Abstract
Heat stress is a major abiotic stress posing a serious threat to plants. Heat-responsive mechanisms in plants are complicated and fine-tuned. Heat signaling transduction and photosynthesis are highly sensitive. Therefore, a thorough understanding of the molecular mechanism in heat stressed-signaling transduction and photosynthesis is necessary to protect crop yield. Current high-throughput proteomics investigations provide more useful information for underlying heat-responsive signaling pathways and photosynthesis modulation in plants. Several signaling components, such as guanosine triphosphate (GTP)-binding protein, nucleoside diphosphate kinase, annexin, and brassinosteroid-insensitive I-kinase domain interacting protein 114, were proposed to be important in heat signaling transduction. Moreover, diverse protein patterns of photosynthetic proteins imply that the modulations of stomatal CO2 exchange, photosystem II, Calvin cycle, ATP synthesis, and chlorophyll biosynthesis are crucial for plant heat tolerance.
Keywords: heat response, proteomics, photosynthesis, signaling
1. Introduction
Heat is one of the most severe environmental stress factors limiting crop growth and productivity [1,2,3]. Crop-based modeling and empirical studies in tropical and subtropical regions have reported 2.5–16% direct yield losses of major crops for every 1 °C increase in seasonal temperature [4]. Global warming leads to the continual increase of global mean temperature with 0.3 °C per decade [5], which has a serious threat to crop production. Photosynthesis contributes more than 90% of crop biomass, and thus enhancement of photosynthesis would increase crop yields [6]. However, photosynthesis is very sensitive to heat and easy to be inhibited under heat conditions. Alteration of any photosynthetic processes, such as photosystem II (PS II), PS I, electron transport chains, ATP synthesis, and carbon fixation always happened when plants were subjected to heat stress [6,7,8]. Therefore, improvement of photosynthesis efficiency is vital for crop production and could be a promising approach for increasing crop yield under heat stress.
Photosynthesis alteration in response to heat stress has been well reviewed in the past years [1,6,7,9,10,11,12]. Plant response to heat is a complex trait, dependent on heat intensity, duration time and plant species. Heat stress affects photosynthesis in the levels of physiological, biochemical and molecular aspects, such as destacking of thylakoid membrane, photosystem II damage, inhibition of cytochrome b6/f complex and ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO), as well as reactive oxygen species (ROS) production. Accordingly, plants have developed several heat avoidant or tolerant strategies, such as stomatal closure, accumulation of osmoprotectants, modulation of fatty acids composition and saturation, increase of heat shock proteins, activation of ROS-scavenging enzyme activity, as well as synthesis of secondary metabolites [6]. Efforts have also been conducted to improve photosynthetic efficiency by engineering photosynthesis-related genes, such as chloroplastic glutamine synthetase (GS2), chlorplastic Fe superoxide dismutase (FeSOD), ω-3 fatty acid desaturase (FAD7), sedoheptulose-1,7-bisphosphatase (SBPase), C4-carboxylase phosphoenolpyruvate (C4-PEPC), and transcription factors ABA-responsive 17 (ABR17) [7,13].
Heat-responsive signaling is also crucial for the protection of photosynthesis apparatus under heat stress. Although the mechanism of plant heat sensing and signaling are still unclear, many signaling molecules (e.g., Ca2+ and ROS), hormones (e.g., ABA), protein kinases (e.g., calcium-dependent protein kinases (CDPK), and mitogen-activated protein kinase (MAPK)), and transcription factors (e.g., heat shock factor (HSF)) are known to participate in heat signal transduction. In addition, some candidate heat-responsive genes encoding heat shock protein 110 (HSP101), HSP90, HSF, abscisic aicd-responsive element-binding factor (AREB1) and WRKY11 have a high potential to be used for crop breeding with heat tolerance [13,14,15,16]. In spite of the intensive studies on plant heat tolerance, and the great progress by transgenic engineering and quantitative trait locus (QTL) molecular assisted breeding in recent years, a complete understanding of thermostolerant mechanism remains elusive. Moreover, there are still no economically practical technological means to improve photosynthetic capability and facilitate crop production under heat stress.
Because of the posttranscriptional and posttranslational modifications, the changes of protein abundance can provide direct understanding of heat adaptive mechanism. Two-dimensional electrophoretic (2DE) and two-dimensional fluorescence difference in gel (DIGE) approaches have been widely used for separating heat-responsive proteins in plant. 2DE is a powerful technology with high resolution in protein separation, especially for protein isoform analysis, and DIGE overcomes some of the limitations of 2DE (e.g., throughput, reproducibility and sensitivity) [17]. The differentially expressed protein spots on 2DE/DIGE can be identified by matrix-assisted laser desorption/ionization tandem time-of-flight mass (MALDI-TOF/TOF MS) and liquid chromatography electrospray ionization (ESI) MS system (LC-MS) [18]. Importantly, a gel-free quantitative proteomics strategy, isobaric tags for relative and absolute quantitation (iTRAQ), has been applied for proteomic quantitation with a high throughput, sensitivity and efficiency, especially for identifying low abundant proteins (e.g., transmembrane receptors, intracellular kinases, and transcription factors) [18]. In previous studies, 2DE/DIGE-based proteomics was the main approach popularly employed in investigation of plant heat-responsive proteins (Table 1). Among them, more than 500 heat-responsive photosynthetic and signaling protein species have been identified in leaves from 16 plant species, including eight dicots (i.e., Brassica oleracea, Carissa spinarum, Glycine max, Medicago sativa, Portulaca oleracea, Raphanus sativus, Arabidopsis thaliana, and Vitis vinifera) and eight monocots (i.e., Agave americana, Agrostis sp., Hordeum sp., Miscanthus sinensis, Oryza sp., Pinellia ternata, Zea mays and Triticum aestivum) (Table 1 and Table S1). Here, we review the critical heat-responsive signaling and photosynthesis mechanisms in leaves revealed from these proteomic publications.
Table 1.
Summary of current publications on heat-responsive proteomics.
| Plant Species | Tissue/Organ | Variety | Treatment Conditions | Platforms | Protein Species (a) | Unique Proteins (b) | Reference |
|---|---|---|---|---|---|---|---|
| Agave americana | leaf chloroplast | nd | 55 °C; 4 h | 2DE, ESI-Q-Trap MS | 58 | 58(26/32) | [19] |
| Agrostis scabra/Agrostis stolonifera | root | nd; Penncross | 20 °C, 30 °C, 40 °C; 2 d, 10 d | 2DE, MALDI-TOF/TOF MS | 70 | 67(23/44) | [20] |
| Agrostis scabra/Agrostis stolonifera | leaf | nd; Penncross | 40 °C/35 °C day/night; 2 d, 10 d | 2DE-DIGE | 71 | 71(57/14) | [21] |
| Arabidopsis thaliana | leaf | Columbia (Col-0) | 40 °C; 6 h | 2DE, MALDI-TOF MS, ESI-Q-Trap MS | 37 | 33(12/21) | [22] |
| Avena sativa | seed | nd | 35 °C, 45 °C, 50 °C; 24 h, 2 d | 2DE, ESI-Orbitrap MS | 21 | 21(2/19) | [23] |
| Brassica oleracea | leaf | TSS-AVRDC-2; B-75 | 40 °C; 3 d | 2DE, MALDI-TOF MS | 24 | 24(10/13/1) | [24] |
| Carissa spinarum | leaf | nd | 42 °C/35 °C day/night; 48 h, 120 h | 2DE, MALDI-TOF/TOF MS | 49 | 26(13/13) | [25] |
| Glycine max | leaf, stem, root | Enrei | 40 °C; 6, 12, 24 h | 2DE, MALDI-TOF MSESI-Orbitrap MS | 150 | 150(122/28) | [26] |
| Glycine max | leaf | Surge; Davison | 40 °C/35 °C day/night; 6 d | 2DE-DIGE, MALDI-TOF MS | 88 | 44(19/25) | [27] |
| Glycine max | seed | Ningzhen No. 1 | 40 °C/30 °C day/night; 24, 96, 168 h | 2DE, MALDI-TOF MS | 42 | 42(22/20) | [28] |
| Hordeum spontaneum | leaf | nd | 42 °C, 2 h | 2DE-DIGE, MALDI-TOF/TOF MS | 20 | 20(12/8) | [29] |
| Hordeum vulgare | leaf | Arta; Keel | 36 °C/32 °C day/night; 7 d | 2DE, MALDI-TOF/TOF MS | 99 | 99(67/32) | [30] |
| Medicago sativa | leaf | Huaiyin | 36 °C; 24, 48, 72 h | 2DE, MALDI-TOF/TOF MS | 81 | 81 | [31] |
| Miscanthus sinensis | leaf | Kosung | 42 °C; 24, 48 h | 2DE, MALDI-TOF MS, 2DE, MALDI-TOF/TOF MS | 55 | 55(30/25) | [32] |
| Oryza meridionalis | leaf | nd | 45 °C; 24 h | 2DE, ESI-Q-Trap MS | 50 | 38(22/16) | [33] |
| Oryza sativa | leaf | Dongjin | 40 °C; 12, 24 h | 2DE, MALDI-TOF MS | 73 | 56(47/9) | [34] |
| Oryza sativa | leaf | nd | 35, 40, 45 °C; 48 h | 2DE, MALDI-TOF MS | 63 | 52(28/24) | [35] |
| Oryza sativa | leaf | N22 | 42 °C/32 °C day/night; 24 h | 2DE, MALDI-TOF MS | 111 | 52(37/15) | [36] |
| Oryza sativa | leaf, spikelet | N22; Gharib | 28 °C; 12 h | 2DE, MALDI-TOF MS | 36 | 36 | [37] |
| Oryza sativa | cell suspension cultures | Doongara | 44 °C; 3 d | 1DE, ESI-Q-Trap MS | 139 | 139 | [38] |
| Oryza sativa | grain | Khao Dawk Mali 105 | 40 °C; 3 d | ESI-Orbitap MS | 822 | 822 | [39] |
| Oryza sativa | leaf | Nipponbare | 42 °C, 12 h, 24 h | 2DE, MALDI-TOF/TOF MS | 12 | 12(9/3) | [40] * |
| Pinellia ternata | leaf | nd | 38 °C; 24 h | 2DE, MALDI-TOF/TOF MS | 27 | 24(17/7) | [41] |
| Portulaca oleracea | leaf | nd | 35 °C; 6h, 12 h, 24 h | 2D, MALDI-TOF/TOF MS | 154 | 51(36/15) | [42] |
| Prunus persica | mesocarp | nd | 39 °C; 3 d | 2DE, MALDI-TOF/TOF MS | 44 | 33(15/18) | [43] |
| Raphanus sativus | leaf | NAU-08Hr-10 | 40 °C; 0 h, 12 h, 24 h | 2DE, MALDI-TOF/TOF MS | 11 | 11(4/1/6) | [44] |
| Triticum aestivum | endosperm | Récital | 34 °C/10 °C day/night | 2DE, MALDI-TOF MS | 37 | 23(22/1) | [45] |
| Triticum aestivum | non-prolamins | Thésée | 34 °C/10 °C day/night | 2DE, MALDI-TOF MS | 42 | 24(16/8) | [46] |
| Triticum aestivum | seed | Svevo | 37 °C/17 °C day/night; 5 d | 2DE, MALDI-TOF/TOF MS | 47 | 47(37/10) | [47] |
| Triticum aestivum | spikelet | Vinjett | 32 °C/24 °C day/night; 10 d | 2DE, MALDI-TOF/TOF MS | 57 | 57(36/21) | [48] |
| Triticum aestivum | leaf | 810; 1039 | 35 °C/26 °C day/night; 5 d | 2DE, MALDI-TOF MS | 49 | 49(32/11, 12/21) | [49] |
| Vitis vinifera | leaf | Cabernet Sauvignon | 43 °C; 6 h | iTRAQ, ESI-Q-TOF MS | 113 | 113(48/65) | [50] |
| Zea mays | leaf | Zhengdan 958 | heat from 28 to 42 °C, total 8 h | iTRAQ, ESI-Orbitrap MS | 172 | 172(77/95) | [51] * |
(a) The number of identified protein identities included all the protein isoforms generated from gene variable splicing and post-translational modifications in these original publications. (b) The number of non-redundant protein identities whose members have similar protein sequence homology and functional domain (increased protein number/decreased protein number). The references labeled with * are phosphoproteomic studies. The information of these heat-responsive proteins is listed in Tables S1 and S2. 2DE, two-dimensional electrophoretic; d, days; DIGE, two-dimensional fluorescence difference in gel; ESI-Q-TOF MS, electrospray ionization quadrupole time-of-flight mass; iTRAQ, isobaric tags for relative and absolute quantitation; MALDI-TOF MS, matrix-assisted laser desorption/ionization time-of-flight mass; MALDI-TOF/TOF MS, matrix-assisted laser desorption/ionization tandem time-of-flight mass; nd, not detected.
2. Heat-Responsive Signaling Transduction
The heat signal perception and transduction are very important steps for plant stress tolerance. At least four sensors are proposed to trigger heat stress response, including a plasma membrane channel that initiates an inward calcium flux, a histone sensor in the nucleus, and two unfolded protein sensors in the endoplasmic reticulum and the cytosol [52]. The downstream heat-responsive signaling pathways are predicted to be Ca2+ signaling, G protein-mediated signaling, and kinase signaling. Recent proteomic studies found several signaling proteins, which provided new clues for understanding the complex heat stress signal transduction (Table 2).
Table 2.
Heat stress-responsive proteins involved in photosynthesis, carbon metabolism and signaling identified in leaves by proteomics studies.
| Protein Name | Abbreviation | Plant Species |
|---|---|---|
| 1. Photoreaction | ||
| Magnesium chelatase subunit | CHLI | Po; Gm; Vv |
| Chlorophyll a/b binding protein | LHC | Ta; Pt; Os; Vv; MS |
| Oxygen-evolving enhancer protein 1 | OEE1 | Gm; Aa; Ta; Os; Cs; Asc; Ast; At |
| Oxygen-evolving enhancer protein 2 | OEE2 | Asc; Rs; Gm; Os; Gm; At; Ms |
| Photosystem I PsaA subunit | PsaA | Aa; Vv |
| Photosystem I PsaB subunit | PsaB | Aa |
| Photosystem I PsaD subunit | PsaD | Aa; At; Vv; Ms |
| Photosystem I PsaE subunit | PsaE | Asc; Ast |
| Photosystem I PsaN subunit | PsaN | Aa; Asc; Vv |
| Photosystem I PsaL subunit | PsaL | Vv |
| Photosystem I PsaF subunit | PsaF | Vv |
| Photosystem I PsaH subunit | PsaH | Vv |
| Photosystem II PsbA subunit | PsbA | Vv |
| Photosystem II PsbP subunit | PsbP | Os |
| Photosystem II PsbO subunit | PsbO | Os |
| Photosystem II PsbS subunit | PsbS | Vv |
| Photosystem II PsbR subunit | PsbR | Vv |
| 2. Calvin cycle | ||
| RuBisCO activase | RCA | Os; Ta; Om; Asc; Ast; Os; At; Vv; Ms |
| RuBisCO large subunit | Rubisco LS | Gm; Os; Bo; Aa; Ta; Po; Cs; Cs; Om; Asc; Ast; At; Vv; Ms |
| RuBisCO small subunit | Rubisco SS | Ta; Gm; Os; Bo; Asc; Ast; At; Vv; Ms |
| Phosphoribulokinase | PRK | Os; Om; Asc; Ast; Vv |
| Transketolase | TK | Os; Om |
| Sedoheptulose-1,7-bisphosphatase | SBPase | Gm; Ta; Om |
| Chloroplast fructose-bisphosphate aldolase | FBPA | At; Asc; Ast; Gm; Ta; Vv |
| C4-specific pyruvate orthophosphate dikinase | PPDK | Ta |
| Carbonic anhydrase | CA | Asc; Ast; Gm; Vv |
| Phosphoglycerate kinase | PGK | Om |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | At; Ast; Asc; Ta; Ms Vv, |
| 3. Photorespiration | ||
| Glycolate oxidase | GO | Po |
| Glycine dehydrogenase | GLDC | Os; Om |
| Glycine decarboxylase | GDC | Ta; Os |
| Glutamine synthetase | GS | Os |
| 4. ATP synthesis and electrical transport chain | ||
| ATP synthase CF (0) b subunit | CF0 | Vv |
| ADP, ATP carrier protein 1 | AAC | Aa |
| ATP synthase α subunit | α | Os; Ta |
| ATP synthase β subunit | β | Aa; Asc; Ast; Bo; Gm; Om; Os; Ta; Vv |
| ATP synthase γ subunit | γ | Asc; Ast; At; Vv |
| ATP synthase δ subunit | δ | Cs; Vv |
| Cytochrome c oxidase assembly protein | COX | Po |
| Rieske Fe/S protein of cytochrome b6/f complex | Fe/S | Pt |
| Ferredoxin-NADP(H) oxidoreductase | FNR | Om; Gm; Vv |
| Plastocyanin | PC | Ms |
| 5. Glycolysis | ||
| Triosephosphate isomerase | TPI | Gm; Ta; Cs; Os; At |
| Alcohol dehydrogenase | ADH | Gm; Po |
| Fructokinase | FK | Gm |
| Fructose-bisphosphate aldolase | FBPA | Gm; Asc; Ast; Os |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | Gm; Gm; Ta; Asc; Ast; Os; At |
| Phosphoglycerate kinase | PGK | Gm; Asc; Ast; Os |
| Phosphoglycerate mutase | PGM | Ta |
| Pyruvate kinase | PK | Ast |
| Enolase | Ta; Asc; Ast; Ms | |
| 6. Pentose phosphate pathway | ||
| Phosphogluconate dehydrogenase | PGD | Asc; Ast; Vv |
| Transketolase | TK | Os |
| 7. TCA cycle | ||
| Malate dehydrogenase | MDH | Os; Gm; Asc; Ast; Vv; Ms |
| Isocitrate dehydrogenase | IDH | Ta |
| Cytoplasmic aconitate hydratase | AH | Asc; Ast |
| Phosphoenolpyruvate carboxylase | PEPC | Po; Gm; Aa |
| Dihydrolipoyl dehydrogenase | DLDH | Ta |
| Pyruvate dehydrogenase | PDH | Os; Cs; Gm |
| Citrate synthase | CS | Ms; Vv |
| Fumarate hydratase | FH | Ms |
| 8. Signaling | ||
| Phospholipase C | PLC | Ta |
| GTP-binding protein | G protein | Po |
| Ras-related nuclear protein 1A | Ran 1A | Asc; Ast |
| Ras-related protein | Rab | Gm |
| Ca2+-transporting ATPase-like protein | Aa | |
| Calcium/calmodulin-dependent protein kinase | CDPK | Gm |
| Calcium-binding protein | CaB | Aa |
| BRI1-KD interacting protein 114 | Ta | |
| Nucleoside diphosphate kinase | NDPK | As; Gm; Ms; Os |
| WRKY transcriptional factor | WRKY | Po |
| MYB family transcription factor | MYB | Po |
Aa, Agave americana; Asc, Agrostis scabra; Ast, Agrostis stolonifera; At, Arabidopsis thaliana; Cs, Carissa spinarum; Gm, Glycine max; Ms, Medicago sativa; Om, Oryza meridionalis; Os, Oryza sativa; Pt, Pinellia ternata; Po, Portulaca oleracea; Ta, Triticum aestivum. Vv, Vitis vinifera. Please refer to Table S1 for details.
2.1. Ca2+ Signaling Pathways
It is well known that the inward flux of calcium serves as one of the primary heat sensors of plants [52], and regulates multiple signaling pathways in plants in response to heat stress [53]. In proteomics results, the abundances of calcium-binding protein and a Ca2+-transporting ATPase-like protein were increased in chloroplasts from A. americana leaves under 55 °C heat treatment [19]. Calcium-binding proteins participate in calcium cell signaling pathways by binding to Ca2+, which are involved in the activation of multiple kinases and transcription factors in heat stress. Importantly, a calmodulin-binding family protein showed a significant increase of phosphorylation level [51] activating Ca2+-calmodulin-dependent processes in response to heat stress [54]. In contrast, an isoform of annexin in A. thaliana leaves was declined in response to heat stress [22]. Annexin is a conserved family of eukaryotic Ca2+-dependent phospholipid-binding protein, which participates in the response of environmental stress [55,56]. A. thaliana annexin 1 mediates a plasma membrane calcium-permeable conductance in roots activated by ROS. An O. sativa annexin interacted with MAPK and was involved in Ca2+-dependent MAPK signaling pathway [55,57]. However, the role of annexin in heat signaling is still unclear.
2.2. G Protein-Mediated Signaling
Proteomics also found several G proteins showed increased trends in leaves in response to heat stress, such as an isoform of G protein in P. oleracea [42], a Rab1C in G. max [26] and four isoforms of Ran1A in Agrostis sp. [20]. It is well known that G proteins constitute one of the most important cell signaling cascades and participate in multiple signaling pathways [58,59]. Previous studies have shown that some isoforms of G proteins (e.g., G alpha, G beta, G gamma, and Rab7) are associated with plant heat tolerance [60,61,62,63]. These findings indicate the possible function of these G proteins/small G proteins in heat sensing, thus bringing novel perspectives in understanding the complex heat stress signal transduction.
2.3. Kinase Signaling Pathways
The heat signals transmitted by second messengers activate multiple protein kinases (e.g., CDPK and MAPK). Current proteomic studies found the increased abundances of nucleoside diphosphate kinases (NDPKs) in heat-stressed leaves of G. max [26] and O. sativa [34]. NDPK can interact with H2O2-mediated mitogen-activated protein kinase (MAPK) signaling and associate with plant heat tolerance [64]. The increase of NDPK has also been reported in plants to cope with other environmental stresses, such as drought, salt and cold [34,65]. We therefore propose that the accumulation of NDPK is important for plant stress tolerance. Similarly, a BRI1-KD interacting protein 114, which contains NDPK group I like domain, was increased in heat-tolerant T. aestivum cultivar, but did not change in heat-sensitive cultivar [49]. Previous studies have shown that the increased BRI1-KD interacting protein 114 play roles in alleviating effects of salt stress in a tolerant T. aestivum cultivar [66]. Their detailed roles in plant heat signaling still need to be further investigated.
In addition, the phosphorylation levels of two protein kinases (i.e., serine threonine-protein kinase wnk4-like and protein kinase superfamily protein) and phospholipase C (PLC) were also changed in response to heat stress [51]. It is well known that serine/threonine (Ser/Thr) phosphorylation plays key roles in the regulation of plant stress response. PLC is a major membrane phospholipid hydrolyzing enzyme, and its phosphorylated form is involved in the regulation of various cellular processes in plants in response to stress conditions, such as heat, salt and drought [67,68,69,70].
2.4. Heat-Responsive Transcription Factors
Proteomic studies also found several transcription factors involved in plant heat signal transduction, such as WRKY and MYB. Both of them were increased in P. oleracea leaves upon 35 °C treatment [42]. P. oleracea is a typical C4 and thermotolerant plant species widely distributed in tropical regions. The increase of WRKY and MYB in response to heat stress may be correlated with the great heat tolerance of P. oleracea. MYB proteins are involved in regulating signal transduction and biosynthesis of secondary metabolites [71]. Overexpression of OsMYB55 improves O. sativa tolerance to heat by enhancing amino acid metabolism through transcription activation [72]. WRKY acts as key component in abscisic acid signaling and play roles in multiple stress tolerances [71,73]. OsWRKY11-overexpressing O. sativa exhibited enhanced tolerance to drought and heat [74]. All these indicate that MYB proteins and WRKY can act as very promising targets to improve crop heat stress tolerance.
Additionally, the activity of heat shock transcription factor A1 (HsfA1) was negatively regulated by HSP 70 and HSP90 [75]. Proteomics studies reported that several HSPs (e.g., HSP70s, HSP90s, and small HSPs) were induced in many plant species under heat treatments [22,26,41,45] (Table S1). HSPs are well known for their critical role in maintaining protein folding under heat stress. A schematic model of the heat signaling pathway mediated by novel signaling components discovered in proteomic studies is shown in Figure 1.
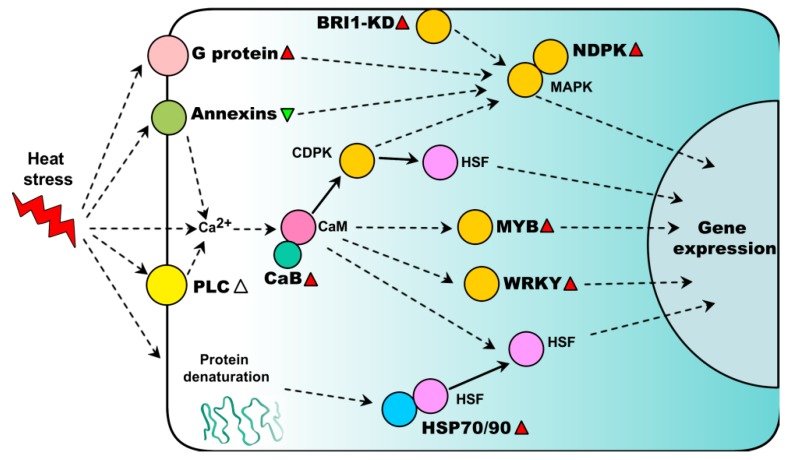
Figure 1.
Schematic representation of heat-responsive proteins involved in signal transduction pathway. Heat stress results in an inward flux of calcium and activation of G protein by phospholipase C (PLC), annexins and other proteins. Calcium binds the calmodulin (CaM) and calcium-binding protein (CaB), and then activates multiple kinases (e.g., CDPK and MAPK) and transcriptional regulators (e.g., MYB, WRKY and HSF). HSP70 and HSP90 negatively regulate the activity of HSFA1. BRI1-KD and annexin participate in the heat signal transduction through MAPK-activated stress response. Red and green triangles represent heat-increased and heat-decreased abundance of proteins, respectively. White triangle represents heat-increased phosphorylation level. Partly adopted from Mittler et al. [52].
3. Chlorophyll Synthesis Is Disturbed by Heat Stress
Chloroplasts are the major organelles for photosynthesis, and chlorophyll (Chl) is the most important pigment for light absorbance during photosynthesis. It has been reported that Chl biosynthesis is inhibited when plants were exposed to heat stress, which is probably due to the heat destruction of several enzymes in Chl biosynthesis [76,77], such as protochlorophyllide reductase (POR) and magnesium-chelatase subunit. POR is responsible for the photoreduction of protochlorophyllide to chlorophyllide. Previous study indicated that the abundance of POR was decreased in heat-stressed T. aestivum seedlings associated with the reduced accumulation of Chl [78]. Moreover, in heat-stressed Z. mays, the phosphorylation level of POR was decreased by heat stress [51] (Table S2). It has been suggested that POR phosphorylation can facilitate the aggregation of protochlorophyllide and POR, while the aggregation is needed for the next catalytic reaction for POR [79]. These suggested that not only the abundance but also the phosphorylation level of POR may contribute to the Chl biosynthesis. Magnesium-chelatase catalyzes the insertion of magnesium ion into protoporphyrin IX to yield Mg-protoporphyrin IX. A magnesium-chelatase subunit ChlI-2 identified in V. vinifera leaves were repressed after heat stress [50]. However, an isoform of magnesium-chelatase subunit of POR was significantly increased in leaves of P. oleracea under 35 °C heat stresses for 6–24 h [42]. The increased abundance of magnesium-chelatase subunit may be related to the excellent photosynthetic capability of P. oleracea under heat stress. Further functional analyses of magnesium-chelatase subunit and chlorophyll-binding proteins in heat-treated P. oleracea leaves are needed.
4. Photosystem (PS) II and PS I Are Disrupted under Heat Stress
PSII is one of the most thermosensitive components of photosynthetic apparatus [80,81]. The water oxidizing complex (WOC), PSII reaction center, and light harvesting complexes are all initially disrupted under heat stress. Some of the heat-responsive proteins involved in PSII are summarized in Figure 2. Among them, chlorophyll-binding proteins are involved in harvesting light energy and transferring it to photochemical reaction centers. As revealed by proteomics studies, chlorophyll-binding proteins were generally decreased in heat-treated H. vulgare [29] and V. vinifera [50] ((Table 2 and Table S1). In the chloroplasts of leaves from A. americana, the protein abundance and transcript of light-harvesting chlorophyll a/b binding protein (LHC) were all decreased under heat stress [19]. However, chlorophyll-binding protein species in leaves of P. ternata exhibited a substantial increase in response to 38 °C heat stress for 24 h [41]. In heat stressed leaves of M. sativa, three chlorophyll a/b binding proteins were increased at 24 h, but decreased in the prolonged heat stress course (72 h) [31]. These results indicate that enhanced abundances of chlorophyll-binding proteins may be somehow related to heat tolerance, depending on the stress intensity, duration, and plant species.
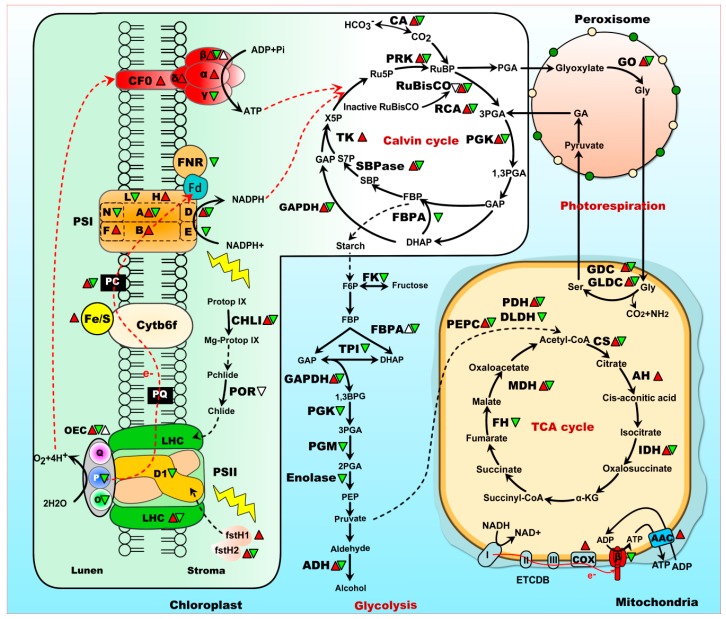
Figure 2.
Schematic representation of heat-responsive photosynthesis and carbon metabolism revealed from proteomics. Red triangle and green triangle represent heat-increased protein abundance and heat-decreased protein abundance, respectively. White regular triangle and white inverted triangles represent heat-increased phosphorylation level and heat-decreased phosphorylation level, respectively. The solid line indicates single-step reactions, and the dashed line indicates multi-step reactions. ADH, alcohol dehydrogenase; AH, aconitate hydratase; AMY, β-amylase; CA, carbonic anhydrase; CHLI, magnesium-chelatase subunit; COX, cytochrome c oxidase assembly protein; Cytb6f, cytochrome b6/f complex; D1, photosystem II reaction-center D1 protein; DLDH, dihydrolipoyl dehydrogenase; FBPA, fructose-bisphosphate aldolase; Fe/S, Rieske Fe/S protein of cytochrome b6/f complex; FK, fructokinase; FNR, ferredoxin—NADP reductase; FPK, fructose-6-phosphate-2-kinase; fstH, filamentation temperature-sensitive H; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GDC, glycine decarboxylase; GLDC, glycine dehydrogenase; GO, glycolate oxidase; IDH, isocitrate dehydrogenase; LHC, chlorophyll a/b binding protein; MDH, malate dehydrogenase; NDUFV, NADH2 dehydrogenase; OEC, oxygen-evolving enhancer protein; PDH, pyruvate dehydrogenase; PEP, phosphoenolpyruvate carboxylase; PGK, phosphoglycerate kinase; PGM, phosphoglycerate mutase; PK, pyruvate kinase; PRK, phosphoribulokinase; PSI, photosystem I; PSII, photosystem II; RCA, ribulose-1,5-bisphosphate carboxylase/oxygenase activase; Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; SBPase, sedoheptulose-1,7-bisphosphatase; SHMT, serine hydroxymethyltransferase; SUS, sucrose synthase; TPI, triosephosphate isomerase; UPGase, UDP-glucose pyrophosphorylase.
Heat inactivation of PSII is mainly due to the dissociation of oxygen-evolving complex (OEC) [6]. OEC is involved in the photo-oxidation of water during the light reactions of photosynthesis. It is more sensitive and easy to be inhibited than the reaction center of PSII, when plants were exposed to heat stress even for a short time. In proteomics studies, the two most important members of OEC, oxygen-evolving enhancer protein 1 (OEC1) and OEC2, normally showed a decrease in some plant species, such as G. max [26], A. americana [19], M. sinensis [32], O. sativa [35], Agrostis sp. [21], R. sativus [44], and C. spinarum [25], which was supposed to be the main reason of the inhibition of photosynthesis. Additionally, two OEC subunits, PsbP and PsbO, also significantly decreased in O. sativa when exposed to heat (e.g., 35 °C, 40 °C, and 45 °C) treatment for 48 h [35], which may contribute to the decreased abundance of OEC [80,82,83]. In addition, an isoform of OEC1 with significantly heat-induced phosphorylation level at Ser-3 site was found in leaves of Z. mays [51]. Previous studies suggest that phosphorylation partially suppressed the release of three OEC subunits, PsbO, PsbP and PsbQ (17KD), from PSII membranes under light stress [84]. Thus, we suppose that the increased OEC1 phosphorylation would alleviate the damage of OEC1, but its regulatory mechanism remains to be investigated.
To coping with heat stress, plants have developed a sophisticated mechanism to repair PSII damage [10]. PSII reaction-center D1 protein turnover plays a key role in the PSII repair cycle. Chloroplastic degradation (Deg) proteases, filamentation temperature-sensitive H (fstH) proteases and other proteases participate in the proteolysis of D1 protein [85,86]. Proteomics studies found an increased abundance of cysteine proteinase in leaves of A. americana [19], and fstH1 protease in Hordenm spontaneu [29] and Hordenm vulgare [30] when exposed to heat stress (Figure 2 and Table S1). Moreover, fstH2 was increased in heat-tolerant T. aestivum cultivar but decreased in heat-sensitive one [49], implying fstH2 would be an interesting target for crop breeding. In A. thaliana, fstH11 has been suggested to have a direct role in thermotolerance, which can protect the photosynthesis apparatus from heat stress [87].
It has been reported that PSI activity is much more heat stable than PSII. However, several PSI subunits (e.g., PsaA, PsaD, PsaE, and PsaN) were also generally heat-decreased in A. americana, A. thaliana, A. stolonifera, and A. scabra, respectively [19,21,22](Figure 2). The abundances of PsaB were increased in 55 °C heat-treated leaf chloroplasts of A. americana, but its transcript level was induced at 45 °C then suddenly decreased at 55 °C and 65 °C [19]. PsaA and PsaB are both involved in binding electron transport cofactors P700. PsaD and PsaE provide the docking sites for soluble electron transporter ferredoxin on the stromal side of the thylakoid membrane. Their decrease in abundance implies that the energy transfer in PSI is inhibited under heat stress. However, because PSI activity is difficult to assay [88], the detailed function of these proteins in PSI in response to heat stress is still unknown.
5. CO2 Fixation Is Inhibited Under Heat Stress
Heat-inhibition of photosynthetic CO2 fixation has been documented in many studies. The heat reduction of CO2 fixation is due to low production of ATP and NADPH from the light reactions, as well as decreased intercellular CO2 concentration under heat stress. Both of these in turn significantly affect the activities of key photosynthetic enzymes, such as carbonic anhydrase (CA), RuBisCO, RuBisCO activase (RCA), and phosphoribulokinase.
CA is a zinc-containing metalloenzyme, which catalyzes the reversible hydration of CO2 and functions in CO2 exchange by influencing the internal stomatal conductance [89]. Under heat stress, five isoforms of CA were decreased in G. max cv Enrei under 40 °C for 12 h [26]. However, CAs was heat-increased in heat-tolerant G. max variety, but decreased in heat-sensitive one when expose to 42 °C heat stress for six days [27]. In addition, four isoforms of CA in Agrostis sp. were heat-increased [21]. These suggest that CAs may play important roles in various plant species/cultivars with different tolerance abilities. Moreover, functional analyses have shown that overexpressing O. sativa CA gene significantly improved the heat tolerance of E. coli recombinant [90], which reinforces the functional evidence for the potential role of CA in heat tolerance.
Proteomic studies revealed that some isoforms of the RuBisCO large subunits and small subunits were decreased in several plants under heat stress [25,26,49] (Table 2 and Table S1). Moreover, heat stress also induced dephosphorylation of RuBisCO [40], which is suspected to decline RuBisCO activity [68]. RuBisCO activity is regulated by RCA. RCA functions as maintenance and acclimatization of photosynthetic CO2 fixation, increases the photosynthetic rate during heat stress [31]. RCA is easy to be dissociated by heat stress, which causes a reduction in the photosynthetic capacity [91]. Proteomic studies revealed the abundances of RCAs in V. vinifera and Agrostis sp. were significant decreased [21,50], which in is in accordance with the inhibition of the activities of RCAs [92,93]. Interestingly, several isoforms of RCA were heat-increased in O. sativa [35], C. spinarum [25], and T. aestivum [45,49]. Most plants have two forms (α- and β-isoform) of RCA which results from alternative splicing of one RCA pre-mRNA. Both α- and β-isoform are capable of promoting RuBisCO activation but they differ markedly in their enzyme activity and sensitivity to thermal denaturation [94,95]. For instance, the heat-induced RCA α-isoform in O. sativa plays an important role in photosynthetic acclimation to moderate heat stress, while RCA small isoform (β-isoform) plays a major role in maintaining RuBisCO initial activity under normal conditions [94].
Moreover, several enzymes involved in the regeneration of ribulose bisphosphate (RuBP) were widely decreased under heat stress, such as phosphoribulokinase (PRK) in O. sativa, M. sinensis, A. stolonifera, and A. scabra, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) A/B subunits in A. stolonifera and A. scabra, fructose-bisphosphate aldolase (FBPA) in G. max and M. sinensis, as well as sedoheptulose-1,7-bisphosphatase (SBPase) in G. max and M. sinensis [26,32,34,49]. These enzymes play key roles in carbon flux in Calvin cycle, determining carbon assimilation and photosynthesis rate [96]. The heat-decreased abundances of these enzymes imply carbon fixation or assimilation is highly disturbed in plants under heat stress. Among these enzymes, SBPase is a major control point in the C3 cycle [91], and it is possible to increase photosynthesis carbon fixation by increasing the level of SBPase under heat stress conditions. The decrease of SBPase in transgenic tobacco resulted in the decrease of photosynthetic capacity [97], while overexpression of SBPase enhanced the photosynthesis in transgenic O. sativa plants under heat stress [98]. An isoform of SBPase in heat-tolerant plant O. meridionalis consistently increased in protein abundance, while its gene expression was declined under heat stress [33], indicating SBPase may be a transient response to protect the photosynthetic machinery during heat stress. The similar observation also found in another two photosynthesis-related enzymes (i.e., phosphoglycerate kinase and PRK) in O. meridionalis [33], which indicated that the heat-responsive function was regulated at both transcript and translational levels. Besides, GAPDH is a key enzyme for the conversion of glycerate-3-phosphate to glyceraldehyde-3-phosphate interacting with ATP and NADPH, where glycerate-3-phosphate can accept electrons from NADPH, preventing the ROS-induced deceleration of PSII repair. In proteomics studies, a heat-increased chloroplastic GAPDH A subunit was found in tolerant T. aestivum cultivar under 35 °C/26 °C (day/night) heat treatment for five days, which was in contrast to that in sensitive T. aestivum cultivar [49]. In A. thaliana, overexpressing ThGAPB exhibited higher recycling rates of ADP and NADP+, which helps to maintain photosynthetic efficiency by reducing ROS production under stress conditions [99]. In addition, several isoforms of chloroplast transketolase (TK) in O. meridionalis and O. sativa were generally increased in heat-stressed leaves, which would facilitate to recover the activity of RuBisCO to cope with heat stress [34].
Additionally, C4-specific pyruvate orthophosphate dikinase (C4-PPDK) was found increased during heat stress and heat recovery processes in leaves of C4 M. sinensis [32]. C4-PPDK catalyzes the formation of phosphoenol pyruvate, the initial acceptor of CO2 in the C4 photosynthetic pathway. The increased abundance of C4-PPDK plays an important role in enhancing the photosynthesis of C4 plant to cope with heat stress [100]. In heat stressed Z. mays, the phosphorylation level of phosphoenolpyruvate carboxykinase (PEPCK) was decreased, whereas its protein abundance did not change [51]. The PEPCK involved in C4 photosynthesis catalyzes the release of CO2 from oxaloacetate for Calvin cycle. The phosphorylation of PEPCK may contribute to the coordinate regulation of the activity of PEPCK [101,102], which performs notably the initial fixation of CO2 in the photosynthetic carbon metabolism of C4 plants. Transferring the C4 enzymes to C3 plants is often thought as a useful strategy to improve the photosynthetic performance of C3 plants. Some C4 enzymes encoding genes, such as C4-PPDK [103], C4-PEPC [104], and C4-SBPase [105], have been conducted on generating transgenic plant to improving C3 photosynthetic performance under abiotic stresses. However, now more and more researchers found that transferring single C4 enzyme to improve photosynthetic capacity is limited. Generating transgenic plant with multiple C4 genes or other photosynthesis-related genes should be considered for enhance of plant tolerance.
Heat stress not only affects carbon fixation, but also adjusts respiratory carbon metabolism in leaves. Among the 74 heat-responsive proteins involved in various carbon metabolism pathways (e.g., glycolysis, pentose phosphate pathway and TCA cycle) from ten plant species, 56 protein species were heat-decreased, while 18 protein species were heat-increased (Table 2 and Table S1). This implies that respiratory carbon metabolism is significantly inhibited in plants under heat stress. However, the heat response of glycolysis and TCA cycle were various in different plant species. For example, most proteins were decreased in Agrostis sp., implying carbon metabolism was heat-inhibited. GAPDHs were generally decreased in heat-sensitive cultivars, but increased in heat-tolerant ones. In addition, four enzymes (i.e., GAPDH, malate dehydrogenase, pyruvate dehydrogenase and transketolase) were generally heat-increased in leaves of O. sativa [34,35], but four enzymes in TCA cycle (i.e., dihydrolipoyl dehydrogenase, aconitate hydratase, malate dehydrogenase, and citrate synthase) were heat-decreased in V. vinifera leaves [48]. This is consistent with what was found in transcriptome analysis, i.e., that the glycolysis-related genes (e.g., phosphofructokinase and pyruvate decarboxylase) were up-regulated in rice leaves, but most TCA cycle-related genes were down-regulated [106].
6. Photorespiration Is Enhanced to Cope with Heat Stress
Photorespiration can protect photosynthesis from photoinhibition and prevent ROS accumulation in green tissues [82], and is believed to play various roles in plants stress resistance amino acid metabolism and signal transduction. In proteomics results, a key photorespiration-related protein, glycolate oxidase (GO), catalyzing the oxidation of glycolate to glyoxylate, was increased in P. oleracea [42] but decreased in leaves of V. vinifera [50] under heat stress. It is known that P. oleracea is a typical C4 plant which has higher heat tolerance and excellent photosynthetic capability, wherase V. vinifera is a C3 plant. The different GO abundances under heat stress between the two species may be related to their different photosynthetic capability. Previous studies have shown that rice photosynthesis and growth was positively correlated with GO activity [107]. In O. sativa, overexpression of GO significantly improved photosynthesis under high light and heat stress [107], whereas, suppression of GO inhibited photosynthesis through deactivating RuBisCO in rice [108]. All these results imply that increase of GO facilitates maintaining photosynthetic activity, which could be taken as a candidate gene for improving plant photosynthesis.
In addition, glycine decarboxylase (GDC) in T. aestivum [49] and glycine dehydrogenases (GLDC) in O. sativa [35] and O. meridionalis [33] were also heat-responsive (Figure 2). GDC is important in maintaining electron flow in order to prevent photoinhibition under stress conditions. As revealed by proteomics studies, GDC was increased in heat-tolerant genotype of T. aestivum, but decreased in heat-sensitive genotypes [49]. Similarly, GLDCs were increased in leaves of heat tolerant O. meridionalis, but decreased in rice. This suggests that proteins involved in photorespiration were more stable in heat-tolerant cultivars/species, which could be necessary for leaves to cope with heat stress. The glycine cleavage system H protein in rice leaves was also increased [35]. Overexpression of glycine decarboxylase H could considerably enhance the net-photosynthesis and growth of A. thaliana [109]. Besides, chloroplastic glutamine synthetase precursor was significantly increased in rice seedlings under 40 °C and 45 °C treatments. Chloroplastic glutamine synthetase is also a key enzyme involved in photorespiration, which catalyzes the ATP-dependent condensation of ammonia with glutamate to yield glutamine. The increases of these photorespiration-related proteins indicated that plant was partly protected from the photo-oxidation damage when exposed to heat stress.
7. Energy Supply Is Essential for Heat Tolerance
As revealed by proteomics studies, some heat-responsive proteins involved in energy metabolism were altered by heat stress (Table 2 and Table S1). The abundances of α, β, γ, and δ subunits of ATP synthase were heat-changed in plant species/genotypes (Figure 2). For example, ATP synthase γ subunit showed significantly decrease in leaves of Agrostis sp. and A. thaliana, while ATP synthase α subunit (in T. aestivum) and β subunit (in T. aestivum and B. oleracea) were increased in heat-tolerant plant genotypes but decreased in heat-sensitive genotypes [24,49]. The ATP synthase γ subunit is believed to be important in regulating ATP synthase activity and the flow of protons through the CF0 complex. The decreased ATP synthase γ subunit indicated the reduction of ATP synthesis in these plants under heat stress. The changes in abundances of these ATP synthase subunits exposed to heat stress indicated that ATP synthase is important in maintaining the energy metabolism under heat stress conditions. In heat-stressed rice leaves, ATP synthase activity were reduced, along with an decrease abundance of ATP synthase β subunit but an increased abundance of α subunit [34]. Moreover, an increased phosphorylation level of ATPase β subunit was found in heat-stressed rice leaves [40]. It has been reported that phosphorylation reduced the activity of ATP synthase [110]. The CF1 α subunit is the largest subunit of the ATP synthase, which displays an organizing function in the assembly of the multi-subunits of ATP synthase upon thermal denaturation [50]. Interestingly, all the identified ATP synthase subunits (i.e., β, γ, and δ subunit of CF0) in heat-stressed V. vinifera were induced under heat stress, and all of them recovered to their control levels after subsequent recovery [50]. The increase of these subunits may be a strategy for V. vinifera to adaption and recovery from heat stress. Besides, the abundance of ATP synthase δ subunit was significantly increased in heat-tolerant C. spinarum [25]. All these indicated that maintenance of ATPase abundance and activity is crucial for energy supply in heat-tolerant genotypes.
The electron transport in photosynthetic process was also significantly affected by heat stress. Ferredoxin-NADP(H) oxidoreductase (FNR), a key enzyme in photosynthetic electron transport, which catalyzes the last enzymatic step of the noncyclic photosynthetic light reaction responsible for the reduction of NADP+ in the PSI complex, were found decreased in heat-stressed leave of G. max [26] and V. vinifera [50]. In addition, FNR exhibited both heat-reduced abundance and gene expression in leaves of O. meridionalis [33], implying photosynthetic electron transport was highly inhibited by heat stress. Another photosynthetic electron transport related protein, plastocyanin, was increased in leaves of M. sativa under short-term (24 h and 48 h) heat stress, and then decreased under long-term (72 h) heat stress [31]. However, Rieske Fe/S protein of cytochrome b6/f complex was heat-increased in leaves of P. ternata [41]. The Rieske Fe/S protein of the cytochrome b6/f complex is an indispensable component of photosynthetic electron transport chain in chloroplasts [111]. All these indicate that heat tolerance in plants is a cost-intensive process and needs considerable cellular energy to cope with adversaries of heat [112].
8. Conclusions and Perspectives
Heat-responsive signaling and photosynthetic modulation are fine-tuned and critical pathways for plant tolerance. Previous transcriptomics and molecular genetics studies have identified and characterized some heat-responsive genes underlying the signaling and photosynthetic mechanisms. Current proteomics provided more protein abundance information for interpreting the alteration of these pathways, such as heat signal transduction, PSII repair, electron transport, PSI activity, CO2 fixation, ATP synthesis, and photorespiration. Moreover, the heat-responsive phosphorylation levels of some important proteins (e.g., POR, OEC, RuBisCO and PEPCK) implied that post translational modification (PTM) is crucial during the processes of plant heat tolerance. Importantly, some representative heat-responsive proteins (e.g., HSP, ftsH11, MYB, WRKY, and SBPase) have potential to improve heat stress tolerance in plants, although most of the heat-responsive proteins identified from proteomics approaches still need to be characterized by molecular genetics. In addition, most of the protein information is generated from 2DE gel-based proteomics approach, the throughput and sensitivity of which are much lower than those of gel-free approaches such as iTRAQ or tandem mass tag (TMT) label and label-free methods. The limitation of protein separation and label method probably resulted in most low abundant proteins, such as signal molecules, transcription factors, thylakoid membrane protein, and kinase, were missed in identification. Therefore, the high-throughput labeling proteomic and PTM analysis (e.g., S-nitrosylation, glycosylation and ubiquitination) would facilitate by providing more information for heat-response signaling and photosynthetic networks in plants [113].
Acknowledgments
This work was supported by the Natural Science Foundation of Shanghai (No. 15ZR1431300), National Natural Science Foundation of China (No. 31601744), Capacity Construction Project of Local Universities, Shanghai (No. 14390502700), Project of Prospering Agriculture with Science and Technology, Shanghai, China (2015, No. 9), and Development and Collaborative Innovation Center of Shanghai (No. ZF1205).
Abbreviations
| 2DE | Two-dimensional electrophoretic |
| BRI1-KD | Brassinosteroid-insensitive I-kinase domain |
| C4-PPDK | C4-specific pyruvate orthophosphate dikinase |
| CA | Carbonic anhydrase |
| CDPK | Calcium-dependent protein kinases |
| Chl | Chlorophyll |
| DIGE | Two-dimensional fluorescence difference in gel |
| ESI-Q-TOF | Electrospray ionization quadrupole time-of-flight |
| fstH | Filamentation temperature-sensitive H |
| FBPA | Fructose-bisphosphate aldolase |
| G protein | GTP-binding protein |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GDC | Glycine decarboxylase |
| GLDC | Glycine dehydrogenases |
| GO | Glycolate oxidase |
| HSF | Heat shock factor |
| HSPs | Heat shock proteins |
| iTRAQ | Isobaric tags for relative and absolute quantitation |
| LHC | Light-harvesting chlorophyll a/b binding protein |
| MALDI | Matrix-assisted laser desorption/ionization |
| MAPK | Mitogen-activated protein kinase |
| MAPK | Mitogen-activated protein kinase |
| NDPK | Nucleoside diphosphate kinase |
| OEC | Oxygen-evolving complex |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PLC | Phospholipase C |
| POR | Protochlorophyllide reductase |
| PS | Photosystem |
| ROS | Reactive oxygen species |
| RCA | Rubisco activase |
| RuBisCO | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| RuBP | Ribulose bisphosphate |
| SBPase | Sedoheptulose-1,7-bisphosphatase |
| TK | Chloroplast transketolase |
| TOF | Time-of-flight |
| TOF/TOF | Tandem time-of-flight |
| WOC | Water oxidizing complex |
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/10/2191/s1.
Author Contributions
Xiaoli Wang and Shaojun Dai designed this work and collected the data. Xiaoli Wang and Shaojun Dai wrote the manuscript. Xiaofeng Cai, Chenxi Xu, and Quanhua Wang collected the data and helped edit the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bita C.E., Gerats T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013;4 doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasanuzzaman M., Nahar K., Alam M.M., Roychowdhury R., Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013;14:9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long S.P., Ort D.R. More than taking the heat: Crops and global change. Curr. Opin. Plant Biol. 2010;13:241–248. doi: 10.1016/j.pbi.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Lobell D.B., Burke M., Tebaldi C., Mastrandrea M.D., Falcon W.P., Naylor R.L. Prioritizing climate change adaptation needs for food security in 2030. Science. 2008;319:607–610. doi: 10.1126/science.1152339. [DOI] [PubMed] [Google Scholar]
- 5.Jones P.D., New M., Parker D.E., Martin S., Rigor I.G. Surface air temperature and its changes over the past 150 years. Rev. Geophys. 1999;37:173–199. doi: 10.1029/1999RG900002. [DOI] [Google Scholar]
- 6.Mathur S., Agrawal D., Jajoo A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B. 2014;137:116–126. doi: 10.1016/j.jphotobiol.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Ashraf M., Harris P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica. 2013;51:163–190. doi: 10.1007/s11099-013-0021-6. [DOI] [Google Scholar]
- 8.Ashraf M. Some important physiological selection criteria for salt tolerance in plants. Flora. 2004;199:361–376. doi: 10.1078/0367-2530-00165. [DOI] [Google Scholar]
- 9.Nouri M.-Z., Moumeni A., Komatsu S. Abiotic stresses: Insight into gene regulation and protein expression in photosynthetic pathways of plants. Int. J. Mol. Sci. 2015;16:20392–20416. doi: 10.3390/ijms160920392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gururani M.A., Venkatesh J., Tran L.-S.P. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant. 2015;8:1304–1320. doi: 10.1016/j.molp.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Qu A.-L., Ding Y.-F., Jiang Q., Zhu C. Molecular mechanisms of the plant heat stress response. Biochem. Biophys. Res. Commun. 2013;432:203–207. doi: 10.1016/j.bbrc.2013.01.104. [DOI] [PubMed] [Google Scholar]
- 12.Johnova P., Skalak J., Saiz-Fernandez I., Brzobohaty B. Plant responses to ambient temperature fluctuations and water-limiting conditions: A proteome-wide perspective. BBA-Proteins Proteom. 2016;1864:916–931. doi: 10.1016/j.bbapap.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Grover A., Mittal D., Negi M., Lavania D. Generating high temperature tolerant transgenic plants: Achievements and challenges. Plant Sci. 2013;205:38–47. doi: 10.1016/j.plantsci.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Zou J., Liu C.F., Chen X.B. Proteomics of rice in response to heat stress and advances in genetic engineering for heat tolerance in rice. Plant Cell Rep. 2011;30:2155–2165. doi: 10.1007/s00299-011-1122-y. [DOI] [PubMed] [Google Scholar]
- 15.Huang B.R., Xu C.P. Identification and characterization of proteins associated with plant tolerance to heat stress. J. Integr. Plant Biol. 2008;50:1230–1237. doi: 10.1111/j.1744-7909.2008.00735.x. [DOI] [PubMed] [Google Scholar]
- 16.Ohama N., Sato H., Shinozaki K., Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017;22:53–65. doi: 10.1016/j.tplants.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Abreu I.A., Farinha A.P., Negrao S., Goncalves N., Fonseca C., Rodrigues M., Batista R., Saibo N.J.M., Oliveira M.M. Coping with abiotic stress: Proteome changes for crop improvement. J. Proteom. 2013;93:145–168. doi: 10.1016/j.jprot.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Dai S., Chen S. Understanding information processes at the proteomics level. In: Kasabov N., editor. Springer Handbook of Bio-/Neuroinformatics. Springer; Berlin/Heidelberg, Germany: 2014. pp. 57–72. [Google Scholar]
- 19.Shakeel S.N., Aman S., Haq N.N., Heckathorn S.A., Luthe D. Proteomic and transcriptomic analyses of Agave americana in response to heat stress. Plant Mol. Biol. Rep. 2013;31:840–851. doi: 10.1007/s11105-013-0555-6. [DOI] [Google Scholar]
- 20.Xu C.P., Huang B.R. Root proteomic responses to heat stress in two Agrostis grass species contrasting in heat tolerance. J. Exp. Bot. 2008;59:4183–4194. doi: 10.1093/jxb/ern258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu C.P., Huang B.R. Differential proteomic response to heat stress in thermal Agrostis scabra and heat-sensitive Agrostis stolonifera. Physiol. Plant. 2010;139:192–204. doi: 10.1111/j.1399-3054.2010.01357.x. [DOI] [PubMed] [Google Scholar]
- 22.Rocco M., Arena S., Renzone G., Scippa G.S., Lomaglio T., Verrillo F., Scaloni A., Marra M. Proteomic analysis of temperature stress-responsive proteins in Arabidopsis thaliana rosette leaves. Mol. Biosyst. 2013;9:1257–1267. doi: 10.1039/c3mb70137a. [DOI] [PubMed] [Google Scholar]
- 23.Chen L.L., Chen Q.Z., Kong L.Q., Xia F.S., Yan H.F., Zhu Y.Q., Mao P.S. Proteomic and physiological analysis of the response of oat (Avena sativa) seeds to heat stress under different moisture conditions. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin H.H., Lin K.H., Chen S.C., Shen Y.H., Lo H.F. Proteomic analysis of broccoli (Brassica oleracea) under high temperature and waterlogging stresses. Bot. Stud. 2015;56 doi: 10.1186/s40529-015-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M.H., Li G.W., Huang W., Bi T., Chen G.Y., Tang Z.C., Su W.A., Sun W.N. Proteomic study of Carissa spinarum in response to combined heat and drought stress. Proteomics. 2010;10:3117–3129. doi: 10.1002/pmic.200900637. [DOI] [PubMed] [Google Scholar]
- 26.Ahsan N., Donnart T., Nouri M.Z., Komatsu S. Tissue-specific defense and thermo-adaptive mechanisms of soybean seedlings under heat stress revealed by proteomic approach. J. Proteome Res. 2010;9:4189–4204. doi: 10.1021/pr100504j. [DOI] [PubMed] [Google Scholar]
- 27.Das A., Eldakak M., Paudel B., Kim D.-W., Hemmati H., Basu C., Rohila J.S. Leaf proteome analysis reveals prospective drought and heat stress response mechanisms in soybean. BioMed Res. Int. 2016;2016:6021047. doi: 10.1155/2016/6021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L., Ma H., Song L., Shu Y., Gu W. Comparative proteomics analysis reveals the mechanism of pre-harvest seed deterioration of soybean under high temperature and humidity stress. J. Proteom. 2012;75:2109–2127. doi: 10.1016/j.jprot.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Ashoub A., Baeumlisberger M., Neupaertl M., Karas M., Bruggemann W. Characterization of common and distinctive adjustments of wild barley leaf proteome under drought acclimation, heat stress and their combination. Plant Mol. Biol. Rep. 2015;87:459–471. doi: 10.1007/s11103-015-0291-4. [DOI] [PubMed] [Google Scholar]
- 30.Rollins J.A., Habte E., Templer S.E., Colby T., Schmidt J., von Korff M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.) J. Exp. Bot. 2013;64:3201–3212. doi: 10.1093/jxb/ert158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W., Wei Z., Qiao Z., Wu Z., Cheng L., Wang Y. Proteomics analysis of alfalfa response to heat stress. PLoS ONE. 2013;8:e82725. doi: 10.1371/journal.pone.0082725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharmin S.A., Alam I., Rahman M.A., Kim K.H., Kim Y.G., Lee B.H. Mapping the leaf proteome of Miscanthus sinensis and its application to the identification of heat-responsive proteins. Planta. 2013;238:459–474. doi: 10.1007/s00425-013-1900-6. [DOI] [PubMed] [Google Scholar]
- 33.Scafaro A.P., Haynes P.A., Atwell B.J. Physiological and molecular changes in Oryza meridionalis Ng., a heat-tolerant species of wild rice. J. Exp. Bot. 2010;61:191–202. doi: 10.1093/jxb/erp294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee D., Ahsan N., Lee S., Kang K.Y., Bahk J.D., Lee I., Lee B. A proteomic approach in analyzing heat-responsive proteins in rice leaves. Proteomics. 2007;7:3369–3383. doi: 10.1002/pmic.200700266. [DOI] [PubMed] [Google Scholar]
- 35.Han F., Chen H., Li X., Yang M., Liu G., Shen S. A comparative proteomic analysis of rice seedlings under various high-temperature stresses. Biochim. Biophys. Acta. 2009;1794:1625–1634. doi: 10.1016/j.bbapap.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Das S., Krishnan P., Mishra V., Kumar R., Ramakrishnan B., Singh N.K. Proteomic changes in rice leaves grown under open field high temperature stress conditions. Mol. Biol. Rep. 2015;42:1545–1558. doi: 10.1007/s11033-015-3923-5. [DOI] [PubMed] [Google Scholar]
- 37.Shi W.J., Muthurajan R., Rahman H., Selvam J., Peng S.B., Zou Y.B., Jagadish K.S.V. Source-sink dynamics and proteomic reprogramming under elevated night temperature and their impact on rice yield and grain quality. New Phytol. 2013;197:825–837. doi: 10.1111/nph.12088. [DOI] [PubMed] [Google Scholar]
- 38.Gammulla C.G., Pascovici D., Atwell B.J., Haynes P.A. Differential metabolic response of cultured rice (Oryza sativa) cells exposed to high- and low-temperature stress. Proteomics. 2010;10:3001–3019. doi: 10.1002/pmic.201000054. [DOI] [PubMed] [Google Scholar]
- 39.Timabud T., Yin X., Pongdontri P., Komatsu S. Gel-free/label-free proteomic analysis of developing rice grains under heat stress. J. Proteom. 2016;133:1–19. doi: 10.1016/j.jprot.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Chen X., Zhang W., Zhang B., Zhou J., Wang Y., Yang Q., Ke Y., He H. Phosphoproteins regulated by heat stress in rice leaves. Proteome Sci. 2011;9 doi: 10.1186/1477-5956-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y.H., Zhu G.S., Guo Q.S., Zhu Z.B., Wang C.L., Liu Z.Y. A comparative proteomic analysis of Pinellia ternata leaves exposed to heat stress. Int. J. Mol. Sci. 2013;14:20614–20634. doi: 10.3390/ijms141020614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y., Chen J., Liu Q., Ben C., Todd C.D., Shi J., Yang Y., Hu X. Comparative proteomic analysis of the thermotolerant plant Portulaca oleracea acclimation to combined high temperature and humidity stress. J. Proteome Res. 2012;11:3605–3623. doi: 10.1021/pr300027a. [DOI] [PubMed] [Google Scholar]
- 43.Lara M.V., Borsani J., Budde C.O., Lauxmann M.A., Lombardo V.A., Murray R., Andreo C.S., Drincovich M.F. Biochemical and proteomic analysis of Dixiland peach fruit (Prunus persica) upon heat treatment. J. Exp. Bot. 2009;60:4315–4333. doi: 10.1093/jxb/erp267. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y.Y., Xu L., Zhu X.W., Gong Y.Q., Xiang F., Sun X.C., Liu L.W. Proteomic analysis of heat stress response in leaves of radish (Raphanus sativus L.) Plant Mol. Biol. Rep. 2013;31:195–203. doi: 10.1007/s11105-012-0486-7. [DOI] [Google Scholar]
- 45.Majoul-Haddad T., Bancel E., Martre P., Triboi E., Branlard G. Effect of short heat shocks applied during grain development on wheat (Triticum aestivum L.) grain proteome. J. Cereal Sci. 2013;57:486–495. doi: 10.1016/j.jcs.2013.02.003. [DOI] [Google Scholar]
- 46.Majoul T., Bancel E., Triboi E., Hamida J.B., Branlard G. Proteomic analysis of the effect of heat stress on hexaploid wheat grain: Characterization of heat-responsive proteins from total endosperm. Proteomics. 2003;3:175–183. doi: 10.1002/pmic.200390026. [DOI] [PubMed] [Google Scholar]
- 47.Laino P., Shelton D., Finnie C., De Leonardis A.M., Mastrangelo A.M., Svensson B., Lafiandra D., Masci S. Comparative proteome analysis of metabolic proteins from seeds of durum wheat (cv. Svevo) subjected to heat stress. Proteomics. 2010;10:2359–2368. doi: 10.1002/pmic.200900803. [DOI] [PubMed] [Google Scholar]
- 48.Yang F., Jorgensen A.D., Li H., Sondergaard I., Finnie C., Svensson B., Jiang D., Wollenweber B., Jacobsen S. Implications of high-temperature events and water deficits on protein profiles in wheat (Triticum aestivum L. cv. Vinjett) grain. Proteomics. 2011;11:1684–1695. doi: 10.1002/pmic.201000654. [DOI] [PubMed] [Google Scholar]
- 49.Wang X., Dinler B.S., Vignjevic M., Jacobsen S., Wollenweber B. Physiological and proteome studies of responses to heat stress during grain filling in contrasting wheat cultivars. Plant Sci. 2015;230:33–50. doi: 10.1016/j.plantsci.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Liu G.-T., Ma L., Duan W., Wang B.-C., Li J.-H., Xu H.-G., Yan X.-Q., Yan B.-F., Li S.-H., Wang L.-J. Differential proteomic analysis of grapevine leaves by iTRAQ reveals responses to heat stress and subsequent recovery. BMC Plant Biol. 2014;14 doi: 10.1186/1471-2229-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu X., Wu L., Zhao F., Zhang D., Li N., Zhu G., Li C., Wang W. Phosphoproteomic analysis of the response of maize leaves to drought, heat and their combination stress. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mittler R., Finka A., Goloubinoff P. How do plants feel the heat. Trends Biochem. Sci. 2012;37:118–125. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Goraya G.K., Kaur B., Asthir B., Bala S., Kaur G., Farooq M. Rapid injuries of high temperature in plants. J. Plant Biol. 2017;60:298–305. doi: 10.1007/s12374-016-0365-0. [DOI] [Google Scholar]
- 54.Graff J.M., Young T.N., Johnson J.D., Blackshear P.J. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J. Biol. Chem. 1989;264:21818–21823. [PubMed] [Google Scholar]
- 55.Jami S.K., Clark G.B., Ayele B.T., Ashe P., Kirti P.B. Genome-wide comparative analysis of annexin superfamily in plants. PLoS ONE. 2012;7:e47801. doi: 10.1371/journal.pone.0047801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davies J.M. Annexin-mediated calcium signalling in plants. Plants. 2014;3:128–140. doi: 10.3390/plants3010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohila J.S., Chen M., Chen S., Chen J., Cerny R.L., Dardick C., Canlas P.E., Xu X., Gribskov M., Kanrar S. Protein–protein interactions of tandem affinity purification-tagged protein kinases in rice. Plant J. 2006;46:1–13. doi: 10.1111/j.1365-313X.2006.02671.x. [DOI] [PubMed] [Google Scholar]
- 58.Kim W.Y., Cheong N.E., Lee D.C., Je D.Y., Bahk J.D., Cho M.J., Lee S.Y. Cloning and sequencing analysis of a full-length cDNA encoding a G protein α subunit, SGA1, from soybean. Plant Physiol. 1995;108:1315–1316. doi: 10.1104/pp.108.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tuteja N. Signaling through G protein coupled receptors. Plant Signal. Behav. 2009;4:942–947. doi: 10.4161/psb.4.10.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng X., Zeng X., Ding X., Li S., Yu C., Zhu Y. Ectopic expression of a vesicle trafficking gene, OsRab7, from Oryza sativa, confers tolerance to several abiotic stresses in Escherichia coli. Afr. J. Biotechnol. 2011;10:6941–6946. [Google Scholar]
- 61.Yadav D.K., Islam S.M.S., Tuteja N. Rice heterotrimeric G-protein gamma subunits (RGG1 and RGG2) are differentially regulated under abiotic stress. Plant Signal. Behav. 2012;7:733–740. doi: 10.4161/psb.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Misra S., Wu Y., Venkataraman G., Sopory S.K., Tuteja N. Heterotrimeric G-protein complex and G-protein-coupled receptor from a legume (Pisum sativum): Role in salinity and heat stress and cross-talk with phospholipase C. Plant J. 2007;51:656–669. doi: 10.1111/j.1365-313X.2007.03169.x. [DOI] [PubMed] [Google Scholar]
- 63.Kang C.H., Lee S.Y., Park J.H., Lee Y., Jung H.S., Chi Y.H., Jung Y.J., Chae H.B., Shin M.R., Kim W.Y., et al. Stress-driven structural and functional switching of Ypt1p from a GTPase to a molecular chaperone mediates thermo tolerance in Saccharomyces cerevisiae. FASEB J. 2015;29:4424–4434. doi: 10.1096/fj.15-270140. [DOI] [PubMed] [Google Scholar]
- 64.Moon H., Lee B., Choi G., Shin D., Prasad D.T., Lee O., Kwak S.-S., Kim D.H., Nam J., Bahk J., et al. NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc. Natl. Acad. Sci. USA. 2003;100:358–363. doi: 10.1073/pnas.252641899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dooki A.D., Mayer-Posner F.J., Askari H., Zaiee A.A., Salekdeh G.H. Proteomic responses of rice young panicles to salinity. Proteomics. 2006;6:6498–6507. doi: 10.1002/pmic.200600367. [DOI] [PubMed] [Google Scholar]
- 66.Maleki M., Naghavi M.R., Alizadeh H., Poostini K., Mishani C.A. Comparison of protein changes in the leaves of two bread wheat cultivars with different sensitivity under salt stress. Annu. Res. Rev. Biol. 2014;4:1784–1797. doi: 10.9734/ARRB/2014/7795. [DOI] [Google Scholar]
- 67.Singh A., Bhatnagar N., Pandey A., Pandey G.K. Plant phospholipase C family: Regulation and functional role in lipid signaling. Cell Calcium. 2015;58:139–146. doi: 10.1016/j.ceca.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Li L., Wang F., Yan P., Jing W., Zhang C., Kudla J., Zhang W. A phosphoinositide-specific phospholipase C pathway elicits stress-induced Ca2+ signals and confers salt tolerance to rice. New Phytol. 2017;214:1172–1187. doi: 10.1111/nph.14426. [DOI] [PubMed] [Google Scholar]
- 69.Wang C.-R., Yang A.-F., Yue G.-D., Gao Q., Yin H.-Y., Zhang J.-R. Enhanced expression of phospholipase C 1 (ZmPLC1) improves drought tolerance in transgenic maize. Planta. 2008;227:1127–1140. doi: 10.1007/s00425-007-0686-9. [DOI] [PubMed] [Google Scholar]
- 70.Krckova Z., Brouzdova J., Danek M., Kocourkova D., Rainteau D., Ruelland E., Valentova O., Pejchar P., Martinec J. Arabidopsis non-specific phospholipase C1: Characterization and its involvement in response to heat stress. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samad A.F.A., Sajad M., Nazaruddin N., Fauzi I.A., Murad A.M.A., Zainal Z., Ismail I. MicroRNA and transcription factor: Key players in plant regulatory network. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El-kereamy A., Bi Y.-M., Ranathunge K., Beatty P.H., Good A.G., Rothstein S.J. The rice R2R3-MYB transcription factor OsMYB55 is involved in the tolerance to high temperature and modulates amino acid metabolism. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0052030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rushton D.L., Tripathi P., Rabara R.C., Lin J., Ringler P., Boken A.K., Langum T.J., Smidt L., Boomsma D.D., Emme N.J., et al. WRKY transcription factors: Key components in abscisic acid signalling. Plant Biotechnol. J. 2012;10:2–11. doi: 10.1111/j.1467-7652.2011.00634.x. [DOI] [PubMed] [Google Scholar]
- 74.Wu X., Shiroto Y., Kishitani S., Ito Y., Toriyama K. Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep. 2009;28:21–30. doi: 10.1007/s00299-008-0614-x. [DOI] [PubMed] [Google Scholar]
- 75.Nakashima K., Yamaguchi-Shinozaki K., Shinozaki K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014;5 doi: 10.3389/fpls.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Efeoglu B., Ekmekci Y., Cicek N. Physiological responses of three maize cultivars to drought stress and recovery. S. Afr. J. Bot. 2009;75:34–42. doi: 10.1016/j.sajb.2008.06.005. [DOI] [Google Scholar]
- 77.Farhad M., Babak A.M., Reza Z.M., Hassan R.M., Afshin T. Response of proline, soluble sugars, photosynthetic pigments and antioxidant enzymes in potato (Solanum tuberosum L.) to different irrigation regimes in greenhouse condition. Aust. J. Crop Sci. 2011;5:55–60. [Google Scholar]
- 78.Mohanty S., Grimm B., Tripathy B.C. Light and dark modulation of chlorophyll biosynthetic genes in response to temperature. Planta. 2006;224:692–699. doi: 10.1007/s00425-006-0248-6. [DOI] [PubMed] [Google Scholar]
- 79.Selstam E., Schelin J., Brain T., Williams W.P. The effects of low pH on the properties of protochlorophyllide oxidoreductase and the organization of prolamellar bodies of maize (Zea mays) FEBS J. 2002;269:2336–2346. doi: 10.1046/j.1432-1033.2002.02897.x. [DOI] [PubMed] [Google Scholar]
- 80.Berry Bjorkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Biol. 2003;31:491–543. doi: 10.1146/annurev.pp.31.060180.002423. [DOI] [Google Scholar]
- 81.Srivastava A., Guisse B., Greppin H., Strasser R.J. Regulation of antenna structure and electron transport in Photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim. Biophys. Acta. 1997;1320:95–106. doi: 10.1016/S0005-2728(97)00017-0. [DOI] [Google Scholar]
- 82.Takahashi S., Bauwe H., Badger M.R. Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiol. 2007;144:487–494. doi: 10.1104/pp.107.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Havaux M. Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ. 1993;16:461–467. doi: 10.1111/j.1365-3040.1993.tb00893.x. [DOI] [Google Scholar]
- 84.Chen L., Jia H., Du L., Tian Q., Gao Y., Liu Y. Release of the oxygen-evolving complex subunits from photosystem II membranes in phosphorylation condition under light stress. Chin. J. Chem. 2011;29:2631–2636. doi: 10.1002/cjoc.201180434. [DOI] [Google Scholar]
- 85.Che Y., Fu A., Hou X., Mcdonald K.L., Buchanan B.B., Huang W., Luan S. C-terminal processing of reaction center protein D1 is essential for the function and assembly of photosystem II in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2013;110:16247–16252. doi: 10.1073/pnas.1313894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schuhmann H., Adamska I. Deg proteases and their role in protein quality control and processing in different subcellular compartments of the plant cell. Physiol. Plant. 2012;145:224–234. doi: 10.1111/j.1399-3054.2011.01533.x. [DOI] [PubMed] [Google Scholar]
- 87.Chen J., Burke J.J., Velten J., Xin Z. FtsH11 protease plays a critical role in Arabidopsis thermotolerance. Plant J. 2006;48:73–84. doi: 10.1111/j.1365-313X.2006.02855.x. [DOI] [PubMed] [Google Scholar]
- 88.Sonoike K. Photoinhibition of photosystem I. Physiol. Plant. 2011;142:56–64. doi: 10.1111/j.1399-3054.2010.01437.x. [DOI] [PubMed] [Google Scholar]
- 89.Majeau N., Arnoldo M., Coleman J.R. Modification of carbonic anhydrase activity by antisense and over-expression constructs in transgenic tobacco. Plant Mol. Biol. 1994;25:377–385. doi: 10.1007/BF00043867. [DOI] [PubMed] [Google Scholar]
- 90.Tianpei X., Mao Z., Zhu Y., Li S. Expression of rice mature carbonic anhydrase gene increase E. coli tolerance to heat stress. Appl. Biochem. Biotechnol. 2015;176:625–635. doi: 10.1007/s12010-015-1600-8. [DOI] [PubMed] [Google Scholar]
- 91.Raines C.A. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: Current and future strategies. Plant Physiol. 2011;155:36–42. doi: 10.1104/pp.110.168559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Craftsbrandner S.J., Salvucci M.E. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002;129:1773–1780. doi: 10.1104/pp.002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salvucci M.E., Craftsbrandner S.J. Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol. 2004;134:1460–1470. doi: 10.1104/pp.103.038323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang D., Li X., Zhou Z., Feng X., Yang W.X., Jiang D. Two Rubisco activase isoforms may play different roles in photosynthetic heat acclimation in the rice plant. Physiol. Plant. 2010;139:55–67. doi: 10.1111/j.1399-3054.2009.01344.x. [DOI] [PubMed] [Google Scholar]
- 95.Kurek I., Chang T.K., Bertain S., Madrigal A., Liu L., Lassner M., Zhu G. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell. 2007;19:3230–3241. doi: 10.1105/tpc.107.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rokka A., Zhang L., Aro E. Rubisco activase: An enzyme with a temperature-dependent dual function? Plant J. 2001;25:463–471. doi: 10.1046/j.1365-313x.2001.00981.x. [DOI] [PubMed] [Google Scholar]
- 97.Harrison E.P., Willingham N.M., Lloyd J.C., Raines C.A. Reduced sedoheptulose-1,7-bisphosphatase levels in transgenic tobacco lead to decreased photosynthetic capacity and altered carbohydrate accumulation. Planta. 1998;204:27–36. doi: 10.1007/s004250050226. [DOI] [Google Scholar]
- 98.Feng L., Wang K., Li Y., Tan Y., Kong J., Li H., Li Y., Zhu Y. Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Rep. 2007;26:1635–1646. doi: 10.1007/s00299-006-0299-y. [DOI] [PubMed] [Google Scholar]
- 99.Chang L., Guo A., Jin X., Yang Q., Wang D., Sun Y., Huang Q., Wang L., Peng C., Wang X. The beta subunit of glyceraldehyde 3-phosphate dehydrogenase is an important factor for maintaining photosynthesis and plant development under salt stress—Based on an integrative analysis of the structural, physiological and proteomic changes in chloroplasts in Thellungiella halophila. Plant Sci. 2015;236:223–238. doi: 10.1016/j.plantsci.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 100.Ishimaru K., Ichikawa H., Matsuoka M., Ohsugi R. Analysis of a C4 maize pyruvate, orthophosphate dikinase expressed in C3 transgenic Arabidopsis plants. Plant Sci. 1997;129:57–64. doi: 10.1016/S0168-9452(97)00154-4. [DOI] [Google Scholar]
- 101.Walker R.P., Paoletti A., Leegood R.C., Famiani F. Phosphorylation of phosphoenolpyruvate carboxykinase (PEPCK) and phosphoenolpyruvate carboxylase (PEPC) in the flesh of fruits. Plant Physiol. Biochem. 2016;108:323–327. doi: 10.1016/j.plaphy.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 102.Monreal J., Feria A.B., Vinardell J.M., Vidal J., Echevarria C., Garciamaurino S. ABA modulates the degradation of phosphoenolpyruvate carboxylase kinase in sorghum leaves. FEBS Lett. 2007;581:3468–3472. doi: 10.1016/j.febslet.2007.06.055. [DOI] [PubMed] [Google Scholar]
- 103.Fukayama H., Tsuchida H., Agarie S., Nomura M., Onodera H., Ono K., Lee B.H., Hirose S., Toki S., Ku M.S., et al. Significant accumulation of C4-specific pyruvate, orthophosphate dikinase in a C3 plant, rice. Plant Physiol. 2001;127:1136–1146. [PMC free article] [PubMed] [Google Scholar]
- 104.Ku M.S.B., Agarie S., Nomura M., Fukayama H., Tsuchida H., Ono K., Hirose S., Toki S., Miyao M., Matsuoka M. High-level expression of maize phosphoenolpyruvate carboxylasein transgenic rice plants. Nat. Biotechnol. 1999;17:76–80. doi: 10.1038/5256. [DOI] [PubMed] [Google Scholar]
- 105.Feng L., Han Y., Liu G., An B., Yang J., Yang G., Li Y., Zhu Y. Overexpression of sedoheptulose-1,7-bisphosphatase enhances photosynthesis and growth under salt stress in transgenic rice plants. Funct. Plant Biol. 2007;34:822–834. doi: 10.1071/FP07074. [DOI] [PubMed] [Google Scholar]
- 106.Zhang X., Rerksiri W., Liu A., Zhou X., Xiong H., Xiang J., Chen X., Xiong X. Transcriptome profile reveals heat response mechanism at molecular and metabolic levels in rice flag leaf. Gene. 2013;530:185–192. doi: 10.1016/j.gene.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 107.Cui L.-L., Lu Y., Li Y., Yang C., Peng X.-X. Overexpression of glycolate oxidase confers improved photosynthesis under high light and high temperature in rice. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.01165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu Y., Li Y., Yang Q., Zhang Z., Chen Y., Zhang S., Peng X. Suppression of glycolate oxidase causes glyoxylate accumulation that inhibits photosynthesis through deactivating Rubisco in rice. Physiol. Plant. 2014;150:463–476. doi: 10.1111/ppl.12104. [DOI] [PubMed] [Google Scholar]
- 109.Timm S., Florian A., Arrivault S., Stitt M., Fernie A.R., Bauwe H. Glycine decarboxylase controls photosynthesis and plant growth. FEBS Lett. 2012;586:3692–3697. doi: 10.1016/j.febslet.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 110.Bunney T.D., van Walraven H.S., de Boer A.H. 14-3-3 protein is a regulator of the mitochondrial and chloroplast ATP synthase. Proc. Natl. Acad. Sci. USA. 2001;98:4249–4254. doi: 10.1073/pnas.061437498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Molik S., Karnauchov I., Weidlich C., Herrmann R.G., Klosgen R.B. The Rieske Fe/S protein of the cytochromeb 6 /f complex in chloroplasts. J. Biol. Chem. 2001;276:42761–42766. doi: 10.1074/jbc.M106690200. [DOI] [PubMed] [Google Scholar]
- 112.Wahid A., Gelani S., Ashraf M., Foolad M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 113.Kumar V., Khare T., Sharma M., Wani S.H. Engineering crops for future: A phosphoproteomics approach. Curr. Protein Pept. Sci. 2017;18 doi: 10.2174/1389203718666170209152222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.