Abstract
Aviscumine, a recombinant lectin I, has been identified as an immunomodulatory agent within a new class of ribotoxic stress-inducing anticancer substances that have demonstrated efficacy in phase I/II trials. The aim of the present study was to elucidate the presumed effect of aviscumine on enhancing human natural killer (NK) cell antitumor cytotoxicity. To measure the effect of aviscumine on human NK cell cytotoxicity, chromium-51-release assays against K-562 cells were performed with isolated NK cells from the whole blood of 34 healthy volunteers. Two effector-to-target cell ratios (12.5:1 and 25:1) were used by two independent investigators with a focus on the concentration-dependent effect (0.5 vs. 1 ng/ml aviscumine), reproducibility (first vs. second investigator) and the specificity of the effect by comparison to a heat-inactivated aliquot and interleukin 2 (IL-2) stimulation (10 ng/ml). The mediation of the effect via degranulation was demonstrated by flow cytometric analyses of CD107α expression. Statistics were performed with SPSS using Student's t-tests for normally distributed data. Aviscumine induced a significant and reproducible, concentration-dependent increase in NK cell cytotoxicity (n=22; P<0.01 for both concentrations and ratios), which was also demonstrated when administered in combination with IL-2 (n=12; 12.5:1 ratio, P<0.001; 25:1 ratio, P=0.025) and when compared with the heat-inactivated aliquots (n=12; 12.5:1, P=0.004; 25:1 ratio, P=0.007). The mediation of its effect via interferon γ degranulation was demonstrated by significantly enhanced CD107α expression (n=7; P=0.005). Taken together, the results indicate that aviscumine induced an increase in NK cell anticancer cytotoxicity. These results highlight its clinical potential as an immunostimulatory agent, particularly with regard to combined use with chemotherapeutics or immune checkpoint inhibitors. However, further studies are required.
Keywords: ADCC, immunotherapy, natural killer cells, antitumor cytotoxicity
Introduction
In recent years immunoactive agents have raised the bar for cancer therapies (1,2). In addition to immune checkpoint inhibitors [such as inhibitors of programmed cell death 1 (PD-1) and PD-ligand 1, which predominantly target the T-cell crosstalk, as well as inhibitors of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)] (3), chimeric antigen receptor T cells, vaccines (4) and plant-derived proteins have been extensively studied for their immunomodulatory activity (5,6). The present study focuses on one of such plant-derived proteins: A synthesized plant lectin I named aviscumine. Aviscumine is a heterodimer, which is composed of a toxic A-chain, representing a site-specific type-II ribosome-inactivating N-glycosidase, and a carbohydrate-binding subunit B, responsible for its cellular uptake (7–9). The N-glycosidase-mediated catalytic inactivation of ribosomes leads to a time- and dose-dependent inhibition of protein translation and synthesis (GI50, 1 ng/ml) in various human tumor cell lines (10–12), independently of cell cycle or proliferation status (9,12–14). As well as its direct cytotoxic effect, immunomodulatory activity of aviscumine was suggested in early clinical trials in which aviscumine demonstrated a clinical efficacy in various types of solid tumor with tolerable toxicity profiles (15–18); this immunomodulatory effect was presumed based on increased levels of interleukin (IL) 1β, tumor necrosis factor a (TNF-α) and interferon g (IFN-γ) detected in patient sera during treatment (15). Consistently, the phase I trial (19) of subcutaneous, low-dose (nanogram range) aviscumine application demonstrated a clinical benefit in patients with progressive solid tumors subsequent to standard treatment failure with a stable disease rate of 31% (8/26 patients; median duration, 17 weeks); this trial detected elevated IL-1β, TNF-α and IFN-γ and decreased IL-6 and IL-10 levels in patient sera during the aviscumine treatment (19). The recently published phase II trial supports the clinical efficacy of aviscumine, as well as its immunostimulatory activity and potential for combined use with chemotherapeutics (17,20); however, limited data concerning its immunological activity, particularly on the innate immune system, are available.
It has been shown that lectins represent pathogen-associated molecular patterns (PAMPs) and thereby activate pattern recognition receptors (PRRs) causing the activation of the immune system via type-I phagocytic cells (21,22). Müthing et al (23) found a preferential binding of lectin I to Neu5Aca2-6Galβ1-4GlcNAc epitopes, and glycosphingolipids were also described to be overexpressed in various tumors and associated with cellular stress induction, causing cytokine release. A number of mechanisms, involving PRR-like receptors on NK cells, stress induction and crosstalk with other immune cells, may be responsible for the immunostimulatory activity of aviscumine and warrant further investigation.
Based on prior investigations, the present study focused on the immunostimulatory activity of the recombinant mistletoe lectin aviscumine on human natural killer (NK) cells via a standardized, functional assessment. The results demonstrated a significant and reproducible increase in NK cell antitumor activity via degranulation.
Materials and methods
Healthy volunteers
The study was approved by the regional ethics board (no. AN1460 294/4.15) and all healthy volunteers provided their written informed consent. In total, 34 healthy individuals, who had no major illness, coagulation disorders or acute infections at time of blood withdrawal were included in the present study (median age, 30 years; age range, 22–67 years; male vs. female: 18 vs. 16).
Recombinant mistletoe lectin
Aviscumine was provided by CYTAVIS BioPharma GmbH (Hamburg, Germany) as a pure powder. It was dissolved and diluted according to the company's manual.
Isolation of peripheral blood mononuclear cells (PBMCs) and NK cells
PBMC isolation from whole blood samples was performed via density gradient centrifugation using Lymphoprep™ (Fresenius KabiNorge AS, Oslo, Norway) according to the manufacturer's protocol. NK cells were subsequently isolated by negative depletion with magnetic cell sorting using an NK Cell Isolation kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) following the manufacturer's protocol. NK cell purity was assessed by flow cytometric analyses via quantification of CD3/CD56+-stained cells (catalog nos., 332771 and 345811; BD Pharmingen™; BD Biosciences, Heidelberg, Germany) following standard staining procedures, revealing a purity of ≥95% (data not shown). The isolated NK cells were immediately subjected to viability assessment and cellular cytotoxicity (CC) assays, including chromium-51 (51Cr)-release and degranulation analyses.
Viability assessment of NK cells under aviscumine
A standard trypan blue exclusion assay (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to assess the effects of different concentrations of aviscumine (0.1, 0.5, 1, 3 and 6 ng/ml) on NK cell viability with and without IL-2 stimulation (10 ng/ml). Aviscumine was added in the different concentrations to 25,000 NK cells seeded in triplicates in 96-well plates (n=3) and viability was assessed after 24, 36 and 72 h via manual counting in a Neubauer plate by light microscopy. Thereby, the appropriate aviscumine concentrations for use in the 51Cr-release and degranulation assays were determined.
NK cell-mediated cellular cytotoxicity
51Cr-release assay. NK cell-mediated cellular cytotoxicity was measured with a standard 51Cr-release assay (24) against K-562 cells (a chronic myeloid leukemia in blast crisis cell line; Leibniz-Institute DSMZ; German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) by two independent investigators. In brief, two different amounts of isolated NK cells (12,500 or 25,000 cells/well) were seeded in 96-well cell culture plates and incubated with the different concentrations of aviscumine (0.5 and 1 ng/ml) with or without IL-2 (10 ng/ml) in complete RPMI medium with 10% fetal calf serum, 2 mM L-glutamine and 1% penicillin/streptomycin (all from PAA Laboratories; GE Healthcare Bio-Sciences Austria GmbH, Pasching, Austria) at 37°C and 5% CO2 for 24 h. Subsequently, 51Cr-labeled [0.96 TBq (26.00 Ci)/mmol; 37 MBq (1 mCi)/ml; Hartmann Analytic, Braunschweig, Germany] K-562 cells (1,000 cells/well, pre-incubated with 100 µCi at 37°C and 5% CO2 for 1 h) were added to the pre-seeded NK cells. After 4 h of co-incubation at 37°C, the amount of 51Cr released into the supernatant was measured with a WIZARD 25 Wallac Automatic Gamma Counter (PerkinElmer, Inc., Waltham, MA, USA). All experiments were run in triplicate.
Percentage of specific lysis was calculated according to the formula reported by Ströhlein et al (25): Specific lysis (%) = 100 × (mean experimental release - mean spontaneous release)/(mean maximal release-mean spontaneous release).
The first investigator analyzed two concentrations of aviscumine (0.5 and 1 ng/ml) to determine concentration-dependent effects. The second investigator extended the experimental setting by the addition of IL-2 stimulation and analysis of a heat-inactivated batch of aviscumine. For IL-2 stimulation 10 ng/ml IL-2 (Sigma-Aldrich; Merck KGaA) was used. The heat inactivation of aviscumine was performed for 60 min at 90°C.
NK cell degranulation assay
NK cell function via degranulation was assesed by measurement of CD107α expression levels (n=7) on a flow cytometer. In short, 50,000 natural killer cells per tube were treated with or without aviscumine (1 ng/ml) in RPMI (PAA Laboratories; GE Healthcare Bio-Sciences Austria GmbH) overnight at 37°C in 5% CO2. Subsequent to washing with washing buffer [phosphate-buffered saline (PBS) + 0.5% bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA) + 2 nM EDTA], 1,000 K-562 cells were added and co-cultured for 4 h at 37°C and 5% CO2 together with 5 µl of CD107α (phycoerythrin-conjugated; catalog no., 555801; BD Pharmingen™; BD Biosciences) diluted in 20 µl of staining buffer [PBS + 0.5% BSA and 0.1% NaN3] for 4 h in the dark at 37°C, with the addition of 5 µl of CD56 (fluorescein isothiocyanate-conjugated; catalog no., 332771; BD Pharmingen™; BD Biosciences) and CD3 (peridinin chlorophyll-Cy5.5-conjugated; catalog no., 345811; BD Pharmingen™; BD Biosciences) for the last 25 min. This was followed by washing with the previously described wash buffer and immediate measurement via flow cytometry (FACSCalibur; BD Biosciences). Analyses were performed with Flowing Software version 2.5.0 (Perttu Terho; Cell Imaging Core, Turku Center for Biotechnology, University of Turku, Finland) based on CD107α expression levels in histogram plots of CD3− and CD56+ NK cells.
Statistical analyses
For statistical analyses SPSS Statistics version 20 (IBM SPSS, Armonk, NY, USA) was used. Following the assessment of normal data distribution via Kolmogorov-Smirnov-test, paired Student's t-tests were performed to test for significant differences between treated and untreated (control) populations. The statistical significance threshold was set at P<0.05; P<0.01 was considered to indicate high significance; 0.05<P<0.1 was referred to as a non-significant trend. Graphs show the mean values and error bars indicate one standard error of the mean.
Results
Effect of IL-2 addition under aviscumine treatment on NK cell viability
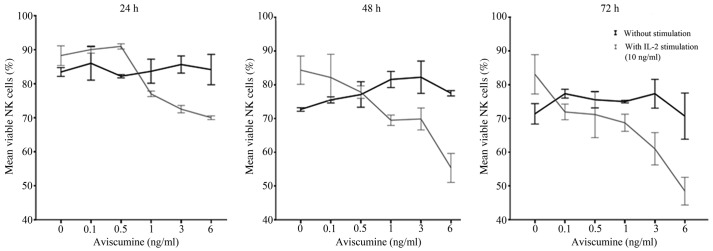
Dose-finding for subsequent immunomodulatory activity testing was performed prior to further immunological evaluations due to aviscumine's reported direct cytotoxic effects. Different aviscumine concentrations (0.1–6 ng/ml) were tested on human NK cells for various incubation times (24, 36 and 72 h) to assess these direct toxic effects. At concentrations ≤6 ng/ml no direct toxic effects on the NK cells by aviscumine were detected (Fig. 1). As further immunological testing would include IL-2 stimulation of the NK cells, viability was also assessed under the combined use of IL-2 and aviscumine. For the standard IL-2 concentration (10 ng/ml) no toxic effects were observed in the experiments (Fig. 1; 0 ng aviscumine). With the combined application of IL-2 and aviscumine a time- and concentration-dependent decrease in viability was observed (Fig. 1). Based on these results aviscumine was used at concentrations of 0.5 or 1 ng/ml in all subsequent functional assays for the assessment of its immunomodulatory capacity.
Figure 1.
Direct cytotoxic effects of aviscumine on NK cells. These investigations were run by the first investigator. Graphs show the time- and concentration-dependent changes in NK cell viability as a percentage of total counted cells under aviscumine treatment with and without IL-2 stimulation (10 ng/ml), as determined by trypan blue dye exclusion assay (n=3). Data are presented as the mean ± standard error of the mean. NK, natural killer; IL-2, interleukin 2.
Increased NK-cell mediated antitumor cytotoxicity under aviscumine
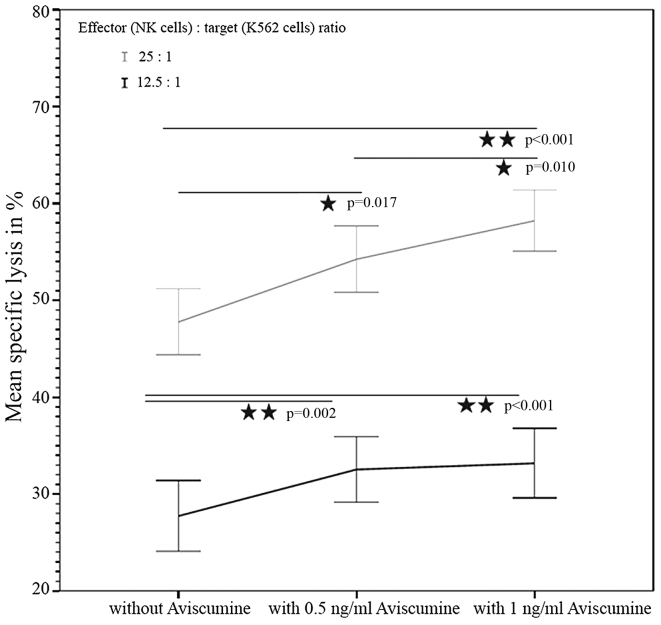
51Cr-release assay. The first investigator assessed the concentration-dependent effect of aviscumine on NK-cell mediated cytotoxicity (n=22) using a standard 51Cr-release assay. The test revealed a concentration-dependent, statistically significant increase in NK cell-mediated cytotoxicity against tumor cells following treatment with aviscumine at the two tested concentrations (0.5 and 1 ng/ml) and effector-to-target (NK:K-562) cell ratios (12.5:1 and 25:1). The mean percentages of specific lysis with 0, 0.5 and 1 ng/ml aviscumine stimulation were 27.44, 32.54 and 33.18% for the 12.5:1 effector: target ratio, and 47.76, 54.24 and 58.22% for the 25:1 effector: target ratio, respectively (Fig. 2).
Figure 2.
Increased NK cell-mediated cytotoxicity by aviscumine: Concentration-dependent effect. These experiments were performed by the first investigator. The graph shows the aviscumine-concentration-dependent increase of NK-mediated cytotoxicity against K-562 cells evaluated in 22 samples for two different concentrations. Data are presented as the mean ± standard error of the mean. Statistical results represent Student's t-test for normally distributed data. NK, natural killer.
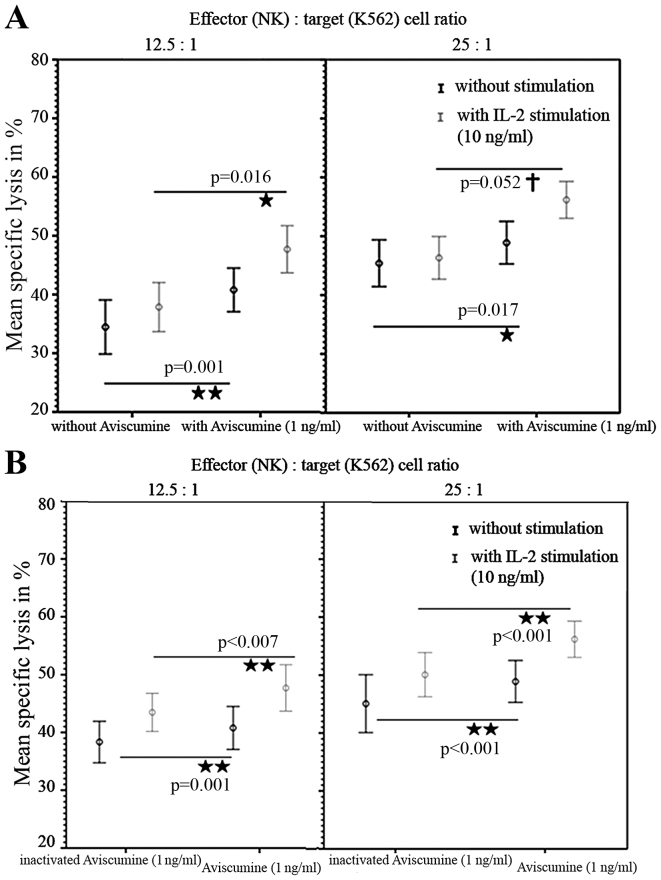
A second investigator repeated these 51Cr-release assays and confirmed the increased cytotoxic capacity of NK-cells under 1 ng/ml aviscumine stimulation (vs. no aviscumine) with 40.77 vs. 34.56% specific lysis for the 12.5:1 effector: target ratio and 48.9 vs. 45.4% for the 25:1 effector: target ratio, respectively (Fig. 3A, black lines). Furthermore, when IL-2 was used as an internal stimulation control, specific lysis in cells treated with 1 ng/ml aviscumine (vs. no aviscumine) was measured as 47.7 vs. 37.86% for the 12.5:1 ratio and 56.17 vs. 46.32% for the 25:1 ratio (Fig. 3A, gray lines) and thus demonstrated no impairment of aviscumine efficacy. In summary aviscumine treatment induced an increase in specific cell lysis of 5–10%. Although this increase was moderate, it was reproducible and reached statistical significance in various settings (Fig 2. and Fig. 3A).
Figure 3.
Increased NK cell-mediated cytotoxicity by aviscumine. Reproducible and substance-specific effects-data acquired by a second observer compared with Fig. 2. (A) Aviscumine induced a significant increase of NK cellular cytotoxicity against K-562 cells evaluated in 12 cases without and with IL-2, for two effector:target cell ratios (12.5:1 and 25:1). (B) Aviscumine induced a significant increase of NK cellular cytotoxicity against K-562 cells evaluated in 12 cases compared with a heat-inactivated aviscumine batch as a negative control, with or without IL-2 stimulation, for two effector:target cell ratios. All data are presented as the mean ± standard error of the mean. P-values were calculated via Student's t-test for normally distributed data. NK, natural killer; IL-2, interleukin 2.
To exclude any non-specific effects of aviscumine heat-inactivation (90°C for 30 min) was performed. Significant differences between the effects of aviscumine vs. its heat-inactivated form confirmed the specificity of the measured activity with 40.78 vs. 38.35% (without IL-2) and 47.7 vs. 43.48% (with IL-2) for the 12.5:1 effector:target ratio and 48.9 vs. 45.07% (without IL-2) and 56.17 vs. 50.07% (with IL-2) for the 25:1 effector:target ratio (Fig. 3B). Nevertheless, these differences were less distinct than those observed in the comparison of aviscumine with media alone (Fig. 3).
NK cell degranulation assay
The flow cytometric analyses of the expression of the degranulation marker CD107α confirmed the results of the 51Cr-release assay. The increase in CD107α expression following 1 ng/ml aviscumine treatment reached statistical significance compared with a control setting without aviscumine (69.83 vs. 57.07%; n=7; Student's t-test, P=0.005; Fig. 4).
Figure 4.
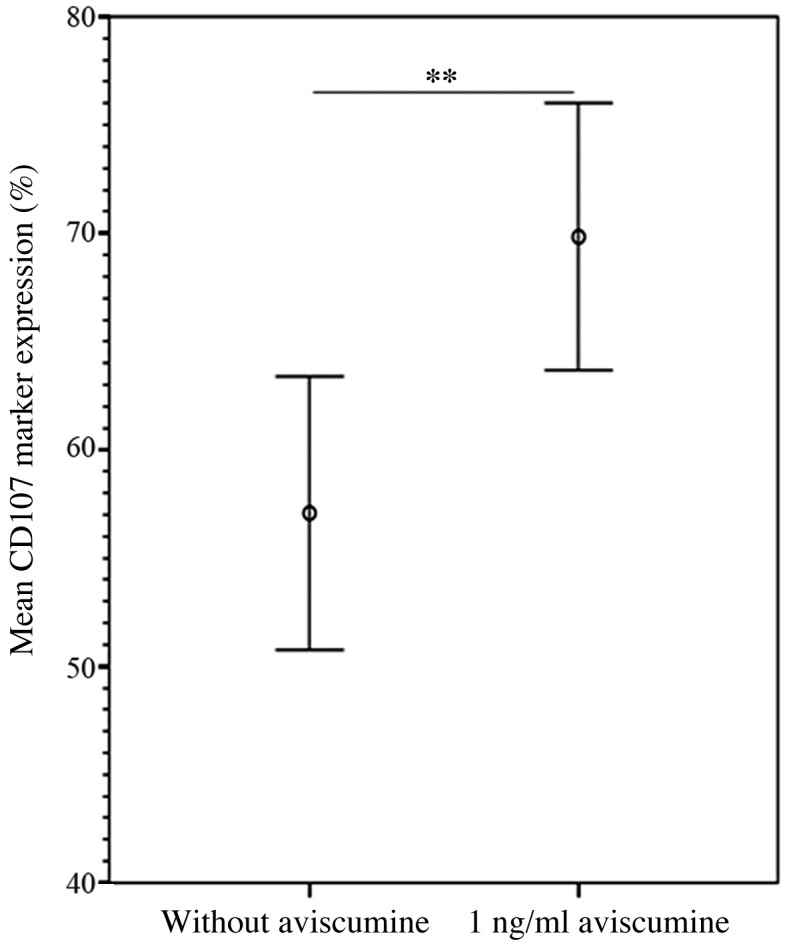
Increased NK cell-mediated cytotoxicity via CD107α-associated degranulation following aviscumine treatment. Flow cytometric analyses of the effect of aviscumine (1 ng/ml) on CD107α degranulation marker expression on natural killer cells (n=7), measured as a percentage of CD107α expression level in NK cells. Data are presented as the mean ± standard error of the mean. P-values were calculated via Student's t-test for normally distributed data (**P<0.01). NK, natural killer.
Discussion
NK cells serve a key role in tumor immunology (26–28) and NK cell cytotoxicity assays have demonstrated an impairment of NK cell activity dependent on clinical stage in numerous types of malignancy (26). Recently, immune checkpoint inhibitors, which predominantly target the T-cell population, such as PD-1 and PD-L1 inhibitors but also, CTLA-4 inhibitors were found to be capable of releasing the ‘brake’ on anticancer immunity (2). Nevertheless, with 20–30% of durable remissions with long-term survival for various cancer entities, such as lung cancer (29) or melanoma (30) under these treatment strategies, there remains a need to further improve the efficacy of therapies. Thus, combinations with other immunostimulatory agents may gain clinical interest with regard to the restoration of antitumor immunity. Besides cell therapy, few immunostimulatory agents are under clinical investigation (31). One of them is a plant-derived recombinant lectin I, aviscumine, that has demonstrated disease stabilizations in a number of solid tumors with tolerable toxicity for its immunostimulatory dose range in early clinical trials (17,19). The measured changes in patient plasma cytokine levels (increased IL-1β, TNF-α and IFN-γ and decreased IL-6 and IL-10 levels) indicate the activation of NK and T cells (19). A very recent study revealed that lectin structures represent PAMPs and thereby activate the immune system via PRRs (21–23). By focusing on NK cells, the present study was able to demonstrate a reproducible stimulation of NK cell antitumor activity for a non-toxic concentration range of aviscumine (Figs. 1–3). To the best of our knowledge, this is the first functional study to reveal these postulated effects in a standardized ex vivo human model and thereby support the prior published works.
Notably, the evaluation of aviscumine's direct toxic effects revealed a time- and concentration-dependent decrease in NK cell viability in combination with IL-2 (Fig. 1). The underlying mechanism of this observation is unknown. One potential mechanism may be activation-induced cell death, wherein an IL-2-induced upregulation of Fas ligand (an apoptosis ligand) combined with the activation of the NK cell receptor induces apoptosis (32).
The specificity of aviscumine's effect on NK cell stimulation was also confirmed by comparison with the effect of a heat-inactivated aliquot (Fig. 3), even though differences were smaller. Furthermore, the results were reassessed by flow cytometric analysis of CD107α expression, highlighting the capacity of aviscumine to enhance NK cell activity via degranulation (Fig. 4).
These data, in line with the clinical findings in early clinical trials (15,17,19), support the potential of this plant-derived recombinant lectin I as an anticancer agent, particularly with regard to its combined use with immune checkpoint inhibitors or chemotherapeutics, as postulated in case reports and clinical investigations (22,33). Nevertheless, further studies to validate the present findings and assess the detailed mechanisms and clinical efficacy of aviscumine are warranted.
Acknowledgements
The present study was financially supported by the Austrian Cancer Society Tyrol (Österreichische Krebshilfe-Krebsgesellschaft Tirol) and TEXO (Tyrolean Association of Experimental Oncology). The authors declare the following conflicts of interest: Dr Heinz Zwierzina is involved in the phase II trial of the drug as a national principal investigator; Dr Hans Lentzen is the managing director of MELEMA Pharma GmbH, Hamburg, Germany (and formerly of CYTAVIS BioPharma GmbH).
References
- 1.Brower V. Checkpoint blockade immunotherapy for cancer comes of age. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv069. pii: djv069. [DOI] [PubMed] [Google Scholar]
- 2.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Gracia JL, Labiano S, Rodriguez-Ruiz ME, Sanmamed MF, Melero I. Orchestrating immune check-point blockade for cancer immunotherapy in combinations. Curr Opin Immunol. 2014;27:89–97. doi: 10.1016/j.coi.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Gardner TA, Elzey BD, Hahn NM. Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccin Immunother. 2012;8:534–539. doi: 10.4161/hv.19795. [DOI] [PubMed] [Google Scholar]
- 5.Jiang QL, Zhang S, Tian M, Zhang SY, Xie T, Chen DY, Chen YJ, He J, Liu J, Ouyang L, Jiang X. Plant lectins, from ancient sugar-binding proteins to emerging anti-cancer drugs in apoptosis and autophagy. Cell Prolif. 2015;48:17–28. doi: 10.1111/cpr.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eck J, Langer M, Möckel B, Baur A, Rothe M, Zinke H, Lentzen H. Cloning of the mistletoe lectin gene and characterization of the recombinant A-chain. Eur J Biochem. 1999;264:775–784. doi: 10.1046/j.1432-1327.1999.00638.x. [DOI] [PubMed] [Google Scholar]
- 8.Eck J, Langer M, Möckel B, Witthohn K, Zinke H, Lentzen H. Characterization of recombinant and plant-derived mistletoe lectin and their B-chains. Eur J Biochem. 1999;265:788–797. doi: 10.1046/j.1432-1327.1999.00784.x. [DOI] [PubMed] [Google Scholar]
- 9.Langer M, Möckel B, Eck J, Zinke H, Lentzen H. Site-specific mutagenesis of mistletoe lectin: The role of RIP activity in apoptosis. Biochem Biophys Res Commun. 1999;264:944–948. doi: 10.1006/bbrc.1999.1610. [DOI] [PubMed] [Google Scholar]
- 10.Möckel B, Burger A, Schultz RJ, Wilhelm-Ogunbiyi K, Langer M, Zinke H, Fiebig HH, Lentzen H. Assessing the cancerostatic potency of rViscumin towards human tumor xenografts and cell lines in vitro. European J Cancer. 2001;37:S12. doi: 10.1016/S0959-8049(01)80352-X. [DOI] [Google Scholar]
- 11.Langer M.MB, Wilhelm-Ogunbiyi K, Witthohn K, Lentzen H. Antitumour activity of rViscumin in vitro and in vivo. 2003;26:394. [Google Scholar]
- 12.Wilhelm-Ogunbiyi K, Möckel B, Burger A, Langer M, Zinke H, Fiebig HH, et al. rViscumin, a novel anticancer agent-preclinical and clinical development status. Eur J Cancer. 2001;37(Supplement 3):S5. doi: 10.1016/S0959-8049(01)80333-6. [DOI] [Google Scholar]
- 13.Abuharbeid S, Apel J, Sander M, Fiedler B, Langer M, Zuzarte ML, Czubayko F, Aigner A. Cytotoxicity of the novel anti-cancer drug rViscumin depends on HER-2 levels in SKOV-3 cells. Biochem Biophys Res Commun. 2004;321:403–412. doi: 10.1016/j.bbrc.2004.06.160. [DOI] [PubMed] [Google Scholar]
- 14.Hostanska K, Vuong V, Rocha S, Soengas MS, Glanzmann C, Saller R, Bodis S, Pruschy M. Recombinant mistletoe lectin induces p53-independent apoptosis in tumour cells and cooperates with ionising radiation. Br J Cancer. 2003;88:1785–1792. doi: 10.1038/sj.bjc.6600982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schöffski P, Riggert S, Fumoleau P, Campone M, Bolte O, Marreaud S, Lacombe D, Baron B, Herold M, Zwierzina H, et al. Phase I trial of intravenous aviscumine (rViscumin) in patients with solid tumors: A study of the European Organization for Research and Treatment of Cancer New Drug Development Group. Ann Oncol. 2004;15:1816–1824. doi: 10.1093/annonc/mdh469. [DOI] [PubMed] [Google Scholar]
- 16.Schöffski P, Breidenbach I, Krauter J, Bolte O, Stadler M, Ganser A, Wilhelm-Ogunbiyi K, Lentzen H. Weekly 24 h infusion of aviscumine (rViscumin): A phase I study in patients with solid tumours. Eur J Cancer. 2005;41:1431–1438. doi: 10.1016/j.ejca.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Trefzer U, Gutzmer R, Wilhelm T, Schenck F, Kähler KC, Jacobi V, Witthohn K, Lentzen H, Mohr P. Treatment of unresectable stage IV metastatic melanoma with aviscumine after anti-neoplastic treatment failure: A phase II, multi-centre study. J Immunother Cancer. 2014;2:27. doi: 10.1186/s40425-014-0027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zwierzina H, Bergmann L, Fiebig H, Aamdal S, Schöffski P, Witthohn K, Lentzen H. The preclinical and clinical activity of aviscumine: A potential anticancer drug. Eur J Cancer. 2011;47:1450–1457. doi: 10.1016/j.ejca.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Bergmann L, Aamdal S, Marreaud S, Lacombe D, Herold M, Yamaguchi T, Wilhelm-Ogunbiyi K, Lentzen H, Zwierzina H. European Organisation for Research and Treatment of Cancer: Phase I trial of r viscumin (INN: Aviscumine) given subcutaneously in patients with advanced cancer: A study of the European Organisation for Research and Treatment of Cancer (EORTC protocol number 13001) Eur J Cancer. 2008;44:1657–1662. doi: 10.1016/j.ejca.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Rose A, El-Leithy T, vom Dorp F, Zakaria A, Eisenhardt A, Tschirdewahn S, Rübben H. Mistletoe Plant Extract in Patients with Nonmuscle Invasive Bladder Cancer: Results of a Phase Ib/IIa Single Group Dose Escalation Study. J Urol. 2015;194:939–943. doi: 10.1016/j.juro.2015.04.073. [DOI] [PubMed] [Google Scholar]
- 21.Kutikhin AG, Yuzhalin AE. Editorial: Pattern Recognition Receptors and Cancer. Frontiers in Immunology. 2015;6:1–2. doi: 10.3389/fimmu.2015.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirsch A, Hajto T. Case reports of sarcoma patients with optimized lectin-oriented mistletoe extract therapy. J Altern Complement Med. 2011;17:973–979. doi: 10.1089/acm.2010.0596. [DOI] [PubMed] [Google Scholar]
- 23.Müthing J, Meisen I, Bulau P, Langer M, Witthohn K, Lentzen H, Neumann U, Peter-Katalinić J. Mistletoe lectin I is a sialic acid-specific lectin with strict preference to gangliosides and glycoproteins with terminal Neu5Ac alpha 2–6Gal beta 1–4GlcNAc residues. Biochemistry. 2004;43:2996–3007. doi: 10.1021/bi0301892. [DOI] [PubMed] [Google Scholar]
- 24.Kiessling R, Klein E, Pross H, Wigzell H. ‘Natural’ killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 25.Ströhlein MA, Grützner KU, Schildberg FW, Heiss MM. Induction of cytotoxicity against autologous tumour cells by interleukin-12: Evidence for intrinsic anti-tumor immune capacity in curatively resected gastrointestinal tumour patients. Cancer Immunol Immunother. 2002;51:505–512. doi: 10.1007/s00262-002-0310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konjevic G, Jurisic V, Jovic V, Vuletic A, Martinovic Mirjacic K, Radenkovic S, Spuzic I. Investigation of NK cell function and their modulation in different malignancies. Immunol Res. 2012;52:139–156. doi: 10.1007/s12026-012-8285-7. [DOI] [PubMed] [Google Scholar]
- 27.Fregni G, Perier A, Avril MF, Caignard A. NK cells sense tumors, course of disease and treatments: Consequences for NK-based therapies. Oncoimmunology. 2012;1:38–47. doi: 10.4161/onci.1.1.18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol. 2014;44:1582–1592. doi: 10.1002/eji.201344272. [DOI] [PubMed] [Google Scholar]
- 29.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDowell KA, Hank JA, DeSantes KB, Capitini CM, Otto M, Sondel PM. NK Cell-based immunotherapies in pediatric oncology. J Pediatr Hematol Oncol. 2015;37:79–93. doi: 10.1097/MPH.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poggi A, Massaro AM, Negrini S, Contini P, Zocchi MR. Tumor-induced apoptosis of human IL-2-activated NK cells: Role of natural cytotoxicity receptors. J Immunol. 2005;174:2653–2660. doi: 10.4049/jimmunol.174.5.2653. [DOI] [PubMed] [Google Scholar]
- 33.Hajto T, Baranyai L, Kirsch A, Kuzma M, Perjési P. Can a synergistic activation of pattern recognition receptors by plant immunomodulators enhance the effect of oncologic therapy? Case Report of a patient with uterus and ovary sarcoma. Clin Case Rep Rev. 2015;1:235–238. doi: 10.15761/CCRR.1000176. [DOI] [Google Scholar]