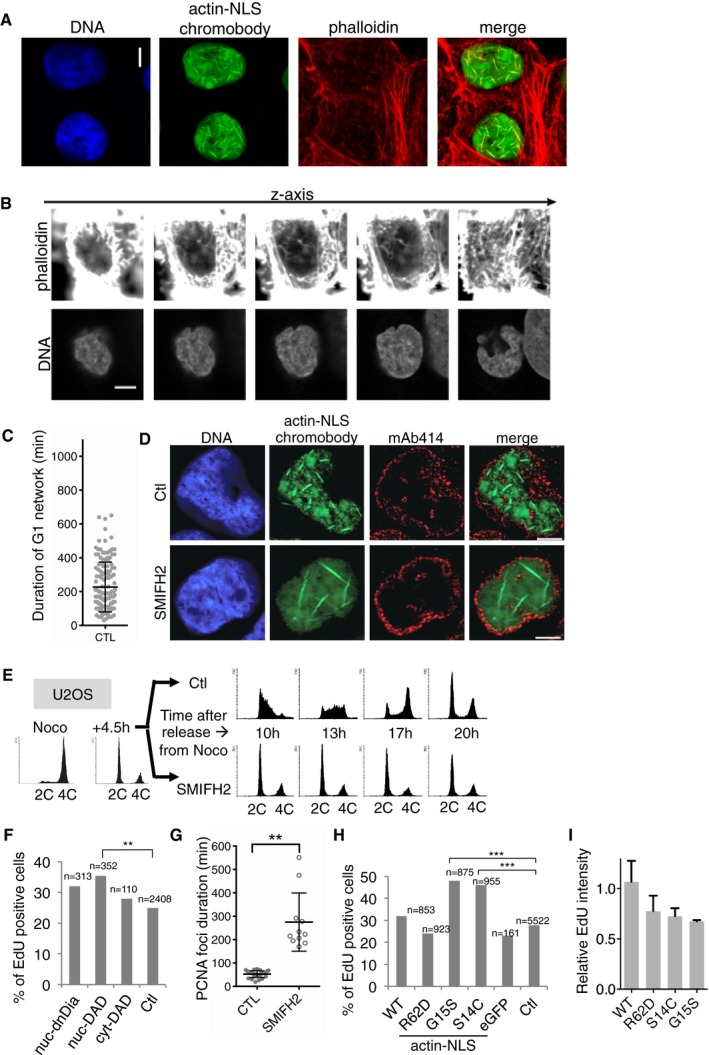
- Early G1 U2OS cells expressing actin‐NLS chromobody co‐stained with phalloidin and DAPI (DNA). Scale bar, 5 μm.
- Serial confocal planes of an early G1 U2OS cell fixed with glutaraldehyde and stained with phalloidin and DAPI. Scale bar, 5 μm.
- Duration of early G1 nuclear actin network (mean ± SD, n = 135 cells from three independent experiments).
- Interphase U2OS cells expressing actin‐NLS chromobody, treated with DMSO (Ctl) or SMIFH2 (50 μM) for 2 h, stained with mAb414 and DAPI. Scale bar, 5 μm.
- FACS analysis of U2OS cells synchronised in M‐phase, released into G1 and treated with DMSO (Ctl) or SMIFH2 (50 μM).
- Quantification of EdU incorporation from two independent experiments after a 1‐h pulse in U2OS cells expressing formin constructs (**P‐value < 0.001; Student's t‐test).
- Duration of PCNA foci in U2OS cells expressing PCNA chromobody, control or SMIFH2‐treated (each dot represents the mean of 5–7 foci per cell, mean ± SD of 29 and 11 cells, respectively, per condition from two independent experiments; **P‐value < 0.001; Student's t‐test).
- Quantification of EdU incorporation from two independent experiments after a 1‐h pulse in U2OS cells expressing actin‐NLS mutants (***P‐value < 0.0001; Student's t‐test).
- Quantification (mean ± SEM) of EdU intensity from (H) normalised to the non‐transfected cells.
Next, in living cells, we investigated whether formins are involved in the regulation of nuclear actin dynamics. Specific formin inhibition with SMIFH2 induced stabilisation of long nuclear actin filaments in most cells (Fig 1D, Movies EV2 and EV3). Similar effects of SMIFH2 on actin filaments have been seen in a defined system with purified components in vitro (Rizvi et al, 2009), suggesting that formin inhibition prevents nucleation of new filaments but allows elongation of existing filaments.
Actin dynamics is required for initiation and progression of DNA replication
Since nuclear actin filaments were disassembled prior to S‐phase, we surmised that their stabilisation by formin inhibition might affect DNA replication. To test this, we synchronised U2OS or HeLa cells in M‐phase and released into mid‐G1 before adding SMIFH2, and analysed entry into S‐phase by flow cytometry (FACS). S‐phase entry was dose dependently inhibited by SMIFH2 (Figs 1E and EV1A and B). We then altered endogenous formin activity by expressing a GFP‐tagged mDia2 diaphanous autoregulatory domain (DAD), either specifically in the nucleus (nuc‐DAD) or in the cytoplasm (cyt‐DAD), or a nuclear‐localised dominant negative mDia2 (nuc‐dnDia; Baarlink et al, 2013). These constructs differentially affect formin activity and function: DAD overactivates endogenous mDia2, whereas dnDia2 inhibits it. Nuc‐DAD increased the fraction of cells in S‐phase significantly (Fig 1F), whereas cyt‐DAD did not, implying that over‐activating nuclear formins lengthens S‐phase. Nuc‐dnDia also slightly increased the S‐phase fraction but this was not statistically significant (P = 0.08). These results suggest that interfering with nuclear formin activity might impede progression of DNA replication. To confirm this, we analysed the effects of SMIFH2 on dynamics of PCNA foci. In cells that were in S‐phase, PCNA foci persisted upon SMIFH2 addition or nuc‐DAD expression (Fig 1G; Movies EV4 and EV5). Arrested PCNA foci might indicate replication fork stalling, which can generate DNA damage. Indeed, immunofluorescence analysis showed that nuc‐DAD expression or SMIFH2 treatment strongly increased the fraction of cells with γ‐H2AX foci. Importantly, 100% of cells synchronised in S‐phase and treated with SMIFH2 were γ‐H2AX‐positive (Fig EV1C and D), indicative of S‐phase arrest.
Figure EV1. Blocking formins inhibits DNA replication and general transcription and causes replication stress in human cells.
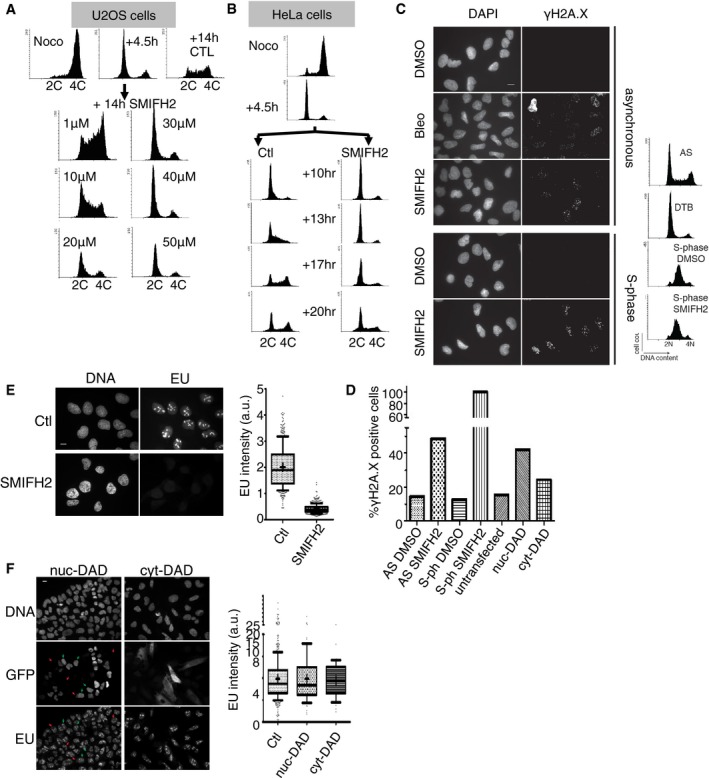
- FACS analysis of M‐phase‐synchronised U2OS cells, released into G1 in the presence of increasing concentrations of SMIFH2. Cells were collected when control cells were in S‐phase (+14 h).
- FACS analysis of HeLa cells, synchronised in M‐phase and released into G1 as in Fig 1E, and treated with DMSO (Ctl) or SMIFH2, collected at the time points indicated.
- Asynchronous (AS) or double‐thymidine block (DTB) S‐phase‐synchronised U2OS cells were treated for 2 h with DMSO, bleomycin (Bleo) or SMIFH2, and stained for γH2A.X. Corresponding FACS profiles are shown. Scale bar, 5 μm.
- Quantification of γH2A.X‐positive cell number from experiment presented in (C), and in cells transiently transfected with mDia2 nuc‐DAD or cyt‐DAD constructs. Cells with > 10 foci were considered positive.
- Left: Immunofluorescent images of control or SMIFH2‐treated (1 h pre‐treatment, 1 h co‐incubation) U2OS cells pulsed for 1 h with EU. Scale bar, 5 μm. Right: Quantification of EU signal intensity (n > 400). Crosses, mean values; whiskers, 10th and 90th percentiles.
- Left: Immunofluorescent images of U2OS cells transfected with GFP‐mDia2 nuc‐DAD or cyt‐DAD constructs, pulsed for 1 h with EU. Right: Quantification of EU incorporation (n > 100, n = 61 and n = 47, respectively). Scale bar, 5 μm. Red arrows: examples of non‐transfected cells; green arrows: examples of GFP‐expressing (transfected) cells. Positions of arrows are the same in both panels. Graphs: crosses, mean values; whiskers, 10th and 90th percentiles.
Source data are available online for this figure.
To test whether altering nuclear actin dynamics without interfering with formins also affected DNA replication, we expressed nuclear‐localised actin: wild‐type (WT), or mutants S14C and G15S, which favour polymerisation, or the polymerisation‐defective R62D (Appendix Fig S1). Nuclear WT and R62D actin expression led to a non‐significant increase or decrease, respectively, in the S‐phase fraction. In contrast, nuclear S14C and G15S mutants strongly increased the number of cells in S‐phase (Fig 1H). Interestingly, cells expressing high levels of S14C and G15S also had significantly lower EdU signal intensity (Fig 1I). Thus, altering nuclear actin dynamics, derepressing nuclear formins, or inhibiting formins all impede S‐phase progression.
Since entry into S‐phase requires E2F‐dependent transcription of replication factors, we next analysed whether interfering with formin activity would affect global transcription, as assessed by 5‐ethynyl‐uridine (EU) incorporation into newly synthesised RNA. Indeed, SMIFH2 totally abolished general transcription (Fig EV1E), whereas neither nuc‐DAD nor cyt‐DAD constructs had significant effects (Fig EV1F). This suggests that the inhibition of global transcription by SMIFH2 might be responsible for the block in S‐phase entry, but the observed defects in S‐phase progression upon expression of nuclear formin mutants depend on a different mechanism.
Inhibiting formins disrupts importin‐dependent nuclear transport
While SMIFH2 inhibits transcription, we also noticed that the nuclei were often smaller and misshapen after a 4‐h treatment or longer (Fig 2A and B). This suggested that actin dynamics might be required for nuclear growth or transport, both of which are essential for DNA replication. To investigate this further, we used a well‐established nuclear translocation assay.
Figure 2. Formin inhibition abolishes nuclear import.
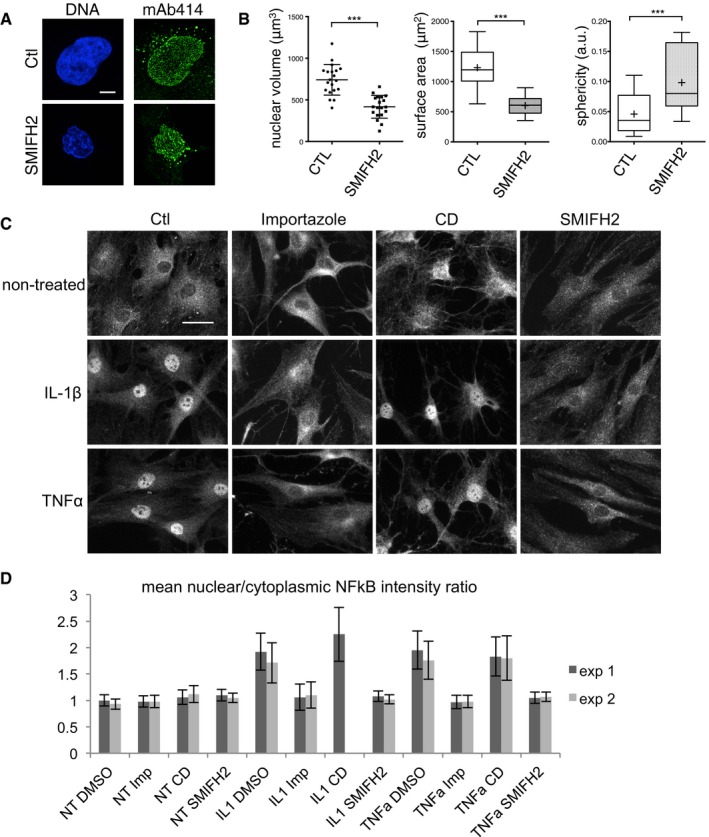
- Confocal planes of nuclei of cells treated with DMSO (Ctl) or SMIFH2 (50 μM) for 4 h, stained with mAb414 and DAPI (DNA). Scale bar, 5 μm.
- Characterisation of nuclear morphology from (A) (mean ± SD of 20 cells from two independent experiments; ***P‐value < 0.0001; Student's t‐test). Scatter plot, lines are mean ± SD; box plots, whiskers: min and max values, crosses are means.
- Immunofluorescence images of RA‐FLS fibroblasts, treated for 1 h with DMSO (Ctl), importazole (50 μM), CytD (40 μM) or SMIFH2 (50 μM), subsequently stimulated or not with IL‐1β or TNF‐α, stained for NF‐κB. Scale bar, 20 μm.
- Quantification of the data presented in (C). Mean nuclear/cytoplasmic NF‐κB intensity ratio (± SD) of two independent experiments using two different fibroblast sources; n ≥ 400 for each condition; CytD sample was lost in exp 2.
In primary human fibroblasts, the p65 subunit of the ubiquitously expressed nuclear factor‐κB (NF‐κB) translocates to the nucleus upon stimulation with interleukin‐1 beta (IL‐1β) or tumour necrosis factor alpha (TNF‐α), which cause degradation of the cytoplasmic inhibitor IκB, releasing the NLS of NF‐κB. Cytokine treatment therefore bypasses effects of cytoplasmic actin disruption on cell shape and NF‐κB regulation, which also impact on IκB (Németh et al, 2004; Sero et al, 2015). This allowed us to study effects of formin inhibition on NF‐κB nuclear translocation itself. We also tested cytochalasin D (CytD), which binds the barbed (plus)‐end of F‐actin, arresting both polymerisation and depolymerisation (Schliwa, 1982). As a positive control for inhibition of nuclear transport, we used importazole, which distorts Ran–importin‐β interactions, thereby inhibiting nuclear accumulation of importin and cargo (Soderholm et al, 2011). Cells were treated with drugs for 1 h, followed by 30‐min cytokine stimulation. As expected, importazole strongly reduced NF‐κB nuclear translocation (Fig 2C and D). CytD had no effect, whereas SMIFH2 did not significantly change cell or nuclear shape, but almost completely abolished NF‐κB nuclear translocation (Fig 2C and D).
Collectively, these data suggest that general nuclear transport is acutely sensitive to reduction in formin activity.
Actin dynamics in Xenopus egg extracts
To further characterise the defects in nuclear transport and DNA replication upon disruption of nuclear actin dynamics, we switched to Xenopus egg extracts (XEE). The advantage of this system is that the nuclear processes can be studied in a context that is independent of both transcription and cytoskeleton–environment interactions. First, to characterise nuclear actin regulators in this system, we analysed the combined nucleoskeleton and chromatin proteome of nuclei assembled in XEE by label‐free high‐resolution mass spectrometry. To identify proteins that associate with this fraction independently of DNA replication, we compared replicating nuclei with non‐replicating nuclei assembled in the presence of purvalanol A (PA) to inhibit CDKs (Fig EV2A). We chose PA since it has high affinity for both CDK1 and CDK2 (Gray et al, 1998) and completely abolishes DNA replication in XEE (Echalier et al, 2012).
Figure EV2. The proteome of replicating nuclei in XEE .
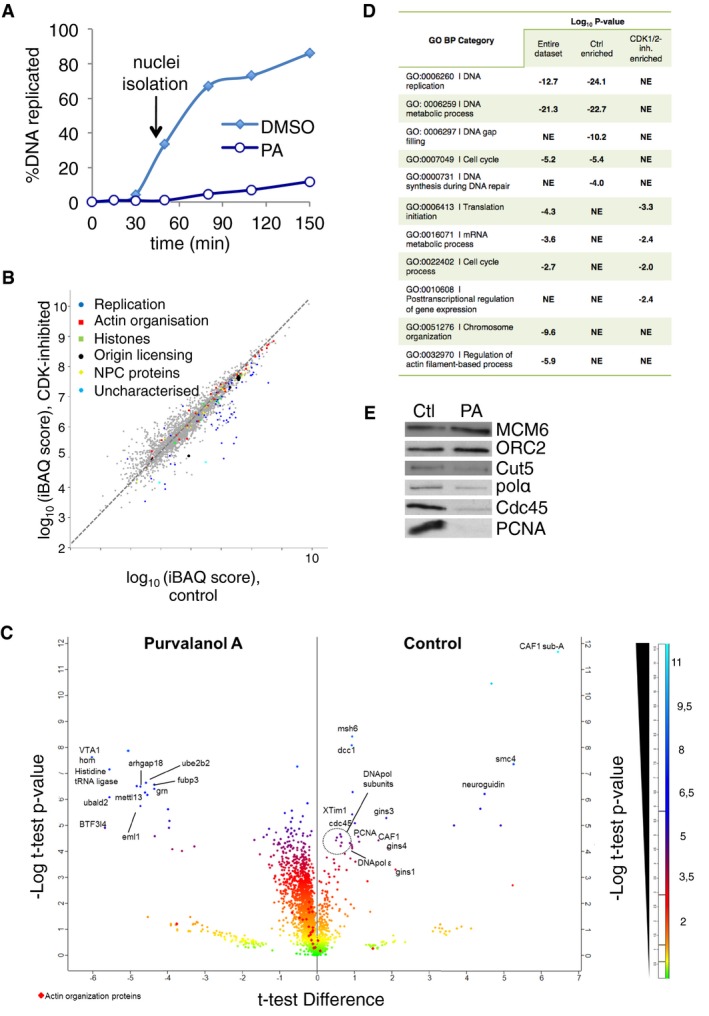
- Replication time course of sperm chromatin in control and purvalanol A (PA)‐treated egg extracts, with nuclei isolated for MS analysis at 50 min.
- Graphical representation of the identified proteome with relative quantitation data (mean values from three replicates). Full dataset, Dataset EV1.
- Volcano plot combining the fold change between control and CDK‐inhibited conditions with their log10 P‐values (Student's t‐test). The most significantly differentially abundant proteins are highlighted.
- GO analysis using DAVID, showing the most highly enriched GO biological processes in each condition (full GO analysis, Dataset EV2). NE, not enriched.
- Western blots of chromatin fractions from control and PA‐treated nuclei used for MS analysis.
We identified 2610 non‐redundant proteins (Fig EV2B and C; Appendix Fig S1B, Dataset EV1). Enriched biological processes included DNA metabolism, chromatin organisation and regulation of actin polymerisation (Fig EV2D; Dataset EV2). Specifically, we identified 55 actin regulators (Appendix Table S1), including actin filament nucleating factors such as formins and the Arp2/3 complex. Three formin homologues, diaph1 (mDia1), diaph3 (mDia2) and formin 2, were additionally found by homology searching using a database of the highly related Xenopus tropicalis (Dataset EV1, Appendix Table S2). These actin regulators did not require CDK activity for localisation to the insoluble fraction of nuclei, unlike chromatin recruitment of proteins involved in DNA replication, DNA repair and the S‐phase checkpoint (Fig EV2B–E). Immunofluorescence analysis confirmed that many actin polymerisation regulators localised to replicating nuclei (Fig 3A). We also observed that actin factors were loaded onto chromatin at the pre‐RC formation stage of DNA replication (Fig 3B), while nuclear actin was mostly insoluble (Fig 3C). The absence of tubulin (Fig 3C and Dataset EV1) confirmed the purity of our sample preparations.
Figure 3. Dynamic nature of nuclear actin in Xenopus egg extract.
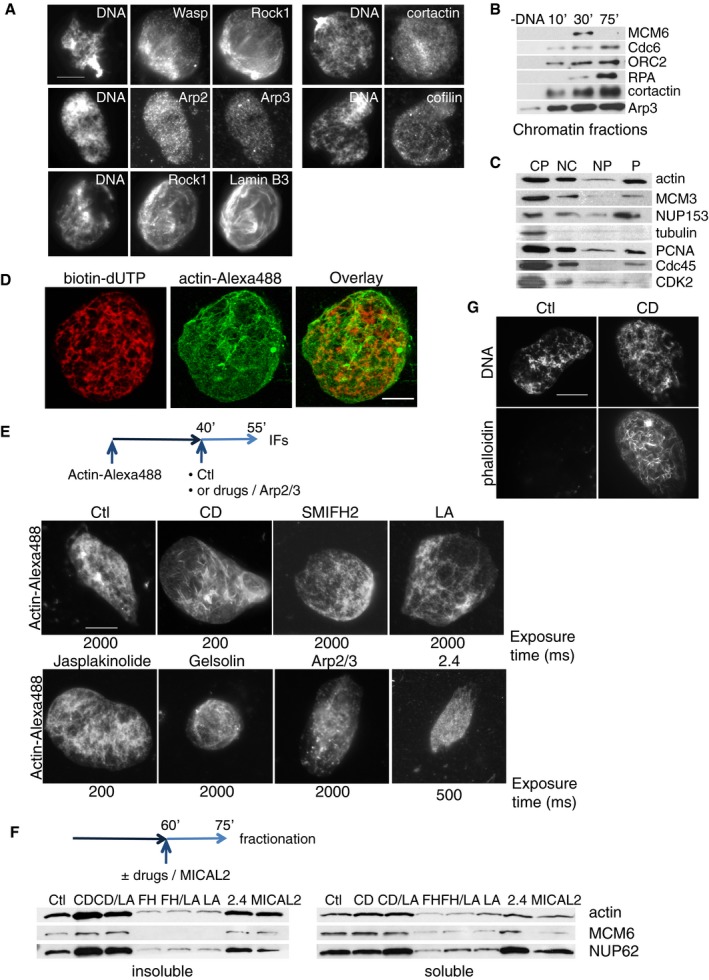
- Immunofluorescence images of the actin regulators indicated, analysed 60 min after sperm head addition. Scale bar, 10 μm.
- Western blot analysis of the indicated replication and actin factors loaded onto chromatin at the indicated time points, in control conditions.
- Western blot analysis of cytoplasm (CP), whole nuclear (NC), nucleoplasmic (NP) and insoluble (P) fraction at 60‐min time point during DNA replication, probed with antibodies against proteins indicated.
- Confocal images a control nucleus, formed in the presence of actin–Alexa Fluor 488 and stained for incorporated biotin‐dUTP. Scale bar, 10 μm.
- Extract was supplemented with sperm nuclei and actin–Alexa Fluor 488; at 40 min, indicated drugs or Arp2/3 and VCA domain of WASP were added, and nuclei were analysed for fluorescent actin at 55 min. Long exposure time (2,000 ms) was needed to visualise nuclear actin in all conditions with the exception of CytD, jasplakinolide (exposure time 200 ms) and the formin inhibitor 2.4 (500 ms). Scale bar, 10 μm.
- Nuclei were allowed to form for 60 min before drugs (CytD, CD; SMIFH2, FH; latrunculin A, LA; 2.4 compound) or MICAL2 recombinant protein was added, then purified at 75 min. Soluble and insoluble nuclear fractions were blotted for the proteins indicated. Equal number of nuclei was used in each condition.
- Extract was supplemented with sperm nuclei; at 45 min, CytD (CD) was added and nuclei were analysed at 60 min and stained with phalloidin. Scale bar, 10 μm.
Source data are available online for this figure.
To visualise nuclear actin directly as well as the effects of treatments modifying actin dynamics, we added trace concentrations (that are negligible compared with endogenous nuclear actin concentrations; see Materials and Methods) of fluorescently labelled actin protein to the extracts, prior to sperm chromatin addition. This revealed both diffuse and patterned intra‐nuclear staining (Fig 3D and E), but, in contrast to a previous study (Krauss et al, 2003), we did not observe phalloidin‐stained nuclear filaments, consistent with their absence in cells in S‐phase.
Next, we investigated the effects of recombinant actin regulatory proteins, as well as different drugs that modify actin dynamics on nuclear actin in XEE (Figs 3E and F, and EV3A). XEE contain around 50 mg/ml protein, of which 5–10% is actin. Thus, there is at least 100 μM actin in extracts. Since effective drug concentrations depend on adsorption, distribution and metabolism, we expected that several hundred micromolar concentrations of actin drugs would be required to elicit phenotypic effects in this system. In contrast, in cells, due to active import, drugs can routinely attain 1,000‐fold higher concentrations than in the medium (Martinez Molina et al, 2013).
Figure EV3. Actin dynamics is required for DNA replication.
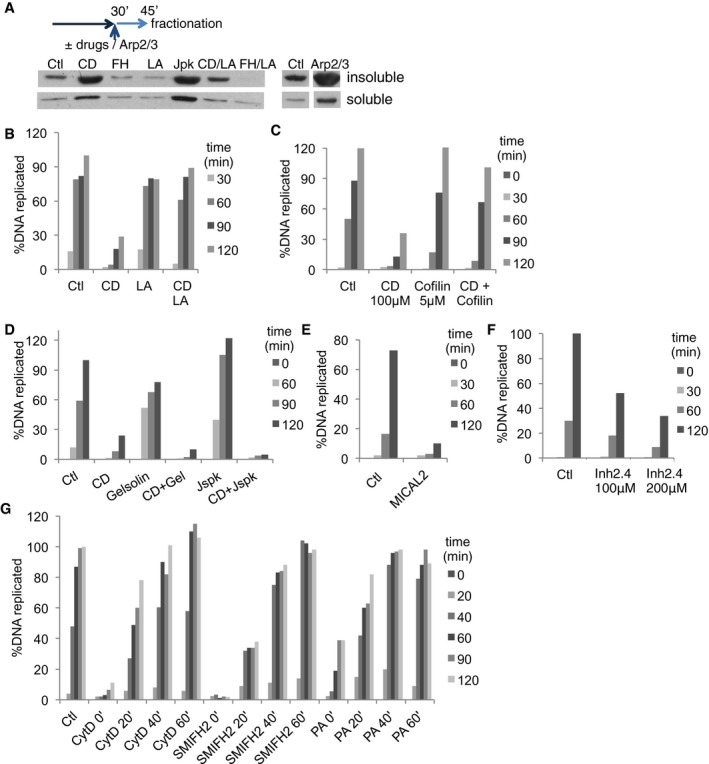
-
ANuclei were allowed to form for 30 min before drugs (CytD, CD; SMIFH2, FH; latrunculin A, LA; jasplakinolide, Jpk; CytD and latrunculin A, CD/LA; SMIFH2 and latrunculin A, FH/LA) or Arp2/3 recombinant protein (in combination with VCA domain of WASP) was added, then purified at 45 min. Soluble and insoluble nuclear fractions were blotted for actin. Equal number of nuclei was used in each condition.
-
B–FDNA replication assessed in control extract, or extracts supplemented with CytD (CD), with or without latrunculin A (LA) (B); CytD (CD) with or without cofilin (C); CytD (CD), gelsolin, CytD and gelsolin (CD + Gel), jasplakinolide (Jspk), or CytD and jasplakinolide (CD + Jspk) (D); recombinant MICAL2 protein (E), or formin inhibitor 2.4 (F). Each panel is representative of multiple experiments: (B) 5 experiments; (C–F) 2 experiments.
-
GDNA replication analysed in control extract, or extracts supplemented with CytD, SMIFH2 or PA that was added at the time points indicated. Representative of two independent experiments.
Source data are available online for this figure.
SMIFH2 reduced levels of nuclear actin and control proteins in a similar manner to latrunculin A, which potently binds actin monomers and impedes filament assembly, while an unrelated formin inhibitor, compound 2.4 (Gauvin et al, 2009; Baarlink et al, 2013), had the opposite effect (Fig 3F). Therefore, different modes of formin inhibition may have contrasting effects on actin dynamics (of note: like phalloidin, 2.4 cannot penetrate into living cells and thus could not be used in the experiments described above). Cytochalasin D, jasplakinolide (Jpk) and purified Arp2/3 plus GST‐WASP‐VCA proteins or recombinant MICAL‐2 protein (Lee et al, 2013) all strongly increased total levels of nuclear actin, which was mostly insoluble (Figs 3F and EV3A). Indeed, CytD caused the formation of stable nuclear actin filaments that were readily visualised with phalloidin (Fig 3G). Interestingly, the effects of CytD were partially reversed by latrunculin A, which reduced nuclear actin levels (Figs 3F and EV3A). These data suggest that nuclear actin polymerisation, depolymerisation and nucleocytoplasmic shuttling exist in a dynamic equilibrium in XEE.
Actin dynamics is required for DNA replication
We next analysed the effects of manipulating actin dynamics on DNA replication in XEE. The absolute percentage of added sperm DNA replicated at each time point was quantified by radioactive nucleotide incorporation (Blow & Laskey, 1986 and Materials and Methods).
Increasing both absolute and polymeric nuclear actin with CytD inhibited DNA replication (Fig 4A), while latrunculin A had no effect on replication alone, and indeed reversed the effect of CytD (Fig EV3B). Recombinant cofilin, which dissociates actin monomers, also rescued the block to replication caused by CytD (Fig EV3C). Alone, Jpk or recombinant gelsolin (that binds to the barbed end of filaments, arresting their dynamics and severs filaments, increasing the free‐end concentration) had no effect on DNA replication. However, combining Jpk or gelsolin with lower CytD concentrations synergised to inhibit DNA replication (Fig EV3D). Recombinant MICAL protein also abolished DNA replication (Fig EV3E). Thus, treatments that promote increased nuclear actin levels and/or polymerisation all prevent DNA synthesis in XEE.
Figure 4. DNA replication requires actin dynamics in XEE .
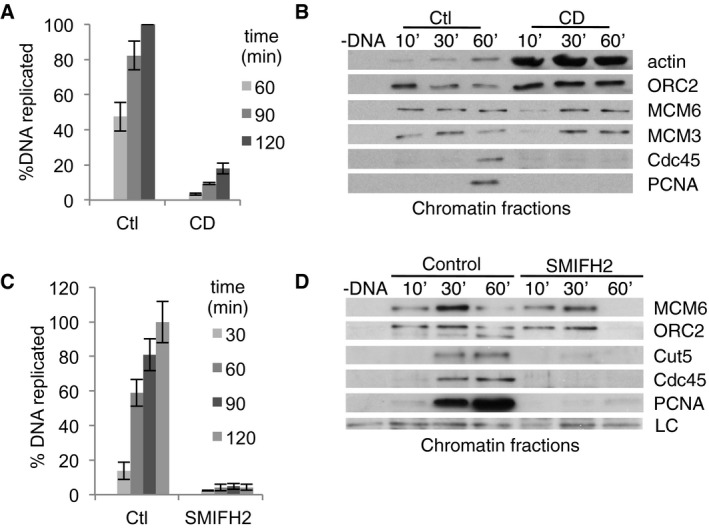
- Chromosomal DNA replication determined by 33P‐dCTP incorporation assay in control conditions or with CytD (CD); mean ± SEM of eight independent experiments.
- Chromatin loading of pre‐RC and pre‐IC factors in control conditions (Ctl) or with CytD (CD).
- DNA replication assays in control (Ctl) or formin‐inhibited extracts: SMIFH2 (500 μM; mean ± SEM of eight independent experiments).
- Chromatin loading of pre‐RC and pre‐IC factors in control conditions or in the presence of SMIFH2 (500 μM). LC, loading control (unspecific band).
Source data are available online for this figure.
We studied the step at which DNA replication was arrested by purifying chromatin from successive time points and Western blotting for several components of pre‐replication complexes (ORC2, MCM3 and MCM6) or pre‐initiation complexes (Cdc45, PCNA). While pre‐RC components were present on the chromatin in both control and CytD‐treated extracts, CytD prevented assembly of the pre‐IC (Fig 4B).
We next investigated the effects of formin inhibition. SMIFH2 and the unrelated, highly specific inhibitor 2.4 (Gauvin et al, 2009) caused dose‐dependent inhibition of replication (Figs 4C and EV3F), providing further evidence for formin involvement in DNA replication and suggesting that differences between these two inhibitors are quantitative rather than qualitative. SMIFH2, like CytD, prevented conversion of pre‐RC to pre‐IC (Fig 4D). Consistent with these results, adding either CytD or SMIFH2 at different time points during ongoing DNA replication had a similar effect to inhibition of CDK with PA (Fig EV3G): inhibition of DNA synthesis if the inhibitors were added before the pre‐IC formation, and the lesser the effect, the later the inhibitors were introduced. Thus, disruption of actin dynamics or inhibition of formins prevents DNA replication in a mechanism independent of transcription.
Actin dynamics is required for NPC formation and nuclear transport
In XEE, whereas pre‐RC formation is independent of a nuclear envelope, the latter is required to efficiently assemble pre‐ICs (Krasinska & Fisher, 2009), and DNA replication requires active transport through nuclear pore complexes (NPC; Cox, 1992). In XEE, inhibiting actin dynamics prevented DNA decondensation and growth of nuclei (Figs 5A and EV4A; Appendix Fig S2), similar to the misshapen nuclear phenotype that we observed in human somatic cells upon SMIFH2 treatment, which also blocked nuclear transport.
Figure 5. NPC formation and function requires actin dynamics.
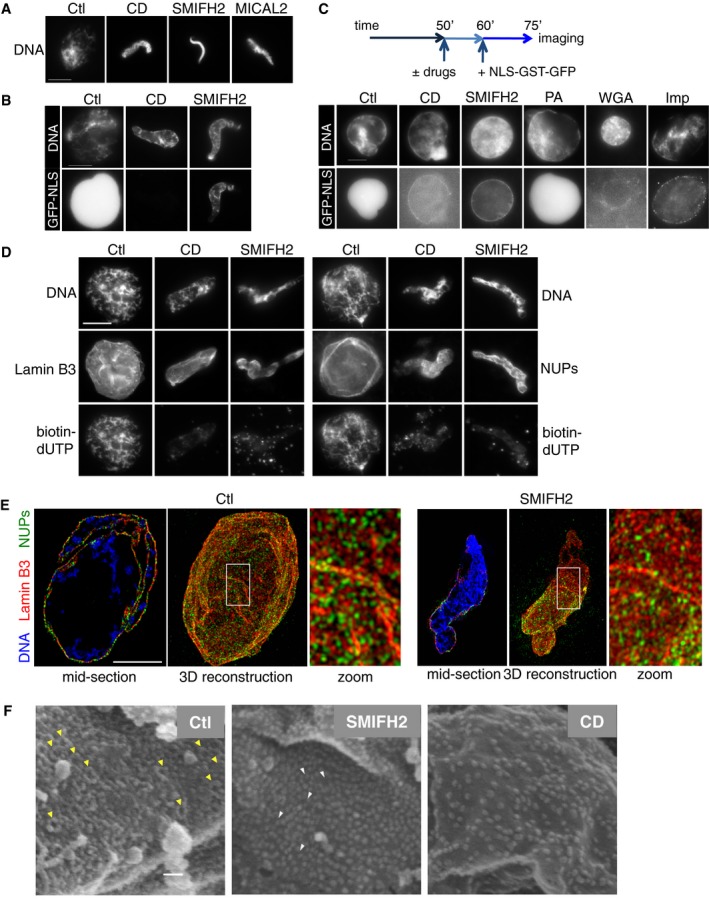
- Immunofluorescence images of nuclei formed either in control extracts or in the presence of indicated drugs or MICAL2, analysed at 60 min. Scale bar, 10 μm.
- Immunofluorescence images of nuclei formed in control or CytD (CD)‐ or SMIFH2‐treated extracts. Nuclear transport was assayed with NLS‐tagged GST‐GFP protein added at the onset of experiment. Nuclei were analysed at 60 min. Scale bar, 10 μm.
- Top, scheme: Nuclei were supplemented which CytD (CD), SMIFH2, PA, WGA or importazole (Imp) at 50 min; NLS‐GST‐GFP was added at 60 min and nuclei were imaged at 75 min. Scale bar, 10 μm.
- Immunofluorescence images of nuclei formed in control extract, or in the presence of CytD (CD) or SMIFH2, stained at 60 min for lamin B3 and NUPs (mAb414); DNA replication was assessed as biotin‐dUTP incorporation. Scale bar 10 μm.
- 3D‐SIM images of the nuclear lamina (lamin B3, red), NUPs (mAb414, green), in control or formin‐inhibited (SMIFH2) conditions. A reconstructed 3D image and a section of the same nucleus are shown. In sections, DNA is shown (blue). Scale bar, 5 μm.
- Scanning electron microscopy (FEISEM) images of nuclei formed in the presence of DMSO (Ctl), CytD (CD) or SMIFH2 at 50 min. Representative NPCs (yellow arrowheads) or incompletely formed NPCs (white arrowheads). Magnification ×40,000. Scale bar, 100 nm.
Figure EV4. Active nuclear transport requires actin dynamics.
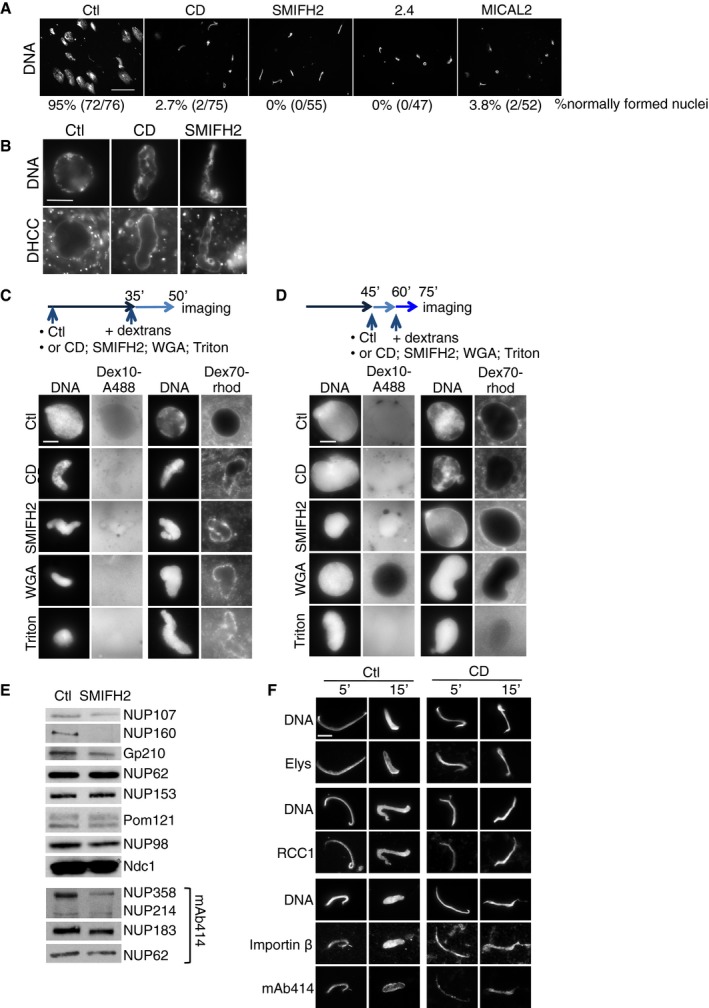
- Immunofluorescence images of nuclei formed in control extracts, or extracts supplemented with the indicated drug or MICAL2 recombinant protein. DNA was stained with Hoechst and the number of normally assembled nuclei was determined. Scale bar, 50 μm.
- Immunofluorescence images of nuclei formed in control or CytD (CD)‐ or SMIFH2‐treated extracts as in Fig 5B. Nuclear membranes were visualised with the lipid dye DHCC. Nuclei were analysed at 60 min. Scale bar, 10 μm.
- Scheme: Nuclei were formed in control extract or in the presence of CytD (CD), SMIFH2, WGA or Triton; at 35 min Dextran10‐Alexa488 (Dex10‐A488) or Dextran70‐rhodamine (Dex70‐rhod) were added; nuclei were imaged directly at 50 min. Scale bar, 10 μm.
- Scheme: Nuclei were formed in control extract; at 45 min, CytD (CD), SMIFH2, WGA or Triton was added, followed by the addition of Dextran10‐Alexa488 (Dex10‐A488) or Dextran70‐rhodamine (Dex70‐rhod); nuclei were imaged directly at 75 min. Scale bar, 10 μm.
- Western blots of total nuclear fractions in control and formin‐inhibited (SMIFH2) extracts, probed with antibodies to the NUPs indicated; the same number of nuclei was analysed for each condition.
- Immunofluorescence images of nuclei formed for 5 and 15 min in control or cytochalasin D‐treated extracts, stained for DNA, Elys, RCC1, importin‐β and FG‐NUPs (mAb414). Scale bar, 10 μm.
Source data are available online for this figure.
We therefore examined nuclear transport in the nuclei formed in XEE using an NLS‐tagged GST‐GFP probe (Talcott & Moore, 2000). SMIFH2 or CytD treatment prevented the accumulation of the probe inside nuclei (Fig 5B), showing that nuclear transport was inhibited. Nevertheless, the nuclear membrane remained intact, as verified by staining with the membrane dye DHCC (Fig EV4B) and by exclusion of fluorescently labelled high molecular weight (MW) dextrans (Fig EV4C). To exclude the possibility that NPCs might not be fully formed if actin dynamics is disrupted, we first allowed nuclei to assemble normally before adding CytD or SMIFH2. As positive controls for the inhibition of nuclear transport, we added the lectin wheat germ agglutinin (WGA), which blocks NPC function (Finlay et al, 1987), and importazole. As a negative control, we used the CDK inhibitor PA, which blocks DNA replication but has not been reported to affect nuclear transport. We then added NLS‐GST‐GFP and assessed its nuclear accumulation. As expected, control and PA conditions allowed the probe to concentrate inside the nuclei, whereas it was excluded by WGA, importazole, CytD and SMIFH2 (Fig 5C). We again verified nuclear integrity by analysing dextran nuclear accumulation in the same conditions. This showed that the membrane was intact as high MW dextrans were excluded in all conditions but in the presence of detergent. NPCs were present since low MW dextrans, which passively diffuse through NPCs, accumulated inside nuclei in all conditions except WGA (Fig EV4D). Collectively, these experiments confirm that in XEE, abrogating actin dynamics or inhibiting formins does not affect nuclear envelope integrity but prevents nuclear transport.
Since nuclear assembly and growth require active nuclear transport, we asked whether CytD or SMIFH2 would also affect initial NPC assembly. Under these conditions, while nuclear morphology was disrupted, immunofluorescence of lamin B3 and NUPs (using the monoclonal antibody mAb414) showed the presence of these proteins in nuclei (Fig 5D). However, whereas NUP staining in control nuclei showed punctate staining, this was less apparent in CytD‐ or SMIFH2‐treated extracts. We therefore analysed nuclear pores in nuclei formed in XEE using 3D structured illumination microscopy (3D‐SIM). In control XEE, immunofluorescence analysis of the NUPs showed evenly dispersed punctate staining. In contrast, upon SMIFH2 treatment, while lamina staining remained homogeneous, NUP staining was reduced overall, with dense NUP clusters between NUP‐free regions (Fig 5E). We tested the levels of individual NUPs in the nuclei by Western blotting. In the presence of SMIFH2, NUP160 was not detected, while the levels of NUP107, Gp210, NUP358, NUP214 and NUP183 were decreased (Fig EV4E). We then used whole‐mount field‐emission scanning electron microscopy (FEISEM) to analyse morphology of nuclear pores in nuclei formed in the presence of SMIFH2 or CytD. Whereas they were readily visible in control conditions, no morphologically normal nuclear pores could be detected in nuclei from SMIFH2‐ or CytD‐treated XEE (Fig 5F and Appendix Fig S3).
Arresting actin dynamics hinders cargo release from importin
Since nuclear transport and NPC assembly were disrupted by SMIFH2 or CytD, we investigated which steps in NPC assembly were affected. We first assayed by immunofluorescence the kinetics of association of Elys, importin‐β, FG‐NUPs and RCC1 with sperm DNA that initiates NPC formation. There was no difference between drug treatments and controls (Fig EV4F). The subsequent step in NPC assembly involves RanGTP‐mediated release of nucleoporins from importin‐β (Bai et al, 2014). We thus compared FG‐NUP–importin interactions in control and CytD‐ or importazole‐treated extracts by assaying mAb414 immunoprecipitates. Neither treatment decreased signal of importin‐β, showing that NUP–importin‐β binding is not inhibited, and if anything was increased, suggesting that NUP release onto chromatin might be less efficient (Fig 6A). This should interfere with NPC formation.
Figure 6. Disruption of actin dynamics hinders cargo release from importin.
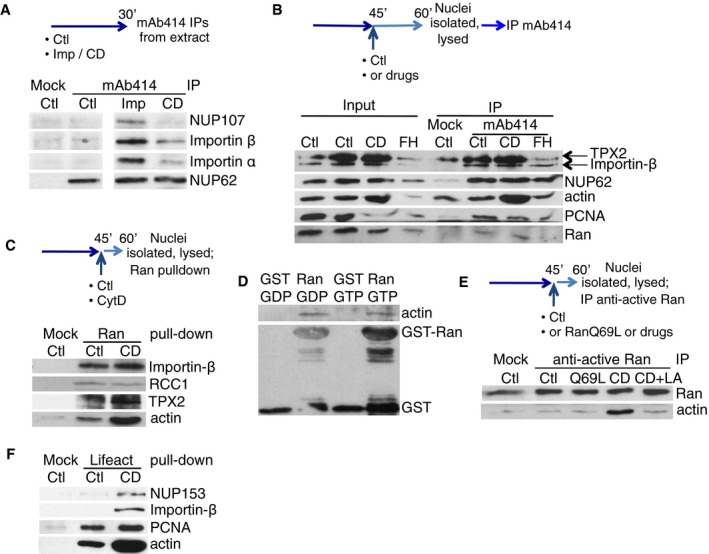
- Scheme: Control extract (Ctl) or extract treated with CytD (CD) or importazole (Imp) was incubated for 30 min and immunoprecipitated with mAb414 (Mock IP, no antibody added). Beads were blotted for the proteins indicated.
- Scheme: Nuclei, formed in control extract, or with CytD (CD) or SMIFH2 (FH) added at 45 min, were purified at 60 min and used for immunoprecipitation with mAb414; 10% of lysed nuclei were used as input. Beads were blotted with the proteins indicated.
- Scheme: Nuclei were formed in extract supplemented at 45 min with CytD (CD), then lysed, and pull‐down was performed using GST‐Ran WT covalently coupled to glutathione beads, or control beads (Mock; equal volumes of beads were used in each condition). Beads were blotted with the proteins indicated.
- In vitro pull‐down between actin–biotin and glutathione‐immobilised GST or GST‐Ran WT, pre‐loaded with either GTP or GDP; beads were blotted for actin or GST.
- Scheme: Nuclei, formed in control extract, supplemented at 45 min with RanQ69L, CytD (CD), without or with latrunculin A (LA), were lysed and immunoprecipitated with anti‐active Ran antibodies (Mock IP, no antibody added). Beads were blotted for actin and Ran.
- Proteins binding to actin within nuclei formed in control conditions (Ctl) or with CytD (CD) were pulled down using biotinylated Lifeact peptide and immobilised streptavidin, and analysed by Western blotting for the indicated proteins. Mock: Lifeact peptide was omitted.
Source data are available online for this figure.
The interaction of cargo–importin complex with NUPs at the NPCs is one of the steps crucial for effective nuclear import. We therefore next analysed the interaction between nuclear NUPs and importin–cargos by performing immunoprecipitation of FG‐NUPs using mAb414 from lysed nuclei formed in control XEE, where CytD or SMIFH2 was subsequently added (Fig 6B, scheme). As examples of importin‐dependent cargo, we chose the key DNA replication factor PCNA (Kim & Lee, 2008), which was absent from chromatin upon SMIFH2 or CytD treatment, and, as a control, TPX2 (targeting protein for kinesin 2), a nuclear protein which is not involved in DNA replication. As observed before (Fig 3E), SMIFH2 treatment strongly decreased the abundance of all tested proteins in the nuclei (Fig 6B), providing further evidence for a block of nuclear import, which also probably explains why formin inhibition did not increase nuclear actin levels nor polymerisation. While CytD did not affect importin‐β or TPX2 levels, it strongly decreased PCNA levels in nuclei, suggesting that nuclear import of PCNA no longer counterbalances its export. Interestingly, neither CytD nor SMIFH2 altered the amount of importin‐β interacting with NUPs, but CytD increased actin binding to NUPs. However, SMIFH2, but not CytD, strongly decreased the load of both TPX2 and PCNA. These results argue that SMIFH2 treatment resulted in a defective interaction between importin and its cargo, whereas CytD did not quantitatively affect NUP–importin–cargo interactions but increased NUP–actin binding.
We therefore next tested whether CytD affects importin–cargo release within nuclei. The release step involves RanGTP binding to importin‐β (Lowe et al, 2010). We performed Ran pull‐downs from nuclei and analysed binding of RCC1, its GTP exchange factor and importin‐β (Fig 6C). Although neither interaction was affected by CytD, Ran interactions with actin and TPX2 were increased, suggesting that excessive actin binding might hinder cargo release. Actin binding to Ran could potentially occur directly as it could be reconstituted with purified proteins, irrespective of whether Ran was in its GDP‐ or GTP‐loaded form (Fig 6D). CytD had no effect on RanGTP levels but promoted nuclear RanGTP–actin binding, and latrunculin A reversed this effect (Fig 6E). These results suggest that increased binding of RanGTP to actin inhibits cargo release from importins. To confirm that actin binds to import complexes, we performed a complementary experiment, by pulling down proteins that interact with actin from nucleoplasm using biotinylated Lifeact peptide (Riedl et al, 2008) with immobilised streptavidin, and analysing them by Western blot. Indeed, Lifeact bound actin, importin‐β, NUPs and the cargo PCNA, which were all increased in the presence of CytD, and none of which were pulled down if the Lifeact peptide was absent (Fig 6F).
Formins act in parallel with CDK to promote pre‐IC formation
While nuclear transport is required for nuclear assembly and S‐phase onset, our results in human cells indicated that inhibiting formins impairs DNA replication even after nuclear assembly. It was thus important to establish whether there is a continued requirement for nuclear transport throughout DNA replication. To do this, we designed experiments to determine execution points for biochemical activities at successive phases of DNA synthesis. This involves nuclear transfer experiments in which nuclei are isolated from one extract and transferred to another with different conditions. We first verified that WGA addition, to block existing NPC function, or depleting NUPs with WGA (WGA‐bpΔ) to prevent new NPC formation, both blocked DNA replication (Fig EV4A–C). We also checked that nuclear transfers did not affect integrity of the nuclei, by confirming that they could still exclude high MW dextrans (Fig EV4D).
We then tested whether DNA replication requires formins or further NPC formation once nuclei have been correctly formed. To do this, we allowed nuclei to form in an extract where replication licensing was prevented by adding recombinant geminin (Fig 7A, scheme; Fig EV4E and F). These nuclei were then transferred into a second extract with added recombinant Cdt1, to overcome the geminin licensing block. Second extracts contained SMIFH2, WGA or vehicle, or were mock‐depleted or depleted of NUPs. Figure 7A shows that in nuclei transferred into mock‐depleted extracts, replication was efficient, confirming that the procedure did not damage nuclei. In nuclei transferred into mock‐depleted extracts containing SMIFH2, or into WGA‐containing extracts, DNA replication was abolished. However, in NUP‐depleted extracts, DNA replication occurred, albeit less efficiently (WGA‐bp∆), suggesting that further NPC formation is dispensable. Nevertheless, in extracts where NUPs were depleted and SMIFH2 was present, replication was totally blocked (WGA‐bp∆ +SMIFH2). This experiment shows that both formin activity and nuclear transport, but probably not new NPC formation, are still required for DNA replication even once the nucleus has been fully assembled.
Figure 7. Formins promote pre‐IC formation in parallel with CDK .
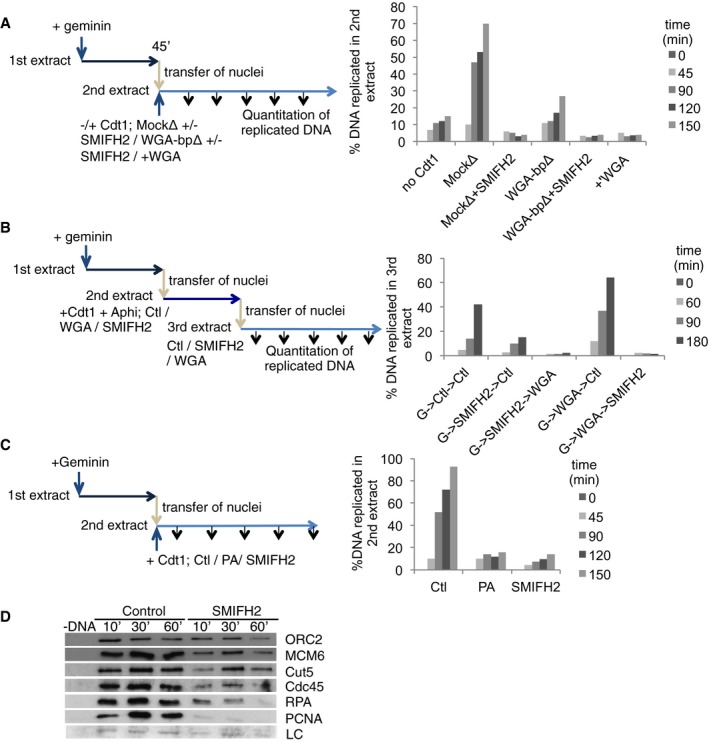
- Scheme: Nuclei were formed in the first extract containing geminin, then transferred to a second extract, with Cdt1, which was either mock (MockΔ)‐ or WGA‐binding protein (WGA‐bpΔ)‐depleted, with or without addition of SMIFH2, or where WGA was added (+WGA). Replication efficiency was measured in the second extract.
- Scheme: Double‐reciprocal nuclear transfer, from geminin‐treated extract into either SMIFH2‐ or WGA‐treated Cdt1‐containing extract, with aphidicolin; and then into a third extract with the alternative condition. DNA replication was assessed in the third extract.
- Scheme: Nuclear transfer experiment, in which first extracts contained geminin; second extracts contained Cdt1 and were controls (Ctl), or CDKs (PA) or formins (SMIFH2) were inhibited. DNA replication was assessed in the second extract.
- Chromatin was purified from second extracts of experiment in (C) and blotted for the proteins indicated. LC, loading control (unspecific band).
Source data are available online for this figure.
One possibility consistent with our results would be that formins have a role in the continued function of NPCs in DNA replication. If so, then requirements for formin activity should not be accomplished in the absence of nuclear transport, and vice versa. To test this, we performed a double‐reciprocal nuclear transfer experiment. As before, first extracts contained geminin and second extracts contained Cdt1, to release the licensing block, plus either SMIFH2 or WGA, as well as aphidicolin, to prevent replication elongation. Nuclei were further transferred into aphidicolin‐free third extracts with the alternative conditions, or to a control extract (scheme, Fig 7B). Transfer from a WGA extract into a control extract allowed efficient replication, but transfer from a SMIFH2 extract into a control extract replicated less well, suggesting that formin inhibition is not fully reversible by nuclear transfer. Neither transfer from WGA‐into‐SMIFH2 nor from SMIFH2‐into‐WGA allowed any DNA replication in the third extract (Fig 7B). Therefore, ongoing nuclear transport and formin activity are required in parallel to promote DNA replication in fully formed nuclei.
Given the above results, assuming that formins control nuclear transport, continued formin activity post‐nuclear formation should be essential for pre‐IC formation. As a control, we inhibited CDK activity, which is also required for pre‐IC formation and should thus also be required post‐nuclear formation. Therefore, in this experiment, we transferred nuclei from geminin‐containing extract to a second extract, treated with either PA, SMIFH2 or vehicle, and quantified DNA replication. As expected, both PA and SMIFH2 prevented replication in preassembled nuclei (Fig 7C). Chromatin‐bound PCNA was essentially undetectable in SMIFH2‐treated nuclei (Fig 7D), showing that formin activity is required after nuclear assembly in XEE to allow pre‐IC formation and DNA replication.
Nuclear formin activity controls chromatin loading of PCNA and CDKs
Finally, we asked whether nuclear formins have additional roles in DNA replication. To bypass formin requirement for nuclear transport, we concentrated replication factors inside nuclei by inhibiting CRM1‐dependent nuclear export with leptomycin B. During this step, we blocked DNA replication by inhibiting CDKs with PA. Then, we isolated the nuclei and transferred them to new extracts without PA, to allow replication, but containing either vehicle or SMIFH2 (Fig 8A). This procedure worked as expected, and nuclei transferred to control second extracts replicated efficiently (Fig 8B). To test whether nuclear transport remained superfluous, we compared conditions with and without leptomycin B in the second extract. In the absence of leptomycin B, SMIFH2 treatment prevented replication (Fig 8B) and caused loss of nuclear CDK and PCNA (Fig 8C). Thus, formin‐dependent nuclear transport is required throughout DNA replication to maintain nuclear levels of replication factors. However, although nuclear levels of CDKs and PCNA remained similar between control and SMIFH2 treatments when leptomycin B was present, SMIFH2 prevented their localisation onto chromatin and DNA replication remained blocked (Fig 8B and C). This indicates that nuclear formin activity is required for pre‐IC formation independently of its roles in nuclear transport.
Figure 8. Nuclear formin activity controls chromatin loading of PCNA and CDKs.

-
A–CScheme: Nuclear transfer from the first extract, where active nuclear export (leptomycin B, LB) and CDK (PA) were inhibited, into the second extract, either control or treated with SMIFH2, containing leptomycin B or not. (B) DNA replication was assessed in the second extract. (C) Total nuclear and chromatin‐associated replication factors in nuclei isolated at 60 min were analysed by Western blotting. PCNA and PSTAIR antibodies were sequentially used to blot the same area of membrane.
-
DChromosomal DNA replication in NPE determined by 33P‐dCTP incorporation assay in control conditions (Ctl) or in the presence of SMIFH2; mean ± SEM of four independent experiments.
-
ETime course of chromatin loading of indicated replication factors in NPE, in control (Ctl) or SMIFH2‐treated extracts (500 μM); SE, short exposure; LE, long exposure.
Source data are available online for this figure.
To confirm this, we used nucleoplasmic extracts (NPE). They are highly concentrated and DNA can replicate in the absence of a nuclear envelope and thus nuclear transport (Walter et al, 1998). DNA in control NPE replicated efficiently, but replication was totally abolished when formin activity was inhibited with SMIFH2 (Fig 8D). A similar effect was observed with the 2.4 formin inhibitor (Fig EV5G). Importantly, ORC was strongly diminished on the chromatin, and loading of the pre‐IC components, including Cdc45, RPA and PCNA, was almost completely inhibited by SMIFH2 (Fig 8E).
Figure EV5. Ongoing nuclear transport is required to promote DNA replication.
-
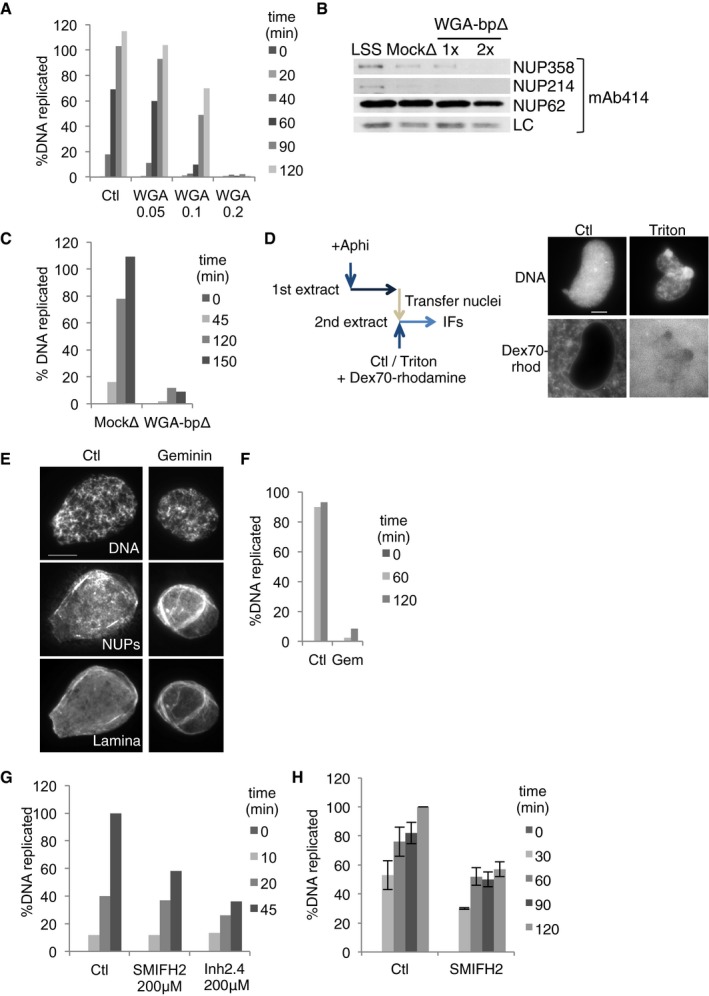
AReplication assay in control extract and in extract supplemented with WGA at the concentrations indicated (mg/ml).
-
B, CXEE was control (Mock) or WGA‐depleted (WGA‐bp∆), and assayed for depletion efficiency (after one (1x) and two (2x) rounds of depletion) by Western blot with mAb414 (B) and replication (C).
-
DScheme: Nuclei were formed in extract containing aphidicolin to block initiation of DNA replication, then transferred to a second extract, control or treated with 0.1% Triton X‐100, and supplemented with Dextran70‐rhodamine (Dex70‐rhod); nuclei were imaged directly 15 min later. Scale bar, 10 μm.
-
EImmunofluorescence images of nuclei formed for 60 min in control and geminin (40 nM)‐containing extracts, stained for DNA, NUPs (mAb414) and lamin B3. Scale bar, 10 μm.
-
FReplication time course of control and geminin‐treated extract.
-
GReplication time course of sperm chromatin in NPE extract treated with SMIFH2 or 2.4 formin inhibitor (mean values of two replicates), both used at 200 μM.
-
HReplication time course of ssDNA in high‐speed extract, control (Ctl) or in the presence of SMIFH2 (mean ± SEM; n = 3 and 4, respectively).
Source data are available online for this figure.
Finally, we tested the effect of formin inhibition on elongation of DNA replication. To this end, we used high‐speed egg extracts (HSS) that are devoid of membranes and thus incapable of forming nuclei and replicating dsDNA, but which efficiently assemble chromatin and synthesise the complementary DNA strand (Méchali & Harland, 1982). SMIFH2 significantly inhibited ssDNA synthesis (Fig EV5H), corroborating the immobilisation of PCNA foci that we observed in human somatic cells (Fig 1G). In summary, formin inhibition specifically prevents chromatin loading of replication components and interferes with ssDNA synthesis, thus revealing additional downstream formin‐dependent steps in the initiation and elongation of DNA replication.
Discussion
Our study uncovers transcription‐independent roles for actin dynamics and mDia formins in nuclear transport and DNA replication in both Xenopus egg extracts and somatic human cells. Disrupting actin dynamics in XEE prevented decondensation of chromatin and nuclear growth. Concomitantly, nuclear transport of NLS‐bearing cargo was inhibited. This abolished nuclear pore complex formation and function, both of which involve importin‐α/β‐mediated cargo binding and subsequent release, which is dependent on the interaction with Ran. Arresting actin dynamics resulted in increased actin binding to RanGTP, preventing cargo release from importin‐β. A similar phenotype was previously observed with importazole, which alters Ran–importin interactions without preventing their binding (Soderholm et al, 2011). Similarly, a single site K37D/K152A Ran mutation can affect importin‐β–Ran interactions, impeding cargo release (Lee et al, 2005). Future studies will be required to map the exact interaction sites and determine conformational changes induced by actin binding to Ran, and how this modifies Ran–importin functions.
While chemical inhibition is not specific for nuclear formins, this is currently the only way to inhibit formins in synchronised cell cultures, and allowed us to show that S‐phase entry of G1‐synchronised cells requires formin activity. This may well be the result of a global transcription defect. However, treatment of cells with formin inhibitor during S‐phase recapitulated the results observed with expression of nuclear‐localised constructs that disrupt formin activity, and which do not affect global transcription. In addition, SMIFH2 also inhibited replication complex assembly in the transcription‐free XEE, and use of nucleoplasmic extracts allowed us to confirm that this is independent of the requirement for formins in nuclear transport.
In addition, using live‐cell imaging tools specific for nuclear actin, we present physiological nuclear actin filament polymerisation in early G1 of somatic cell cycles. Interestingly, these filaments must disassemble before entry into S‐phase for the cell cycle to continue. Differences between nuclear actin structures in XEE and somatic cells can be explained by the different nature of these systems. Somatic nuclei in G1 are formed by reassembly of the nuclear envelope around post‐mitotic chromatin and are transcriptionally competent, whereas XEE nuclei are formed de novo from naked sperm chromatin, and support DNA replication but not transcription. We did not expect to observe nuclear actin filaments in XEE as they do not have a G1 phase. Thus, while we do not currently know the role of the transient early G1 nuclear filaments in somatic cells, we speculate that they might sequester monomeric nuclear actin to allow activation of transcriptional programmes (Vartiainen et al, 2007; Baarlink et al, 2013).
Both the nuclear DAD construct, which activates endogenous nuclear mDia, and the polymerisation‐favouring nuclear actin mutants prolonged S‐phase. This is likely a result of the impairment of pre‐IC formation, which may result from a combination of two effects: perturbed import of PCNA (and possibly other replication factors) into nuclei and subsequent requirement of mDia for CDK2 and PCNA chromatin loading. Moreover, the elongation step of DNA synthesis is also affected when formins are inhibited. Uncovering the detailed mechanism of formin action in nuclear transport and replication complex assembly and elongation will require further work. This may not only depend on alteration of actin dynamics. Interestingly, mDia2 has been recently reported to have actin nucleation‐independent roles in proteasome function and p53 transcriptional activity (Isogai et al 2015, 2016). In the same studies, mDia2 was also shown to directly interact with CDK2, which might point towards the role in localisation of the kinase to the chromatin during the initiation of DNA replication. Other connections between formins and CDKs exist, since formin 2 was identified by mass spectrometry in nucleoli, and it stabilises the CDK inhibitor p21 (Yamada et al, 2013).
Nucleocytoplasmic shuttling of actin (Dopie et al, 2012) means that drug effects on cytoplasmic actin have knock‐on effects on nuclear actin levels. Indeed, CytD and jasplakinolide both greatly increased nuclear actin levels, and CytD promoted nuclear actin filament stabilisation in XEE. However, adding recombinant Arp2/3 and WASP‐VCA also increased nuclear actin levels but did not promote similar filament formation nor affect nuclear transport and DNA replication. Conversely, two different formin inhibitors had opposite effects on nuclear actin levels without filament stabilisation, yet inhibited both nuclear transport and DNA replication. Furthermore, addition of purified proteins MICAL2 or gelsolin was inhibitory for replication, while addition of recombinant cofilin could rescue the effects of CytD. Finally, hyperactivation of nuclear formins inhibited S‐phase progression. These results imply that deregulated nuclear actin dynamics, rather than an increase in nuclear actin levels or filament formation per se, prevents DNA replication.
Results from this study and previous work suggest that both cytoplasmic and nuclear actin dynamics control cell proliferation by multiple mechanisms, potentially providing a link between the extracellular environment and nuclear functions. For example, cell anchorage and the cytoskeleton are involved in growth factor‐dependent transcription, for example, of cyclin D1 in mammalian cells (Assoian & Zhu, 1997), as well as degradation of the CDK inhibitor CDKN1A (Densham et al, 2009). We and others (Serebryannyy et al, 2016) find that treatments that induce nuclear actin filaments eliminate global transcription. We show here that manipulating nuclear actin also arrests DNA replication in a transcription‐independent manner.
In conclusion, together with accumulating evidence for important roles in chromatin regulation and transcription, our study strongly reinforces the notion that actin dynamics and formins have critical roles in essential nuclear processes.
Materials and Methods
Antibodies
Antibodies used are as follows: XCdc45, XCdc6, XRPA, XMCM3 (gifts from M. Méchali); XORC2, XMCM6 (gifts from J. Maller); XCut5 (gift from D. Maiorano); PCNA (Abcam; ab18197, or Oncogene Science NA03); PSTAIR (Sigma‐Aldrich; P7962); human Cdc6 (H‐304, Santa Cruz Biotechnology; SC‐8341); actin (Sigma‐Aldrich, clones A2066 or AC‐15; Hypermol, clone 2G2); Ran (Santa Cruz Biotechnology, C29; SC‐1156); active Ran (NewEast Biosciences; 26915); cofilin (Abcam; ab42824); Arp2 (Abcam; ab47654); mDia2 (One World Lab; 11016); NF‐κB p65 (A) (Santa Cruz Biotechnology; SC‐109); XNUP107, XNUP62, XNUP153 (gifts from B. Hülsmann); Elys (gift from J. Blow); γH2A.X pSer129 (Millipore, clone JBW301); TPX2, XRCC1, HS importin‐β (Bompard et al, 2010). In‐house rabbit polyclonal antibodies against His‐tagged Xenopus importin‐α were raised and affinity‐purified. The original construct for His‐tagged human importin‐α was a gift from D. Görlich; XLaminB3 (gift from B. Goldman); WASP (Abcam; ab74904); mAb414 (Abcam; ab50008); ROCK1 (Abcam; ab58305); Arp3 (Abcam; ab49671); cortactin (Millipore; clone 4F11); tubulin (Santa Cruz Biotechnology; SC‐9104); GST (Pierce; MA4‐004); biotin (Cell Signaling; D5A7); digoxigenin (Roche, clone 1.71.256).
Plasmids
The PCNA‐TagRFP and actin‐TagGFP chromobodies were purchased from Chromotek®. To allow endogenous nuclear actin detection, the SV40 nuclear localisation sequence (NLS, ccgcctaagaaaaagcggaag gtg) was added at the C‐terminus of the actin chromobody, or in between the actin Vhh sequence and the TagGFP. The former is essentially identical to the nAC recently published (Plessner et al, 2015) but with a different stop codon. Both of our nuclear actin chromobodies gave identical results but only the former was used in this study. The actin‐NLS R62D mutant (Baarlink et al, 2013) was used as template to generate the actin‐NLS WT form and that was subsequently mutated to S14C or G15S. Formin mutants, mDia2‐DAD constructs, actin‐NLS R62D and Lifeact‐GFP‐NLS were gifts from R. Grosse.
Xenopus egg extracts and replication reactions
Interphase egg extracts, chromatin isolation and replication assays were prepared and performed essentially as described (Blow & Laskey, 1986), with minor modifications. In brief, eggs laid overnight in 150 mM NaCl were dejellied in degellying buffer (29 mM Tris pH 8.5, 110 mM NaCl, 5 mM DTT); rinsed several times in High Salt Barths solution (15 mM Tris pH 7.6, 110 mM NaCl, 2 mM KCl, 1 mM MgSO4, 0.5 mM Na2HPO4, 2 mM NaHCO3), twice in MMR (5 mM HEPES‐KOH pH 7.6, 100 mM NaCl, 2 mM KCl, 0.1 mM EDTA, 1 mM MgCl2, 2 mM CaCl2), before activation with 0.3 μg/ml calcimycin ionophore in MMR. Subsequently, two rinses in MMR and two more in SB (50 mM HEPES‐KOH pH 7.6, 50 mM KCl, 2.5 mM MgCl2, 5% sucrose, 0.014% β‐mercaptoethanol) followed, while during the last rinse the eggs were transferred on ice and SB was supplemented with protease inhibitors (10 μg/ml leupeptin, pepstatin and aprotinin). Eggs were spun down at 200 g for 1 min and excess of buffer was removed before being centrifuged at 16,000 g, 4°C for 10 min. Protease inhibitors and 10 μg/ml cytochalasin B were added to the cytoplasmic fraction. This concentration of cytochalasin B, a much weaker actin drug than cytochalasin D, is required to reduce the viscosity sufficiently that extracts can be obtained by centrifugation but has no effect on DNA replication and does not provoke nuclear actin stabilisation. Extracts were further centrifuged in SW55Ti rotor for 20 min at 20k rpm (48,000 g) at 4°C. The cytoplasmic layer was extracted with a large‐bore needle and syringe, and supplemented with glycerol 3% and ATP regenerating system (10 mM creatine phosphate, 10 μg/ml creatine kinase, 1 mM ATP, 1 mM MgCl2) added from a 20× stock. Aliquots were frozen in liquid nitrogen. Where indicated, a 1:100 dilution of cytochalasin D (at final concentration of 400 μM, unless otherwise indicated; Enzo); SMIFH2 (500 μM, unless otherwise stated; Calbiochem); purvalanol A (200 μM; Sigma‐Aldrich); latrunculin A (100 μM, unless otherwise indicated; Enzo); jasplakinolide (100 μM; Enzo); importazole (500 μM, unless otherwise indicated; Sigma‐Aldrich); 2.4 formin inhibitor (at indicated concentrations; K216‐0385, ChemDiv); or DMSO solvent only was added to the Xenopus egg extracts. Where indicated, extract was supplemented with recombinant geminin and Cdt1 (40 nM; gift from M. Lutzmann); recombinant MICAL2 (48 ng/μl of extract; gift from V.N. Gladyshev); WGA (0.2 mg/ml; Calbiochem); aphidicolin (25 μg/ml; Sigma‐Aldrich); recombinant cofilin (5 μM; Hypermol; 8419‐01); recombinant Arp2/3 complex and GST‐VCA (200 nM; Hypermol; 84101 and 8416‐01, respectively); recombinant His‐Ran WT and Q96L (used at 5 μM; purified as described previously: Bompard et al, 2005); gelsolin (80 ng/μl; Sigma‐Aldrich, G8032); dextran‐Alexa Fluor 488 10,000 MW and dextran‐rhodamine B 70,000 MW (used at 2.5 μl/μl; Life Technologies, D‐22910 and D‐1841). For mass spectrometry analysis, sperm heads were added at concentration of 2,800/μl and the insoluble fraction of nucleoskeleton and chromatin was isolated at 50 min from 1 ml of extract per condition. Nucleoplasmic extracts (NPE) preparation, analysis of DNA replication efficiency and chromatin loading of replication factors were performed as described (Tutter & Walter, 2006). The NPE was supplemented with DMSO, SMIFH2 or 2.4 compound at a final vehicle concentration of 1.6% (SMIFH2 final concentration 800 μM, unless otherwise stated).
Cell culture
Cells (U2OS or HeLa) were cultured in DMEM Glutamax (Invitrogen) supplemented with 10% heat inactivated foetal bovine serum (FBS; Invitrogen) and 1× antibiotic mixture (complete medium). Cell lines were tested for mycoplasma contamination regularly. For cell cycle synchronisation, cells were incubated in complete medium containing 2 mM thymidine for 14–16 h. After an 8‐ to 10‐h release in complete medium, 2 mM thymidine was added again for 20 h. Cells were released and 6–7 h later nocodazole (50–100 ng/ml) was added for additional 5 h. This resulted in a homogeneous G2/M population with minimal exposure to nocodazole. Cells were washed with PBS and complete medium was added for 4.5 h, at which time DMSO or SMIFH2 (50 μM) was added. At indicated time points, cells were detached by trypsinisation, washed with ice‐cold PBS and pellets were collected for FACS and/or immunoblotting analysis. For transient transfections of plasmid DNA, jetPEI or Lipofectamine 2000 or 3000 was used, according to the manufacturer's instructions (Polyplus Transfection or Invitrogen, respectively).
U2OS cells stably expressing the nuclear actin or PCNA chromobody were obtained upon Lipofectamine 2000 transfection and selection with 2 μg/ml puromycin. Clones were obtained by serial dilution.
To analyse NF‐κB translocation, RA‐FLS (rheumatoid arthritis, fibroblast‐like synovicites) were prepared as described (Morel et al, 2005). Cells were seeded at 10,000 per well on coverslips in 12‐well plates in RPMI medium/5% FBS, allowed to adhere for 24 h, then starved overnight in RPMI/1% FBS. The following day, fresh medium/1% FBS was supplemented with 0.1% DMSO, importazole (50 μM) or SMIFH2 (50 μM) for 1 h, followed by stimulation with IL‐1β (10 ng/ml final; Miltenyi Biotec) or TNF‐α (10 ng/ml final; Miltenyi Biotec) for 30 min. Cells were then washed in PBS, fixed in 3.7% formaldehyde/PBS and proceeded for NF‐κB immunostaining.
Immunoprecipitations, pull‐downs and nuclear transfers
Glutathione‐immobilised GST‐Ran wild‐type and Q69L mutant were produced as previously described (Bompard et al, 2010). For IPs, 10 μl of beads [glutathione‐Sepharose (GE Healthcare) beads were used as mock] were washed in PBS and incubated with lysed nuclei (corresponding to 25 μl of extract, lysed at 55 min; drugs were added at 40 min) for 2 h at 4°C, washed in 150 mM NaCl/PBS, resuspended in Laemmli buffer and analysed by Western blotting.
For mAb414 and importin‐β IPs from egg extract, 10 μl of DynaBeads (for mAb414) or 10 μl packed protein G‐agarose (Roche) beads (for importin‐β) were washed with PBS and incubated with antibody for 2 h at 4°C, subsequently washed in PBS and incubated with 25 μl extract diluted with SB buffer for 2 h at 4°C. Beads were then processed as above.
For anti‐active Ran IP, 10 μl packed protein G‐agarose (Roche) beads were incubated with 1 μg of antibody for 2 h at 4°C, blocked in 10 mg/ml BSA/PBS, washed in PBS and incubated with lysed nuclei (corresponding to 25 μl of extract) for 2 h at 4°C, washed in 0.1% Triton X‐100/150 mM NaCl/PBS, resuspended in Laemmli buffer and analysed by Western blotting. For actin‐Ran in vitro pull‐down, glutathione‐Sepharose (GE Healthcare) beads were pre‐incubated with recombinant GST protein (Bompard et al, 2010); 10 μl of glutathione‐GST and glutathione‐GST‐Ran beads were washed and blocked in 10 mg/ml BSA/PBS, washed in PBS and incubated with 1 μg of actin–biotin (Cytoskeleton) for 2 h at 4°C, then proceeded as above. For Lifeact‐NLS‐actin pull‐down, 10 μl packed streptavidin–agarose (Novagen) beads were incubated with 5 nmol Lifeact‐NLS‐biotin peptide (MG‐VADLIKKFESISKEEGDPP‐VATPPKKKRK‐V‐biotin; synthesised by Cambridge Research Biochemicals) for 2 h at 4°C, washed in PBS and incubated with lysed sonicated nuclei (corresponding to 40 μl of extract) for 2 h at 4°C, washed in 150 mM NaCl/PBS, resuspended in Laemmli buffer and analysed by Western blotting.
GTP/GDP nucleotide exchange assay with glutathione‐immobilised recombinant GST‐Ran was performed as previously described (Bompard et al, 2010). Beads were subsequently washed in wash buffer (20 mM Tris pH 7.5, 50 mM NaCl, 5 mM MgCl2) and incubated with 1 μg of actin–biotin (Cytoskeleton)/10 μl of beads for 2 h at 4°C; washed in wash buffer, resuspended in Laemmli buffer and analysed by Western blotting.
For nuclear transfer experiments, sperm heads were added to egg extract supplemented as indicated, and at time points indicated, nuclei were diluted 10× in CPB buffer (50 mM KCl; 20 mM HEPES pH 7.6; 2% sucrose; 5 mM MgCl2) with protease inhibitors, layered onto 1 ml sucrose cushion (0.7 M sucrose in CPB) and centrifuged for 5 min at 6,000 g at 4°C and resuspended in the recipient extract. For nuclear fractionation, the pellet was further resuspended in CPB containing 0.3% Triton X‐100, then recentrifuged, supernatant recovered as nucleoplasmic fraction and pellet resuspended directly in Laemmli buffer as insoluble nuclear fraction. For immunoblot analysis, fractions corresponding to the same number of nuclei were loaded on gel.
Immunofluorescence microscopy
Immunofluorescence microscopy using Xenopus egg extract nuclei and preparation of samples for visualising actin was performed as described (Krauss et al, 2003). Where indicated, 20 μM biotin‐dUTP or digoxigenin‐dUTP (Roche), and inhibitors or DMSO, was used. Actin–Alexa Fluor and actin–biotin conjugates were obtained from Life Technologies and Cytoskeleton, respectively, and used at 25 μg/ml. DHCC was used at 2 μM. pGEX 4T1 GST‐GFP‐NLS plasmid was a gift from Dale Shumaker (Northwestern University, Chicago; Talcott & Moore, 2000). DNA was stained with 1 μg/ml Hoechst 33258. TRITC‐ or rhodamine‐conjugated phalloidin (Invitrogen) was used at 1/500. Secondary antibodies and streptavidin were Alexa Fluor conjugates and were used at 1/500. Images were taken with upright Zeiss AxioimagerZ1 (100×; 1.4NA) microscope operated with Metamorph 6.2.6. software (Molecular Devices), using constant exposure time for each filter setting. Superresolution images were taken using 3D‐SIM with a Deltavision OMX microscope, with Olympus UPSLAPO oil objective (100×; 1.4NA), and analysed using OMERO.insight application. Confocal images were taken using Leica SP5‐SMD microscope. The Duolink in situ PLA was performed according to the manufacturer's instructions (Olink Bioscience, Uppsala, Sweden).
Cultured cells were seeded on gelatin‐coated coverslips, synchronised and treated as described for each experiment. EdU (5‐ethynyl‐2′‐deoxyuridine; 10 μM) or EU (5‐ethynyl‐uridine; 1 mM for 1 h) was detected with click reaction using the Alexa Fluor® 647 Imaging Kit, according to the manufacturer's instructions (Invitrogen), and images were acquired as described above using identical settings between the tested conditions. For nuclear actin imaging, cells were transfected and fixed 24–48 h later either with 3.7% formaldehyde in cytoskeleton buffer (10 mM MES, 150 mM NaCl, 5 mM EGTA, 5 mM glucose and 5 mM MgCl2) at pH 6.2 (Small et al, 1999) or with glutaraldehyde essentially as described (Baarlink et al, 2013). TRITC‐conjugated phalloidin was used at 1/1,000 for 1.5 h. For “phalloidin alone” staining, cells were fixed with glutaraldehyde as above, and phalloidin was used at 1/200 for 20 min after three quenching steps with sodium borohydride (1 mg/ml; Small et al, 1999). Coverslips were mounted with DAPI‐containing Prolong Gold or Diamond (Thermo Fisher). Image analysis, γH2A.X foci counting and signal intensity measurement were performed in Fiji‐ImageJ (Schindelin et al, 2012) using identical parameters for all conditions. The NucleusJ plug‐in (Poulet et al, 2015) was used to measure parameters of nuclear morphology.
For the analysis of NF‐κB translocation, images were acquired using a Carl Zeiss AxioimagerZ2 microscope, a plan‐apochromat 40× 1.4 NA oil immersion lens and FS49 (Hoechst) and FS45 HQ (Texas Red) fluorescence filter sets and a grid projection illumination system (aka. Apotome). The high signal‐to‐noise ratio and out of focus removal proved to be important for the analysis.
To increase the sample size, a large‐field Hamamatsu Orca Flash4.0 LT sCMOS camera was used and 5 × 5 mosaic acquisitions were performed.
Individual tiles were analysed using a custom‐designed Cell Profiler analysis routine. Briefly, nuclei masks were identified using an intensity‐based automatic Otsu threshold on the Hoechst images. Cut objects at the edges of the image, as well as non‐nuclear small objects, were discarded. Rare, fused nuclei were segmented using an intensity algorithm. Subsequently, the nuclear masks were expanded by 10 pixels. NF‐κB staining integrated intensity and masked areas were then measured in both nucleus and expanded nucleus masks. Cytoplasm integrated intensities and areas were derived using expanded nucleus mask minus nucleus mask values. Mean intensity values (integrated intensity/area) and nucleus/cytoplasm mean intensity ratios were calculated.
Statistics
Graphs were created and statistical analyses (two‐tailed unpaired t‐test) were performed in Microsoft Excel 2011 or GraphPad Prism 6. The number of cells counted in each condition and P‐values (*P ≤ 0.05; **P ≤ 0.001; ***P ≤ 0.0001) are indicated in the figures.
Timelapse microscopy
For live videomicroscopy, cells were seeded in glass bottom 35‐mm dishes with one or four compartments, transfected as above, and image analysis was initiated 10–15 min after addition of drugs. Z‐stacks (10 μm in five planes) were acquired every 10 min using an inverted microscope (Nikon) equipped with confocal spinning disc CSU‐X1 Andor, 60×/1.4 oil objective using the software Andor iQ3. Stacks were processed and movies generated in Fiji. To measure the duration of nuclear actin network and PCNA foci, timelapse videos with images taken at 10‐min intervals were used. Duration of nuclear actin network and replication foci were measured based on their appearance and disappearance in the timelapse images taken at 10‐min intervals. Outliers were removed with the ROUT method (Q = 1%) in Prism 6.
Electron microscopy
Ten or 20 μl of interphase Xenopus egg extract was supplemented with sperm DNA as described above; nuclei were allowed to assemble in the presence or absence of actin inhibitors (SMIFH2 or cytochalasin D). Sample preparation for scanning electron microscopy (SEM) was performed as described (Allen et al, 2007), with minor modifications. Briefly, reactions were stopped by diluting 25‐fold with cold CPB buffer supplemented with protease inhibitor cocktail (Sigma‐Aldrich) and centrifuged at 1,000 × g for 2 min at 4°C. Nuclei were resuspended in 0.5 ml CPB, layered onto 0.5–1 ml sucrose cushion (0.7 M in CPB) and centrifuged at 3,000 × g for 15 min at 4°C onto acetone‐washed silicon chips (Agar Scientific). Nuclei were fixed in fixation buffer (80 mM PIPES, pH 6.8, 30 mM KCl, 1 mM MgCl2, 0.25% glutaraldehyde, 2% formaldehyde, 5% w/v sucrose) for 30 min at room temperature, washed in 0.2 M sodium cacodylate and post‐fixed with 1% osmium tetroxide solution in 0.2 M sodium cacodylate. After a wash in H2O, samples were dehydrated with increasing concentrations of ethanol (30, 50, 70, 90%, and three times in absolute ethanol) followed by 10‐min incubation in graded ethanol–hexamethyldisilazane. After one wash with hexamethyldisilazane, the samples were sputter‐coated with approximately 3‐ to 10‐nm‐thick gold film and examined under a scanning electron microscope (Hitachi S4000 or S4800). Images were obtained using a lens detector with an acceleration voltage of 20 kV at calibrated magnifications, with Axone software (version 2013; Newtec) and processed in ImageJ or Photoshop.
Fluorescence‐activated cell sorting (FACS) analysis
Cells (0.5–1 × 106) were suspended in cold PBS; then, pure ethanol was added to reach 70% (v/v) and fixed cells were stored in −20°C until FACS analysis. DNA was stained in PBS solution containing 2.5 μg/ml propidium iodide (PI) and 500 μg/ml RNase (Sigma‐Aldrich). FACS data were obtained using FacsCalibur BD flow cytometer and visualised using Flowing software (http://www.flowingsoftware.com/—versions 2.4.1 and above).
Subcellular fractionation and immunoblotting
Chromatin and nucleoplasmic fractions were prepared from cell pellets essentially as described and protein concentrations were determined with the BCA method (Pierce). For immunoblotting, 10 μg of chromatin and 15 μg of soluble nuclear material were loaded on 10 or 12% polyacrylamide gels and transferred onto PVDF membranes. After blocking with 2% BSA, the corresponding antibodies were incubated for 14–16 h at 4°C.
Mass spectrometry
Protein samples containing the nucleoskeleton and chromatin were resuspended in 2× Laemmli buffer and sonicated. Proteins (corresponding to 0.5 ml of extract) were reduced, alkylated and separated by SDS–PAGE in 4–20% gradient gels (Bio‐Rad), each lane was sliced in 15 pieces and in‐gel trypsin (Gold, Promega) digestion, and peptide extraction was performed essentially as described (Shevchenko et al, 2006). Obtained peptides were analysed online by nano‐flow HPLC‐nanoelectrospray ionisation using a LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific) coupled to an Ultimate 3000 HPLC (Dionex, Thermo Fisher Scientific). Desalting and pre‐concentration of samples were performed online on a Pepmap® pre‐column (0.3 × 10 mm, Dionex). A gradient consisting of 0–40% B in A for 60 min, followed by 80% B/20% A for 15 min (A = 0.1% formic acid, 2% acetonitrile in water; B = 0.1% formic acid in acetonitrile) at 300 nl/min, was used to elute peptides from the capillary reverse‐ phase column (0.075 × 150 mm, Pepmap®, Dionex). Eluted peptides were electrosprayed online at a voltage of 2.2 kV. A cycle of one full‐scan mass spectrum (400–2,000 m/z) at a resolution of 60,000 (at 400 m/z), followed by five data‐dependent MS/MS spectra, was repeated continuously throughout the nanoLC separation. All MS/MS spectra were recorded using normalised collision energy (35%, activation Q 0.25 and activation time 30 ms) with an isolation window of 3 m/z. Raw data analysis was performed using the MaxQuant software (v. 1.3.0.5). Peak lists were searched against the NCBI Xenopus laevis (release 130117; http://www.ncbi.nlm.nih.gov), 255 frequently observed contaminants as well as reversed sequences of all entries. The X. laevis genome is not fully sequenced and this results in several “uncharacterised proteins”. Therefore, we also searched against the X. tropicalis database, which is fully sequenced. The following settings were applied: spectra were searched with a mass tolerance of 7 ppm (MS) and 0.5 m/z (MS/MS). Enzyme specificity was set to trypsin/P. Up to two missed cleavages were allowed and only peptides with at least six amino acids in length were considered. Carbamidomethylation of Cys was selected as fixed modification. Oxidation on methionine, phosphorylation on serine, threonine or tyrosine and acetylation on Protein N‐term were set as a variable modification. Peptide identifications were accepted based on their false discovery rate (< 1%). Accepted peptide sequences were subsequently assembled by MaxQuant into proteins to achieve a false discovery rate of 1% at the protein level. Only proteins identified by at least one unique peptide or two peptides of at least six amino acids and in at least two of the three replicates were selected for further analyses.
For quantitation, the log10 of median intensity‐based absolute quantification (iBAQ) was used. To compensate for the incomplete X. laevis database, MaxQuant.txt files were modified as follows: matchgroups were created with protein groups identified by similar peptides; GO categories for identified X. laevis proteins were downloaded from Uniprot after converting the GI IDs into UniprotKB accession numbers and added manually in the MaxQuant files before being loaded onto Perseus; information on “uncharacterised proteins” was extracted by assigning the UniRef90 or Uniref50 cluster. In Perseus, two groups were defined: “IBAQ D” for DMSO (control) and “IBAQ P” for purvalanol A‐treated (CDK‐inhibited) sample. “NaN values” were converted to “0”, so that proteins identified in only one of the two conditions would be included in the plots. Statistical analysis was performed in Perseus selecting the two‐sample t‐test with Benjamini–Hochberg and FDR 1% as parameters.
For Gene Ontology analysis in DAVID (Huang et al, 2009), gene names were loaded using X. laevis as background. All terms are presented in Dataset EV2 but only GO BP with P‐value < 0.01 were further analysed in REVIGO to remove redundant GO terms (for Fig EV2E) with the following settings: SimRel method, whole Uniprot database (default settings) and Small Similarity (0.5).
The proteomics data were deposited into the MassIVE database (University of California Sand Diego), with accession number MSV000081201.
Author contributions
NP and LK conceived, performed and analysed most experiments and wrote the manuscript. BH performed other experiments. SU and NP performed proteomics. MR co‐supervised NP for proteomics. AC provided an important intellectual contribution. JD supervised NPE experiments. NM co‐supervised nuclear transport experiments. DF conceived and directed the study and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Movie EV5
Source Data for Expanded View
Review Process File
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 6
Source Data for Figure 7
Source Data for Figure 8
Acknowledgements
This work was supported by ANR Grant ANR‐09‐BLAN‐0252 and Ligue Nationale Contre le Cancer, “Equipe labellisée”, grants EL2010. LNCC/DF and EL2013. LNCC/DF and the Région Languedoc Roussillon. Thanks to the laboratories of J. Blow, M. Méchali, D. Görlich, B. Huelsmann, D. Maiorano and M. Hahne for providing antibodies, and R. Grosse for the Lifeact‐GFP‐NLS, the actin‐NLS R62D and all formin vectors. Thanks to B.C. Lee and V. Gladyshev for MICAL reagents, and H. Hochegger, I. Hagan, P. Jay, V. Dulic, J. Hutchins and A. Crevenna for comments on the manuscript. Thanks to Puck Knipscheer at the Hubrecht Institute in Utrecht, the Netherlands, for sharing the experience with the NPE (Cancéropôle GSO Mobility Grant, convention No. 2016‐M02). Thanks to M. Goldberg for advice on sample preparation for electron microscopy, C. Cazevieille of the CRIC‐IURS, D. Cot of the IEM, V. Georget, J. M. Langerak and J. Cau of MRI imaging facilities, Montpellier, for assistance with electron and light microscopy and analysis. Thanks to R. Audo for help with NF‐κB experiments.
The EMBO Journal (2017) 36: 3212–3231
References
- Allen TD, Rutherford SA, Murray S, Gardiner F, Kiseleva E, Goldberg MW, Drummond SP (2007) Visualization of the nucleus and nuclear envelope in situ by SEM in tissue culture cells. Nat Protoc 2: 1180–1184 [DOI] [PubMed] [Google Scholar]
- Assoian RK, Zhu X (1997) Cell anchorage and the cytoskeleton as partners in growth factor dependent cell cycle progression. Curr Opin Cell Biol 9: 93–98 [DOI] [PubMed] [Google Scholar]
- Arias EE, Walter JC (2004) Initiation of DNA replication in Xenopus egg extracts. Front Biosci 9: 3029–3045 [DOI] [PubMed] [Google Scholar]
- Baarlink C, Wang H, Grosse R (2013) Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science 340: 864–867 [DOI] [PubMed] [Google Scholar]
- Bai L, Michael WM, Yan S (2014) Importin β‐dependent nuclear import of TopBP1 in ATR‐Chk1 checkpoint in Xenopus egg extracts. Cell Signal 26: 857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Laskey RA (1986) Initiation of DNA replication in nuclei and purified DNA by a cell‐free extract of Xenopus eggs. Cell 47: 577–587 [DOI] [PubMed] [Google Scholar]
- Bompard G, Sharp SJ, Freiss G, Machesky LM (2005) Involvement of Rac in actin cytoskeleton rearrangements induced by MIM‐B. J Cell Sci 118: 5393–5403 [DOI] [PubMed] [Google Scholar]
- Bompard G, Rabeharivelo G, Frank M, Cau J, Delsert C, Morin N (2010) Subgroup II PAK‐mediated phosphorylation regulates Ran activity during mitosis. J Cell Biol 190: 807–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A, Lorca T, Castro A (2012) Quantitative live imaging of endogenous DNA replication in mammalian cells. PLoS One 7: e45726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TG, Rosenbaum JL (1979) An actin filament matrix in hand‐isolated nuclei of X. laevis oocytes. Cell 18: 1101–1108 [DOI] [PubMed] [Google Scholar]
- Conaway RC, Conaway JW (2009) The INO80 chromatin remodeling complex in transcription, replication and repair. Trends Biochem Sci 34: 71–77 [DOI] [PubMed] [Google Scholar]
- Cox LS (1992) DNA replication in cell‐free extracts from Xenopus eggs is prevented by disrupting nuclear envelope function. J Cell Sci 101(Pt 1): 43–53 [DOI] [PubMed] [Google Scholar]
- Densham RM, O'Neill E, Munro J, König I, Anderson K, Kolch W, Olson MF (2009) MST kinases monitor actin cytoskeletal integrity and signal via c‐Jun N‐terminal kinase stress‐activated kinase to regulate p21Waf1/Cip1 stability. Mol Cell Biol 29: 6380–6390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopie J, Skarp K‐P, Rajakylä EK, Tanhuanpää K, Vartiainen MK (2012) Active maintenance of nuclear actin by importin 9 supports transcription. Proc Natl Acad Sci USA 109: E544–E552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopie J, Rajakylä EK, Joensuu MS, Huet G, Ferrantelli E, Xie T, Jäälinoja H, Jokitalo E, Vartiainen MK (2015) Genome‐wide RNAi screen for nuclear actin reveals a network of cofilin regulators. J Cell Sci 128: 2388–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echalier A, Cot E, Camasses A, Hodimont E, Hoh F, Jay P, Sheinerman FB, Krasinska L, Fisher D (2012) An integrated chemical biology approach provides insight into Cdk2 functional redundancy and inhibitor sensitivity. Chem Biol 19: 1028–1040 [DOI] [PubMed] [Google Scholar]
- Feric M, Brangwynne CP (2013) A nuclear F‐actin scaffold stabilizes ribonucleoprotein droplets against gravity in large cells. Nat Cell Biol 15: 1253–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay DR, Newmeyer DD, Price TM, Forbes DJ (1987) Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol 104: 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin TJ, Fukui J, Peterson JR, Higgs HN (2009) Isoform‐selective chemical inhibition of mDia‐mediated actin assembly. Biochemistry (Mosc) 48: 9327–9329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounon P, Karsenti E (1981) Involvement of contractile proteins in the changes in consistency of oocyte nucleoplasm of the newt Pleurodeles waltlii . J Cell Biol 88: 410–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NS, Wodicka L, Thunnissen AM, Norman TC, Kwon S, Espinoza FH, Morgan DO, Barnes G, LeClerc S, Meijer L, Kim SH, Lockhart DJ, Schultz PG (1998) Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281: 533–538 [DOI] [PubMed] [Google Scholar]
- Grosse R, Vartiainen MK (2013) To be or not to be assembled: progressing into nuclear actin filaments. Nat Rev Mol Cell Biol 14: 693–697 [DOI] [PubMed] [Google Scholar]
- Hetzer MW, Walther TC, Mattaj IW (2005) Pushing the envelope: structure, function, and dynamics of the nuclear periphery. Annu Rev Cell Dev Biol 21: 347–380 [DOI] [PubMed] [Google Scholar]
- Hofmann WA, Stojiljkovic L, Fuchsova B, Vargas GM, Mavrommatis E, Philimonenko V, Kysela K, Goodrich JA, Lessard JL, Hope TJ, Hozak P, de Lanerolle P (2004) Actin is part of pre‐initiation complexes and is necessary for transcription by RNA polymerase II. Nat Cell Biol 6: 1094–1101 [DOI] [PubMed] [Google Scholar]
- Hu P, Wu S, Hernandez N (2004) A role for beta‐actin in RNA polymerase III transcription. Genes Dev 18: 3010–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ghisletti S, Saijo K, Gandhi M, Aouadi M, Tesz GJ, Zhang DX, Yao J, Czech MP, Goode BL, Rosenfeld MG, Glass CK (2011) Coronin 2A mediates actin‐dependent de‐repression of inflammatory response genes. Nature 470: 414–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57 [DOI] [PubMed] [Google Scholar]
- Huet G, Skarp K‐P, Vartiainen MK (2012) Nuclear actin levels as an important transcriptional switch. Transcription 3: 226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K, Iida H, Yahara I (1986) Heat shock induction of intranuclear actin rods in cultured mammalian cells. Exp Cell Res 165: 207–215 [DOI] [PubMed] [Google Scholar]
- Isogai T, van der Kammen R, Goerdayal SS, Heck AJR, Altelaar AFM, Innocenti M (2015) Proteomic analyses uncover a new function and mode of action for mouse homolog of Diaphanous 2 (mDia2). Mol Cell Proteomics MCP 14: 1064–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai T, van der Kammen R, Bleijerveld OB, Goerdayal SS, Argenzio E, Altelaar AFM, Innocenti M (2016) Quantitative proteomics illuminates a functional interaction between mDia2 and the proteasome. J Proteome Res 15: 4624–4637 [DOI] [PubMed] [Google Scholar]
- Johnson MA, Sharma M, Mok MTS, Henderson BR (2013) Stimulation of in vivo nuclear transport dynamics of actin and its co‐factors IQGAP1 and Rac1 in response to DNA replication stress. Biochim Biophys Acta 1833: 2334–2347 [DOI] [PubMed] [Google Scholar]
- Kalendová A, Kalasová I, Yamazaki S, Uličná L, Harata M, Hozák P (2014) Nuclear actin filaments recruit cofilin and actin‐related protein 3, and their formation is connected with a mitotic block. Histochem Cell Biol 142: 139–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor P, Chen M, Winkler DD, Luger K, Shen X (2013) Evidence for monomeric actin function in INO80 chromatin remodeling. Nat Struct Mol Biol 20: 426–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoudoli GA, Gillespie PJ, Stewart G, Andersen JS, Swedlow JR, Blow JJ (2008) Temporal profiling of the chromatin proteome reveals system‐wide responses to replication inhibition. Curr Biol 18: 838–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Lee H (2008) Lys‐110 is essential for targeting PCNA to replication and repair foci, and the K110A mutant activates apoptosis. Biol Cell 100: 675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiseleva E, Drummond SP, Goldberg MW, Rutherford SA, Allen TD, Wilson KL (2004) Actin‐ and protein‐4.1‐containing filaments link nuclear pore complexes to subnuclear organelles in Xenopus oocyte nuclei. J Cell Sci 117: 2481–2490 [DOI] [PubMed] [Google Scholar]
- Krasinska L, Fisher D (2009) Replication initiation complex formation in the absence of nuclear function in Xenopus . Nucleic Acids Res 37: 2238–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss SW, Chen C, Penman S, Heald R (2003) Nuclear actin and protein 4.1: essential interactions during nuclear assembly in vitro . Proc Natl Acad Sci USA 100: 10752–10757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurat CF, Yeeles JTP, Patel H, Early A, Diffley JFX (2017) Chromatin controls DNA replication origin selection, lagging‐strand synthesis, and replication fork rates. Mol Cell 65: 117–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labib K (2010) How do Cdc7 and cyclin‐dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev 24: 1208–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lanerolle P (2012) Nuclear actin and myosins at a glance. J Cell Sci 125: 4945–4949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Matsuura Y, Liu SM, Stewart M (2005) Structural basis for nuclear import complex dissociation by RanGTP. Nature 435: 693–696 [DOI] [PubMed] [Google Scholar]
- Lee BC, Péterfi Z, Hoffmann FW, Moore RE, Kaya A, Avanesov A, Tarrago L, Zhou Y, Weerapana E, Fomenko DE, Hoffmann PR, Gladyshev VN (2013) MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation. Mol Cell 51: 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AR, Siegel JJ, Kalab P, Siu M, Weis K, Liphardt JT (2010) Selectivity mechanism of the nuclear pore complex characterized by single cargo tracking. Nature 467: 600–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist MR, Storaska AJ, Liu TC, Larsen SD, Evans T, Neubig RR, Jaffrey SR (2014) Redox modification of nuclear actin by MICAL‐2 regulates SRF signaling. Cell 156: 563–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Molina D, Jafari R, Ignatushchenko M, Seki T, Larsson EA, Dan C, Sreekumar L, Cao Y, Nordlund P (2013) Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341: 84–87 [DOI] [PubMed] [Google Scholar]
- McDonald D, Carrero G, Andrin C, de Vries G, Hendzel MJ (2006) Nucleoplasmic beta‐actin exists in a dynamic equilibrium between low‐mobility polymeric species and rapidly diffusing populations. J Cell Biol 172: 541–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méchali M, Harland RM (1982) DNA synthesis in a cell‐free system from Xenopus eggs: priming and elongation on single‐stranded DNA in vitro . Cell 30: 93–101 [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Pasque V, Jullien J, Gurdon JB (2011) Nuclear actin polymerization is required for transcriptional reprogramming of Oct4 by oocytes. Genes Dev 25: 946–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Teperek M, Yusa K, Allen GE, Bradshaw CR, Gurdon JB (2013) Nuclear wave1 is required for reprogramming transcription in oocytes and for normal development. Science 341: 1002–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel J, Audo R, Hahne M, Combe B (2005) Tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL) induces rheumatoid arthritis synovial fibroblast proliferation through mitogen‐activated protein kinases and phosphatidylinositol 3‐kinase/Akt. J Biol Chem 280: 15709–15718 [DOI] [PubMed] [Google Scholar]
- Németh ZH, Deitch EA, Davidson MT, Szabó C, Vizi ES, Haskó G (2004) Disruption of the actin cytoskeleton results in nuclear factor‐kappaB activation and inflammatory mediator production in cultured human intestinal epithelial cells. J Cell Physiol 200: 71–81 [DOI] [PubMed] [Google Scholar]
- Obrdlik A, Percipalle P (2011) The F‐actin severing protein cofilin‐1 is required for RNA polymerase II transcription elongation. Nucleus 2: 72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philimonenko VV, Zhao J, Iben S, Dingová H, Kyselá K, Kahle M, Zentgraf H, Hofmann WA, de Lanerolle P, Hozák P, Grummt I (2004) Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat Cell Biol 6: 1165–1172 [DOI] [PubMed] [Google Scholar]
- Plessner M, Melak M, Chinchilla P, Baarlink C, Grosse R (2015) Nuclear F‐actin formation and reorganization upon cell spreading. J Biol Chem 290: 11209–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet A, Arganda‐Carreras I, Legland D, Probst AV, Andrey P, Tatout C (2015) NucleusJ: an ImageJ plugin for quantifying 3D images of interphase nuclei. Bioinformatics 31: 1144–1146 [DOI] [PubMed] [Google Scholar]
- Rando OJ, Zhao K, Janmey P, Crabtree GR (2002) Phosphatidylinositol‐dependent actin filament binding by the SWI/SNF‐like BAF chromatin remodeling complex. Proc Natl Acad Sci USA 99: 2824–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich‐Soldner R (2008) Lifeact: a versatile marker to visualize F‐actin. Nat Methods 5: 605–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SA, Neidt EM, Cui J, Feiger Z, Skau CT, Gardel ML, Kozmin SA, Kovar DR (2009) Identification and characterization of a small molecule inhibitor of formin‐mediated actin assembly. Chem Biol 16: 1158–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM, Kreis TE, Jockusch BM (1980) Reversible translocation of cytoplasmic actin into the nucleus caused by dimethyl sulfoxide. Proc Natl Acad Sci USA 77: 5268–5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J‐Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M (1982) Action of cytochalasin D on cytoskeletal networks. J Cell Biol 92: 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebryannyy LA, Parilla M, Annibale P, Cruz CM, Laster K, Gratton E, Kudryashov D, Kosak ST, Gottardi CJ, de Lanerolle P (2016) Persistent nuclear actin filaments inhibit transcription by RNA polymerase II. J Cell Sci 129: 3412–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sero JE, Sailem HZ, Ardy RC, Almuttaqi H, Zhang T, Bakal C (2015) Cell shape and the microenvironment regulate nuclear translocation of NF‐κB in breast epithelial and tumor cells. Mol Syst Biol 11: 790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M (2006) In‐gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1: 2856–2860 [DOI] [PubMed] [Google Scholar]
- Small J, Rottner K, Hahne P, Anderson KI (1999) Visualising the actin cytoskeleton. Microsc Res Tech 47: 3–17 [DOI] [PubMed] [Google Scholar]
- Soderholm JF, Bird SL, Kalab P, Sampathkumar Y, Hasegawa K, Uehara‐Bingen M, Weis K, Heald R (2011) Importazole, a small molecule inhibitor of the transport receptor importin‐beta. ACS Chem Biol 6: 700–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer VA, Costes S, Inman JL, Xu R, Chen J, Hendzel MJ, Bissell MJ (2011) Depletion of nuclear actin is a key mediator of quiescence in epithelial cells. J Cell Sci 124: 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüven T, Hartmann E, Görlich D (2003) Exportin 6: a novel nuclear export receptor that is specific for profilin.actin complexes. EMBO J 22: 5928–5940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talcott B, Moore MS (2000) The nuclear import of RCC1 requires a specific nuclear localization sequence receptor, karyopherin alpha3/Qip. J Biol Chem 275: 10099–10104 [DOI] [PubMed] [Google Scholar]
- Tutter AV, Walter JC (2006) Chromosomal DNA replication in a soluble cell‐free system derived from Xenopus eggs. Methods Mol Biol 322: 121–137 [DOI] [PubMed] [Google Scholar]
- Vartiainen MK, Guettler S, Larijani B, Treisman R (2007) Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316: 1749–1752 [DOI] [PubMed] [Google Scholar]
- Walter J, Sun L, Newport J (1998) Regulated chromosomal DNA replication in the absence of a nucleus. Mol Cell 1: 519–529 [DOI] [PubMed] [Google Scholar]
- Wu X, Yoo Y, Okuhama NN, Tucker PW, Liu G, Guan J‐L (2006) Regulation of RNA‐polymerase‐II‐dependent transcription by N‐WASP and its nuclear‐binding partners. Nat Cell Biol 8: 756–763 [DOI] [PubMed] [Google Scholar]
- Yamada K, Ono M, Perkins ND, Rocha S, Lamond AI (2013) Identification and functional characterization of FMN2, a regulator of the cyclin‐dependent kinase inhibitor p21. Mol Cell 49: 922–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo Y, Wu X, Guan J‐L (2007) A novel role of the actin‐nucleating Arp2/3 complex in the regulation of RNA polymerase II‐dependent transcription. J Biol Chem 282: 7616–7623 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Movie EV5
Source Data for Expanded View
Review Process File
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 6
Source Data for Figure 7
Source Data for Figure 8


