Abstract
Gastric cancer (GC), one of the types of tumor most prone to malignancy, is characterized by high lethality. Numerous molecular mediators of GC have been identified, including transcription factors, signaling molecules and non-coding RNAs. Recently, inhibition of angiogenesis has emerged as a potential strategy for GC therapy. In the present study, the levels of vascular endothelial growth factor (VEGF), peroxisome proliferator-activated receptor-α (PPARα) and miR-21 in GC patients and individuals without cancer, and the correlation between VEGF and miR-21, and PPARα and miR-21 expression were analyzed. In addition, the GC MKN45 cell line was treated with apatinib (a tyrosine kinase inhibitor) and aspirin (an activator of the transcription factor, PPARα) to investigate the effects of these compounds on tumorigenesis. Furthermore, the present study attempted to elucidate the molecular mechanisms of alteration of GC tumorigenesis by aspirin and apatinib. The results of the current study demonstrated that there was a higher expression of VEGF and miR-21 in GC tissues compared with that in morphologically adjacent normal tissues whereas PPARα expression was decreased. These results were confirmed in vitro, as treatment of MKN45 cells with VEGF resulted in a significant increase in miR-21 expression and a significant reduction in PPARα protein expression. Furthermore, the inhibitory effects of VEGF on PPARα mRNA and protein expression were demonstrated to be mediated by miR-21. Suppression of PPARα protein expression attenuated the inhibitory effects of miR-21 on the level of PPARα mRNA, thereby enhancing tumorigenesis in gastric cancer. Treatment of MKN45 cells with aspirin reduced the levels of phosphorylated AKT by activating PPARα, whereas treatment with apatinib inhibited the phosphorylation of vascular endothelial growth factor receptor 2 and phosphoinositide-3 kinase in MKN45 cells. Finally, treatment of MKN45 cells with apatinib and aspirin suppressed tumorigenesis by inhibiting cell proliferation, migration, invasion and colony formation. Taken together, the results of the present study indicate that treatment with a combination of aspirin and apatinib may be a potential therapeutic strategy for GC treatment.
Keywords: gastric cancer, aspirin, apatinib, phosphoinositide-3 kinase/AKT signaling pathway, peroxisome proliferator-activated receptor-α, microRNA-21
Introduction
Although the incidence of gastric cancer (GC) has declined, it remains the third-leading cause of cancer-associated mortality (1). Despite the use of numerous treatment modalities, including surgery, chemotherapy, radiotherapy and immunotherapy, disease prognosis and treatment efficacy remain poor (2,3). Evidence indicates that vascular endothelial growth factor receptor 2 (VEGFR2) serves a critical role in GC oncogenesis and angiogenesis, suggesting that this molecule may represent a potential therapeutic target (4). Stimulation of VEGER2 by VEGF can simultaneously activate several molecular pathways, including the Raf/Mitogen-activated protein kinase kinase/extracellular-related signal kinase, p38-mitogen activated protein kinase and phosphoinositide-3 kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin signaling pathways, which mediate cell proliferation, migration, and survival, respectively (5,6). Apatinib targets VEGFR2 in chemoresistant GC, improving the survival of GC patients (7,8).
MicroRNAs (miRNAs/miRs) are ~22 nucleotide in length, non-coding RNA molecules that regulate gene expression at the post-transcriptional level by binding to the 3′ untranslated region (3′UTR) of their target mRNAs (9). Numerous studies have demonstrated roles for miRNAs in human cancer (10). In particular, miR-21 was found to be aberrantly overexpressed in numerous cancer types, including those of the prostate, breast and lung (11). miR-21 also mediates tumor cell growth and metastasis by activating AKT signaling (12). Moreover, VEGF upregulates miR-21 expression in human umbilical vein endothelial cells (13) by an unknown mechanism. However, miR-21 was also found to regulate peroxisome proliferator-activated receptor-α (PPARα) in the process of endothelial inflammation (14).
PPARα is a pleiotropic molecule that transcriptionally regulates genes involved in lipid and glucose homeostasis (15). PPARα also exhibits anti-inflammatory properties, with previous studies implicating it in the progression of several types of cancer, including hepatic, kidney, breast and lung cancer (16–19). However, the role of PPARα in GC has not been studied. The anti-inflammatory effects of aspirin are reported to be mediated by PPARα activation (20). Moreover, aspirin has also been demonstrated to reduce the risk of developing GC (21), although the mechanisms underlying this effect remain unknown.
In the present study, aspirin was used to activate PPARα as a step towards elucidating the effects of miR-21 and PPARα in GC. The present study analyzed the association between PPARα, miR-21 and VEGF in patients with GC. It further identified the effect of apatinib and aspirin on GC cell proliferation. Treatment with a combination of apatinib and aspirin may represent a novel strategy to treat gastric cancer.
Materials and methods
Oligonucleotides, antibodies, reagents and kits
Oligonucleotides encoding miR-21 mimics (hsa-miR-21 mimics; cat no. HMI0371; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), a non-coding (NC) miRNA (miR-control: AGUACUGCUUACGAUACGGTT), miR-21 inhibitor (anti-miR-21; cat no. HSTUD0371; Sigma-Aldrich; Merck KGaA), and NC inhibitor (cat no. B04003; anti-miR-control; Shanghai GenePharma Co., Ltd., Shanghai, China). The pcDNA3.1-PPARα plasmid, a PPARα-specific siRNA (cat no. AM16708; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the pGL3-PPARα-3′UTR plasmid (containing miR-21-binding sequences) were gifts from Dr Han-Yang Hu (Wuhan University, Hubei, China). Mouse monoclonal antibodies (mAbs) against β-actin (cat no. sc70319, 1:4,000), PPARα (cat no. sc130640, 1:2,000) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Phosphorylated (p)-VEGFR2 antibody (cat no. ab38473, 1:1,000) was purchased from Abcam (Cambridge, MA, USA). Mouse mAbs against p-AKT (cat no. 4051, 1:2,000), AKT (cat no. 2920, 1:2,000), rabbit mAb against p-PI3K (cat no. 4228, 1:2,000) and PI3K (cat no. 4249, 1:2,000) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin (Ig)G (cat no. sc2005, 1:3,000) and HRP-conjugated goat anti-rabbit IgG (cat no. sc2004, 1:3,000) secondary antibodies were obtained from Santa Cruz Biotechnology, Inc. VEGF, aspirin and apatinib were purchased from Sigma-Aldrich; Merck KGaA. For cell transfection, Lipofectamine 2000 was purchased from Invitrogen; Thermo Fisher Scientific, Inc. Finally, the human peroxisome proliferators activator receptors α ELISA kit (CSB-E09754h) and, human vascular endothelial cell growth factor ELISA kit (CSB-E11718h) were purchased from Cusabio (College Park, MD, USA).
Human brain tissue, cell culture, and transfection
A total of 30 patients (19 male, 11 female; age range, 45–60 years; mean age, 55.7) undergoing GC surgery at Inner Mongolia Autonomous Region People's Hospital (Hohhot, China) were enrolled in the present study. Tumor tissues and cancer-adjacent normal tissues, as well as a blood sample, were obtained for use in the study. Written informed consent was obtained from all participants. In addition, 30 blood samples from individuals without cancer were used as controls in ELISA detection. All tissue samples divided into two parts. One section was used for RNA isolation, while the other was used for protein extraction. The human GCMKN1, MKN45, MKN74, and IM95 cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin (EMD Millipore, Billerica, MA, USA) at 37°C in a humidified chamber with 5% CO2.
Transfection procedures were performed using Lipofectamine 2000 reagent according to the manufacturer's protocol. Briefly, MKN45 cells were cultured in 6-well plates in RPMI-1640 medium at 37°C in a humidified chamber with 5% CO2. When the cells were 80% confluent, the culture medium was changed for OPTI-MEM (cat no. 31985088; Gibco; Thermo Fisher Scientific, Inc.), and MKN45 cells transfected with miR-21 mimics, miR-21 inhibitors, or NC controls at a concentration of 150 pmol/ml. To analyze the effect of PPARα on AKT expression, PPARα plasmid (7 µg/ml) and PPARα specific siRNA (150 pmol/ml) were transfected into MKN45 cells. After 48 h, the cells were harvested for the luciferase reporter assay and western blot analysis. All experiments were approved by the Inner Mongolia Autonomous Region People's Hospital Ethics Committee.
ELISA
The serum was obtained from patients with GC and healthy individuals, and the levels of VEGF and PPARα in the peripheral blood were detected using ELISA kits according to the manufacturer's instructions. Absorbance was detected at 450 nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each assay was performed in triplicate, and the results were averaged over three independent experiments.
Reverse transcription-quantitative PCR (RT-qPCR) and western blotting
Tumor tissues and cancer-adjacent normal tissues were washed twice with ice-cold TBS, and RNA was extracted with TRIzol reagent (Takara Bio, Inc., Otsu, Japan) according to the manufacturer's instructions. VEGF (8 ng/ml)-treated MKN45 cells or transfected MKN45 cells were used in PCR analysis. cDNA was synthesized using a Mir-X™ miRNA FirstStrand Synthesis kit (cat no. 638315; Takara Bio, Inc.) according to the manufacturer's protocol. A Mir-X™ miRNA RT-qPCR SYBR® kit (cat no. 638314; Takara Bio, Inc.) was used to amplify mature miR-21. The sequences of the PCR primers used are as follows: miR-21 forward, 5′-ACGTTGTGTAGCTTATCAGTG-3′ (the reverse primer was supplied in the Mir-X™ miRNA RT-qPCR SYBR® kit); U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The expression levels of miR-21 were normalized to the U6 RNA. Thermocycling conditions were as follows and according to the manufacturer's protocol: Denaturation at 95°C for 10 sec followed by 40 cycles at 95°C for 5 sec and at 65°C for 20 sec. Dissociation curve conditions were as follows; 95°C for 60 sec, 55°C for 30 sec and 95°C for 30 sec. Data were analyzed using the 2−ΔΔCq method (22).
For protein detection, the cells were lysed using ice-cold RIPA buffer (cat no. 89900; Thermo Fisher Scientific, Inc.) and centrifuged at 4°C, 12,000 × g, for 5 min. Protein concentrations were determined using a BCA protein assay kit (Beyotime Biotechnology, Shanghai, China). Samples were heated at 95°C for 5 min and then supersonicated. Total cellular proteins (30 µg/lane) were subjected to electrophoresis in 12% polyacrylamide gel. The proteins in the gel were then transferred to a polyvinylidene difluoride membrane. Following the transfer, the membrane was blocked at 37°C for 1 h with 5% non-fat milk in tris-buffered saline (pH 7.6), with 0.05% Tween-20. The blots were then separately incubated with primary mouse anti-human β-actin (1:4,000), PPARα (1:2,000), VEGFR (1:1,000), AKT (1:2,000) and PI3K (1:2,000) antibodies, and then with HRP-conjugated goat anti-mouse IgG secondary antibody (1:3,000). Chemiluminescence signals were detected using an enhanced chemiluminescence western blotting kit (cat no. 32106; Thermo Fisher Scientific, Inc.). Scanned western blot images were analyzed semi-quantitatively using QuantityOne software (cat no. 1709600; Bio-Rad Laboratories, Inc.). The relative intensity values of bands were calculated using FluorChem 2.0 software (ProteinSimple, San Jose, CA, USA) and normalized to β-actin. Each assay was performed in triplicate, and the results were averaged over three independent experiments.
Luciferase activity assay
To elucidate the regulatory effects of miR-21 on PPARα, bioinformatic methods were used to identify the targets of miR-21 in GC cells. The binding target of miR-21 was predicted using the online software Target Scan Human 7.0 (http://www.targetscan.org/vert_71/).
For the luciferase reporter assay, 2×105 MKN45 cells were seeded in 24-well plates, and PGL3-PPARα-3′UTR plasmid and Renilla luciferase vector (cat no. E1751; Promega Corporation, Madison, WI, USA) were used to co-transfect MKN45 cells for 48 h. Renilla luciferase was used as an internal control for normalization. Luciferase activity was detected using the Dual-Luciferase® Reporter Assay system (cat no. E1910; Promega Corporation) according to the manufacturer's protocol. In brief, the cells were co-transfected with 14 µg pGL3-PPARα-3′UTR plasmid and 150 pmol either miR-21mimic, inhibitor or negative control using Lipofectamine 2000. The cells were lysed 12 h after transfection, and luciferase activity was measured using the Dual-Luciferase Reporter Assay system (Promega Corporation) according to the manufacturer's protocol. Additionally, MKN45 cells were transfected with miRNA mimics or inhibitor (150 pmol/ml) and collected 48 h later for analysis of PPARα by western blotting. Each assay was performed in triplicate, and the results were averaged over three independent experiments.
Immunofluorescence and confocal microscopy
MKN45 cells (1×106) were cultured in culture dishes in RPMI-1640, and the cells were treated with aspirin (1 mM) or apatinib (0.1 mM) for 24 h. The control cells were treated with DMSO at 37°C. Immunofluorescence was performed as reported by Zhang et al (23). Briefly, the cells were fixed in 4% paraformaldehyde at room temperature for 15 min and then washed with PBS, permeabilized with 0.1% Triton X-100 and subsequently blocked with 10% normal goat serum for 1 h at 37°C. The cells were subsequently incubated for 1 h at 37°C with primary antibodies against PPARα (1:200), p-AKT (1:200), p-VEGFR2 (1:100), p-PI3K (1:500) followed by 3 washes with PBS. Subsequently, the cells were incubated with fluorescein isothiocyanate-labeled goat anti-mouse IgG (1:300) and phycoerythrin-labeled goat anti-rabbit IgG (1:200) at 37°C for 1 h. The cells were then washed with PBS, and Hoechst (cat no. 23491-52-3; Sigma-Aldrich; Merck KGaA) staining was performed to visualize the nuclei at room temperature for 10 min. The stained cells were analyzed using confocal microscopy (magnification, ×600; Leica Microsystems, Inc., Buffalo Grove, IL, USA).
Cell proliferation, migration, invasion and colony formation assays
Briefly, cell proliferation was detected using the Cell Proliferation Assay kit (Promega Corporation). The 50% inhibitory concentration (IC50) values of aspirin and apatinib were calculated using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). MKN45 cells (1×105) were cultured in Biocoat™ 24-well chambers (BD Biosciences, San Jose, CA, USA) in RPMI-1640 medium and treated with aspirin (1 mM) or apatinib (0.1 mM) for 72 h. Migration assays were performed, and cell invasion assays were performed using Biocoat Matrigel invasion chambers with 8-µm pores and polycarbonate membranes (BD Biosciences). The cells in the lower chamber were counted under a light microscope (magnification, ×200) For the colony formation assay, the MKN45 cells were cultured in 12-well plates (200 cells/well) and treated with aspirin (1 mM), apatinib (0.1 mM), or aspirin (1 mM) together with apatinib (0.1 mM). Colonies >75 µm in diameter or containing >50 cells were counted as 1 positive colony. The cells were grown for 10 days (37°C with 5% CO2), and colony formation was visualized with crystal violet staining in less than 30 min at room temperature and counted using an inverted light microscope. Plate clone formation efficiency was calculated as (number of colonies/number of cells inoculated) ×100. All experiments were performed according to the manufacturer's protocol. Each assay was performed in triplicate, and the results were averaged over three independent experiments.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) or GraphPad Prism 5 (GraphPad Software, Inc.). Spearman's rank correlation coefficient analyses were performed to test for a statistically significant positive or negative correlation between VEGF, PPARα and miR-21 using GraphPad Prism. Data are expressed as the mean ± standard deviation. Statistical analysis of all examined variables was performed using analysis of variance. Post-hoc t-tests were performed using an unpaired Student's t-test or one-way analysis of variance. P<0.05 was considered to indicate a statistically significant result.
Results
VEGF and PPARα expression are associated with miR-21 levels in patients with GC
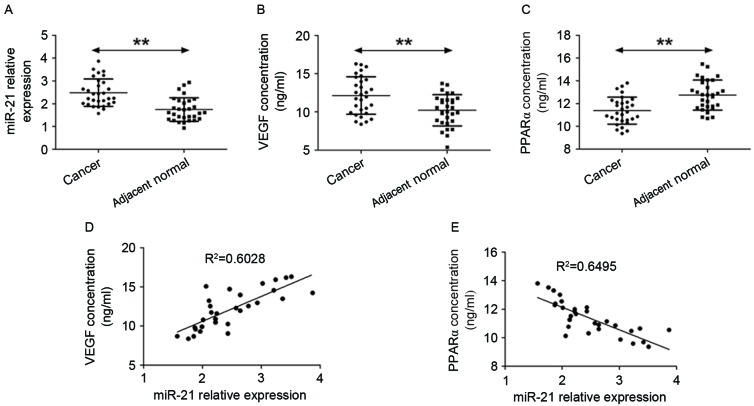
The levels of miR-21 expression were determined in GC and normal-adjacent tissue specimens by RT-qPCR. It was observed that miR-21 expression levels were higher in GC samples compared with cancer-adjacent normal tissues (Fig. 1A). Subsequently, ELISA was performed to quantify the concentrations of VEGF and PPARα in the peripheral blood of gastric cancer patients and healthy controls (Fig. 1B and C). The data show that VEGF expression was higher in GC patients compared with individuals without cancer. By contrast, the levels of PPARα in the peripheral blood of GC patients were lower compared with healthy controls. Next, the association between VEGF, PPARα and miR-21 expression was assessed and R-values were evaluated using linear regression with GraphPad Prism 5 software. It was identified thatmiR-21 levels were positively correlated with levels of VEGF expression (Fig. 1D) but negatively correlated with levels of PPARα expression (Fig. 1E).
Figure 1.
VEGF and PPARα expression are associated with the levels of miR-21 in patients with GC. (A) Reverse transcription-quantitative polymerase chain reaction analysis was performed to detect the expression of miR-21 in GC and cancer-adjacent normal tissues. (B and C). ELISA was used to analyze the expression of VEGF and PPARα in the peripheral blood of healthy controls and patients with gastric cancer. **P<0.01, cancer tissue vs. adjacent normal tissue. (D) Association between miR-21 levels and VEGF concentration. (E) Association between miR-21 levels and PPARα concentration. VEGF, vascular endothelial growth factor; PPARα, peroxisome proliferator-activated receptor-α; miR-21, microRNA-21; GC, gastric cancer.
VEGF inhibits PPARα by inducing miR-21 expression in GC cells
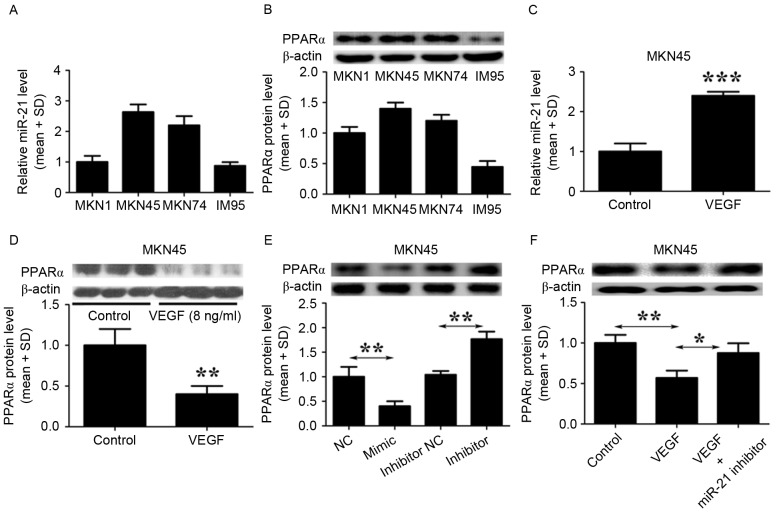
To investigate the effects of VEGF expression on the levels of miR-21 and PPARα, the levels of miR-21 and PPARα in GC cell lines (MKN1, MKN45, MKN74 and IM95) were quantified using RT-qPCR and immunoblot analysis. The basal levels of miR-21 were not significantly different across the four GC cell lines (Fig. 2A). However, three out of the four cell lines (MKN1, MKN45 and MKN74) tested expressed high levels of PPARα protein, with only IM95 cells expressing negligible levels of PPARα (Fig. 2B). Furthermore, treatment of MKN45 cells with VEGF (8 ng/ml) significantly increased miR-21 expression (Fig. 2C) but reduced the levels of PPARα protein (Fig. 2D). Subsequently, MKN45 cells were transfected with a miR-21 mimic to determine whether miR-21 is able to regulate the expression of PPARα. The expression of PPARα protein was significantly suppressed by the miR-21 mimic, and PPARα expression increased when miR-21 was inhibited (Fig. 2E). To confirm whether VEGF is able to inhibit PPARα via induction of miR-21, MKN45 cells were treated with VEGF alone, or a combination of VEGF and amiR-21 inhibitor. Levels of PPARα protein were lower in cells treated with VEGF alone but this inhibitory effect was attenuated when treatment of VEGF was combined with that of the miR-21 inhibitor (Fig. 2F). Taken together, these findings indicate that VEGF inhibits PPARα via induction of miR-21.
Figure 2.
VEGF inhibits PPARα expression by inducing miR-21 in GC cells. (A) RT-qPCR was performed to detect the baseline levels of miR-21 expression in four GC cell lines. (B) Western blotting was performed to detect the baseline levels of PPARα protein in four GC cell lines. (C) RT-qPCR was performed to detect the effect of 8 ng/ml VEGF on the expression of miR-21 in MKN45 cells. The control cells were treated with PBS. ***P<0.001, VEGF treated group vs. control group. (D) Western blotting was used to assess the levels of PPARα protein in MKN45 cells treated with VEGF. The control cells were treated with PBS. **P<0.01, VEGF treated group vs. control group. (E) Western blotting was used to evaluate the levels of PPARα protein in MKN45 cells treated with a miR-21 mimic, a miR-21 inhibitor or negative control. **P<0.01, miR-21 mimic group vs. negative control group; miR-21 inhibitor group vs. miR-21 inhibitor inhibitor group. (F) Western blotting was used to analyze the levels of PPARα protein in MKN45 cells treated either with VEGF, or with VEGF together with a miR-21 inhibitor. The control cells were treated with PBS. *P<0.05, miR-21 inhibitor with VEGF group; **P<0.01, VEGF group vs. control group. The results shown are from three representative independent experiments. VEGF, vascular endothelial growth factor; PPARα, peroxisome proliferator-activated receptor-α; miR-21, microRNA-21; GC, gastric cancer; RT-qPCR, reverse transcription-quantitative polymerase chain reaction analysis; SD, standard deviation.
Aspirin and apatinib induced PPARα and attenuated PI3K/AKT signaling in GC cells
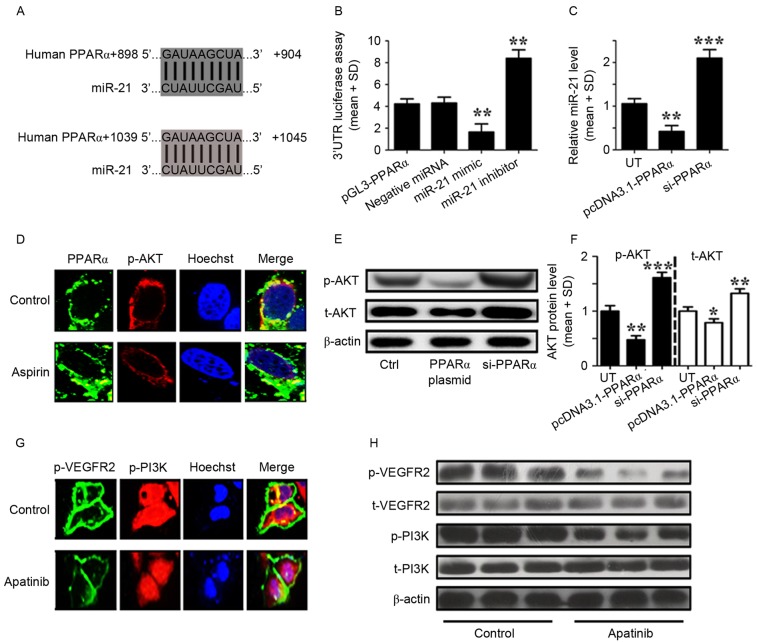
To elucidate the regulatory effects of miR-21 on PPARα, bioinformatic methods were used to identify the targets of miR-21 in GC cells. Using this approach, the 3′UTR of PPARα was identified to contain two miR-21 binding sites (Fig. 3A). A luciferase reporter assay was subsequently performed to verify the functional interaction of miR-21 with the PPARα 3′UTR, and it was demonstrated that miR-21 significantly inhibited the luciferase activity of the PPARα 3′UTR luciferase reporter in MKN45 cells (Fig. 3B). Next, MKN45 cells were transfected with a PPARα expression plasmid and found that PPARα overexpression was able to suppress the levels of miR-21 expression (Fig. 3C).
Figure 3.
Aspirin and apatinib induced PPARα expression and attenuated PI3K/AKT signaling in GC cells, respectively. (A) The PPARα 3′UTR contains binding sites for miR-21. (B) The luciferase activity of MKN45 cells was measured following co-transfection with the indicated PPARα 3′UTR reporter constructs and a miR-21 mimic, miR-21 inhibitor or negative control for 12 h. **P<0.01, miR-21 mimic group vs. negative miRNA group; miR-21 inhibitor group vs. negative miRNA group. (C) RT-qPCR was performed to detect the expression of miR-21 in MKN45 cells transfected with PPARα plasmid or siPPARα for 24 h. **P<0.01, pcDNA3.1-PPARα transfected group vs. UT group; ***P<0.001, siPPARα transfected group vs. UT group. (D) Immunofluorescence demonstrates the effects of aspirin on PPARα (green) and p-AKT (red) expression in MKN45 cells. (E) Western blotting was used to analyze the levels of p-AKT in MKN45 cells transfected with a PPARα plasmid or siPPARα. (F) The levels of p-AKT relative to the internal control (β-actin), according to western blotting results. *P<0.05, pcDNA3.1-PPARα transfected group vs. UT. (G) Immunofluorescence images showingthe effects of apatinib on p-VEGFR2 (green) and p-PI3K (red) expression in MKN45 cells. (H) Western blotting was used to analyze the levels of phosphorylated and total VEGFR2 and PI3K in MKN45 cells treated with apatinib. Control cells were treated with DMSO. The results depicted are from three representative independent experiments. VEGF, vascular endothelial growth factor; PPARα, peroxisome proliferator-activated receptor-α; miR-21, microRNA-21; PI3K, phosphoinositide-3 kinase; AKT, protein kinase B; 3′UTR, 3′ untranslated region; GC, gastric cancer; RT-qPCR, reverse transcription-quantitative polymerase chain reaction analysis; SD, standard deviation; si, small interfering; t, total; p, phosphorylated; UT, untreated cells.
As aforementioned, aspirin and apatinib induce PPARα expression and inhibit the phosphorylation of VEGFR2, respectively. In addition, it was reported that apatinib may inhibit VEGFR2 as a tyrosine kinase inhibitor (24). However, the mechanisms by which aspirin and apatinib induce PPARα expression remain to be fully understood. MKN45 cells were treated with aspirin and immunofluorescence microscopy was performed to visualize the effects of aspirin on the levels of PPARα and p-AKT. Following aspirin treatment (Fig. 3D), PPARα expression was induced (green) and the levels of p-AKT (red) were suppressed, compared with the control cells (Fig. 3D). Subsequently, western blot analysis was performed to quantify the levels of p-AKT protein in MKN45 cells following PPARα overexpression or knockdown. PPARα expression was able to reduce the levels of phosphorylated and total AKT in MKN45 cells (Fig. 3E and F). The effects of apatinib treatment on p-VEGFR2 and p-PI3K were further assessed in MKN45 cells. Following apatinib treatment (Fig. 3G), the levels of p-VEGFR2 expression in the cell membrane (green), as well as the levels of p-PI3K (red) were suppressed compared with the control cells (Fig. 3G). Finally, western blot analysis was performed to quantify the levels of VEGFR2 and PI3K protein in MKN45 cells (Fig. 3H). Levels of total VEGFR2 and PI3K were affected by apatinib treatment. However, the levels of p-VEGFER2 and p-PI3K were lower following apatinib treatment. Taken together, these results suggest that aspirin and apatinib induce PPARα and attenuate PI3K/AKT signaling in GC cells, respectively.
Aspirin and apatinib inhibit tumorigenesis of GC cells
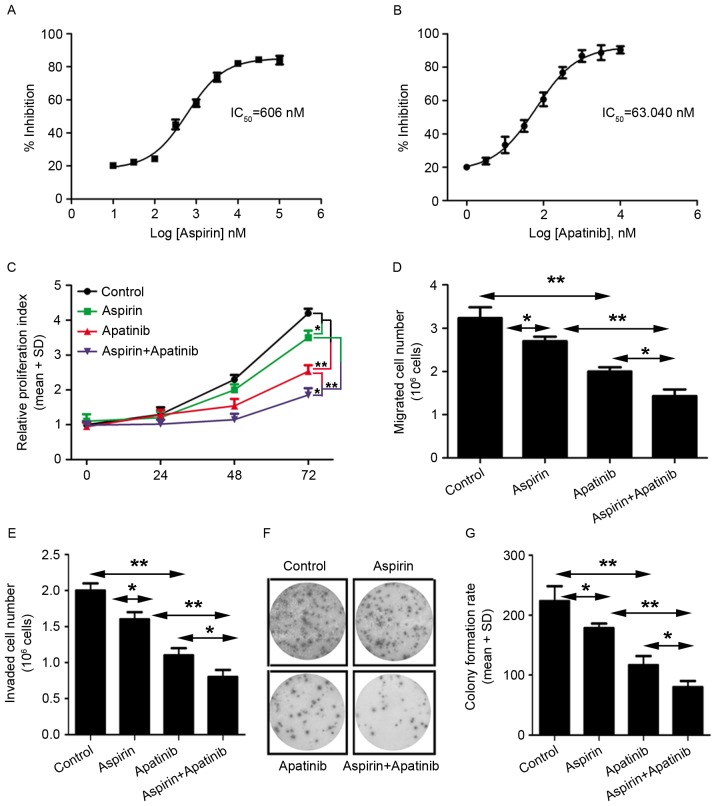
Next, the roles of aspirin and apatinib in tumorigenesis were evaluated. First, to evaluate the effects of these compounds on viability of gastric cancer cells, cell proliferation assay was performed using MKN45 cells. IC50 was calculated using GraphPad Prism 5 software. It was observed that the cells were more sensitive to apatinib treatment (IC50=63.04 nM) compared with aspirin treatment (IC50=606 nM) (Fig. 4A and B). Next, the effect of aspirin and apatinib on MKN45 cell proliferation was assessed at various time-points.
Figure 4.
Aspirin and apatinib inhibit tumorigenesis of GC cells. (A and B) The IC50 values of aspirin and apatinib were detected using a cell proliferation assay kit. (C) The proliferation of aspirin and/or apatinib-treated MKN45 cells was determined after 0, 24, 48 and 72 h. Experiments were performed in triplicate for each group. (D and E) Migration and invasion of MKN45 cells following treatment with aspirin and/or apatinib. (F) Representative micrographs and (G) quantification of crystal violet-stained cell colonies. The control cells were treated with dimethyl sulfoxide. *P<0.05, **P<0.01, aspirin or apatinib treated group vs. the control group. GC, gastric cancer; IC50, 50% inhibitory concentration; SD, standard deviation.
Treatment of GC cells with a combination of aspirin and apatinib inhibited cell proliferation, compared with treatment with either aspirin or apatinib alone (Fig. 4C). Furthermore, the effect of aspirin and apatinib on migration, colony formation and viability of MKN45 cells was evaluated (Fig. 4D-G). It was observed that treatment with a combination of aspirin and apatinib decreased migration (Fig. 4D), viability (Fig. 4E) and colony formation (Fig. 4F and G) of MKN45 cells. Taken together, these results reveal that apatinib combined with aspirin is able to inhibit proliferation and migration of gastric cancer cells, suggesting that these two agents may have potential as a combination therapy in GC.
Discussion
In the present study, aspirin and apatinib were demonstrated to exert antitumor effects in GC cells. Recently, inhibition of angiogenesis has emerged as a potential therapeutic strategy for GC (25). However, anti-angiogenic agents, including bevacizumab, sunitinib and sorafenib, have failed to increase patient survival (26). Apatinib, a novel tyrosine kinase inhibitor that targets VEGFR2 kinase, has generated positive results in initial preclinical and clinical studies involving GC patients (26,27). There is dispute about the efficacy and side effects of apatinib; it was associated with significant survival prolongation compared with placebo in a chemorefractory Chinese population (24). However, contrasting results have been obtained in different populations (28,29). Numerous studies have demonstrated that p-VEGFR2 is able to activate the PI3K/AKT signaling pathway, which regulates critical tumorigenic processes, including cellular proliferation, survival, growth and motility (30). In addition, other studies have indicated a role for miR-21 in cancer (11). Specifically, miR-21 was reported to inhibit PPARα, a transcriptional activator and known regulator of fatty acid metabolism (31). In recent years, PPARα has also been demonstrated to mediate the development of several types of cancer, including those of the lung, liver and colon (19,32). Although VEGF and miR-21 serve important functions in cancer development, the association between them in GC remains to be fully understood. The present study reported a novel treatment against gastric cancer that suggested combining aspirin and apatinib might inhibit GC growth in vitro. That aspirin, an inhibitor of the enzyme cyclooxygenase, may be used as an antitumor drug is supported by the present study. Aspirin may inhibit GC cell proliferation in vitro. Apatinib may be used as a treatment for heavily pretreated patients with GC by targeting VEGFR (33,34). However, VEGF, a ligand of VEGFR, may promote GC cell proliferation by inhibiting PPARα. The present study provided a novel strategy in which aspirin may be combined with a certain specific antitumor drug to improve the curative effect.
In the present study, miR-21expression and the concentration of VEGF protein were higher in tissue from gastric cancer patients, whereas the levels of PPARα were lower compared with adjacent normal tissues. A correlation analysis revealed that miR-21 levels were positively correlated with the levels of VEGF, and negatively correlated with the levels of PPARα. In addition, treatment of MKN45 GC cells with VEGF (8 ng/ml) significantly increased the expression of miR-21. The level of PPARα protein was significantly decreased by VEGF treatment, and this inhibitory effect was attenuated by treatment with a miR-21 inhibitor. Furthermore, two miR-21 binding sequences were identified in the 3′UTR of the PPARα gene. It was demonstrated that miR-21 was able to directly suppress PPARα expression by binding to these sites. Notably, miR-21 expression was decreased when PPARα was overexpressed in MKN45 cells, whereas the levels of total and p-AKT protein were significantly reduced. These results indicate the presence of a signaling loop consisting of miR-21 and PPARα. Specifically, miR-21 may repress PPARα by directly targeting its 3′UTR. In turn, decreased PPARα expression reduces the inhibition of AKT activation and increases the expression of miR-21. Ultimately, miR-21 promotes AKT signaling and increases the inhibitory effects of miR-21 on PPARα.
To investigate the role of PPARα in gastric cancer, MKN45 cells were treated with aspirin. In the clinic, aspirin is widely used to treat fever, pain and inflammation (35), and may effectively prevent certain types of cancer (36–38). However, the molecular mechanisms by which aspirin alters cancer risk remain unknown. Recently, aspirin was reported to be a potential PPARα activator (19). In agreement with these findings, the results of the present study demonstrated that aspirin was able to increase PPARα protein expression and reduce levels of p-AKT in MKN45 cells. Additionally, the levels of p-VEGFR2 and p-PI3K were decreased in apatinib-treated MKN45 cells. These results suggest that treatment with aspirin and apatinib was able to increase PPARα protein expression and reduce the levels of p-VEGFR2 and p-PI3K, respectively, thereby blocking PI3K/AKT signaling. Finally, to observe the effects of aspirin and apatinib on MKN45 cell tumorigenesis, MKN45 cells were treated with aspirin or apatinib alone, or the two compounds in combination. Following treatment with aspirin and apatinib together, proliferation, migration, invasion and colony formation of MKN45 cells were inhibited.
In summary, the results of the present study indicate that aspirin and apatinib are able to stimulate PPARα and inhibit VEGFR2 phosphorylation, respectively. Activated PPARα reduced the levels of phosphorylated and total AKT, and decreased the levels of miR-21 in GC cells. These effects attenuated the inhibitory effect of miR-21 on PPARα, ultimately leading to persistent PPARα-mediated inhibition of p-AKT. Treatment with apatinib suppressed VEGFR2 phosphorylation, which further reduced the protein levels of p-PI3K and p-AKT. In conclusion, aspirin and apatinib exert antitumor effects by blocking PI3K/AKT signaling in GC cells.
Acknowledgements
The authors would like to thank Dr Hanyang Hu (Wuhan University, Wuhan, China) for providing the pGL3-PPARα-3′UTR plasmid and analyzing miRNA targets and transfection, Dr Yushan Ren (Lunan Pharmaceutical Group Co., Linyi, China) for discussion and Dr Jie Li (Lunan Pharmaceutical Group Co.) for technical support.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;17:CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Petrioli R, Francini E, Roviello F, Marrelli D, Fiaschi AI, Laera L, Rossi G, Bianco V, Brozzetti S, Roviello G. Sequential treatment with epirubicin, oxaliplatin and 5FU (EOF) followed by docetaxel, oxaliplatin and 5FU (DOF) in patients with advanced gastric or gastroesophageal cancer: A single-institution experience. Cancer Chemother Pharmacol. 2015;75:941–947. doi: 10.1007/s00280-015-2715-x. [DOI] [PubMed] [Google Scholar]
- 4.Ebos JM, Bocci G, Man S, Thorpe PE, Hicklin DJ, Zhou D, Jia X, Kerbel RS. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res. 2004;2:315–326. [PubMed] [Google Scholar]
- 5.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 6.Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signaling and therapeutic inhibition. Cell Signal. 2007;19:2003–2012. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 8.Ilson DH. Targeting the vascular endothelial growth factor pathway in gastric cancer: A hit or a miss? J Clin Oncol. 2016;34:1431–1432. doi: 10.1200/JCO.2015.65.8666. [DOI] [PubMed] [Google Scholar]
- 9.Chang H, Kim N, Park JH, Nam RH, Choi YJ, Lee HS, Yoon H, Shin CM, Park YS, Kim JM, Lee DH. Different microRNA expression levels in gastric cancer depending on Helicobacter pylori infection. Gut Liver. 2015;9:188–196. doi: 10.5009/gnl13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suárez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009;104:442–454. doi: 10.1161/CIRCRESAHA.108.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH. miR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS One. 2011;6:e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang FS, Tian SS, Lu JJ, Ding XH, Qian CD, Ding B, Ding ZS, Jin B. Cardamonin Regulates miR-21 expression and suppresses angiogenesis induced by vascular endothelial growth factor. Biomed Res Int. 2015;2015:501581. doi: 10.1155/2015/501581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, Li JY, Chien S. MicroRNA-21 targets peroxisome proliferators-activated receptor alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci USA. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Kim T, Qu A, Gonzalez FJ. Abstract 23: Nuclear receptor PPARα activation triggers hepatic cell death in Ikkβ-deficient mice. Cancer Res. 2015;75:23. doi: 10.1158/1538-7445.AM2015-23. [DOI] [Google Scholar]
- 17.Aboud Abu O, Donohoe D, Bultman S, Fitch M, Riiff T, Hellerstein M, Weiss RH. PPARα inhibition modulates multiple reprogrammed metabolic pathways in kidney cancer and attenuates tumor growth. Am J Physiol Cell Physiol. 2015;308:C890–C898. doi: 10.1152/ajpcell.00322.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandran K, Goswami S, Sharma-walia N. Implications of a peroxisome proliferator-activated receptor alpha (PPARα) ligand clofibrate in breast cancer. Oncotarget. 2016;7:15577–15599. doi: 10.18632/oncotarget.6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skrypnyk N, Chen X, Hu W, Su Y, Mont S, Yang S, Gangadhariah M, Wei S, Falck JR, Jat JL, et al. PPARα activation can help prevent and treat non-small cell lung cancer. Cancer Res. 2014;74:621–631. doi: 10.1158/0008-5472.CAN-13-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Planagumà A, Titos E, López-Parra M, Gaya J, Pueyo G, Arroyo V, Clària J. Aspirin (ASA) regulates 5-lipoxygenase activity and peroxisome proliferator-activated receptor alpha-mediated CINC-1 release in rat liver cells: Novel actions of lipoxin A4 (LXA4) and ASA-triggered 15-epi-LXA4. FASEB J. 2002;16:1937–1939. doi: 10.1096/fj.02-0224fje. [DOI] [PubMed] [Google Scholar]
- 21.Søgaard KK, Farkas DK, Pedersen L, Lund JL, Thomsen RW, Sørensen HT. Long-term risk of gastrointestinal cancers in persons with gastric or duodenal ulcers. Cancer Med. 2016;5:1341–1351. doi: 10.1002/cam4.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SS, Nam JS, Cho HJ, Won JH, Kim JW, Ji JH, Yang MJ, Park JH, Noh CK, Shin SJ, et al. Plasma microRNA-122 as a predictive marker for treatment response following transarterial chemoembolization in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2017;32:199–207. doi: 10.1111/jgh.13448. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Li Y. miR-148a downregulates the expression of transforming growth factor-β2 and SMAD2 in gastric cancer. Int J Oncol. 2016;48:1877–1885. doi: 10.3892/ijo.2016.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontana E, Smyth EC. Novel targets in the treatment of advanced gastric cancer: A perspective review. Ther Adv Med Oncol. 2016;8:113–125. doi: 10.1177/1758834015616935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research Network: Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roviello G, Petrioli R, Marano L, Polom K, Marrelli D, Perrella A, Roviello F. Angiogenesis inhibitors in gastric and gastroesophageal junction cancer. Gastric Cancer. 2016;19:31–41. doi: 10.1007/s10120-015-0537-5. [DOI] [PubMed] [Google Scholar]
- 27.Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase withpotent activity in vitro and in vivo. Cancer Sci. 2011;102:1374–1380. doi: 10.1111/j.1349-7006.2011.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazăr DC, Tăban S, Cornianu M, Faur A, Goldiş A. New advances in targeted gastric cancer treatment. World J Gastroenterol. 2016;22:6776–6799. doi: 10.3748/wjg.v22.i30.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornaro L, Vasile E, Falcone A. Apatinib in advanced gastric cancer: A doubtful step forward. J Clin Oncol. 2016 Aug 15; doi: 10.1200/JCO.2016.68.6931. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/S1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed W, Ziouzenkova O, Brown J, Devchand P, Francis S, Kadakia M, Kanda T, Orasanu G, Sharlach M, Zandbergen F, Plutzky J. PPARs and their metabolic modulation: New mechanisms for transcriptional regulation? J Intern Med. 2007;262:184–198. doi: 10.1111/j.1365-2796.2007.01825.x. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez FJ, Shah YM. PPARalpha: Mechanism of species differences and hepatocarcinogenesis of peroxisome proliferators. Toxicology. 2012;246:2–8. doi: 10.1016/j.tox.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, Tong Z, Zhang Q, Wang X, Feng J, Pang D, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135:1961–1969. doi: 10.1002/ijc.28829. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 35.Sweetman SC, editor. Martindale: The Complete Drug Reference. 37th. Pharmaceutical Press; London, UK: 2011. [Google Scholar]
- 36.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 37.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 38.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]