Mosquitoes that transmit malaria have developed resistance to all classes of insecticides. The informed implementation of resistance-management strategies and the development of new insecticides are needed.
Abstract
Malaria vectors have developed resistance to all classes of insecticides that are used to target the adult mosquito to prevent parasite transmission. The number of resistant mosquito populations has increased dramatically in recent years, most likely as a result of the scale-up of vector control activities, and the intensity of this resistance is increasing rapidly and compromising the performance of vector control tools. Bednets and indoor residual spray formulations containing alternative active ingredients have shown promise in field trials but are still several years away from implementation. As existing insecticides become less effective at killing mosquitoes in the countries with the highest burden of malaria, there is growing concern that the advances made in reducing malaria transmission will be eroded by insecticide resistance. The likelihood of this scenario, and strategies that may help mitigate against this, are reviewed below.
USE OF INSECTICIDES IN MALARIA CONTROL
Wide-scale implementation of tools to prevent malaria transmission by the mosquito vectors has achieved dramatic results across Africa. It is estimated that nearly half of the population at risk of malaria in Africa are protected by insecticide-treated nets (ITNs) and approximately 7% live in houses that have received indoor residual spraying (IRS). The scale-up in coverage with these preventative measures has contributed to an approximate halving of malaria mortality in Africa between 2000 and 2013 (WHO 2014a). Outside of Africa, the feeding patterns of malaria vectors mean that ITNs are generally less effective and, hence, usage patterns are lower. IRS is reportedly used in over half of the countries in the Americas and Asia that have ongoing malaria transmission (WHO 2014a).
Only pyrethroid insecticides are approved for bednet treatment and with hundreds of millions of ITNs in use today, this imposes a major selection pressure on mosquitoes. Until recently, the majority of IRS programs were also reliant on this insecticide class although an increasing number of countries are switching to organophosphates (principally primiphos-methyl) or, in some cases, carbamates (bendiocarb) in response to emerging pyrethroid resistance. The organochlorine, DDT, is also still used in some IRS programs in Africa and in India.
THE EMERGENCE AND SPREAD OF RESISTANCE
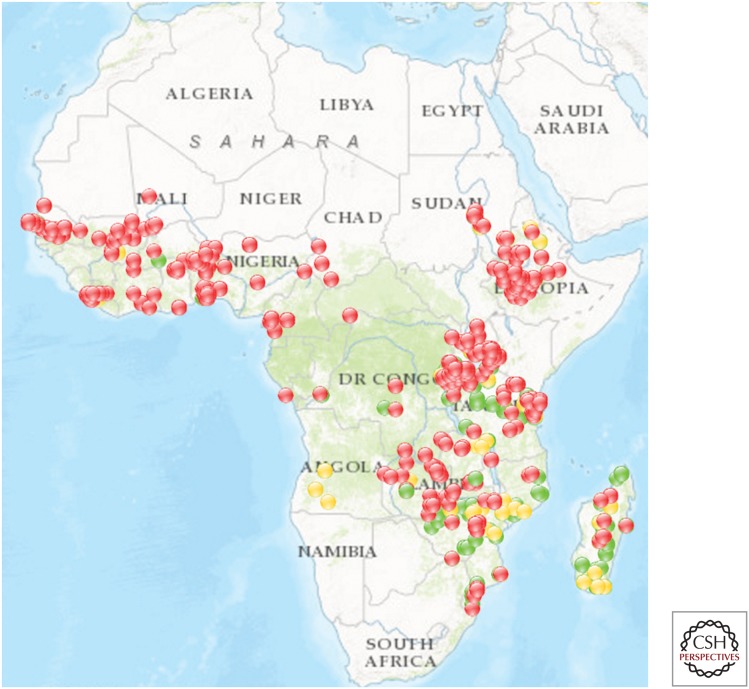
Pyrethroid resistance was first detected in African malaria vectors in Sudan in the 1970s and later in West Africa in the early 1990s (Brown 1986; Elissa et al. 1993). These early instances of resistance were likely selected for by exposure of mosquitoes to pyrethroids used to protect agricultural crops against insect damage and pyrethroid resistance remained relatively rare until the end of the 20th century (Ranson and Lissenden 2016). But in recent years, reports of pyrethroid resistance in the major African malaria vectors have increased markedly and it is now becoming increasingly difficult to find a population of Anopheles gambiae s.l. that is fully susceptible to this insecticide class (Fig. 1). Pyrethroid resistance is also widespread in Anopheles funestus in southern Africa and resistance has been detected in West and East African populations of this vector species (Riveron et al. 2013; Mulamba et al. 2014).
Figure 1.
Pyrethroid resistance in malaria vectors. Flags indicate mortality in WHO bioassays (red <90% mortality, orange 90%–97% mortality, green >97% mortality). Data shown are from 2011 to 2016 (Source: IR Mapper [www.irmapper.com], September 2016).
DDT resistance is also prevalent across Africa. In An. gambiae s.s. and An. coluzzi cross resistance between DDT and pyrethroids, caused by alterations in the common target site of both insecticide classes, the voltage-gated sodium channel (known as kdr mutations or alleles), is common (Donnelly et al. 2009). Kdr is less common in An. arabiensis and absent in An. funestus but metabolic resistance can confer DDT resistance in both these species (see below).
Resistance to carbamates and organophosphates is also on the increase (Ranson and Lissenden 2016). Cross resistance between these insecticide classes can be caused by mutations in their shared target site. Of increasing concern is the emergence of mosquito populations that are resistant to all four classes of insecticide. This can arise when multiple resistance mechanisms are selected for in the same population (e.g., An. gambiae and An. coluzzi in West Africa containing multiple target site resistance alleles have been reported [Dabire et al. 2008; Edi et al. 2012; Essandoh et al. 2013]) or when a single resistance mechanism can cause resistance to multiple insecticide classes (e.g., elevated activity of enzymes that detoxify pyrethroids, DDT, organophosphates, and/or carbamates [Mitchell et al. 2012; Edi et al. 2014]).
RESISTANCE MECHANISMS
Understanding the genetic basis of insecticide resistance can help elucidate patterns of cross resistance, as described above, and also lead to field-based diagnostics to track and manage resistance (Donnelly et al. 2015).
Target Site Resistance
The best-characterized resistance mechanisms are mutations in the insecticide target sites that reduce the binding of the toxicant. In An. gambiae, two alternative substitutions in the same codon of the voltage-gated sodium channel, the 1014F and 1014S kdr alleles, are now widely distributed across the continent (Donnelly et al. 2009; Djegbe et al. 2011). An additional mutation (N1575Y) in the same target site gene is present in West Africa but is only found in mosquitoes that contain the 1014F kdr mutation (Jones et al. 2012a). In vitro binding studies have shown that the 1575Y allele alone does not confer pyrethroid resistance but it acts synergistically with 1014F to confer a stronger level of resistance in mosquitoes with this double mutation (Wang et al. 2015). This example highlights a common pattern in the emergence of resistance—the overlayering of multiple resistance mutations, each of which serves to further increase the level of resistance of the individual to the toxicant.
Carbamates and organophosphates both target the acetylcholine esterase (AchE) enzyme, which is responsible for degrading the neurotransmitter acetylcholine in the nerve synapses. By inhibiting this enzyme, the insecticides cause a toxic level of actetylcholine to accumulate. A glycine to serine substitution in codon 119 of AchE is strongly associated with resistance to carbamates and/or organophosphates in some West African countries (Essandoh et al. 2013; Edi et al. 2014). The frequency of this mutation is increasing, which is of great concern, particularly in areas where these insecticide classes are replacing pyrethroids for IRS.
Metabolic Resistance
Metabolic resistance refers to the ability of the mosquito to detoxify the insecticide before it reaches the target site. Several enzyme families have been implicated in insecticide detoxification (Li et al. 2007), but the two families most strongly associated with resistance in malaria vectors are the glutathione transferases (GSTs) and the cytochrome P450s. Overexpression of, or amino acids substitutions in, the GSTE2 enzyme can confer DDT resistance in An. gambiae and An. funestus and molecular diagnostics have been used to demonstrate the association between genotype and phenotype at this locus (Ranson et al. 2001; Mitchell et al. 2014; Riveron et al. 2014).
Increased expression of multiple cytochrome P450s have been associated with pyrethroid resistance. Several Anopheles P450 enzymes have been expressed in vitro and their ability to metabolize pyrethroids demonstrated, with some also metabolizing other insecticide classes (Muller et al. 2008; Edi et al. 2014; Mitchell et al. 2014). Nevertheless, the genetic mechanism responsible for elevated P450 gene expression in mosquitoes has proved elusive so far. In An. funestus, there is strong genetic evidence to implicate a mutation in a regulatory element in the vicinity of the CYP9P9 genes (Riveron et al. 2013) and, thus, it is hopeful that a molecular diagnostic for this resistance mechanism will be available soon. The situation is less clear in An. gambiae s.l. Although genetic mapping, transcriptomic studies, and whole-genome sequencing have identified several strong candidates for P450-mediated resistance, they have so far failed to coalesce on a single major locus controlling the overexpression of P450s. This may indicate that P450-based resistance has emerged independently in multiple An. gambiae populations with different enzymes responsible for resistance in separate populations. Although the search for DNA markers for resistance continues (Donnelly et al. 2015), robust qPCR diagnostic tools to detect the major pyrethroid-resistance-associated P450s are now available.
Reduced Penetration
Until recently, reduced penetration, sometimes referred to as cuticular resistance, was considered a minor, secondary resistance mechanism in mosquitoes. However, several indirect lines of evidence suggest this mechanism may be evolving in African malaria vectors. Measurements of the cuticle in An. funestus and An. gambiae have found a significant thickening in pyrethroid-resistant mosquitoes (Wood et al. 2010; Balabanidou et al. 2016) and a reduced uptake of pyrethroids has been demonstrated in a resistant An. gambiae strain (Balabanidou et al. 2016). Multiple genes implicated in cuticular hydrocarbon synthesis have been found elevated in pyrethroid-resistant strains of Anopheles arabiensis in Zanzibar (Jones et al. 2013) and An. coluzzi in Burkina Faso (Toe et al. 2015). Both of these populations have very high pyrethroid-resistance phenotypes, which cannot be attributed to other known resistance mechanisms alone. Two cytochrome P450s genes (CYP4G16 and CYP4G17) are elevated in these populations but these P450s do not metabolize insecticides. Instead CYP4G16, and its ortholog in Drosophila melanogaster CYP4G1, catalyzes a critical step in the production of CHCs (Qiu et al. 2012; Balabanidou et al. 2016). Finally, several members of the ABC transporter family belonging to a subfamily that, in other insects, has been implicated in transport of lipids to the cuticle are elevated in insecticide-resistant populations of An. gambiae (Broehan et al. 2013; Jones et al. 2013). Ongoing work measuring insecticide uptake rates and cuticle composition will hopefully clarify the importance of reduced penetration in insecticide resistance in malaria vectors.
Behavioral Resistance
Increased use of insecticides in the domestic environment may be selecting for genetic changes in the behavior of malaria vectors that increase the rates of outdoor feeding and/or resting, making them less amenable to control by ITNs or IRS. Outdoor transmission is a major obstacle to effective vector control in South East Asia and South America (Santos et al. 2009; Gryseels et al. 2015). In Africa, outdoor transmission has previously been thought to account for only a very small percentage of malaria cases and it is clear that, even in countries that have had sustained coverage with ITNs over many years, the majority of malaria transmission still occurs indoors (Bayoh et al. 2014). However, if African malaria vectors do change their behavior as a result of intensive indoor use of insecticide the implications for malaria control could be catastrophic (Gatton et al. 2013; WHO 2014b). This is clearly something that needs careful monitoring.
LIMITATIONS IN INSECTICIDE-RESISTANCE MONITORING
Understanding the extent and magnitude of resistance in the local vector population is an essential requirement for the design and implementation of effective resistance management strategies. Yet, less than half the 96 countries reporting the use of ITNs and IRS for malaria control, were able to produce any data on resistance for the previous year (WHO 2014a), suggesting monitoring is intermittent at best. However, before criticizing programs for the lack of data on resistance, it is worth reflecting on the utility of the data routinely collected for evidence-based decision making in malaria control.
The vast majority of insecticide resistance monitoring, including the data in the map in Figure 1, relies entirely on the use of discriminating dose bioassays using World Health Organization tube assays or, in a small number of cases, CDC bottle bioassays. Data are reported as percentage mortality and a threshold of less than 90% mortality is used to define resistance (WHO 2013). This standardized methodology is useful for tracking the spread of resistance but does not provide information on the strength of this resistance or its impact. It is not uncommon to find close to zero mortality after exposure of the main African malaria vectors, An. gambiae or An. funestus, to the discriminating dose of pyrethroids but simply collating data on the prevalence of resistance may mask important changes in the strength of this resistance. For example, 3 years of monitoring insecticide resistance in An. gambiae from Vallé de Kou in Southwest Burkina Faso using discriminating dose assays showed no significant difference in percentage mortality between the years, but when a more quantitative measure was used to assess the strength of this resistance, resistance was found to have increased 10× in a single year (Toe et al. 2014).
Several bioassays that measure the strength of resistance have been described and are compared in a recent publication (Bagi et al. 2015). A consensus on the most suitable methodology would facilitate geographic comparisons and also aid evaluations of the efficacy of resistance-management strategies. However, measuring the intensity of resistance is only the first step. As discussed below, agreement is also needed on what level of resistance has an operational impact. In the agricultural sector, definitions of operationally significant resistance are often related to the field dose of insecticide by dividing the insecticide concentration required to achieve 50% mortality (LC50) by the field dose (Zimmer and Nauen 2011). This is not so straightforward in vector control as formulation issues can have a major impact on the bioavailability of insecticide making the field dose difficult to determine. Furthermore, as discussed below, to assess the public health impact of resistance, we are not only interested in whether or not a mosquito survives exposure to insecticide, but also how this exposure impacts the vectorial capacity. The disconnect between the data generated by resistance monitoring and the data needed to assess the impact of this resistance on malaria control activities must be addressed if this information is going to be used to drive decision making.
IMPACT OF RESISTANCE ON VECTOR CONTROL
The data shown in Figure 1 provide a snapshot of the location of known pyrethroid-resistant malaria vectors in Africa. From the limited number of studies assessing the strength of resistance it is clear that pyrethroid-resistance levels have reached extremely high levels in some sites, with LC50s 100- or even 1000-fold higher than standard laboratory susceptible strains being reported (Edi et al. 2012; Mawejje et al. 2013; Toe et al. 2014). However, the evidence for the impact of this resistance on current control activities is less clear. Part of the reasons for this uncertainty is methodological. As discussed above, the use of discriminating dose assays, which bear no resemblance to the application rates and measure resistance prevalence rather than intensity makes correlations between bioassays and control failure difficult to infer. Inclusion of cone bioassays, in which mosquitoes are exposed directly to a sprayed surface or an ITN can partially help fill these gaps and several studies have now shown that the level of resistance in the field is sufficient to compromise the performance of currently available ITNs (Ochomo et al. 2013; Toe et al. 2014). It is important that these cone bioassays are not just performed on freshly sprayed surfaces or new ITNs as the public health impact of resistance is most likely to manifest itself as insecticide rates decay (Churcher et al. 2016). Studies in Burkina Faso and Kenya both found ITNs that had been in use for a year or more in the field were much less effective at killing resistant mosquitoes than new nets. (Toe et al. 2014; Wanjala et al. 2015).
Experimental Hut Studies Have Been Used to Demonstrate Reduced Personal Protection from ITNs in Areas of Resistance
One of the first such studies was conducted in Benin, in an era when the malaria vectors in the North of the country were still fully susceptible to insecticides but in the South the majority of the vectors were resistant to pyrethroids. Using experimental huts containing volunteers sleeping under holed nets, the study found that ITNs reduced blood-feeding by 96% at the Northern site with susceptible vectors, but had almost no impact in the Southern site with high levels of pyrethroid resistance (N’Guessan et al. 2007). Furthermore the mortality of mosquitoes entering huts at the susceptible site was nearly three times as high as that at the site with high levels of pyrethroid resistance. This was followed up with household studies in 2007, which showed that sleeping under an ITN was no more protective than sleeping under an untreated net in areas with high pyrethroid resistance (Asidi et al. 2012).
It is important to remember that ITNs work by providing both personal protection to users and community protection to non-ITN users and, assuming the ITNs are of good physical quality, it is the latter effect that is most likely to be reduced as resistance increases (Thomas and Read, 2016). Experimental hut studies can measure how personal protection is impacted by resistance (see, for example, Strode et al. 2014) but modeling approaches are needed to extrapolate from this to assess the public health impact of resistance on the entire community. Using a meta-analysis of experimental hut data and a mathematical model of malaria transmission Churcher found that personal protection remained substantial until pyrethroid resistance reached high levels in the population however, the community protection dissipated much more rapidly as resistance increased (Churcher et al. 2016). Although ITN use has increased greatly in recent years, most countries are still a long way from universal coverage and use of ITNs. It is, therefore, critical that the impact of resistance on both ITN used and nonusers is considered when measuring the public health impact of resistance.
In addition, it is important that models of malaria transmission also consider other possible impacts of the resistance phenotype, the majority of which are woefully under studied. Most assays record mortality 24-h post-exposure as the end point; there is very little data on the long-term impact on the mosquito after surviving exposure to insecticides. Yet to quantify the public health impact of insecticide resistance, it is necessary to measure the impact of resistance on all traits that impact the ability of the mosquito to transmit the parasite. As an example, a recent laboratory study found that exposure to ITNs reduced the mean lifespan of adult mosquitoes. Including this delayed mortality in models of malaria transmission suggested that ITNs would still dramatically reduce the malaria transmission potential even in areas where the mosquito population was largely resistant to the immediate killing effect of the pyrethroids (Viana et al. 2016). Furthermore, it is already established that older mosquitoes are less resistant then their younger counterparts (on which bioassays are routinely conducted). If resistance declines with mosquito age, to the extent that mosquitoes old enough to transmit malaria are still killed by field doses of insecticide, the impact of resistance will again be diminished (Lines and Nassor 1991; Jones et al. 2012b). Other studies have found that exposure of insecticide-resistant mosquitoes to ITNs reduces the likelihood of parasite development (Kristan et al. 2016). These are all important parameters that must be investigated and quantified. However, it is already abundantly clear that, although these factors may possibly reduce the impact of resistance, they will not remove the threat altogether. In Kenya (Ochomo et al. 2013) and Benin (Gnanguenon et al. 2013), mosquitoes with fully developed sporozoites have been found resting inside ITNs, clearly unperturbed by the presence of the insecticide.
The epidemiological impact of resistance will be influenced by a large number of non-vector factors (e.g., efficacy of case management approaches, drug resistance, etc.). Thus, it is complex to attribute an effect directly to insecticide resistance, and even harder to extrapolate between different ecological settings. Longitudinal studies, with accurate records of malaria transmission and resistance levels, afford one of the best chances of demonstrating the impact of resistance. The most widely cited evidence for the impact of resistance comes from such a study in KwaZulu Natal, which demonstrated a correlation between the emergence of pyrethroid resistance and a spike in malaria cases, which was later contained by the reintroduction of DDT (Barnes et al. 2005). More recently, a similar conclusion was reached in Senegal, where reduction in ITN efficacy was attributed to resistance, although the absence of longitudinal resistance data makes this conclusion difficult to validate (Trape et al. 2011). Unfortunately, the opportunity for initiating new studies of this nature, at least for pyrethroid resistance in Africa, may have passed, unless good historical data sets already exist.
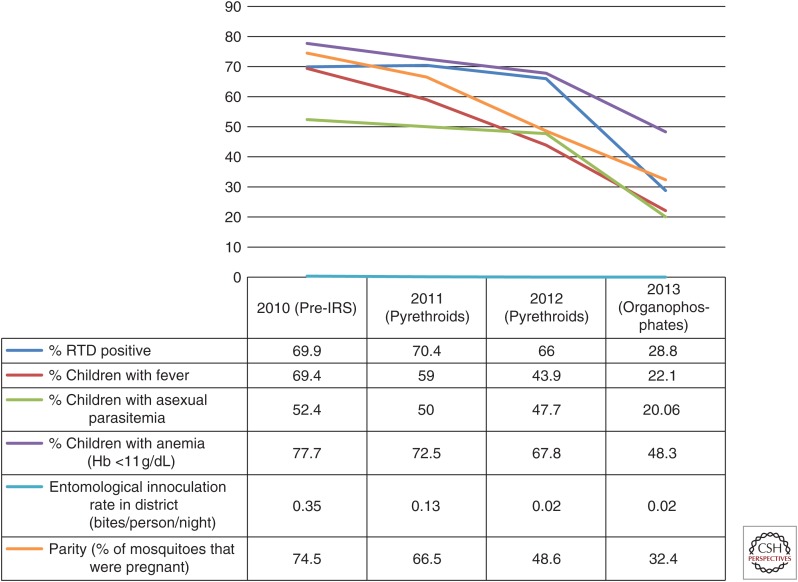
Indirect evidence that insecticide resistance is impacting on malaria transmission can be obtained from retrospective analysis in countries that have changed insecticide class in IRS programs (usually either in response to reports of resistance or increases in malaria cases) and seen an improvement in control. As an example, DDT and pyrethroids were being used for IRS in Uganda, despite the known presence of resistance. When these insecticides were replaced with bendiocarb, a marked improvement in slide positivity rates was observed (Kigozi et al. 2012). Similarly, in Ghana, pyrethroid resistance triggered a switch to use of the organophosphate insecticide Actellic (primiphos-methyl) for IRS, which was associated with a noticeable impact on key indicators (Fig. 2) (President's Malaria Initiative 2015).
Figure 2.
Improvement in malaria indicators in Bunkpurugu-Yunyoo, Ghana between 2010 and 2013 after a switch in insecticide class from pyrethroids to carbamates for indoor residual spraying. (Source: President’s Malaria Initiative 2015.) RTD, Rapid diagnostic test; IRS, indoor residual spraying.
RESISTANCE-MANAGEMENT STRATEGIES
Although there are many challenges to definitively linking insecticide resistance with increased malaria transmission most stakeholders remain in no doubt that if the selection pressure on malaria mosquitoes is allowed to continue unchecked, resistance will eventually result in the failure of existing tools. In 2012, WHO published the Global Plan for Insecticide Resistance Management (GPIRM) (WHO 2012). This document outlined a number of strategies designed to prevent this scenario becoming a reality and provided guidelines to vector control programs on how best to respond to the presence of resistance.
Resistance-management strategies for programs reliant on ITNs are clearly very limited given that WHO recommends universal coverage with bednets (WHO 2014c) and yet there are currently no alternative insecticides to pyrethroids for net impregnation. Some net manufacturers have introduced new nets to the market that contain pyrethroids, plus the synergist piperonyl butoxide (PBO): PermaNet 3.0, is a mosaic long-lasting insecticide treated net with PBO on the roof of the net and deltamethrin on the sides, while Olyset Plus has PBO and permethrin combined throughout the net. Both of these ITNs have interim approval from the WHO pesticide evaluation scheme (WHOPES) as conventional LLINs and the Vector Control Advisory Group (VCAG) have supported the PermaNet 3.0 manufacturers’ claim that this net has increased bioefficacy against mosquitoes that have developed metabolic resistance to insecticides (WHO 2014d). Despite this, these LLINs are not in widespread use against resistant populations and instead WHO recommends pilot implementations only (WHO 2015a).
Bednets containing alternative insecticides with new modes of action that differ to the currently available adulticides are under evaluation in the field. Interceptor G2, from BASF, contains the slow acting insecticide chlorfenapyr and the pyrethroid alphacypermethrin and is currently being reviewed by WHOPES (WHO 2015b). Olyset Duo, from Sumitomo Chemicals, contains permethrin and an insect growth regulator, pyriproxifen. This is also undergoing WHOPES evaluation and, as the idea of sterilizing the resistant mosquitoes that survive contact with the bi-treated net is a novel paradigm, this net is being evaluated in a clinical trial in Burkina Faso (Tiono et al. 2015). It is anticipated that one or both of these new nets with novel modes of action will be on the market by 2017–2018.
For malaria control programs using IRS, it should theoretically be possible to manage insecticide resistance by careful preplanned rotation of insecticide classes with different modes of action (i.e., alternating between DDT or pyrethroids and carbamates or organophosphates). This can be effective if implemented in a proactive manner to prevent the emergence of resistance. However, in reality, rotation of insecticide class is usually triggered by reports of resistance or perceived failures of the current product (e.g., increases in malaria cases, increased reports of mosquitoes inside homes). If resistance to one or more classes of insecticide to be used in rotation has already developed, effective resistance management will only be achieved if resistance has a fitness cost. In the worst-case scenario, the end result may be a more rapid selection of resistance to both chemicals. Little is known about the fitness costs of different resistance mechanisms in mosquitoes and this raises questions over the long-term efficacy of current IRM strategies. Furthermore, simply changing insecticide class may not be sufficient to relieve the selection pressure on the mosquito. Three of the four insecticide classes used in public health are widely used in agriculture and hence mosquitoes may be continually exposed to low doses of these chemicals even if their use in vector control is suspended. For programs including pyrethroids in their IRS rotations, the reality of continued selection pressure from ITNs and the use of coils and pyrethroid-based repellents and aerosols must be factored in. Finally, the possibility that increased expression of a single enzyme could render three or more insecticide classes obsolete is obviously a major impediment to even the best-planned IRM strategies (Edi et al. 2014).
As with ITNs, new products for IRS are under development. However, for these to be implemented in the timeframe necessary to prevent resistance from derailing malaria control, a more coordinated approach to their evaluation, regulation, and to the production of guidelines on when and where they should be deployed is essential (Hemingway et al. 2016).
FUTURE PERSPECTIVES
It is vital that the lessons learned from the current resistance crisis are used to inform future insecticide development and deployment. This will be dependent on the coordinated action of a wide range of stakeholders (Hemingway et al. 2016) and will also require brave decisions to be made to ensure the stewardship of these new products. The introduction of a single new insecticide into the market place would provide a very short-term solution and inevitably lead to the same issues now being faced with pyrethroid resistance. Selection pressure might be reduced if this product was not widely used in agriculture but, if this new insecticide met the desired criteria (reviewed in Vontas et al. 2014), it would likely be used at scale very rapidly, exerting intense selection pressure on mosquito populations. Resistance-management strategies, including plans for monitoring, recording, and sharing data on resistance evolution, must be planned from the outset and should be evidence-based.
CONCLUDING REMARKS
With resistance to one or more insecticides in African malaria vectors now becoming the norm, rather than the exception, it is time to switch the emphasis from simply describing the problem to providing effective, practical solutions. This includes revisiting the way we monitor for resistance to provide information that is of more direct value to implementers and funders, providing a more robust set of evidence on the current and projected impact of resistance to aid in planning and budgeting malaria control activities, evaluating current options to tackle resistance, and, finally, synthesizing lessons learned to develop guidelines for the effective stewardship of new insecticide products as they are introduced into the market. It may be too late to preserve the pyrethroids for future generations but now is the time to start planning evidence-based insecticide-resistance-management strategies for new public health insecticides.
REFERENCES
- Asidi A, N’Guessan R, Akogbeto M, Curtis C, Rowland M. 2012. Loss of household protection from use of insecticide-treated nets against pyrethroid-resistant mosquitoes, benin. Emerg Infect Dis 18: 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagi J, Grisales N, Corkill R, Morgan JC, N’Fale S, Brogdon WG, Ranson H. 2015. When a discriminating dose assay is not enough: Measuring the intensity of insecticide resistance in malaria vectors. Malaria J 14: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanidou V, Kampouraki A, MacLean M, Blomquist GJ, Tittiger C, Mijailovsky SJ, Chalepakis G Anthousi A, Lynd A, Sanou A, Hemingway J, et al. 2016. Cytochrome P450 associated with insecticide resistance catalyses cuticular hydrocarbon production in An. gambiae. Proc Natl Acad Sci 113: 9268–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KI, Durrheim DN, Little F, Jackson A, Mehta U, Allen E, Dlamini SS, Tsoka J, Bredenkamp B, Mthembu DJ, et al. 2005. Effect of artemether–lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med 2: e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoh MN, Walker ED, Kosgei J, Ombok M, Olang GB, Githeko AK, Killeen GF, Otieno P, Desai M, Lobo NF, et al. 2014. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasit Vectors 7: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broehan G, Kroeger T, Lorenzen M, Merzendorfer H. 2013. Functional analysis of the ATP-binding cassette transporter family in Tribolium castaneum. BMC Genomics 14: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AW. 1986. Insecticide resistance in mosquitoes: A pragmatic review. J Am Mosq Control Assoc 2: 123–140. [PubMed] [Google Scholar]
- Churcher TS, Lissenden N, Griffin JT, Worrall E, Ranson H. 2016. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. eLife 5: e16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabire KR, Diabate A, Djogbenou L, Ouari A, N’Guessan R, Ouedraogo JB, Hougard JM, Chandre F, Baldet T. 2008. Dynamics of multiple insecticide resistance in the malaria vector Anopheles gambiae in a rice growing area in South-Western Burkina Faso. Malaria J 7: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djegbe I, Boussari O, Sidick A, Martin T, Ranson H, Chandre F, Akogbeto M, Corbel V. 2011. Dynamics of insecticide resistance in malaria vectors in Benin: First evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malaria J 10: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly MJ, Corbel V, Weetman D, Wilding CS, Williamson MS, Black WCt. 2009. Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends Parasitol 25: 213–219. [DOI] [PubMed] [Google Scholar]
- Donnelly MJ, Issacs AT, Weetman D. 2015. Identification, validation and application of molecular diagnostics for insecticide resistance in malaria vectors. Trends Parasitol 32: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edi CV, Koudou BG, Jones CM, Weetman D, Ranson H. 2012. Multiple-insecticide resistance in Anopheles gambiae mosquitoes, Southern Cote d’Ivoire. Emerg Infect Dis 18: 1508–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edi CV, Djogbenou L, Jenkins AM, Regna K, Muskavitch MA, Poupardin R, Jones CM, Essandoh J, Ketoh GK, Paine MJ, et al. 2014. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet 10: e1004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elissa N, Mouchet J, Riviere F, Meunier JY, Yao K. 1993. Resistance of Anopheles gambiae s.s. to pyrethroids in Cote d’Ivoire. Ann Soc Belg Med Trop 73: 291–294. [PubMed] [Google Scholar]
- Essandoh J, Yawson AE, Weetman D. 2013. Acetylcholinesterase (Ace-1) target site mutation 119S is strongly diagnostic of carbamate and organophosphate resistance in Anopheles gambiae s.s. and Anopheles coluzzii across southern Ghana. Malaria J 12: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HC, Gould F, Hastings I, Marshall J, Ranson H, et al. 2013. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution: Int J Organ Evol 67: 1218–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanguenon V, Azondekon R, Oke-Agbo F, Sovi A, Osse R, Padonou G, Aikpon R, Akogbeto MC. 2013. Evidence of man-vector contact in torn long-lasting insecticide-treated nets. BMC Public Health 13: 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryseels C, Durnez L, Gerrets R, Uk S, Suon S, Set S, Phoeuk P, Sluydts V, Heng S, Sochantha T, et al. 2015. Re-imagining malaria: Heterogeneity of human and mosquito behaviour in relation to residual malaria transmission in Cambodia. Malaria J 14: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, Coetzee M, Simard F, Roch DK, Hinzoumbe CK, et al. 2016. Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet 387: 1785–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Liyanapathirana M, Agossa FR, Weetman D, Ranson H, Donnelly MJ, Wilding CS. 2012a. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci 109: 6614–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Sanou A, Guelbeogo WM, Sagnon N, Johnson PC, Ranson H. 2012b. Aging partially restores the efficacy of malaria vector control in insecticide-resistant populations of Anopheles gambiae s.l. from Burkina Faso. Malaria J 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Toe HK, Sanou A, Namountougou M, Hughes A, Diabate A, Dabire R, Simard F, Ranson H. 2012c. Additional selection for insecticide resistance in urban malaria vectors: DDT resistance in Anopheles arabiensis from Bobo-Dioulasso, Burkina Faso. PLoS ONE 7: e45995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Haji KA, Khatib BO, Bagi J, Mcha J, Devine GJ, Daley M, Kabula B, Ali AS, Majambere S, et al. 2013. The dynamics of pyrethroid resistance in Anopheles arabiensis from Zanzibar and an assessment of the underlying genetic basis. Parasit Vectors 6: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigozi R, Baxi SM, Gasasira A, Sserwanga A, Kakeeto S, Nasr S, Rubahika D, Dissanayake G, Kamya MR, Filler S, et al. 2012. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS ONE 7: e42857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristan M, Lines J, Nuwa A, Ntege C, Meek SR, Abeku TA. 2016. Exposure to deltamethrin affects development of Plasmodium falciparum inside wild pyrethroid resistant Anopheles gambiae s.s. mosquitoes in Uganda. Parasites Vectors 9: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Schuler MA, Berenbaum MR. 2007. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol 52: 231–253. [DOI] [PubMed] [Google Scholar]
- Lines JD, Nasssor NS. 1991. DDT resistance in Anopheles gambiae declines with mosquito age. Med Vet Entomol 5: 261–265. [DOI] [PubMed] [Google Scholar]
- Mawejje HD, Wilding CS, Rippon EJ, Hughes A, Weetman D, Donnelly MJ. 2013. Insecticide resistance monitoring of field-collected Anopheles gambiae s.l. populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Med Vet Entomol 27: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SN, Stevenson BJ, Muller P, Wilding CS, Egyir-Yawson A, Field SG, Hemingway J, Paine MJ, Ranson H, Donnelly MJ. 2012. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proc Natl Acad Sci 109: 6147–6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SN, Rigden DJ, Dowd AJ, Lu F, Wilding CS, Weetman D, Dadzie S, Jenkins AM, Regna K, Boko P, et al. 2014. Metabolic and target-site mechanisms combine to confer strong DDT resistance in Anopheles gambiae. PLoS ONE 9: e92662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulamba C, Riveron JM, Ibrahim SS, Irving H, Barnes KG, Mukwaya LG, Birungi J, Wondji CS. 2014. Widespread pyrethroid and DDT resistance in the major malaria vector Anopheles funestus in East Africa is driven by metabolic resistance mechanisms. PLoS ONE 9: e110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Warr E, Stevenson BJ, Pignatelli PM, Morgan JC, Steven A, Yawson AE, Mitchell SN, Ranson H, Hemingway J, et al. 2008. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet 4: e1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Guessan R, Corbel V, Akogbeto M, Rowland M. 2007. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis 13: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochomo EO, Bayoh NM, Walker ED, Abongo BO, Ombok MO, Ouma C, Githeko AK, Vulule J, Yan G, Gimnig JE. 2013. The efficacy of long-lasting nets with declining physical integrity may be compromised in areas with high levels of pyrethroid resistance. Malaria J 12: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- President’s Malaria Initiative. 2015. Ghana: Malaria operational plan FY 2015. USAID, Washington, DC.
- Qiu Y, Tittiger C, Wicker-Thomas C, Le Goff G, Young S, Wajnberg E, Fricaux T, Taquet N, Blomquist GJ, Feyereisen R. 2012. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc Natl Acad Sci 109: 14858–14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, Lissenden N. 2016. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parastitol 32: 187–196. [DOI] [PubMed] [Google Scholar]
- Ranson H, Rossiter L, Ortelli F, Jensen B, Wang X, Roth CW, Collins FH, Hemingway J. 2001. Identification of a novel class of insect glutathione S–transferases involved in resistance to DDT in the malaria vector Anopheles gambiae. Biochem J 359: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveron JM, Irving H, Ndula M, Barnes KG, Ibrahim SS, Paine MJ, Wondji CS. 2013. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc Natl Acad Sci 110: 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveron JM, Yunta C, Ibrahim SS, Djouaka R, Irving H, Menze BD, Ismail HM, Hemingway J, Ranson H, Albert A, et al. 2014. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol 15: R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RL, Padilha A, Costa MD, Costa EM, Dantas-Filho Hde C, Povoa MM. 2009. Malaria vectors in two indigenous reserves of the Brazilian Amazon. Rev Saude Publica 43: 859–868. [DOI] [PubMed] [Google Scholar]
- Strode C, Donegan S, Garner P, Enayati AA, Hemingway J. 2014. The impact of pyrethroid resistance on the efficacy of insecticide treated bednets against African anopheline mosquitoes: Systematic review and meta analysis. Plos Med 18: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MB, Read AF. 2016. The threat (or not) of insecticide resistance for malaria control. Proc Natl Acad Sci 113: 8900–8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiono AB, Pinder M, N’Fale S, Faragher B, Smith T, Silkey M, Ranson H, Lindsay SW. 2015. The AvecNet Trial to assess whether addition of pyriproxyfen, an insect juvenile hormone mimic, to long-lasting insecticidal mosquito nets provides additional protection against clinical malaria over current best practice in an area with pyrethroid-resistant vectors in rural Burkina Faso: Study protocol for a randomised controlled trial. Trials 16: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toe KH, Jones CM, N’Fale S, Ismail HM, Dabire RK, Ranson H. 2014. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg Infect Dis 20: 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toe KH, N’Fale S, Dabire RK, Ranson H, Jones CM. 2015. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genomics 16: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trape JF, Tall A, Diagne N, Ndiath O, Ly AB, Faye J, Dieye-Ba F, Roucher C, Bouganali C, Badiane A, et al. 2011. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: A longitudinal study. Lancet Infect Dis 11: 925–932. [DOI] [PubMed] [Google Scholar]
- Viana M, Hughes A, Matthiopoulos J, Ranson H, Ferguson HM. 2016. Delayed mortality effects cut the malaria transmission potential of insecticide-resistant mosquitoes. Proc Natl Acad Sci 113: 8975–8980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vontas J, Moore S, Kleinschmidt I, Ranson H, Lindsay S, Lengeler C, Hamon N, McLean T, Hemingway J. 2014. Framework for rapid assessment and adoption of new vector control tools. Trends Parasitol 30: 191–204. [DOI] [PubMed] [Google Scholar]
- Wang L, Nomura Y, Du Y, Liu N, Zhorov BS, Dong K. 2015. A mutation in the intracellular loop III/IV of mosquito sodium channel synergizes the effect of mutations in helix IIS6 on pyrethroid resistance. Molec Pharmacol 87: 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanjala CL, Zhou G, Mbugi J, Simbauni J, Afrane YA, Ototo E, et al. 2015. Insecticidal decay effects of long-lasting insecticide nets and indoor residual spraying on Anopheles gambiae and Anopheles arabiensis in Western Kenya. Parasit Vectors 8: 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood O, Hanrahan S, Coetzee M, Koekemoer L, Brooke B. 2010. Cuticle thickening associated with pyrethroid resistance in the major malaria vector Anopheles funestus. Parasit Vectors 3: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2012. Global plan for insecticide resistance management in malaria vectors. Global Malaria Programme, World Health Organization, Geneva. [DOI] [PMC free article] [PubMed]
- WHO. 2013. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. World Health Organization, Geneva.
- WHO. 2014a. World malaria report. World Health Organization, Geneva.
- WHO. 2014b. Control of residual malaria parasite transmission. WHO/HTM/GMP/MPAC/2014.5. World Health Organization, Geneva.
- WHO. 2014c. WHO recommendations for achieving universal coverage with long-lasting insecticidal nets in malaria control September 2013 (revised March 2014). World Health Organization, Geneva.
- WHO. 2014d. Second meeting of the vector control advisory group. World Health Organization, Geneva.
- WHO. 2015a. Conditions for use of long-lasting insecticidal nets treated with a pyrethroid and piperonyl butoxide. World Health Organization, Geneva.
- WHO. 2015b. Pesticide products under WHOPES laboratory and or field testing and evaluation. World Health Organization, Geneva.
- Zimmer C, Nauen R. 2011. Pyrethroid resistance and thiacloprid baseline susceptibility of European populations of Meligethes aeneus (Coleoptera: Nitidulidae collected in winter oilseed rape. Pest Manag Sci 67: 599–608. [DOI] [PubMed] [Google Scholar]