Abstract
Plant growth promoting endophytic bacteria (PGPB) isolated from Brassica napus were inoculated in two cultivars of Helianthus tuberosus (VR and D19) growing on sand supplemented with 0.1 mM Cd or 1 mM Zn. Plant growth, concentrations of metals and thiobarbituric acid (TBA) reactive compounds were determined. Colonization of roots of H. tuberosus D19 by Pseudomonas sp. 262 was evaluated using confocal laser scanning microscopy. Pseudomonas sp. 228, Serratia sp. 246 and Pseudomonas sp. 262 significantly enhanced growth of H. tuberosus D19 exposed to Cd or Zn. Pseudomonas sp. 228 significantly increased Cd concentrations in roots. Serratia sp. 246, and Pseudomonas sp. 256 and 228 resulted in significantly decreased contents of TBA reactive compounds in roots of Zn exposed D19 plants. Growth improvement and decrease of metal-induced stress were more pronounced in D19 than in VR. Pseudomonas sp. 262-green fluorescent protein (GFP) colonized the root epidermis/exodermis and also inside root hairs, indicating that an endophytic interaction was established. H. tuberosus D19 inoculated with Pseudomonas sp. 228, Serratia sp. 246 and Pseudomonas sp. 262 holds promise for sustainable biomass production in combination with phytoremediation on Cd and Zn contaminated soils.
Keywords: metal contaminated soil, Helianthus tuberosus, phytoremediation, high biomass crop, green fluorescent protein, plant growth promoting bacteria
1. Introduction
During the last two decades, the potential use of plants to remediate metal contaminated soils has been intensively investigated. For application of phytotechnologies on metal contaminated soils, and especially in the case of phytoextraction, metal availability, uptake and phytotoxicity are the main limiting factors [1,2,3,4,5]. The interactions between plants and beneficial bacteria may increase the efficiency of phytoextraction because of increased biomass, metal uptake and plant tolerance to toxic metals [6,7,8,9]. Plant growth can be enhanced: (1) indirectly, by preventing growth and activity of plant pathogens through the production of antibiotics or through competition for space and nutrients [10]; and (2) directly, by increasing available nutrients through different mechanisms such as nitrogen fixation [11], solubilization of minerals such as phosphorous and iron [12,13], and production of phytohormones (as IAA, indole-3-acetic acid) [14] and 1-aminocyclopropane-1-carboxylate (ACC) deaminase [15,16]. Metal and nutrient availability can be enhanced by excreting organic acids that decrease pH in the rhizosphere or by enhancing the Fe(III) mobility and other cations through production of siderophores [9,17,18]. Some microorganisms are equipped with metal-resistance/sequestration systems that can contribute to metal detoxification [19]. Plant-associated bacteria can also adsorb metals by binding them to anionic functional groups or to extracellular polymeric substances of the cell wall [20,21,22]. This leads to a reduced metal uptake and translocation inside the plant, improving its growth through decreasing phytotoxicity [8,23]. In a previous study, we showed that bacterial strains isolated from a Zn contaminated soil increased root length of Brassica napus seedlings in the presence of Cd and Zn under in vitro conditions [24].
Many studies have evaluated the interactions between plants and their associated bacteria for the removal or stabilization of metals in contaminated soils [25]. In some cases, bacteria isolated from metal tolerant plants promoted the growth of plants from different taxonomic groups [23,26,27,28] and demonstrated high levels of colonization in plant species different from the original host. Several studies have been performed under hydroponic conditions to evaluate the effects of bacteria on growth, metal uptake and production of thiobarbituric acid (TBA) reactive compounds for different plants and metals [29,30,31]. However, most studies tested the use of single inocula on the same plant species. In the light of bacteria-stimulated phytoremediation, it is however important to assess the bacterial colonization of more than one plant cultivar under different metal pollution contexts.
The strategy of bacterial inoculation is one of the most critical steps in phytotechnology applications [32]. The colonization must be effective in order to achieve beneficial effects on plant growth and metal uptake [33]. A profound knowledge about plant growth promoting endophytic bacteria (PGPB) colonization routes and plant–bacteria interactions is essential to develop an effective method of inoculation [34]. The use of fluorescent proteins in non-invasive microscopy is a well-established and valuable tool in biology and biotechnology [35]. Labeling with enhanced green fluorescent protein (EGFP) can be adopted to observe the colonization patterns of bacteria [36,37,38]. GFP has been described to be a good marker for studying bacterial behavior in the rhizosphere and the endosphere [39,40]. Recently, Ma et al. [3] pointed out that endophytes could be a more reliable source of natural biocenosis than rhizobacteria because of their intimate association with plants, although their effects in phytotechnologies still should be investigated more in depth.
Helianthus tuberosus L. (Asteraceae) is a high biomass crop used for bio-ethanol production. It is vegetatively propagated by tubers [41] with low production costs and negligible pests and disease problems [42,43]. Several studies have demonstrated the tolerance of this crop to metals such as Cd, Pb and Zn [44,45,46,47,48]. All these characteristics make H. tuberosus a promising candidate for phytoremediation of metal contaminated soils, as well as to produce renewable energy. Therefore, the aim of this work was to evaluate the effects of PGPB strains, isolated from B. napus growing on a metal contaminated soil, on growth, metal uptake and TBA reactive compounds, in two cultivars of H. tuberosus (VR and D19) exposed to Cd and Zn. Of one particular interesting endophytic strain, Pseudomonas sp. 262, the colonization of the roots of H. tuberosus was studied using confocal laser scanning microscopy.
2. Results and Discussion
2.1. Plant Growth and Metal Uptake
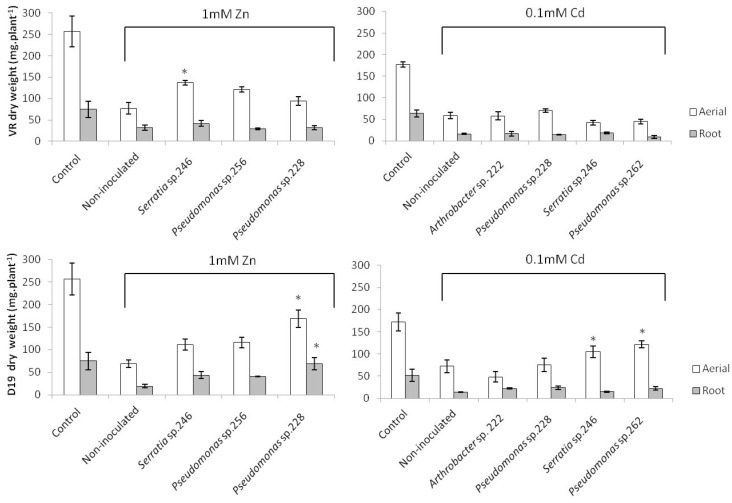
Exposure to Cd and Zn significantly decreased the weight of H. tuberosus in comparison to the non-exposed control plants (Figure 1). In particular, the shoot weight decreased by 57% and the root weight by 67% when plants were exposed to 0.1 mM Cd; the reductions reached 70% and 50% in shoot and root weights in the case plants were grown in presence of 1 mM Zn. Some of the inoculated bacterial strains significantly improved growth of metal exposed plants. In the presence of Zn, inoculation of Pseudomonas sp. 228 significantly increased both shoot and root weights of the D19 cultivar, by 145% and 263% respectively (Figure 1). Serratia sp. 246 increased the shoot weight of the VR cultivar under Zn exposure by 78%. In Cd exposed plants of the D19 cultivar, inoculation of Pseudomonas sp. 262 and Serratia sp. 246 significantly increased the shoot weight by 68% and 46%, respectively. These beneficial effects on weight are in line with earlier studies in which positive effects of inoculation with PGPB on growth of plants exposed to metals were reported [49,50,51]. However, these positive effects of the endophytes Pseudomonas sp. and Serratia sp. have been not described before in a tuberous plant exposed to metals.
Figure 1.
Dry weight (mg·plant−1) of the H. tuberosus cultivars VR and D19 after three weeks of growth in presence of 1 mM Zn or 0.1 mM Cd. * Significant differences between inoculated and non-inoculated after Tukey’s test, p < 0.05; mean values ± SE; n = 4.
In vitro, the inoculated bacterial strains demonstrated plant growth-promoting characteristics like production of IAA, acetoin and ACC deaminase activity that can improve the growth of their host plant (Table 1). Production of IAA and acetoin can stimulate root formation [52,53], and thereby increase the nutrient absorption capacity of the plant. ACC deaminase activity can reduce the ethylene levels generated due to stress, improving the growth of plants in presence of toxic concentrations of metals [16]. It is important to mention that the endophytic bacterial strains that increased the growth of H. tuberosus also increased the length of the roots of Brassica napus seedlings in vertical agar plates containing toxic concentrations of Cd and Zn [24]. This suggests that these endophytes are beneficial for plants from different families.
Table 1.
Metal tolerance and plant growth promoting (PGP) characteristics of selected bacterial strains for inoculation in H. tuberosus under hydroponic conditions with Cd and Zn, modified from [24].
| Comp. 1 | Strain | Identification | Accesion | Zn | Cd | Fe 0 µM | Fe 0.25 µM | OA | ACC | IAA | Ace | Psol | N fix |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil | 222 | Arthrobacter sp. | KT461847 | +++ | +++ | − | − | ++ | +++ | − | − | − | + |
| Root | 228 | Pseudomonas sp. | KT461831 | ++ | ++ | + | + | + | ++ | + | − | ++ | − |
| Root | 246 | Serratia sp. | KT461863 | +++ | +++ | + | + | ++ | +++ | ++ | − | − | − |
| Root | 256 | Pseudomonas sp. | KT461831 | +++ | + | + | + | - | + | ++ | + | +++ | ++ |
| Root | 262 | Pseudomonas sp. | KT461831 | + | + | ++ | − | + | +++ | ++ | + | − | − |
1 Compartment (Comp.), growth in the presence of Zn (1 mM) and Cd (0.8 mM), siderophores (Fe 0 µM and Fe 0.25 µM), Organic acids (OA), ACC (ACC deaminase activity), IAA (indole-3-acetic acid), Ace (Acetoin), phosphate solubilization (Psol), nitrogen fixation (N fix). + low, ++ medium, +++ high production, − absence of production.
The bacterial inoculation also affected the metal concentrations in both cultivars of H. tuberosus (Table 2). The Zn concentration significantly decreased in roots of the VR cultivar inoculated with Pseudomonas sp. 228. No significant differences were found between inoculated and non-inoculated plants of the D19 cultivar. In the case of Cd exposure, the effects were different. Inoculation of Pseudomonas sp. 228 significantly increased the Cd concentration in roots of the D19 cultivar in comparison to non-inoculated plants. In contrast, inoculation of Pseudomonas sp. 262 and Arthrobacter sp. 222 decreased the Cd concentration in roots of the VR cultivar. Serratia sp. 246 and Pseudomonas sp. 262 also decreased the concentrations of Cd in the shoots of, respectively, the VR and the D19 cultivar. In metal contaminated nutrient solutions, metals are almost entirely available to plants. Therefore, the effects of the bacteria on the plant uptake could be masked because of the high metal uptake that usually occurs in these cases. Wan et al. [30] did not observe significant differences in Cd uptake by hydroponically grown Solanum nigrum after inoculation of Serratia nematodiphila LRE07 in the presence of high Cd concentrations. These authors concluded that the effect of the strain was more significant at lower concentrations (10 µM of Cd). Moreover, the decreases in Cd and Zn concentrations in inoculated plants could be due to the capacity of some bacteria to adsorb and immobilize toxic ions from the solution through the production of extracellular polysaccharides and proteins that can bind and precipitate metals [54]. It this way, bacteria can reduce the phytotoxic effects of the metals improving the growth of the host plant [21,23,29]. Several authors have reported such effect in different plant species and diverse growth conditions. Marques et al. [55] observed that the Cd and Zn concentrations in roots of Helianthus annuus decreased after inoculation with Chrysiobacterium humi, isolated from a Cd-Zn contaminated soil. They attributed this effect to the fact that some bacteria can share the metal load with the plant, thereby decreasing the metal uptake in the plant. Tripathi et al. [56] described that the growth of Phaseolus vulgaris improved after inoculation of Pseudomonas putida KNP9 in a soil spiked with Cd and Pb. They suggested that the improved growth was possibly due to a decreased metal uptake by the plant. Vivas et al. [22] reported that the inoculation of Brevibacillus sp. alleviated the toxicity of Zn in Trifolium repens by reducing the metal uptake by plants growing on a Zn contaminated soil. Inoculation with Serratia sp. MSMC541 decreased the metal translocation of Lupinus luteus when growing in a soil spiked with As, Cd, Pb and Zn [57]. They concluded that this strain protects the plants against metal toxicity by reducing their uptake and, in this way promoting plant growth. In our work, the weight of D19 plants increased in presence of Pseudomonas sp. 228 under Zn exposure, and after addition of Pseudomonas sp. 262 and Serratia sp. 246 in presence of Cd. In the case of the VR cultivar, the weight also increased in plants inoculated with Serratia sp. 246 in presence of Zn. The concentration of metals tended to decrease in roots of plants inoculated with Pseudomonas sp. 228 and Serratia sp. 246, although this decrease was only significant in the case of the D19 cultivar, after inoculation with Pseudomonas sp. 262 in presence of Cd. Taking this into account, our data support the hypothesis that bacteria have cultivar-dependent and metal-specific effects on plant growth. Pseudomonas sp. 262 is a promising endophyte because it can lower metal uptake in presence of Cd, decrease phytotoxicity, and improve plant growth.
Table 2.
Total metal concentrations (mg·kg−1 dry matter) in two cultivars of H. tuberosus grown in absence (control) and in presence of 1 mM Zn or 0.1 mM Cd.
| Treatments | VR | D19 | |||
|---|---|---|---|---|---|
| Zn | |||||
| Aerial | Root | Aerial | Root | ||
| Zn | Control | 58 ± 14a | 40 ± 10a | 75 ± 23a | 41 ± 5a |
| Non-inoculated | 1533 ± 149b | 4533 ± 945c | 1097 ± 175b | 3862 ± 1063bc | |
| Serratia sp. 246 | 1155 ± 23b | 4195 ± 355bc | 1283 ± 207b | 3455 ± 1767b | |
| Pseudomonas sp. 256 | 1349 ± 183b | 4368 ± 442bc | 1554 ± 299b | 3484 ± 651b | |
| Pseudomonas sp. 228 | 975 ± 154b | 2237 ± 368b | 1317 ± 177b | 3504 ± 1167b | |
| Cd | |||||
| Cd | Control | 0.43 ± 0.09a | 1.2 ± 0.2a | 0.6 ± 0.1a | 0.5 ± 0.2a |
| Non-inoculated | 152 ± 10c | 1118 ± 177def | 106 ± 44bc | 889 ± 196cde | |
| Arthrobacter sp. 222 | 83 ± 6bc | 492 ± 85bc | 58 ± 8b | 631 ± 140bc | |
| Pseudomonas sp. 228 | 112 ± 23bc | 1250 ± 320ef | 106 ± 18bc | 1365 ± 145f | |
| Serratia sp. 246 | 24 ± 4b | 908 ± 314cde | 129 ± 45c | 798 ± 65bcd | |
| Pseudomonas sp. 262 | 145 ± 29c | 487 ± 57bc | 81 ± 5bc | 383 ± 107b | |
Different letters represent significant differences per column, cultivar and metal after Tukey’s test, p < 0.05; mean values ± SE; n = 4.
2.2. Nutrient Status
In general, inoculation of bacterial strains did not have clear effects on the nutrient concentrations in both cultivars (Tables S1–S4). Macronutrients as Na and Ca were significantly lower in roots of, respectively, VR and D19 plants when plants were inoculated with Serratia sp. 246, Pseudomonas sp. 228 and 256 in presence of 1 mM of Zn (Table S1). In the case of exposure of the plants to 0.1 mM Cd, inoculation of Arthrobacter sp. 222, Serratia sp. 246, Pseudomonas sp. 228 and 262 led to lower K concentrations in shoots of the VR cultivar (Table S2).
Micronutrient concentrations changed in some cases after bacterial inoculation. When plants were grown in the presence of Zn, inoculation of Serratia sp. 246, Pseudomonas sp. 228 and 256 significantly decreased the Cu and Fe concentrations in roots of respectively the VR and D19 cultivars (Table S3). The concentrations of Cu in roots of Cd exposed plants of the VR cultivar were also lower after inoculation with Arthrobacter sp. 222, Serratia sp. 246 and Pseudomonas sp. 262 (Table S4). However, Serratia sp. 246 increased the Fe content in the shoots of the VR cultivar in presence of Zn (Table S3). The latter strain also increased the weight of VR plants exposed to Zn.
The lower Cu and Fe concentrations in roots of both cultivars when inoculated with Serratia sp. 246 and Pseudomonas (262 and 256) can be due to the above-mentioned bacterial mechanisms of metal sequestration and/or biosorption. Microorganisms indeed have developed complex mechanisms of metal resistance that can affect the availability of metals and nutrients [58,59]. PGPB can sequestrate elements through extracellular production of polysaccharides, by fixing elements such as Fe or Cu on the membrane or cell wall or they can precipitate them in the form of hydroxides or other insoluble metal salts [23,60,61]. Bacterial surfaces hold polar functional groups that can interact with cations [62]. In our work, the excess of Cd and Zn might induce the bacterial mechanisms of metal resistance that lower the availability of metals and also the solubility of other nutrients that might be precipitated on the cell surface.
The synthesis of siderophores is stimulated in presence of toxic metals in order to supply the appropriate amounts of ions to the plant and diminish the phytotoxicity symptoms [17,29]. This PGP characteristic plays an important role under soil conditions in which the nutrients are mainly present for the plants in unavailable chemical forms. Therefore, it can be expected that, under the growth conditions used in this study (sand moistened with half-strength Hoagland whether or not supplemented with Zn or Cd), the effects of the bacteria on nutrient uptake are less pronounced, since Fe is supplied in an appropriate concentration with the nutrient solution. Thus, the differences observed in the nutrient concentrations in the plants might also be due to the imbalance of nutrients generated by the presence of metals in the solution.
2.3. Lipid Peroxidation
TBA reactive compounds are produced as a result of peroxidation of membrane lipids. This process is initiated by excess of free radicals in consequence of oxidative stress. Increased levels of TBA reactive compounds are an indicator for physiological stress [63]. Many studies reported that levels of TBA reactive compounds increased in plants exposed to toxic concentrations of metals such as Cd, Zn, and Pb [64,65,66,67].
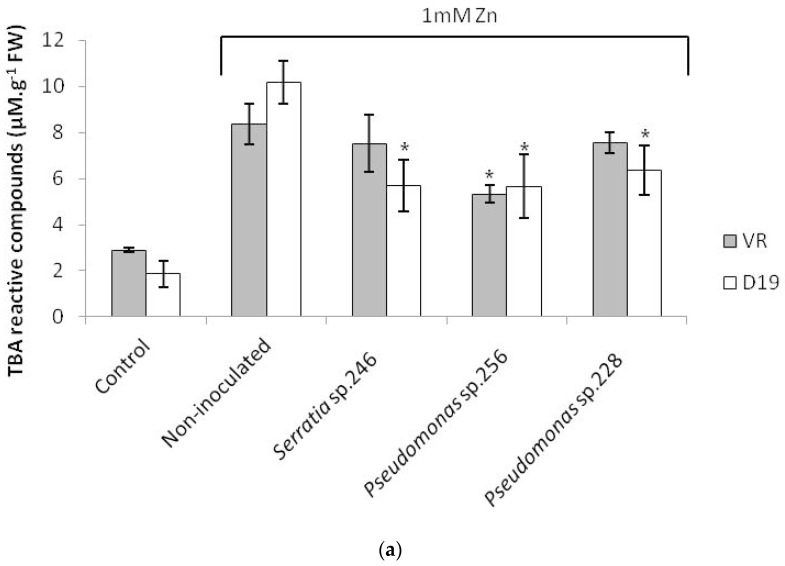
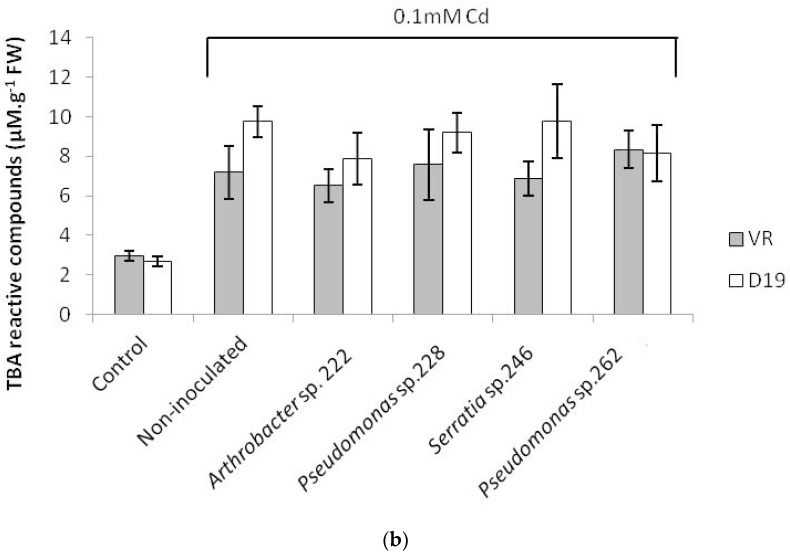
In the present work, exposure to 1 mM Zn and 0.1 mM Cd significantly increased the levels of TBA reactive compounds in roots of both cultivars of H. tuberosus (Figure 2). No significant differences in TBA-levels were found in leaves of metal-exposed plants compared to non-exposed plants. Nouairi et al. [68] obtained similar results for leaves of Brassica juncea exposed to 50 µM Cd. According to them, this result could be related with a tolerance mechanism of the plant to avoid oxidative stress generated by the presence of metals in the leaves. A reduction of the concentrations of TBA reactive compounds has been reported to result from increased activities of anti-oxidative enzymes, which limit H2O2 levels and membrane damage [69].
Figure 2.
Thiobarbituric acid reactive compounds (µM·g−1 fresh weight) in roots of H. tuberosus cultivars VR and D19 after three weeks of exposure to: 1 mM of Zn (a); and 0.1 mM Cd (b). * Significant differences between inoculated and non-inoculated after Tukey’s test, p < 0.05; mean values ± SE; n = 4.
Interestingly, the inoculation of Serratia sp. 246, Pseudomonas sp. 256 and 228 significantly decreased the amounts of TBA reactive compounds in roots of the D19 cultivar grown in the presence of Zn (Figure 2a). The roots of the VR cultivar also contained lower levels of TBA reactive compounds when plants were inoculated with Pseudomonas sp. 228. In Cd exposed plants, no significant differences in TBA reactive compounds were observed between inoculated and non-inoculated plants (Figure 2b). Decreases of TBA reactive compounds after inoculation of PGPB were reported by several authors in different plant species. Pandey et al. [31] described that inoculation of Ochrobactrum strain CdSP9 lowered the content of TBA reactive compounds in hydroponically grown Oryza sativa exposed to Cd. Wan et al. [30] also observed that the inoculation of Serratia nematodiphila LRE07 decreased the concentration of TBA reactive compounds in Solanum nigrum exposed to Cd under hydroponic conditions.
These results suggest that the inoculated bacteria can assist metal exposed plants to keep the oxidative stress under control. In the inoculated plants, the Zn concentrations tended to decrease which could at least partially explain the lowering of TBA reactive compounds in inoculated plants.
2.4. Colonization of Enhanced Green Fluorescent Protein (EGFP): Tetracycline® Pseudomonas sp. 262 in the Roots of H. tuberosus
Pseudomonas sp. 262 was able to grow in the presence of 0.8 mM Cd and showed in vitro the capacity to produce siderophores (in absence of iron), organic acids, indole acetic acid, acetoin and ACC deaminase (Table 1). Moreover, this bacterial strain increased the shoot weight of the D19 cultivar of H. tuberosus exposed to 0.1 mM Cd. Taking this into account, Pseudomonas sp. 262 was selected to be labeled with the EGFP: tetracycline® plasmid to study the bacterial colonization of the roots of the H. tuberosus D19 cultivar.
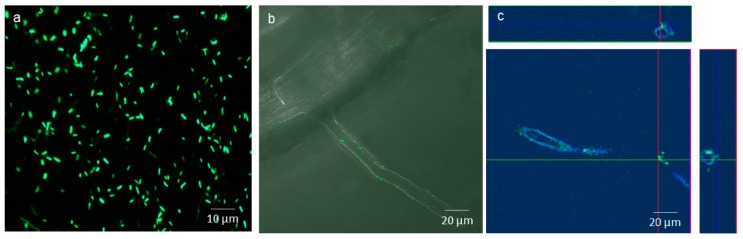
Figure 3a demonstrates that the conjugation was effective, since Pseudomonas sp. 262 showed fluorescence after blue light (488 nm) excitation, and was able to grow in presence of tetracycline (20 µg·mL−1). In Figure 3b, EGFP-Pseudomonas sp. 262 can be seen as single cells attached to the surfaces of root hairs. Two days after inoculation, bacterial cells were also found inside the root hair which can be observed from the orthogonal plot (Figure 3c). These results support that Pseudomonas sp. 262 is an endophytic strain.
Figure 3.
Confocal images of EGFP-labeled Pseudomonas sp. 262 colonising the root hairs of one-week-old seedlings of H. tuberosus D19 cultivar: (a) solution with EGFP-labeled Pseudomonas sp. strain 262 with blue light (488 nm) excitation; (b) single cells attached to a root hair; and (c) ortho-image of the root hair, showing bacterial cells (green) inside plant cells (in blue).
Ma et al. [70] reported that another Pseudomonas sp. A3R3 isolated from roots of Alyssum serpyllifolium showed a high level of colonization in root and shoot interior of Brassica juncea. He et al. [27] also observed that Rahnella sp. JN6, originally isolated from Polygonum pubescens, could colonize the root, stem and leaf tissues of Brassica napus. Rahnella aquatilis SPb, an endophytic bacterial strain from Ipomoea batatas was inoculated in hybrid poplar and increased the growth of the cuttings in comparison with non-inoculated conditions, illustrating the beneficial effects of the strains in growth of another plant species not related with the initial host plant [71].
In our study, the EGFP-labeled bacterial strain was found in the root interior of H. tuberosus in the studied conditions. Since the inoculated bacterial cells were also found attached to the root hair surface, we suggest this one of the entry routes of the bacterial cells to the plant. Moreover, after inoculation of this strain, the growth of the H. tuberosus D19 cultivar improved significantly when exposed to Cd. This beneficial effect on plant growth, together with the visualization of the bacteria on the root hair surfaces and inside roots, indicates that a beneficial plant–microbe interaction was established.
3. Materials and Methods
3.1. Plant Material
Tubers of two cultivars of H. tuberosus (Violet de Rennes abbreviated as VR, and Blanc Précoce commonly named D19) were collected in spring in the field collection of IMIDRA (Instituto Madrileño de Investigación y Desarrollo Rural, Agrario y Alimentario; Madrid, Spain) to perform the experiments. The tubers were kept during two weeks at 4 °C for vernalization. After this period and before starting the experiments, the tubers were vigorously washed in tap water to remove the adhered soil.
3.2. PGPB Strains
Cultivable bacteria were isolated from soil, rhizosphere and plant-endosphere of Brassica napus growing on a Zn-contaminated site in Belgium [24]. Based on their PGP characteristics (Table 1), 3 Zn-tolerant strains (Serratia sp. strain 246, Pseudomonas sp. strain 228, and Pseudomonas sp. strain 256) and 4 Cd-tolerant strains (Arthrobacter sp. strain 222, Pseudomonas sp. strain 228, Pseudomonas sp. strain 262, and Serratia sp. strain 246) were selected to inoculate H. tuberosus. Serratia sp. strain 246 and Pseudomonas sp. strain 228 were inoculated in the presence of Zn and Cd because both strains showed high tolerance to grow with both metals. The strains were grown in 869 liquid medium [72] at 30 °C under shaking conditions.
3.3. Inoculation of PGPB Strains in H. tuberosus
Tuber slices with buds were incubated in 1 L plastic pots filled with moist quartz sand that were placed in a growth chamber at 25/12 °C, 14/12 h of photoperiod. The following conditions were established after one week of growth: (i) control plants grown in sand without metal and bacteria; (ii) non-inoculated, metal-exposed plants grown in the presence of metals (Cd or Zn), but without bacteria; (iii) plants inoculated with bacterial strains (Arthrobacter sp. 222, Pseudomonas sp. 228, 262 and Serratia sp. 246) and grown in presence of Cd; and (iv) plants inoculated with bacterial strains (Pseudomonas sp. 228, 256 and Serratia sp. 246) and grown in presence of Zn.
Half strength modified Hoagland’s solution (1 mM Ca (NO3)2·4H2O, 1.5 mM KNO3, 0.5 mM NH4H2PO4, 0.25 mM MgSO4·7H2O, 1 µM MnSO4·H2O, 12.5 µM H3BO3, 0.25 µM (NH4)6Mo7O4, 0.05 µM CuSO4·5H2O, 1 µM ZnSO4·7H2O, 10 µM NaFeIII-EDTA, and demineralized water buffered with 1 mM of 2-(N-morpholino) ethanesulfonic acid, at pH 5.5 ± 0.5) was added to the sand until saturation. Metal exposures were performed by adding 0.1 mM of Cd (added as CdSO4·8H2O) or 1 mM of Zn (added as ZnSO4·7H2O) to the nutrient solution. Plants were watered every two days with the nutrient solution supplemented with metals. The metal concentrations used in this experiment were chosen based on a former study [73]. Two plants were put per pot with four independent replicates per treatment. The bacterial suspension (108 cfu·mL−1) in buffer (10 mM MgSO4) was added into the pots. Buffer (10 mM MgSO4) without bacteria was added to the controls. After 3 weeks of growth, plants were harvested.
3.4. Plant Analysis
After harvest, the roots were rinsed in 10 mM sodium ethylenediaminetetraacetic acid (Na2EDTA) to remove the adhering metal-containing particles, and subsequently washed in distilled water. Plants were subdivided into leaves, stems and roots, weighed and dried in a forced air oven for 48 h at 60 °C to determine the dry weights. Subsequently, the dried tissues were individually ground and digested (30 mg) according to [47]. Total concentrations of metals and macro/micronutrients were determined by flame atomic absorption spectrometry (Fast Sequential Model AA240FS, Varian, Santa Clara, CA, USA). The quality of the digestion and analytical methods was verified by including blanks and certified reference materials (NCS DC73348 Brush Branches and Leaves, China National Analysis Center for Iron and Steel, and CTA-VTL-2 Virginia Tobacco Leaves, Polish Academy of Sciences and Institute of Nuclear Chemistry and Technology) with every set of samples. The recovery percentages for metals were: Cd (~95%) and Zn (~101%).
The membrane lipid peroxidation in the plant tissues was estimated in terms of the content of thiobarbituric acid reactive (TBA) compounds according to the method of [74], modified by [75]. The calibration curve was carried out with every set of samples, using 1,1,3,3-Tetraethoxypropane (TEP) as precursor of malondialdehyde (MDA). Absorbances were determined with a UV–Vis light spectrophotometer (Thermo Spectronic Helios Alpha, Thermo Fisher Scientific, Madison, WI, USA).
3.5. Evaluation of the Colonization Process: Localization of Inoculated EGFP Labeled Pseudomonas sp. 262
3.5.1. Bacterial Strains and Growth Conditions
The receptor, Pseudomonas sp. 262 was grown in 284 minimal medium [76] supplemented with 0.4 mM of Cd (added as CdSO4·8H2O) at 30 °C. The donor, Escherichia coli strain dH5a, carrying the EGFP pMP4655 plasmid, was grown in 869 medium [72] supplemented with 20 µg·mL−1 tetracycline at 30 °C. The helper, E. coli strain dH5a, carrying the pRK2013 plasmid, was grown in 869 medium at 30 °C. Donor and helper were constructed in the Institute of Biology Leiden, Leiden University (The Netherlands) [39].
3.5.2. Introduction of the EGFP: Tetracycline into Pseudomonas sp. 262
Triparental mating was carried out to label Pseudomonas sp. 262 with the EGFP: tetracycline® plasmid. The strains were grown in 869 medium at 30 °C under shaking conditions. Growth curves were obtained by diluting an overnight culture in order to verify the time needed to reach the appropriate optical density (OD) for conjugation (donor and helper OD 0.3–0.4, and receptor OD 0.7). The OD was measured at 660 nm every 30 min using a Visible Diode Array Spectrophotometer, Novaspec Plus, Amersham Biosciences, Piscataway, New York, United States. Once the appropriate OD was reached, the bacterial strains were centrifuged at 3000 rpm during 10 min, and then added to the mating filter in a Petri dish with 869 medium. After the conjugation, 284 minimal medium supplemented with 0.4 mM of Cd (added as CdSO4·8H2O) and tetracycline (20 µg·mL−1) was used to isolate the receptor labeled strains. Fluorescence of the strains was checked using a Nikon 80i fluorescence microscope (High-pressure Mercury Lamp; Excitation filters: 465–495 nm, dichroic mirror 505 nm, emission filter 515–555 nm. Objectives used: 40×/0.95 Air Plan Apo WD 0.14 mm and 100×/1.25 Oil Plan Apo WD 0.17 mm).
3.5.3. Inoculation of EGFP Pseudomonas sp. 262 on Roots of H. tuberosus
Tuber slices with buds of H. tuberosus cultivar D19 were grown on coarse perlite moistened with a half strength modified Hoagland’s solution (see above) under greenhouse conditions (25–30 °C temperature and 70–90% relative humidity). The bacterial suspension (108 cfu·mL−1) was added to the pots (0.2 L) after appearance of the first roots (at 5 days). Four repetitions were used.
3.5.4. Confocal Laser Scanning Microscopy
After 48 h of incubation, one-week-old plant roots were washed to remove weakly adhered bacterial cells and, subsequently, intact root preparations (at 25 °C) were observed with a Zeiss LSM510 confocal laser scanning microscope (Carl Zeiss, Jena, Germany) mounted on an Axiovert 200M. The objective used was 40×/1.1 water immersion (Zeiss LD C-Apochomat 40×/1.1 WKorr UV–VIS-IR, Carl Zeiss).
Excitation was performed at 488 nm using an Argon laser source. Backward GFP signal was filtered using a 500–550 nm band pass filter. Images were edited using the software Zen 2009 Light Edition (Carl Zeiss MicroImaging GmbH, Jena, Germany).
3.6. Statistical Analysis
Statistical analysis of data was performed using the IBM SPSS Statistics 19.0 software (Armonk, NY, USA). Two-way analysis of variance (ANOVA) and Tukey’s test were applied. Differences at p < 0.05 levels were considered significant.
4. Conclusions
The effects of the bacterial strains on the growth of H. tuberosus differed in function of the metal, the inoculated bacterial strain and the plant cultivar. The improvement of growth and the decrease of the metal-induced stress were more pronounced in the D19 cultivar than in the VR cultivar. Three endophytes of Brassica napus enhanced the growth of the D19 cultivar exposed Cd or Zn. Only Pseudomonas sp. 228 increased Cd uptake. Using confocal microscopy, we observed that, two days after inoculation, EGFP-labeled Pseudomonas sp. 262 colonized the root surface and interior of H. tuberosus. In combination with the growth promotion that was observed after inoculation, this demonstrates an established plant–microbe interaction. Therefore, use of the D19 cultivar in combination with Pseudomonas sp. 228, Serratia sp. 246 and Pseudomonas sp. 262 holds promise for application in phytoremediation strategies on Cd-Zn contaminated soils.
Acknowledgments
We would like to acknowledge Rik Paesen from the Biomedical Research Institute of Hasselt University for his assistance in the confocal laser microscope and Marta Letón Rojo from Agro-environmental department of Instituto Madrileño de Investigación y Desarrollo Rural, Agrario y Alimentario (IMIDRA) for her help in Flame Atomic Absorption measurements. We would like also to thank to Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA) to support the grant Formación de Personal Investigador FPI-INIA 2010 of B. Montalbán, EIADES Project S2009/AMB-1478 (Comunidad de Madrid) and the UHasselt Methusalem project 08M03VGRJ. S. Thijs and N. Weyens are grateful to the FWO (Fund for Scientific Research Flanders) for, respectively, a PhD and post-doc grant.
Abbreviations
| PGPB | Plant Growth Promoting Bacteria |
| TBA | Thiobarbituric Acid |
| EGFP | Enhanced Green Fluorescent Protein |
| EDTA | Ethylenediaminetetraacetic Acid |
| MDA | Malondialdehyde |
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/10/2026/s1.
Author Contributions
Blanca Montalbán, Araceli Pérez-Sanz, Mª Carmen Lobo, Jaco Vangronsveld and Nele Weyens conceived and designed the plant experiments. Blanca Montalbán, Araceli Pérez-Sanz and Mª Carmen Lobo analyzed the data. Sofie Thijs, Blanca Montalbán and Marcel Ameloot designed, performed and analyzed the colonization assays and localization of inoculated EGFP labeled bacteria with confocal laser scanning microscopy. Blanca Montalbán wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Mulligan C.N., Yong R.N., Gibbs B.F. Heavy metal removal from sediments by biosurfactants. J. Hazard Mater. 2001;85:111–125. doi: 10.1016/S0304-3894(01)00224-2. [DOI] [PubMed] [Google Scholar]
- 2.Weyens N., van der Lelie D., Taghavi S., Newman L., Vangronsveld J. Exploiting plant-microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 2009;27:591–598. doi: 10.1016/j.tibtech.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Ma Y., Rajkumar M., Zhang C.H., Freitas H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016;174:14–25. doi: 10.1016/j.jenvman.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 4.Coninx L., Martinova V., Rineau F. Mycorrhiza-Assisted Phytoremediation. In: Cuypers A., Vangronsveld J., editors. Phytoremediation. Advances in Botanical Research. Volume 83. Elsevier; Amsterdam, The Netherlands: 2017. pp. 127–188. [Google Scholar]
- 5.Kidd P.S., Álvarez-López V., Becerra-Castro C., Cabello-Conejo M., Prieto-Fernández Á. Potential role of plant-associated bacteria in plant metal uptake and implications in phytotechnologies. In: Cuypers A., Vangronsveld J., editors. Phytoremediation. Advances in Botanical Research. Volume 83. Elsevier; Amsterdam, The Netherlands: 2017. pp. 87–126. [Google Scholar]
- 6.Germida J.J., Siciliano S.D., Renato de Freitas J., Seib A.M. Diversity of root-associated bacteria associated with field-grown canola (Brassica napus L.) and wheat (Triticuma estivum L.) FEMS Microbiol. Ecol. 1998;26:43–50. doi: 10.1111/j.1574-6941.1998.tb01560.x. [DOI] [Google Scholar]
- 7.Genrich I., Burd D., George D., Glick B.R. Plant growth promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol. 2000;46:237–245. doi: 10.1139/w99-143. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar M., Sandhya S., Prasad M.N.V., Freitas H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012;30:1562–1574. doi: 10.1016/j.biotechadv.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Ullah A., Heng S., Munis M.F.H., Fahads S., Yang X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015;117:28–40. doi: 10.1016/j.envexpbot.2015.05.001. [DOI] [Google Scholar]
- 10.Lugtenberg B., Kamilova F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- 11.Roper M.M., Ladha J.K. Biological N2-fixation by heterotrophic and phototrophic bacteria in association with straw. Plant Soil. 1995;174:211–224. doi: 10.1007/BF00032248. [DOI] [Google Scholar]
- 12.Kim K.Y., Jordan D., McDonald G.A. Enterobacter agglomerans, phosphate solubilizing bacteria, and microbial activity in soil: Effect of carbon sources. Soil Biol. Biochem. 1998;30:995–1003. doi: 10.1016/S0038-0717(98)00007-8. [DOI] [Google Scholar]
- 13.Ryan R.P., Germaine K., Franks A., Ryan D.J., Dowling D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008;278:1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 14.Dobbelaere S., Croonenborghs A., Thys A., Vandebroek A., Vanderleyden J. Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil. 1999;212:155–164. doi: 10.1023/A:1004658000815. [DOI] [Google Scholar]
- 15.Glick B.R., Penrose D.M., Li J. A model for the lowering of plant ethylene concentrations by plant growth promoting bacteria. J. Theor. Biol. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- 16.Glick B.R. Phytoremediation: Synergistic use of plants and bacteria to clean up the environment. Biotechnol. Adv. 2003;21:383–393. doi: 10.1016/S0734-9750(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 17.Glick B.R., Bashan Y. Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol. Adv. 1997;15:353–378. doi: 10.1016/S0734-9750(97)00004-9. [DOI] [PubMed] [Google Scholar]
- 18.Fasim F., Ahmed N., Parsons R., Gadd G.M. Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol. Lett. 2002;213:1–6. doi: 10.1111/j.1574-6968.2002.tb11277.x. [DOI] [PubMed] [Google Scholar]
- 19.Diels L., De Smet M., Hooyberghs L., Corbisier P. Heavy metals bioremediation of soil. Mol. Biotechnol. 1999;12:149–158. doi: 10.1385/MB:12:2:149. [DOI] [PubMed] [Google Scholar]
- 20.Rouch D.A., Lee T.O.B., Morby A.P. Understanding cellular responses to toxic agents: A model for mechanisms-choice in bacterial resistance. J. Ind. Microbiol. 1995;14:132–141. doi: 10.1007/BF01569895. [DOI] [PubMed] [Google Scholar]
- 21.Madhaiyan M., Poonguzhali S., Sa T. Influence of plant species and environmental conditions on epiphytic and endophytic pink-pigmented facultative methylotrophic bacterial populations associated with field-grown rice cultivars. J. Microbiol. Biotechnol. 2007;17:1645–1654. [PubMed] [Google Scholar]
- 22.Vivas A., Biró B., Ruíz-Lozano J.M., Barea J.M., Azcón R. Two bacterial strains isolated from a Zn-polluted soil enhance plant growth and mycorrhizal efficiency under Zn-toxicity. Chemosphere. 2006;62:1523–1533. doi: 10.1016/j.chemosphere.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y., Prasad M.N.V., Rajkumar M., Freitas H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011;29:248–258. doi: 10.1016/j.biotechadv.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Montalbán B., Croes S., Weyens N., Lobo M.C., Pérez-Sanz A., Vangronsveld J. Characterization of bacterial communities associated with Brassica napus L. growing on a Zn-contaminated soil and their effects on root growth. Int. J. Phytoremediat. 2016;18:985–993. doi: 10.1080/15226514.2016.1183566. [DOI] [PubMed] [Google Scholar]
- 25.Chen B., Shen J., Zhang X., Pan F., Yang X., Feng Y. The endophytic bacterium, Sphingomonas SaMR12, improves the potential for zinc phytoremediation by its host, Sedum alfredii. PLoS ONE. 2014;9:e106826. doi: 10.1371/journal.pone.0106826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheng X., Sun L., Huang Z., He L., Zhang W., Chen Z. Promotion of growth and Cu accumulation of bio-energy crop (Zea mays) by bacteria: Implications for energy plant biomass production and phytoremediation. J. Environ. Manag. 2012;103:58–64. doi: 10.1016/j.jenvman.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 27.He H., Ye Z., Yang D., Yan J., Xiao L., Zhong T., Yuan M., Cai X., Fang Z., Jing Y. Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Chemosphere. 2013;90:1960–1965. doi: 10.1016/j.chemosphere.2012.10.057. [DOI] [PubMed] [Google Scholar]
- 28.Sessitsch A., Kuffner M., Kidd P., Vangronsveld J., Wenzel W.W., Fallmann K., Puschenreiter M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013;60:182–194. doi: 10.1016/j.soilbio.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajkumar M., Ae N., Freitas H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere. 2009;77:153–160. doi: 10.1016/j.chemosphere.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 30.Wan Y., Luo S., Chen J., Xiao X., Chen L., Zeng G., Liu C., He Y. Effect of endophyte-infection on growth parameters and Cd-induced phytotoxicity of Cd-hyperaccumulator Solanum nigrum L. Chemosphere. 2012;89:743–750. doi: 10.1016/j.chemosphere.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Pandey S., Ghosh P.K., Ghosh S., Kumar D.T., Maiti T.K. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013;5:7–11. doi: 10.1007/s12275-013-2330-7. [DOI] [PubMed] [Google Scholar]
- 32.Weyens N., van der Lelie D., Taghavi S., Vangronsveld J. Phytoremediation: Plant-endophyte partnerships take the challenge. Curr. Opin. Biotechnol. 2009;20:248–254. doi: 10.1016/j.copbio.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Lugtenberg B.J.J., Dekkers L., Bloemberg G.V. Molecular determinants of rhizosphere colonization by Pseudomonas. Annu. Rev. Phytopathol. 2001;39:461–490. doi: 10.1146/annurev.phyto.39.1.461. [DOI] [PubMed] [Google Scholar]
- 34.Compant S., Clément C., Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010;42:669–678. doi: 10.1016/j.soilbio.2009.11.024. [DOI] [Google Scholar]
- 35.Lagendijk E.L., Validov S., Lamers G.E.M., de Weert S., Bloemberg G.V. Genetic tools for tagging Gram-negative bacteria with mCherry for visualization in vitro and in natural habitats, biofilm and pathogenicity studies. FEMS Microbiol. Lett. 2010;305:81–90. doi: 10.1111/j.1574-6968.2010.01916.x. [DOI] [PubMed] [Google Scholar]
- 36.Bloemberg G.V., Lugtenberg B.J.J. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 2001;4:343–350. doi: 10.1016/S1369-5266(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 37.Germaine K., Keogh E., Borremans B., van der Lelie D., Barac T., Oeyen L., Vangronsveld J., Moore F.P., Moore E.R.B., Campbel C.D., et al. Colonisation of poplar trees by gfp expressing endophytes. FEMS Microbiol. Ecol. 2004;48:109–118. doi: 10.1016/j.femsec.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Weyens N., Boulet J., Adriaensen D., Timmermans J.P., Prinsen E., Van Oevelen S., D’Haen J., Smeets K., van der Lelie D., Taghavi S., et al. Contrasting colonization and plant growth promoting capacity between wild type and a gfp-derative of the endophyte Pseudomonas putida W619 in hybrid poplar. Plant Soil. 2012;356:217–230. doi: 10.1007/s11104-011-0831-x. [DOI] [Google Scholar]
- 39.Bloemberg G.V., Wijfjes A.H., Lamers G.E., Stuurman N., Lugtenberg B.J. Simultaneous imaging of Pseudomonas fluorescens WCS365 populations expressing three different autofluorescent proteins in the rhizosphere: New perspectives for studying microbial communities. Mol. Plant Microbe Interact. 2000;13:1170–1176. doi: 10.1094/MPMI.2000.13.11.1170. [DOI] [PubMed] [Google Scholar]
- 40.Newman K.L., Almeida R.P.P., Purcell A.H., Lindow S.E. Use of a green fluorescent strain for analysis of Xylella fastidiosa colonization of Vitis vinifera. Appl. Environ. Microbiol. 2003;69:7319–7327. doi: 10.1128/AEM.69.12.7319-7327.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serieys H., Souyris I., Gil A., Poinso B., Berville A. Diversity of Jerusalem artichoke clones (Helianthus tuberosus L.) from the INRA-Montpellier collection. Genet. Resour. Crop Evol. 2010;57:1207–1215. doi: 10.1007/s10722-010-9560-x. [DOI] [Google Scholar]
- 42.Denoroy P. The crop physiology of Helianthus tuberosus L.: A model oriented view. Biomass Bioenergy. 1996;11:11–32. doi: 10.1016/0961-9534(96)00006-2. [DOI] [Google Scholar]
- 43.Kays S.J., Nottingham S.F. Biology and Chemistry of Jerusalem Artichoke Helianthus tuberosus L. Taylor and Francis Group; Boca Raton, FL, USA: Abingdon, UK: New York, NY, USA: 2007. pp. 1–496. [Google Scholar]
- 44.Cui S., Zhou Q., Chao L. Potential hyperaccumulation of Pb, Zn, Cu and Cd in endurant plants distributed in an old smeltery, northeast China. Environ. Geol. 2007;51:1043–1048. doi: 10.1007/s00254-006-0373-3. [DOI] [Google Scholar]
- 45.Chen L., Long X., Zhang Z., Zheng X., Rengel Z., Liu Z. Cadmium accumulation and translocation in two Jerusalem Artichoke (Helianthus tuberosus L.) Cultivars. Pedosphere. 2011;21:573–580. doi: 10.1016/S1002-0160(11)60159-8. [DOI] [Google Scholar]
- 46.Long X., Ni N., Wang L., Wang X., Wang Y., Zhang Z., Zed R., Liu Z., Shao H. Phytoremediation of cadmium-contaminated soil by two Jerusalem Artichoke (Helianthus tuberosus L.) genotypes. CLEAN Soil Air Water. 2013;41:202–209. doi: 10.1002/clen.201100668. [DOI] [Google Scholar]
- 47.Montalbán B., Lobo M.C., Alonso J., Pérez-Sanz A. Metal(loid)s uptake and effects on the growth of Helianthus tuberosus cultivar-clones under multi-polluted hydroponic cultures. CLEAN Soil Air Water. 2016;44:1368–1374. doi: 10.1002/clen.201400630. [DOI] [Google Scholar]
- 48.Willscher S., Jablonski L., Fona Z., Rahmi R., Wittig J. Phytoremediation experiments with Helianthus tuberosus under different pH and heavy metal soil concentrations. Hydrometallurgy. 2016;168:153–158. doi: 10.1016/j.hydromet.2016.10.016. [DOI] [Google Scholar]
- 49.Zaidi S., Usmani S., Singh B.R., Musarrat J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere. 2006;64:991–997. doi: 10.1016/j.chemosphere.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 50.Sheng X.F., Xia J.J. Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere. 2006;64:1036–1042. doi: 10.1016/j.chemosphere.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 51.Sheng X.F., Xia J.J., Jiang C.Y., He L.Y., Qian M. Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ. Pollut. 2008;156:1164–1170. doi: 10.1016/j.envpol.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Duan J., Jiang W., Cheng Z., Heikkila J.J., Glick B.R. The Complete Genome Sequence of the Plant Growth-Promoting Bacterium Pseudomonas sp. UW4. PLoS ONE. 2013;8:e58640. doi: 10.1371/journal.pone.0058640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glick B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010;28:367–374. doi: 10.1016/j.biotechadv.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Burd G.I., Dixon D.G., Glick B.R. A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl. Environ. Microbiol. 1998;64:3663–3668. doi: 10.1128/aem.64.10.3663-3668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marques A.P.G.C., Moreira H., Franco A.R., Rangel A.O.S.S., Castro P.M.L. Inoculating Helianthus annuus (sunflower) grown in zinc and cadmium contaminated soils with plant growth promoting bacteria—Effects on phytoremediation strategies. Chemosphere. 2013;92:74–83. doi: 10.1016/j.chemosphere.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 56.Tripathi M., Munot H.P., Shouche Y., Meyer J.M., Goel R. Isolation and functional characterization of siderophore-producing lead- and cadmium-resistant Pseudomonas putida KNP9. Curr. Microbiol. 2005;50:233–237. doi: 10.1007/s00284-004-4459-4. [DOI] [PubMed] [Google Scholar]
- 57.Aafi N.E., Brhada F., Dary M., Maltouf A.F., Pajuelo E. Rhizostabilization of metals in soils using Lupinus luteus inoculated with the metal resistant rhizobacterium Serratia sp. MSMC 541. Int. J. Phytoremediat. 2012;14:261–274. doi: 10.1080/15226514.2011.604693. [DOI] [PubMed] [Google Scholar]
- 58.Nies D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999;51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- 59.Bruins M.R., Kapil S., Oehme F.W. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Saf. 2000;45:198–207. doi: 10.1006/eesa.1999.1860. [DOI] [PubMed] [Google Scholar]
- 60.Chen H., Cutright T.J. Preliminary evaluation of microbiallymediated precipitation of cadmium, chromium, and nickel by rhizosphere consortium. J. Environ. Eng. 2003;129:4–9. doi: 10.1061/(ASCE)0733-9372(2003)129:1(4). [DOI] [Google Scholar]
- 61.Kidd P., Barceló J., Bernal M.P., Navari-Izzo F., Poschenrieder C., Shilev S., Clemente R., Monterroso C. Trace element behaviour at the root–soil interface: Implications in Phytoremediation. Environ. Exp. Bot. 2009;67:243–259. doi: 10.1016/j.envexpbot.2009.06.013. [DOI] [Google Scholar]
- 62.Vecchio A., Finoli C., Di Simine D., Andreoni V. Heavy metal biosorption by bacterial cells. Fresen. J. Anal. Chem. 1998;361:338–342. doi: 10.1007/s002160050899. [DOI] [Google Scholar]
- 63.Li Y., Zhang S., Jiang W., Liu D. Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistiastratiotes L. Environ. Sci. Pollut. Res. 2013;20:1117–1123. doi: 10.1007/s11356-012-1054-2. [DOI] [PubMed] [Google Scholar]
- 64.Smeets K., Cuypers A., Lambrechts A., Semane B., Hoet P., Van Laere A., Vangronsveld J. Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after Cd application. Plant Physiol. Biochem. 2005;43:437–444. doi: 10.1016/j.plaphy.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 65.Wang C., Zhang S.H., Wang P.F., Hou J., Zhang W.J., Li W., Lin Z.P. The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere. 2009;75:1468–1476. doi: 10.1016/j.chemosphere.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 66.Bauddh K., Singh R.P. Growth, tolerance, efficiency and phytoremediation potential of Ricinuscommunis (L.) and Brassica juncea (L.) in salinity and drought affected cadmium contaminated soil. Ecotoxicol. Environ. Saf. 2012;85:13–22. doi: 10.1016/j.ecoenv.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 67.Jozefczak M., Keunen E., Schat H., Bliek M., Hernández L.E., Carleer R., Remans T., Bohler S., Vangronsveld J., Cuypers A. Differential response of Arabidopsis leaves and roots to cadmium: Glutathione-related chelating capacity vs antioxidant capacity. Plant Physiol. Biochem. 2014;83:1–9. doi: 10.1016/j.plaphy.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Nouairi I., Ammar W.B., Youssef N.B., Miled D.D.B., Ghorbal M.H., Zarrouk M. Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol. Plant. 2009;31:237–247. doi: 10.1007/s11738-008-0224-9. [DOI] [Google Scholar]
- 69.Zhang F.Q., Wang Y.S., Lou Z.P., Dong J.D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza) Chemosphere. 2007;67:44–50. doi: 10.1016/j.chemosphere.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 70.Ma Y., Rajkumar M., Luo Y.M., Freitas H. Inoculation of endophytic bacteria on host and non-host plants—Effects on plant growth and Ni uptake. J. Hazard. Mater. 2011;195:230–237. doi: 10.1016/j.jhazmat.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 71.Khan Z., Doty S.L. Characterization of bacterial endophytes of sweet potato plants. Plant Soil. 2009;322:197–207. doi: 10.1007/s11104-009-9908-1. [DOI] [Google Scholar]
- 72.Mergeay M., Nies D., Schlegel H.G., Gerits J., Charles P., Van Gijsegem F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistgance to heavy metals. J. Bacteriol. 1985;162:328–334. doi: 10.1128/jb.162.1.328-334.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montalbán B., García-Gonzalo P., Pradas del Real A.E., Alonso J., Lobo M.C., Pérez-Sanz A. Brachypodium distachyon tolerance to metals under in vitro conditions: A comparison with two metal-tolerant energy crops. Fresen. Environ. Bull. 2014;23:2086–2092. [Google Scholar]
- 74.Reilly C.A., Aust S.D. Measurement of lipid peroxidation. Curr. Protoc. Toxicol. 2001;28:659–671. doi: 10.1002/0471140856.tx0204s00. [DOI] [PubMed] [Google Scholar]
- 75.Catalá M., Gasulla F., Pradas del Real A.E., Garcia-Breijo F., Reig-Arminana J., Barreno E. Fungal-associated NO is involved in the regulation of oxidative stress during rehydration in lichen symbiosis. BMC Microbiol. 2010;10:297. doi: 10.1186/1471-2180-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlegel H.G., Kaltwasser H., Gottschalk G. Ein Submersverfahren zur Kultur wassers toffoxy dieren der Bakterien: Wachstums physiologische Untersuchungen. Arch. Mikrobiol. 1961;38:209–222. doi: 10.1007/BF00422356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.