Abstract
Clostridium difficile infection (CDI) is a major source of morbidity and mortality for hospitalized patients. Although most patients have a clinical response to existing antimicrobial therapies, recurrent infection develops in up to 30% of patients. Fecal microbiota transplant is a novel approach to this complex problem, with an efficacy rate of nearly 90% in the setting of multiple recurrent CDI. This review covers the current epidemiology of CDI (including toxigenic and nontoxigenic strains, risk factors for infection, and recurrent infection), methods of diagnosis, existing first-line therapies in CDI, the role of fecal microbiota transplant for multiple recurrent CDIs, and the potential use of fecal microbial transplant for patients with severe or refractory infection.
Keywords: Clostridium difficile infection, recurrent C difficile infection, fecal microbiota transplant, fecal transplant
Clostridium difficile is a gram-positive, toxin-producing anaerobic bacillus bacterium. When it was initially described in infants in 1935, the bacterium was difficult to culture and subsequently named Bacillus difficilis.1 C difficile is ubiquitous in the environment, being found in river water, soil, and meats.2 C difficile is also a spore-forming type of bacteria that can tolerate extreme environments.3 The spectrum in clinical presentation of C difficile infection (CDI) can vary widely in humans, ranging from asymptomatic colonization of the gastrointestinal tract to severe disease leading to toxic megacolon or intestinal perforation. Transmission of CDI occurs horizontally via the fecal-oral route. In health care settings, this is commonly through hand carriage (health care providers, patients' visitors) and environmental contamination (stethoscopes, thermometers, commodes).4-6 In this article, we review the epidemiology of C difficile infection, clinical presentations of infection, diagnosis, existing therapies, and fecal microbiota transplant (FMT) as an emerging therapy.
Epidemiology of C difficile Infection
Colonization Versus Infection
C difficile colonization is found in up to 15% of healthy adults, and its prevalence is even higher in hospitalized patients and residents of long-term care facilities.7,8 However, colonization does not mean infection. For example, the majority of infants experience transient colonization with C difficile without colitis developing.9 This transient colonization may be due to lack of a receptor that can bind the C difficile toxin, development of antibodies to C difficile toxin, protective mechanisms associated with breast-feeding, or development of intestinal bile acid metabolism.9-11 Infection due to C difficile is defined as symptoms (diarrhea) with either (1) confirmatory testing of toxigenic C difficile or (2) colonoscopic or histopathologic confirmation of pseudomembranous colitis.12 However, even this definition can be problematic because (1) it does not distinguish diarrhea from another cause along with C difficile colonization and (2) pseudomembranous colitis can have other origins. Diagnostic testing and therapeutic intervention are not recommended in asymptomatic patients because they may complicate diagnostic decision-making, and inappropriate antimicrobial therapy in CDI may lead to unnecessary alteration in the gut microbiome.
Intestinal Ecology and Dysbiosis
The microbiome of the gastrointestinal tract is integral to the overall health of its human hosts. The microbiome of the gut has coevolved in host-bacterial mutualism over time. The predominant phyla in the human gut are the Bacteroidetes (includes genus Bacteroides) and the Firmicutes (includes genera Clostridium and Eubacterium), each of which comprise about 30% of the colonic bacterial ecology.13
Disruption of the symbiotic relationship of these bacteria can lead to opportunistic organisms, including pathogens, moving into the gut flora and a phenomenon known as dysbiosis. Dysbiosis is a general term to characterize an intestinal (predominantly colonic) microbiome that is altered from its normal state, generally a decreased diversity and abundance of bacteria. Adults, even when colonized, tend not to have overt CDI develop without dysbiosis developing first. With a disruption of the intestinal microbiota, most commonly by antibiotics, C difficile can take advantage of the dysbiotic state and cause infection. With the increased use of antibiotics, the problem of CDI has reached epidemic proportions.
Incidence and Prevalence of CDI
In the past decade, the United States has seen a dramatic increase in the rates of CDI with a disproportionate increase in occurrence in elderly persons. More recently, populations such as otherwise-healthy peripartum women and healthy adults living in the community without health care or antibiotic exposure who previously have not been at risk are getting CDIs.14 In the United States in 2011, the estimated incidence of community-acquired CDI was 51.9 cases per 100 000 population, whereas the incidence of CDI associated with health care was 95.3 cases per 100 000 population.15 In the community, the rate of first recurrence was 13.5% (estimated 21 600 cases) whereas the rate of death within 30 days was 1.3% (estimated 2000 deaths). However, health care–associated CDI had a first recurrence rate of 20.9% (estimated 61 400 cases) and a rate of death within 30 days of 9.3% (estimated 27 300 deaths). Notably at this point, the majority of cases of CDI are occurring in the community, and thus CDI should no longer be thought of as a disease associated only with hospitals or health care.
In the United States and Canada, outbreaks of CDI have been attributed to a single strain of C difficile known as NAP1/BI/027 (North American NAPF type 1).16 NAP1 is thought to have increased virulence because it produces toxin A, toxin B, and binary toxin and is resistant to fluoroquinolones. Patients infected with the NAP1 strain had more severe CDIs than did patients infected with other strains.17 The NAP1 strain is also associated with decreased cure rates and increased recurrence rates of CDI.18 Although NAP1 is more common in elderly patients and patients in long-term nursing facilities, some studies have shown a lack of association between NAP1 strains and severe disease.19 The actual incidence of the NAP1/BI/027 strain varies by geographic region, and testing for the strain is useful for an epidemiologic understanding of CDI, but not for the practical care of an individual.
The burden of CDI is immense and leads to substantial health care expenditures. Between 2000 and 2002, the estimated hospital cost in the United States for CDI alone was more than $3.2 billion per year.20 In the clinical setting, CDI is the leading cause of hospital-associated infections.21
Symptoms and Severity of C difficile Infection
Signs and Symptoms
Recognizing the signs and symptoms of CDI is crucial to early intervention and therapy. The range of symptoms in CDI is broad, from mild diarrhea to fulminant colitis complicated by toxic megacolon or colonic perforation.22 The classic signs and symptoms of CDI are nonspecific and related to colitis: frequent, semiformed or watery nonbloody diarrhea with crampy abdominal pain, generally following an antibiotic trigger.23 Typically patients with CDI have nonbloody diarrhea; however, patients with inflammatory bowel disease may have bloody diarrhea.24,25 Although commonly noted, an antibiotic trigger for CDI is not required, particularly in elderly persons, hospitalized patients, and people with chronic or severe illnesses.
Severity Classification
The American College of Gastroenterology classifies CDI as follows: mild—only diarrhea; moderate—diarrhea and abdominal pain; severe—signs and symptoms meeting the criteria for systemic inflammatory response syndrome, low albumin, admission to the intensive care unit (ICU), or evidence of end-organ failure.26 Multiple classification schemes exist to classify CDI (Table 1), but no consensus has been reached on classification of severity of disease.
Table 1. Classification Schemes for Severity of Clostridium difficile Infection (CDI).
| Society | Definition of Severe Disease |
|---|---|
| American College of Gastroenterology26 | Any one of the following associated with C difficile infection:
|
| Infectious Disease Society of Americab |
|
| ATLAS criteriac |
|
Abbreviations: CDI, Clostridium difficile infection; ICU, intensive care unit; SIRS, systemic inflammatory response syndrome.
SIRS criteria: body temperature ≤ 36°C or ≥ 38°C, heart rate ≥ 90/min, respiratory rate ≥ 20/min or Paco2 < 32 mm Hg, white blood cell count ≥ 12 000/μL or ≤ 4000/μL or > 10% bands.
Expert opinion.12
Multiple Recurrent C difficile Infections
As noted, the first recurrence rate for CDI is between 13% and 20%.15 Recurrent CDI is typically defined as recurrent infection within 8 weeks of completion of antimicrobial therapy. With each subsequent recurrence in CDI, relapse rates increase significantly. Following a first recurrence, the rate for a second recurrence increases to 40% and subsequently to more than 60% for further recurrences.29 Patients with recurrent CDI are deficient in the bacterial phyla that normally dominate the colon, which may predispose them to multiple recurrences.30
Risk Factors for C difficile Infection
The major identified risk factor for CDI is antibiotic use. CDI is responsible for up to 30% of antibiotic-associated diarrhea.31 Antibiotics lead to dysbiosis, characterized by decreased diversity of the colonic microbiota. In this setting, C difficile has the potential to thrive.32 Broad-spectrum antibiotics (including therapy with multiple antimicrobial agents) and long-term antibiotic use are associated with an increased risk of CDI; however, even a single dose of antibiotics (eg, surgical prophylaxis, empiric antimicrobial therapy before establishing infectious diagnosis) can lead to CDI.33,34
Other well-established risk factors include advanced age (> 65 years), health care exposure (including hospitalization and residence in long-term care facilities), and particularly longer durations of health care exposure.35-37 In general, gastric acid suppression is thought to be a potential risk factor for CDI because of the loss of the protective mechanism against ingested bacteria and spores.38 Several meta-analyses correlated use of proton pump inhibitors with CDI, especially in critically ill patients.39,40 A complete list of risk factors for CDI can be found in Table 2.
Table 2. Risk Factors for Clostridium difficile Infection.
| Antibiotic exposure |
| Exposure to C difficile |
| Age > 65 years |
| Gastric acid suppression |
| Human immunodeficiency virus infection |
| Chemotherapy |
| Gastrointestinal tract manipulation (eg, enteric tube feeding) |
| Gastrointestinal tract surgery |
| Gastrointestinal tract disease (eg, inflammatory bowel disease) |
| Health care exposure (hospitalization, long-term care facilities) |
Patients with impaired immune response are more susceptible to CDI. Although not clinically measured, antibody responses to CDI have been studied, and patients with a decreased immunoglobulin G immune response to toxin A of C difficile have decreased cure rates and increased rates of recurrent infection.7 Immunosuppressed patients, such as those undergoing chemotherapy for malignant neoplasms or those infected with human immunodeficiency virus, are reported to be at increased risk for CDI.41,42 This greater risk may be due to increased health care exposure, increased antimicrobial exposure, or decreased immune response.
Recent gastrointestinal tract surgery or manipulation (eg, enteric tube feedings) may also be risk factors for CDI, most likely related to changes in gut microflora related to these procedures.43,44 Patients with inflammatory bowel disease (IBD) are at increased risk of CDI developing, and CDI may worsen IBD. Up to 50% of patients with IBD who had CDI develop required hospitalization, and 20% of patients ultimately required colectomy.45
Diagnosis
With advanced diagnostic testing, the presence or absence of C difficile is easily discernible. However, distinguishing colonization with C difficile from infection requires a careful history and physical examination. This distinction can present a difficult diagnostic dilemma as more than 30% of hospitalized patients may be colonized and could test positive for C difficile or its toxins on diagnostic evaluation.46,47 It is therefore recommended that testing for CDI should be performed only on loose (diarrheal) stools, unless there is concern for CDI-induced ileus.48
It is important to consider other possible causes of diarrhea, even in patients with risk factors for CDI. Other possible causes include infections with other bacteria or viruses (although these are less likely when a patient is hospitalized for longer than 72 hours), non-CDI antibiotic-related diarrhea (70% of antibiotic-related diarrhea cases), IBD, ischemic colitis, and food allergens. Patients with underlying gastrointestinal disease and CDI may present differently than otherwise “healthy” patients. For example, patients with IBD often lack pseudomembrane formation.45
Culture
Traditionally stool culture is the gold-standard diagnostic study for the identification of C difficile. However, stool culture is often not feasible in clinical practice because the culture times are impractical.49 Additionally, not all strains of C difficile produce toxin, and thus culture must be followed by specific toxigenic testing.50 Testing with stool culture is most useful in epidemiologic studies for identifying bacterial isolates.12
Enzyme Immunoassay
Enzyme immunoassay is a rapid test with a sensitivity of 75% to 94% and a specificity of 83% to 98% for the identification of C difficile toxins A and B in the stool.51 With its quick turnaround time and low cost, enzyme immunoassay was previously the most frequently used test by hospital laboratories. Unfortunately, its low sensitivity makes it a less preferable method of diagnosis because further diagnostic studies may be required in negative tests with high clinical suspicion of infection.51,52 Historically when enzyme immunoassay was used, 3 negative tests on 3 consecutive days were required to fully exclude C difficile as the cause of diarrhea; however, the clinical utility of this approach has been debated.53
Polymerase Chain Reaction
Nucleic acid amplification tests, such as polymerase chain reaction (PCR), for C difficile toxin genes are superior to toxin A and B enzyme immunoassay testing for identifying CDI.26 Most hospitals today use C difficile PCR toxin testing. PCR is highly sensitive and specific. This form of testing is recommended by the American Gastroenterological Association as a standard diagnostic test for CDI.26 With stool PCR toxin testing, a single sample is adequate. Often the turnaround time for this test is the same day or the next day. Testing on repeat days is not recommended given the high sensitivity and specificity of this test. Isothermal amplification is another promising nucleic acid amplified test similar to PCR that has been approved by the Food and Drug Administration (FDA) but currently lacks sufficient data for recommendation as a clinical diagnostic tool in CDI.26 One concern with nucleic acid amplification testing, such as PCR for toxin gene expression, is overdiagnosis because these tests do not distinguish active infection from colonization, underscoring the importance of testing only when clinically appropriate.
Other Testing
Other available laboratory, procedural, and imaging studies in the evaluation of CDI are not recommended as standard diagnostic studies. Pseudomembranes are detected in only 51% to 55% of confirmed CDIs via direct visualization with colonoscopy, sigmoidoscopy, or histopathology and may not be identified in patients with IBD.45,54 Additionally, pseudomembranes may be present in infections not related to C difficile.55,56 Computed tomography of the abdomen is neither sensitive nor specific for the identification of CDI, but it is recommended for evaluation of complications from CDI.26,57
After the diagnosis of CDI has been established, repeat testing for C difficile, via any mechanism, is not recommended during the same episode of diarrhea. Any toxin-based testing can remain positive despite treatment for several weeks.58 Similarly, testing for eradication of C difficile toxin after treatment is not recommended.26
Diagnosing Multiple Recurrent C difficile Infection
Following an initial diagnosis and treatment of CDI, diagnosis of recurrent CDI can be challenging. Often patients have diarrhea while undergoing treatment for CDI. Many of the antibiotics used for treatment of CDI (eg, vancomycin) can cause diarrhea; however, as of this writing, C difficile does not have resistance to the typical antibiotics used to treat CDI. Additionally, patients often experience post-infectious irritable bowel syndrome. Distinguishing C difficile colonization with irritable bowel syndrome from recurrent CDI can be extremely difficult and requires collection of a thorough and accurate history of symptom response and antibiotic use. Patients should demonstrate a clinical response to antibiotics against C difficile, then experience a clinical recurrence of prior symptoms within 8 weeks of cessation of antibiotics. In general for outpatients who do not have a clinical response to vancomycin or fidaxomicin, an alternative diagnosis (such as microscopic colitis, IBD, or irritable bowel syndrome) should be sought. Rather than repeating C difficile toxin testing, which may still remain positive, endoscopic evaluation while the patient is being treated with antibiotics can be helpful to determine other causes of diarrhea.
Current Therapies
The first step in CDI therapy is to identify the patient's CDI trigger and mitigate that if possible. Most commonly, the trigger is an antibiotic therapy, and consideration should be given to deescalating or discontinuing triggering antibiotics before treating CDI. In theory, decreasing or stopping antibiotic treatment allows the gut microbiota to be restored. It is also important to consider the route of administration of anti-CDI medications and other clinical variables that may be barriers to initiating therapy, such as ileus or anatomic variations in the gastrointestinal tract postoperatively. In patients with high pretest probability of infection, treatment can be initiated before laboratory confirmation of CDI, although doing so is not widely recommended, particularly given the rapid turnaround of PCR-based testing. Antiperistaltic medications are not recommended for therapy because they can mask symptoms and lead to adverse outcomes.12
Initial Therapy
Although many antibiotics can treat CDI, current guidelines suggest that initial treatment of CDI involves 1 of 2 antibiotics. For mild to moderate disease, metronidazole 500 mg orally 3 times daily for 10 days remains the first-line treatment. If the patient cannot take metronidazole, or a trial of metronidazole is done and no clinical improvement is seen within 5 to 7 days, vancomycin 125 mg orally 4 times daily for 10 days may be substituted.26 In mild to moderate CDI, fidaxomicin 200 mg by mouth twice daily for 10 days is an effective alternative to vancomycin.59 In cases of severe disease, vancomycin 125 mg orally 4 times daily for 10 days is recommended. For fulminant or complicated disease, vancomycin 500 mg orally 4 times daily plus metronidazole 500 mg intravenously every 8 hours and vancomycin 500 mg in 500 mL saline as enema 4 times daily with surgical consultation is the recommended regimen.26
Metronidazole is commonly used as the initial antibiotic of choice for the first episode of CDI, most likely due to the cost and perceived benefit of decreasing vancomycin-resistant bacteria. However, liquid vancomycin can be inexpensively compounded, and encapsulated forms of vancomycin have decreased in price. In 2012, the estimated cost of 10 days of compounded vancomycin was $25 compared with $35 for metronidazole.60 Additionally bacterial resistance rates to vancomycin appear to be similar regardless of initial metronidazole use or vancomycin use.61 Last, evidence has been reported of metronidazole failure in cases of more severe CDI, most likely related to increased prevalence of the NAP1 strain, which predisposes to more severe disease.26,62 For these reasons, oral vancomycin is becoming the antibiotic of choice for an initial episode of CDI for many providers and currently reflects the practice of the authors.
Recurrent CDI
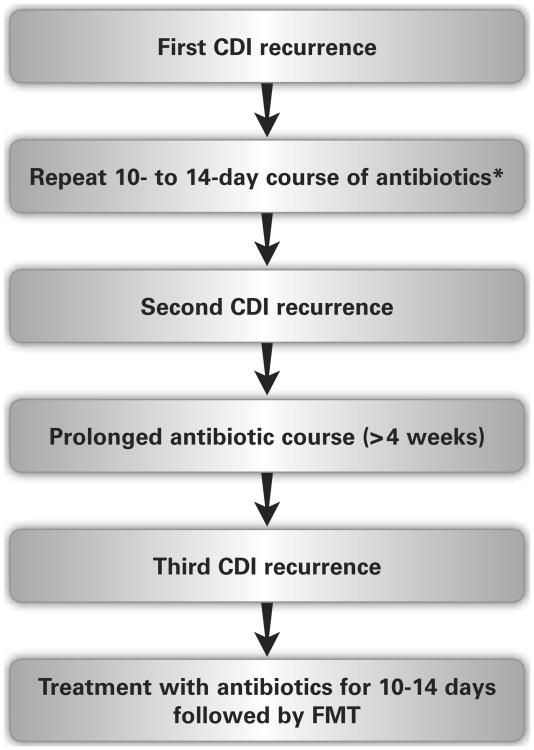
It is important to distinguish between a spontaneous recurrence and an antibiotic-triggered recurrence. A spontaneous recurrence is more likely to lead to multiple recurrent CDIs. The importance of the patient's history cannot be overstated in identifying recurrent disease because C difficile toxin can remain positive in the setting of postinfectious irritable bowel syndrome. Thus a keen practitioner must clinically determine CDI recurrence versus colonization and another cause of diarrhea. Once the presence of CDI is again established, guidelines recommend the first episode of recurrent CDI be treated with the same antibiotic chosen for initial therapy (metronidazole or vancomycin). However, it is the authors' practice to recommend vancomycin for the first spontaneous recurrence if metronidazole was used for the initial CDI. Fidaxomicin is a reasonable choice for a first recurrence following CDI treated with oral vancomycin; however, this option is often limited by cost. Recurrences beyond the second episode should not be treated with metronidazole as there is concern for neurotoxic effects with prolonged use, and there is decreased effectiveness of metronidazole in multiple recurrent CDIs.26,63 Any additional recurrence (third episode) should be treated with a prolonged (> 4 week) antibiotic course: most commonly a vancomycin taper or pulse regimen.26 Rifaximin 400 mg twice daily for 14 days after a vancomycin taper or pulse regimen had promising results; but has little role in the era of FMT (described in the following section).64 FMT should be considered for a spontaneous recurrence of CDI following a prolonged antibiotic course.26 In certain cases, FMT may be considered before the third recurrence of CDI. Immunosuppressed patients, particularly those with IBD, are at an increased risk of recurrence and consideration should be given to earlier use of FMT (see Figure).
Figure.
Proposed clinical algorithm for the management of recurrent Clostridium difficile infection (CDI). Note that the specific choice of antibiotics may vary. The most common prolonged antibiotic course would be a 6-week oral vancomycin taper; however, other prolonged courses may be acceptable as well. Before fecal microbiota transplant (FMT), the patient should be treated with antibiotics for at least 10 to 14 days to control the infection.
*Although guidelines suggest that the same antibiotics can be used for the first recurrence, we recommend using vancomycin or fidaxomicin.
When recurrences of CDI are not spontaneous (eg, multiple antibiotics for urinary tract infections), prolonged courses of antibiotics for CDI may not be needed. In this setting, treating with 10 to 14 days of oral vancomycin, followed by C difficile suppression with daily oral vancomycin until the antibiotic treatment is completed may be necessary. In some patients who require lifelong or frequent, multiple courses of antibiotics, a low dose of vancomycin can be used indefinitely for C difficile suppression. In general, FMT should not be performed when CDI follows only antibiotic use, as repeat antibiotic use will have the same effect after FMT as it did before FMT.
Probiotics and Other Therapies
Probiotics are frequently considered for CDI with regard to prevention, treatment, and as supplements to therapy. Saccharomyces boulardii and Lactobacillus have been reported to decrease the incidence of antibiotic- associated diarrhea in a recent meta-analysis and several previous studies.65,66 Although researchers in initial reports found that Saccharomyces boulardii decreased CDI recurrence when used as adjunctive therapy with vancomycin, this result was not confirmed in subsequent trials.67-69 In critically ill patients, probiotics may be detrimental because cases of fungemia or invasive infections with Lactobacillus have been reported.70,71 Last, as with many other supplements, probiotics are not regulated by the FDA. Although the theory of probiotics holds promise, the current lack of sufficient evidence and risk of adverse reactions with their use has led professional societies to recommend against the use of probiotics in the treatment of CDI.26 Other therapies for CDI, including intravenous immunoglobulin and vaccines to toxin A and B, are being studied but currently lack the therapeutic efficacy for widespread adoption.72
Fecal Microbiota Transplant
History
FMT has been present since long before modern medicine. The first documented case of ingested fecal material for medicinal purposes dates back to fourth-century Chinese medicine, when highly regarded physician Ge Hong used that technique to treat severe diarrhea or food poisoning as well as malaria. It was again documented as “yellow soup” in 16th-century China by Li Shizhen for the treatment of gastrointestinal diseases in his book, Bencao Gangmu.73 A similar practice to FMT, called “rumen transfaunation,” is widely used in veterinary medicine and was first documented in Sweden in 1776. The process involves transfer of cud (partially digested food from the first stomach) of a healthy donor animal to treat indigestion in a sick recipient animal.74 In modern medicine, the first published research on the concept of FMT was by Eiseman et al75 in 1958 and involved fecal enemas as an adjunctive treatment for antibiotic-induced pseudomembranous colitis. However, between 1958 and 2010, almost no reports have been published on this technique in the medical literature. Since 2010, FMT has become increasingly recognized as an effective therapy for multiple recurrent CDI.
FMT as a Therapy for CDI
The principle behind FMT consists of restoring a healthy gut microbiota (symbiosis) from an altered gut microbiota state (dysbiosis). This restoration is done via transfer of donor feces from a presumably healthy microbiome to that of a recipient with an altered microbiome.76 As C difficile is considered an opportunistic bacterium that causes disease in settings of dysbiosis, restoring healthy gut microflora allows competition of normal occurring microflora with that of the toxigenic strain of C difficile and subsequent resolution of infection.77 A 2010 consensus document identified 3 primary indications for considering FMT: (1) multiple recurrent CDI, (2) moderate CDI with no response to standard therapy (vancomycin or fidaxomicin) for at least 1 week, and (3) severe or fulminant CDI with no response to standard therapy in 48 hours.78
FMT for Multiple Recurrent CDI
As perpetual dysbiosis seems to be the key driver in multiple recurrent CDI, restoring a healthy colonic microbiota following treatment of CDI can break the dysbiotic cycle. With multiple courses of antimicrobials, the colonic microbiome loses its diversity and its function. Subsequent alterations in bile acids, sugar alcohols, and fatty acids can promote growth of C difficile.79,80 The bulk of evidence for FMT exists for multiple recurrent CDI. In this setting, FMT is highly effective for treating multiple recurrent CDIs with a nearly 90% cure rate in many observational studies.81,82 In the single randomized control trial for FMT, recurrent CDI was resolved in 81% of patients compared with 31% who received nontapered/nonpulsed vancomycin.83 FMT performed via lower routes of administration (colonoscopy or enema) appear to be more successful than upper routes (gastroscopy, or nasogastric and nasointestinal tubes).82 The reason for this difference in effectiveness is unclear but may be related to FMT dose or inactivation by gastric acid. FMT capsules are a promising option, and researchers in an uncontrolled study81 reported a 90% response rate in patients with recurrent CDI. FMT in special populations is largely yet to be studied. In a recent study, Khoruts et al84 noted IBD as an independent risk factor for FMT failure.
FMT for Nonresponsive CDI
In the outpatient setting, nonresponsive CDI is typically due to an alternative diagnosis for diarrhea in the presence of C difficile colonization. Rather than move quickly to FMT in this setting, an extensive search for alternative causes of diarrhea should be performed. As vancomycin can cause diarrhea in some people, a trial of another antibiotic against C difficile is reasonable. However, in severely ill patients, often in the ICU, CDI may appear to be “vancomycin resistant.” Although C difficile itself is not known to be resistant to vancomycin, antibiotic therapy may be inadequate because of overwhelming toxin production and subsequent immune response or inability of antibiotics to reach the colon due to ileus or surgical anatomy (eg, a colonic diversion or Hartmann pouch). In settings such as these, consideration of FMT as the next step in therapy may be appropriate, particularly if a patient is not a surgical candidate. FMT for antibiotic-refractory CDI has shown promise in small studies. Weingarden et al85 reported on 4 ICU patients who received colonoscopic FMT for severe CDI that was not responding to antibiotics. FMT provided short-term resolution of symptoms, with a short recurrence leading the authors to recommend FMT followed by resumption of antibiotics and a plan for a second FMT. Neemann et al86 reported a single case of CDI refractory to pharmacological treatment following allogenic stem cell transplant that was treated with nasojejunal FMT. Although other anecdotes exist for using FMT for CDI refractory to pharmacological treatment, no controlled trials have been performed and the exact clinical protocol is unknown. We recommend that in such cases the treating physicians consider consultation with a gastroenterologist who is experienced in using FMT for CDI.
Sources of Microbiota and the Donor Screening Process
The optimal donor for FMT is not known. In addition to potential infectious risks (which are most likely very small if stool is collected from asymptomatic persons), there are concerns for passing a microbiome that predisposes to other diseases, such as diabetes or heart disease. Although the magnitude of these risks is unknown, the FDA has set forth regulations regarding FMT that are focused on allowing FMT to be performed when needed, while limiting potential side effects.87
As FMT has no FDA indication, it technically requires an Investigational New Drug (IND) application in order to be performed. However the FDA has announced that for the indication of CDI not responding to standard therapy, they will exercise enforcement discretion, that is, if providers follow general ethical guidelines, FMT can be performed by a physician without an IND approval. Use of FMT for other indications still requires an IND application. Practitioners not experienced in FMT should consult the FDA guidelines before performing FMT.
FMT donors can be anyone over the age of 18, known or unknown to the patient, and willing to be a donor. These donors can be a family member, friend, significant other, or an unrelated volunteer. There are pros and cons to each type of donor. Family members, particularly maternal-line first-degree relatives may share the highest number of microbial species with the recipient. Significant others to the recipient may have the advantage of sharing environmental risk factors. Unrelated volunteers are preferred in blood donation and may be also preferred in FMT because risk factors for infectious disease may be minimized or not shared with the recipient in the case of fecal microbiota donors who are family members or loved ones.88
FMT donors should undergo rigorous screening to minimize the potential for infectious transmission. The current guidelines on FMT recommend using a donor questionnaire similar to those used with blood donation followed by serologic and stool assessment for infectious risk (Table 3) and exclusion of other conditions that could potentially be related to transmission of disease (Table 4).78,89 Much of the exclusion criteria are speculative, based on correlation with altered intestinal microflora without clear link to causation.
Table 3. Broadened Screening of Fecal Microbiota Transplant Donorsa.
| Depending on recipient's comorbid conditions or donor's exposure, consider screening for the following: |
|---|
| Giardia |
| Cryptosporidium |
| Isospora and Cyclospora |
| Escherichia coli 0157 |
| Rotavirus |
| Listeria |
| Vibrio |
| Norovirus |
| Cytomegalovirus |
| Human T-cell lymphotropic virus |
| Epstein-Barr virus |
| Dientamoeba fragilis |
| Blastocystis hominis |
| Strongyloides stercoralis |
| Entamoeba histolytica |
| Helicobacter pylori |
| Schistosoma |
| JC virus |
| Vancomycin-resistant enterococci |
| Methicillin-resistant Staphylococcus aureus |
As outlined by Kelly et al.89
Table 4. Exclusion Criteria for Fecal Microbiota Transplant Donorsa.
| Antimicrobial therapy within past 3 months |
History of gastrointestinal disease
|
| History of autoimmune disease |
| Ongoing immunomodulatory therapy |
| History of chronic pain syndromes |
| History of neurological/neurodevelopmental disorders |
| Metabolic syndrome |
| Obesity (defined as body mass index > 30) |
| Malnutrition (moderate to severe) |
| History of malignant neoplasia |
| Ongoing oncologic therapy (chemotherapy, irradiation) |
As outlined by Kelly et al.89
Donor screening can be prohibitively rigorous for physicians to perform without local experience. Because of this need, stool banking has become a common practice, although the regulatory aspects of this process are still being delineated. As part of the guidance for donor screening, the FDA stipulates that the donor be known to either the physician or the patient. In the case of banked stool, the donor is anonymous to both parties. At the time of this writing, frozen, banked stool is clinically available for physicians to use. It is likely that the FDA will impose modest regulations on stool banking in the future to limit potential side effects, while maintaining access to this clearly lifesaving intervention.
Methods of Fecal Transplant
Fecal transplant protocols are not standardized. The initial steps in preparing donor stool for FMT include diluting the specimen, usually with normal saline, followed by homogenization and filtration of the feces, if required. The prepared feces can then be used directly or even frozen for future use.81
No standardized protocol or recommendations regarding the administration of fecal microbiota for transplant are available either. Each patient's clinical presentation and personal preferences may assist with deciding on a method of administration. Methods of transplant currently used include the upper gastrointestinal tract (with endoscopy, nasointestinal tubes, or pill ingestion), the proximal part of the colon by colonoscopy, or the distal part of the colon by enema, rectal tube, or sigmoidoscopy. A combined method of administration may also be preferable in more complex cases (such as ileus or complex gastrointestinal anatomy).
Fresh or frozen stool can be used for the transplant process. Frozen stool maintains its molecular integrity and is effective in FMT.90 FMT via oral capsule with frozen feces has a efficacy rate similar to that of FMT via fresh stool.81 Nasointestinal (nasogastric or nasojejunal) tube FMT requires placement of the tube, which involves risk of vomiting (and aspiration) as well as radiation exposure while confirming placement of the tube before donor feces are administered.91 FMT via endoscopy, colonoscopy, or sigmoidoscopy not only carries risk of the transplant itself, but procedural risks such as perforation or aspiration and respiratory failure with sedation. In patients who are not procedural candidates, nasointestinal routes or enemas for FMT may be more suitable.
Cost
Another consideration in methods of fecal transplant is cost. Although an endoscopic approach to transplant may be preferable to the patient over nasogastric tube placement, it carries an added burden of expense. Interestingly, endoscopic FMT is more cost-effective than treatment with vancomycin for initial CDI.92
Safety and Patient Concerns in FMT
Although generally perceived as safe, the safety profile of FMT is not well studied owing to the lack of large cohort trials. When FMT is performed via colonoscopy, postprocedural symptoms include abdominal pain, bloating, flatulence with borborygmus, diarrhea, constipation, vomiting, transient fever, and belching. These symptoms are often transient and resolve within a few hours.82 Major adverse reactions after FMT include procedural risks in addition to risks related to the fecal transplant itself, such as pathogen exposure. Although overall the risk of pathogen exposure is thought to be low, potential transmitted pathogens include norovirus and Escherichia coli.93,94 Another concern in patients with IBD recently reported by Khoruts et al84 was flare of disease in more than 25% of patients who underwent FMT.
More prospective studies are required to identify long-term concerns related to the safety and potential risks of FMT. FMT has been studied in immunocompromised patients, and was used in 1 retrospective trial95 with no infectious complications. Another potential concern is changes in gut microbiota of the recipient after transplant. Many disease processes have been attributed to alterations in the microbiome of the gastrointestinal tract and posttransplant alterations in the microbiome may theoretically predispose patients to these conditions. Theoretical conditions that may be transmitted include obesity, diabetes mellitus type 2, atherosclerosis, IBD, nonalcoholic fatty liver disease, irritable bowel syndrome, asthma, and autism.89
Nursing Implications
CDI has particular implications for nursing, especially in the setting of FMT in an ICU. Enteric precautions with proper isolation strategies for patients with CDI are some of the most integral pieces of nursing care for these patients.96 According to the Centers for Disease Control and Prevention, precautions should include contact isolation for the duration of disease.97 Contact isolation also includes gloves and gowns for all health care staff and visitors, discontinuing antibiotics when appropriate, not sharing electronic thermometers, and ensuring consistent environmental cleaning and disinfection. Handwashing hygiene with soap and water after each patient encounter with CDI is also important as alcohol in waterless antiseptic hand cleaners lacks sporicidal activity against C difficile.12 Nursing staff should educate patients, patients' families, and patients' visitors on the importance of hand-washing hygiene.
Another consideration in the nursing care of patients is antimicrobial therapy after FMT. Populations who are inclined to have CDI develop often require antibiotics (eg, surgical prophylaxis, ongoing infection which was cause of hospitalization, susceptibility to infection given immunosuppression and advanced age). If required, these antibiotics will most likely alter the patient's gut microbiome in the future. Nurses should be alert that after FMT, antibiotic exposure can still lead to recurrent CDI and thus nurses should continue to maintain a high level of suspicion for CDI if a patient is being treated with antibiotics.
Last, FMT performed in the hospital requires particular nursing care after FMT. Patients undergoing transplant are often bedridden and are most likely in an environment that is highly contaminated with C difficile spores. Immediately before FMT, attempts should be made to thoroughly clean the patient's room with an alcohol-based cleaner. Alcohol-based cleaners are typically sufficient because the reservoir of C difficile spores is the patient rather than the environment. In outbreak-type situations, bleach-based cleaner may be preferred, however, the risks of bleach-based cleaning (eg, corrosion) must be weighed against the benefits.98 If possible, a new (or thoroughly cleaned bed) and fresh sheets should be obtained. Minimizing the C difficile spore burden may improve FMT effectiveness rates for inpatients.
Conclusion
CDI has reached epidemic proportions in the United States and in many places around the world. Reducing the burden of CDI requires judicious use of antibiotics and improved health care precautions to decrease transmission. CDI can pose a diagnostic dilemma because tests do not allow infection to be distinguished from colonization. Although many antibiotics can treat C difficile, oral vancomycin is most likely the most cost-effective therapy. FMT has revolutionized the treatment of CDI and is becoming a more widely used therapeutic option for multiple recurrent CDI. It is also an option for CDI not responding to standard therapy, although significantly more research needs to be done in this area before it can be routinely recommended. The future of CDI treatment will most likely involve more advanced forms of FMT such as capsules, advanced probiotics, and prebiotics.
Acknowledgments
Byron P. Vaughn is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. He has received research support from Genentech-Roche and speaking/consulting fees from Abbvie and Janssen.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Alyssa Liubakka, Department of Medicine, University of Minnesota, 420 Delaware Street SE, MMC 284, Minneapolis, MN 55455.
Byron P. Vaughn, Division of Gastroenterology, Hepatology, and Nutrition, and part of the Microbiota Therapeutics Program, University of Minnesota, Minneapolis, Minnesota.
References
- 1.Hall IC, O'Toole E. Intestinal flora in new-born infants: with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49(2):390–402. [Google Scholar]
- 2.al Saif N, Brazier JS. The distribution of Clostridium difficile in the environment of South Wales. J Med Microbiol. 1996;45(2):133–137. doi: 10.1099/00222615-45-2-133. [DOI] [PubMed] [Google Scholar]
- 3.Rao A, Jump RL, Pultz NJ, Pultz MJ, Donskey CJ. In vitro killing of nosocomial pathogens by acid and acidified nitrite. Antimicrob Agents Chemother. 2006;50(11):3901–3904. doi: 10.1128/AAC.01506-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rupnik M. Is Clostridium difficile-associated infection a potentially zoonotic and foodborne disease? Clin Microbiol Infect. 2007;13:457–459. doi: 10.1111/j.1469-0691.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- 5.Sehulster L, Chinn RY. Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 2003;52(Rr-10):1–42. [PubMed] [Google Scholar]
- 6.Loo VG. Environmental interventions to control Clostridium difficile. Infect Dis Clin North Am. 2015;29(1):83–91. doi: 10.1016/j.idc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342(6):390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 8.Ozaki E, Kato H, Kita H, et al. Clostridium difficile colonization in healthy adults: transient colonization and correlation with enterococcal colonization. J Med Microbiol. 2004;53(Pt 2):167–172. doi: 10.1099/jmm.0.05376-0. [DOI] [PubMed] [Google Scholar]
- 9.Jangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J Pediatr Gastroenterol Nutr. 2010;51(1):2–7. doi: 10.1097/MPG.0b013e3181d29767. [DOI] [PubMed] [Google Scholar]
- 10.Castagliuolo I, Riegler M, Pasha A, et al. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile–induced enteritis. J Clin Invest. 1998;101(8):1547–1550. doi: 10.1172/JCI2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penders J, Vink C, Driessen C, London N, Thijs C, Stobberingh EE. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol Lett. 2005;243(1):141–147. doi: 10.1016/j.femsle.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 13.Manson JM, Rauch M, Gilmore MS. The commensal microbiology of the gastrointestinal tract. Adv Exp Med Biol. 2008;635:15–28. doi: 10.1007/978-0-387-09550-9_2. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Severe Clostridium difficile-associated disease in populations previously at low risk: four states, 2005. MMWR Morbid Mortal Wkly Rep. 2005;54(47):1201–1205. [PubMed] [Google Scholar]
- 15.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 17.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 18.Petrella LA, Sambol SP, Cheknis A, et al. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain. Clin Infect Dis. 2012;55(3):351–357. doi: 10.1093/cid/cis430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scardina T, Labuszewski L, Pacheco SM, Adams W, Schreckenberger P, Johnson S. Clostridium difficile infection (CDI) severity and outcome among patients infected with the NAP1/BI/027 strain in a non-epidemic setting. Infect Control Hosp Epidemiol. 2015;36(3):280–286. doi: 10.1017/ice.2014.45. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien JA, Lahue BJ, Caro JJ, et al. The emerging infectious challenge of Clostridium difficile–associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–1227. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- 21.He M, Miyajima F, Roberts P, et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet. 2013;45:109–113. doi: 10.1038/ng.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drapkin MS, Worthington MG, Chang TW, Razvi SA. Clostridium difficile colitis mimicking acute peritonitis. Arch Surg. 1985;120(11):1321–1322. doi: 10.1001/archsurg.1985.01390350097021. [DOI] [PubMed] [Google Scholar]
- 23.Manabe YC, Vinetz JM, Moore RD, et al. Clostridium difficile colitis: an efficient clinical approach to diagnosis. Ann Intern Med. 1995;123:835–840. doi: 10.7326/0003-4819-123-11-199512010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Caroff DA, Edelstein PH, Hamilton K, Pegues DA. The Bristol Stool Scale and its relationship to Clostridium difficile infection. J Clin Microbiol. 2014;52(9):3437–3439. doi: 10.1128/JCM.01303-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berg AM, Kelly CP, Farraye FA. Clostridium difficile infection in the inflammatory bowel disease patient. Inflamm Bowel Dis. 2013;19(1):194–204. doi: 10.1002/ibd.22964. [DOI] [PubMed] [Google Scholar]
- 26.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–498. doi: 10.1038/ajg.2013.4. quiz 499. [DOI] [PubMed] [Google Scholar]
- 27.Miller MA, Louie T, Mullane K, et al. Derivation and validation of a simple clinical bedside score (ATLAS) for Clostridium difficile infection which predicts response to therapy. BMC Infect Dis. 2013;13:148. doi: 10.1186/1471-2334-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chopra T, Miller M, Severson R, Marchaim D, Kaye KS, Alangaden G. ATLAS-A bedside scoring system: predicting mortality due to Clostridium difficile infection (CDI) in elderly hospitalized patients. Paper presented at: 48th Annual Meeting of the Infectious Diseases Society of America (IDSA); October 21–24, 2010; Vancouver, BC. Abstract 452. [Google Scholar]
- 29.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 30.Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile–associated diarrhea. J Clin Gastroenterol. 2010;44(5):354–360. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- 31.Barbut F, Petit JC. Epidemiology of Clostridium difficile–associated infections. Clin Microbiol Infect. 2001;7(8):405–410. doi: 10.1046/j.1198-743x.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilson KH, Sheagren JN, Freter R, Weatherbee L, Lyerly D. Gnotobiotic models for study of the microbial ecology of Clostridium difficile and Escherichia coli. J Infect Dis. 1986;153:547–551. doi: 10.1093/infdis/153.3.547. [DOI] [PubMed] [Google Scholar]
- 33.Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol. 2009;15(13):1554–1580. doi: 10.3748/wjg.15.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yee J, Dixon CM, McLean AP, Meakins JL. Clostridium difficile disease in a department of surgery: the significance of prophylactic antibiotics. Arch Surg. 1991;126(2):241–246. doi: 10.1001/archsurg.1991.01410260131019. [DOI] [PubMed] [Google Scholar]
- 35.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis. 2006;12(3):409–415. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vesteinsdottir I, Gudlaugsdottir S, Einarsdottir R, Kalaitzakis E, Sigurdardottir O, Bjornsson ES. Risk factors for Clostridium difficile toxin-positive diarrhea: a population-based prospective case-control study. Eur J Clin Microbiol Infect Dis. 2012;31(10):2601–2610. doi: 10.1007/s10096-012-1603-0. [DOI] [PubMed] [Google Scholar]
- 37.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 38.Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium difficile–associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103(9):2308–2313. doi: 10.1111/j.1572-0241.2008.01975.x. [DOI] [PubMed] [Google Scholar]
- 39.Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 40.Barletta JF, Sclar DA. Proton pump inhibitors increase the risk for hospital-acquired Clostridium difficile infection in critically ill patients. Crit Care. 2014;18(6):714. doi: 10.1186/s13054-014-0714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17:109–113. doi: 10.1093/clinids/17.1.109. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez TH, Brooks JT, Sullivan PS, et al. Bacterial diarrhea in persons with HIV infection, United States, 1992-2002. Clin Infect Dis. 2005;41:1621–1627. doi: 10.1086/498027. [DOI] [PubMed] [Google Scholar]
- 43.Bliss DZ, Johnson S, Savik K, et al. Acquisition of Clostridium difficile and Clostridium difficile–associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129:1012–1019. doi: 10.7326/0003-4819-129-12-199812150-00004. [DOI] [PubMed] [Google Scholar]
- 44.Thibault A, Miller MA, Gaese C. Risk factors for the development of Clostridium difficile–associated diarrhea during a hospital outbreak. Infect Control Hosp Epidemiol. 1991;12:345–348. doi: 10.1086/646354. [DOI] [PubMed] [Google Scholar]
- 45.Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5(3):345–351. doi: 10.1016/j.cgh.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 46.Samore MH, DeGirolami PC, Tlucko A, et al. Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin Infect Dis. 1994;18:181–187. doi: 10.1093/clinids/18.2.181. [DOI] [PubMed] [Google Scholar]
- 47.Koo HL, Van JN, Zhao M, et al. Real-time polymerase chain reaction detection of asymptomatic Clostridium difficile colonization and rising C. difficile-associated disease rates. Infect Control Hosp Epidemiol. 2014;35(6):667–673. doi: 10.1086/676433. [DOI] [PubMed] [Google Scholar]
- 48.Dubberke ER, Han Z, Bobo L, et al. Impact of clinical symptoms on interpretation of diagnostic assays for Clostridium difficile infections. J Clin Microbiol. 2011;49(8):2887–2893. doi: 10.1128/JCM.00891-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilkins TD, Lyerly DM. Clostridium difficile testing: after 20 years, still challenging. J Clin Microbiol. 2003;41(2):531–534. doi: 10.1128/JCM.41.2.531-534.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenover FC, Novak-Weekley S, Woods CW, et al. Impact of strain type on detection of toxigenic Clostridium difficile: comparison of molecular diagnostic and enzyme immunoassay approaches. J Clin Microbiol. 2010;48:3719–3724. doi: 10.1128/JCM.00427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Planche T, Aghaizu A, Hollman R, et al. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. Lancet Infect Dis. 2008;8(12):777–784. doi: 10.1016/S1473-3099(08)70233-0. [DOI] [PubMed] [Google Scholar]
- 52.Turgeon DK, Novicki TJ, Quick J, et al. Six rapid tests for direct detection of Clostridium difficile and its toxins in fecal samples compared with the fibroblast cytotoxicity assay. J Clin Microbiol. 2003;41(2):667–670. doi: 10.1128/JCM.41.2.667-670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemat H, Khan R, Ashraf MS, et al. Diagnostic value of repeated enzyme immunoassays in Clostridium difficile infection. Am J Gastroenterol. 2009;104(8):2035–2041. doi: 10.1038/ajg.2009.174. [DOI] [PubMed] [Google Scholar]
- 54.Gerding DN, Olson MM, Peterson LR, et al. Clostridium difficile–associated diarrhea and colitis in adults: a prospective case-controlled epidemiologic study. Arch Intern Med. 1986;146:95–100. [PubMed] [Google Scholar]
- 55.Kendrick JB, Risbano M, Groshong SD, Frankel SK. A rare presentation of ischemic pseudomembranous colitis due to Escherichia coli O157:H7. Clin Infect Dis. 2007;45(2):217–219. doi: 10.1086/518990. [DOI] [PubMed] [Google Scholar]
- 56.Janvier J, Kuhn S, Church D. Not all pseudomembranous colitis is caused by Clostridium difficile. Can J Infect Dis Med Microbiol. 2008;19(3):256–257. doi: 10.1155/2008/613573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartlett JG. Clinical practice: antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–349. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 58.Trudel JL. Clostridium difficile colitis. Clin Colon Rectal Surg. 2007;20(1):13–17. doi: 10.1055/s-2007-970195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 60.Patel D, Goldman-Levine D. Fidaxomicin (Dificid) for Clostridium difficile infection. STEPS: New Drug Reviews. American Academy of Family Physicians. [Accessed May 3, 2016]; http://www.aafp.org/afp/2013/0201/p211.pdf. Published February 1, 2013.
- 61.Al-Nassir WN, Sethi AK, Li Y, Pultz MJ, Riggs MM, Donskey CJ. Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile–associated disease. Antimicrob Agents Che-mother. 2008;52(7):2403–2406. doi: 10.1128/AAC.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 63.Kapoor K, Chandra M, Nag D, et al. Evaluation of metronidazole toxicity: a prospective study. Int J Clin Pharmacol Res. 1999;19:83–88. [PubMed] [Google Scholar]
- 64.Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile–associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44:846–848. doi: 10.1086/511870. [DOI] [PubMed] [Google Scholar]
- 65.Hickson M, D'Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335(7610):80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guarino A, Guandalini S, Lo Vecchio A. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol. 2015;49(suppl 1):S37–S45. doi: 10.1097/MCG.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 67.McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913–1918. [PubMed] [Google Scholar]
- 68.Pochapin M. The effect of probiotics on Clostridium difficile diarrhea. Am J Gastroenterol. 2000;95(suppl 1):S11–S13. doi: 10.1016/s0002-9270(99)00809-6. [DOI] [PubMed] [Google Scholar]
- 69.Lawrence SJ, Korzenik JR, Mundy LM. Probiotics for recurrent Clostridium difficile disease. J Med Microbiol. 2005;54:905–906. doi: 10.1099/jmm.0.46096-0. [DOI] [PubMed] [Google Scholar]
- 70.Niault M, Thomas F, Prost J, et al. Fungemia due to Saccharomyces species in a patient treated with enteral Saccharomyces boulardii. Clin Infect Dis. 1999;28:930. doi: 10.1086/517255. [DOI] [PubMed] [Google Scholar]
- 71.Salminen MK, Tynkkynen S, Rautelin H, et al. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhanmous GG in Finland. Clin Infect Dis. 2002;35:1155–1160. doi: 10.1086/342912. [DOI] [PubMed] [Google Scholar]
- 72.Abourgergi MS, Kwon JH. Intravenous immunoglobulin for the treatment of Clostridium difficile infection: a review. Dig Dis Sci. 2011;56:19–26. doi: 10.1007/s10620-010-1411-2. [DOI] [PubMed] [Google Scholar]
- 73.Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107(11):1755–1756. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 74.DePeters EJ, George LW. Rumen transfaunation. Immunol Lett. 2014;162(2 pt A):69–76. doi: 10.1016/j.imlet.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–859. [PubMed] [Google Scholar]
- 76.Seekatz AM, Aas J, Gessert CE, et al. Recovery of the gut microbiome following fecal microbiota transplantation. MBio. 2014;5(3):e00893–14. doi: 10.1128/mBio.00893-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shahinas D, Silverman M, Sittler T, et al. Toward an understanding of changes in diversity associated with fecal microbiome transplantation based on 16S rRNA gene deep sequencing. MBio. 2012;3(5):e00338–12. doi: 10.1128/mBio.00338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Theriot CM, Koenigsknecht MJ, Carlson PE, Jr, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772–1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 82.Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48(8):693–702. doi: 10.1097/MCG.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 83.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 84.Khoruts A, Rank KM, Newman KM, et al. Inflammatory bowel disease affects the outcome of fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2016.02.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weingarden AR, Hamilton MJ, Sadowsky MJ, Khoruts A. Resolution of severe Clostridium difficile infection following sequential fecal microbiota transplantation. J Clin Gastroenterol. 2013;47(8):735–737. doi: 10.1097/MCG.0b013e31829004ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neemann K, Eichele DD, Smith PW, Bociek R, Akhtari M, Freifeld A. Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transpl Infect Dis. 2012;14(6):E161–165. doi: 10.1111/tid.12017. [DOI] [PubMed] [Google Scholar]
- 87.US Food and Drug Administration. Draft Guidance for Industry: Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. [Accessed May 2, 2016]; http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm387023.htm. Published March 2014.
- 88.Dorsey KA, Moritz ED, Steele WR, Eder AF, Stramer SL. A comparison of human immunodeficiency virus, hepatitis C virus, hepatitis B virus, and human T-lymphotropic virus marker rates for directed versus volunteer blood donations to the American Red Cross during 2005 to 2010. Transfusion. 2013;53(6):1250–1256. doi: 10.1111/j.1537-2995.2012.03904.x. [DOI] [PubMed] [Google Scholar]
- 89.Kelly CR, Kahn S, Kashyap P, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology. 2015;149(1):223–237. doi: 10.1053/j.gastro.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4(2):125–135. doi: 10.4161/gmic.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baxter M, Ahmad T, Colville A, Sheridan R. Fatal aspiration pneumonia as a complication of fecal microbiota transplant. Clin Infect Dis. 2015;61(1):136–137. doi: 10.1093/cid/civ247. [DOI] [PubMed] [Google Scholar]
- 92.Varier RU, Biltaji E, Smith KJ, et al. Cost-effectiveness analysis of treatment strategies for initial Clostridium difficile infection. Clin Microbiol Infect. 2014;20(12):1343–1351. doi: 10.1111/1469-0691.12805. [DOI] [PubMed] [Google Scholar]
- 93.Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol. 2013;108(8):1367. doi: 10.1038/ajg.2013.164. [DOI] [PubMed] [Google Scholar]
- 94.Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn's disease and recurrent Clostridium difficile infection. J Crohns Colitis. 2014;8(3):252–253. doi: 10.1016/j.crohns.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 95.Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guillemin I, Marrel A, Beriot-Mathiot A, et al. How do Clostridium difficile infections affect nurses' everyday hospital work: a qualitative study. Int J Nurs Pract. 2015;21(suppl 2):38–45. doi: 10.1111/ijn.12166. [DOI] [PubMed] [Google Scholar]
- 97.Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. Am J Infect Control. 2007;35(10 suppl 2):S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rutala WA, Weber DJ Healthcare Infection Control Practices Advisory Committee. Guideline for Disinfection and Sterilization in Healthcare Facilities. [Accessed May 2, 2016];2008 http://www.cdc.gov/hicpac/Disinfection_Sterilization/3_2contaminatedDevices.html#3.