Structure-based vaccine design strategies for three viruses—RSV, influenza A, and HIV—have shown promising results. Strategies that incorporate B-cell ontogenies and viral evasion mechanisms appear exceptionally powerful.
Abstract
The ability of structure-based design to control the shape and reactivity—the atomic-level chemistry—of an immunogen argues for it being one of the “most powerful” immunogen-design strategies. But antigenic reactivity is only one of the properties required to induce a protective immune response. Here, a multidimensional approach is used to exemplify the enabling role atomic-level information can play in the development of immunogens against three viral pathogens, respiratory syncytial virus, influenza A virus, and human immunodeficiency virus (HIV), which have resisted standard approaches to vaccine development. Overall, structure-based strategies incorporating B-cell ontogenies and viral evasion mechanisms appear exceptionally powerful.
With less than 30 pathogens with vaccines licensed for human use, the list of desired vaccines is long and ever growing (www.who.int/immunization/diseases/en). The semi-yearly occurrences of viral outbreaks—with H1N1 influenza A virus (2009), Middle East respiratory syndrome coronavirus (2012), Ebola virus (2014), and Zika virus (2016) dominating headlines—emphasize the need for additional vaccines. All the while, more established pathogens such as respiratory syncytial virus (RSV) and human immunodeficiency virus (HIV) continue to kill millions (Murray and Lopez 1997), and a divergent airborne-transmitted influenza A virus such as the 1918 “Spanish flu,” which killed an estimated 50 million worldwide (Webster et al. 1992), remains on the watch list of most feared pandemics.
Great Debates.
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
What are the most powerful immunogen design strategies? The three “I”s of classical vaccinology—“Isolate, Inactivate, and Inject”—represent a strategy that has afforded the development of more than half the currently licensed vaccines (Hilleman 1998). But “most powerful” does not seem to be concerned with how successful nor how efficacious a particular strategy might be, but rather, which strategy succeeds, when others fail. Thus it seems reasonable to consider a strategy “most powerful” if it is able to induce a protective response against pathogens, for which other strategies have failed. Here I describe recent results from the Vaccine Research Center against three viral pathogens that have resisted vaccine development. These involve the aforementioned enveloped viruses, RSV, influenza A virus, and HIV, their exposed type 1 fusion machines (Colman and Lawrence 2003), and structure-based strategies that have recently yielded encouraging results.
STRUCTURE-BASED CONFORMATIONAL STABILIZATION STRATEGY FOR RSV
RSV is a member of the paramyxoviradae family of viruses. It infects most children during their first year of life, is responsible for 7% of deaths 1 month to 1 year of age, and is the greatest cause of hospitalization for children under 5 years of age (Shay et al. 1999; Nair et al. 2010; Hall 2012). In the elderly, it induces the same degree of excess mortality as influenza virus (Thompson et al. 2003) and has been on the list of “most desired vaccines” by large pharma for >20 years. A failed vaccine trial in the 1960s induced a number of deaths (Ottolini and Hemming 1997), and decades of RSV-vaccine research achieved an increase of only two- to fourfold in RSV-neutralizing titers in healthy adults (Paradiso et al. 1994; Tristram et al. 1994; Langley et al. 2009; Swanson et al. 2011; Karron et al. 2013; Glenn et al. 2016).
Vaccine interest in the type 1 fusion machine of RSV, its fusion (F) glycoprotein, has been heighted by the advent of palizumab (Synagis), a humanized monoclonal whose passive infusion in high-risk infants reduces the incidence of disease (The IMpact-RSV Study Group 1998; Homaira et al. 2014). Over the last 10 years, antibodies of substantially higher potency than palivizumab have been identified that recognize F in its prefusion conformation (Beaumont et al. 2012; McLellan et al. 2013b). Analysis of human serum, moreover, indicates neutralization responses elicited by natural RSV infection to target preferentially the prefusion state of F (Ngwuta et al. 2015). We determined the prefusion structure of RSV F, in complex with the D25 antibody (McLellan et al. 2013b), and used structure-based design to stabilize the metastable prefusion state (McLellan et al. 2013a) by addition of a disulfide bond (DS) and cavity-filling mutations (Cav1). Immunization indicated the prefusion F-stabilized “DS-Cav1” variant to elicit highly protective titers in mice and macaques, a finding hailed as one of the top breakthroughs of 2013 (Cohen 2013). Phase I clinical trials with DS-Cav1 have begun in 2016, and second-generation versions with up to 20-fold increased stability and fourfold increased immunogenicity have recently been developed through a strategy of iterative structure-based improvement (Joyce et al. 2016a).
Altogether, these results provide a paradigm for immunogen design based on conformational stabilization and iterative structure-based optimization (Fig. 1).
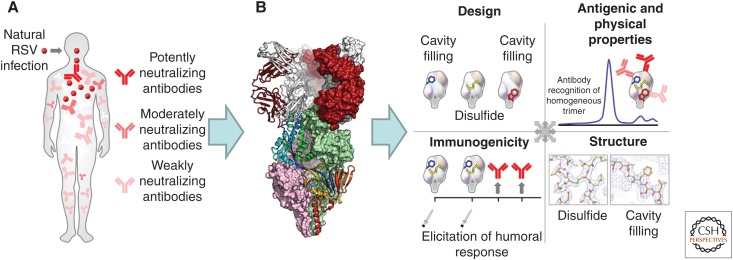
Figure 1.
A structure-based paradigm for vaccine development. (A) Natural infection induces a variety of responses. In the first step of structure-based vaccine design, a potently neutralizing and frequently elicited response is selected as a template for vaccine design. (B) The second step involves the structural determination of the selected response, in complex with the eliciting antigen. Shown is the apex-directed antigen-binding fragment of the D25 antibody (white and red) binding to the trimeric RSV-fusion glycoprotein (pink and green surface representation, with one protomer in rainbow-colored ribbon). (C) An information matrix comprising immunogen design, antigenic and physical properties, atomic-level structures, and immunogenicity is used to determine properties that correlate with improved immunogenicity, and these properties are iteratively optimized (Joyce et al. 2016b). (Panel from McLellan et al. 2013a; adapted, with permission, from The American Association for the Advancement of Science © 2013.)
STRUCTURE-BASED B-CELL-ONTOGENY STRATEGY FOR INFLUENZA A VIRUS
While the structure-based strategies developed for RSV may succeed with viruses that evade by conformational change, many viruses do not use structural rearrangements to evade the humoral immune response. Unlike the metastable RSV F glycoprotein, which rearranges to its postfusion conformation even on infectious virus (Liljeroos et al. 2013), the type 1 fusion machine of influenza A virus, its hemagglutinin (HA) glycoprotein, is stable under physiological conditions; as long as the influenza HA glycoprotein does not encounter acidic pH, it resides in a stable prefusion state. Immune evasion by the HA glycoprotein appears not to involve conformational change, but sequence diversity: either antigenic drift (Carrat and Flahault 2007), responsible for the ability of seasonal strains, such as the H1 and H3 subtypes of influenza A that currently cocirculate, to infect an estimated 10% of the human population each year; or antigenic shift (Alexander and Brown 2000), potentially involving zoonotic crossovers from a highly diverse reservoir of influenza subtypes, which reside in domesticated livestock and migratory birds.
One potential approach to overcoming sequence diversity involves clues from antibodies identified from natural infection: an antibody to vaccine approach (Burton 2002). In such an approach, antibodies with broad neutralization activity unhindered by sequence diversity would serve as templates for vaccine development. Structure-based approaches can be used to define the epitopes recognized by broadly neutralizing antibodies and to create immunogens that focus the immune response these sites of viral vulnerability. At the Vaccine Research Center, we have proposed the expansion of this approach to include considerations of the B-cell ontogeny by which effective antibodies are generated (Kwong and Mascola 2012). In such an expanded approach, it is critical to understand not only what antibodies are made of and which sites of viral vulnerability they target, but also the immunological pathways by which antibodies are generated. In this approach, one seeks to elicit effective antibodies by replicating the pathway or pathways by which the template antibodies were generated.
One potential flaw in this approach: antibodies are created by stochastic processes of recombination and somatic hypermutation, with an estimated diversity of greater than 1012 (Boyd et al. 2009). If protective immunity occurs through different mechanisms of recognition or are created by different immunological processes, then B-cell ontogeny strategies based on “re-eliciting” similar antibodies in the general population may flounder.
The identification of multidonor antibodies, with the same mechanism of recognition and the same B-cell development pathway, however, raises hope for B-cell ontogeny strategies (Scheid et al. 2011; Wu et al. 2011; Zhou et al. 2013; Jackson et al. 2014; Truck et al. 2015). Such reproducible, “convergent,” or “public” clonal types have been observed against a number of different pathogens, and—for influenza A virus—the most well-known example involves HA-stem directed antibodies derived from the VH1-69 germline gene (Throsby et al. 2008; Ekiert et al. 2009; Sui et al. 2009; Kashyap et al. 2010). These VH1-69-derived antibodies, however, generally neutralize only group 1 subtypes of influenza A (Dreyfus et al. 2012), and it has not been clear how to induce greater neutralization breadth.
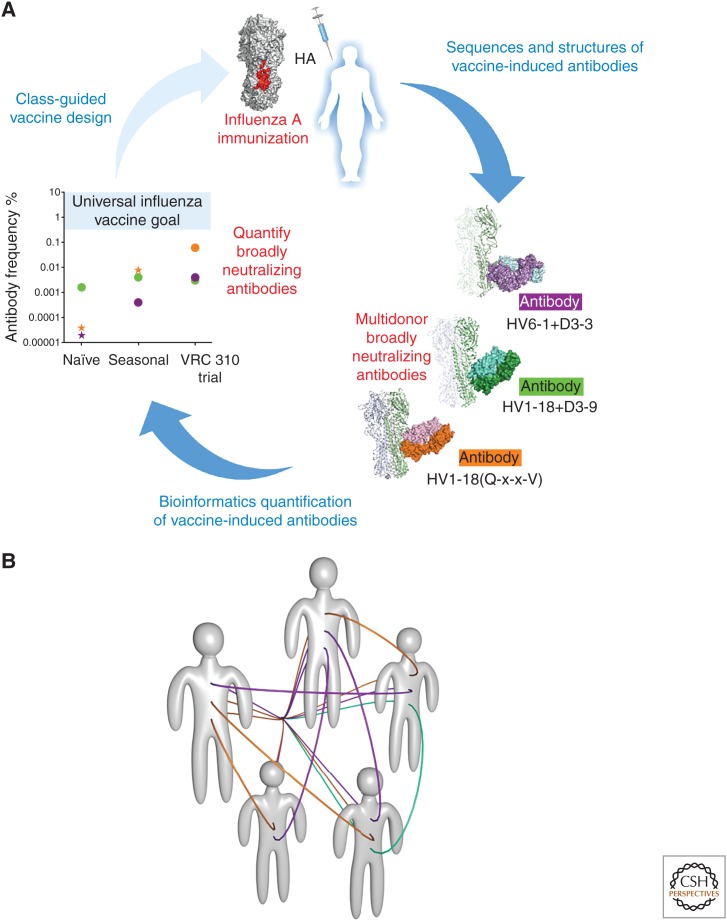
In examining cross-reactive B cells from a vaccine trial (VRC 310) involving immunizations with a divergent H5 strain of influenza A (Ledgerwood et al. 2011, 2013), however, we identified three multidonor classes of antibodies capable of neutralizing diverse strains of influenza A from both group 1 and group 2 subtypes (Fig. 2A) (Joyce et al. 2016a). Two of these multidonor classes, one derived from the VH6-1 germline gene with a D3-3 recombination and a second from the VH1-18 germline gene with a (Q-x-x-V) motif appeared to be substantially induced by both seasonal and divergent H5 immunization (Joyce et al. 2016a). Antibodies from both of these multidonor classes recognize overlapping epitopes in the conserved HA stem, thereby enabling structure-based strategies involving stem-only immunogens (Impagliazzo et al. 2015; Yassine et al. 2015), chimeric immunogens (Krammer et al. 2015), or immunization with diverse HAs (Ledgerwood et al. 2011). Notably, the VRC 310 regimen appeared to increase the frequency of the VH1-18 (Q-x-x-V) class by over 1000-fold. B-cell ontogeny-based immunogens—specific for the VH6-1 + D3-3 germline or the VH1-18 (Q-x-x-V) germline—may induce even higher frequencies, as has been shown for VRC01-class antibodies with knockin mice (Dosenovic et al. 2015; Jardine et al. 2015). Potential solutions to the universal vaccine can thus be found at select B-cell ontogenies in multiple donors (Fig. 2B). Overall, the identification and quantification of multidonor flu antibodies, coupled with structure-based strategies of germline priming, provides an exciting approach to developing a universal influenza A vaccine.
Figure 2.
A B-cell ontogeny-based paradigm for vaccine development. (A) Reproducible antibodies, observed in multiple donors, represent vaccine solutions potentially available to the general population. Three multidonor classes of antibody (purple, orange, and green) were identified in subjects from the VRC 310 trial, which involved immunization with a diverse H5 influenza strain. Bioinformatics-delineated sequencing signatures allowed for the quantification of transcripts corresponding to these signatures, which should aid in the class-guided elicitation of these antibodies. (Panel from Joyce et al. 2016a; adapted, with permission, from Elsevier © 2016.) (B) Schematic of five humans with reproducible classes of broadly neutralizing antibodies, as represented by purple, orange, and green lines.
STRUCTURE-BASED EVASION-MECHANISM STRATEGY FOR HIV
What about viruses for which antibody templates from natural infection are exceedingly difficult to elicit? This appears to be the case for HIV, wherein antibodies from natural infection have been identified that neutralize over 90% of circulating HIV isolates, but ontogeny-based analyses indicate these antibodies may take years to mature (Liao et al. 2013; Bonsignori et al. 2016; MacLeod et al. 2016) or to require unusual recombination (Briney et al. 2012; Doria-Rose et al. 2014; Gorman et al. 2016). Would it be possible for immunization to induce antibodies through pathways distinct or more efficient than natural infection?
We hypothesized that an effective vaccine might be identified through a structure-based mechanistic strategy involving (1) identification of the evasion mechanism(s) the target pathogen uses to confound the humoral immune response, (2) use of structural biology to create immunogens that overcome each of these evasion mechanisms, and (3) combination of individual solutions into a single immunogen or immunization regime.
With HIV, the Env glycoproteins, gp120 and gp41, comprise the type 1 fusion machine that makes up the Env spike, the sole virion component to extend beyond the protective viral membrane. These assemble into a trimeric closed state recognized by most neutralizing antibodies (Munro et al. 2014). Viral mechanisms of immune evasion that shield the HIV envelope (Env) spike from antibody-mediated neutralization involve sequence variation (Starcich et al. 1986), conformational change (Kwong et al. 2002), and N-linked glycosylation (Fig. 3, left) (Wei et al. 2003). Structure-based solutions to each of these viral mechanisms of evasion have been proposed including a focus on conserved neutralization epitopes, conformational fixation of the critical prefusion closed conformation of Env, and epitope-specific deglycosylation (Fig. 3, right). However, the combination of all of these approaches into a single immunogen or immunization regimen has only recently been attempted (Kwong 2016). It remains to be seen whether such a combination structure-based mechanistic approach will succeed in eliciting HIV-neutralizing antibodies effective on diverse viral strains.
Figure 3.
A structure-based mechanistic approach to HIV vaccine development. HIV evades the humoral immune response by Env mechanisms of sequence variation, N-linked glycosylation, and conformational change (left). Solutions to each of the mechanisms of evasion have been identified by structure-based design (right). (Figure from Pancera et al. 2014; adapted, with permission, from Nature Publishing Group © 2014.)
CONCLUDING REMARKS
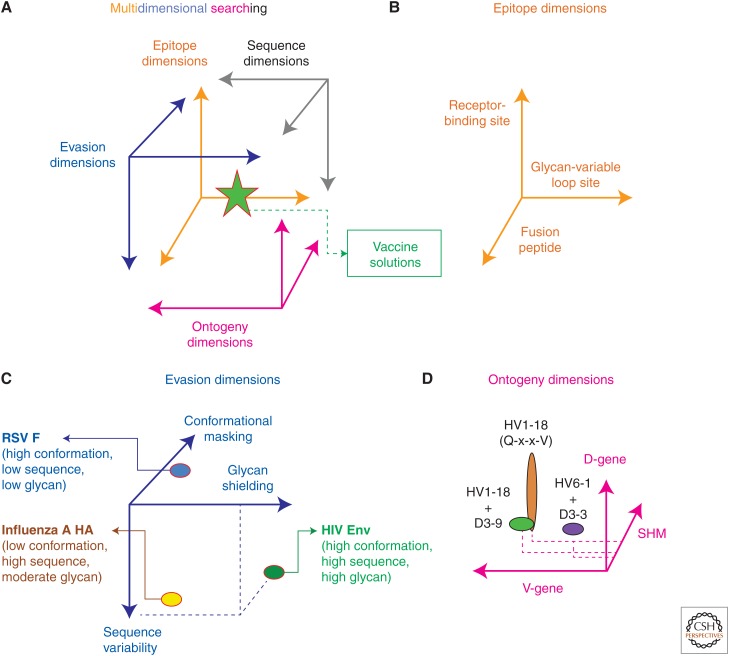
In attempting to identify the “most powerful” immunogen design strategy, it seems pertinent to inquire as to the efficiency of various search strategies, which can involve hypothesis-driven searches as well as game theory, resolution-enhancing strategies, and multidimensional searching (Nabel 2009). In truth, hypotheses can always be formulated to include all solutions (e.g., the union of a hypothesis and its null hypothesis), but such inclusiveness says little about the efficiency of the hypothesis in identifying a solution. Similarly, multidimensional searching can be all-encompassing, but this says little about which dimensions provide the most efficient means to find a solution. For example, a 600-residue glycoprotein might be the solution to the HIV vaccine problem; however, it may be exceedingly difficult to identify this solution in the 20600 potential solutions that comprise the sequence space for a 600-residue protein made up of the 20 naturally occurring amino acids. Rather, other dimensions such as those that comprise neutralization epitopes, evasion mechanism, or B-cell ontogenies might allow for more efficient identification of vaccine solutions (Fig. 4).
Figure 4.
Coordinates of effective vaccines. (A) The search for an effective vaccine may involve multiple dimensions, each of which provide a different representation of the vaccine solution (Nabel 2009). (B) Promising targets for vaccine design include known epitopes of broadly neutralizing antibodies, such as fusion peptide (Kong et al. 2016) or supersites of vulnerability represented by the clusters of epitopes around the receptor-binding site (Zhou et al. 2015) or a glycan-variable loop site (Kong et al. 2013). (C) Searches of evasion dimensions may more efficiently allow for the identification of vaccine roadblocks and their solutions. Evasion mechanisms used by type 1 fusion machines of RSV, influenza A virus, and HIV are shown. (Structure-based solutions to evasion mechanisms for RSV are shown in Fig. 1 and for HIV in Fig. 3.) (D) Ontogeny dimensions comprising immunoglobulin-origin genes and SHM of multidonor-antibody classes represent reproducible vaccine solutions available to the general population (Joyce et al. 2016a), as described in the text and in Figure 2.
How important are combination approaches? In searching for efficient strategies to identify solutions to another multidimensional problem, that of protein crystallization, we observed (1) that the most important parameter governing protein solubility was the concentration of the precipitant, and (2) that combinations of mechanistically distinct precipitants identified crystallization conditions capable of inducing lattice formation for proteins that had otherwise resisted crystallization (Majeed et al. 2003).
Similarly, the most important parameter governing the elicitation of neutralizing antibody against a particular pathogen is likely to be the immune-evasion parameter preventing antibodies from being elicited by that pathogen. Combinations of solutions to mechanistically distinct immune-evasion parameters might similarly provide a means to identify vaccine solutions for pathogens such as HIV, which otherwise resist typical strategies of vaccine design. It will be fascinating to determine which immunogen design strategy allows for the elicitation of broadly neutralizing antibodies against HIV. In light of the difficulty of the HIV vaccine problem, whichever strategy succeeds will likely be deemed most powerful.
ACKNOWLEDGMENTS
I thank J. Stuckey for assistance with figures, G. Nabel and N. Noinaj for discussions on multidimensional searching, B. Graham, A. McDermott, and J. Mascola for vaccine design with RSV, influenza A virus, and HIV, and members of the Structural Biology Section and the Structural Bioinformatics Core Section, Vaccine Research Center, the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institutes of Health (NIH) for discussions and comments on the manuscript. Funding is provided by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, and the National Institutes of Health.
REFERENCES
- Alexander DJ, Brown IH. 2000. Recent zoonoses caused by influenza A viruses. Rev Sci Technol 19: 197–225. [DOI] [PubMed] [Google Scholar]
- Beaumont T, Bakker AQ, Yasuda E. 2012. RSV specific binding molecule. U.S. Patent 20120070446 A1. [Google Scholar]
- Bonsignori M, Zhou TQ, Sheng ZZ, Chen L, Gao F, Joyce MG, Ozorowski G, Chuang GY, Schramm CA, Wiehe K, et al. 2016. Maturation pathway from germline to broad HIV-1 neutralizer of a CD4-mimic antibody. Cell 165: 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd SD, Marshall EL, Merker JD, Maniar JM, Zhang LN, Sahaf B, Jones CD, Simen BB, Hanczaruk B, Nguyen KD, et al. 2009. Measurement and clinical monitoring of human lymphocyte clonality by massively parallel V-D-J pyrosequencing. Sci Transl Med 1: 12ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briney BS, Willis JR, Crowe JE. 2012. Human peripheral blood antibodies with long HCDR3s are established primarily at original recombination using a limited subset of germline genes. PLoS ONE 7: e36750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR. 2002. Antibodies, viruses and vaccines. Nat Rev Immunol 2: 706–713. [DOI] [PubMed] [Google Scholar]
- Carrat F, Flahault A. 2007. Influenza vaccine: The challenge of antigenic drift. Vaccine 25: 6852–6862. [DOI] [PubMed] [Google Scholar]
- Cohen J. 2013. In vaccine design, looks do matter. Science 342: 1442–1443. [DOI] [PubMed] [Google Scholar]
- Colman PM, Lawrence MC. 2003. The structural biology of type I viral membrane fusion. Nat Rev Mol Cell Biol 4: 309–319. [DOI] [PubMed] [Google Scholar]
- Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, et al. 2014. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 509: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenovic P, von Boehmer L, Escolano A, Jardine J, Freund NT, Gitlin AD, McGuire AT, Kulp DW, Oliveira T, Scharf L, et al. 2015. Immunization for HIV-1 broadly neutralizing antibodies in human Ig knockin mice. Cell 161: 1505–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, et al. 2012. Highly conserved protective epitopes on influenza B viruses. Science 337: 1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiert DC, Bhabha G, Elsliger MA, Friesen RHE, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324: 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn GM, Fries LF, Thomas DN, Smith G, Kpamegan E, Lu HX, Flyer D, Jani D, Hickman SP, Piedra PA. 2016. A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis 213: 411–422. [DOI] [PubMed] [Google Scholar]
- Gorman J, Soto C, Yang MM, Davenport TM, Guttman M, Bailer RT, Chambers M, Chuang GY, DeKosky BJ, Doria-Rose NA, et al. 2016. Structures of HIV-1 Env V1V2 with broadly neutralizing antibodies reveal commonalities that enable vaccine design. Nat Struct Mol Biol 23: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB. 2012. The burgeoning burden of respiratory syncytial virus among children. Infect Disorders Drug Targets 12: 92–97. [DOI] [PubMed] [Google Scholar]
- Hilleman MR. 1998. Six decades of vaccine development—A personal history. Nat Med 4: 507–514. [DOI] [PubMed] [Google Scholar]
- Homaira N, Rawlinson W, Snelling TL, Jaffe A. 2014. Effectiveness of Palivizumab in preventing RSV hospitalization in high risk children: A real-world perspective. Int J Pediatr 10.1155/2014/571609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu XY, Hoffman RMB, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, et al. 2015. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 349: 1301–1306. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Liu Y, Roskin KM, Glanville J, Hoh RA, Seo K, Marshall EL, Gurley TC, Moody MA, Haynes BF, et al. 2014. Human responses to influenza vaccination show seroconversion signatures and convergent antibody rearrangements. Cell Host Microbe 16: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine JG, Ota T, Sok D, Pauthner M, Kulp DW, Kalyuzhniy O, Skog PD, Thinnes TC, Bhullar D, Briney B, et al. 2015. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 349: 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MG, Wheatley AK, Thomas PV, Chuang GY, Soto C, Bailer RT, Druz A, Georgiev IS, Gillespie RA, Kanekiyo M, et al. 2016a. Vaccine-induced antibodies that neutralize group 1 and group 2 influenza A viruses. Cell 166: 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MG, Zhang B, Ou L, Chen M, Chuang GY, Druz A, Kong WP, Lai YT, Rundlet EJ, Tsybovsky Y, et al. 2016b. Iterative structure-based improvement of a fusion-glycoprotein vaccine against RSV. Nat Struct Mol Biol 23: 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron RA, Buchholz UJ, Collins PL. 2013. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol 372: 259–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap AK, Steel J, Rubrum A, Estelles A, Briante R, Ilyushina NA, Xu L, Swale RE, Faynboym AM, Foreman PK, et al. 2010. Protection from the 2009 H1N1 pandemic influenza by an antibody from combinatorial survivor-based libraries. PLoS Pathog 6: e1000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Lee JH, Doores KJ, Murin CD, Julien JP, McBride R, Liu Y, Marozsan A, Cupo A, Klasse PJ, et al. 2013. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat Struct Mol Biol 20: 796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong R, Xu K, Zhou TQ, Acharya P, Lemmin T, Liu K, Ozorowski G, Soto C, Taft JD, Bailer RT, et al. 2016. Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Science 352: 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Palese P, Steel J. 2015. Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol 386: 301–321. [DOI] [PubMed] [Google Scholar]
- Kwong PD. 2016. Structure-based immunogen design: Building on our understanding of Env-immune evasion. In HIV-1 Vaccines, Keystone Symposia on Molecular and Cellular Biology, p. 56 Olympic Valley, CA. [Google Scholar]
- Kwong PD, Mascola JR. 2012. Human antibodies that neutralize HIV-1: Identification, structures, and B cell ontogenies. Immunity 37: 412–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, et al. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420: 678–682. [DOI] [PubMed] [Google Scholar]
- Langley JM, Sales V, McGeer A, Guasparini R, Predy G, Meekison W, Li MR, Capellan J, Wang E. 2009. A dose-ranging study of a subunit Respiratory Syncytial Virus subtype A vaccine with and without aluminum phosphate adjuvantation in adults ≥65 years of age. Vaccine 27: 5913–5919. [DOI] [PubMed] [Google Scholar]
- Ledgerwood JE, Wei CJ, Hu ZH, Gordon IJ, Enama ME, Hendel CS, McTamney PM, Pearce MB, Yassine HM, Boyington JC, et al. 2011. DNA priming and influenza vaccine immunogenicity: Two phase 1 open label randomised clinical trials. Lancet Infect Dis 11: 916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood JE, Zephir K, Hu ZH, Wei CJ, Chang L, Enama ME, Hendel CS, Sitar S, Bailer RT, Koup RA, et al. 2013. Prime-boost interval matters: A randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. J Infect Dis 208: 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Lynch R, Zhou TQ, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang ZH, et al. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeroos L, Krzyzaniak MA, Helenius A, Butcher SJ. 2013. Architecture of respiratory syncytial virus revealed by electron cryotomography. Proc Natl Acad Sci 110: 11133–11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod DT, Choi NM, Briney B, Garces F, Ver LS, Landais E, Murrell B, Wrin T, Kilembe W, Liang CH, et al. 2016. Early antibody lineage diversification and independent limb maturation lead to broad HIV-1 neutralization targeting the Env high-mannose patch. Immunity 44: 1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed S, Ofek G, Belachew A, Huang CC, Zhou TQ, Kwong PD. 2003. Enhancing protein crystallization through precipitant synergy. Structure 11: 1061–1070. [DOI] [PubMed] [Google Scholar]
- McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, et al. 2013a. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, et al. 2013b. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340: 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro JB, Gorman J, Ma XC, Zhou Z, Arthos J, Burton DR, Koff WC, Courter JR, Smith AB, Kwong PD, et al. 2014. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science 346: 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD. 1997. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet 349: 1498–1504. [DOI] [PubMed] [Google Scholar]
- Nabel GJ. 2009. The coordinates of truth. Science 326: 53–54. [DOI] [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, et al. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 375: 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, Yassine HM, Moin SM, Killikelly AM, Chuang GY, et al. 2015. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 7: 309ra162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini MG, Hemming VG. 1997. Prevention and treatment recommendations for respiratory syncytial virus infection—Background and clinical experience 40 years after discovery. Drugs 54: 867–884. [DOI] [PubMed] [Google Scholar]
- Pancera M, Zhou TQ, Druz A, Georgiev IS, Soto C, Gorman J, Huang JH, Acharya P, Chuang GY, Ofek G, et al. 2014. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso PR, Hildreth SW, Hogerman DA, Speelman DJ, Lewin EB, Oren J, Smith DH. 1994. Safety and immunogenicity of a subunit respiratory syncytial virus-vaccine in children 24 to 48 months old. Pediatr Infect Dis J 13: 792–798. [DOI] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, et al. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333: 1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. 1999. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 282: 1440–1446. [DOI] [PubMed] [Google Scholar]
- Starcich BR, Hahn BH, Shaw GM, Mcneely PD, Modrow S, Wolf H, Parks ES, Parks WP, Josephs SF, Gallo RC, et al. 1986. Identification and characterization of conserved and variable regions in the envelope gene of Htlv-III/Lav, the retrovirus of AIDS. Cell 45: 637–648. [DOI] [PubMed] [Google Scholar]
- Sui JH, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, et al. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 16: 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A. 2011. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci 108: 9619–9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The IMpact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102: 531–537. [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289: 179–186. [DOI] [PubMed] [Google Scholar]
- Throsby M, van den Brink E, Jongeneelen M, Poon LLM, Alard P, Cornelissen L, Bakker A, Cox F, van Deventer E, Guan Y, et al. 2008. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ Memory B Cells. PLoS ONE 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristram DA, Welliver RC, Hogerman DA, Hildreth SW, Paradiso P. 1994. 2nd-year surveillance of recipients of a respiratory syncytial virus (RSV) F-protein subunit vaccine, PFP-1—Evaluation of antibody persistence and possible disease enhancement. Vaccine 12: 551–556. [DOI] [PubMed] [Google Scholar]
- Truck J, Ramasamy MN, Galson JD, Rance R, Parkhill J, Lunter G, Pollard AJ, Kelly DF. 2015. Identification of antigen-specific B cell receptor sequences using public repertoire analysis. J Immunol 194: 252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992. Evolution and ecology of influenza A viruses. Microbiol Rev 56: 152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XP, Decker JM, Wang SY, Hui HX, Kappes JC, Wu XY, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, et al. 2003. Erratum: Antibody neutralization and escape by HIV-1. Nature 423: 197. [DOI] [PubMed] [Google Scholar]
- Wu X, Zhou TQ, Zhu J, Zhang BS, Georgiev I, Wang C, Chen XJ, Longo NS, Louder M, McKee K, et al. 2011. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333: 1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine HM, Boyington JC, McTamney PM, Wei CJ, Kanekiyo M, Kong WP, Gallagher JR, Wang LS, Zhang Y, Joyce MG, et al. 2015. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med 21: 1065. [DOI] [PubMed] [Google Scholar]
- Zhou T, Zhu J, Wu XL, Moquin S, Zhang BS, Acharya P, Georgiev IS, Altae-Tran HR, Chuang GY, Joyce MG, et al. 2013. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity 39: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Lynch RM, Chen L, Acharya P, Wu XL, Doria-Rose NA, Joyce MG, Lingwood D, Soto C, Bailer RT, et al. 2015. Structural repertoire of HIV-1-neutralizing antibodies targeting the CD4 supersite in 14 donors. Cell 161: 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]